1. Introduction
Primary ITP is defined as a platelet count <100×10
9/L whose cause has not been identified. The concept “secondary ITP” arises from scenarios where the low platelet counts are subsequent to diagnosed diseases known to cause immune destruction of platelets. The incidence of primary ITP is 2-4 cases per 100,000 individuals per year, in both adults and children. The prevalence is higher in adults (10 per 100,000
vs. 5 per 100,000 individuals in children) because the rate of chronicity is greater in this population [
1].
There are many pathophysiological mechanisms playing a causal role in primary ITP, which explains the heterogeneity of the disease. Nevertheless, the consequences are basically of two types. On the one hand, there is an increase in platelet destruction, which is mainly, but not solely, caused by the onset of autoantibodies able to opsonize the cell surface for the subsequent complement- or phagocyte-mediated cell killing. The increased rate of platelet desialylation, which accelerates liver clearance, and a greater apoptosis rate also contribute to the low counts. On the other hand, platelet turnover decreases because of a lower rate of cell production. This is due to autoantibodies targeting thrombopoietin (TPO) receptor and preventing TPO from stimulating proliferation and differentiation of megakaryocytes, as well as to an apoptotic imbalance concerning these cells [
2].
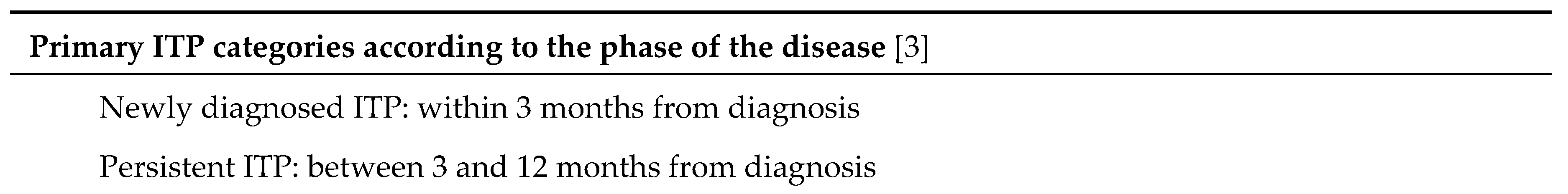
The autoantibodies identified in patients with primary ITP can bind a large variety of targets, which are frequently located at the platelet surface, for instance glycoproteins (GP) GPIIb/IIIa or GPIb/IX. Bleeding manifestations are not the only symptoms of primary ITP. Asthenia is also frequently found, and some patients are predisposed to experience thromboembolic events, infection or other autoimmune diseases. Primary ITP shows a self-limiting course in the majority of children and in one third of adults. According to the standardized terminology [
3], the disease can be defined according to the time elapsed since diagnosis: i) “newly diagnosed primary ITP” encompasses all cases at diagnosis; ii) “persistent primary ITP” refers to the period lasting between 3 and 12 months from diagnosis; iii) “chronic primary ITP” is the term reserved for patients with primary ITP lasting for more than 12 months.
In contrast to the relevant advances achieved in the therapeutic field in the last decade, there has been little progress in the diagnosis of the disease, and reliable markers or solid confirmatory tests are lacking. Diagnosis continues to be essentially clinical and based on excluding other causes of thrombocytopenia. When a patient is suspected to have primary ITP, once the clinical history, physical examination, peripheral blood smear, immunoglobulin level and viral serology have allowed us to rule out other processes, the general rule consists of limiting tests to a minimum, since most of them will not be very informative. The paradigmatic laboratory finding is isolated thrombocytopenia, and careful examination of the peripheral blood smear is mandatory. In primary ITP patients, platelets are usually large and granular, with elevated mean platelet volumes and immature fractions. The systematic analysis of bone marrow is not recommended, except in the event that treatment response is inadequate, or when other abnormalities in the peripheral blood smear or in the clinical presentation lead to the suspicion of other disorders. These limitations may result in an incorrect diagnosis in one out of 7 patients identified as having primary ITP [
4]. Further attempts to overcome this challenge are required.
Quality of life (QoL) of primary ITP patients is reduced to a similar extent to that seen in patients with other chronic diseases such as cancer, arthritis or diabetes mellitus [
5]. The proper treatment of primary ITP not only has to pursue the recovery of platelet counts and cessation of bleeding. Minimizing the impact of the disease on the patient’s QoL is highly advisable.
Table 1 summarizes the most relevant topics regarding pathophysiology and diagnosis of primary ITP, and
Table 2 compiles a list of definitions and concepts that have reached consensus and should be well known [
3,
6].
2. Methods
The AGREE methodology was followed for the compilation of these recommendations. The PICO (Population, Intervention, Comparison, and Outcome) framework was used to select the questions to be addressed regarding ITP management. These were as follows: first-line, second-line and multirefractory ITP treatment; follow-up of patients with primary ITP; primary ITP in selected patient populations; secondary ITP; primary ITP and thrombosis; ITP and COVID-19.
Each author was assigned one topic to perform a comprehensive literature search, especially focusing on the last 5 years and guided by relevant MeSH terms. In order to establish the recommendations, the reliability of the compiled information was evaluated, according to strengths and limitations. Finally, peer reviews of each topic were performed until a general consensus was reached.
3. First-Line, Second-Line and Multirefractory ITP Treatment
Table 3 summarizes the main issues to consider regarding first and second-line treatment, as well as those scenarios of recurrent refractoriness, and sets out the response rates expected for each therapeutic strategy [
1,
6,
7,
8,
9].
First-Line Treatment
The aim of the treatment is to achieve hemorrhage cessation and prevent future bleeding events. Treatment must be started in newly diagnosed adult patients with active bleeding or when they present with platelet counts <20×10
9/L (<30×10
9/L when they are >65 years or present with hemorrhage risk factors). These criteria are not necessarily to be applied for a second treatment, since the patient´s opinion is particularly important in such a situation. First-line treatment has not evolved dramatically.
Table 4 shows the main therapeutic options with their expected response rates. Glucocorticoids remain the cornerstone, although treatment duration has been reduced to minimize side effects. The initial dose of prednisone (0.5-1 mg/kg/day, not exceeding 80 mg daily) should not be kept beyond 3 weeks (two weeks in the event of no response). The dose must be progressively reduced and treatment must be terminated no later than 8 weeks from the start. Dexamethasone, at no more than 3-4 cycles consisting of 40 mg/day for 4 days each 2-4 weeks, is a validated alternative. Although the recovery of platelet counts is faster with the latter, long-term response rates are not different for the two therapies [
10]. Intravenous (i.v.) immunoglobulins (IVIg) are recommended for patients with active bleeding or when steroids are contraindicated. The more widely used regimens are 1 g/kg administered 1 or 2 days, or 0.4 g/kg administered 3-5 days in patients >65 years. Nevertheless, alternative patterns have been suggested, such as a single dose of 0.2-0.4/kg, which could be repeated again 3 days afterwards in the event of no response. This last strategy has been shown to be effective and, furthermore, more sustainable [
11].
In the event of severe hemorrhage, high dose methylprednisolone and platelet transfusion are recommended in addition to IVIg, and the use of TPO receptor agonists (TPO-RAs) can be considered. Among these, romiplostim at 5-10 μg/kg is the most frequently reported option [
12], although there is no reason to think that eltrombopag at a daily dose of 75 mg will not be effective. The efficacy of treatments to stop severe hemorrhage has to be assessed according to blood cessation rather than platelet count recovery [
13]. Finally, the combination of steroids with either rituximab, TPO-RAs or immunosuppressants is not recommended outside of clinical trials [
14,
15,
16,
17].
Prophylaxis with trimetropin-sulfametoxazol at 80 mg/400 mg twice a day 2 to 3 times a week to prevent infection by Pneumocistiis carinii must be administered in the following cases: patients on steroid treatment lasting >4 weeks at daily doses >30 mg; patients on prednisone >8 weeks at 15-30/mg/day; patients combining 15-30/mg/day prednisone with cyclosporine; patients with prednisone at >10 mg/day and meeting ≥2 of the following criteria: age >65 years, pulmonary disease, concomitant use of another immunosuppressant. Prophylaxis against herpes virus with acyclovir at 400 mg/day is advisable for either patients >60 years, patients on prednisone at daily doses >7.5 mg, or patients with history of infection with this pathogen.
Prophylaxis with entecavir at 0.5 mg/day is recommended for those patients with antibodies against VHBc and a positive test for hepatitis B virus (HBV) antigen, who are on treatment with prednisone either at >10 mg/day during ≥8 weeks, or at >20 mg/day during >4 weeks. In the event that the test for antibodies against VHBc was positive but that for HBV antigen was negative, the patient should be periodically monitored [
18].
Prophylaxis to prevent osteoporosis with calcium and vitamin D (colecalciferol at weekly dose of 2,800 IU) is recommended for postmenopausal women, >50 years old (y.o.) male patients being on steroid treatment for >3 months, and in those patients who, having a T-score of bone mineral density (BMD) <-1.5, were being treated or were to be treated with steroids at doses >2.5 mg/day for >3 months. This prophylaxis should be applied to premenopausal women and <50 y.o. male patients only in the event of history of previous fractures or when the T-score was <-1.5 and treatment with steroids at doses >5 mg/day for >3 months was being administered or planned [
19].
Second-Line Treatment
Although initial response rates to glucocorticoids are high, many adult patients will relapse (
Table 3). In these cases, re-exposing patients to these treatments is not suitable unless it is justified by an emergency situation. Personalizing therapy becomes paramount when choosing a second-line treatment option. Thus, each patient’s comorbidities will notably influence the therapeutic decision. The findings observed in studies with TPO-RAs, fostamatinib and rituximab suggest that the first two are the more effective and less toxic therapeutic options to be used as second-line treatment of primary ITP [
6,
7]. Randomized studies to compare them directly have not been reported so far.
TPO-RAs induce platelet production and have an excellent efficacy/safety profile. We recommend using any of the commercially available TPO-RAs as the first option of second-line treatment, although the experience with eltrombopag and romiplostin is longer than that reported with avatrombopag so far. Patients will actively participate in decision-making, and the choice will also be influenced by their priorities and lifestyle. Responses have been reported in >80% cases with these agents [
20,
21], and cross-resistance has not been observed [
22]. Furthermore, many patients will be able to suspend other treatments, even TPO-RAs themselves, without leading to a new drop in platelet counts. In the absence of response to a TPO-RA, switching to another one or to fostamatinib is recommended. If TPO-RA refractoriness is definitely confirmed, the use of other immunosuppressants such as mycophenolate mofetil, azathioprine or low-dose steroids can be considered [
23].
Fostamatinib is another option of second line treatment in ITP. Fostamatinib is a spleen tyrosine kinase (SYK) inhibitor able to reduce the anti-platelet activity of phagocytes. This agent achieves rapid, long-lasting platelet count increases in 40-45% of those patients refractory to previous treatments [
24]. Although the response rate is lower when considering heavily pre-treated patients, the efficacy of fostamatinib goes up to 75% when chosen as the first option of second-line treatment [
25]. Furthermore, some studies have shown encouraging results regarding long-term efficacy [
26,
27]. We recommend fostamatinib as second line treatment even before TPO-RA in patients with high thromboembolic risk. The remarkably low incidence of thromboembolic events together with the absence of platelet peaks associated with fostamatinib, make it particularly suitable to be used by primary ITP patients presenting with either arterial or venous thrombosis or history of previous thromboembolic events, regardless of their severity [
27].
Rituximab is a monoclonal antibody targeting the B-cell surface receptor CD20. The interaction induces B-cell depletion and the subsequent decrease in antibody generation. This agent is the second option of the second-line treatment. The experience with rituximab in primary ITP patients is extensive even though it has no specific approval to treat this disease [
28]. Overall responses have been reported in 60-80% of patients, although the proportion of those achieving long-lasting responses after >3-5 years drops to 20-30% [
29,
30]. The standard and more widely used regimen consists of 4 doses of 375 mg/m
2 administered over 4 consecutive weeks. Nevertheless, the low-dose regimen, which uses 4 doses of 100 mg/m
2 instead of 375 mg/m
2, makes it possible to save costs and is associated with fewer adverse events, while showing a similar efficacy [
31]. There is a third regimen consisting of 1 g/day doses administered at days 1 and 15, whose efficacy is similar to the previous ones [
32]. In any case, vaccination against encapsulated bacteria is required before starting rituxumab. Active or latent HBV infection has also to be discarded, and, when applicable, treated. Finally, it must be beared in mind that progressive multifocal leukoencephalopathy has been classified as a complication of rituximab treatment, although it occurs very rarely [
28].
Another second-line option is splenectomy. Its main advantage is its high efficacy associated with a low cost (
Table 3) [
33]. Nevertheless, there are also major limitations, such as the increased risk of thromboembolism or severe infection, and so the benefit:risk ratio must be carefully assessed. Since the current scenario offers several second-line safe and effective pharmacologic options (with others in the pipeline), GEPTI recommends limiting splenectomy to highly selected patients according to their comorbidities, lifestyle and priorities, as well as delaying the procedure in the hope that the patient may achieve a suboptimal response at least to one of the second-line therapies. In any case, splenectomy must not be performed within the first 12 months after diagnosis. In the event that splenectomy is finally the chosen option, the laparoscopic procedure is preferred, and a proper preoperative vaccine pattern and postoperative thromboembolic prophylaxis must be observed [
1].
Vaccination Prior to Splenectomy
After the procedure, those patients who have not been properly immunized are at a risk of severe bacterial infection which is 50-fold higher than that of non-splenectomized patients. The causal agents are
Streptococcus pneumoniae,
Haemophilus influenzae and
Neisseria meningitidis in 50-90%, 5-15% and 5-15% of cases, respectively [
34]. Vaccination reduces the risk, but this does not disappear completely. Vaccines must be administered at least 2 weeks before the procedure. In the event that the severity of the situation prompts immediate surgical intervention, vaccination will be initiated as soon as possible, within the first 2 weeks after the procedure.
Finally, these patients must also be vaccinated against influenza each year, and serogroup B meningococcal vaccine has to be considered for those younger than 25 years [
35].
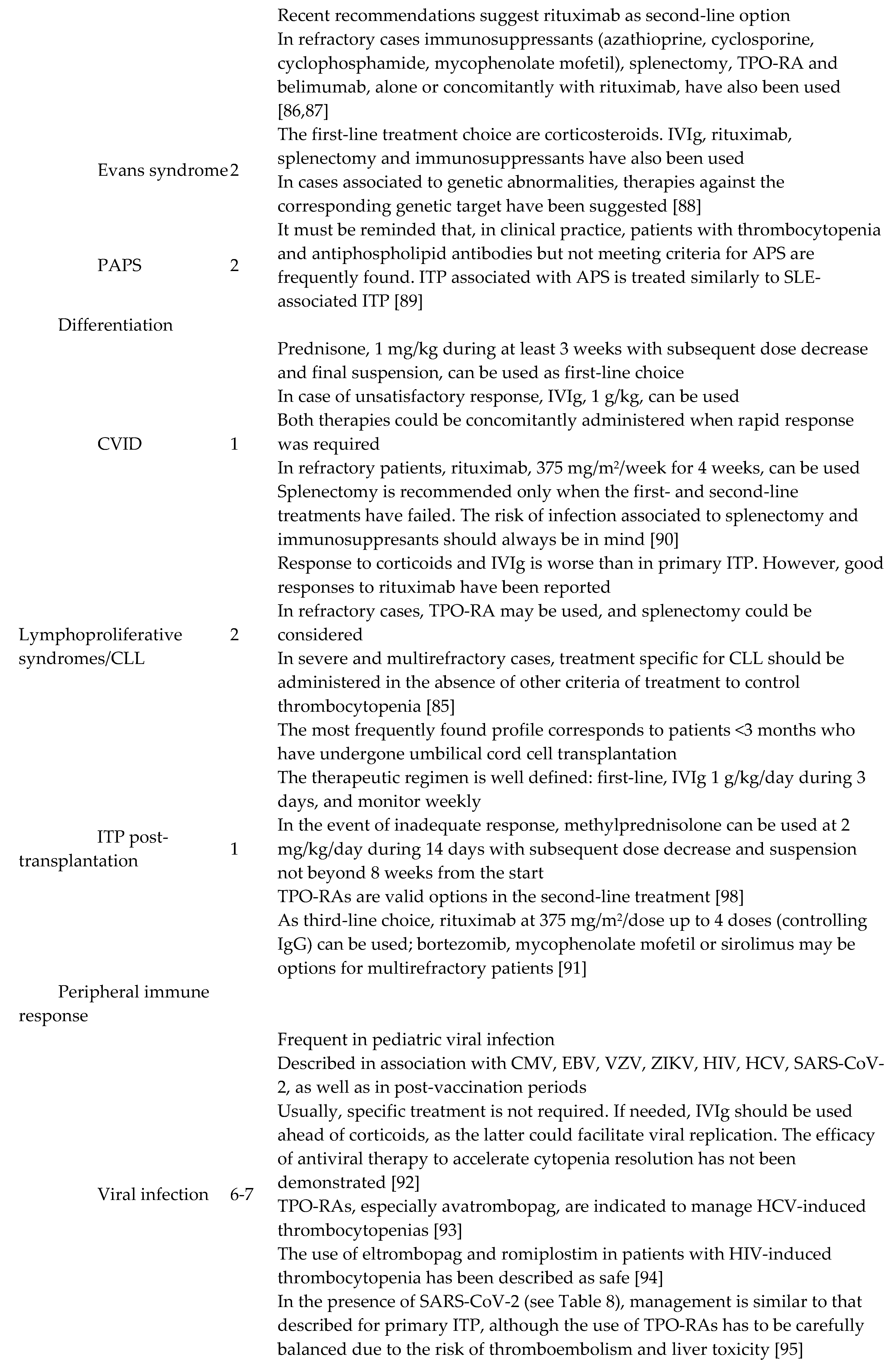
Treatment of Multirefractory Patients
Multirefractory primary ITP is a severe condition that can be experienced by up to 20% of patients. The term “refractoriness” has been controversial. It has been recently defined as the total loss of response to one or more treatments, including rituximab and TPO-RA [
36]. In these cases, reconsidering primary ITP diagnosis is advisable, and bone marrow examination is indicated. As far as the therapeutic attitude is concerned, eradication of
Helicobacter pylori, a gram-negative bacterium which can be detected in the digestive tract of more than half of the total population, can be proposed, since it has been associated with primary ITP in several studies [
37]. On the other hand, treatments consisting of combinations of agents able to induce platelet generation and prevent platelet destruction must be applied. Patient rescues subsequent to administration of steroids concomitantly with rituximab or TPO-RA have been described [
38,
39,
40]. The use of immunosuppressants such as azathioprine, cyclosporine or mycophenolate mofetil, immunomodulators such as danazol or dapsone, or cytostatic agents such as cyclophosphamide or vinca alkaloids (vincristine, vinblastine) can also be envisaged [
36,
41,
42,
43,
44,
45,
46]. Nevertheless, these agents may induce side effects that should prompt a careful examination of the benefit:risk ratio. Some patients may present without active bleeding and with no limitation in their QoL, thus not requiring pharmacologic support while their condition persists. Furthermore, there are no reliable studies either providing support for their use or comparing their efficacy. The
Supplementary Table S1 provides details regarding dose, expected response and side effects associated with the treatments addressed in this section.
4. Follow-Up of Patients with Primary ITP. Scenarios and Recommendations
The fact that the diagnosis of primary ITP is performed by exclusion may lead to situations where the definitive diagnosis has not been made before the initiation of treatment. Patients should be closely followed-up by experienced practitioners, with the aim to rule out other diseases responsible for the symptoms attributed to primary ITP, and to control the subsequent onset of other disorders, especially when patients have persistent or chronic primary ITP, or are elderly. Furthermore, follow-up is required for the early identification of thrombocytopenia-derived complications and side effects of treatments. The scenarios that can be most frequently found throughout the follow-up period are described hereafter.
Hospitalization
The hospital admittance criteria for primary ITP patients are as follows [
1]:
Grade 2 hemorrhage according to the World Health Organization (WHO), and platelets <30×109/L.
Grade ≥3 hemorrhage (requires red blood cell transfusion), regardless of platelet counts.
Adults who are newly diagnosed with primary ITP and present with platelet counts <20x109/L, even if they are asymptomatic or present with minor mucocutaneous hemorrhage. This decision is supported by the following arguments: possible uncertainty regarding diagnosis; requirement to monitor platelet count evolution; possible bleeding complications; need to guarantee that treatment is administered correctly.
-
The following patient profiles could also benefit from hospitalization:
- ◦
those refractory to treatment.
- ◦
those whose diagnosis is not solid enough.
- ◦
those presenting with relevant comorbidities.
- ◦
those using concomitant medication associated with high hemorrhagic risk.
- ◦
those presenting with significant mucosal bleeding.
- ◦
those either with low social support, living far away from hospital or whose follow-up cannot be guaranteed.
Those adult patients with newly diagnosed primary ITP with platelet counts >20x10
9/L who either are asymptomatic or present with minor mucocutaneous bleeding, are recommended to receive ambulatory treatment instead of hospitalization. Tabla 4 summarizes the guidelines to follow with the different profiles of primary ITP patients who are not hospitalized [
1,
6,
8,
9,
47].
Follow-Up of Diseases Frequently Associated with Primary ITP
During follow-up, close monitoring for early detection of diseases classically overrepresented in primary ITP patients is advisable. The prevalence of diabetes, renal failure, hypertension, vascular disease and thyroid disease is 2-2.5-fold higher than that of the normal population, the prevalence of other autoimmune diseases is 5-fold higher, and that of hematological malignancies is up to 6-20 fold higher.
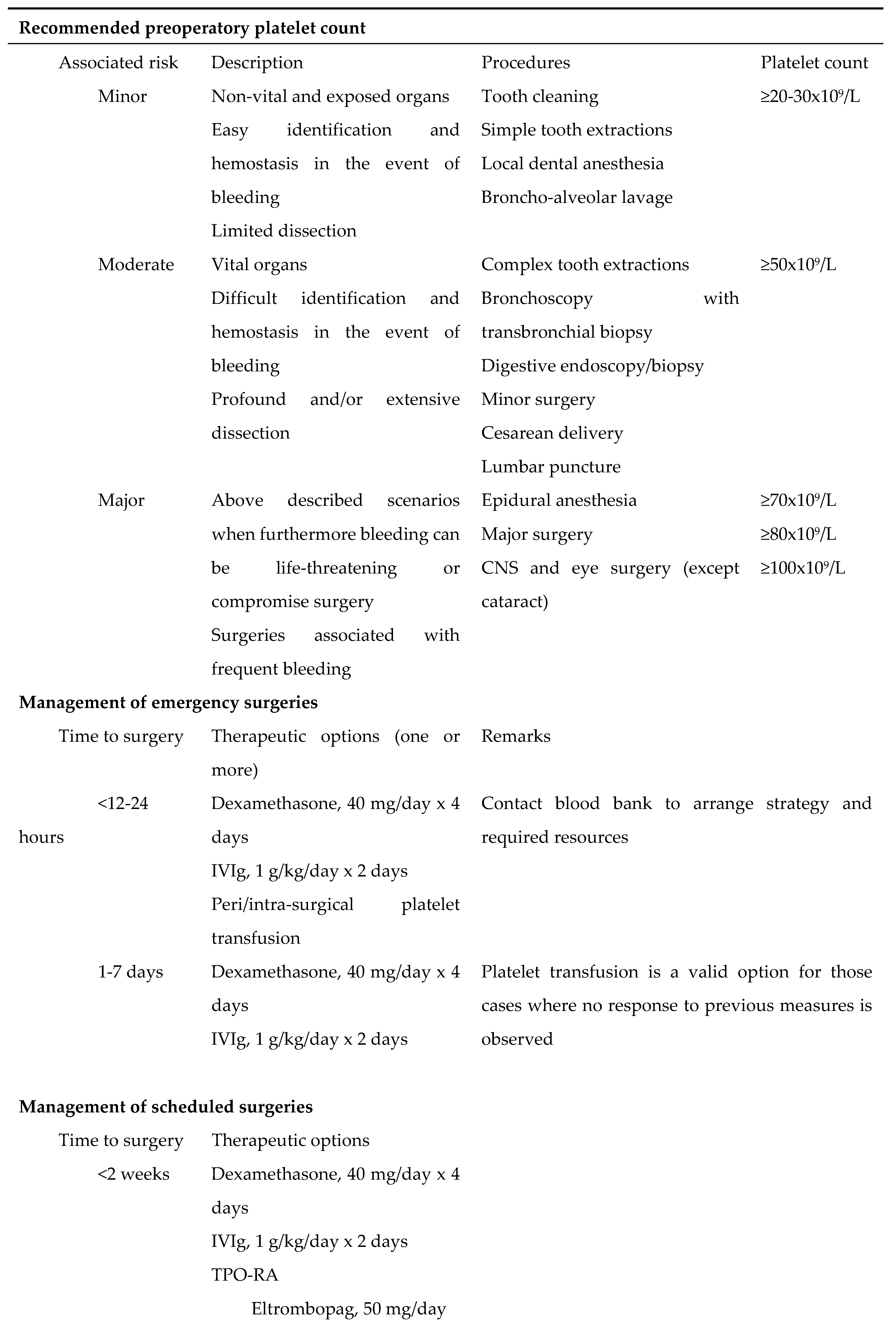
Surgery
The optimal platelet count-target to avoid surgery-associated risk is still controversial. As a general rule, it is accepted that presurgical treatment is required when platelet counts are <50x10
9/L, while it would not be needed with counts >100x10
9/L [
48]. Nevertheless, these values not only are merely indicative, but they are non-directly applicable to primary ITP either, since bleeding manifestations are less frequent in patients with this condition than those observed in patients with other thrombocytopenias [
49]. For minor procedures with a standard bleeding risk, platelet counts >50×10
9/L are recommended, which should increase to >70-100×10
9/L to undergo major surgery or procedures on the central nervous system.
In emergency situations, the approach must be the same as that followed in the scenario of severe bleeding, i.e., one or more of these actions should be taken: administration of IVIg; administration of corticosteroids, preferably dexamethasone to take advantage of its rapid-acting profile; platelet transfusion, ideally after the aforementioned measures have been applied. TPO-RAs are not the best option when surgery is to be performed shortly, since they induce platelet generation at long-term. However, their use could be considered to maintain suitable platelet counts after surgery, especially after complex procedures.
Finally, when surgery is going to be planned, the time spent for an agent to achieve a sufficient platelet count increase must be considered when setting the date of the procedure. There is currently no agent to be definitely chosen ahead of others for presurgical preparation. The same therapies that are suitable for first- and second-line treatment can be used for presurgical preparation with the same hierarchy [
50].
Table 5 summarizes the recommended platelet counts according to surgical risk as well as the guidelines to proceed with urgent or planned surgical procedures.
Suspension of Treatment with TPO-RA
The long-term use of TPO-RA has allowed specialists to report long-lasting responses. This finding, together with the good safety profile associated with these drugs [
51,
52], has prompted their continuous use. Another argument to support this measure is the drop in platelet counts to pre-treatment values as early as 2 weeks after treatment suspension, which has been occasionally described [
53]. Nevertheless, cases of long-term remission after treatment withdrawal [so called sustained remission off-treatment (SROT)] have also been reported [
54,
55,
56,
57], which may be due to immunomodulatory actions performed by this therapeutic group [
57]. This last observation encouraged some practitioners to reduce progressively the TPO-RA dose, and, finally, suspend treatment, provided that a drop in the platelet count was not detected. This procedure not only saves costs but also reduces the risk of TPO-RA-associated adverse events [
56]. Normally, candidates to achieve SROT after progressive dose reduction followed by suspension would be those who had presented with stable platelet counts (50-100x10
9/L) during a 4-6 months period on TPO-RA treatment, regardless of disease stage [
55,
56,
57]. Patients must be properly informed about this therapeutic option, for them to decide after balancing risks and benefits. Several protocols to reduce dosage and suspend treatment have been proposed [
58,
59,
60,
61]. Ours is detailed in the Supplementary
Table 2, where the profiles of SROT candidate and non-candidate patients are also described [
59,
62,
63].
5. Primary ITP in Selected Patient Populations
Pediatric Patients
Primary ITP is usually self-limited in children. The highest incidence is reported in 2-8 y.o. patients, and history of a triggering infectious episode is not an infrequent occurrence. The trend to spontaneous remission is observed even after 2 years’ evolution. The diagnostic approach is similar to that of adults. Although most pediatric patients with newly diagnosed primary ITP do not present with relevant bleeding symptoms and do not require treatment, it is mandatory that parents and children be aware of the risks associated with a severe or potentially fatal hemorrhage.
Hospitalization is recommended for those pediatric patients with active hemorrhage, bleeding risk factors or platelet counts ≤20x10
9/L. In order to make therapeutic decisions, platelet counts should not be the only factor for guidance. Other variables such as the nature of mucocutaneous symptoms, the type of active hemorrhage and the bleeding risk factors should also be considered on a case-by-case basis. The aim of the treatment should focus on the control of clinically relevant hemorrhages rather than the platelet count recovery. First-line treatments are either corticosteroids such as prednisone (oral) or methylprednisolone (i.v.), or high dose IVIg. In the event of no response to the firstly chosen agent, the alternative one can be tried [
64,
65]. TPO-RA can be used as second-line option [
66,
67]. Failure of first and second treatment lines should prompt not only bone marrow examination but also the consideration of other drugs such as mycophenolate mofetil or rituximab, even although the experience with these agents is limited in children. Splenectomy may be an option in scenarios of life-threatening hemorrhage.
Table 6 provides details about these therapeutic guidelines.
Elderly Patients
The incidence of primary ITP goes up to 9 per 100,000 individuals per year in >75 y.o. patients [
1]. Nevertheless, the fact that some comorbidities causing thrombocytopenia can lead to an inaccurate diagnosis due to “ITP imitation” must be kept in mind. Furthermore, the incidence of these entities, such as megaloblastic or iron deficiency anemias, myelodysplastic syndromes (MDS) or acute leukemias, increases with age. For this reason, differential diagnosis is particularly important. When reasonable doubts arise, bone marrow analysis, including cytogenetic and flow cytometry approaches, is recommended.
Elderly primary ITP patients are at higher risk of bleeding, thromboembolism and infection, and they require frequently antiplatelet and anticoagulant therapies. Platelet counts are the main determinants of bleeding risk, and should be maintained at values >30x10
9/L in >75 y.o. patients, as well as in those >60 years with concomitant bleeding risk factors [
68,
69]. TPO-RAs, IVIg and vinca alkaloids can be considered when rapid platelet count increase is required [
68].
The therapeutic attitude with elderly ITP patients with no active bleeding consists of the use of corticosteroids for first-line treatment, still at lower doses (prednisone at 0.5mg/kg/day) and for shorter periods than those used with younger patients [
1,
68]. IVIg are indicated in the event of severe thrombocytopenia only (<10x10
9/L), or with high bleeding risk [
69]. According to patient’s comorbidities, dexamethasone at standard doses may be an option. The choice of the second-line treatment should be made on an individual basis, and the patient should participate actively [
1]. The good safety/efficacy profile of TPO-RAs in elderly patients makes them the main second-line therapeutic option [
1,
70]. Furthermore, their sustained response rates seem to be comparable to those observed with TPO-RAs in other adult populations [
55,
71,
72]. Nevertheless, it must be remarked that the risk of thromboembolism associated with these drugs is higher in the elderly, since the concomitant presence of several other thromboembolic risk factors is not uncommon [
73]. An alternative option for patients at high thromboembolic risk can be fostamatinib [
74,
75]. Rituximab may also be considered, although long-term remissions are scarce and more associated toxicities have been reported [
1,
69]. Finally, immunosuppressants or immunomodulators such as mycophenolate mofetil, cyclosporine, azathioprine, danazol or dapsone may be a valid option for those elderly patients presenting with moderate symptoms, since the safety/efficacy profile of these agents is well known. Nevertheless, many of these drugs require several months to achieve the intended effect [
1,
68]. Splenectomy is not recommended in the elderly except in isolated cases of multirefractory patients, because the procedure is less effective and triggers more bleeding and infectious complications than in other populations.
Table 6 provides details regarding the treatment of primary ITP in the elderly.
Pregnant Patients
When primary ITP is suspected in a pregnant woman, other pregnancy-related causes of thrombocytopenia should be ruled out. In fact, although thrombocytopenia is the second hematologic disorder more frequently found in pregnancy, around 80% of cases are of gestational origin. The hallmark of these is a progressive decrease of platelet counts, starting in the mid-second trimester and persisting in the third one [
76]. The procedure to diagnose primary ITP in pregnancy requires assessment of blood pressure, urine proteins, hemostatic status, and antiphospholipid and antinuclear antibodies (ANA) [
77].
Severe complications are not frequently found in pregnant women with primary ITP, and neonatal incidences of thrombocytopenia or bleeding events are low. Particularly risky scenarios would be those of patients unable to maintain stable platelet counts >30x10
9/L with standard treatments, or patients with history of previous pregnancies with severe neonatal thrombocytopenia. Recommended platelet counts to undergo vaginal delivery are >50×10
9/L. This value goes up to >70×10
9/L in the event that cesarean delivery is required or epidural anesthesia is going to be used. The choice of type of labor will be made according to obstetric criteria only [
78].
Pregnant women with platelets >20-30×10
9/L do not require treatment systematically. With lower values, the first-line options are glucocorticoids and IVIg. Starting with prednisone is recommended. This should be used at doses of 10-20 mg/day, since these are the lowest ones enough to achieve platelet counts in the range of 20-30x10
9/L. In order to avoid fetal risk, dexamethasone should not be used. IVIg has to be administered only in the event of side effects associated with steroids, severe hemorrhage or requirement for particularly rapid platelet count recovery, especially when delivery is close in time [
7]. The usefulness of TPO-RAs as second-line option has not been established yet, since enough clinical evidence is lacking (only isolated cases and one case series have been reported [
79,
80]). The data sheets of these drugs do not include this indication, and any decision concerning this medication should be made in accordance with the patient's wishes, once she has been properly informed. If TPO-RAs are finally chosen, it is advisable to avoid them in the first trimester. Rituximab does not seem to be teratogenic. However, it has been associated with prolonged B-cell lymphocytopenia and the requirement to delay vaccination in neonates exposed in utero. For this reason, this agent should not be used within at least 6 months of planned conception [
81]. Fostamatinib has been associated with fetal mortality in animal models [
82].
Azathioprine and cyclosporin can be used without teratogenic risk. Finally, data regarding safety/efficacy of splenectomy in pregnant patients are limited. If the procedure is finally chosen, it should be performed during the second trimester, keeping in mind that an associated risk of neonatal thrombocytopenia exists [
1].
After labor, platelet counts must be assessed in the neonate. If these are <100×10
9/L, they should be monitored daily. With values <50×10
9/L, cranial ultrasound should be performed and, if hemorrhage was detected, IVIg and steroids should be administered, at minimal doses and for a short period of time, pursuing a platelet count-target of >100×10
9/L. In those neonates presenting with hemorrhagic symptoms or platelet counts <30×10
9/L, one unique dose of IVIg is recommended in order to achieve rapid response. Finally, those neonates with thrombocytopenia lasting beyond 3 weeks from birth should quit breastfeeding [
1].
Table 6 summarizes the most relevant topics regarding management of primary ITP in pregnant women and neonates.
6. Secondary ITP
Secondary forms of ITP account for the 9-20% of all ITP cases in adults. This rate increases with age [
83,
84]. Those pathologies able to induce immune tolerance disorders leading to secondary ITP are varied. Systemic lupus erythematosus (SLE) is the most commonly found entity [
85,
86,
87,
88,
89,
90,
91,
92,
93,
94,
95,
96,
97,
98] (Table 7). Thrombocytopenias secondary to drug use are particular conditions [
99,
100]. Indeed, treatment with the causal agent must be immediately suspended. When this is heparin, another anticoagulant should be started, preferably i.v. administered thrombin direct inhibitors. After platelet count recovery, these can be substituted by coumarins, starting at low doses [
101]. Direct oral anticoagulants might be another option, although there is not enough evidence to recommend them specifically yet.
First-line treatment is similar in most cases of primary and secondary ITP, namely glucocorticoids and/or IVIg. However, the second-line option has to consider seriously the underlying disease when managing secondary ITP. For instance, the benefit:risk ratio regarding TPO-RA use or splenectomy should be balanced in cases of ITP secondary to SLE or antiphospholipid syndrome. Rituximab may be considered in the context of common variable immunodeficiency (CVID). The guidelines to treat secondary ITP are summarized in
Table 7.
Table 7.
Secondary ITP, causes and management.
Table 7.
Secondary ITP, causes and management.
Table 8.
Primary ITP in special scenarios: thrombosis, COVID-19.
Table 8.
Primary ITP in special scenarios: thrombosis, COVID-19.
7. Primary ITP and Thrombosis
Pathophysiology, Risk Associated with the Treatment of Primary ITP
Patients with primary ITP are at twice the risk of venous or arterial thrombosis compared to the normal population, even when platelet counts are markedly low [
102]. The origin is multifactorial, with causal roles played by the classical thromboembolic risk factors and the therapies that are being administered to treat thrombocytopenia [
103]. On the one hand, patients with primary ITP have higher circulating levels of neutrophil extracellular traps (NETs), E-selectin, plasminogen activator inhibitor-1 (PAI-1) and microparticles rich in phosphatidylserine and tissue factor (TF), as well as hyperreactive immature platelets, within a proinflammatory scenario that also promotes coagulation, occasionally boosted by lupus anticoagulant and/or anticardiolipin or anti-β2-glycoprotein-I antibodies [
104,
105,
106]. On the other hand, most primary ITP treatments induce some extent of thrombotic risk. Corticosteroids could increase the expression of TF and factor VIII, reduce that of thrombomodulin, and promote cell adhesion via von Willebrand factor; occasionally, IVIg could trigger thromboembolic venous events in patients with concomitant risk factors and arterial events in patients of advanced age and/or with atherosclerosis; platelets of TPO-RA-treated patients tend to present apoptotic patterns leading to expression of phosphatidylserine on the cell surface, thus promoting the assembly of the prothrombinase complex; finally, splenectomy may also promote thrombosis, either portal or systemic [
58,
106].
Antiplatelet and Anticoagulant Treatments in the Context of Primary ITP
It must be recalled that thrombocytopenia is predictive of a poor prognosis in patients with acute coronary syndromes. In order to minimize the bleeding risk associated with the use of antiplatelet agents in patients with thrombocytopenia, non-steroidal anti-inflammatory drugs and inhibitors of GPIIb/IIIa should be avoided, proton pump inhibitors should be administered, aspirin should be given at low dose, the prolonged use of triple antithrombotic therapy should be avoided and, in those patients undergoing stent placement, double antiplatelet therapy should be limited to one month after the procedure. Treatment should be decided on an individual basis, and should be influenced by the thrombotic risk and the hemorrhagic history of each patient. Aspirin could be used in cases of acute arterial events provided that platelet counts are >10×10
9/L, while the double antiplatelet treatment should be restricted to patients with counts >30×10
9/L [
107].
There are no studies designed to evaluate the safety and efficacy of anticoagulant treatment in primary ITP patients. Nevertheless, the administration of therapeutic doses of anticoagulants to patients with platelet counts >50×109/L is generally accepted. Bleeding risk increases when counts are <50x109/L. In such cases, the options would be suspending anticoagulation or reducing anticoagulant drug dose. In the event of total contraindication for anticoagulation, a vena cava filter could be placed provided that the thrombus is below the placement area.
In those patients with history of thromboembolism, glucocorticoids and fostamatinib would be the first-line and second-line options, respectively. In the event that there is no response to fostamatinib and platelet counts must be maintained to avoid complications associated with anticoagulation or antiplatelet drugs, the use of TPO-RAs could be considered.
Table 8 summarizes the guidelines to follow in the management of patients with primary ITP and thromboembolism or thromboembolic risk.
8. ITP and COVID-19
SARS-CoV-2, like other viral agents, is able to induce ITP. In this case, the diagnosis of secondary ITP is also by exclusion.
The treatment of ITP in patients with COVID-19 and platelet counts <20×10
9/L and/or active bleeding should consist of prednisone at 0.5-1 mg/kg/day for no more than 2 weeks, followed by progressive dose reduction and, finally, suspension no later than 8 weeks from the start. Those patients with severe COVID-19 who are already on corticoids and present with platelet counts <20×10
9/L and/or active bleeding could be additionally administered IVIg, at a total dose of 2 g/kg. In the event that counts continue to be <20×10
9/L and/or active bleeding persists, TPO-RA could be administered, although at the lowest possible dose. An alternative option may be fostamatinib, which could be beneficial not only for platelet count recovery but also to relieve COVID-19-triggered inflammatory processes [
108]. Rituximab, and other immunosuppressants, should be avoided, since these agents reduce the ability to produce antibodies [
109,
110,
111].
Those patients with primary ITP in its chronic phase who are being well controlled with their ITP treatment, should not change their therapeutic regimen if they are infected by SARS-CoV-2. If the infection leads to a relapse of the thrombocytopenia, patients should be administered IVIg if the drop of platelet counts is severe, and platelets should be transfused in the event of bleeding. In those patients who were already being treated with a TPO-RA, an increase of the dose or the addition of another TPO-RA or fostamatinib could be proposed [
112,
113].
When those patients with primary ITP and, furthermore, on anticoagulant treatment, are infected by SARS-CoV-2, even if COVID-19 symptoms are severe, they can continue using low molecular weight heparin (LMWH) at prophylactic dose provided that cell counts are >30×10
9/L. Anticoagulation or antiplatelet agents can be used at therapeutic doses with counts >50x10
9/L [
112].
Finally, it must be remarked that the risk of secondary ITP associated with SARS-CoV-2 vaccination is very low, in the range of that induced by other commercially available vaccines against other viral agents [
114,
115]. There is no contraindication against using COVID-19 vaccines in pregnant women or patients with preexisting ITP [
116,
117].
The most important notions concerning treatment of ITP secondary to COVID-19 and managing SARS-CoV-2 infection in patients with primary ITP are summarized in
Table 8.
9. Limitations
The field of primary ITP is a rapidly changing landscape. Many of the guidelines and recommendations are aimed to provide some guidance only. Large series and/or randomized prospective studies to compare therapeutic approaches or assess reliably the efficacy of treatments are lacking in the primary ITP scenario. This is a common problem to all guides and consensus documents addressing this disorder. Recommendations have thus not been graded because they are taken from expert opinions and non-comparative studies, and therefore have only a low level of evidence. On the other hand, the pathophysiology has not been addressed in depth, since the aim of this article was to provide physicians with an updated reference for their day-to-day practice. Finally, secondary ITP would deserve one updated review focusing exclusively on this complex condition.
10. Conclusions
Primary ITP management remains a challenge. Its diagnosis is performed by exclusion and requires the involvement of experienced practitioners. There is now a consensus on the disease categories, criteria used to define refractoriness and types of treatment response, and all these should be considered when managing patients with thrombocytopenia. Glucocorticoids and IVIg remain the cornerstones of first line of treatment. Regarding second line treatment, TPO-RAs are usually the first choice. Fostamatinib is also a valid option, and has been shown to be even better than the former for those patients with high thromboembolic risk. A variety of immunosuppressants, immunomodulators or cytostatic agents may be considered for multirefractory patients, after carefully weighing up their risks and benefits. The increasing choice of therapeutic options means that splenectomy is now only performed on a very limited set of patients. Treatments may be adjusted in specific subpopulations (pediatric, elderly, pregnant women) or in the presence of concomitant conditions (thrombosis, COVID-19). Primary ITP management is continuously evolving, and regular updates are necessary.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization: MEMC, CPI. Participation in rounds of discussion, literature search, writing and writing review: MEMC, MCH, BSG, MTAR, ABaG, ABeG, SBP, EBC, NBC, GCN, ISCM, LEU, LFFF, LJGF, MCGC, TJGL, CGG, JMGC, IJR, RJB, ELA, DMC, VMR, EMM, JAPF, MMPA, ISO, DVF, CPI. Final supervision: BSG, MEMC, CPI. All authors have read and agreed to this version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Article did not report any data.
Conflicts of Interest
MEMC has received honoraria for speaking at symposia and advisory boards from Amgen, Sobi, Novartis, Takeda, Novo Nordisk, Grifols, Sanofi. MCH declares no conflict of interest. BSG declares no conflict of interest. MTAR has the following financial relationships: advisor on scientific boards for Novo Nordisk, Roche, LFB, CSL Behring, Bayer, Pfizer, Grifols, Novartis and Amgen. Reported receiving consulting fees and/or speaker from Novo Nordisk, Roche, LFB, CSL Behring, Bayer, Pfizer, Grifols, Novartis and Amgen. AGB declares no conflict of interest. ABG declares no conflict of interest. SBP declares no conflict of interest. EBC declares no conflict of interest. NBC has received research grants from Grifols and Novo Nordisk and speaker honoraria from Amgen, Novartis, Sobi, Grifols, and Novo Nordisk. GCN declares no conflict of interest. ISCM declares no conflict of interest. LEU declares no conflict of interest. LFFF declares no conflict of interest. LJGF declares no conflict of interest. MCGC has received speaker honoraria from Amgen, Sobi, Bayer, Takeda, Novo Nordisk, Pfizer y Werfen. TJGL has received research grants from Amgen, Novartis, Sobi and Grifols and speaker honoraria from Amgen, Novartis, Sobi, Grifols, Momenta, Alpine and Argenx. CGG declares no conflict of interest. JMGC has received honoraria as speaker honoraria from Novartis and Sobi. IJR declares no conflict of interest. RJB has received speaker or advisor honoraria from Amgen, CSL Behring, Grifols, Novo Nordisk, Pfyzer, Roche, Sobi. ELA declares no conflict of interest. DMC has participated in advisory activities in collaboration with Sobi. VMR declares no conflict of interest. EMM has received honoraria from AMGEM for speaking at symposia. JAPF declares no conflict of interest. MMPA declares no conflict of interest. ISO has carried out teaching and scientific advisory activities in collaboration with Sobi, CSL Behring, Novo Nordisk, Takeda, Bayer, Pfizer, Boehringher Ingelheim, Bristol-Squibb-Myers; Leo Pharma, Daiichi Sankyo and Sanofi. DVF declares no conflict of interest. CPI declares no conflict of interest.
References
- Lozano:, M.L.; Sanz, M.A.; Vicente, V.; Grupo Español de PTI (GEPTI). Guidelines of the Spanish ITP Group for the diagnosis, treatment and follow-up of patients with immune thrombopenia. Med. Clin. 2021, 157, 191–198. [Google Scholar]
- Provan, D.; Semple, J.W. Recent advances in the mechanisms and treatment of immune thrombocytopenia. EBioMedicine 2022, 76, 103820. [Google Scholar] [CrossRef] [PubMed]
- Rodeghiero, F.; Stasi, R.; Gernsheimer, T.; Michel, M.; Provan, D.; Arnold, D.M.; Bussel, J.B.; Cines, D.B.; Chong, B.H.; Cooper, N.; et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood, 2009, 113, 2386–2393. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.M.; Nazy, I.; Clare, R.; Jaffer, A.M.; Aubie, B.; Li, N.; Kelton, J.G. Misdiagnosis of primary immune thrombocytopenia and frequency of bleeding: lessons from the McMaster ITP Registry. Blood Adv. 2017, 1, 2414–2420. [Google Scholar] [CrossRef] [PubMed]
- McMillan, R.; Bussel, J.B.; George, J.N.; Lalla, D.; Nichol, J.L. Self-reported health-related quality of life in adults with chronic immune thrombocytopenic purpura. Am. J. Hematol. 2008, 83, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Neunert, C.; Terrell, D.R.; Arnold, D.M.; Buchanan, G.; Cines, D.B.; Cooper, N.; Cuker, A.; Despotovic, J.M.; George, J.N.; Grace, R.F.; et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019, 3, 3829–3866. [Google Scholar] [CrossRef] [PubMed]
- Provan, D.; Arnold, D.M.; Bussel, J.B.; Chong, B.H.; Cooper, N.; Gernsheimer, T.; Ghanima, W.; Godeau, B.; González-López, T.J.; Grainger, J.; et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019, 3, 3780–3817. [Google Scholar] [CrossRef] [PubMed]
- Choi, P.Y.; Merriman, E.; Bennett, A.; Enjeti, A.K.; Tan, C.W.; Goncalves, I.; Hsu, D.; Bird, R. Consensus guidelines for the management of adult immune thrombocytopenia in Australia and New Zealand. Med. J. Aust. 2022, 216, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, H.; Kuwana, M.; Hato, T.; Takafuta, T.; Fujimura, K.; Kurata, Y.; Murata, M.; Tomiyama, Y.; Committee for the Revision of “Reference Guide for Management of adult ITP” Blood Coagulation Abnormalities Research Team. Research on Rare and Intractable Disease supported by Health, Labour and Welfare Science Research Grants Reference guide for management of adult immune thrombocytopenia in Japan: 2019 Revision. Int. J. Hematol. 2020, 111, 329–351. [Google Scholar] [CrossRef]
- Mithoowani, S.; Gregory-Miller, K.; Goy, J.; Miller, M.C.; Wang, G.; Noroozi, N.; Kelton, J.G.; Arnold, D.M. High-dose dexamethasone compared with prednisone for previously untreated primary immune thrombocytopenia: a systematic review and meta-analysis. Lancet Haematol. 2016, 3, e489–e496. [Google Scholar] [CrossRef]
- Zhou, Z.; Qiao, Z.; Li, H.; Luo, N.; Zhang, X.; Xue, F.; Yang, R. Different dosages of intravenous immuno- globulin (IVIg) in treating immune thrombocytopenia with long-term follow-up of three years: Results of a prospective study including 167 cases. Autoimmunity 2016, 49, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Roumier, M.; Le Burel, S.; Audia, S.; Chauchet, A.; Gousseff, M.; Hamidou, M.; Liferman, F.; Moulis, G.; Lioger, B.; Galicier, L.; et al. High dose romiplostim as a rescue therapy for adults with severe bleeding and refractory immune thrombocytopenia. Am. J. Hematol. 2021, 96, E43–E46. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Kiesewetter, H.; Kalus, U.; Movassaghi, K.; Meyer, O. Massive platelet transfusion is a rapidly effective emergency treatment in patients with refractory autoimmune thrombocytopenia. Thromb. Haemost. 2008, 100, 762–765. [Google Scholar] [PubMed]
- Zaja, F.; Baccarani, M.; Mazza, P.; Bocchia, M.; Gugliotta, L.; Zaccaria, A.; Vianelli, N.; Defina, M.; Tieghi, A.; Amadori, S.; et al. Dexamethasone plus rituximab yields higher sustained response rates than dexamethasone monotherapy in adults with primary immune thrombocytopenia. Blood 2010, 115, 2755–2762. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, M.; Hou, Y.; Qin, P.; Zeng, Q.; Yu, W.; Guo, X.; Wang, J.; Wang, X.; Liu, G.; et al. High-dose dexamethasone plus recombinant human thrombopoietin vs high-dose dexamethasone alone as frontline treatment for newly diagnosed adult primary immune thrombocytopenia: A prospective, multicenter, randomized trial. Am. J. Hematol. 2020, 95, 1542–1552. [Google Scholar] [CrossRef] [PubMed]
- Pell, J.; Greenwood, R.; Ingram, J.; Wale, K.; Thomas, I.; Kandiyali, R.; Mumford, A.; Dick, A.; Bagot, C.; Cooper, N.; et al. Trial protocol: a multicentre randomised trial of first-line treatment pathways for newly diagnosed immune thrombocytopenia: standard steroid treatment versus combined steroid and mycophenolate. The FLIGHT trial. B.M.J. Open 2018, 8, e024427. [Google Scholar] [CrossRef]
- Chugh, S.; Darvish-Kazem, S.; Lim, W.; Crowther, M.A.; Ghanima, W.; Wang, G.; Heddle, N.M.; Kelton, J.G.; Arnold, D.M. Rituximab plus standard of care for treatment of primary immune thrombocytopenia: a systematic review and meta-analysis. Lancet Haematol. 2015, 2, e75–e81. [Google Scholar] [CrossRef]
- Malpica, L.; Moll, S. Practical approach to monitoring and prevention of infectious complications associated with systemic corticosteroids, antimetabolites, cyclosporine, and cyclophosphamide in nonmalignant hematologic diseases. Hematology Am. Soc. Hematol. Educ. Program 2020, 2020, 319–327. [Google Scholar] [CrossRef]
- González-Macías, J.; Del Pino-Montes, J.; Olmos, J. M.; Nogués, X.; en nombre de la Comisión de Redacción de las Guías de Osteoporosis de la SEIOMM. Clinical practice guidelines for posmenopausal, glucocorticoid-induced and male osteoporosis. Spanish Society for Research on Bone and Mineral Metabolism (3rd updated version 2014). Rev. Clin. Esp. (Barc.) 2015, 215, 515–526. [Google Scholar] [CrossRef]
- Kuter, D.J.; Bussel, J.B.; Newland, A.; Baker, R.I.; Lyons, R.M.; Wasser, J.; Viallard, J.F.; Macik, G.; Rummel, M.; Nie, K.; et al. Long-term treatment with romiplostim in patients with chronic immune thrombocytopenia: safety and efficacy. Br. J. Haematol. 2013, 161, 411–423. [Google Scholar] [CrossRef]
- Saleh, M.N.; Bussel, J.B.; Cheng, G.; Meyer, O.; Bailey, C.K.; Arning, M.; Brainsky, A.; EXTEND Study Group. Safety and efficacy of eltrombopag for treatment of chronic immune thrombocytopenia: results of the long-term, open-label EXTEND study. Blood 2013, 121, 537–545. [Google Scholar] [CrossRef] [PubMed]
- González-Porras, J.R.; Godeau, B.; Carpenedo, M. Switching thrombopoietin receptor agonist treatments in patients with primary immune thrombocytopenia. Ther. Adv. Hematol. 2019, 10, 2040620719837906. [Google Scholar] [CrossRef] [PubMed]
- Al-Samkari, H.; Kuter, D.J. Optimal use of thrombopoietin receptor agonists in immune thrombocytopenia. Ther. Adv. Hematol. 2019, 10, 2040620719841735. [Google Scholar] [CrossRef] [PubMed]
- McBride, A.; Nayak, P.; Kreychman, Y.; Todd, L.; Duliege, A.M.; Mehta, A.R. Fostamatinib disodium hexahydrate: a novel treatment for adult immune thrombocytopenia. Am. J. Manag. Care 2019, 25(19 Suppl), S347–S358. [Google Scholar]
- Kapur, R. Fine-tuning the treatment toolbox of immune thrombocytopenia: fostamatinib as a second-line therapy. Br. J. Haematol. 2020, 190, 817–818. [Google Scholar] [CrossRef] [PubMed]
- Bussel, J.B.; Arnold, D.M.; Boxer, M.A.; Cooper, N.; Mayer, J.; Zayed, H.; Tong, S.; Duliege, A.M. Long-term fostamatinib treatment of adults with immune thrombocytopenia during the phase 3 clinical trial program. Am. J. Hematol. 2019, 94, 546–553. [Google Scholar] [CrossRef]
- Cooper, N.; Altomare, I.; Thomas, M.R.; Nicolson, P.L.R.; Watson, S.P.; Markovtsov, V.; Todd, L.K.; Masuda, E.; Bussel, J.B. Assessment of thrombotic risk during long-term treatment of immune thrombocytopenia with fostamatinib. Ther. Adv. Hematol. 2021, 12, 20406207211010875. [Google Scholar] [CrossRef]
- Lucchini, E.; Zaja, F.; Bussel, J. Rituximab in the treatment of immune thrombocytopenia: what is the role of this agent in 2019? Haematologica 2019, 104, 1124–1135. [Google Scholar] [CrossRef]
- Ghanima, W.; Khelif, A.; Waage, A.; Michel, M.; Tjønnfjord, G.E.; Romdhan, N.B.; Kahrs, J.; Darne, B.; Holme, P.A.; RITP study group. Rituximab as second-line treatment for adult immune thrombocytopenia (the RITP trial): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2015, 385, 1653–1661. [Google Scholar] [CrossRef]
- Marangon, M.; Vianelli, N.; Palandri, F.; Mazzucconi, M.G.; Santoro, C.; Barcellini, W.; Fattizzo, B.; Volpetti, S.; Lucchini, E.; Polverelli, N.; et al. Rituximab in immune thrombocytopenia: gender, age, and response as predictors of long-term response. Eur. J. Haematol. 2017, 98, 371–377. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Y.; He, Z.; Chen, Q.; Liu, Z.; Yu, L.; Wang, C. The efficacy and safety of low-dose rituximab in immune thrombocytopenia: a systematic review and meta-analysis. Platelets 2019, 30, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Khellaf, M.; Charles-Nelson, A.; Fain, O.; Terriou, L.; Viallard, J. F.; Cheze, S.; Graveleau, J.; Slama, B.; Audia, S.; Ebbo, M.; et al. Safety and efficacy of rituximab in adult immune thrombocytopenia: results from a prospective registry including 248 patients. Blood 2014, 124, 3228–3236. [Google Scholar] [CrossRef] [PubMed]
- Radkowiak, D.; Zychowicz, A.; Lasek, A.; Wysocki, M.; Major, P.; Pędziwiatr, M.; Budzyński, P.; Kulawik, J.; Budzyński, A. 20 years' experience with laparoscopic splenectomy. Single center outcomes of a cohort study of 500 cases. Int. J. Surg. 2018, 52, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Holdsworth, R.J.; Irving, A.D.; Cuschieri, A. Postsplenectomy sepsis and its mortalityrate: Actual versus perceived risks. Br. J. Surg. 1991, 78, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Arnold, D.M.; McCrae, K.R. Splenectomy for immune thrombocytopenia: down but not out. Blood 2018, 131, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Cuker, A.; Neunert, C.E. How I treat refractory immune thrombocytopenia. Blood 2016, 128, 1547–1554. [Google Scholar] [CrossRef]
- Stasi, R.; Sarpatwari, A.; Segal, J.B.; Osborn, J.; Evangelista, M.L.; Cooper, N.; Provan, D.; Newland, A.; Amadori, S.; Bussel, J.B. Effects of eradication of Helicobacter pylori infection in patients with immune thrombocytopenic purpura: a systematic review. Blood 2009, 113, 1231–1240. [Google Scholar] [CrossRef]
- Bussel, J.B.; Lee, C.S.; Seery, C.; Imahiyerobo, A.A.; Thompson, M.V.; Catellier, D.; Turenne, I.G.; Patel, V.L.; Basciano, P.A.; Elstrom, R.L.; et al. Rituximab and three dexamethasone cycles provide responses similar to splenectomy in women and those with immune thrombocytopenia of less than two years duration. Haematologica 2014, 99, 1264–1271. [Google Scholar] [CrossRef]
- Choi, P.Y.; Roncolato, F.; Badoux, X.; Ramanathan, S.; Ho, S.J.; Chong, B.H. A novel triple therapy for ITP using high-dose dexamethasone, low-dose rituximab, and cyclosporine (TT4). Blood 2015, 126, 500–503. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.Y.; Fei, H.R.; Wang, L.; Yuan, C.L. Efficacy of low-dose rituximab in combination with recombinant human thrombopoietin in treating ITP. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1583–1588. [Google Scholar]
- Quiquandon, I.; Fenaux, P.; Caulier, M.T.; Pagniez, D.; Huart, J.J.; Bauters, F. Re-evaluation of the role of azathioprine in the treatment of adult chronic idiopathic thrombocytopenic purpura: a report on 53 cases. Br. J. Haematol. 1990, 74, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.R.; Naithani, R.; Mahapatra, M.; Kumar, R.; Mishra, P.; Saxena, R. Efficacy of cyclosporine as a single agent therapy in chronic idiopathic thrombocytopenic purpura. Haematologica 2008, 93, e61–e63. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, C.A.; Pell, J.; Hill, Q.; Bagot, C.; Cooper, N.; Ingram, J.; Breheny, K.; Kandiyali, R.; Rayment, R.; Evans, G.; et al. Mycophenolate Mofetil for First-Line Treatment of Immune Thrombocytopenia. N. Engl. J. Med. 2021, 385, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gu, X.; Fu, R.; Li, Y.; Lv, M.; Sun, T.; Lv, C.; Liu, X.; Xue, F.; Zhang, L.; et al. The Effect of Danazol in Primary Immune Thrombocytopenia: An Analysis of a Large Cohort From a Single Center in China. Clin. Appl. Thromb. Hemost. 2016, 22, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.P.; Patil, A.S. Dapsone for immune thrombocytopenic purpura in children and adults. Platelets 2015, 26, 164–167. [Google Scholar] [CrossRef]
- Park, Y.H.; Yi, H.G.; Lee, M.H.; Kim, C.S.; Lim, J.H. Clinical efficacy and tolerability of vincristine in splenectomized patients with refractory or relapsed immune thrombocytopenia: a retrospective single-center study. Int. J. Hematol. 2016, 103, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Matzdorff, A.; Meyer, O.; Ostermann, H.; Kiefel, V.; Eberl, W.; Kühne, T.; Pabinger, I.; Rummel, M. Immune Thrombocytopenia - Current Diagnostics and Therapy: Recommendations of a Joint Working Group of DGHO, ÖGHO, SGH, GPOH, and DGTI. Oncol. Res. Treat. 2018, 41 (Suppl. 5), 1–30. [Google Scholar] [CrossRef]
- Glance, L.G.; Blumberg, N.; Eaton, M.P.; Lustik, S.J.; Osler, T.M.; Wissler, R.; Zollo, R.; Karcz, M.; Feng, C.; Dick, A.W. Preoperative thrombocytopenia and postoperative outcomes after noncardiac surgery. Anesthesiology 2014, 120, 62–75. [Google Scholar] [CrossRef]
- Ghanima, W.; Gernsheimer, T.; Kuter, D.J. How I treat primary ITP in adult patients who are unresponsive to or dependent on corticosteroid treatment. Blood 2021, 137, 2736–2744. [Google Scholar] [CrossRef]
- Bussel, J.B.; Kuter, D. Preparing patients with immune thrombocytopenia for surgery: what are the options? Lancet. Haematol. 2020, 7, e626–e627. [Google Scholar] [CrossRef]
- Kuter, D.J.; Rummel, M.; Boccia, R.; Macik, B.G.; Pabinger, I.; Selleslag, D.; Rodeghiero, F.; Chong, B.H.; Wang, X.; Berger, D.P. Romiplostim or standard of care in patients with immune thrombocytopenia. N. Engl. J. Med. 2010, 363, 1889–1899. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Saleh, M.N.; Marcher, C.; Vasey, S.; Mayer, B.; Aivado, M.; Arning, M.; Stone, N. L.; Bussel, J.B. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet 2011, 377, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Bussel, J.B.; Kuter, D.J.; George, J.N.; McMillan, R.; Aledort, L.M.; Conklin, G.T.; Lichtin, A.E.; Lyons, R.M.; Nieva, J.; Wasser, J.S.; et al. AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N. Engl. J. Med. 2006, 355, 1672–1681. [Google Scholar] [CrossRef]
- Newland, A.; Godeau, B.; Priego, V.; Viallard, J.F.; López Fernández, M.F.; Orejudos, A.; Eisen, M. Remission and platelet responses with romiplostim in primary immune thrombocytopenia: final results from a phase 2 study. Br. J. Haematol. 2016, 172, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Mahévas, M.; Fain, O.; Ebbo, M.; Roudot-Thoraval, F.; Limal, N.; Khellaf, M.; Schleinitz, N.; Bierling, P.; Languille, L.; Godeau, B.; et al. The temporary use of thrombopoietin-receptor agonists may induce a prolonged remission in adult chronic immune thrombocytopenia. Results of a French observational study. Br. J. Haematol. 2014, 165, 865–869. [Google Scholar] [CrossRef] [PubMed]
- González-López, T.J.; Pascual, C.; Álvarez-Román, M.T.; Fernández-Fuertes, F.; Sánchez-González, B.; Caparrós, I.; Jarque, I.; Mingot-Castellano, M.E.; Hernández-Rivas, J.A.; Martín-Salces, M.; et al. Successful discontinuation of eltrombopag after complete remission in patients with primary immune thrombocytopenia. Am. J. Hematol. 2015, 90, E40–E43. [Google Scholar] [CrossRef] [PubMed]
- Lucchini, E.; Palandri, F.; Volpetti, S.; Vianelli, N.; Auteri, G.; Rossi, E.; Patriarca, A.; Carli, G.; Barcellini, W.; Celli, M.; et al.; for Gruppo Italiano Malattie Ematologiche dell’Adulto (GIMEMA) Eltrombopag second-line therapy in adult patients with primary immune thrombocytopenia in an attempt to achieve sustained remission off-treatment: results of a phase II, multicentre, prospective study. Br. J. Haematol. 2021, 193, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Mingot-Castellano, M.E.; Román, M.T.Á.; Fernández Fuertes, L.F.; González-López, T.J.; Guinea de Castro, J.M.; Jarque, I.; López-Fernández, M.F.; Lozano, M.L.; Sánchez González, B.; Ferreiras, D.V.; et al. Management of Adult Patients with Primary Immune Thrombocytopenia (ITP) in Clinical Practice: A Consensus Approach of the Spanish ITP Expert Group. Adv. Hematol. 2019, 2019, 4621416. [Google Scholar] [CrossRef]
- Cooper, N.; Hill, Q. A.; Grainger, J.; Westwood, J. P.; Bradbury, C.; Provan, D.; Thachil, J.; Ramscar, N.; Roy, A. Tapering and Discontinuation of Thrombopoietin Receptor Agonist Therapy in Patients with Immune Thrombocytopenia: Results from a Modified Delphi Panel. Acta Haematol. 2021, 144, 418–426. [Google Scholar] [CrossRef]
- Carpenedo, M.; Baldacci, E.; Baratè, C.; Borchiellini, A.; Buccisano, F.; Calvaruso, G.; Chiurazzi, F.; Fattizzo, B.; Giuffrida, G.; Rossi, E.; et al. Second-line administration of thrombopoietin receptor agonists in immune thrombocytopenia: Italian Delphi-based consensus recommendations. Ther. Adv. Hematol. 2021, 12, 20406207211048361. [Google Scholar] [CrossRef]
- González-López, T.J.; Provan, D. Sustained Remission Off-Treatment (SROT) of TPO-RAs: The Burgos Ten-Step Eltrombopag Tapering Scheme. Medicina (Kaunas) 2023, 59, 659. [Google Scholar] [CrossRef] [PubMed]
- Carpenedo, M.; Baldacci, E.; Baratè, C.; Borchiellini, A.; Buccisano, F.; Calvaruso, G.; Chiurazzi, F.; Fattizzo, B.; Giuffrida, G.; Rossi, E.; et al. Second-line administration of thrombopoietin receptor agonists in immune thrombocytopenia: Italian Delphi-based consensus recommendations. Ther. Adv. Hematol. 2021, 12, 20406207211048361. [Google Scholar] [CrossRef] [PubMed]
- Cuker, A.; Despotovic, J.M.; Grace, R.F.; Kruse, C.; Lambert, M.P.; Liebman, H.A.; Lyons, R.M.; McCrae, K.R.; Pullarkat, V.; Wasser, J.S.; et al. Tapering thrombopoietin receptor agonists in primary immune thrombocytopenia: Expert consensus based on the RAND/UCLA modified Delphi panel method. Res. Pract. Thromb. Haemost. 2020, 5, 69–80. [Google Scholar] [CrossRef]
- Blanchette, V.; Imbach, P.; Andrew, M.; Adams, M.; McMillan, J.; Wang, E.; Milner, R.; Ali, K.; Barnard, D.; Bernstein, M. Randomised trial of intravenous immunoglobulin G, intravenous anti-D, and oral prednisone in childhood acute immune thrombocytopenic purpura. Lancet 1994, 344, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Heitink-Pollé, K.M.J.; Uiterwaal, C.S.P.M.; Porcelijn, L.; Tamminga, R.Y.J.; Smiers, F.J.; van Woerden, N.L.; Wesseling, J.; Vidarsson, G.; Laarhoven, A.G.; de Haas, M.; et al.; TIKI Investigators Intravenous immunoglobulin vs observation in childhood immune thrombocytopenia: a randomized controlled trial. Blood 2018, 132, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Neunert, C.; Despotovic, J.; Haley, K.; Lambert, M. P.; Nottage, K.; Shimano, K.; Bennett, C.; Klaassen, R.; Stine, K.; Thompson, A.; et al.; Pediatric ITP Consortium of North America (ICON) Thrombopoietin Receptor Agonist Use in Children: Data From the Pediatric ITP Consortium of North America ICON2 Study. Pediatr. Blood Cancer 2016, 63, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, M. D.; Bussel, J. B.; Blanchette, V. S.; Despotovic, J.; Bennett, C.; Raj, A.; Williams, B.; Beam, D.; Morales, J.; Rose, M. J.; et al. Romiplostim in children with immune thrombocytopenia: a phase 3, randomised, double-blind, placebo-controlled study. Lancet 2016, 388, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Mahévas, M.; Michel, M.; Godeau, B. How we manage immune thrombocytopenia in the elderly. Br. J. Haematol. 2016, 173, 844–856. [Google Scholar] [CrossRef]
- Lucchini, E.; Fanin, R.; Cooper, N.; Zaja, F. Management of immune thrombocytopenia in elderly patients. Eur. J. Intern. Med. 2018, 58, 70–76. [Google Scholar] [CrossRef]
- González-López, T. J.; Sánchez-González, B.; Jarque, I.; Bernat, S.; Fernández-Fuertes, F.; Caparrós, I.; Soto, I.; Fernández-Rodríguez, A.; Bolaños, E.; Pérez-Rus, G.; et al. Use of eltrombopag for patients 65 years old or older with immune thrombocytopenia. Eur. J. Haematol. 2020, 104, 259–270. [Google Scholar] [CrossRef]
- Palandri, F.; Rossi, E.; Bartoletti, D.; Ferretti, A.; Ruggeri, M.; Lucchini, E.; Carrai, V.; Barcellini, W.; Patriarca, A.; Rivolti, E.; et al. Real-world use of thrombopoietin receptor agonists in older patients with primary immune thrombocytopenia. Blood 2021, 138, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Bussel, J.B.; Wang, X.; Lopez, A.; Eisen, M. Case study of remission in adults with immune thrombocytopenia following cessation of treatment with the thrombopoietin mimetic romiplostim. Hematology 2016, 21, 257–262. [Google Scholar] [CrossRef] [PubMed]
- González-López, T.J.; Alvarez-Román, M.T.; Pascual, C.; Sánchez-González, B.; Fernández-Fuentes, F.; Jarque, I.; Pérez-Rus, G.; Pérez-Crespo, S.; Bernat, S.; Hernández-Rivas, J.A.; et al. Eltrombopag safety and efficacy for primary chronic immune thrombocytopenia in clinical practice. Eur. J. Haematol. 2016, 97, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Boccia, R.; Cooper, N.; Ghanima, W.; Boxer, M.A.; Hill, Q.A.; Sholzberg, M.; Tarantino, M. D.; Todd, L.K.; Tong, S.; Bussel, J.B.; FIT Clinical Trial Investigators. Fostamatinib is an effective second-line therapy in patients with immune thrombocytopenia. Br. J. Haematol. 2020, 190, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hsia, C.C. The Efficacy and Safety of Fostamatinib in Elderly Patients with Immune Thrombocytopenia: A Single-Center, Real-World Case Series. Adv. Hematol. 2022, 2022, 8119270. [Google Scholar] [PubMed]
- Pishko, A.M.; Levine, L.D.; Cines, D.B. Thrombocytopenia in pregnancy: Diagnosis and approach to management. Blood Rev. 2020, 40, 100638. [Google Scholar] [CrossRef]
- Eslick, R.; McLintock, C. Managing ITP and thrombocytopenia in pregnancy. Platelets 2020, 31, 300–306. [Google Scholar] [CrossRef]
- Poston, J.N.; Gernsheime, T.B. Management of immune thrombocytopenia in pregnancy. Ann. Blood 2021, 6, 5. [Google Scholar] [CrossRef]
- Bussel, J.B.; Cooper, N.; Lawrence, T.; Michel, M.; Vander Haar, E.; Wang, K.; Wang, H.; Saad, H. Romiplostim use in pregnant women with immune thrombocytopenia. Am. J. Hematol. 2023, 98, 31–40. [Google Scholar] [CrossRef]
- Michel, M.; Ruggeri, M.; Gonzalez-Lopez, T.J.; Alkindi, S.; Cheze, S.; Ghanima, W.; Tvedt, T.H.A.; Ebbo, M.; Terriou, L.; Bussel, J.B.; et al. Use of thrombopoietin receptor agonists for immune thrombocytopenia in pregnancy: results from a multicenter study. Blood 2020, 136, 3056–3061. [Google Scholar] [CrossRef]
- Cines, D.B.; Levine, L.D. Thrombocytopenia in pregnancy. Blood 2017, 130, 2271–2277. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ema.europa.eu/en/documents/product-information/tavlesse-epar-product-information_en.pdf (accessed on 13 June 2023).
- Michel, M.; Lega, J.C.; Terriou, L. Secondary ITP in adults. Rev. Med. Interne 2021, 42, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Moulis, G.; Palmaro, A.; Montastruc, J.L.; Godeau, B.; Lapeyre-Mestre, M.; Sailler, L. Epidemiology of incident immune thrombocytopenia: a nationwide population-based study in France. Blood, 2014, 124, 3308–3315. [Google Scholar] [CrossRef] [PubMed]
- Cines, D.B.; Bussel, J.B.; Liebman, H.A.; Luning Prak, E.T. The ITP syndrome: pathogenic and clinical diversity. Blood 2009, 113, 6511–6521. [Google Scholar] [CrossRef] [PubMed]
- Fanouriakis, A.; Tziolos, N.; Bertsias, G.; Boumpas, D.T. Update οn the diagnosis and management of systemic lupus erythematosus. Ann. Rheum. Dis. 2021, 80, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Mahévas, M.; Azzaoui, I.; Crickx, E.; Canoui-Poitrine, F.; Gobert, D.; Languille, L.; Limal, N.; Guillaud, C.; Croisille, L.; Jeljeli, M.; et al. Efficacy, safety and immunological profile of combining rituximab with belimumab for adults with persistent or chronic immune thrombocytopenia: results from a prospective phase 2b trial. Haematologica 2021, 106, 2449–2457. [Google Scholar] [CrossRef]
- Kim, T.O.; Despotovic, J.M. Primary and Secondary Immune Cytopenias: Evaluation and Treatment Approach in Children. Hematol. Oncol. Clin. North. Am. 2019, 33, 489–506. [Google Scholar] [CrossRef]
- Tomasello, R.; Giordano, G.; Romano, F.; Vaccarino, F.; Siragusa, S.; Lucchesi, A.; Napolitano, M. Immune Thrombocytopenia in Antiphospholipid Syndrome: Is It Primary or Secondary? Biomedicines, 2021, 9, 1170. [Google Scholar] [CrossRef]
- Podjasek, J.C.; Abraham, R.S. Autoimmune cytopenias in common variable immunodeficiency. Front. Immunol. 2012, 3, 189. [Google Scholar] [CrossRef]
- Michniacki, T.F.; Ebens, C.L.; Choi, S.W. Immune-Mediated Cytopenias After Hematopoietic Cell Transplantation: Pathophysiology, Clinical Manifestations, Diagnosis, and Treatment Strategies. Curr. Oncol. Rep. 2019, 21, 87. [Google Scholar] [CrossRef]
- Liebman, H.A.; Stasi, R. Secondary immune thrombocytopenic purpura. Curr. Opin. Hematol. 2007, 14, 557–573. [Google Scholar] [CrossRef] [PubMed]
- Abdela, J. Current Advance in Thrombopoietin Receptor Agonists in the Management of Thrombocytopenia Associated With Chronic Liver Disease: Focus on Avatrombopag. Clinical medicine insights. Clin. Med. Insights Blood Disord. 2019, 12, 1179545X19875105. [Google Scholar] [CrossRef] [PubMed]
- Stasi, R. Therapeutic strategies for hepatitis- and other infection-related immune thrombocytopenias. Semin. Hematol. 2009, 46 (Suppl. 2), S15–S25. [Google Scholar] [CrossRef] [PubMed]
- Pavord, S.; Thachil, J.; Hunt, B.J.; Murphy, M.; Lowe, G.; Laffan, M.; Makris, M.; Newland, A. C.; Provan, D.; Grainger, J.D.; et al. Practical guidance for the management of adults with immune thrombocytopenia during the COVID-19 pandemic. Br. J. Haematol. 2020, 189, 1038–1043. [Google Scholar] [CrossRef]
- Arnold, D.M.; Bernotas, A.; Nazi, I.; Stasi, R.; Kuwana, M.; Liu, Y.; Kelton, J.G.; Crowther, M.A. Platelet count response to H. pylori treatment in patients with immune thrombocytopenic purpura with and without H. pylori infection: a systematic review. Haematologica 2009, 94, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P. Rescue therapy after Helicobacter pylori eradication failure. Gastroenterol. Hepatol. 2011, 34, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Bento, L.; Canaro, M.; Bastida, J. M.; Sampol, A. Thrombocytopenia and Therapeutic Strategies after Allogeneic Hematopoietic Stem Cell Transplantation. J. Clin. Med. 2022, 11, 1364. [Google Scholar] [CrossRef]
- Platelets on the Web. Drug-Induced Thrombocytopenia. Available online: https://www.ouhsc.edu/platelets/ditp.html (accessed on 2 June 2023).
- George, J.N.; Aster, R.H. Drug-induced thrombocytopenia: pathogenesis, evaluation, and management. Hematology Am. Soc. Hematol. Educ. Program 2009, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Arepally, G.M.; Cines, D.B. Pathogenesis of heparin-induced thrombocytopenia. Transl. Res. 2020, 225, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Swan, D.; Newland, A.; Rodeghiero, F.; Thachil, J. Thrombosis in immune thrombocytopenia - current status and future perspectives. Br. J. Haematol. 2021, 194, 822–834. [Google Scholar] [CrossRef]
- Le Guenno, G.; Guieze, R.; Audia, S.; Khellaf, M.; Michel, M.; Bonnotte, B.; Ruivard, M.; Godeau, B. Characteristics, risk factors and management of venous thromboembolism in immune thrombocytopenia: a retrospective multicentre study. Intern. Med. J. 2019, 49, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Román, M.T.; Fernández-Bello, I.; Jiménez-Yuste, V.; Martín-Salces, M.; Arias-Salgado, E.G.; Rivas Pollmar, M.I.; Justo Sanz, R.; Butta, N.V. Procoagulant profile in patients with immune thrombocytopenia. Br. J. Haematol. 2016, 175, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Lozano, M.L.; Garabet, L.; Fernandez-Perez, M.P.; De Los Reyes-García, A.M.; Diaz-Lozano, P.; Garcia-Barbera, N.; Aguila, S.; Vicente, V.; Ghanima, W.; Martinez, C.; et al. Platelet activation and neutrophil extracellular trap (NET) formation in immune thrombocytopenia: is there an association? Platelets 2020, 31, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Justo Sanz, R.; Monzón Manzano, E.; Fernández Bello, I.; Teresa Álvarez Román, M.; Martín Salces, M.; Rivas Pollmar, M.I.; Jiménez Yuste, V.; Butta, N.V. Platelet Apoptosis and PAI-1 are Involved in the Pro-Coagulant State of Immune Thrombocytopaenia Patients Treated with Thrombopoietin Receptor Agonists. Thromb. Haemost. 2019, 119, 645–659. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.P.; Steg, G.; Bhatt, D.L. The management of antiplatelet therapy in acute coronary syndrome patients with thrombocytopenia: a clinical conundrum. Eur. Heart. J. 2017, 38, 3488–3492. [Google Scholar] [CrossRef] [PubMed]
- Strich, J.R.; Tian, X.; Samour, M.; King, C.S.; Shlobin, O.; Reger, R.; Cohen, J.; Ahmad, K.; Brown, A.W.; Khangoora, V.; et al. Fostamatinib for the Treatment of Hospitalized Adults With Coronavirus Disease 2019: A Randomized Trial. Clin. Infect. Dis. 2022, 75, e491–e498. [Google Scholar] [CrossRef]
- Schietzel, S.; Anderegg, M.; Limacher, A.; Born, A.; Horn, M. P.; Maurer, B.; Hirzel, C.; Sidler, D.; Moor, M. B. Humoral and cellular immune responses on SARS-CoV-2 vaccines in patients with anti-CD20 therapies: a systematic review and meta-analysis of 1342 patients. R.M.D. open 2022, 8, e002036. [Google Scholar] [CrossRef]
- Jena, A.; Mishra, S.; Deepak, P.; Kumar, M.P.; Sharma, A.; Patel, Y.I.; Kennedy, N.A.; Kim, A.H.J.; Sharma, V.; Sebastian, S. Response to SARS-CoV-2 vaccination in immune mediated inflammatory diseases: Systematic review and meta-analysis. Autoimmun. Rev. 2022, 21, 102927. [Google Scholar] [CrossRef]
- Bahadoram, M.; Saeedi-Boroujeni, A.; Mahmoudian-Sani, M.R.; Hussaini, H.; Hassanzadeh, S. COVID-19-induced immune thrombocytopenic purpura; Immunopathogenesis and clinical implications. Infez. Med. 2022, 30, 41–50. [Google Scholar]
- Mingot-Castellano, M. E.; Alcalde-Mellado, P.; Pascual-Izquierdo, C.; Perez Rus, G.; Calo Pérez, A.; Martinez, M. P.; López-Jaime, F. J.; Abalo Perez, L.; Gonzalez-Porras, J. R.; López Fernández, F.; et al. on behalf GEPTI (Grupo Español de Trombocitopenia Inmune) Incidence, characteristics and clinical profile of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection in patients with pre-existing primary immune thrombocytopenia (ITP) in Spain. Br. J. Haematol. 2021, 194, 537–541. [Google Scholar] [CrossRef]
- Lapietra, G.; Ferretti, A.; Baldacci, E.; Chistolini, A.; Santoro, C. Immune thrombocytopenia management during COVID-19 pandemic: An Italian monocentric experience. EJHaem. 2022, 3, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Moreira, E.D., Jr.; Kitchin, N.; Xu, X.; Dychter, S. S.; Lockhart, S.; Gurtman, A.; Perez, J. L.; Zerbini, C.; Dever, M. E.; Jennings, T. W.; et al. Safety and Efficacy of a Third Dose of BNT162b2 Covid-19 Vaccine. N. Engl. J. Med. 2022, 386, 1910–1921. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef] [PubMed]
- Tormen, M.; Taliento, C.; Salvioli, S.; Piccolotti, I.; Scutiero, G.; Cappadona, R.; Greco, P. Effectiveness and safety of COVID-19 vaccine in pregnant women: A systematic review with meta-analysis. B.J.O.G. 2023, 130, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Visser, C.; Swinkels, M.; van Werkhoven, E. D.; Croles, F. N.; Noordzij-Nooteboom, H. S.; Eefting, M.; Last-Koopmans, S. M.; Idink, C.; Westerweel, P. E.; Santbergen, B.; et al. COVID-19 vaccination in patients with immune thrombocytopenia. Blood Adv. 2022, 6, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
Table 1.
Primary ITP: general aspects, pathophysiology and diagnosis.
Table 1.
Primary ITP: general aspects, pathophysiology and diagnosis.
Table 2.
Consensual definitions and concepts in the context of primary ITP.
Table 2.
Consensual definitions and concepts in the context of primary ITP.
Table 3.
First-line and second-line treatment of primary ITP, and management of refractory patients.
Table 3.
First-line and second-line treatment of primary ITP, and management of refractory patients.
Table 4.
Guidelines for the follow-up of non-hospitalized primary ITP patients.
Table 4.
Guidelines for the follow-up of non-hospitalized primary ITP patients.
Table 5.
Topics of interest regarding surgery in patients with primary ITP.
Table 5.
Topics of interest regarding surgery in patients with primary ITP.
Table 6.
Management of primary ITP in pediatric patients, elderly patients and pregnant women.
Table 6.
Management of primary ITP in pediatric patients, elderly patients and pregnant women.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).