Submitted:
25 October 2023
Posted:
26 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Biomaterials in Neurological Disorders
3. Biomaterials and Their Mechanisms of Action in TBI
3.1. Biomaterials Utilized in TBI Therapy
3.1.1. Hydrogels
Self-Assembling Peptides
3.1.2. Electrospun Nanofibers
3.2. Mechanisms of Repair by Biomaterials in TBI
3.3. Complications, Limitations and Recommendations
| Biomaterial | Characteristic | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Natural hydrogels | Cross-linked macromolecular networks | -No mechanical/spatial restrictions compared to synthetic polymer scaffolds -Mesh size and porosity of hydrogels can be modified -Biocompatible -Injectable -Porous |
-Heterogeneity between batches -May carry natural pathogens -Difficulty in precise modification of the material |
[119,146] |
| Synthetic hydrogels | Can be modified according to need | -Biologically inert -Chemically stable -Easier to control important perimeters |
- Premade, require invasive implantation surgery -Cause more inflammatory response than natural hydrogels |
[223,224,225] |
| Self-assembling peptides SAPNs | Composed of repeating units of amino acids and characterized by the formation of double-β-sheet structures | -High porosity -Increased cell signaling from bioactive peptides that are present in high density at the damaged site -Highly biocompatible -Allow minimally invasive treatments |
-Lack of understanding of their degradability -Lack of data on long-term electroactivity of the scaffold |
[182,226,227] |
| Electrospun nanofibers | A nonwoven mat of micro- and nanofibers is created when fluid filament is stretched in a powerful electric field | -Aligned nanofibers can resemble the topographical characteristics of the extracellular matrix in the brain -Due to large surface-to-volume ratio, electrospun fibers improve cell adhesion, mass transfer characteristics, and drug loading |
-pH difference, local enzymes may degrade the fibers | [150,228] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.-C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed]
- Peeters, W.; van den Brande, R.; Polinder, S.; Brazinova, A.; Steyerberg, E.W.; Lingsma, H.F.; Maas, A.I. Epidemiology of traumatic brain injury in Europe. Acta Neurochir. 2015, 157, 1683–1696. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.; Woessner, D. Sports-related traumatic brain injury. Prim. Care Clin. Off. Pract. 2015, 42, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, B.E.; Stein, C.R.; Bagg, K.; Humphrey, R.J.; Orosco, J. Traumatic brain injury hospitalizations of US army soldiers deployed to Afghanistan and Iraq. Am. J. Prev. Med. 2010, 38, S108–S116. [Google Scholar] [CrossRef] [PubMed]
- Theadom, A.; Mahon, S.; Hume, P.; Starkey, N.; Barker-Collo, S.; Jones, K.; Majdan, M.; Feigin, V.L. Incidence of sports-related traumatic brain injury of all severities: A systematic review. Neuroepidemiology 2020, 54, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.E.; Berry, J.G.; Jamieson, L.M. Head and traumatic brain injuries among Australian youth and young adults, July 2000–June 2006. Brain Inj. 2012, 26, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Peplow, P.V.; Martinez, B.; Gennarelli, T.A. Prevalence, needs, strategies, and risk factors for neurodegenerative diseases. Neurodegener. Dis. Biomark. Towards Transl. Res. Clin. Pract. 2022, 2022, 3–8. [Google Scholar]
- Norup, A.; Kruse, M.; Soendergaard, P.L.; Rasmussen, K.W.; Biering-Sørensen, F. Socioeconomic consequences of traumatic brain injury: A danish nationwide register-based study. J. Neurotrauma 2020, 37, 2694–2702. [Google Scholar] [CrossRef] [PubMed]
- Howlett, J.R.; Nelson, L.D.; Stein, M.B. Mental Health Consequences of Traumatic Brain Injury. Biol. Psychiatry 2022, 91, 413–420. [Google Scholar] [CrossRef]
- DeKosky, S.T.; Blennow, K.; Ikonomovic, M.D.; Gandy, S. Acute and chronic traumatic encephalopathies: Pathogenesis and biomarkers. Nat. Rev. Neurol. 2013, 9, 192–200. [Google Scholar] [CrossRef]
- Crane, P.K.; Gibbons, L.E.; Dams-O’Connor, K.; Trittschuh, E.; Leverenz, J.B.; Keene, C.D.; Sonnen, J.; Montine, T.J.; Bennett, D.A.; Leurgans, S.; et al. Association of Traumatic Brain Injury with Late-Life Neurodegenerative Conditions and Neuropathologic Findings. JAMA Neurol. 2016, 73, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Vespa, P.M. Hormonal dysfunction in neurocritical patients. Curr. Opin. Crit. Care 2013, 19, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Foreman, B.; Lee, H.; Mizrahi, M.A.; Hartings, J.A.; Ngwenya, L.B.; Privitera, M.; Tortella, F.C.; Zhang, N.; Kramer, J.H. Seizures and Cognitive Outcome After Traumatic Brain Injury: A Post Hoc Analysis. Neurocritical Care 2022, 36, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Monsour, M.; Ebedes, D.; Borlongan, C.V. A review of the pathology and treatment of TBI and PTSD. Exp. Neurol. 2022, 351, 114009. [Google Scholar] [CrossRef] [PubMed]
- Alouani, A.T.; Elfouly, T. Traumatic Brain Injury (TBI) Detection: Past, Present, and Future. Biomedicines 2022, 10, 2472. [Google Scholar] [CrossRef] [PubMed]
- Brett, B.L.; Gardner, R.C.; Godbout, J.; Dams-O’Connor, K.; Keene, C.D. Traumatic Brain Injury and Risk of Neurodegenerative Disorder. Biol. Psychiatry 2022, 91, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Haidar, M.A.; Shakkour, Z.; Barsa, C.; Tabet, M.; Mekhjian, S.; Darwish, H.; Goli, M.; Shear, D.; Pandya, J.D.; Mechref, Y. Mitoquinone Helps Combat the Neurological, Cognitive, and Molecular Consequences of Open Head Traumatic Brain Injury at Chronic Time Point. Biomedicines 2022, 10, 250. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention; National Center for Injury Prevention and Control. Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2003. [Google Scholar]
- Dikmen, S.; Machamer, J.; Fann, J.R.; Temkin, N.R. Rates of symptom reporting following traumatic brain injury. J. Int. Neuropsychol. Soc. JINS 2010, 16, 401–411. [Google Scholar] [CrossRef] [PubMed]
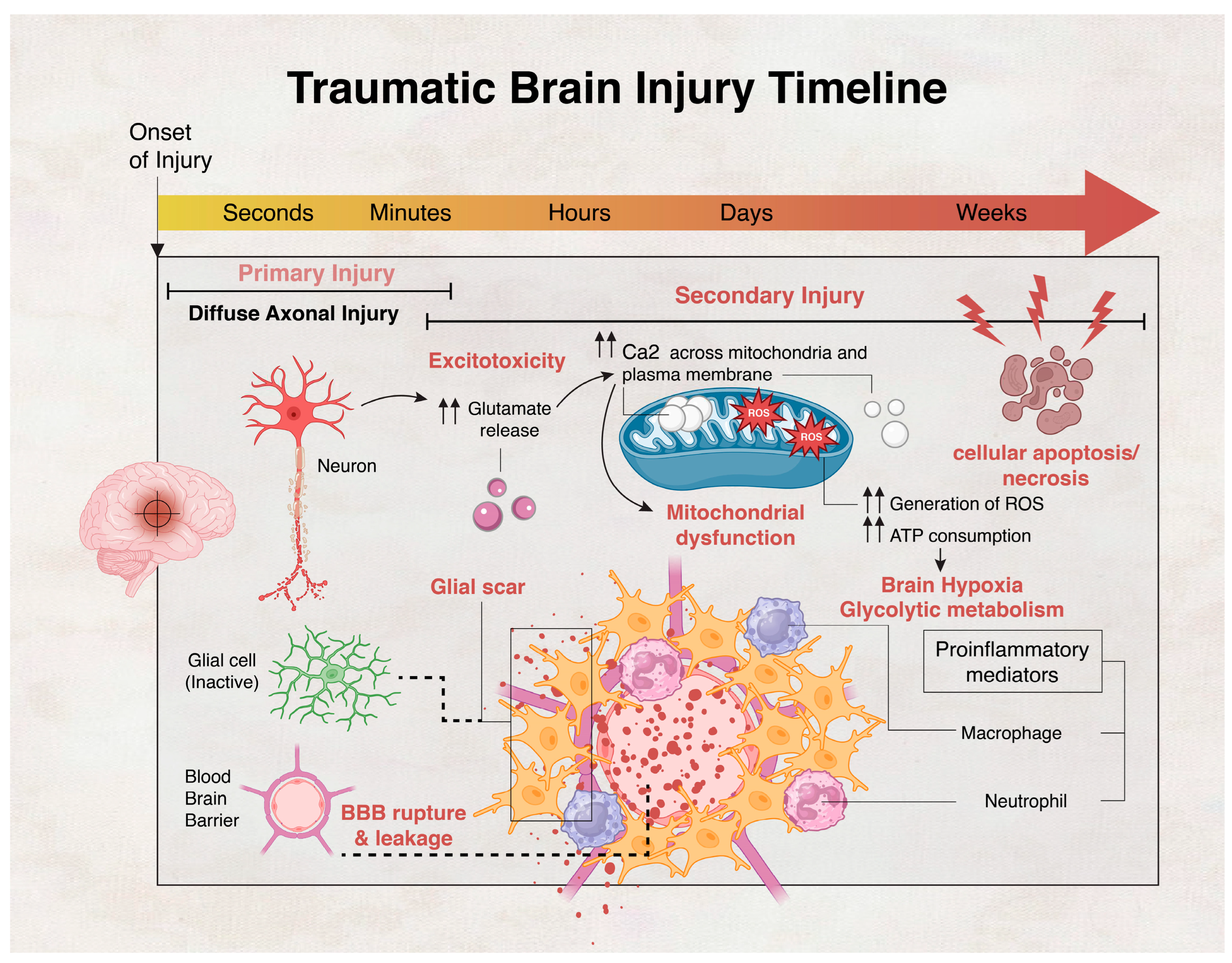
- Dixon, K.J. Pathophysiology of Traumatic Brain Injury. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 215–225. [Google Scholar] [CrossRef]
- McKee, A.C.; Daneshvar, D.H. The neuropathology of traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 45–66. [Google Scholar]
- Kaur, P.; Sharma, S. Recent advances in pathophysiology of traumatic brain injury. Curr. Neuropharmacol. 2018, 16, 1224–1238. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.-C.; Kuan, C.-Y.; Subramaniam, S.; Zhao, J.-Y.; Sivasubramaniam, S.; Chang, H.-Y.; Lin, F.-H. A potent inhibition of oxidative stress induced gene expression in neural cells by sustained ferulic acid release from chitosan based hydrogel. Mater. Sci. Eng. C 2015, 49, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Raad, M.; El Tal, T.; Gul, R.; Mondello, S.; Zhang, Z.; Boustany, R.M.; Guingab, J.; Wang, K.K.; Kobeissy, F. Neuroproteomics approach and neurosystems biology analysis: ROCK inhibitors as promising therapeutic targets in neurodegeneration and neurotrauma. Electrophoresis 2012, 33, 3659–3668. [Google Scholar] [CrossRef] [PubMed]
- Kobeissy, F.H.; Guingab-Cagmat, J.D.; Zhang, Z.; Moghieb, A.; Glushakova, O.Y.; Mondello, S.; Boutté, A.M.; Anagli, J.; Rubenstein, R.; Bahmad, H.; et al. Neuroproteomics and Systems Biology Approach to Identify Temporal Biomarker Changes Post Experimental Traumatic Brain Injury in Rats. Front. Neurol. 2016, 7, 198. [Google Scholar] [CrossRef] [PubMed]
- Ottens, A.K.; Kobeissy, F.H.; Fuller, B.F.; Liu, M.C.; Oli, M.W.; Hayes, R.L.; Wang, K.K. Novel neuroproteomic approaches to studying traumatic brain injury. Prog. Brain Res. 2007, 161, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Stirling, D.P.; Cummins, K.; Wayne Chen, S.; Stys, P. Axoplasmic reticulum Ca2+ release causes secondary degeneration of spinal axons. Ann. Neurol. 2014, 75, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Song, Y.; Zhang, J.; Lin, W.; Dong, H.; Pathology, E. Calcium signaling is implicated in the diffuse axonal injury of brain stem. Int. J. Clin. Exp. Pathol. 2015, 8, 4388. [Google Scholar] [PubMed]
- Arciniegas, D.B.; Silver, J.M. Pharmacotherapy of posttraumatic cognitive impairments. Behav. Neurol. 2006, 17, 25–42. [Google Scholar] [CrossRef]
- Weil, Z.M.; Gaier, K.R.; Karelina, K. Injury timing alters metabolic, inflammatory and functional outcomes following repeated mild traumatic brain injury. Neurobiol. Dis. 2014, 70, 108–116. [Google Scholar] [CrossRef]
- Shaito, A.; Hasan, H.; Habashy, K.J.; Fakih, W.; Abdelhady, S.; Ahmad, F.; Zibara, K.; Eid, A.H.; El-Yazbi, A.F.; Kobeissy, F.H. Western diet aggravates neuronal insult in post-traumatic brain injury: Proposed pathways for interplay. EBioMedicine 2020, 57, 102829. [Google Scholar] [CrossRef]
- Fehily, B.; Fitzgerald, M. Repeated mild traumatic brain injury: Potential mechanisms of damage. Cell Transplant. 2017, 26, 1131–1155. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.G.; Wang, J.A.; Carrico, K.M.; Hall, E.D. Pharmacological inhibition of lipid peroxidation attenuates calpain-mediated cytoskeletal degradation after traumatic brain injury. J. Neurochem. 2011, 117, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Muneer, P.; Chandra, N.; Haorah, J. Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol. Neurobiol. 2015, 51, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Kobeissy, F.H.; Liu, M.C.; Yang, Z.; Zhang, Z.; Zheng, W.; Glushakova, O.; Mondello, S.; Anagli, J.; Hayes, R.L.; Wang, K.K. Degradation of βII-Spectrin Protein by Calpain-2 and Caspase-3 Under Neurotoxic and Traumatic Brain Injury Conditions. Mol Neurobiol 2015, 52, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Warren, M.W.; Zheng, W.; Kobeissy, F.H.; Cheng Liu, M.; Hayes, R.L.; Gold, M.S.; Larner, S.F.; Wang, K.K. Calpain- and caspase-mediated alphaII-spectrin and tau proteolysis in rat cerebrocortical neuronal cultures after ecstasy or methamphetamine exposure. Int. J. Neuropsychopharmacol. 2007, 10, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.L. Blood–brain barrier and traumatic brain injury. J. Neurosci. Res. 2014, 92, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Shlosberg, D.; Benifla, M.; Kaufer, D.; Friedman, A. Blood–brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat. Rev. Neurol. 2010, 6, 393–403. [Google Scholar] [CrossRef]
- Llorens-Bobadilla, E.; Chell, J.M.; Le Merre, P.; Wu, Y.; Zamboni, M.; Bergenstråhle, J.; Stenudd, M.; Sopova, E.; Lundeberg, J.; Shupliakov, O. A latent lineage potential in resident neural stem cells enables spinal cord repair. Science 2020, 370, eabb8795. [Google Scholar] [CrossRef]
- Zhou, L.; Fan, L.; Yi, X.; Zhou, Z.; Liu, C.; Fu, R.; Dai, C.; Wang, Z.; Chen, X.; Yu, P. Soft conducting polymer hydrogels cross-linked and doped by tannic acid for spinal cord injury repair. ACS Nano 2018, 12, 10957–10967. [Google Scholar] [CrossRef]
- Ng, S.Y.; Lee, A.Y.W. Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets. Front. Cell Neurosci. 2019, 13, 528. [Google Scholar] [CrossRef]
- Perez, E.J.; Tapanes, S.A.; Loris, Z.B.; Balu, D.T.; Sick, T.J.; Coyle, J.T.; Liebl, D.J. Enhanced astrocytic d-serine underlies synaptic damage after traumatic brain injury. J. Clin. Investig. 2017, 127, 3114–3125. [Google Scholar] [CrossRef]
- Chen, Y.; Swanson, R.A. Astrocytes and brain injury. J. Cereb. Blood Flow Metab. 2003, 23, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Al-Haj, N.; Issa, H.; Zein, O.E.; Ibeh, S.; Reslan, M.A.; Yehya, Y.; Kobeissy, F.; Zibara, K.; Eid, A.H.; Shaito, A. Phytochemicals as Micronutrients: What Is their Therapeutic Promise in the Management of Traumatic Brain Injury. In Role of Micronutrients in Brain Health; Springer: Berlin/Heidelberg, Germany, 2022; pp. 245–276. [Google Scholar]
- Xiong, B.; Wang, Y.; Chen, Y.; Xing, S.; Liao, Q.; Chen, Y.; Li, Q.; Li, W.; Sun, H. Strategies for structural modification of small molecules to improve blood–brain barrier penetration: A recent perspective. J. Med. Chem. 2021, 64, 13152–13173. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.A. Nanotechnology approaches for the regeneration and neuroprotection of the central nervous system. Surg. Neurol. 2005, 63, 301–306. [Google Scholar] [CrossRef]
- Han, L.; Jiang, C. Evolution of blood-brain barrier in brain diseases and related systemic nanoscale brain-targeting drug delivery strategies. Acta Pharm. Sinica. B 2021, 11, 2306–2325. [Google Scholar] [CrossRef] [PubMed]
- Cash, A.; Theus, M.H. Mechanisms of Blood-Brain Barrier Dysfunction in Traumatic Brain Injury. Int. J. Mol. Sci. 2020, 21, 3344. [Google Scholar] [CrossRef]
- Kochanek, P.M.; Bramlett, H.M.; Dixon, C.E.; Dietrich, W.D.; Mondello, S.; Wang, K.K.; Hayes, R.L.; Lafrenaye, A.; Povlishock, J.T.; Tortella, F.C. Operation brain trauma therapy: 2016 update. Mil. Med. 2018, 183, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Francis, N.L.; Bennett, N.K.; Halikere, A.; Pang, Z.P.; Moghe, P.V. Self-assembling peptide nanofiber scaffolds for 3-D reprogramming and transplantation of human pluripotent stem cell-derived neurons. ACS Biomater. Sci. Eng. 2016, 2, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, F.; Cobianchi, S.; Heimann, C.; Phillips, J.B.; Udina, E.; Navarro, X. Stabilization, rolling, and addition of other extracellular matrix proteins to collagen hydrogels improve regeneration in chitosan guides for long peripheral nerve gaps in rats. Neurosurgery 2017, 80, 465–474. [Google Scholar] [CrossRef]
- Yang, R.; Xu, C.; Wang, T.; Wang, Y.; Wang, J.; Quan, D.; Deng, D.Y. PTMAc-PEG-PTMAc hydrogel modified by RGDC and hyaluronic acid promotes neural stem cells’ survival and differentiation in vitro. RSC Adv. 2017, 7, 41098–41104. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, A.; Huang, Z.; Yin, G.; Pu, X.; Jin, J. Enhancement of neurite adhesion, alignment and elongation on conductive polypyrrole-poly (lactide acid) fibers with cell-derived extracellular matrix. Colloids Surf. B Biointerfaces 2017, 149, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Zhu, H.; Tan, D.; Ren, H.; Gu, X.; Zhao, Y.; Zhang, P.; Sun, Z.; Yang, Y.; Gu, J. Electrospun silk fibroin-based neural scaffold for bridging a long sciatic nerve gap in dogs. J. Tissue Eng. Regen. Med. 2018, 12, e1143–e1153. [Google Scholar] [CrossRef]
- Al-Thani, N.; Haider, M.Z.; Al-Mansoob, M.; Patel, S.; Ahmad, S.M.S.; Kobeissy, F.; Shaito, A. Nano-Engineering in Traumatic Brain Injury. In Impact of Engineered Nanomaterials in Genomics and Epigenomics; John Wiley and Sons Ltd.: West Sussex, UK, 2023; pp. 217–228. [Google Scholar] [CrossRef]
- Khan, J.; Rudrapal, M.; Bhat, E.A.; Ali, A.; Alaidarous, M.; Alshehri, B.; Banwas, S.; Ismail, R.; Egbuna, C. Perspective Insights to Bio-Nanomaterials for the Treatment of Neurological Disorders. Front. Bioeng. Biotechnol. 2021, 9, 724158. [Google Scholar] [CrossRef] [PubMed]
- Hicks, A.U.; Lappalainen, R.S.; Narkilahti, S.; Suuronen, R.; Corbett, D.; Sivenius, J.; Hovatta, O.; Jolkkonen, J. Transplantation of human embryonic stem cell-derived neural precursor cells and enriched environment after cortical stroke in rats: Cell survival and functional recovery. Eur. J. Neurosci. 2009, 29, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Tuladhar, A.; Payne, S.L.; Shoichet, M.S. Harnessing the potential of biomaterials for brain repair after stroke. Front. Mater. 2018, 5, 14. [Google Scholar] [CrossRef]
- Elkin, B.S.; Azeloglu, E.U.; Costa, K.D.; Morrison, B., 3rd. Mechanical heterogeneity of the rat hippocampus measured by atomic force microscope indentation. J. Neurotrauma 2007, 24, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Moshayedi, P.; Nih, L.R.; Llorente, I.L.; Berg, A.R.; Cinkornpumin, J.; Lowry, W.E.; Segura, T.; Carmichael, S.T. Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials 2016, 105, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Biran, R.; Martin, D.C.; Tresco, P.A. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp. Neurol. 2005, 195, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Chen, F.-M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, S.; Liang, M.; Yang, H.; Chang, C. Biomaterials Based Growth Factor Delivery for Brain Regeneration After Injury. Smart Mater. Med. 2022, 3, 352–360. [Google Scholar] [CrossRef]
- Davim, J.P. Biomedical Composites: Materials, Manufacturing and Engineering; Walter de Gruyter: Boston, MA, USA, 2013; Volume 2. [Google Scholar]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Kretlow, J.D.; Klouda, L.; Mikos, A.G. Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hoffman, A.S. Hydrogels. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 153–166. [Google Scholar]
- Gradinaru, V.; Treweek, J.; Overton, K.; Deisseroth, K. Hydrogel-tissue chemistry: Principles and applications. Annu. Rev. Biophys. 2018, 47, 355–376. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.M.; Yacoub, M.H. Practice. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 38. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.; Youssef, G. Dynamic properties of hydrogels and fiber-reinforced hydrogels. J. Mech. Behav. Biomed. Mater. 2018, 85, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Aurand, E.R.; Lampe, K.J.; Bjugstad, K.B. Defining and designing polymers and hydrogels for neural tissue engineering. Neurosci. Res. 2012, 72, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lin, J.; Yan, W. A Prosperous Application of Hydrogels with Extracellular Vesicles Release for Traumatic Brain Injury. Front. Neurol. 2022, 13, 908468. [Google Scholar] [CrossRef]
- Saracino, G.A.; Cigognini, D.; Silva, D.; Caprini, A.; Gelain, F. Nanomaterials design and tests for neural tissue engineering. Chem. Soc. Rev. 2013, 42, 225–262. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering--a review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive polymeric scaffolds for tissue engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Patenaude, M.; Smeets, N.M.; Hoare, T. Designing injectable, covalently cross-linked hydrogels for biomedical applications. Macromol. Rapid Commun. 2014, 35, 598–617. [Google Scholar] [CrossRef] [PubMed]
- Bakarich, S.E.; Pidcock, G.C.; Balding, P.; Stevens, L.; Calvert, P. Recovery from applied strain in interpenetrating polymer network hydrogels with ionic and covalent cross-links. Soft Matter. 2012, 8, 9985–9988. [Google Scholar] [CrossRef]
- Führmann, T.; Obermeyer, J.; Tator, C.H.; Shoichet, M.S. Click-crosslinked injectable hyaluronic acid hydrogel is safe and biocompatible in the intrathecal space for ultimate use in regenerative strategies of the injured spinal cord. Methods 2015, 84, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Koh, L.-D.; Cheng, Y.; Teng, C.-P.; Khin, Y.-W.; Loh, X.-J.; Tee, S.-Y.; Low, M.; Ye, E.; Yu, H.-D.; Zhang, Y.-W. Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Alvarez, G.S.; Hélary, C.; Mebert, A.M.; Wang, X.; Coradin, T.; Desimone, M.F. Antibiotic-loaded silica nanoparticle–collagen composite hydrogels with prolonged antimicrobial activity for wound infection prevention. J. Mater. Chem. B 2014, 2, 4660–4670. [Google Scholar] [CrossRef]
- Desai, R.M.; Koshy, S.T.; Hilderbrand, S.A.; Mooney, D.J.; Joshi, N.S. Versatile click alginate hydrogels crosslinked via tetrazine–norbornene chemistry. Biomaterials 2015, 50, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Zamproni, L.N.; Mundim, M.T.; Porcionatto, M.A. Biology, d. Neurorepair and regeneration of the brain: A decade of bioscaffolds and engineered microtissue. Front. Cell Dev. Biol. 2021, 9, 649891. [Google Scholar] [CrossRef]
- Nih, L.R.; Gojgini, S.; Carmichael, S.T.; Segura, T.J. Dual-function injectable angiogenic biomaterial for the repair of brain tissue following stroke. Nat. Mater. 2018, 17, 642–651. [Google Scholar] [CrossRef]
- Thiele, J.; Ma, Y.; Bruekers, S.M.; Ma, S.; Huck, W.T. 25th anniversary article: Designer hydrogels for cell cultures: A materials selection guide. Adv. Mater. 2014, 26, 125–147. [Google Scholar] [CrossRef] [PubMed]
- Namba, R.; Cole, A.; Bjugstad, K.; Mahoney, M. Development of porous PEG hydrogels that enable efficient, uniform cell-seeding and permit early neural process extension. Acta Biomater. 2009, 5, 1884–1897. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Servidio, C.; Curcio, F.; Cassano, R. Strategies for Hyaluronic Acid-Based Hydrogel Design in Drug Delivery. Pharmaceutics 2019, 11, 407. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.J.; Nguyen, C.; Chun, H.N.; L Llorente, I.; Chiu, A.S.; Machnicki, M.; Zarembinski, T.I.; Carmichael, S.T. Metabolism. Hydrogel-delivered brain-derived neurotrophic factor promotes tissue repair and recovery after stroke. J. Cereb. Blood Flow Metab. 2017, 37, 1030–1045. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.; Holloway, J.L.; Stabenfeldt, S.E. Hyaluronic Acid Biomaterials for Central Nervous System Regenerative Medicine. Cells 2020, 9, 2113. [Google Scholar] [CrossRef] [PubMed]
- Ucar, B.; Humpel, C. Collagen for brain repair: Therapeutic perspectives. Neural Regen. Res. 2018, 13, 595–598. [Google Scholar] [CrossRef]
- Peppas, N.A.; Moynihan, H.J.; Lucht, L.M. The structure of highly crosslinked poly(2-hydroxyethyl methacrylate) hydrogels. J. Biomed. Mater. Res. 1985, 19, 397–411. [Google Scholar] [CrossRef]
- Ahmad, M.B.; Huglin, M.B. DSC studies on states of water in crosslinked poly (methyl methacrylate-co-n-vinyl-2-pyrrolidone) hydrogels. Polym. Int. 1994, 33, 273–277. [Google Scholar] [CrossRef]
- Almany, L.; Seliktar, D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials 2005, 26, 2467–2477. [Google Scholar] [CrossRef]
- Phipps, M.C.; Clem, W.C.; Grunda, J.M.; Clines, G.A.; Bellis, S.L. Increasing the pore sizes of bone-mimetic electrospun scaffolds comprised of polycaprolactone, collagen I and hydroxyapatite to enhance cell infiltration. Biomaterials 2012, 33, 524–534. [Google Scholar] [CrossRef]
- Paşcu, E.I.; Stokes, J.; McGuinness, G.B. Electrospun composites of PHBV, silk fibroin and nano-hydroxyapatite for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4905–4916. [Google Scholar] [CrossRef] [PubMed]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Farrugia, B.L.; Brown, T.D.; Upton, Z.; Hutmacher, D.W.; Dalton, P.D.; Dargaville, T.R. Dermal fibroblast infiltration of poly(ε-caprolactone) scaffolds fabricated by melt electrospinning in a direct writing mode. Biofabrication 2013, 5, 025001. [Google Scholar] [CrossRef]
- Izal, I.; Aranda, P.; Sanz-Ramos, P.; Ripalda, P.; Mora, G.; Granero-Moltó, F.; Deplaine, H.; Gómez-Ribelles, J.L.; Ferrer, G.G.; Acosta, V.; et al. Culture of human bone marrow-derived mesenchymal stem cells on of poly(L-lactic acid) scaffolds: Potential application for the tissue engineering of cartilage. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1737–1750. [Google Scholar] [CrossRef] [PubMed]
- Grad, S.; Kupcsik, L.; Gorna, K.; Gogolewski, S.; Alini, M. The use of biodegradable polyurethane scaffolds for cartilage tissue engineering: Potential and limitations. Biomaterials 2003, 24, 5163–5171. [Google Scholar] [CrossRef] [PubMed]
- Bini, T.; Gao, S.; Wang, S.; Lim, A.; Hai, L.B.; Ramakrishna, S. Electrospun poly (L-lactide-co-glycolide) biodegradable polymer nanofibre tubes for peripheral nerve regeneration. Nanotechnology 2004, 15, 1459. [Google Scholar] [CrossRef]
- Chew, S.Y.; Mi, R.; Hoke, A.; Leong, K.W. Aligned Protein-Polymer Composite Fibers Enhance Nerve Regeneration: A Potential Tissue-Engineering Platform. Adv. Funct. Mater. 2007, 17, 1288–1296. [Google Scholar] [CrossRef]
- Teo, W. A Review on Electrospinning Design and Nanofibre Assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, R.S.; Bachu, R.D.; Boddu, S.H.; Bhaduri, S.J.P. Biomedical applications of electrospun nanofibers: Drug and nanoparticle delivery. Pharmaceutics 2018, 11, 5. [Google Scholar] [CrossRef]
- Feng, X.; Li, J.; Zhang, X.; Liu, T.; Ding, J.; Chen, X. Electrospun polymer micro/nanofibers as pharmaceutical repositories for healthcare. J. Control. Release 2019, 302, 19–41. [Google Scholar] [CrossRef]
- Venugopal, J.; Low, S.; Choon, A.T.; Ramakrishna, S. Interaction of cells and nanofiber scaffolds in tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 84, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Ma, P. Nano-fibrous scaffolds for tissue engineering. Colloids Surf. B Biointerfaces 2004, 39, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, S.; James, R.; Nukavarapu, S.; Laurencin, C. Electrospun nanofiber scaffolds: Engineering soft tissues. Biomed. Mater. 2008, 3, 034002. [Google Scholar] [CrossRef] [PubMed]
- Alhosseini, S.N.; Moztarzadeh, F.; Mozafari, M.; Asgari, S.; Dodel, M.; Samadikuchaksaraei, A.; Kargozar, S.; Jalali, N. Synthesis and characterization of electrospun polyvinyl alcohol nanofibrous scaffolds modified by blending with chitosan for neural tissue engineering. Int. J. Nanomed. 2012, 7, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Nisbet, D.R.; Rodda, A.E.; Horne, M.K.; Forsythe, J.S.; Finkelstein, D.I. Neurite infiltration and cellular response to electrospun polycaprolactone scaffolds implanted into the brain. Biomaterials 2009, 30, 4573–4580. [Google Scholar] [CrossRef] [PubMed]
- Al-Abduljabbar, A.; Farooq, I. Electrospun Polymer Nanofibers: Processing, Properties, and Applications. Polymers 2022, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, X.; He, Y.; Ma, J.; Ni, G.; Zhou, S. From nano to micro to macro: Electrospun hierarchically structured polymeric fibers for biomedical applications. Prog. Polym. Sci. 2018, 81, 80–113. [Google Scholar] [CrossRef]
- Zech, J.; Leisz, S.; Goettel, B.; Syrowatka, F.; Greiner, A.; Strauss, C.; Knolle, W.; Scheller, C.; Maeder, K. Biopharmaceutics. Electrospun Nimodipine-loaded fibers for nerve regeneration: Development and in vitro performance. Eur. J. Pharm. Biopharm. 2020, 151, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Gommans, H.; Cheyns, D.; Aernouts, T.; Girotto, C.; Poortmans, J.; Heremans, P. Electro-Optical Study of Subphthalocyanine in a Bilayer Organic Solar Cell. Adv. Funct. Mater. 2007, 17, 2653–2658. [Google Scholar] [CrossRef]
- Li, W.; Guo, Y.; Wang, H.; Shi, D.; Liang, C.; Ye, Z.; Qing, F.; Gong, J. Electrospun nanofibers immobilized with collagen for neural stem cells culture. J. Mater. Sci. Mater. Med. 2008, 19, 847–854. [Google Scholar] [CrossRef]
- Qian, J.; Lin, Z.; Liu, Y.; Wang, Z.; Lin, Y.; Gong, C.; Ruan, R.; Zhang, J.; Yang, H. Functionalization strategies of electrospun nanofibrous scaffolds for nerve tissue engineering. Smart Mater. Med. 2021, 2, 260–279. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Skolnick, B.E.; Maas, A.I.; Narayan, R.K.; van der Hoop, R.G.; MacAllister, T.; Ward, J.D.; Nelson, N.R.; Stocchetti, N. A Clinical Trial of Progesterone for Severe Traumatic Brain Injury. N. Engl. J. Med. 2014, 371, 2467–2476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ren, Y.; He, Y.; Chang, R.; Guo, S.; Ma, S.; Guan, F.; Yao, M. In situ forming and biocompatible hyaluronic acid hydrogel with reactive oxygen species-scavenging activity to improve traumatic brain injury repair by suppressing oxidative stress and neuroinflammation. Mater. Today Bio. 2022, 15, 100278. [Google Scholar] [CrossRef] [PubMed]
- Bender, E.C.; Kraynak, C.A.; Huang, W.; Suggs, L.J. Cell-Inspired Biomaterials for Modulating Inflammation. Tissue Eng. Part B Rev. 2022, 28, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Babensee, J.E.; McIntire, L.V.; Mikos, A.G. Growth factor delivery for tissue engineering. Pharm. Res. 2000, 17, 497–504. [Google Scholar] [CrossRef]
- He, J.; Wang, X.-M.; Spector, M.; Cui, F.-Z. Scaffolds for central nervous system tissue engineering. Front. Mater. Sci. 2012, 6, 1–25. [Google Scholar] [CrossRef]
- Su, Z.; Niu, W.; Liu, M.-L.; Zou, Y.; Zhang, C.-L. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat. Commun. 2014, 5, 3338. [Google Scholar] [CrossRef] [PubMed]
- Tamariz, E.; Varela-Echavarría, A. The discovery of the growth cone and its influence on the study of axon guidance. Front. Neuroanat. 2015, 9, 51. [Google Scholar] [CrossRef]
- Musah, S.; Morin, S.A.; Wrighton, P.J.; Zwick, D.B.; Jin, S.; Kiessling, L.L. Glycosaminoglycan-binding hydrogels enable mechanical control of human pluripotent stem cell self-renewal. ACS Nano 2012, 6, 10168–10177. [Google Scholar] [CrossRef]
- Li, X.; Duan, L.; Kong, M.; Wen, X.; Guan, F.; Ma, S. Applications and Mechanisms of Stimuli-Responsive Hydrogels in Traumatic Brain Injury. Gels 2022, 8, 482. [Google Scholar] [CrossRef] [PubMed]
- Jurga, M.; Dainiak, M.B.; Sarnowska, A.; Jablonska, A.; Tripathi, A.; Plieva, F.M.; Savina, I.N.; Strojek, L.; Jungvid, H.; Kumar, A. The performance of laminin-containing cryogel scaffolds in neural tissue regeneration. Biomaterials 2011, 32, 3423–3434. [Google Scholar] [CrossRef] [PubMed]
- Cholas, R.H.; Hsu, H.-P.; Spector, M. The reparative response to cross-linked collagen-based scaffolds in a rat spinal cord gap model. Biomaterials 2012, 33, 2050–2059. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ramos, C.; Gómez-Pinedo, U.; Soria, J.M.; Barcia, J.A.; Pradas, M.M. Neural tissue regeneration in experimental brain injury model with channeled scaffolds of acrylate copolymers. Neurosci. Lett. 2015, 598, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Ikada, Y. Challenges in tissue engineering. J. R. Soc. Interface 2006, 3, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, E.D.; Zawada, W.M.; Adams, F.S.; Bell, K.P.; Freed, C.R. Strands of embryonic mesencephalic tissue show greater dopamine neuron survival and better behavioral improvement than cell suspensions after transplantation in parkinsonian rats. Brain Res. 1998, 806, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Sautter, J.; Tseng, J.; Braguglia, D.; Aebischer, P.; Spenger, C.; Seiler, R.; Widmer, H.; Zurn, A. Implants of polymer-encapsulated genetically modified cells releasing glial cell line-derived neurotrophic factor improve survival, growth, and function of fetal dopaminergic grafts. Exp. Neurol. 1998, 149, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Li, M.; Zhang, H.-Q.; Li, G.-L.; Hua, Y.; Shen, Y.; Ji, X.-M.; Wu, C.-J.; An, H.; Ren, M. Collagen-chitosan scaffold impregnated with bone marrow mesenchymal stem cells for treatment of traumatic brain injury. Neural Regen. Res. 2019, 14, 1780. [Google Scholar] [PubMed]
- Latchoumane, C.V.; Betancur, M.I.; Simchick, G.A.; Sun, M.K.; Forghani, R.; Lenear, C.E.; Ahmed, A.; Mohankumar, R.; Balaji, N.; Mason, H.D.; et al. Engineered glycomaterial implants orchestrate large-scale functional repair of brain tissue chronically after severe traumatic brain injury. Sci. Adv. 2021, 7, eabe0207. [Google Scholar] [CrossRef]
- Donaghue, I.E.; Tam, R.; Sefton, M.V.; Shoichet, M.S. Cell and Biomolecule Delivery for Tissue Repair and Regeneration in the Central Nervous System. J. Control Release 2014, 190, 219–227. [Google Scholar] [CrossRef]
- Pettikiriarachchi, J.T.S.; Parish, C.L.; Shoichet, M.S.; Forsythe, J.S.; Nisbet, D.R. Biomaterials for Brain Tissue Engineering. Aust. J. Chem. 2010, 63, 1143–1154. [Google Scholar] [CrossRef]
- Zhang, K.; Feng, Q.; Fang, Z.; Gu, L.; Bian, L. Structurally dynamic hydrogels for biomedical applications: Pursuing a fine balance between macroscopic stability and microscopic dynamics. Chem. Rev. 2021, 121, 11149–11193. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, W.; Deng, C.; Li, G.; Chang, J.; Zhang, Z.; Jiang, X.; Wu, C. 3D Printing of Lotus Root-Like Biomimetic Materials for Cell Delivery and Tissue Regeneration. Adv. Sci. 2017, 4, 1700401. [Google Scholar] [CrossRef]
- Kyburz, K.A.; Anseth, K.S. Synthetic mimics of the extracellular matrix: How simple is complex enough? Ann. Biomed. Eng. 2015, 43, 489–500. [Google Scholar] [CrossRef]
- Ventre, M.; Netti, P.A. Engineering Cell Instructive Materials to Control Cell Fate and Functions through Material Cues and Surface Patterning. ACS Appl. Mater. Interfaces 2016, 8, 14896–14908. [Google Scholar] [CrossRef]
- Wu, R.-X.; Xu, X.-Y.; Wang, J.; He, X.-T.; Sun, H.-H.; Chen, F.-M. Biomaterials for endogenous regenerative medicine: Coaxing stem cell homing and beyond. Appl. Mater. Today 2018, 11, 144–165. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Harn, H.-J.; Chiou, T.-W. The Role of Biomaterials in Implantation for Central Nervous System Injury. Cell Transpl. 2018, 27, 407–422. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Cheng, X.; Liu, P.; Feng, Q.; Wang, S.; Li, Y.; Gu, H.; Zhong, L.; Chen, M.; et al. Integrated printed BDNF-stimulated HUCMSCs-derived exosomes/collagen/chitosan biological scaffolds with 3D printing technology promoted the remodelling of neural networks after traumatic brain injury. Regen. Biomater. 2023, 10, rbac085. [Google Scholar] [CrossRef]
- Li, J.; Zhang, D.; Guo, S.; Zhao, C.; Wang, L.; Ma, S.; Guan, F.; Yao, M. Dual-enzymatically cross-linked gelatin hydrogel promotes neural differentiation and neurotrophin secretion of bone marrow-derived mesenchymal stem cells for treatment of moderate traumatic brain injury. Int. J. Biol. Macromol. 2021, 187, 200–213. [Google Scholar] [CrossRef]
- Tang, W.; Fang, F.; Liu, K.; Huang, Z.; Li, H.; Yin, Y.; Wang, J.; Wang, G.; Wei, L.; Ou, Y.; et al. Aligned Biofunctional Electrospun PLGA-LysoGM1 Scaffold for Traumatic Brain Injury Repair. ACS Biomater. Sci. Eng. 2020, 6, 2209–2218. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, G.; Chen, L.; Zhang, Y.; Luo, Y.; Zheng, Y.; Hu, F.; Forouzanfar, T.; Lin, H.; Liu, B. Neuro-regenerative imidazole-functionalized GelMA hydrogel loaded with hAMSC and SDF-1α promote stem cell differentiation and repair focal brain injury. Bioact. Mater. 2021, 6, 627–637. [Google Scholar] [CrossRef]
- Mahumane, G.D.; Kumar, P.; Pillay, V.; Choonara, Y.E. Repositioning N-Acetylcysteine (NAC): NAC-Loaded Electrospun Drug Delivery Scaffolding for Potential Neural Tissue Engineering Application. Pharmaceutics 2020, 12, 934. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Tu, J.; Fang, G.; Deng, L.; Gao, X.; Guo, K.; Kong, J.; Lv, J.; Guan, W.; Yang, C. Combining PLGA Scaffold and MSCs for Brain Tissue Engineering: A Potential Tool for Treatment of Brain Injury. Stem Cells Int. 2018, 2018, 5024175. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, Z.; Castaño, O.; Castells, A.A.; Mateos-Timoneda, M.A.; Planell, J.A.; Engel, E.; Alcántara, S. Neurogenesis and vascularization of the damaged brain using a lactate-releasing biomimetic scaffold. Biomaterials 2014, 35, 4769–4781. [Google Scholar] [CrossRef] [PubMed]
- Sulejczak, D.; Andrychowski, J.; Kowalczyk, T.; Nakielski, P.; Frontczak-Baniewicz, M.; Kowalewski, T. Electrospun nanofiber mat as a protector against the consequences of brain injury. Folia Neuropathol. 2014, 52, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chopp, M.; Emanuele, M.; Zhang, L.; Zhang, Z.G.; Lu, M.; Zhang, T.; Mahmood, A.; Xiong, Y. Treatment of Traumatic Brain Injury with Vepoloxamer (Purified Poloxamer 188). J. Neurotrauma 2018, 35, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Macks, C.; Jeong, D.; Bae, S.; Webb, K.; Lee, J.S. Dexamethasone-Loaded Hydrogels Improve Motor and Cognitive Functions in a Rat Mild Traumatic Brain Injury Model. Int. J. Mol. Sci. 2022, 23, 11153. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Chang, Z.H.; Chen, C.; Liang, J.; Shi, J.X.; Fan, X.; Shao, Q.; Meng, W.W.; Wang, J.J.; Li, X.H. 3D printing of injury-preconditioned secretome/collagen/heparan sulfate scaffolds for neurological recovery after traumatic brain injury in rats. Stem Cell Res. Ther. 2022, 13, 525. [Google Scholar] [CrossRef]
- Sahab Negah, S.; Oliazadeh, P.; Jahanbazi Jahan-Abad, A.; Eshaghabadi, A.; Samini, F.; Ghasemi, S.; Asghari, A.; Gorji, A. Transplantation of human meningioma stem cells loaded on a self-assembling peptide nanoscaffold containing IKVAV improves traumatic brain injury in rats. Acta Biomater. 2019, 92, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, C.; Zhang, Y.; Chen, S.; Ding, J.; Chen, Z.; Wu, K.; Wu, X.; Zhou, T.; Zeng, M.; et al. Hyaluronan-based hydrogel integrating exosomes for traumatic brain injury repair by promoting angiogenesis and neurogenesis. Carbohydr. Polym. 2023, 306, 120578. [Google Scholar] [CrossRef]
- Tanikawa, S.; Ebisu, Y.; Sedlačík, T.; Semba, S.; Nonoyama, T.; Kurokawa, T.; Hirota, A.; Takahashi, T.; Yamaguchi, K.; Imajo, M.; et al. Engineering of an electrically charged hydrogel implanted into a traumatic brain injury model for stepwise neuronal tissue reconstruction. Sci. Rep. 2023, 13, 2233. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jia, Y.; Wang, S.; Ma, Y.; Huang, G.; Ding, T.; Feng, D.; Genin, G.M.; Wei, Z.; Xu, F. An ECM-Mimicking, Injectable, Viscoelastic Hydrogel for Treatment of Brain Lesions. Adv. Heal. Mater. 2023, 12, e2201594. [Google Scholar] [CrossRef] [PubMed]
- Moisenovich, M.M.; Plotnikov, E.Y.; Moysenovich, A.M.; Silachev, D.N.; Danilina, T.I.; Savchenko, E.S.; Bobrova, M.M.; Safonova, L.A.; Tatarskiy, V.V.; Kotliarova, M.S.; et al. Effect of Silk Fibroin on Neuroregeneration After Traumatic Brain Injury. Neurochem. Res. 2019, 44, 2261–2272. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, X.; Liu, C.; Li, S.; Yang, Z.; Zhang, F.; Chen, X.; Shan, H.; Tao, L.; Zhang, M. Surface-fill H2S-releasing silk fibroin hydrogel for brain repair through the repression of neuronal pyroptosis. Acta Biomater. 2022, 154, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Dai, C.; Liu, X.; Dai, L.; Li, R.; Ma, K.; Xu, H.; Zhao, F.; Zhang, Z.; He, T.; et al. Implantation of regenerative complexes in traumatic brain injury canine models enhances the reconstruction of neural networks and motor function recovery. Theranostics 2021, 11, 768–788. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Han, Y.; Han, Z.; Zhang, D.; Zhang, L.; Zhao, G.; Li, S.; Jin, G.; Yu, R.; Liu, H. In Situ implantable, post-trauma microenvironment-responsive, ROS Depletion Hydrogels for the treatment of Traumatic brain injury. Biomaterials 2021, 270, 120675. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xia, Y.; Zhang, L.; Xu, T.; Yi, Y.; Chen, J.; Liu, Z.; Yang, L.; Chen, S.; Zhou, X.; et al. Loading neural stem cells on hydrogel scaffold improves cell retention rate and promotes functional recovery in traumatic brain injury. Mater. Today Bio. 2023, 19, 100606. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Agas, A.; Siddiqui, Z.; Kim, K.; Iglesias-Montoro, P.; Kalluru, J.; Kumar, V.; Haorah, J. Angiogenic peptide hydrogels for treatment of traumatic brain injury. Bioact. Mater. 2020, 5, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Che, L.; Lei, Z.; Wu, P.; Song, D. A 3D printable and bioactive hydrogel scaffold to treat traumatic brain injury. Adv. Funct. Mater. 2019, 29, 1904450. [Google Scholar] [CrossRef]
- Yang, B.; Liang, C.; Chen, D.; Cheng, F.; Zhang, Y.; Wang, S.; Shu, J.; Huang, X.; Wang, J.; Xia, K.; et al. A conductive supramolecular hydrogel creates ideal endogenous niches to promote spinal cord injury repair. Bioact. Mater. 2022, 15, 103–119. [Google Scholar] [CrossRef]
- Woerly, S.; Fort, S.; Pignot-Paintrand, I.; Cottet, C.; Carcenac, C.; Savasta, M. Development of a sialic acid-containing hydrogel of poly [N-(2-hydroxypropyl) methacrylamide]: Characterization and implantation study. Biomacromolecules 2008, 9, 2329–2337. [Google Scholar] [CrossRef] [PubMed]
- Woerly, S.; Petrov, P.; Sykova, E.; Roitbak, T.; Simonova, Z.; Harvey, A. Neural tissue formation within porous hydrogels implanted in brain and spinal cord lesions: Ultrastructural, immunohistochemical, and diffusion studies. Tissue Eng. 1999, 5, 467–488. [Google Scholar] [CrossRef] [PubMed]
- Lesný, P.; De Croos, J.; Přádný, M.; Vacık, J.; Michalek, J.; Woerly, S.; Syková, E. Polymer hydrogels usable for nervous tissue repair. J. Chem. Neuroanat. 2002, 23, 243–247. [Google Scholar] [CrossRef]
- Zhong, Y.; Bellamkonda, R.V. Biomaterials for the central nervous system. J. R. Soc. Interface 2008, 5, 957–975. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, M.; Sun, M.; Wang, X.; Pei, D.; Lei, B.; Li, A. Engineering antioxidant poly (citrate-gallic acid)-Exosome hybrid hydrogel with microglia immunoregulation for Traumatic Brain Injury-post neuro-restoration. Compos. Part B Eng. 2022, 242, 110034. [Google Scholar] [CrossRef]
- Liu, Y.; Hsu, Y.H.; Huang, A.P.; Hsu, S.H. Semi-Interpenetrating Polymer Network of Hyaluronan and Chitosan Self-Healing Hydrogels for Central Nervous System Repair. ACS Appl. Mater. Interfaces 2020, 12, 40108–40120. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, D.; Ren, Y.; Guo, S.; Li, J.; Ma, S.; Yao, M.; Guan, F. Injectable hyaluronic acid hydrogel loaded with BMSC and NGF for traumatic brain injury treatment. Mater. Today Bio. 2022, 13, 100201. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Chen, Y.; Zhang, J.; Gao, F.; Ma, S.; Guan, F. Chitosan-based thermosensitive composite hydrogel enhances the therapeutic efficacy of human umbilical cord MSC in TBI rat model. Mater. Today Chem. 2019, 14, 100192. [Google Scholar] [CrossRef]
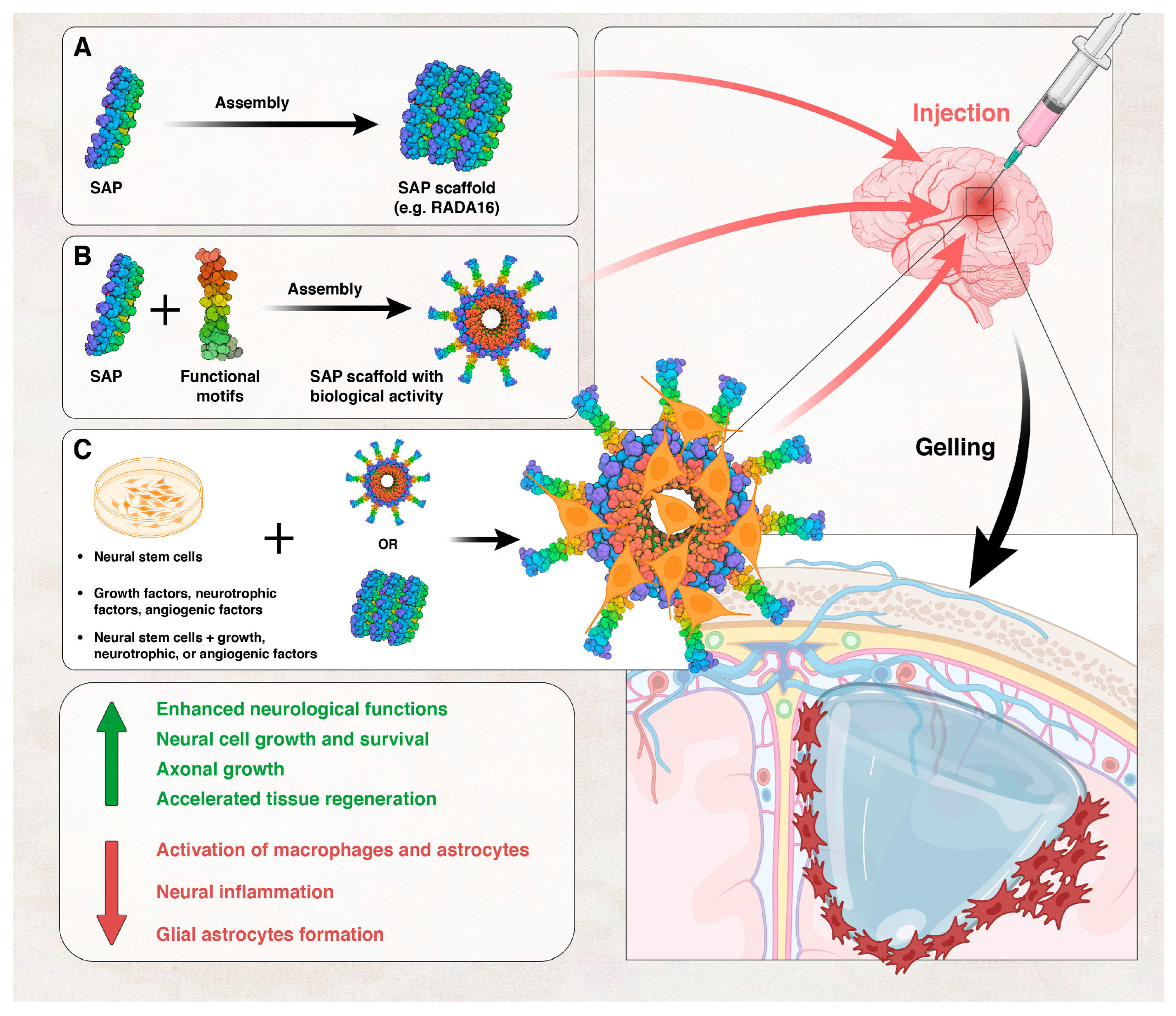
- Chassenieux, C.; Tsitsilianis, C. Recent trends in pH/thermo-responsive self-assembling hydrogels: From polyions to peptide-based polymeric gelators. Soft Matter 2016, 12, 1344–1359. [Google Scholar] [CrossRef]
- Hernandez, A.; Hartgerink, J.D.; Young, S. Self-assembling peptides as immunomodulatory biomaterials. Front. Bioeng. Biotechnol. 2023, 11, 1139782. [Google Scholar] [CrossRef]
- Bakhtiary, N.; Ghalandari, B.; Ghorbani, F.; Varma, S.N.; Liu, C. Advances in Peptide-Based Hydrogel for Tissue Engineering. Polymers 2023, 15, 1608. [Google Scholar] [CrossRef] [PubMed]
- Kartha, K.K.; Babu, S.S.; Srinivasan, S.; Ajayaghosh, A. Attogram sensing of trinitrotoluene with a self-assembled molecular gelator. J. Am. Chem. Soc. 2012, 134, 4834–4841. [Google Scholar] [CrossRef]
- Hosseinkhani, H.; Hong, P.D.; Yu, D.S. Self-assembled proteins and peptides for regenerative medicine. Chem. Rev. 2013, 113, 4837–4861. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.-Y.; Chen, M.-H.; Chang, W.-H.; Huang, M.-Y.; Wang, T.-W. Neural stem cells encapsulated in a functionalized self-assembling peptide hydrogel for brain tissue engineering. Biomaterials 2013, 34, 2005–2016. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.; Li, J.; Chen, C.; Liu, Y. Self-Assembling Peptide-Based Hydrogels for Wound Tissue Repair. Adv. Sci. 2022, 9, e2104165. [Google Scholar] [CrossRef] [PubMed]
- Collier, J.H. Modular self-assembling biomaterials for directing cellular responses. Soft Matter 2008, 4, 2310–2315. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Leung, K.K.G.; Su, H.; Yuan, Q.; Wang, L.; Chu, T.-H.; Zhang, W.; Pu, J.K.S.; Ng, G.K.P.; Wong, W.M. Self-assembling peptide nanofiber scaffold promotes the reconstruction of acutely injured brain. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 345–351. [Google Scholar] [CrossRef]
- Ellis-Behnke, R.; So, K.-F.; Zhang, S. Molecular repair of the brain using self-assembling peptides. Chim. Oggi 2006, 24, 42–45. [Google Scholar]
- Ellis-Behnke, R.; Teather, L.; Schneider, G.; So, K.-F. Using nanotechnology to design potential therapies for CNS regeneration. Curr. Pharm. Des. 2007, 13, 2519–2528. [Google Scholar] [CrossRef]
- Zhang, N.; Luo, Y.; He, L.; Zhou, L.; Wu, W. A self-assembly peptide nanofibrous scaffold reduces inflammatory response and promotes functional recovery in a mouse model of intracerebral hemorrhage. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1205–1217. [Google Scholar] [CrossRef]
- Sarkar, B.; Ma, X.; Agas, A.; Siddiqui, Z.; Iglesias-Montoro, P.; Nguyen, P.K.; Kim, K.K.; Haorah, J.; Kumar, V.A. In vivo neuroprotective effect of a self-assembled peptide hydrogel. Chem. Eng. J. 2021, 408, 127295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Holmes, T.C.; DiPersio, C.M.; Hynes, R.O.; Su, X.; Rich, A. Self-complementary oligopeptide matrices support mammalian cell attachment. Biomaterials 1995, 16, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Leung, G.K.K.; Wang, Y.C.; Wu, W. Peptide nanofiber scaffold for brain tissue reconstruction. Methods Enzymol. 2012, 508, 177–190. [Google Scholar] [PubMed]
- Ellis-Behnke, R.G.; Liang, Y.-X.; You, S.-W.; Tay, D.K.; Zhang, S.; So, K.-F.; Schneider, G.E. Nano neuro knitting: Peptide nanofiber scaffold for brain repair and axon regeneration with functional return of vision. Proc. Natl. Acad. Sci. USA 2006, 103, 5054–5059. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Nakajima, C.; Ajioka, I.; Muraoka, T.; Yaguchi, A.; Fujioka, T.; Akimoto, S.; Matsuo, M.; Lotfy, A.; Nakamura, S.; et al. Amphiphilic peptide-tagged N-cadherin forms radial glial-like fibers that enhance neuronal migration in injured brain and promote sensorimotor recovery. Biomaterials 2023, 294, 122003. [Google Scholar] [CrossRef]
- Maclean, F.L.; Ims, G.M.; Horne, M.K.; Williams, R.J.; Nisbet, D.R. A Programmed Anti-Inflammatory Nanoscaffold (PAIN) as a 3D Tool to Understand the Brain Injury Response. Adv. Mater. 2018, 30, e1805209. [Google Scholar] [CrossRef] [PubMed]
- Maclean, F.L.; Lau, C.L.; Ozergun, S.; O’Shea, R.D.; Cederfur, C.; Wang, J.; Healy, K.E.; Walker, F.R.; Tomas, D.; Horne, M.K.; et al. Galactose-functionalised PCL nanofibre scaffolds to attenuate inflammatory action of astrocytes in vitro and in vivo. J. Mater. Chem. B 2017, 5, 4073–4083. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, M.; Guo, Q.; Liu, N.; Wang, Y.; Wu, T. Modulating axonal growth and neural stem cell migration with the use of uniaxially aligned nanofiber yarns welded with NGF-loaded microparticles. Mater. Today Adv. 2023, 17, 100343. [Google Scholar] [CrossRef]
- Yao, M.; Gao, F.; Xu, R.; Zhang, J.; Chen, Y.; Guan, F. A dual-enzymatically cross-linked injectable gelatin hydrogel loaded with BMSC improves neurological function recovery of traumatic brain injury in rats. Biomater. Sci. 2019, 7, 4088–4098. [Google Scholar] [CrossRef]
- Jeong, D.U.; Bae, S.; Macks, C.; Whitaker, J.; Lynn, M.; Webb, K.; Lee, J.S. Hydrogel-mediated local delivery of dexamethasone reduces neuroinflammation after traumatic brain injury. Biomed. Mater. 2021, 16, 035002. [Google Scholar] [CrossRef]
- Kuan, C.-Y.; Lin, Y.-Y.; Chen, C.-Y.; Yang, C.-C.; Chi, C.-Y.; Li, C.-H.; Dong, G.-C.; Lin, F.-H.J. The preparation of oxidized methylcellulose crosslinked by adipic acid dihydrazide loaded with vitamin C for traumatic brain injury. J. Mater. Chem. B 2019, 7, 4499–4508. [Google Scholar] [CrossRef]
- Lu, J.; Yan, X.; Sun, X.; Shen, X.; Yin, H.; Wang, C.; Liu, Y.; Lu, C.; Fu, H.; Yang, S. Synergistic effects of dual-presenting VEGF-and BDNF-mimetic peptide epitopes from self-assembling peptide hydrogels on peripheral nerve regeneration. Nanoscale 2019, 11, 19943–19958. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.Z.; Lee, K.Y.; Qi, Z.X.; Wang, Z.; Xu, Z.Y.; Wu, X.H.; Mao, Y. Neuroinflammation Following Traumatic Brain Injury: Take It Seriously or Not. Front. Immunol. 2022, 13, 855701. [Google Scholar] [CrossRef]
- Zhang, N.; He, L.; Wu, W. Self-assembling peptide nanofibrous hydrogel as a promising strategy in nerve repair after traumatic injury in the nervous system. Neural Regen. Res. 2016, 11, 717–718. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, F.J.; Cerpa, W. Regulation of Phosphorylated State of NMDA Receptor by STEP(61) Phosphatase after Mild-Traumatic Brain Injury: Role of Oxidative Stress. Antioxidants 2021, 10, 1575. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhou, J.; Huang, T.; Zhang, Z.; Xing, Q.; Zhou, X.; Zhang, K.; Yao, M.; Cheng, T.; Wang, X.; et al. Sodium alginate/collagen/stromal cell-derived factor-1 neural scaffold loaded with BMSCs promotes neurological function recovery after traumatic brain injury. Acta Biomater. 2021, 131, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Huang, C.J.; Xu, X.D.; Jin, G.H.; Huang, R.Q.; Huang, J.F.; Chen, Y.N.; Ju, S.Q.; Wang, Y.; Shi, Y.W.; et al. Transplantation of RADA16-BDNF peptide scaffold with human umbilical cord mesenchymal stem cells forced with CXCR4 and activated astrocytes for repair of traumatic brain injury. Acta Biomater. 2016, 45, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.W.; Chang, K.C.; Chen, L.H.; Liao, S.Y.; Yeh, C.W.; Chuang, Y.J. Effects of an injectable functionalized self-assembling nanopeptide hydrogel on angiogenesis and neurogenesis for regeneration of the central nervous system. Nanoscale 2017, 9, 16281–16292. [Google Scholar] [CrossRef]
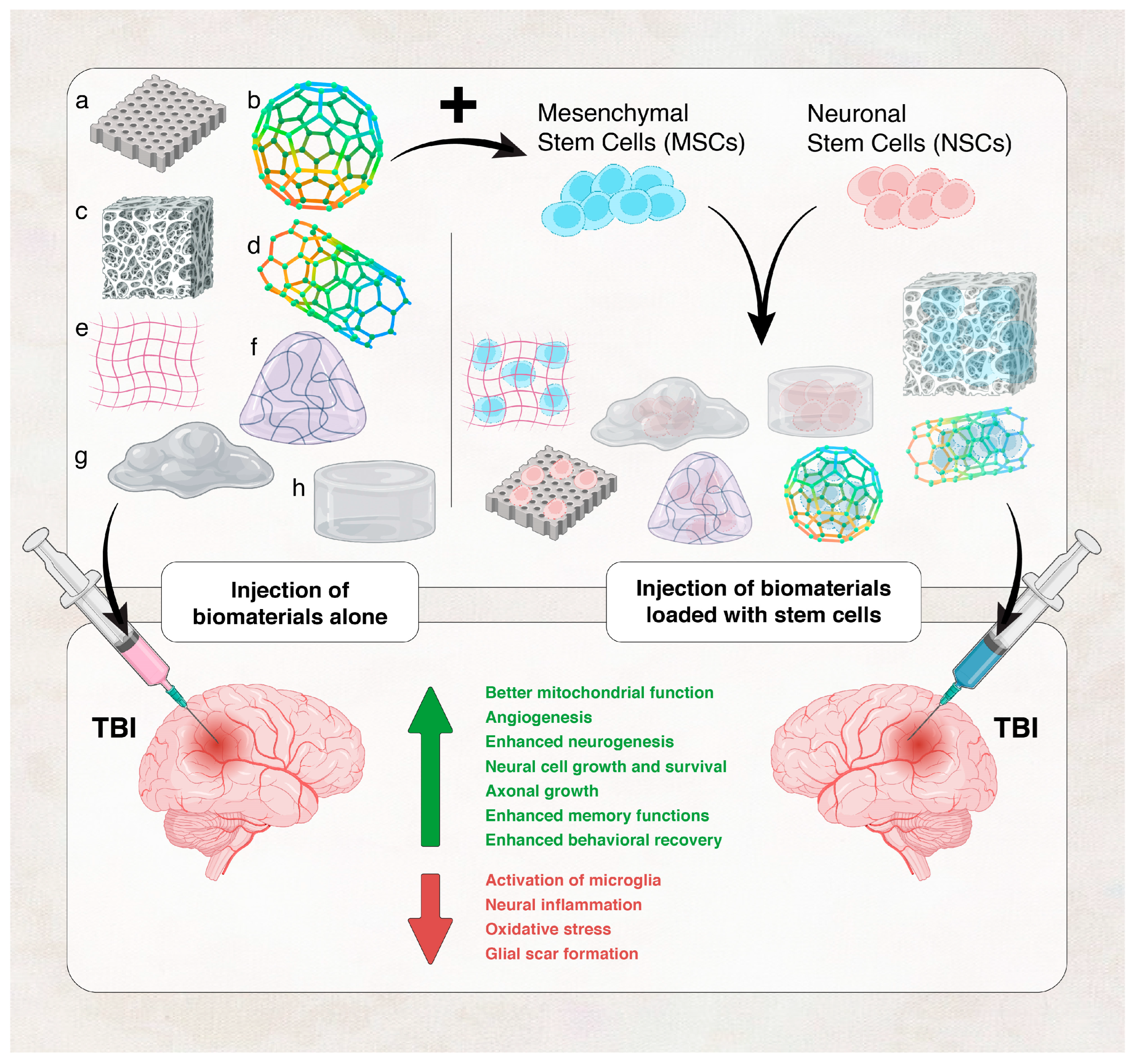
- Tabet, M.; Hasan, H.; Abdelhady, S.; Mahavadi, A.K.; Clervius, H.; Nasrallah, L.; Ahmad, F.; Shaito, N.; Ramakrawala, R.; Zibara, K.; et al. Evaluation of Evidence: Stem Cells as a Treatment Option for Traumatic Brain Injury. eLS 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.; Zoneff, E.; Thomas, J.; Hong, N.; Tan, L.; McGillivray, D.; Perriman, A.; Law, K.; Thompson, L.; Moriarty, N. Hydrogel oxygen reservoirs increase functional integration of neural stem cell grafts by meeting metabolic demands. Nat. Commun. 2023, 14, 457. [Google Scholar] [CrossRef]
- Zimmermann, H.; Zimmermann, D.; Reuss, R.; Feilen, P.; Manz, B.; Katsen, A.; Weber, M.; Ihmig, F.; Ehrhart, F.; Gessner, P. Towards a medically approved technology for alginate-based microcapsules allowing long-term immunoisolated transplantation. J. Mater. Sci. Mater. Med. 2005, 16, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.M.; Oyen, M.L. Hydrogel composite materials for tissue engineering scaffolds. Jom 2013, 65, 505–516. [Google Scholar] [CrossRef]
- Gunatillake, P.A.; Adhikari, R.; Gadegaard, N. Biodegradable synthetic polymers for tissue engineering. Eur. Cell Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bergsma, J.E.; Rozema, F.; Bos, R.; Boering, G.; De Bruijn, W.; Pennings, A. In vivo degradation and biocompatibility study of in vitro pre-degraded as-polymerized polylactide particles. Biomaterials 1995, 16, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Boontheekul, T.; Kong, H.-J.; Mooney, D.J. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials 2005, 26, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Tsuji, H. Effects of molecular weight and small amounts of d-lactide units on hydrolytic degradation of poly (l-lactic acid) s. Polym. Degrad. Stab. 2006, 91, 1665–1673. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Mizuno, A.; Ikada, Y. Properties and morphology of poly (L-lactide). III. Effects of initial crystallinity on long-term in vitro hydrolysis of high molecular weight poly (L-lactide) film in phosphate-buffered solution. J. Appl. Polym. Sci. 2000, 77, 1452–1464. [Google Scholar] [CrossRef]
- Suzui, M.; Futakuchi, M.; Fukamachi, K.; Numano, T.; Abdelgied, M.; Takahashi, S.; Ohnishi, M.; Omori, T.; Tsuruoka, S.; Hirose, A.; et al. Multiwalled carbon nanotubes intratracheally instilled into the rat lung induce development of pleural malignant mesothelioma and lung tumors. Cancer Sci. 2016, 107, 924–935. [Google Scholar] [CrossRef]
- Akhtar, A.; Farzam Rad, V.; Moradi, A.-R.; Yar, M.; Bazzar, M. Emerging polymeric biomaterials and manufacturing-based tissue engineering approaches for neuro regeneration-A critical review on recent effective approaches. Smart Mater. Med. 2023, 4, 337–355. [Google Scholar] [CrossRef]
- Masaeli, R.; Zandsalimi, K.; Tayebi, L. Biomaterials Evaluation: Conceptual Refinements and Practical Reforms. Ther. Innov. Regul. Sci. 2019, 53, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, Y.; Li, X.; Zheng, D.; Gao, J.; Yang, Z. Supramolecular hydrogels of self-assembled zwitterionic-peptides. Chin. Chem. Lett. 2021, 32, 3636–3640. [Google Scholar] [CrossRef]
- Williams, D. Benefit and risk in tissue engineering. Mater. Today 2004, 7, 24–29. [Google Scholar] [CrossRef]
- Hu, H.; Chen, X.; Zhao, K.; Zheng, W.; Gao, C. Recent Advances in Biomaterials-Based Therapies for Alleviation and Regeneration of Traumatic Brain Injury. Macromol. Biosci. 2023, 23, e2200577. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Liu, Z.; Mao, S.; Han, L. Unraveling the Mechanobiology Underlying Traumatic Brain Injury with Advanced Technologies and Biomaterials. Adv. Heal. Mater. 2022, 11, e2200760. [Google Scholar] [CrossRef] [PubMed]
- Bertsch, P.; Diba, M.; Mooney, D.J.; Leeuwenburgh, S.C.G. Self-Healing Injectable Hydrogels for Tissue Regeneration. Chem. Rev. 2023, 123, 834–873. [Google Scholar] [CrossRef]
- Keimpema, E.; Fokkens, M.R.; Nagy, Z.; Agoston, V.; Luiten, P.G.; Nyakas, C.; Boddeke, H.W.; Copray, J.C. Early transient presence of implanted bone marrow stem cells reduces lesion size after cerebral ischaemia in adult rats. Neuropathol. Appl. Neurobiol. 2009, 35, 89–102. [Google Scholar] [CrossRef]
- Hackelbusch, S.; Rossow, T.; Steinhilber, D.; Weitz, D.A.; Seiffert, S. Hybrid microgels with thermo-tunable elasticity for controllable cell confinement. Adv. Healthc. Mater. 2015, 4, 1841–1848. [Google Scholar] [CrossRef]
- Bjugstad, K.; Lampe, K.; Kern, D.; Mahoney, M. Biocompatibility of poly (ethylene glycol)-based hydrogels in the brain: An analysis of the glial response across space and time. J. Biomed. Mater. Res. Part A 2010, 95, 79–91. [Google Scholar] [CrossRef]
- Tamariz, E.; Wan, A.C.; Pek, Y.S.; Giordano, M.; Hernández-Padrón, G.; Varela-Echavarría, A.; Velasco, I.; Castaño, V.M. Delivery of chemotropic proteins and improvement of dopaminergic neuron outgrowth through a thixotropic hybrid nano-gel. J. Mater. Sci. Mater. Med. 2011, 22, 2097–2109. [Google Scholar] [CrossRef]
- Carvalho, A.; Gallo, J.; Pereira, D.M.; Valentão, P.; Andrade, P.B.; Hilliou, L.; Ferreira, P.M.; Bañobre-López, M.; Martins, J.A. Magnetic dehydrodipeptide-based self-assembled hydrogels for theragnostic applications. Nanomaterials 2019, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Koss, K.; Unsworth, L. Neural tissue engineering: Bioresponsive nanoscaffolds using engineered self-assembling peptides. Acta Biomater. 2016, 44, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Zhou, Y.; Chang, J. Electrospun nanofibrous materials for tissue engineering and drug delivery. Sci. Technol. Adv. Mater. 2010, 11, 014108. [Google Scholar] [CrossRef] [PubMed]
| Property | Synthetic Biomaterial | Natural Biomaterial |
|---|---|---|
| Source | Artificially synthesized | Biological sources |
| Biodegradability | Variable, controllable | Naturally degradable |
| Immunogenicity | Generally low | Potential immune response |
| Mechanical properties | Customizable for specific needs | Variable |
| Biocompatibility | Reduced, can be optimized | Good biocompatibility |
| Growth factors | Controlled release | Potential endogenous release |
| Examples | Poly-anhydrides and poly-orthoesters. | Collagen, chitosan, hyaluronic acid |
| Study | Biomaterial | Species | Outcome | References |
|---|---|---|---|---|
| Liu et al. (2023) | Collagen/chitosan/BMExos scaffold | Rat |
|
[143] |
| Li et al. (2021) | Gelatin hydrogel | In vitro & mice |
|
[144] |
| Tang et al. (2020) | aPLGA-LysoGM1 scaffold | In vitro & rat |
|
[145] |
| Zheng et al. (2020) | Gelatin methacrylate hydrogel with polydopamine nanoparticles and hAMSCs | Rat |
|
[146] |
| Mahumane et al. (2020) | N-acetylcysteine (NAC)-loaded poly(lactic-co-glycolic acid) (PLGA) electrospun nanofiber | In vitro & ex vivo (Rat pheochromocytoma PC12 cells) and human glioblastoma multiform A172 cells) |
|
[147] |
| Zhou et al. (2018) | poly(lactic-co-glycolic acid) (PLGA) scaffold | In vitro & in vivo Mesenchymal stem cells (MSCs) and neurons |
|
[148] |
| Álvarez et al. (2014) | poly-L/DL lactic acid (PLA70/30) nanofibers | Mice |
|
[149] |
| Sulejczak et al. (2014) | Electrospun nanofiber/L-lactide-caprolactone copolymer nanofiber net | Rat |
|
[150] |
| Zhang et al. (2018) | Vepoloxamer | Rat |
|
[151] |
| Macks et al. (2022) | poly(Ethylene) glycol-bis-(acryloyloxy acetate) (PEG-bis-AA) with dexamethasone (DX)-conjugated hyaluronic acid (HA-DXM) | Rat |
|
[152] |
| Latchoumane et al. (2021) | Engineered Chondroitin sulfate (eCS) | Rat |
|
[134] |
| Liu et al. (2022) | Secretome/collagen/heparan sulfate scaffold | Rat |
|
[153] |
| Sahab Negah et al. (2019) | Self-assembling peptide hMgSCs + R-GSIK | Rat |
|
[154] |
| Liu et al. (2023) | Bone marrow mesenchymal stem cell-derived exosomes (BME) + hyaluronan-collagen hydrogel (DHC-BME) | Rat |
|
[155] |
| Tanikawa et al. (2023) | Electrically charged hydrogels (C1A1) + VEGF | Mice |
|
[156] |
| Hu et al. (2023) | Self-healing hydrogel (HA-PBA/Gel-Dopa) | Mice |
|
[157] |
| Moisenovich et al. (2019) | Silk fibroin scaffold | Rat |
|
[158] |
| Chen et al. (2022) | Hydrogen sulfide(H2S)-releasing silk fibroin (SF) hydrogel (H2S@SF) | Mice |
|
[159] |
| Jiang et al. (2021) | Collagen/Silk fibroin (SF) scaffold | Canine |
|
[160] |
| Qian et al. (2021) | TM/PC hydrogel(tri-glycerol monostearate, propylene sulfide, and curcumin) | Mice |
|
[161] |
| Zhang et al. (2022) | HT/HGA hydrogel (hyaluronic acid-tyramine + antioxidant gallic acid-grafted hyaluronic acid) | Mice |
|
[119] |
| Chen et al. (2023) | Gelatin methacrylate and sodium alginate hydrogel (GelMA/Alg) | Rat |
|
[162] |
| Ma et al. (2020) | Self-assembling peptide-based hydrogel | Rat |
|
[163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





