1. Introduction
Current recommendations suggest that endovascular aneurysm repair (EVAR) should be the preferred option for patients with abdominal aortic aneurysm (AAA) and a concomitant high perioperative risk, due to lower morbidity and mortality rates, when compared with open conventional management [
1,
2]. Even though open repair is considered as a more durable solution with lower re-intervention rates in the long-term, it has been associated with higher morbidity during the early follow-up period [
3,
4]. That being said, the reported 30-day postoperative morbidity in patients undergoing open repair of AAA may be as high as 50%, with a noticeable lower mortality (5%) [
4,
5].
Over the years, a variety of factors, such as age, female sex, the more complex aneurysm anatomy and the presence of cardiac, pulmonary and renal comorbidities, have been linked to higher rates of postoperative adverse events following the conventional management of AAA [
6]. From the other hand, increased experience and the evolving of dedicated perioperative quality improvement programs have ensured a more favorable outcome, in terms of morbidity and mortality, in patients undergoing either EVAR or open aneurysm repair [
7]. However, patients treated with the endovascular approach should be considered a priori as high risk and, as in open repair, a variety of factors including sex, age and comorbid status may be indicative of future outcomes [
8].
Hence, despite the minimal invasive nature of EVAR, a percentage of patients, ranging from 0 to 40%, may suffer from major or minor postoperative complication [
9,
10]. Of note, the wide range of reported morbidity following EVAR might be indicative of mis-interpreted follow-up outcomes and controversy on the definitions of postoperative complications ensuing EVAR [
9]. As perioperative morbidity may lead to an unfavorable long-term outcome, both meticulous perioperative evaluation and increased awareness for potential predictors of adverse events may enhance a more individualized and optimal perioperative care [
8]. Hence, we ought to assess the 30-day postoperative outcome after elective EVAR and to identify possible predictors for postoperative complications within 30-days post-EVAR.
2. Materials and Methods
2.1. Study cohort
A retrospective analysis of prospectively collected data of consecutive patients treated electively with EVAR was undertaken from March 2016 to February 2019 in a single tertiary referral center. Despite that patients were treated based on the European Society of Vascular Surgery (ESVS) guidelines, the final decision on repair was in surgeon’s discretion and patient’s consent after discussion [
1,
11]. Existing data were recorded in a way that subjects would be unidentifiable. The study was approved by the Institutional Review Board (605/14-02-2017) and was registered (NTC05647486).
2.2. Inclusion and exclusion criteria
Only patients managed with EVAR using standard bifurcated devices in elective setting were included. Patients treated for ruptured, symptomatic, inflammatory or infectious AAA were excluded. Furthermore, any patient needing complex endovascular repair of the proximal landing zone was considered ineligible. Additional exclusion criteria were the presence of any trauma or surgery within two months before EVAR, any autoimmune or systemic inflammatory disease and any malignancy. Of note, any patient with clinical or laboratory signs of infection before the scheduled operation was not offered an EVAR until a complete resolution of the infection was confirmed via a clinical, laboratory and/or imaging evaluation.
2.3. Data collection and postoperative surveillance
A dedicated database existed for the prospective collection of patients’ data, including demographics (age, sex), AAA diameter [after the application of center lumen line using a dedicated software (3mensio, Medical Imaging B.V., Bilthoven, Netherlands], comorbidities (hypertension, dyslipidemia, smoking, chronic renal failure, coronary artery disease, diabetes mellitus, chronic obstructive pulmonary disease, peripheral arterial disease (PAD) and venous thrombosis) and any medications (including antithrombotic agents and lipid lowering agents). Preoperative (within 24 h before the operation) and postoperative (within 24 h after surgery) laboratory values were recorded, including hemoglobin, white blood cells [neutrophils, lymphocytes and neutrophil/lymphocyte ratio (NLR)], platelets, urea, creatinine and C-reactive protein (CRP) [
12]. The length of hospital stay (in days) and the need and length of stay to the intensive care unit (ICU) were also recorded and analyzed.
Early follow-up with clinical and laboratory evaluation took place at the 30rd day postoperatively. Any adverse events, including major adverse cardiovascular events (MACE), acute kidney injury (AKI), post-implantation syndrome (PIS), postoperative delirium (POD), urinary tract infection (UTI), technical graft failure and death of any cause, were recorded. For the purpose of the study MACE, AKI and death were classified as major complications, while PIS, POD, UTI and graft technical failure as minor complications, respectively.
2.4. Definitions
Regarding the preoperative characteristics, hypertension was defined as the presence of systolic or diastolic blood pressure higher than 140mmHg or 90mmHg, respectively or the presence of anti-hypertensive medication [
1]. Dyslipidemia was recorded as the presence of low density lipoprotein cholesterol (LDL) value exceeding 70mg/dl or the use of statins [
13]. Smoking was defined as any current or past tobacco use, regardless of the duration of use. As past smoker were considered all patients reporting smoking cessation at least 6 months before EVAR. Chronic renal failure; according to KDIGO criteria, was any glomerular filtration rate (GFR) <60 mL/h/1.73m
2, using the Cockcroft–Gault equation, or the need for dialysis [
14,
15]. Coronary artery disease included any previous myocardial infarction, percutaneous transcatheter coronary angioplasty and coronary-aortic bypass [
12]. Peripheral arterial disease anamnesis included all patients presenting an ankle-brachial index<0.9 or intermittent claudication or severe atheromatosis (>70% stenosis) of the iliofemoral axis in the preoperative CTA or previous endovascular or open intervention related to lower limb arterial disease [
16].
As far as the postoperative morbidity is concerned, MACE included myocardial infarction (any clinical symptoms associated to acute coronary syndrome and/or any new electrocardiographic sign or high sensitivity troponin elevation), arrhythmia (any event of atrial or ventricular tachycardia with more than 90 pulses per minute or any episode of bradycardia of less than 50 pulses per minute) and stroke (any transient ischemic attack, any stroke; major or minor according to Rankin Score) [
12]. PIS was defined as the presence of at least two of the systemic inflammatory response syndrome (SIRS) criteria including fever >38∘C and leucocytosis >12,000/𝜇L, without any apparent clinical or biochemical sign of infection (negative urine and blood cultures, and chest radiography) [
17]. AKI was defined according to the RIFLE (Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease) criteria, as a two-fold increase in serum creatine or >50% decrease in GFR (estimated with the Cockcroft–Gault equation) [
18]. UTI refers to significant bacteriuria in a patient with symptoms or signs attributable to the urinary tract and no alternate source [
19]. Finally, POD was defined as an acute and fluctuating alteration of mental state of reduced awareness and disturbance of attention based on validated screening tools [
20,
21].
2.5. Outcomes
The assessment of 30-day postoperative morbidity and mortality in patients undergoing elective EVAR and the identification of possible predictors for postoperative complications within 30-days post-EVAR were defined as the outcomes of this study.
2.6. Statistical analysis
Continuous and discrete data were presented using descriptive statistics, including means (and standard deviations) and counts (and percentages). Additionally, the role of potential modifiers was studied using the chi-square test for categorical variables while the T-test and ANOVA (or their non-parametric equivalents, Wilcoxon and Kruskal-Wallis test, when appropriate). Finally, a multivariate logistic regression analysis was carried-out based on statistically important modifiers for each complication group, to verify any actual predictive role. All statistical analyses were conducted using the statistical environment R. The level of statistical significance was set at 0.05.
3. Results
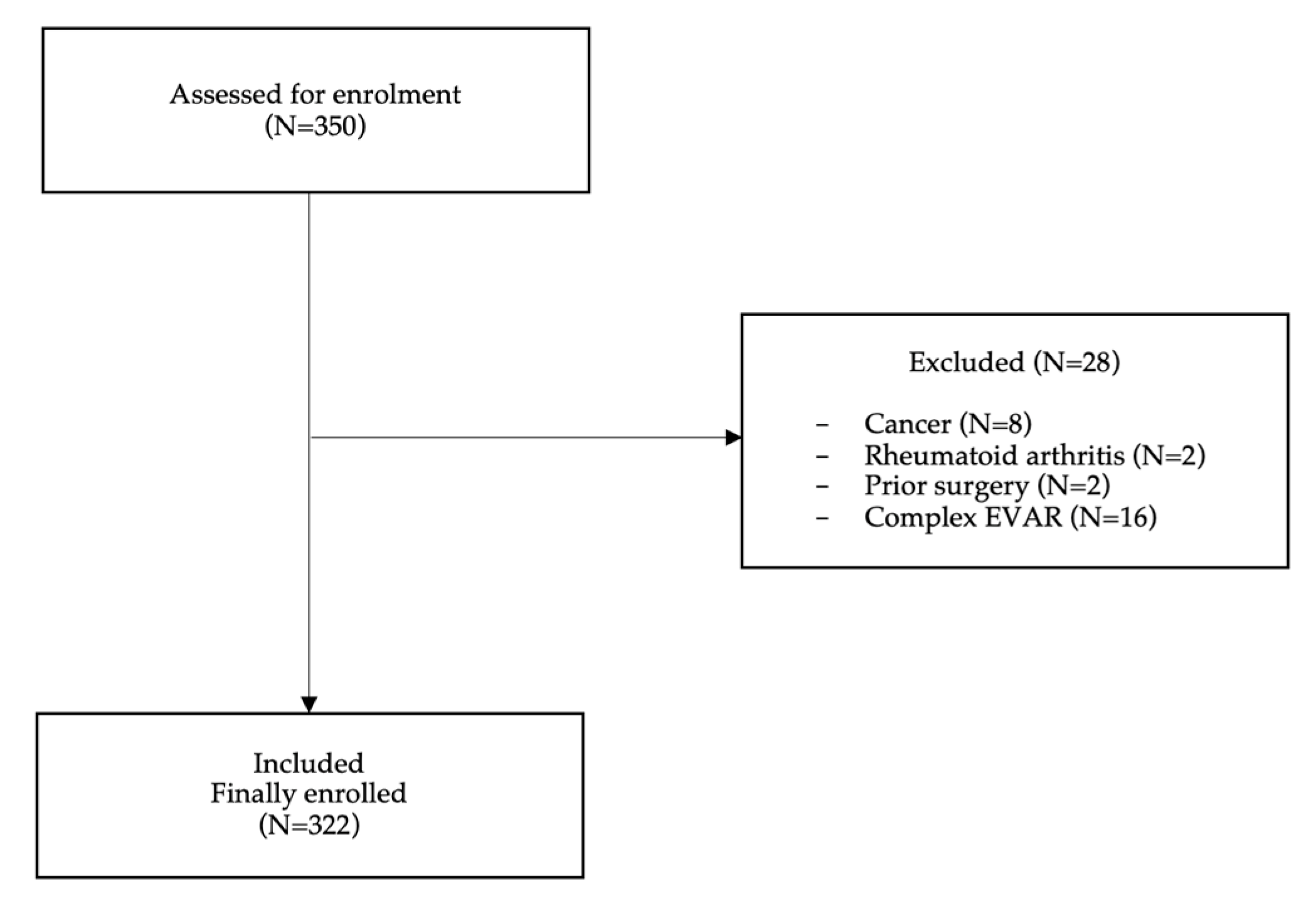
A total of 350 patients were assessed for enrollment. Twenty-eight patients were excluded; 8 patients suffering from cancer and 2 from rheumatoid arthritis, 2 patients due to prior surgery and 16 patients were scheduled for complex EVAR, hence 322 (92%) were finally enrolled (
Figure 1). The mean age of participants was 72.3±7.2 years and the mean aneurysm diameter was 59.2±12.7mm, respectively. The majority of patients were males (98.1%) and were operated under general anesthesia (83%). Hypertension was the most common (86.2%, n=249) comorbidity, followed by dyslipidemia (78.9%, n=228). The median LOH was 4 days (IQR 2 days). The majority of our patients (n=228) were treated with lipid lowering agents, mainly statins, and almost half of them (n=114) with antithrombotics. Patients’ baseline characteristics are presented in
Table 1A,B.
3.1. Primary outcome-overall postoperative morbidity and mortality
Overall, 121 (37.5%) complications, mostly minor (n=103, 31.9%), were recorded
Table 2A. Seventy-seven patients (23.9%) presented with PIS, eleven patients (3.4%) suffered from POD, 11 from UTI and 4 (1.2%) patients from technical graft failure. As far as the major complications are concerned, fortunately the associated morbidity was as low as 5.6% (n=18). Eleven patients (3.4%) evolved MACE, 5 patients (1.6%) AKI and 2 patients (0.6%) unfortunately admitted in ICU and both died; one due to extensive ischemic colitis after hypogastric artery occlusion and the other due to myocardial infarction. Due to the small number of events, no further analysis on death will follow recorded
Table 2B.
3.2. Secondary outcome
3.2.1. Overall complications
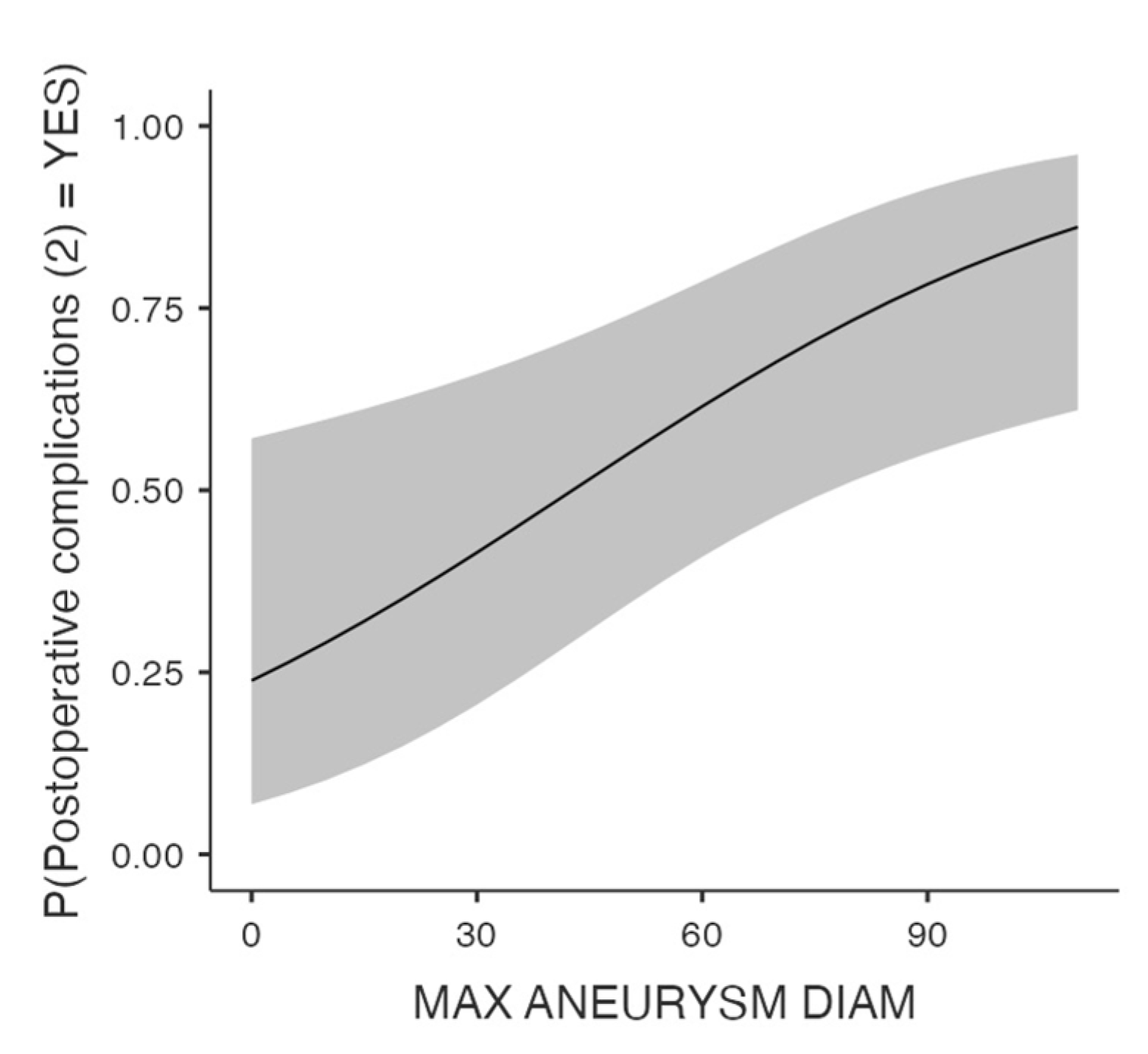
Univariate analysis revealed that postoperative complications including any type of the predefined adverse events, were associated with the initial aneurysm diameter (p=0.016,
Figure 2), past smoking status (p=.004), and PAD (p=.029). The role of maximal aneurysm diameter (OR 1.28, 95% CI 1.01-1.05, p=.01) and past smoking (OR 0.38, 95% CI 0.19-0.71, p=.003) in the development of postoperative complications was further verified by the multivariate model. Of note, no association was found between the administrated pharmaceutical agents, such as the antithrombotics and the lipid lowering factors, and the postoperative morbidity.
3.2.2. Major complications-MACE
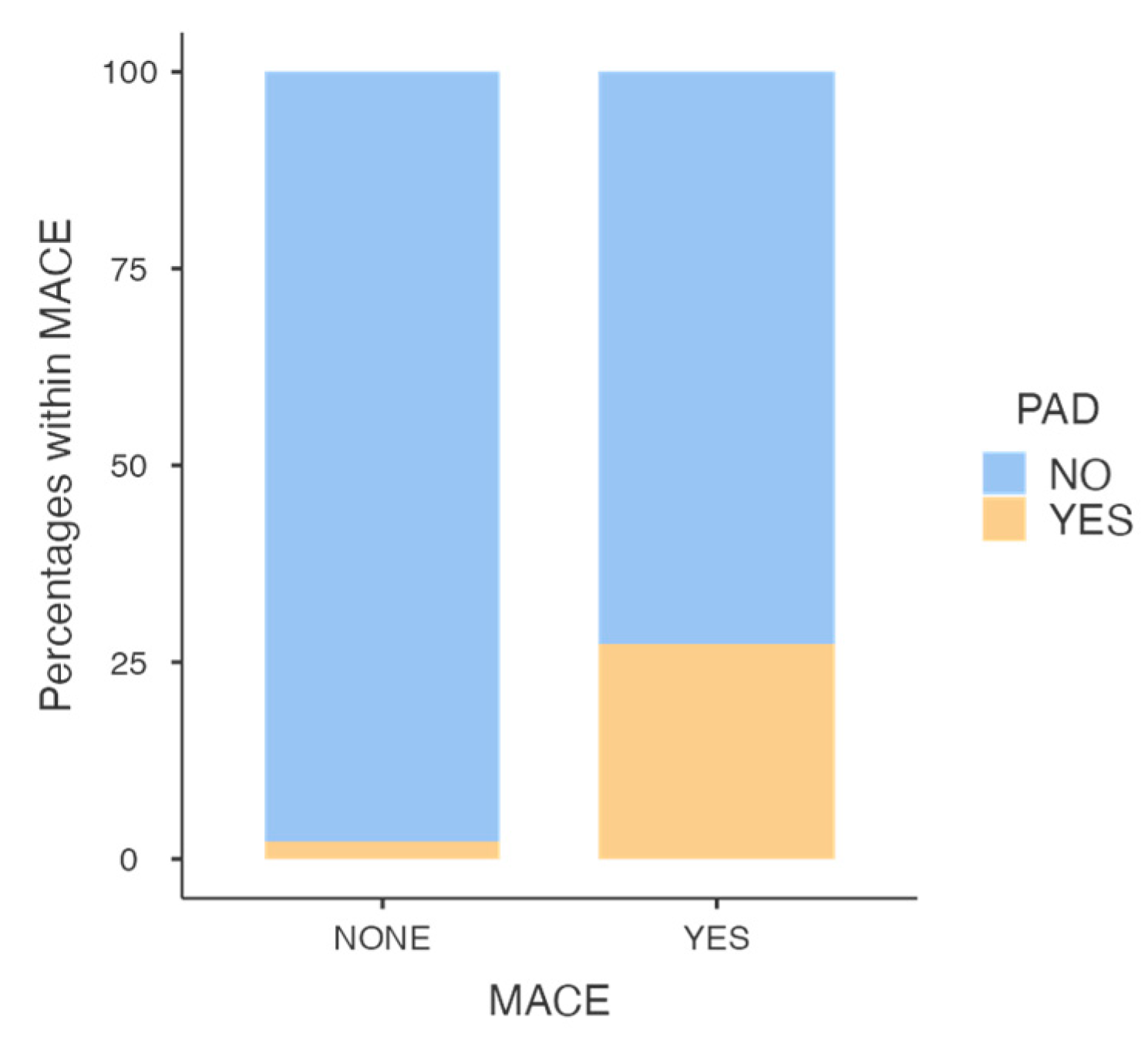
COPD (p=.032), past medical history of venous thrombosis (p=.034), and PAD (p<0.001) were related to MACE evolution. The multivariate analysis showed PAD as an independent predictor for MACE after EVAR (OR 13.94, 95% CI 2.4-81.16, p=.003,
Figure 3).
3.2.3. Major complications-AKI
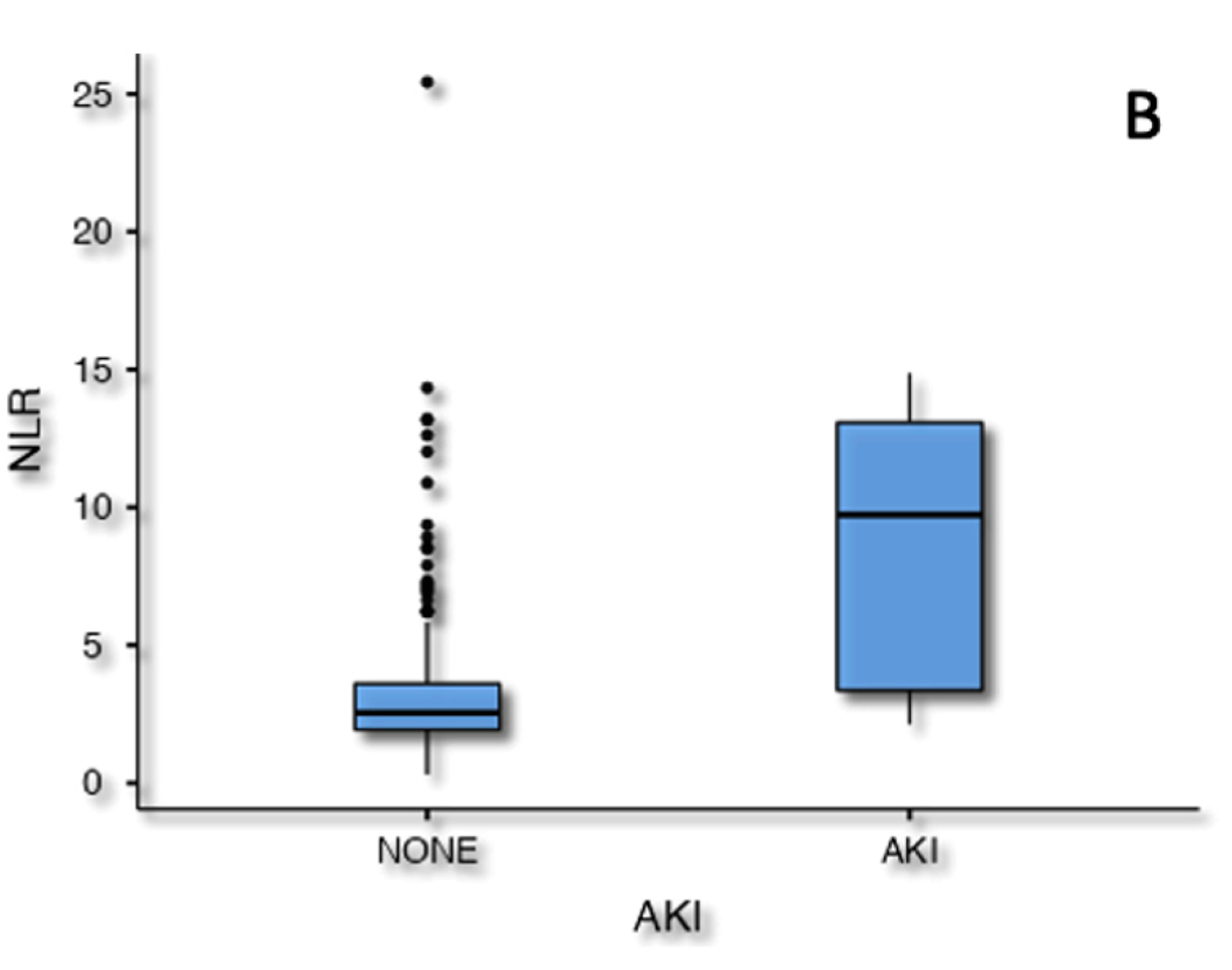
AKI was related to age (p=.024), preoperative lymphocyte titer (p=.025) and estimated NLR (p<.001) in the univariate analysis. Following the multivariate analysis only the preoperative NLR value was still associated to AKI (OR 1.2, 95% CI 1.01-1.43, p=.003,
Figure 4).
3.2.4. Minor complications
As far as the predefined minor complications are concerned, univariate analysis identified predictors only for Preoperative lymphocyte titers (p=.019) and past smoking (p=.01) were associated with a higher incidence of PIS in post-EVAR patients, based on univariate analysis. The multivariate regression analysis revealed that past smoking is an independent predictor of PIS (OR 0.36, 95% CI 0.17-0.76, p=.008).
The results of the multivariate analysis are depicted in
Table 3.
4. Discussion
This study showed that early postoperative morbidity within 30 days following elective standard EVAR under general anaesthesia exceeds 35%. Nonetheless, the vast majority of complications were minor (n=103, 31.9%) and the associated mortality was estimated as null (0.6%). Nowadays, EVAR has been established as a minimally invasive and gold-standard care for high risk patients due to the more favorable early postoperative outcome [
21,
22,
23]. In the United States EVAR accounts for almost 50% of all AAA repairs [
23]. However, the reported rate of major or minor postoperative complications following EVAR may range from 0 to 40%, hence our morbidity rate should not be considered as surprising [
9,
10]. Although, cumulative data have shown that the safety profile of EVAR is improving, many challenges seems to rise ahead and conflicting data still exist due to the utilization of EVAR in everyday clinical practice [
9]. Of note, increased postoperative morbidity has been associated with worse long-term prognosis and mortality rates following the aforementioned invasive procedure [
9,
10,
23].
Moving on to possible predictors of postoperative complications multivariate logistic regression analysis revealed that aneurysm diameter and past smoking should be considered as independent risk factors for postoperative complications. Although, our results support that smoking status should be considered as an independent risk factor for postoperative complications, Peterson et al [
24] found that smoking status does not affect the postoperative prognosis of patients undergoing EVAR. Nonetheless, smoking cessation preoperatively has been implemented in both European [
25] and American [
26] guidelines, since experts suggest that it may reduce the risk of postoperative complications.
As far as, the impact of aneurysm diameter on postoperative prognosis is concerned, conflicting data still exist [
8,
27,
28]. Aneurysm diameter has been previously identified as an indicator of adverse events or even death after EVAR [
8,
28]. Larger aneurysm repair has been related to long-term adverse events, including re-intervention, rupture, mortality and loss to follow-up [
29,
30]. The non-compliance of these patients to the postoperative surveillance, potentially showed patients with less access to medical facilities or decreased ability of self-care. Under this spectrum, the large preoperative diameter might truthfully be a predictor of postoperative morbidity, as indicated from this study, as larger aneurysms may be found and treated in patients with lower compliance to medical instructions or follow-up for other comorbidities.
Although, our results showed that PAD should be considered as a predictor of MACE, surprisingly the past medical history of coronary artery disease was not related to early MACE in our study sample. However, coronary artery disease at baseline has been identified as an strong predictor for MACE postoperatively in the long-term [
31]. Of note, PAD has been recognized as an important factor, maybe of higher prominence than coronary or carotid artery disease, of postoperative cardiovascular complications [
32,
33,
34,
35]. In addition, the presence of disease in multiple vascular territories, has been identified as a malignant condition needing meticulous care and clinical alert [
32]. Among AAA patients the incidence of PAD may exceed 45%, depicting that AAA patients are at high risk of diffuse atherosclerotic disease [
36]. While previous analyses focused on the impact of PAD in EVAR outcomes, especially regarding re-interventions and access complications, we found that patients undergoing EVAR and suffering from PAD were at increased risk for MACE [
35,
37]. This finding highlights the hypothesis that AAA patients with PAD should be considered as suffering from polyvascular disease and a more attentive pre- and intra-operative care should be provided.
AKI after EVAR presented an incidence of up to 3.0% in previous studies, while in this analysis the estimated rate was only 1.5%, despite that the preoperative chronic kidney disease was estimated at 8% [
38]. Previous analyses have shown that preoperative NLR has been related to adverse events [
12,
39,
40]. Especially, an elevated preoperative NLR has been associated to postoperative mortality, regardless of the presence of other comorbidities [
40]. Of note, our team showed in a previous study, with a smaller size sample of the same cohort, that postoperative NLR was also predictive of AKI post-EVAR [
12]. Similar findings were also supported by another recently published data where platelet to lymphocyte ratio was related with post chimney EVAR AKI [
41]. These findings highlight the fact that a simple, inexpensive and routine preoperative marker might be used and evaluated as a predictor of postoperative morbidity and mortality.
Finally, PIS represented almost the 25% of total postoperative complications in this cohort. Previous studies showed a range between 11.2% to 35% [
42,
43,
44,
45]. Although non-infectious inflammatory response after EVAR is considered a benign condition, literature has linked PIS to major cardiovascular adverse events in the long-term [
46]. Multiple factors were related to PIS evolution after EVAR, including thrombus formation and stent-graft material [
35,
36,
37]. In the current analysis, PIS was related to preoperative smoking status; a finding not reported previously. Probably, the higher inflammatory burden of the arterial wall existing in previous smokers than nonsmokers might be used as an explanatory mechanism of a more aggressive postoperative inflammatory reaction [
47].
Limitations
Despite the prospective collection of patients’ data, the retrospective nature is an important limitation of this analysis. Furthermore, the limited number of patients and the short period of follow-up does not permit firm conclusions. However, none of the patients was lost to the initial follow-up. Most patients were males and the findings should be taken into consideration cautiously when applied to female patients. Additionally, a variety of devices were used and some of them were related in the past with higher incidence of PIS. Hence, this confounder may have potentially affected the outcome. Lastly, some anatomic characteristics such as thrombus formation during the early follow-up were not examined regarding their impact on postoperative morbidity and especially PIS.
5. Conclusions
Thirty-day morbidity after elective EVAR under general anaesthesia exceeded 35% in this analysis. However, the associated mortality was relatively low, less than 1%. Past smoking and AAA diameter seemed to be independent predictors of postoperative complications at 30-day follow-up, while PAD and elevated preoperative NLR were independent predictors for postoperative major complications.
6. Patents
Not applicable.
Supplementary Materials
Not applicable.
Author Contributions
“Conceptualization, Maria P Ntalouka and Eleni Arnaoutoglou; Data curation, Maria P Ntalouka, Petroula Nana, Alexandros Brotis, Athanasios Chatzis, Maria Mermiri, Metaxia Bareka and Athanasios Giannoukas; Formal analysis, Alexandros Brotis; Investigation, Petroula Nana; Methodology, Maria P Ntalouka, Petroula Nana, Alexandros Brotis, Athanasios Chatzis, Maria Mermiri, Konstantinos Stamoulis, Metaxia Bareka, Athanasios Giannoukas, Miltiadis Matsagkas and Eleni Arnaoutoglou; Project administration, Maria P Ntalouka, Alexandros Brotis, Athanasios Chatzis, Maria Mermiri, Konstantinos Stamoulis, Metaxia Bareka, Athanasios Giannoukas, Miltiadis Matsagkas and Eleni Arnaoutoglou; Resources, Athanasios Giannoukas and Miltiadis Matsagkas; Software, Alexandros Brotis; Supervision, Miltiadis Matsagkas and Eleni Arnaoutoglou; Validation, Maria P Ntalouka, Petroula Nana, Alexandros Brotis, Athanasios Chatzis, Konstantinos Stamoulis, Metaxia Bareka, Athanasios Giannoukas, Miltiadis Matsagkas and Eleni Arnaoutoglou; Visualization, Maria P Ntalouka, Petroula Nana, Alexandros Brotis, Athanasios Chatzis, Maria Mermiri, Konstantinos Stamoulis, Metaxia Bareka, Athanasios Giannoukas, Miltiadis Matsagkas and Eleni Arnaoutoglou; Writing – original draft, Maria P Ntalouka and Petroula Nana; Writing – review & editing, Maria P Ntalouka, Petroula Nana, Alexandros Brotis, Athanasios Chatzis, Maria Mermiri, Konstantinos Stamoulis, Metaxia Bareka, Athanasios Giannoukas, Miltiadis Matsagkas and Eleni Arnaoutoglou.All authors have read and agreed to the published version of the manuscript.”.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of University Hospital of Larissa (protocol code 605 and date of approval 14-02-2017).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethics and general data protection regulation.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; Van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice – European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-Iliac Artery Aneurysms. Eur J Vasc Endovasc Surg 2019, 57, 8–93. [Google Scholar] [CrossRef] [PubMed]
- Spanos, K.; Nana, P.; Behrendt, C.-A.; Kouvelos, G.; Panuccio, G.; Heidemann, F.; Matsagkas, M.; Debus, S.; Giannoukas, A.; Kölbel, T. Management of Abdominal Aortic Aneurysm Disease: Similarities and Differences Among Cardiovascular Guidelines and NICE Guidance. J Endovasc Ther 2020, 27, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Stather, P.W.; Sidloff, D.; Dattani, N.; Choke, E.; Bown, M.J.; Sayers, R.D. Systematic Review and Meta-Analysis of the Early and Late Outcomes of Open and Endovascular Repair of Abdominal Aortic Aneurysm. Br J Surg 2013, 100, 863–872. [Google Scholar] [CrossRef]
- Landry, G.J.; Liem, T.K.; Abraham, C.Z.; Jung, E.; Moneta, G.L. Predictors of Perioperative Morbidity and Mortality in Open Abdominal Aortic Aneurysm Repair. Am J Surg 2019, 217, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Katsargyris, A.; Lenhardt. Michael Florian, C.; Marques De Marino, P.; Botos, B.; Verhoeven, E.L. Reasons for and Outcomes of Open Abdominal Aortic Repair in the Endovascular Era. Ann Vasc Surg 2021, 73, 417–422. [Google Scholar] [CrossRef]
- Alberga, A.J.; Karthaus, E.G.; Van Zwet, E.W.; De Bruin, J.L.; Van Herwaarden, J.A.; Wever, J.J.; Verhagen, H.J.M.; Van Den Akker, P.J.; Akkersdijk, G.J.; Akkersdijk, G.P.; et al. Outcomes in Octogenarians and the Effect of Comorbidities After Intact Abdominal Aortic Aneurysm Repair in the Netherlands: A Nationwide Cohort Study. Eur J Vasc Endovasc Surg 2021, 61, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Alberga, A.J.; Karthaus, E.G.; Wilschut, J.A.; De Bruin, J.L.; Akkersdijk, G.P.; Geelkerken, R.H.; Hamming, J.F.; Wever, J.J.; Verhagen, H.J.M.; Van Den Akker, P.J.; et al. Treatment Outcome Trends for Non-Ruptured Abdominal Aortic Aneurysms: A Nationwide Prospective Cohort Study. Eur J Vasc Endovasc Surg 2022, 63, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Nejim, B.; Zarkowsky, D.; Hicks, C.W.; Locham, S.; Dakour Aridi, H.; Malas, M.B. Predictors of In-Hospital Adverse Events after Endovascular Aortic Aneurysm Repair. J Vasc Surg 2019, 70, 80–91. [Google Scholar] [CrossRef]
- Noori, V.J.; Healey, C.T.; Eldrup-Jorgensen, J.; Blazick, E.; Hawkins, R.E.; Bloch, P.H.S.; Nolan, B.W. Comparison of Major Adverse Event Rates after Elective Endovascular Aneurysm Repair in New England Using a Novel Measure of Complication Severity. J Vasc Surg 2019, 70, 74–79. [Google Scholar] [CrossRef]
- Castiglione, D.; Easwaran, A.; Prashar, A.; La Grutta, L.; Krokidis, M.; Shaida, N. Assessment of EVAR Complications Using CIRSE Complication Classification System in the UK Tertiary Referral Centre: A ∼6-Year Retrospective Analysis (2014–2019). Cardiovasc Intervent Radiol 2021, 44, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Moll, F.L.; Powell, J.T.; Fraedrich, G.; Verzini, F.; Haulon, S.; Waltham, M.; Van Herwaarden, J.A.; Holt, P.J.E.; Van Keulen, J.W.; Rantner, B.; et al. Management of Abdominal Aortic Aneurysms Clinical Practice Guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg 2011, 41, S1–S58. [Google Scholar] [CrossRef] [PubMed]
- Ntalouka, M.P.; Nana, P.; Kouvelos, G.N.; Stamoulis, K.; Spanos, K.; Giannoukas, A.; Matsagkas, M.; Arnaoutoglou, E. Association of Neutrophil–Lymphocyte and Platelet–Lymphocyte Ratio with Adverse Events in Endovascular Repair for Abdominal Aortic Aneurysm. J Clin Med 2021, 10, 1083. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin Pract 2012, 120, c179–c184. [Google Scholar] [CrossRef] [PubMed]
- MD+CALC. Available online: https://www.mdcalc.com/creatinine-clearance-cockcroft-gault-equation (accessed on March 2016).
- Aboyans, V.; Ricco, J.-B.; Bartelink, M.-L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.-P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in Collaboration with the European Society for Vascular Surgery (ESVS). Eur Heart J 2018, 39, 763–816. [Google Scholar] [CrossRef]
- Arnaoutoglou, E.; Kouvelos, G.; Papa, N.; Kallinteri, A.; Milionis, H.; Koulouras, V.; Matsagkas, M. Prospective Evaluation of Post-Implantation Inflammatory Response After EVAR for AAA: Influence on Patients’ 30 Day Outcome. Eur J Vasc Endovasc Surg 2015, 49, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Walther, C.P.; Podoll, A.S.; Finkel, K.W. Summary of Clinical Practice Guidelines for Acute Kidney Injury. Hosp Pract 2014, 42, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Hooton, T.M.; Bradley, S.F.; Cardenas, D.D.; Colgan, R.; Geerlings, S.E.; Rice, J.C.; Saint, S.; Schaeffer, A.J.; Tambayh, P.A.; Tenke, P.; et al. Diagnosis, Prevention, and Treatment of Catheter-Associated Urinary Tract Infection in Adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis 2010, 50, 625–663. [Google Scholar] [CrossRef] [PubMed]
- Aldecoa, C.; Bettelli, G.; Bilotta, F.; Sanders, R.D.; Audisio, R.; Borozdina, A.; Cherubini, A.; Jones, C.; Kehlet, H.; MacLullich, A.; et al. European Society of Anaesthesiology Evidence-Based and Consensus-Based Guideline on Postoperative Delirium. Eur J Anaesthesiol 2017, 34, 192–214. [Google Scholar] [CrossRef]
- Powell, J.T.; Sweeting, M.J.; Ulug, P.; Blankensteijn, J.D.; Lederle, F.A.; Becquemin, J.-P.; Greenhalgh, R.M.; Greenhalgh, R.M.; Beard, J.D.; Buxton, M.J.; et al. Meta-Analysis of Individual-Patient Data from EVAR-1, DREAM, OVER and ACE Trials Comparing Outcomes of Endovascular or Open Repair for Abdominal Aortic Aneurysm over 5 Years. Br J Surg 2017, 104, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Donas, K.P.; Torsello, G. Complications and Reinterventions after EVAR: Are They Decreasing in Incidence? J Cardiovasc Surg (Torino) 2011, 52, 189–192. [Google Scholar]
- Qrareya, M.; Zuhaili, B. Management of Postoperative Complications Following Endovascular Aortic Aneurysm Repair. Surg Clin North Am 2021, 101, 785–798. [Google Scholar] [CrossRef]
- Peterson, L.; Schweitzer, G.; Simone, A.; Zielke, T.; DeJong, M.; Penton, A.; Blecha, M. The Effect of Smoking Status on Perioperative Morbidity and Mortality after Open and Endovascular Abdominal Aortic Aneurysm Repair. Ann Vasc Surg 2023, 88, 373–384. [Google Scholar] [CrossRef]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; Van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice – European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-Iliac Artery Aneurysms. Eur J Vasc Endovasc Surg 2019, 57, 8–93. [Google Scholar] [CrossRef] [PubMed]
- Chaikof, E.L.; Dalman, R.L.; Eskandari, M.K.; Jackson, B.M.; Lee, W.A.; Mansour, M.A.; Mastracci, T.M.; Mell, M.; Murad, M.H.; Nguyen, L.L.; et al. The Society for Vascular Surgery Practice Guidelines on the Care of Patients with an Abdominal Aortic Aneurysm. J Vasc Surg 2018, 67, 2–77.e2. [Google Scholar] [CrossRef]
- Ramos, C.; Pujari, A.; Rajani, R.R.; Escobar, G.A.; Rubin, B.G.; Jordan, W.D.; Benarroch-Gampel, J. Perioperative Outcomes for Abdominal Aortic Aneurysm Repair Based on Aneurysm Diameter. Vasc Endovascular Surg 2020, 54, 341–347. [Google Scholar] [CrossRef]
- Mastracci, T.M.; Greenberg, R.K.; Hernandez, A.V.; Morales, C. Defining High Risk in Endovascular Aneurysm Repair. J Vasc Surg 2010, 51, 1088–1095.e1. [Google Scholar] [CrossRef]
- Kim, S.; Jeon-Slaughter, H.; Chen, X.; Ramanan, B.; Kirkwood, M.L.; Timaran, C.H.; Modrall, J.G.; Tsai, S. Effect of Abdominal Aortic Aneurysm Size on Mid-Term Mortality After Endovascular Repair. J Surg Res 2021, 267, 443–451. [Google Scholar] [CrossRef]
- De Guerre, L.E.V.M.; Dansey, K.; Li, C.; Lu, J.; Patel, P.B.; Van Herwaarden, J.A.; Jones, D.W.; Goodney, P.P.; Schermerhorn, M.L. Late Outcomes after Endovascular and Open Repair of Large Abdominal Aortic Aneurysms. J Vasc Surg 2021, 74, 1152–1160. [Google Scholar] [CrossRef]
- Diender, E.; Vermeulen, J.J.M.; Pisters, R.; Van Schaik, P.M.; Reijnen, M.M.P.J.; Holewijn, S. Major Adverse Cardiac Events after Elective Infrarenal Endovascular Aortic Aneurysm Repair. J Vasc Surg 2022, 76, 1527–1536.e3. [Google Scholar] [CrossRef] [PubMed]
- Aday, A.W.; Matsushita, K. Epidemiology of Peripheral Artery Disease and Polyvascular Disease. Circ Res 2021, 128, 1818–1832. [Google Scholar] [CrossRef] [PubMed]
- Poredos, P.; Poredos, P. Peripheral Arterial Occlusive Disease and Perioperative Risk. Int Angiol 2018, 37. [Google Scholar] [CrossRef] [PubMed]
- Poredoš, P. Comment on Tsialtas et al., p. 443 Peripheral Arterial Occlusive Disease Increases the Risk of Perioperative Complications. Vasa 2014, 43, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Sirignano, P.; Speziale, F.; Capoccia, L.; Menna, D.; Mansour, W.; Montelione, N.; Setacci, F.; Galzerano, G.; Setacci, C. Iliac and Femoro-Popliteal Arteries Morphological CTA Features as Determinants of Outcome after Standard EVAR Procedures. J Cardiovasc Surg (Torino) 2019, 60. [Google Scholar] [CrossRef] [PubMed]
- Paraskevas, K.I.; Nordon, I.M.; Baxter, S.J.; Shearman, C.P.; Phillips, M.J. Abdominal Aortic Aneurysms, Peripheral Arterial Disease, and Carotid Artery Stenosis: Different Sides of the Same Coin? Angiology 2016, 67, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; O’Donnell, T.F.X.; Swerdlow, N.J.; Li, C.; Lee, A.; Wyers, M.C.; Hamdan, A.D.; Schermerhorn, M.L. Preoperative Risk Score for Access Site Failure in Ultrasound-Guided Percutaneous Aortic Procedures. J Vasc Surg 2019, 70, 1254–1262.e1. [Google Scholar] [CrossRef]
- Novak, Z.; Zaky, A.; Spangler, E.L.; McFarland, G.E.; Tolwani, A.; Beck, A.W. Incidence and Predictors of Early and Delayed Renal Function Decline after Aortic Aneurysm Repair in the Vascular Quality Initiative Database. J Vasc Surg 2021, 74, 1537–1547. [Google Scholar] [CrossRef]
- Octeau, D.; Faries, C.; Barnes, H.; Nakazawa, K.R.; Rao, A.J.; Ting, W.; Marin, M.L.; Vouyouka, A.G.; Faries, P.L.; Tadros, R.O. Neutrophil-to-Lymphocyte Ratio Associated With Adverse Events After Endovascular Aneurysm Repair (EVAR). Ann Vasc Surg 2021, 75, 45–54. [Google Scholar] [CrossRef]
- King, A.H.; Schmaier, A.H.; Harth, K.C.; Kumins, N.H.; Wong, V.L.; Zidar, D.A.; Kashyap, V.S.; Cho, J.S. Elevated Neutrophil-Lymphocyte Ratio Predicts Mortality Following Elective Endovascular Aneurysm Repair. J Vasc Surg 2020, 72, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Caradu, C.; Coatsaliou, Q.; Colacchio, E.C.; Ducasse, E.; Lareyre, F.; Raffort, J. Incidence of Contrast-Induced Nephropathy and Post-Operative Outcomes in Patients Undergoing Chimney Endovascular Aortic Aneurysm Repair. Angiology 2022, 73, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, O.; Di Girolamo, A.; Irace, L.; Baratta, F.; Gossetti, B.; Gattuso, R. Post-Implantation Syndrome: The Impact of Different Devices for Endovascular Abdominal Aortic Aneurysm Repair and Related Etiopathogenetic Implications. Int Angiol 2020, 39. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Choi, J.-H.; Kim, E.-J. Volume of Mural Thrombus Plays a Role in the Elevation of Inflammatory Markers after Endovascular Aortic Repair. J Cardiothorac Surg 2018, 13, 27. [Google Scholar] [CrossRef]
- Oddi, F.M.; Vacca, F.; Ciattaglia, R.; Fresilli, M.; Fazzini, S.; Ippoliti, A. Polyester Stent Graft Devices and Higher Risk of Post-Implantation Syndrome after EVAR: Single-Center Analysis of 367 Patients. Ann Vasc Surg 2021, 75, 455–460. [Google Scholar] [CrossRef]
- Arnaoutoglou, E.; Kouvelos, G.; Papa, N.; Kallinteri, A.; Milionis. H.; Koulouras, V.; Matsagkas, M. Prospective evaluation of post-implantation inflammatory response after EVAR for AAA: influence on patients' 30 day outcome. Eur J Vasc Endovasc Surg. 2015, 49, 175–83. [Google Scholar] [CrossRef]
- Bradley, N.A.; Roxburgh, C.; Khan, F.; Guthrie, G. Postimplantation Syndrome in Endovascular Aortic Aneurysm Repair – a Systematic Review. Vasa 2021, 50, 174–185. [Google Scholar] [CrossRef]
- Siasos, G.; Tsigkou, V.; Kokkou, E.; Oikonomou, E.; Vavuranakis, M.; Vlachopoulos, C.; Verveniotis, A.; Limperi, M.; Genimata, V.; Papavassiliou, A.; et al. Smoking and Atherosclerosis: Mechanisms of Disease and New Therapeutic Approaches. Curr Med Chem 2014, 21, 3936–394. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).