Submitted:
05 September 2023
Posted:
06 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
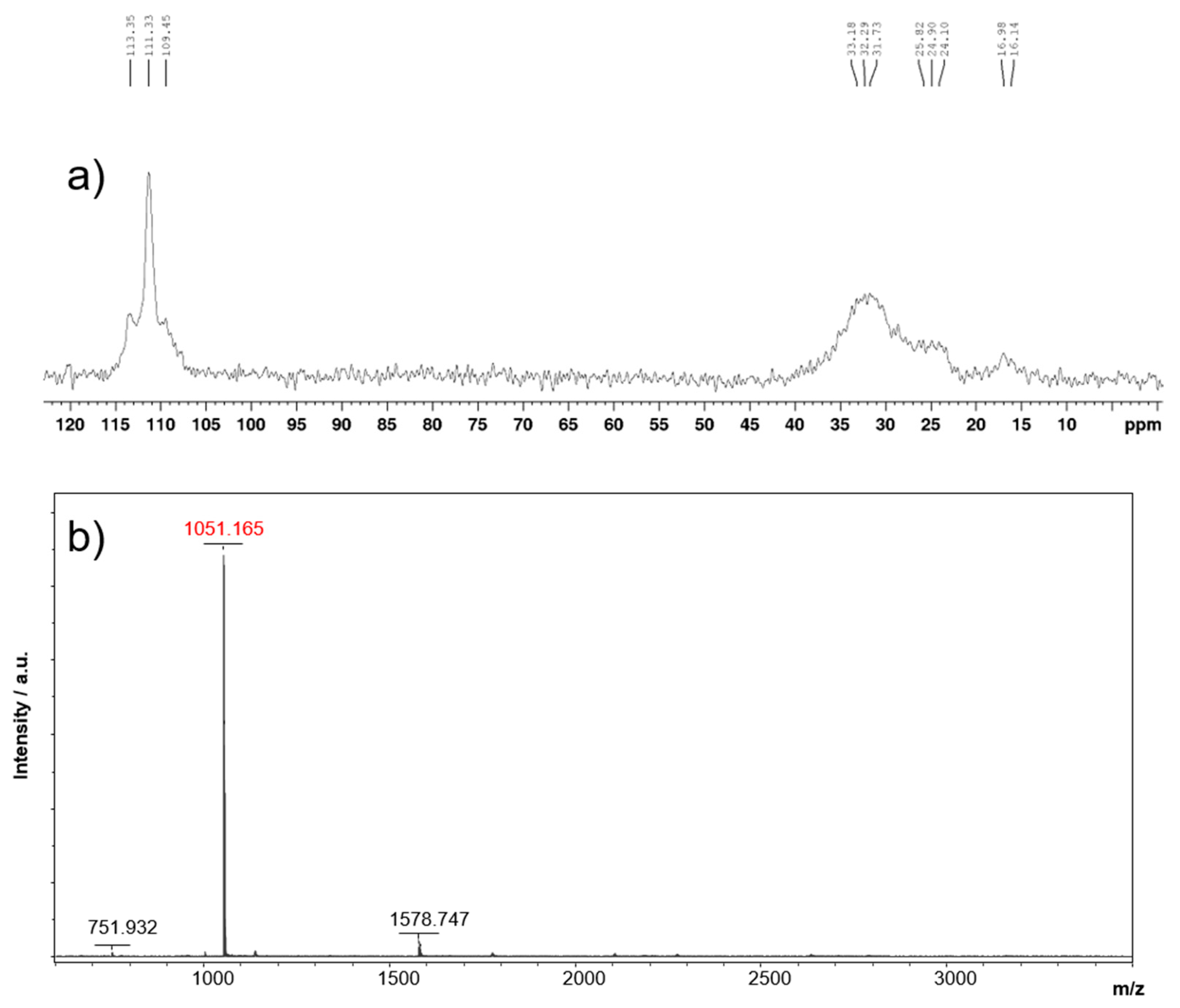
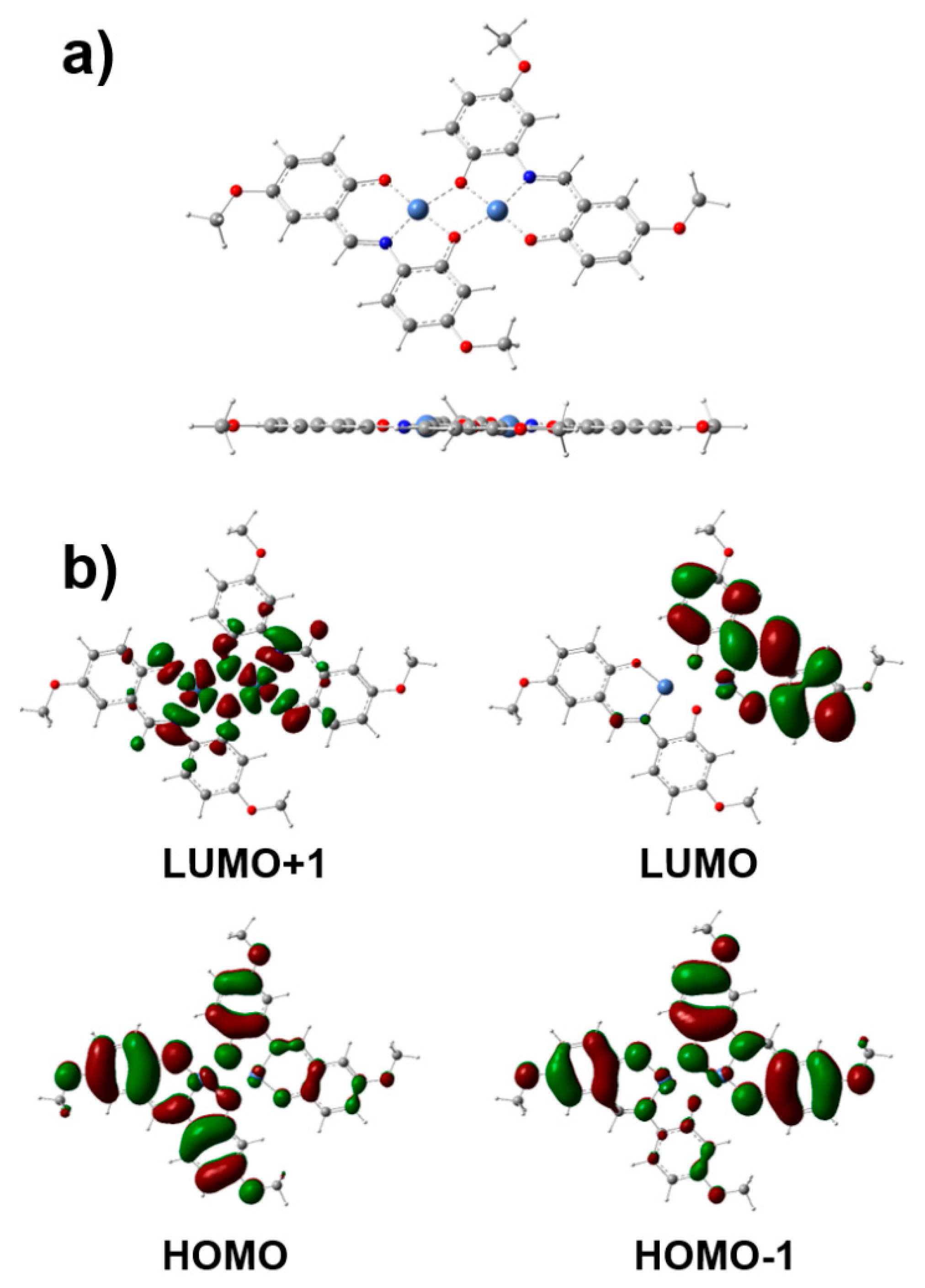
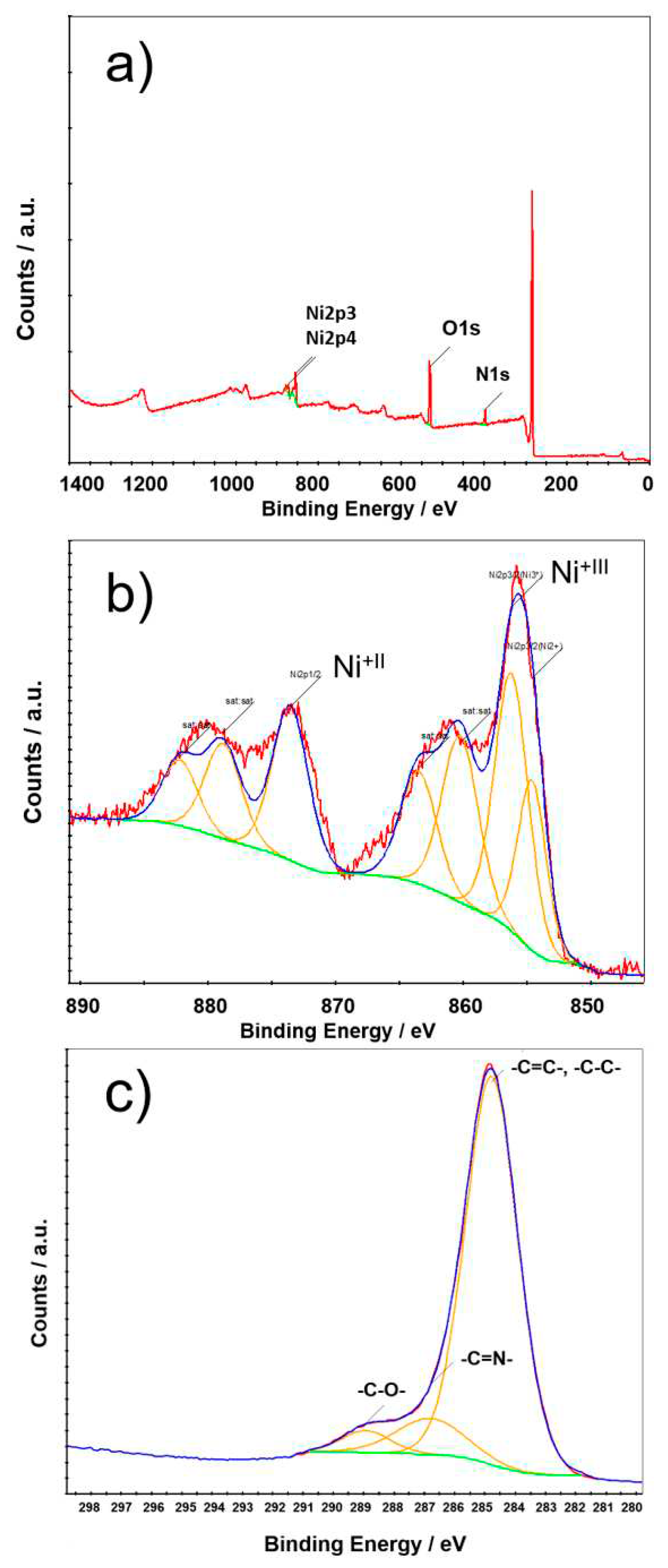
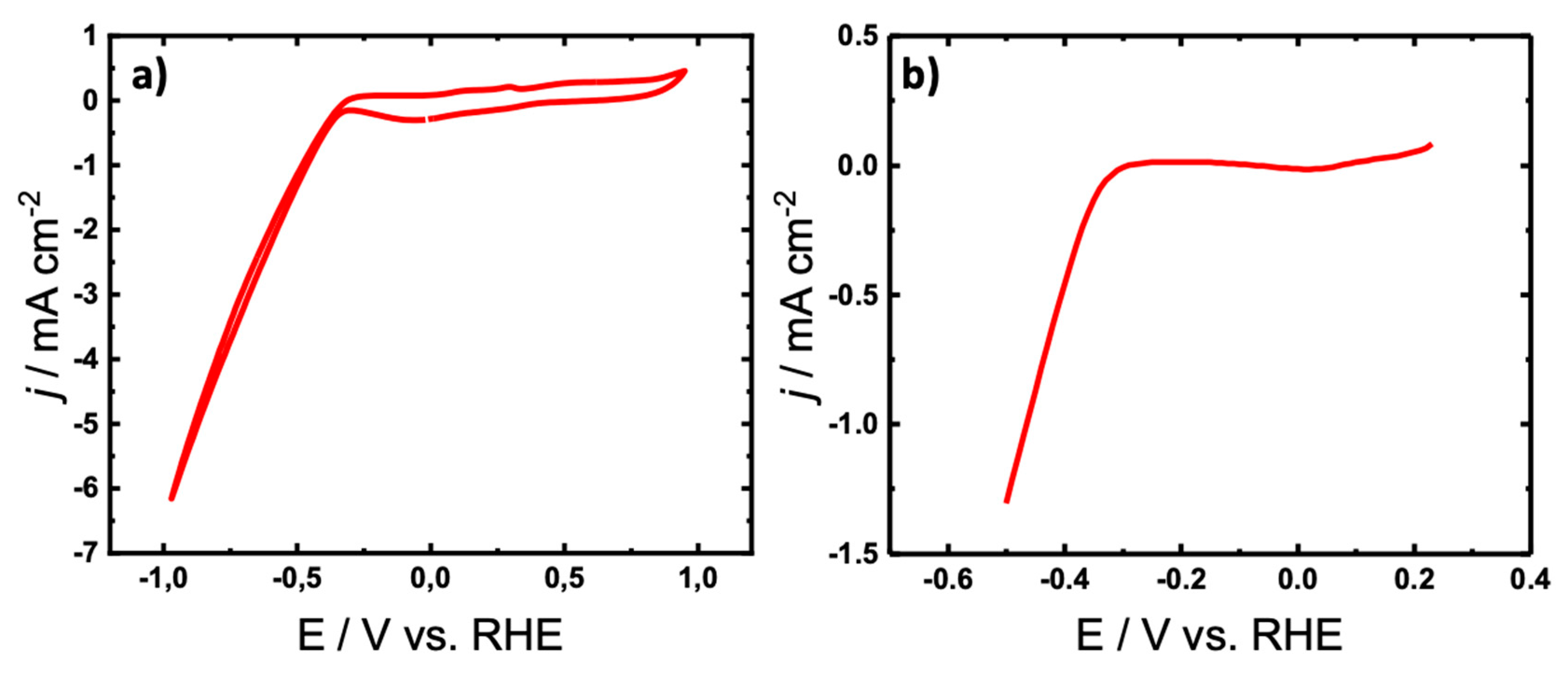
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Synthesis and Characterization
4.1.1. Synthesis of 4-(octyloxy)phenol
4.1.2. Synthesis of 2-hydroxy-5-(octyloxy)benzaldehyde
4.1.3. Synthesis of 2-nitro-4-(octyloxy)phenol
4.1.4. Synthesis of 2-amino-4-(octyloxy)phenol
4.1.5. Synthesis of (Z)-2-((2-hydroxy-5-(octyloxy)benzylidene)amino)-4-(octyloxy)phenol 1
4.1.6. Synthesis of [Ni]2[L]2 Complex 2
DFT Calculations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dresselhaus, M.S.; Thomas, I.L. Alternative Energy Technologies. 2001, 414.
- Gu, S.; Xu, B.; Yan, Y. Electrochemical Energy Engineering: A New Frontier of Chemical Engineering Innovation. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 429–454. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Majumdar, A. Opportunities and Challenges for a Sustainable Energy Future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.; Yan, F.; Zhang, X.; He, Y.; Zhu, C.; Chou, S.; Zhang, X.; Chen, Y. Conductive CuCo-Based Bimetal Organic Framework for Efficient Hydrogen Evolution. Adv. Mater. 2021, 33, 2106781. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhao, S.; Zhao, K.; Muqsit, A.; Tang, H.; Chang, L.; Zhao, H.; Gao, Y.; Tang, Z. Ultrathin Platinum Nanowires Grown on Single-Layered Nickel Hydroxide with High Hydrogen Evolution Activity. Nat. Commun. 2015, 6, 6430. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Bigi, J.P.; Piro, N.A.; Tang, M.L.; Long, J.R.; Chang, C.J. Molecular Cobalt Pentapyridine Catalysts for Generating Hydrogen from Water. J. Am. Chem. Soc. 2011, 133, 9212–9215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, M.; Yang, Y.; Yao, T.; Sun, L. A Molecular Copper Catalyst for Electrochemical Water Reduction with a Large Hydrogen-Generation Rate Constant in Aqueous Solution. Angew. Chem. Int. Ed. 2014, 53, 13803–13807. [Google Scholar] [CrossRef] [PubMed]
- Karunadasa, H.I.; Chang, C.J.; Long, J.R. A Molecular Molybdenum-Oxo Catalyst for Generating Hydrogen from Water. Nature 2010, 464, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, M.; Yang, Y.; Zheng, D.; Han, K.; Sun, L. Highly Efficient Molecular Nickel Catalysts for Electrochemical Hydrogen Production from Neutral Water. Chem Commun 2014, 50, 14153–14156. [Google Scholar] [CrossRef] [PubMed]
- Greeley, J.; Jaramillo, T.F.; Bonde, J.; Chorkendorff, I.; Nørskov, J.K. Computational High-Throughput Screening of Electrocatalytic Materials for Hydrogen Evolution. Nat. Mater. 2006, 5, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Ji, S.; Wang, H.; Linkov, V.; Gai, H.; Liu, F.; Liu, Q.; Wang, R. N-Doped 3D Porous Ni/C Bifunctional Electrocatalysts for Alkaline Water Electrolysis. ACS Sustain. Chem. Eng. 2019, 7, 3974–3981. [Google Scholar] [CrossRef]
- Karunadasa, H.I.; Montalvo, E.; Sun, Y.; Majda, M.; Long, J.R.; Chang, C.J. A Molecular MoS2 Edge Site Mimic for Catalytic Hydrogen Generation. Science 2012, 335, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Abdel Aziz, A.A.; Salem, A.N.M.; Sayed, M.A.; Aboaly, M.M. Synthesis, Structural Characterization, Thermal Studies, Catalytic Efficiency and Antimicrobial Activity of Some M(II) Complexes with ONO Tridentate Schiff Base N-Salicylidene-o-Aminophenol (SaphH2). J. Mol. Struct. 2012, 1010, 130–138. [Google Scholar] [CrossRef]
- Kasumov, V.T. Oxovanadium(IV), Nickel(II) and Palladium(II) Complexes of Tridentate Salicylaldiminates Derived from 2,4-Di-Ter-Butyl-6-Aminophenol. Z. Für Naturforschung B 2001, 56, 263–270. [Google Scholar] [CrossRef]
- Setia, S.; Pal, S.K. Unsymmetrically Substituted Room Temperature Discotic Liquid Crystals Based on Hexa–Peri–Hexabenzocoronene Core. ChemistrySelect 2016, 1, 880–885. [Google Scholar] [CrossRef]
- Kong, L.; Wang, L.; Sun, D.; Meng, S.; Xu, D.; He, Z.; Dong, X.; Li, Y.; Jin, Y. Aggregation-Morphology-Dependent Electrochemical Performance of Co3O4 Anode Materials for Lithium-Ion Batteries. Molecules 2019, 24, 3149. [Google Scholar] [CrossRef] [PubMed]
- Nestke, S.; Stubbe, J.; Koehler, R.; Ronge, E.; Albold, U.; Vioel, W.; Jooss, C.; Sarkar, B.; Siewert, I. A Binuclear Cobalt Complex in the Electrochemical Water Oxidation Reaction. Z. Für Anorg. Allg. Chem. 2022, 648, e202200119. [Google Scholar] [CrossRef]
- Patrício, S.; Cruz, A.I.; Biernacki, K.; Ventura, J.; Eaton, P.; Magalhães, A.L.; Moura, C.; Hillman, A.R.; Freire, C. Novel Layer-by-Layer Interfacial [Ni(Salen)]−Polyelectrolyte Hybrid Films. Langmuir 2010, 26, 10842–10853. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, X.; Deng, F.; Li, J. Electropolymerization and Electrochemical Behavior of Nickel Schiff Base Complexes with Different Groups between Imine Linkages. RSC Adv. 2016, 6, 79894–79899. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, X.; Zhang, R.; Yu, S.; Levell, Z.H.; Wang, C.; Ma, S.; Zou, P.; Han, L.; Qin, J.; et al. Highly Selective Oxygen Reduction to Hydrogen Peroxide on a Carbon-Supported Single-Atom Pd Electrocatalyst. ACS Catal. 2022, 12, 4156–4164. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




