1. Introduction
Skin provides a natural barrier to protect the body against injuries, infections, and fluid loss. The biological and physical characteristics of the skin determine the responsiveness and resistance to stressful environmental factors [
1]. As the outermost layer of the skin and constantly exposed to UV rays from sunlight and other sources, the epidermis is highly susceptible to DNA damage [
2]. However, the innermost layer of the skin, the stratum corneum, offers a potent barrier against substance penetration, which makes effective topical treatments difficult. Currently, researchers are looking for new strategies to improve the performance of drug delivery through the skin. The main challenge is to increase the cutaneous penetration capacity of drugs in the effective concentration to produce tumor cytotoxicity [
3]. For a drug to be effective, especially in the deeper layers of the skin, a higher concentration is required, which may cause adverse reactions.
Many studies have been carried out to improve the drug delivery system through the skin. Drug carrier systems are a rapidly developing area of research with the potential to enhance the efficacy and safety of skin cancer treatment. These systems could be used to deliver drugs directly to cancer cells, which can increase treatment effectiveness and reduce side effects. Drug delivery systems could also used to protect drugs from the effects of the immune system, increasing the effectiveness of treatment. In addition, they control drug release over time, which improves safety.
The nanoformulation is a strategy developed to break this barrier. Pharmacological compounds that are nanoformulated with surfactants, lipids, or other agents can be better targeted more selectively to the deeper layers of the skin. These nanoparticles can cross the skin barrier and deliver drugs directly to target cells. Another technique that attenuates the skin's barrier effect is iontophoresis, which applies a low-intensity galvanic current. This technique can be associated with nanoformulation to enhance the development of penetration of substances through the skin. Photodynamic therapy is a technology that uses thermoactive substances and light at a specific frequency to facilitate the absorption of the drug, increasing skin permeability.
Drug delivery systems can improve the performance of drugs at deep skin level. These systems could allow for the delivery of increasingly effective topical medications for various conditions, including skin cancer treatment. In addition, it is worth noting that in recent years, there has been an increase in interest from pharmaceutical companies and researchers in using natural products in medicines. Several advantages are offered, such as fewer adverse reactions, greater efficiency, greater tolerance, accessibility, and bioavailability compared to synthetic substances.
This review consolidated information from scientific papers on the most studied and published delivery techniques in the last 15 years. Bibliographical research was conducted on Pubmed, Scielo, and Google Scholar database platforms. The main keywords were delivery, carrier, phototherapy, photodynamic therapy, iontophoresis, nanoformulation, nanoemulsion, and nanotechnology.
2. Skin cancer
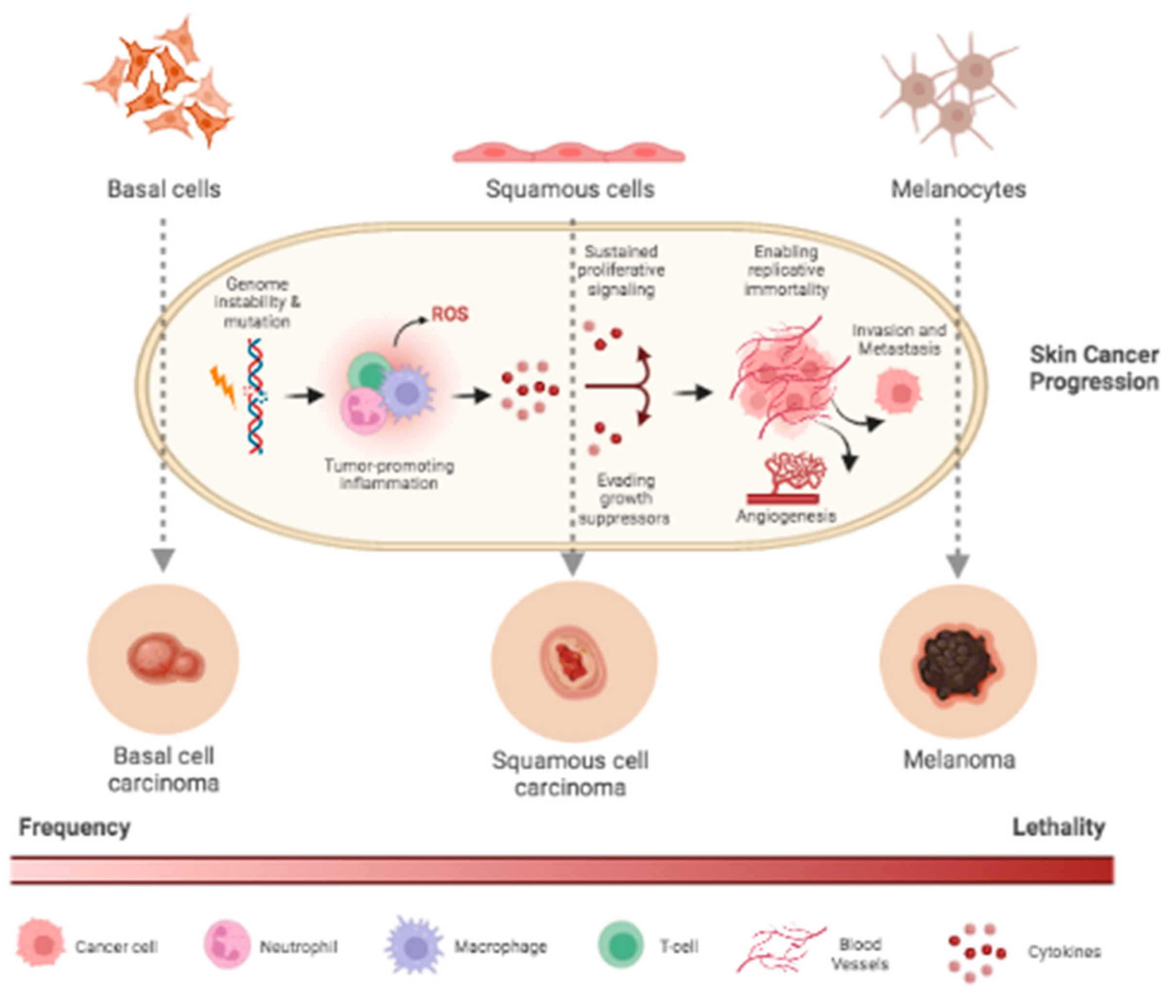
Among cancer diagnoses, one in three is skin cancer. Skin cancer is a multifactorial disease and by far the most common of all cancers worldwide, with increasing frequency over the past three decades. Types of skin cancer include non-melanoma, such as basal cell carcinoma (BCC), squamous cell carcinoma (SCC), and melanoma [
4] (
Figure 1).
There are several etiological attributions for the appearance of cancer exposure with UV rays being the main one. UV radiation is responsible for DNA damage and genetic mutations. Formation of cyclobutane pyrimidine dimers, alterations in p53 tumor suppressor genes, and induction of the immunosuppressive cytokines’ interleukin-1 and tumor necrosis factor-alpha (TNF-α) increase levels of oxidative stress and related inflammatory responses [
6]. All these effects play an essential role in photoaging the skin and increase the initiation and promotion of skin cancer. The risk tends to be greater for residents of places with high solar irradiance closer to the equator and those with greater open air exposure. In addition, those with markers of ultraviolet susceptibility, such as fair skin, eye and hair color, or an inability to tan, and those suffering from benign sun-related skin conditions [
7].
2.1. Melanoma
Melanoma is a highly metastatic, drug-resistant, aggressive, malignant skin cancer that begins in transformed melanocytes. Melanocytes are cells distributed throughout the body in the basal layer of the epidermis with the function of producing melanin. In their normal state, they constitute a population of only 1500 cells per square millimeter [
8] and rarely divide. With the incidence of some stimulating factors, keratinocytes start to produce melanocyte-stimulating hormone α. These cells bind to the melanocortin receptor to induce melanin production, which is transported to the keratinocytes. The melanin accumulated in keratinocytes acts as a barrier to protect their nuclei against mutagenic effects, defending the cells against damage. When keratinocytes mature, a phenomenon called keratinization occurs before they die. Both dead cells and melanin perform a skin-protective barrier process [
9]. Clinically and pathologically, several melanoma subtypes could be distinguished based on genetic differences. Amplification of melanocytes, abnormal growth with presentation of tissue invasion and metastasis, unlimited replicative potential, delay of cellular apoptosis, self-sufficiency of growth factors, insensitivity to growth inhibitors, and sustained angiogenesis are some characteristics presented by melanoma. These coefficients can be stimulated by oncogenic factors or by the suppression of genes to inhibit the tumor. Some alterations in signaling pathways, such as MAPK, PI3K/PTEN/PKB, and MITF, are essential contributors to the pathogenesis of melanoma. The pathogenesis involves the selective growth of cells with favorable mutations, genetic instability, and other factors such as mutagenesis, genetic predisposition, and suppression of the host's immune response [
10].
3. Delivery strategies
3.1. Nanoemulsion
In the outermost layer of the skin, corneocytes and enucleated keratinocytes are the first limiting line of the barrier to the diffusion of substances, playing an essential role in protecting and hydrating the body. However, the transposition of agents through the skin can occur through intercellular permeation, when the compounds pass between the cells of the epidermis; trans follicular, when penetration takes place through the orifice of the hair follicle, and transcellular, when the actives pass through the cells themselves [
14].
Therefore, in a chemotherapy treatment by topical administration, the systemic toxicity is reduced. Consequently, applying a substance on the skin's surface has its effectiveness considerably reduced by encountering the lipophilic layer. Current studies highlight that nanotechnology has excellent potential in biomedicine, such as diagnosis, drug administration, and molecular imaging, offering promising results. Recently, greater attention has been given to nanocarrier technology for the transcutaneous delivery of bioactives, which enhance the penetration of substances through the skin, in which the size of these nanoparticles is a preeminent factor in promoting the transport of drugs through the skin [
15].
Nanoparticles are an engineering that has been extensively explored in research in skin cancer treatment [
16]. Science has made remarkable progress in nano-oncology, with improvements in the bioactivity of chemotherapy drugs for the most varied types of cancer [
17], providing greater efficiency in tumor treatments and reducing the toxicity of traditional systemic treatments.
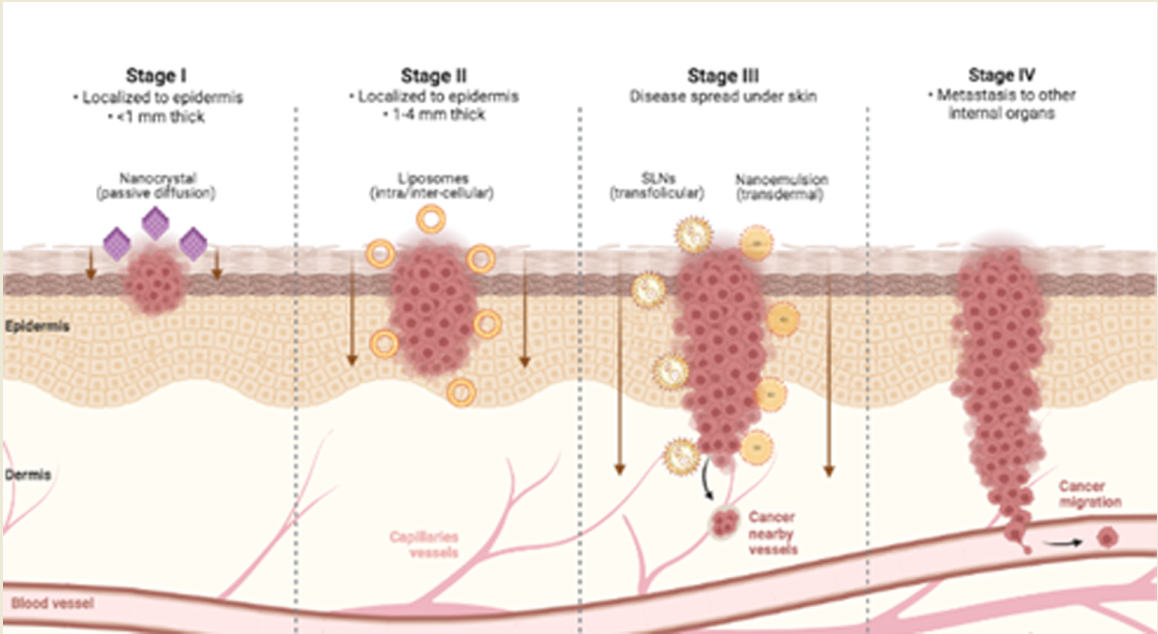
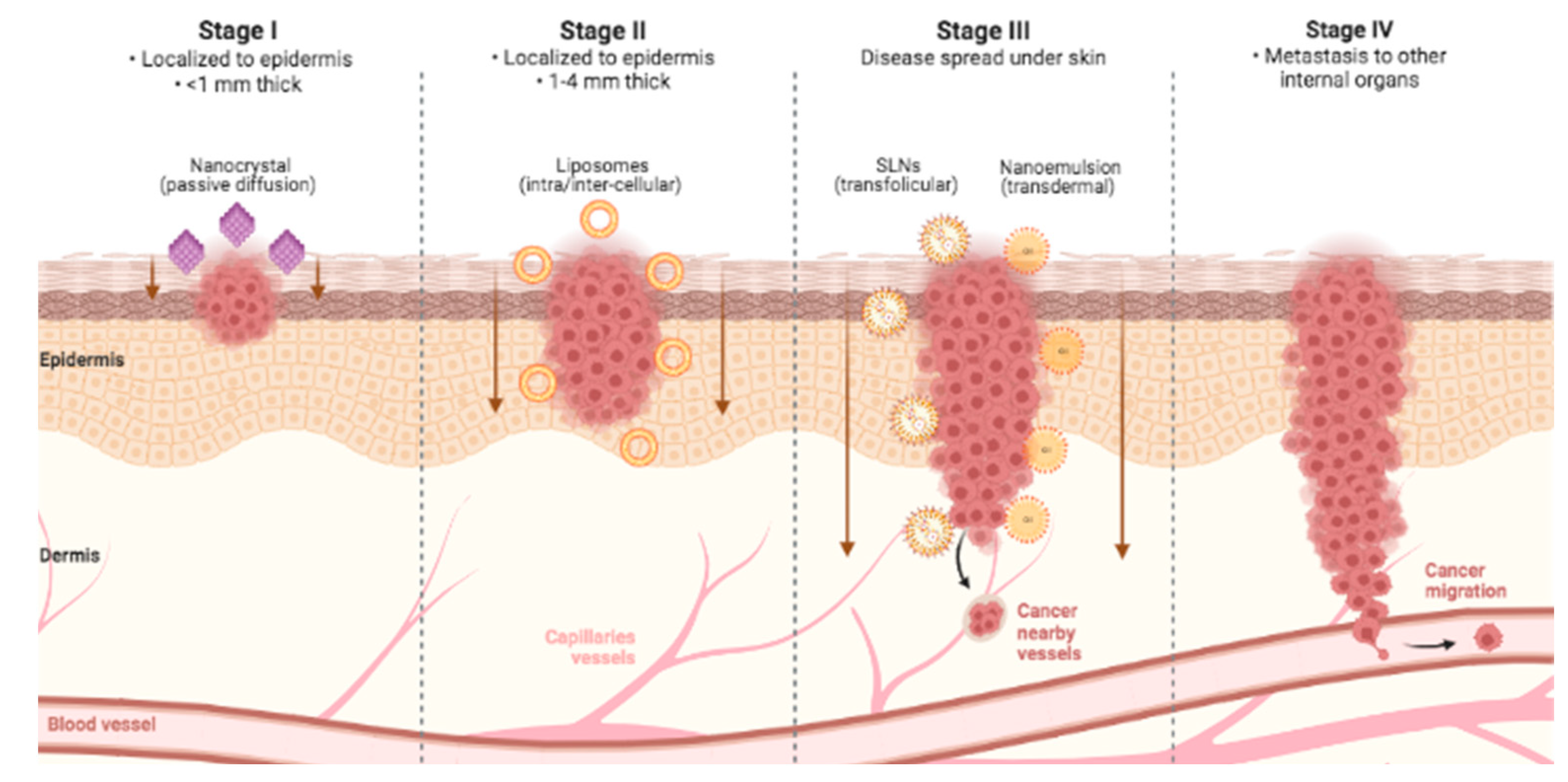
Nanoemulsion is a nanotechnology that transforms droplets of substances on a nanometric scale that can vary in size from 1 to 100 nm to improve the penetration of bioactives through the skin. Its composition has three main parts: water, oil, and surfactant, which act as an emulsifier to reduce the interfacial tension between oil and water [
18], improving the stability and bioavailability of the substance [
19]. Nanoparticles are protected from degradation and can be adapted to deliver to target tissues efficiently, thus characterizing their biospecificity and bioavailability [
20]. This feature provides an important mechanism of action in anticancer treatment [
21]. The overall theme revolves around the ability of nanosystem vehicles to strongly interact with the stratum corneum and manipulate its barrier properties, allowing the particles themselves or the released drugs to navigate through the lipid domains for accumulation and diffusion deeper into the skin (
Figure 2). However, differences in anatomical site, hair follicle density, skin hydration, pH, sebum production, tumor microenvironment, and interindividual responses can alter dermal delivery [
22].
3.2. Iontophoresis
Iontophoresis is a non-invasive technique that uses an electronic device to generate a low-intensity galvanic current. Electrodes are positioned on a particular substance on the skin's surface to provide, in a controlled manner, an increase in the transdermal load capacity [
23]. In applying the iontophoresis technique, two electrodes are used: an anodic electrode, charged with positive charges, and a cationic electrode, charged with a negative charge. One of the electrodes is positioned over an ionized substance, while the other is placed over an electroconductive gel parallel to the paired electrode. During application, the direction of displacement of electrons occurs from the negative pole to the positive pole, so the anodic cathode, which is positively charged, tends to attract negative charges. In this sense, the substance under the electrode must have the same ionic amount. Thus, it will undergo an electro-repulsion process, and, on the other hand, the oppositely charged cathode will promote the electro-attraction process. Another phenomenon that occurs at the site is electroosmosis, when the body tends to seek equilibrium in a gradient of different concentrations, enabling the entry of an ionically charged substance [
24]
3.3. Photodynamic therapy (PDT)
Photodynamic therapy (PDT) is a technique that has gained significant prominence as a new therapy, mainly for treating skin cancer, due to its minimally invasive therapeutic modality. Treatment with PDT consists of topical or intravenous administration of PS (photosensitizers), which selectively accumulates in tumor tissue during a drug-light interval. This is followed by subsequent exposure to an appropriate wavelength of light, usually in the red spectral region λ ≥ 600 nm). Illumination transfers light energy to molecular oxygen to generate reactive oxygen species such as singlet oxygen (1O2), superoxide radical (O2−•), hydroxyl radical (HO•), and hydrogen peroxide (H2O2). These cytotoxic photoproducts initiate a cascade of biochemical events that can induce target tissue damage and death. There are three main mechanisms by which PDT mediates tumor destruction: ROS produced by PDT's photochemical reactions, which can directly destroy tumor cells by inducing apoptosis and necrosis pathways; to indirect cell death due to hypoxia, where PDT affects tumor-associated vasculature and surrounding healthy vessels, leading to a disruption of oxygen and nutrient supply; by inducing an inflammatory response that activates an immune response against tumor cells, leading to immunosuppression in topical treatments. The outcome of PDT depends on all these mechanisms, the contribution of each being determined by the treatment regimen used [
25].
New strategies in the future to increase the potential of PDT in skin cancer include the association of multifunctional photosensitizers with drug delivery systems, such as nanoparticles, to improve selectivity and efficacy through targeted and controlled release of the agent [
26,
27]. Another strategy is using natural rather than artificial light to treat skin lesions. Daylight photodynamic therapy (dPDT) uses sunlight as a light source to treat superficial skin cancer. dPDT may be a less painful, more convenient, and effective alternative than conventional PDT. In Europe, aminolevulinic acid-mediated dPDT has been approved to treat patients with actinic keratosis [
28]. However, the real-time measurement of tissue oxygen levels before and during PDT is one of the main challenges, allowing the optimization of the therapeutic result of PDT by adjusting the luminous fluency rate or using fractional light dose.
4. Natural Products
According to the FDA, natural products (NP) are substances obtained from plants, animals, or microorganisms, whether extracted, purified, or processed in any other way, but that have not had their chemical structure significantly altered. For this review, we treat all compounds in their original form, semisynthetic derivatives, or synthetic compounds of natural origin as NP. Despite the many discoveries of drugs derived from NP, there are still excellent prospects for exploring new bioactives of natural origin. This field of research is still being improved with new sources of compounds, such as new microorganisms and marine organisms. It is important to note that even with significant investments by pharmaceutical industries in modern technologies for discovering new drugs, such as high-throughput automated screening and combinatorial chemistry, natural products remain an important source of new structural entities for developing new drugs [
29]. NP provides the molecular model for synthesizing many substances produced synthetically from medicinal compounds, but most species have not yet been explored systematically. Even traditional medicines from plants must be studied more thoroughly [
30].
NP has the advantage over synthetic compounds of being natural metabolites, presenting fewer side effects, being better tolerated by the body, providing more excellent safety in treatments, and being less expensive. An analysis of all new drugs approved by the FDA shows that natural products and their derivatives represent more than a third of them and play a fundamental role in discovering new drugs [
31].
Natural compounds have significant chemopreventive potential with anticancer pharmacological activities. Bioactive compounds derived from medicinal plants can be inexpensive, well tolerated in the body, and accessible, which encourages the development of strategies to optimize their mechanisms of action. However, many researchers' main challenge is promoting these substances' good bioavailability, solubility, and selectivity. Some research offers these factors that are dominant in treatments with NP. The main natural compounds commonly used in nanoencapsulation systems include chitosan, pectin, alginates, cellulose, starches, and gums. Currently, efforts are being made to improve drug delivery systems to expand the applications of NP, improving pharmacokinetics, targeting, cellular absorption, and the effectiveness of anticancer properties, minimizing side effects [
32]. In addition to optimizing the properties of natural compounds, nanoengineering-based delivery systems demonstrate improved penetration of bioactive molecules through the skin and into the tumor. This procedure increases drug retention in the skin and tumor microenvironment, resulting in reduced dosage, minimal toxicity, and better patient acceptance of treatment [
33]. Physical methods such as iontophoresis and PDT can be associated with nanotechnology strategies to increase the topical penetration power. The general theme revolves around the ability of nanosystem vehicles to strongly interact with the stratum corneum and manipulate its barrier properties, allowing the particles themselves or the released drugs to navigate through the lipid domains for accumulation and diffusion deeper into the skin [
22].
5. Drug delivery strategies for natural products in melanoma topical treatments
Drug delivery systems research is a rapidly evolving area with the potential to revolutionize skin cancer treatment. New delivery systems could deliver drugs directly to cancer cells, improving current therapies' effectiveness and safety and making them more effective and less harmful for patients (
Table 1).
There are few studies for non-invasive strategy treatment using topical therapy with natural products for skin cancer, most of which use nanoparticle strategy. There is scientific evidence for the applicability of nanostructured hybrid films in topical administration traction, using Simvastatin and other compounds, which could be a valuable basis for developing a new adjuvant treatment for skin cancer [
45].
The surface load of Nanostructured lipid carriers greatly influences cutaneous permeation and the pharmacodynamics of tripterin. Nanostructured lipid carriers loaded with cationic tripterin may improve percutaneous level penetration and antimelanoma efficacy of tripterin and offer several advantages over tripterin alone. Therefore, they are promising carriers of tripterin for topical antimelanoma therapy [
34]. Immobilization on f aminopropyl functionalized mesoporous sil- 34 ica nanoparticles (NH2-MSN) improved the photostability of Quercetin (Q), and the protective effect was more evident when the nanometric complex was dispersed in a biphasic system. The grafting of aminopropyl groups on the mesoporous silica resulted in a value strategy to increase the penetration of Q into the skin, as can be deduced from the results obtained in ex vivo experiments with pig skin. These positive findings indicate that NH2-MSN can be considered a potential topical carrier to encapsulate derived flavonoids, improving its intrinsic stability and biological activity after topical application. Cytotoxicity studies, although very preliminary, show that these mesoporous Q-charged nanoparticles appear to be a very promising approach for the delivery of flavonoids as chemopreventive agents in obtained organs and cells; in particular, they showed good biocompatibility and the ability to increase the antiproliferative activity of Q [
35]. The high anti-melanoma effect of transdermal Met-loaded cubic phases with metformin hydrochloride was also shown by the significant decrease in tumor volume and the improvement of melanoma cell apoptosis in the B16 melanoma mice [
17].
The data demonstrate that nano-vesicular formulations effectively delivered artemisone at the destination delivery location, the skin layers essential for melanoma development. SLN formulations were superior to niosome formulations, as the formerly distributed artemisone in stratum corneum-epidermis and epidermis-dermis. In addition, SLNs delivered a higher statistically significant concentration of artemisone in the stratum corneum epidermis than niosomes, which may be due to the greater efficiency of encapsulation of SLNs. In general, nanovesicles help retain artemisone in the skin layers, and therefore, they are likely to increase the activity of artemisone in treating melanoma. Thus, the evaluation of artemisone formulated in nanovesicles for the treatment of melanoma then needs to be performed [
36]. The ethosomes were shown to deliver BBR and EVO to the basal layers of human skin in vitro following topical in vitro application to human skin. Moreover, BBR and EVO co-loaded in ethosomes exhibited improved anti-melanoma effects on B16 cells in tissue culture. This result confirms the suitability of the ethosomes as potential topical carriers for the treatment of melanoma [
31]. The data obtained highlighted the different capacities of ethosomes® and transfersomes® to administer sulforaphane through the skin effectively. Ethosomes® caused increased percutaneous permeation in vitro of sulforaphane compared to transferosomal formulations and drug solutions. These findings are encouraging and suggest that ethosomes® can be effective carriers for topical administration of sulforaphane and a real opportunity for developing an appropriate and innovative therapeutic strategy for treating skin cancer diseases, such as melanoma [
48].
The liposomal design vectors exhibited excellent siRNA load and protection degradation properties. Compared to siRNA isolated or conventional liposomal vectors, the anti-melanoma activity of HIF-1α siRNA was achieved through fc-LPs. The potentiation of antitumor activity can be attributed to the intensified siRNA-mediated transfection by fc-LPs. These findings provided new insights into using HIF-1α SiRNA to improve MM therapy via RNA intervention when delivered with non-toxic cationic liposomes [
37]. The volume of large tumors increased significantly, reduced from 5.02 to 3.05 mm 3, the levels of IL-1α and TNF-α were significantly lower and levels of superoxide dismutase, catalase, and glutathione significantly increased in the group treated with silymarin-nanostructured lipid carrier gel. In addition, on skin treated with placebo and conventional gels, basal squamous cell carcinoma and squamous cell carcinoma were noted, respectively. The silymarin-nanostructured lipid carrier gel showed better treatment results than the traditional gel of silymarin [
42].
The results highlighted the advantage of the selected nanoemulsion over the microemulsion for targeting the epidermis and colocation of paclitaxel and C6 ceramide. The choice of drugs and their combination is important since synergism optimizes therapeutic potential without increasing the dose of individual agents. For paclitaxel, this was relevant to improve the stability of the formulation and may decrease the incidence of adverse effects resulting from concentrations of systemic exposure to the drug [
38]. The antitumor effects of paclitaxel-loaded nanoparticles were evaluated by the MTT assay in vitro and by a xenograft tumor model in vivo, demonstrating that star-shaped paclitaxel-loaded cholic acid/functionalized star-shaped poly/d -α-tocopheryl polyethylene glycol 1000 succinate nanoparticles were significantly superior to commercial paclitaxel formulation Taxol ®. Such drug delivery nanocarriers are potentially applicable to improving clinical MM therapy [
39]. Paclitaxel-Cts (transfersomes) with superior deformability can squeeze efficiently through the channels in the stratum coreum, and the surfactant components improved the fluidity of the lipid molecules in the stratum corneum to increase skin penetration further. In addition, modifying the penetrating peptide also made Paclitaxel-transfersomes greater penetration into the skin and tumor stroma and efficient transport in tumor cells. Paclitaxel-transfersomes have effectively slowed tumor growth in combination with systemic chemotherapy using Taxol, the commercial formulation of Paclitaxel in the mouse model with B10F16 xenograft melanoma [
43].
Cationic liposomes of DOTAP and DOPE can be used as a nanocarrier to co-delate curcumin and siRNA. The cationic liposomes can be transported through the skin after applying anodal iontophoresis. The combination of curcumin and STAT3 siRNA resulted in more significant B16F10 cancer cell growth inhibition than individual agents, while the blank cationic liposomes did not show cytotoxicity. Similarly, co-administration of curcumin and STAT3 siRNA effectively controlled melanoma tumor progression compared with either liposomal curcumin or STAT3 siRNA alone. Furthermore, the topical non-invasive application of curcumin-loaded cationic liposome-STAT3 siRNA complex using iontophoresis showed similar tumor suppression compared with intratumoral administration [
40].
Histology of the skin reveals penetration dependent on size, with framework nucleic acids ≤75 nm effectively reaching the dermis layer. 17 nm tetrahedral framework nucleic acids show greater penetration at 350 μm from the periphery of the skin. It is important to note that structural integrity is maintained during penetration into the skin. Employing a topical mouse melanoma model, the application of doxorubicin-loaded framework nucleic acids accommodates ≥2-fold improvement in drug accumulation and tumor inhibition compared to topically applied free doxorubicin or doxorubicin-loaded in liposomes and polymeric nanoparticles. Programmable penetration with minimal systemic biodistribution highlights the potential of framework nucleic acids as transporters of localized transdermal delivery [
44]. The iontophoresis associated with the nanoparticle delivered more significant quantities of substances through the skin than delivery without these technologies. The high amount of DOX delivered to the deep layers of the skin after the DOX-SLN iontophoresis indicates significant potential for use in topical skin cancer treatment [
33]. The results demonstrated the effect of the structure of the carboxymethylcellulose hydrogel network with a different degree of substitution of carboxymethyl groups in the cellulose structure about the bioconjugation process and in the adaptation of doxorubicin hydrochloride release kinetics in vitro and cytotoxicity about melanoma cancer. cells in vitro. To this end, an innovative platform based on polysaccharide-drug was developed for hydrogels that offer promising applications for skin diseases associated with topical melanoma chemotherapy treatments [
41].
The conjugated nanotransfersomal gel improved both in-vitro and in-vivo permeation and skin deposition of apigenin compared to commercial formulations and retentive in the entire depth of the skin for a sustainable action. These conjugates significantly improved the therapeutic effect and safety and had small vesicle sizes, and an in vitro drug release study indicated sustained prolongation of contained release. Con-A closed nanotransfersomal gel showed reduced cytotoxicity in A375 cells compared to HaCaT cell lines, and histology logical studies confirm that this approach can be used to combat the deleterious effects of ultraviolet radiation. This concludes that it developed conjugated delivery of natural drugs. The system could potentially be effective for melanoma [
46].
The results demonstrated that the modified nanoemulsion with chitosan and sodium alginate improved the penetration of piplartine, indicating that the negative charge of the nanocarrier was not harmful to the penetration compared with the cationic. Adding oleic acid to the chitosan-containing NE increased the penetration of piplartine into the skin. Although the transdermal distribution increased simultaneously, the absolute amount of the drug in the skin (SC + ED) was ~ 15 times greater, suggesting a more excellent localization in the skin. The selected nanoemulsion, which contained chitosan and oleic acid, efficacy shown in skin cancer models 2 and 3D; although the effect was more pronounced on melanoma tissue, the equivalent of healthy skin was also affected, demonstrating the importance of topical application of piplartine in tumor lesions to avoid cytotoxic effects in healthy places [
49]. Chitosan coating of liposomes stabilized the encapsulated unstable photosensitizer, indocyanine green. In addition, the positively charged chitosan coating of liposomes facilitated the cellular uptake, photo-cytotoxicity, and skin permeation of indocyanine green. These findings emphasize indocyanine green-loaded chitosan-coated liposomes' promising potential as topical photodynamic therapy [
47] (
Table 1).
6. Conclusions
Drug delivery systems research is a rapidly evolving area with the potential to revolutionize skin cancer treatment. New delivery systems deliver drugs directly to cancer cells, improving the effectiveness and safety of current therapies, making them more effective and less harmful for patients.
In this review we present results of articles that exhibited new strategies most commonly studied carriers of natural substances. The results found demonstrated that through the application of nanotechnology methods, the iontophoresis technique and photodynamic therapy, the distribution of substances in the carcinogenic microenvironment is increased. In this way, reducing the amount of medication needed to treat skin cancer is possible, thus increasing the efficiency of the bioactive.
New modalities for transporting substances through the skin are still under development, but with perspectives that the near future will bring significant advances for treating skin cancer.
Author Contributions
IOA and CMC participated mainly in the draft, organizing
the topics, and actualizing the literature and writing. IVGS and GSP wrote data
of skin cancer. RAC revised the cancer topics. RE and MAFS analyzed data and revised
drug delivery topics. GPFL contributed to the study's conception and design, and
supervised, and revised the study. All the authors revised and approved the final
version to be published.
Funding
This work was supported by the National Council for Scientific and Technological Development (CNPq); Carlos Chagas Filho Research Support Foundation (FAPERJ); Programa de Oncobiologia - Fundação do Câncer.
Conflict of interest
The authors have no competing interests to declare relevant to this article's content.
Abbreviations
| BCC |
Basal Cell Carcinoma |
| SCC |
Squamous Cell Carcinoma |
| DFSP |
Dermatofibrosarcoma Protuberans |
| dPDT |
daylight Photodynamic Therapy |
| MAPK |
Mitogen-Activated Protein Kinases |
| MITF |
Microphthalmia-associated Transcription Factor
|
| NMSC |
Non-Melanoma Skin Cancer |
| PDT |
Photodynamic Therapy |
| PI3K |
Phosphoinositide 3-Kinase |
| PKB |
Protein Kinase B |
| PS |
Photo Sensitive |
| PTEN |
phosphatase and Tensin homolog |
| ROS |
Reactive Oxygen Species |
| TNF |
Tumor Necrosis Factor |
| UV |
Ultra Violet |
References
- MURPHREY, M. B.; MIAO, J. H.; ZITO, P. M. Histology, Stratum Corneum. National Library Medicine, 07 2021.
- Laikova, Oberemok, Krasnodubets, Gal’chinsky, Useinov, Novikov, Kubyshkin. (2019). Advances in Understanding Skin Cancer: Ultraviolet Radiation, Mutations, and Antisense Oligonucleotides as Anticancer Drugs. Molecules, 24(8), 1516. [CrossRef]
- TAVEIRA, S. F. et al. Effect of iontophoresis on topical delivery of doxorubicin-loaded solid lipid nanoparticles. Journal of biomedical nanotechnology, v. 10, p. 1382 – 90, 5 2014.19. [CrossRef]
- TAVEIRA, S. F.; NOMIZO, A.; LOPEZ, R. F. V. Effect of the iontophoresis of a chitosan gel on doxorubicin skin penetration and cytotoxicity. Journal of controlled release: official journal of the Controlled Release Society, v. 134, p. 35 – 40, 12 2008. [CrossRef]
- Atlanta: American Cancer Society, c2023a. Disponível em: https://www.cancer.org/cancer/leukemia.html. Acesso em: 19 ago. 2023.
- POLLACK, L. A. et al. Melanoma survival in the United States, 1992 to 2005. Journal of the *American Academy of Dermatology, v. 65, p. S78 – 86, 10 2011. [CrossRef]
- NEALE, R. E. et al. Basal cell carcinoma on the trunk is associated with excessive sun exposure. Journal of the American Academy of Dermatology, v. 56, n. 3, p. 380-386, 2007. [CrossRef]
- NGUYEN, Tri H. Mechanisms of metastasis. Clinics in dermatology, v. 22, n. 3, p. 209-216, 2004.
- DAVIS, L. E.; SHALIN, S. C.; TACKETT, A. J. Current state of melanoma diagnosis and treatment. Cancer biology & therapy, v. 20, p. 1366 – 1379, 8 2019. [CrossRef]
- CHESSA, C. et al. Antiviral and Immunomodulatory Properties of Antimicrobial Peptides Produced by Human Keratinocytes. Frontiers in microbiology, v. 11, p. 1155 –, 6 2020.17. [CrossRef]
- LUGOVIc-MIHI ´ c, L. ´ et al. Melanoma Development: Current Knowledge on Melanoma Pathogenesis. Acta dermatovenerologica Croatica : ADC, v. 27, p. 163 – 168, 9 2019.
- CARNEIRO J; JUNQUEIRA, L. C. U. Basic histology: text, atlas. Guanabara-Koogan, 2008.
- RUBIN, A. I et al. Basal-cell carcinoma. New England Journal of Medicine, v. 353, n. 21, p. 2262-2269, 2005.
- MAYO CLINIC,2021. Skin Cancer. Symtomps & causes, diagnosis & treatment. Available at: https://www.mayoclinic.org/ . Acessed: 06/10/2021.
- DHOTE, V. et al. Iontophoresis: a potential emergence of a transdermal drug delivery system. Scientia pharmaceutica, v. 80, p. 1 – 28, 3 2012.
- LABATE, C. et al. Biomechanical Strengthening of the Human Cornea Induced by Nanoplatform-Based Transepithelial Riboflavin/UV-A Corneal Cross-Linking. Investigative ophthalmology & visual science, v. 58, p. 179 – 184, 1 2017.
- MISHRA, H. et al. Melanoma treatment: from conventional to nanotechnology. Journal of Cancer Research and Clinical Oncology, v. 144, p. 2283 – 2302, 8 2018.
- YUAN, Y. et al. Nucleic Acid-Based Functional Nanomaterials as Advanced Cancer Therapeutics. Small (Weinheim an der Bergstrasse, Germany), v. 15, p. e1900172 –, 4 2019.
- MUSTAFA, I. F.; HUSSEIN, M. Z. Synthesis and Technology of Nanoemulsion-Based Pesticide Formulation. Nanomaterials (Basel, Switzerland), v. 10, 8 2020.
- BARKAT, M. A. et al. Therapeutic Nanoemulsion: Concept to Delivery. Current pharmaceutical design, v. 26, p. 1145 – 1166, 3 2020.
- FONSECA-SANTOS, B.; GREMIÃO, M. P. D.; CHORILLI, M. Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. International journal of nanomedicine, v. 10, p. 4981 – 5003, 9 2015.
- OMBREDANE, A. S. et al. Nanoemulsion-based systems as a promising approach for enhancing the antitumoral activity of pequi oil (Caryocar brasilense Cambess.) in breast cancer cells. Journal of Drug Delivery Science and Technology, v. 58, 2020. ISSN 1773-2247. Disponível em: https://www:sciencedirect:com/science/article/pii/ S1773224720301751.
- KRISHNAN V, MITRAGOTRI S. Nanoparticles for topical drug delivery: Potential for skin cancer treatment. Adv Drug Deliv Rev. 2020 Jan 1;153:87-108. [CrossRef]
- LUZ, D. C. da et al. Iontophoresis in lateral epicondylitis: a randomized, double-blind clinical trial. Journal of shoulder and elbow surgery, v. 28, p. 1743 – 1749, 8 2019.
- ROBERTSON, V. et al. Eletroterapia explicada: princípios e prática. 4. ed. Rio de Janeiro: Elsevier, 2009. 491 p.
- CORREIA JH, RODRIGUES JA, PIMENTA S, DONG T, YANG Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics. 2021 Aug 25;13(9):1332. [CrossRef]
- SIMÕES, J. C. S., SARPAKI, S., PAPADIMITROULAS, P., THERRIEN, B., & LOUDOS, G. (2020). Conjugated Photosensitizers for Imaging and PDT in Cancer Research. Journal of Medicinal Chemistry. [CrossRef]
- ZHENG Y, LI Z, CHEN H, GAO Y. Nanoparticle-based drug delivery systems for controllable photodynamic cancer therapy. Eur J Pharm Sci. 2020 Mar 1;144:105213. [CrossRef]
- LEE CN, HSU R, CHEN H, WONG TW. Daylight Photodynamic Therapy: An Update. Molecules. 2020 Nov 8;25(21):5195. [CrossRef]
- Chopra, B., & Dhingra, A. K. (2021). Natural products: A lead for drug discovery and development. Phytotherapy Research. [CrossRef]
- Harvey AL, Edrada-Ebel R, Quinn RJ. The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov. 2015 Feb;14(2):111-29. [CrossRef]
- Patridge E, Gareiss P, Kinch MS, Hoyer D. An analysis of FDA-approved drugs: natural products and their derivatives. Drug Discov Today. 2016 Feb;21(2):204-7. [CrossRef]
- LAGOA R, SILVA J, RODRIGUES JR, BISHAYEE A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnol Adv. 2020 Jan-Feb;38:107382. [CrossRef]
- Labala, S., Mandapalli, P. K., Kurumaddali, A., & Venuganti, V. V. K. (2015). Layer-by-Layer Polymer Coated Gold Nanoparticles for Topical Delivery of Imatinib Mesylate To Treat Melanoma. Molecular Pharmaceutics, 12(3), 878–888. [CrossRef]
- Hongmei Lin, Longfei Lin, Yoonsun Choi, Bozena Michniak-Kohn, Development and in-vitro evaluation of co-loaded berberine chloride and evodiamine ethosomes for treatment of melanoma, International Journal of Pharmaceutics, Volume 581, 2020, 119278, ISSN 0378-5173. [CrossRef]
- Tham, H. P., Xu, K., Lim, W. Q., Chen, H., Zheng, M., Thng, T. G. S., … Zhao, Y. (2018). Microneedle-Assisted Topical Delivery of Photodynamically Active Mesoporous Formulation for Combination Therapy of Deep-Seated Melanoma. ACS Nano. [CrossRef]
- Chen, Y., Zhou, L., Yuan, L., Zhang, Z., & Wu, Q. (2012). Formulation, Characterization and Evaluation of the in Vitro Skin Permeation of Nanostructured Lipid Carriers Encapsulated Tripterine. 2012 International Conference on Biomedical Engineering and Biotechnology. [CrossRef]
- Siu KS, Chen D, Zheng X, Zhang X, Johnston N, Liu Y, Yuan K, Koropatnick J, Gillies ER, Min WP. Non-covalently functionalized single-walled carbon nanotube for topical siRNA delivery into melanoma. Biomaterials. 2014 Mar;35(10):3435-42. [CrossRef]
- Dwivedi A, Mazumder A, Fox LT, Brümmer A, Gerber M, du Preez JL, Haynes RK, du Plessis J. In vitro skin permeation of artemisone and its nano-vesicular formulations. Int J Pharm. 2016 Apr 30;503(1-2):1-7. [CrossRef]
- Naves LB, Dhand C, Venugopal JR, Rajamani L, Ramakrishna S, Almeida L. Nanotechnology for the treatment of melanoma skin cancer. Prog Biomater. 2017 May;6(1-2):13-26. [CrossRef]
- Hafeez A, Kazmi I. Dacarbazine nanoparticle topical delivery system for the treatment of melanoma. Sci Rep. 2017 Nov 28;7(1):16517. [CrossRef]
- Cao J., Wang R., Gao N., Li M., Tian X., Yang W., Ruan Y., Zhou C., Wang G., Liu X., Tang S., Yu Y., Liu Y., Sun G., Peng H., Wang Q., (2015) A7RC peptide modified paclitaxel liposomes dually target breast cancer, Biomater. Sci. 3, 1545–1554.
- Jose A, Labala S, Venuganti VV. Co-delivery of curcumin and STAT3 siRNA using deformable cationic liposomes to treat skin cancer. J Drug Target. 2017 Apr;25(4):330-341. [CrossRef]
- Capanema NSV, Mansur AAP, Carvalho SM, Carvalho IC, Chagas P, de Oliveira LCA, Mansur HS. Bioengineered carboxymethyl cellulose-doxorubicin prodrug hydrogels for topical chemotherapy of melanoma skin cancer. Carbohydr Polym. 2018 Sep 1;195:401-412. [CrossRef]
- Jiang, T., Wang, T., Li, T., Ma, Y., Shen, S., He, B., & Mo, R. (2018). Enhanced Transdermal Drug Delivery by Transfersome-Embedded Oligopeptide Hydrogel for Topical Chemotherapy of Melanoma. ACS Nano. [CrossRef]
- Nuhn, L., Van Hoecke, L., Deswarte, K., Schepens, B., Li, Y., Lambrecht, B. N. De Geest, B. G. (2018). Potent anti-viral vaccine adjuvant based on pH-degradable nanogels with covalently linked small molecule imidazoquinoline TLR7/8 agonist. Biomaterials, 178, 643–651. [CrossRef]
- Schlich M, Fornasier M, Nieddu M, Sinico C, Murgia S, Rescigno A. 3-hydroxycoumarin loaded vesicles for recombinant human tyrosinase inhibition in topical applications. Colloids Surf B Biointerfaces. 2018 Nov 1;171:675-681. [CrossRef]
- Wiraja, C., Zhu, Y., Lio, D. et al. Framework nucleic acids as programmable carrier for transdermal drug delivery. Nat Commun 10, 1147 (2019). [CrossRef]
- Barone A, Mendes M, Cabral C, Mare R, Paolino D, Vitorino C. Hybrid Nanostructured Films for Topical Administration of Simvastatin as Coadjuvant Treatment of Melanoma. J Pharm Sci. 2019 Oct;108(10):3396-3407. [CrossRef]
- Lee EH, Lim SJ, Lee MK. Chitosan-coated liposomes to stabilize and enhance transdermal delivery of indocyanine green for photodynamic therapy of melanoma. Carbohydr Polym. 2019 Nov 15;224:115143. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).