Submitted:
05 September 2023
Posted:
06 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
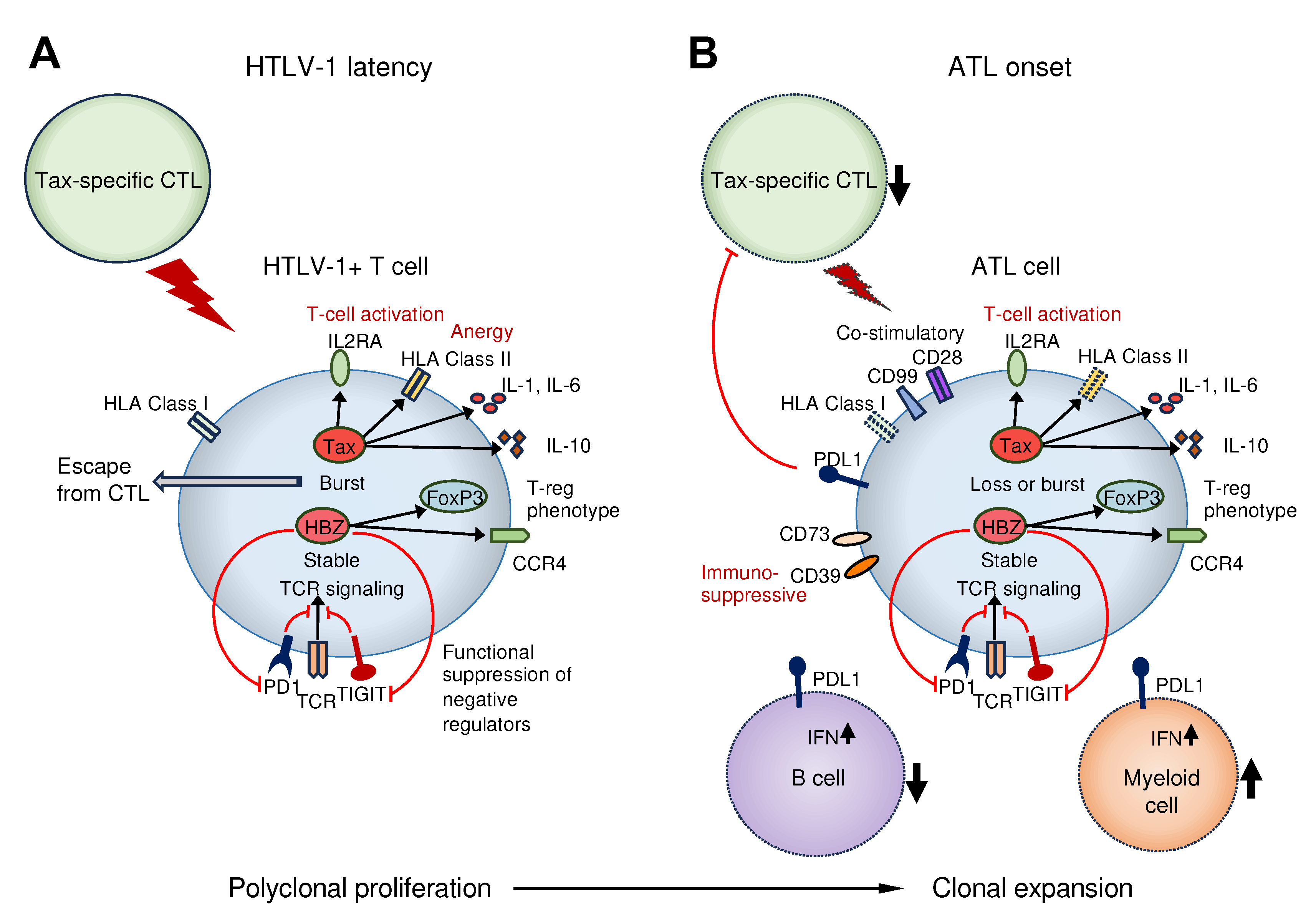
2. Immune disruption because of HTLV-1 infection
2.1. T cell anergy induced by HTLV-1 infection
2.2. Acquisition of regulatory T cell phenotype by HTLV-1 infection
3. Immune abnormalities in ATL
3.1. Genetic alterations
3.2. Tumor immune microenvironment
4. Clinical aspects of HTLV-1 infection and ATL related to immunological alterations
4.1. Mother-to-child Transmission
4.2. Coinfections
4.2.1. HIV
4.2.2. Strongyloides
4.3. Prognosis
4.4. Prognostic factors in aggressive ATL-subtypes.
4.5. Anti-HTLV-1 immunity
4.5.1. Tax -specific CTLs
4.5.2. HBZ -specific CTLs
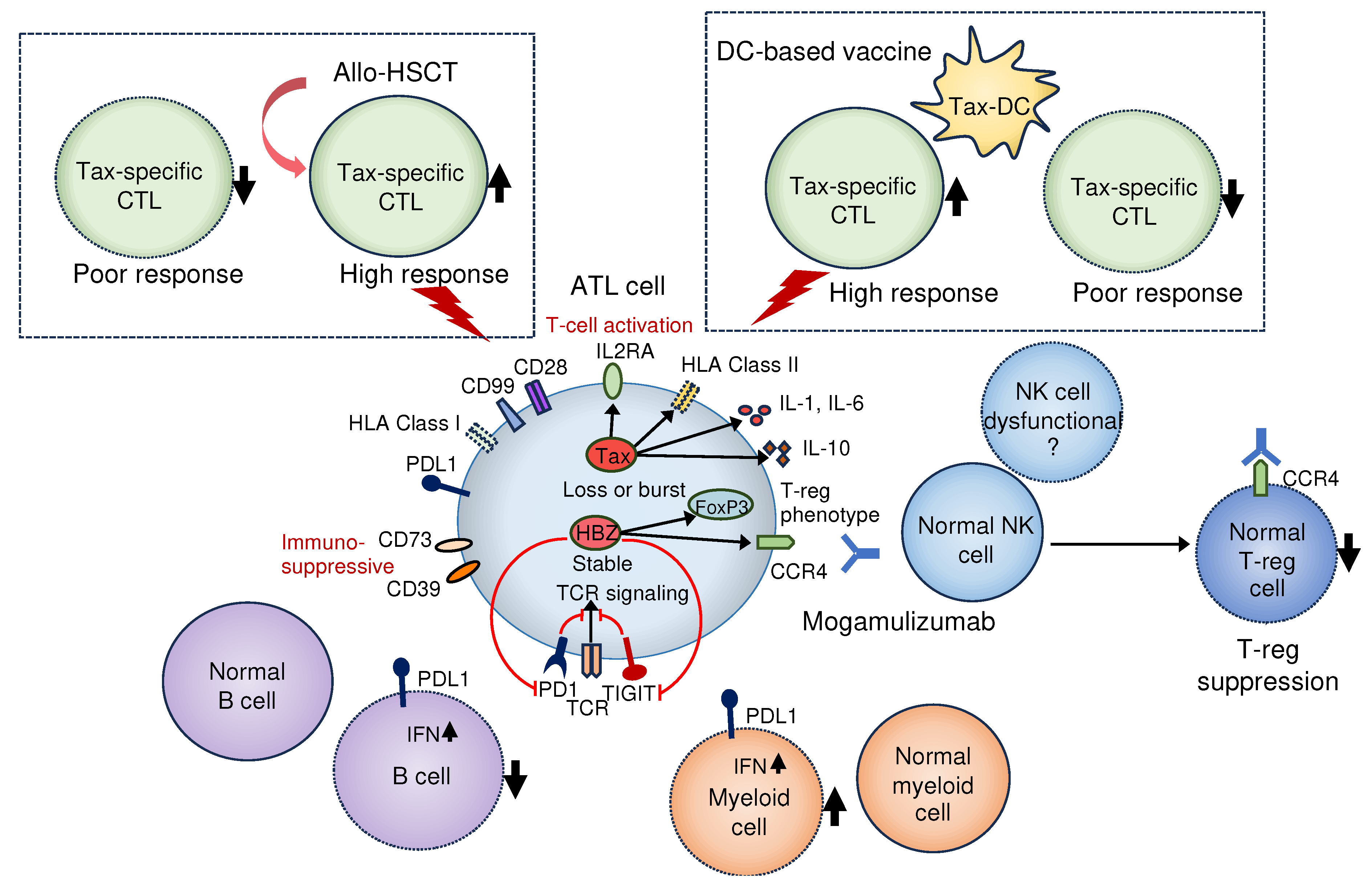
5. Development of therapeutic drugs targeting immunity for ATL
5.1. Anti-CCR4 antibody (mogamulizumab)
5.2. Anti-PD-1 antibody (nivolumab)
5.3. Lenalidomide
5.4. Dendritic cell-based vaccine
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bangham, C.R. Human T Cell Leukemia Virus Type 1: Persistence and Pathogenesis. Annu. Rev. Immunol. 2018, 36, 43–71. [Google Scholar] [CrossRef] [PubMed]
- Hirons, A.; Khoury, G.; Purcell, D.F.J. Human T-cell lymphotropic virus type-1: a lifelong persistent infection, yet never truly silent. Lancet Infect. Dis. 2021, 1, e2–e10. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, K.; Marçais, A.; Nasr, R.; Kato, K.; Fukuda, T.; Hermine, O.; Bazarbachi, A. Diagnostic Approaches and Established Treatments for Adult T Cell Leukemia Lymphoma. Front. Microbiol. 2020, 11, 1207. [Google Scholar] [CrossRef] [PubMed]
- Katsuya, H. Current and emerging therapeutic strategies in adult T-cell leukemia–lymphoma. Int. J. Hematol. 2023, 117, 512–522. [Google Scholar] [CrossRef] [PubMed]
- A Phillips, A. Advances in the treatment of HTLV-1-associated adult T-cell leukemia lymphoma. Curr. Opin. Virol. 2023, 58, 101289. [Google Scholar] [CrossRef]
- Malpica, L.; Enriquez, D.J.; Castro, D.A.; Peña, C.; Idrobo, H.; Fiad, L.; Prates, M.; Otero, V.; Biglione, M.; Altamirano, M.; et al. Real-World Data on Adult T-Cell Leukemia/Lymphoma in Latin America: A Study From the Grupo de Estudio Latinoamericano de Linfoproliferativos. JCO Glob. Oncol. 2021, 7, 1151–1166. [Google Scholar] [CrossRef]
- Legrand, N.; McGregor, S.; Bull, R.; Bajis, S.; Valencia, B.M.; Ronnachit, A.; Einsiedel, L.; Gessain, A.; Kaldor, J.; Martinello, M. Clinical and Public Health Implications of Human T-Lymphotropic Virus Type 1 Infection. Clin. Microbiol. Rev. 2022, 35, e0007821. [Google Scholar] [CrossRef]
- Nosaka, K.; Iwanaga, M.; Imaizumi, Y.; Ishitsuka, K.; Ishizawa, K.; Ishida, Y.; Amano, M.; Ishida, T.; Uike, N.; Utsunomiya, A.; et al. Epidemiological and clinical features of adult T-cell leukemia-lymphoma in Japan, 2010-2011: A nationwide survey. Cancer Sci. 2017, 108, 2478–2486. [Google Scholar] [CrossRef]
- Ramassamy, J.-L.; Tortevoye, P.; Ntab, B.; Seve, B.; Carles, G.; Gaquière, D.; Madec, Y.; Fontanet, A.; Gessain, A. Adult T-cell leukemia/lymphoma incidence rate in French Guiana: a prospective cohort of women infected with HTLV-1. Blood Adv. 2020, 4, 2044–2048. [Google Scholar] [CrossRef]
- Oliveira, P.D.; De Carvalho, R.F.; Bittencourt, A.L. Adult T-cell leukemia/lymphoma in South and Central America and the Caribbean: systematic search and review. Int. J. STD AIDS 2017, 28, 217–228. [Google Scholar] [CrossRef]
- Itabashi, K.; Miyazawa, T.; Uchimaru, K. How Can We Prevent Mother-to-Child Transmission of HTLV-1? Int J Mol Sci. 2023, 24, 6961. [Google Scholar] [CrossRef]
- Nunes, D.; Boa-Sorte, N.; Grassi, M.F.R.; Taylor, G.P.; Teixeira, M.G.; Barreto, M.L.; Dourado, I.; Galvão-Castro, B. HTLV-1 is predominantly sexually transmitted in Salvador, the city with the highest HTLV-1 prevalence in Brazil. PLOS ONE 2017, 12, e0171303. [Google Scholar] [CrossRef] [PubMed]
- Ramassamy, J.L.; Bilounga Ndongo, C. ; Nnuka, P; Antunes, M. ; Le Mener, M.; Betsem A Betsem, E.; Njouom, R.; Cassar, O.; Fontanet, A.; et al. Epidemiological Evidence of Nosocomial and Zoonotic Transmission of Human T-Cell Leukemia Virus-1 in a Large Survey in a Rural Population of Central Africa. J Infect Dis. 2023, 227, 752–760. [Google Scholar]
- Matutes, E. Adult T-cell leukaemia/lymphoma. J Clin Pathol. 2007, 60, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Kalinichenko, S.; Komkov, D.; Mazurov, D. HIV-1 and HTLV-1 Transmission Modes: Mechanisms and Importance for Virus Spread. Viruses 2022, 14, 152. [Google Scholar] [CrossRef] [PubMed]
- Mulherkar, T.H.; Gómez, D.J.; Sandel, G.; Jain, P. Co-Infection and Cancer: Host-Pathogen Interaction between Dendritic Cells and HIV-1, HTLV-1, and Other Oncogenic Viruses. Viruses 2022, 14, 2037. [Google Scholar] [CrossRef]
- Maartens, G.; Celum, C.; Lewin, S.R. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet 2014, 384, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.T.T.; Harab, R.C.; Leite, A.C.; Schor, D.; Araújo, A.; Andrada-Serpa, M.J. Human T Lymphotropic Virus Type 1 (HTLV-1) Proviral Load in Asymptomatic Carriers, HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis, and Other Neurological Abnormalities Associated with HTLV-1 Infection. Clin. Infect. Dis. 2007, 44, 689–692. [Google Scholar] [CrossRef]
- Iwanaga, M.; Watanabe, T.; Utsunomiya, A.; Okayama, A.; Uchimaru, K.; Koh, K.-R.; Ogata, M.; Kikuchi, H.; Sagara, Y.; Uozumi, K.; et al. Joint Study on Predisposing Factors of ATL Development investigators. Human T-cell leukemia virus type I (HTLV-1) proviral load and disease progression in asymptomatic HTLV-1 carriers: a nationwide prospective study in Japan. Blood 2010, 116, 1211–1219. [Google Scholar] [CrossRef]
- El Hajj, H.; Bazarbachi, A. Interplay between innate immunity and the viral oncoproteins Tax and HBZ in the pathogenesis and therapeutic response of HTLV-1 associated adult T cell leukemia. Front. Immunol. 2022, 13, 957535. [Google Scholar] [CrossRef]
- Kannagi, M.; Harashima, N.; Kurihara, K.; Ohashi, T.; Utsunomiya, A.; Tanosaki, R.; Masuda, M.; Tomonaga, M.; Okamura, J. Tumor immunity against adult T-cell leukemia. Cancer Sci. 2005, 96, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, Y.; Yasunaga, J.-I.; Matsuoka, M. HTLV-1's Foxy Strategy for Survival and Transmission. Front. A J. Women Stud. 2022, 1, 792659. [Google Scholar] [CrossRef]
- Mahgoub, M.; Yasunaga, J.-I.; Iwami, S.; Nakaoka, S.; Koizumi, Y.; Shimura, K.; Matsuoka, M. Sporadic on/off switching of HTLV-1 Tax expression is crucial to maintain the whole population of virus-induced leukemic cells. Proc. Natl. Acad. Sci. USA 2018, 115, E1269–E1278. [Google Scholar] [CrossRef]
- Kiik, H.; Ramanayake, S.; Miura, M.; Tanaka, Y.; Melamed, A.; Bangham, C.R.M. Time-course of host cell transcription during the HTLV-1 transcriptional burst. PLOS Pathog. 2022, 18, e1010387. [Google Scholar] [CrossRef] [PubMed]
- Ramanayake, S.; Moulding, D.A.; Tanaka, Y.; Singh, A.; Bangham, C.R.M. Dynamics and consequences of the HTLV-1 proviral plus-strand burst. PLOS Pathog. 2022, 18, e1010774. [Google Scholar] [CrossRef]
- Matsuoka, M.; Mesnard, J.-M. HTLV-1 bZIP factor: the key viral gene for pathogenesis. Retrovirology 2020, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Miyoshi, H.; Ohshima, K. Tumor microenvironment of adult T-cell leukemia/lymphoma. J. Clin. Exp. Hematop. 2021, 61, 202–209. [Google Scholar] [CrossRef]
- Komohara, Y.; Niino, D.; Saito, Y.; Ohnishi, K.; Horlad, H.; Ohshima, K.; Takeya, M. Clinical significance of CD163⁺ tumor-associated macrophages in patients with adult T-cell leukemia/lymphoma. Cancer Sci. 2013, 104, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Yamada, Y.; Kamihira, S.; Atogami, S.; Tsukasaki, K.; Momita, S.; Amagasaki, T.; Sadamori, N.; Tomonaga, M.; Kinoshita, K.-I.; et al. Frequency of eosinophilia in adult T-cell leukemia/lymphoma. Cancer 1992, 69, 966–971. [Google Scholar] [CrossRef]
- Yashiki, S.; Fujiyoshi, T.; Arima, N.; Osame, M.; Yoshinaga, M.; Nagata, Y.; Tara, M.; Nomura, K.; Utsunomiya, A.; Hanada, S.; et al. HLA-A*26, HLA-B*4002, HLA-B*4006, and HLA-B*4801 alleles predispose to adult T cell leukemia: the limited recognition of HTLV type 1 tax peptide anchor motifs and epitopes to generate anti-HTLV type 1 tax CD8(+) cytotoxic T lymphocytes. AIDS Res Hum Retroviruses. 2001, 17, 1047–1061. [Google Scholar] [CrossRef]
- Gillet, N.A.; Cook, L.; Laydon, D.J.; Hlela, C.; Verdonck, K.; Alvarez, C.; Gotuzzo, E.; Clark, D.; Farré, L.; Bittencourt, A.; et al. Strongyloidiasis and Infective Dermatitis Alter Human T Lymphotropic Virus-1 Clonality in vivo. PLOS Pathog. 2013, 9, e1003263. [Google Scholar] [CrossRef]
- Plumelle, Y.; Gonin, C.; Edouard, A.; Bucher, B.J.; Thomas, L.; Brebion, A.; Panelatti, G. Effect ofStrongyloides stercoralisInfection and Eosinophilia on Age at Onset and Prognosis of Adult T-Cell Leukemia. Am. J. Clin. Pathol. 1997, 107, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Hleihel, R.; Akkouche, A.; Skayneh, H.; Hermine, O.; Bazarbachi, A.; El Hajj, H. Adult T-Cell Leukemia: a Comprehensive Overview on Current and Promising Treatment Modalities. Curr. Oncol. Rep. 2021, 23, 141. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.U.; Proietti, F.A.; Ribas, J.G.R.; Araújo, M.G.; Pinheiro, S.R.; Guedes, A.C.; Carneiro-Proietti, A.B.F. Epidemiology, Treatment, and Prevention of Human T-Cell Leukemia Virus Type 1-Associated Diseases. Clin. Microbiol. Rev. 2010, 23, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, K.; Hermine, O.; Bazarbachi, A.; Ratner, L.; Ramos, J.C.; Harrington, W. Jr.; O'Mahony, D.; Janik, J.E.; Bittencourt, A.L.; Taylor, G.P. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J Clin Oncol. 2009, 27, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Babazono, A.; Nishi, T.; Yasui, M.; Matsuda, S.; Fushimi, K.; Fujimori, K. The Impact of Opportunistic Infections on Clinical Outcome and Healthcare Resource Uses for Adult T Cell Leukaemia. PLOS ONE 2015, 10, e0135042. [Google Scholar] [CrossRef]
- White, J.D.; Zaknoen, S.L.; Kasten-Sportès, C.; Top, L.E.; Navarro-Roman, L.; Nelson, D.L.; Waldmann, T.A. Infectious complications and immunodeficiency in patients with human T-cell lymphotropic virus I-associated adult T-cell leukemia/lymphoma. Cancer 1995, 75, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.J.; Jaffe, E.S.; Ehrlich, G.D.; Korman, N.J.; Poiesz, B.J.; Waldmann, T.A. Kaposi's sarcoma in human T-cell leukemia virus type I-associated adult T-cell leukemia. Blood 1990, 76, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Kamachi, K.; Shindo, T.; Miyahara, M.; Kitaura, K.; Akashi, M.; Shin, T.; Suzuki, R.; Oshima, K.; Kimura, S. Epstein–Barr virus-related diffuse large B-cell lymphoma in mogamulizumab-treated adult T-cell leukemia with incomplete T-cell reconstitution. Int. J. Hematol. 2019, 109, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Kawano, N.; Nagahiro, Y.; Yoshida, S.; Tahara, Y.; Himeji, D.; Kuriyama, T.; Tochigi, T.; Nakaike, T.; Shimokawa, T.; Yamashita, K.; et al. Clinical features and treatment outcomes of opportunistic infections among human T-lymphotrophic virus type 1 (HTLV-1) carriers and patients with adult T-cell leukemia-lymphoma (ATL) at a single institution from 2006 to 2016. J. Clin. Exp. Hematop. 2019, 59, 156–167. [Google Scholar] [CrossRef]
- Gabet, A.-S.; Mortreux, F.; Talarmin, A.; Plumelle, Y.; Leclercq, I.; Leroy, A.; Gessain, A.; Clity, E.; Joubert, M.; Wattel, E. High circulating proviral load with oligoclonal expansion of HTLV-1 bearing T cells in HTLV-1 carriers with strongyloidiasis. Oncogene 2000, 19, 4954–4960. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Taylor, G.P.; Klose, R.J.; Schofield, C.J.; Bangham, C.R. Histone H2A monoubiquitylation and p38-MAPKs regulate immediate-early gene-like reactivation of latent retrovirus HTLV-1. J. Clin. Investig. 2018, 3, e123196. [Google Scholar] [CrossRef]
- Kulkarni, A.; Mateus, M.; Thinnes, C.C.; McCullagh, J.S.; Schofield, C.J.; Taylor, G.P.; Bangham, C.R. Glucose Metabolism and Oxygen Availability Govern Reactivation of the Latent Human Retrovirus HTLV-1. Cell Chem. Biol. 2017, 24, 1377–1387.e3. [Google Scholar] [CrossRef]
- Miyazato, P.; Matsuo, M.; Tan, B.J.Y.; Tokunaga, M.; Katsuya, H.; Islam, S.; Ito, J.; Murakawa, Y.; Satou, Y. HTLV-1 contains a high CG dinucleotide content and is susceptible to the host antiviral protein ZAP. Retrovirology 2019, 16, 38. [Google Scholar] [CrossRef] [PubMed]
- Grassmann, R.; Dengler, C.; Müller-Fleckenstein, I.; Fleckenstein, B.; McGuire, K.; Dokhelar, M.C.; Sodroski, J.G.; A Haseltine, W. Transformation to continuous growth of primary human T lymphocytes by human T-cell leukemia virus type I X-region genes transduced by a Herpesvirus saimiri vector. Proc. Natl. Acad. Sci. USA 1989, 86, 3351–3355. [Google Scholar] [CrossRef]
- Tanaka, A.; Takahashi, C.; Yamaoka, S.; Nosaka, T.; Maki, M.; Hatanaka, M. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc. Natl. Acad. Sci. USA 1990, 87, 1071–1075. [Google Scholar] [CrossRef]
- Sugata, K.; Yasunaga, J.-I.; Mitobe, Y.; Miura, M.; Miyazato, P.; Kohara, M.; Matsuoka, M. Protective effect of cytotoxic T lymphocytes targeting HTLV-1 bZIP factor. Blood 2015, 126, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Portis, T.; Harding, J.C.; Ratner, L. The contribution of NF-kappa B activity to spontaneous proliferation and resistance to apoptosis in human T-cell leukemia virus type 1 Tax-induced tumors. Blood 2001, 98, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.J.; Sugata, K.; Reda, O.; Matsuo, M.; Uchiyama, K.; Miyazato, P.; Hahaut, V.; Yamagishi, M.; Uchimaru, K.; Suzuki, Y.; et al. HTLV-1 infection promotes excessive T cell activation and transformation into adult T cell leukemia/lymphoma. J. Clin. Investig. 2021, 131, e150472. [Google Scholar] [CrossRef]
- Shiohama, Y.; Naito, T.; Matsuzaki, T.; Tanaka, R.; Tomoyose, T.; Takashima, H.; Fukushima, T.; Tanaka, Y.; Saito, M. Absolute quantification of HTLV-1 basic leucine zipper factor (HBZ) protein and its plasma antibody in HTLV-1 infected individuals with different clinical status. Retrovirology 2016, 13, 29. [Google Scholar] [CrossRef]
- Satou, Y.; Yasunaga, J.; Yoshida, M.; Matsuoka, M. HTLV-I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc. Natl. Acad. Sci. USA 2006, 103, 720–725. [Google Scholar] [CrossRef]
- Toyoda, K.; Matsuoka, M. Functional and Pathogenic Roles of Retroviral Antisense Transcripts. Front. Immunol. 2022, 13, 875211. [Google Scholar] [CrossRef]
- Yasunaga, J.-I. Strategies of Human T-Cell Leukemia Virus Type 1 for Persistent Infection: Implications for Leukemogenesis of Adult T-Cell Leukemia-Lymphoma. Front. Microbiol. 2020, 11, 979. [Google Scholar] [CrossRef] [PubMed]
- Satou, Y.; Yasunaga, J.-I.; Zhao, T.; Yoshida, M.; Miyazato, P.; Takai, K.; Shimizu, K.; Ohshima, K.; Green, P.L.; Ohkura, N.; et al. HTLV-1 bZIP Factor Induces T-Cell Lymphoma and Systemic Inflammation In Vivo. PLOS Pathog. 2011, 7, e1001274. [Google Scholar] [CrossRef] [PubMed]
- Kinosada, H. ; Yasunaga, J-I. ; Shimura, K.; Miyazato, P.; Onishi, C.; Iyoda, T.; Inaba, K.; Matsuoka, M. HTLV-1 bZIP Factor Enhances T-Cell Proliferation by Impeding the Suppressive Signaling of Co-inhibitory Receptors. PLoS Pathog. 2017, 13, e1006120. [Google Scholar]
- Johnson, J.M.; Nicot, C.; Fullen, J.; Ciminale, V.; Casareto, L.; Mulloy, J.C.; Jacobson, S.; Franchini, G. Free major histocompatibility complex class I heavy chain is preferentially targeted for degradation by human T-cell leukemia/lymphotropic virus type 1 p12(I) protein. J Virol. 2001, 75, 6086–6094. [Google Scholar] [CrossRef]
- Datta, A.; Sinha-Datta, U.; Dhillon, N.K.; Buch, S.; Nicot, C. The HTLV-I p30 Interferes with TLR4 Signaling and Modulates the Release of Pro- and Anti-inflammatory Cytokines from Human Macrophages. J. Biol. Chem. 2006, 281, 23414–23424. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Nagata, Y.; Kitanaka, A.; Shiraishi, Y.; Shimamura, T.; Yasunaga, J.; Totoki, Y.; Chiba, K.; Sato-Otsubo, A.; Nagae, G.; et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat. Genet. 2015, 47, 1304–1315. [Google Scholar] [CrossRef]
- Kataoka, K.; Shiraishi, Y.; Takeda, Y.; Sakata, S.; Matsumoto, M.; Nagano, S.; Maeda, T.; Nagata, Y.; Kitanaka, A.; Mizuno, S.; et al. Aberrant PD-L1 expression through 3′-UTR disruption in multiple cancers. Nature 2016, 534, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Iwanaga, M.; Yasunaga, J.-I.; Nagata, Y.; Kitanaka, A.; Kameda, T.; Yoshimitsu, M.; Shiraishi, Y.; Sato-Otsubo, A.; Sanada, M.; et al. Prognostic relevance of integrated genetic profiling in adult T-cell leukemia/lymphoma. Blood 2018, 131, 215–225. [Google Scholar] [CrossRef]
- Koya, J.; Saito, Y.; Kameda, T.; Kogure, Y.; Yuasa, M.; Nagasaki, J.; McClure, M.B.; Shingaki, S.; Tabata, M.; Tahira, Y.; et al. Single-Cell Analysis of the Multicellular Ecosystem in Viral Carcinogenesis by HTLV-1. Blood Cancer Discov. 2021, 2, 450–467. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Saeed, A.F.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef]
- Troiano, G.; Caponio, V.C.A.; Adipietro, I.; Tepedino, M.; Santoro, R.; Laino, L.; Russo, L.L.; Cirillo, N.; Muzio, L.L. Prognostic significance of CD68+ and CD163+ tumor associated macrophages in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol. 2019, 93, 66–75. [Google Scholar] [CrossRef]
- Shen, H.; Liu, J.; Chen, S.; Ma, X.; Ying, Y.; Li, J.; Wang, W.; Wang, X.; Xie, L. Prognostic Value of Tumor-Associated Macrophages in Clear Cell Renal Cell Carcinoma: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 657318. [Google Scholar] [CrossRef]
- Komohara, Y.; Niino, D.; Saito, Y.; Ohnishi, K.; Horlad, H.; Ohshima, K.; Takeya, M. Clinical significance of CD163⁺ tumor-associated macrophages in patients with adult T-cell leukemia/lymphoma. Cancer Sci. 2013, 104, 945–951. [Google Scholar] [CrossRef]
- Veillette, A.; Chen, J. SIRPα-CD47 Immune Checkpoint Blockade in Anticancer Therapy. Trends Immunol. 2018, 39, 173–184. [Google Scholar] [CrossRef]
- Yanagida, E.; Miyoshi, H.; Takeuchi, M.; Yoshida, N.; Nakashima, K.; Yamada, K.; Umeno, T.; Shimasaki, Y.; Furuta, T.; Seto, M.; et al. Clinicopathological analysis of immunohistochemical expression of CD47 and SIRPα in adult T-cell leukemia/lymphoma. Hematol. Oncol. 2020, 38, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shao, X.; Zhang, Y.; Zhu, M.; Wang, F.X.C.; Mu, J.; Li, J.; Yao, H.; Chen, K. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Med. 2023, 12, 11149–11165. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, H.; Kiyasu, J.; Kato, T.; Yoshida, N.; Shimono, J.; Yokoyama, S.; Taniguchi, H.; Sasaki, Y.; Kurita, D.; Kawamoto, K.; et al. PD-L1 expression on neoplastic or stromal cells is respectively a poor or good prognostic factor for adult T-cell leukemia/lymphoma. Blood 2016, 128, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Azakami, K.; Sato, T.; Araya, N.; Utsunomiya, A.; Kubota, R.; Suzuki, K.; Hasegawa, D.; Izumi, T.; Fujita, H.; Aratani, S.; et al. Severe loss of invariant NKT cells exhibiting anti–HTLV-1 activity in patients with HTLV-1–associated disorders. Blood 2009, 114, 3208–3215. [Google Scholar] [CrossRef]
- Itabashi, K.; Miyazawa, T. Mother-to-Child Transmission of Human T-Cell Leukemia Virus Type 1: Mechanisms and Nutritional Strategies for Prevention. Cancers 2021, 13, 4100. [Google Scholar] [CrossRef]
- Fuchi, N.; Miura, K.; Tsukiyama, T.; Sasaki, D.; Ishihara, K.; Tsuruda, K.; Hasegawa, H.; Miura, S.; Yanagihara, K.; Masuzaki, H. Natural Course of Human T-Cell Leukemia Virus Type 1 Proviral DNA Levels in Carriers During Pregnancy. J. Infect. Dis. 2018, 217, 1383–1389. [Google Scholar] [CrossRef]
- Plancoulaine, S.; Gessain, A.; Tortevoye, P.; Boland-Auge, A.; Vasilescu, A.; Matsuda, F.; Abel, L. A major susceptibility locus for HTLV-1 infection in childhood maps to chromosome 6q27. Hum. Mol. Genet. 2006, 15, 3306–3312. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Takahashi, M.; Norose, Y.; Takeshita, T.; Fukunaga, Y.; Takahashi, H. Transformation of breast milk macrophages by HTLV-I: implications for HTLV-I transmission via breastfeeding. Biomed. Res. 2010, 31, 53–61. [Google Scholar] [CrossRef]
- Gotuzzo, E.; Moody, J.; Verdonck, K.; Cabada, M.M.; González, E.; Van Dooren, S.; Vandamme, A.-M.; Terashima, A.; Vermund, S.H. Frequent HTLV-1 infection in the offspring of Peruvian women with HTLV-1-associated myelopathy/tropical spastic paraparesis or strongyloidiasis. Rev Panam Salud Publica. 2007, 22, 223–230. [CrossRef]
- Montaño-Castellón, I.; Marconi, C.S.C.; Saffe, C.; Brites, C. Clinical and Laboratory Outcomes in HIV-1 and HTLV-1/2 Coinfection: A Systematic Review. Front Public Health. 2022, 10, 820727. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.J.; Julien-Serrette, K.; Edwards, J.; Boyce, G. HTLV-1 Coinfection among Patients Attending a Large HIV Treatment Centre in Trinidad. Microorganisms 2022, 10, 2207. [Google Scholar] [CrossRef]
- Geddes, V.E.V.; José, D.P.; Leal, F.E.; Nixon, D.F.; Tanuri, A.; Aguiar, R.S. HTLV-1 Tax activates HIV-1 transcription in latency models. Virology 2017, 504, 45–51. [Google Scholar] [CrossRef]
- Abad-Fernández, M.; Hernández-Walias, F.J.; de León, M.J.R.; Vivancos, M.J.; Pérez-Elías, M.J.; Moreno, A.; Casado, J.L.; Quereda, C.; Dronda, F.; Moreno, S.; et al. HTLV-2 Enhances CD8+ T Cell-Mediated HIV-1 Inhibition and Reduces HIV-1 Integrated Proviral Load in People Living with HIV-1. Viruses 2022, 14, 2472. [Google Scholar] [CrossRef] [PubMed]
- Richey, J.D.; Chen, B.J.; Deng, A.C. Indolent, waxing and waning cutaneous presentation of HTLV-1-associated adult T-cell leukemia/lymphoma in an HIV-1-positive patient. J Cutan Pathol. 2018, 45, 171–175. [Google Scholar] [CrossRef]
- Schär, F.; Trostdorf, U.; Giardina, F.; Khieu, V.; Muth, S.; Marti, H.; Vounatsou, P.; Odermatt, P. Strongyloides stercoralis: Global Distribution and Risk Factors. PLOS Negl. Trop. Dis. 2013, 7, e2288. [Google Scholar] [CrossRef]
- Carvalho, E.M.; Porto, A.D.F. Epidemiological and clinical interaction between HTLV-1 and Strongyloides stercoralis. Parasite Immunol. 2004, 26, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Toma, H.; Sugahara, K.; Etoh, K.; Shiroma, Y.; Kiyuna, S.; Takara, M.; Matsuoka, M.; Yamaguchi, K.; Nakada, K.; et al. Involvement of IL-2/IL-2R system activation by parasite antigen in polyclonal expansion of CD4(+)25(+) HTLV-1-infected T-cells in human carriers of both HTLV-1 and S. stercoralis. Oncogene 2002, 21, 2466–2475. [Google Scholar] [CrossRef] [PubMed]
- Dykie, A.; Wijesinghe, T.; Rabson, A.B.; Madugula, K.; Farinas, C.; Wilson, S.; Abraham, D.; Jain, P. Human T-cell Leukemia Virus Type 1 and Strongyloides stercoralis: Partners in Pathogenesis. Pathogens 2020, 9, 904. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Nomura, S.; Shimoyama, M.; Shibata, T.; Imaizumi, Y.; Moriuchi, Y.; Tomoyose, T.; Uozumi, K.; Kobayashi, Y.; Fukushima, N.; et al. Japan Clinical Oncology Group (JCOG) prognostic index and characterization of long-term survivors of aggressive adult T-cell leukaemia-lymphoma (JCOG0902A). Br. J. Haematol. 2014, 166, 739–748. [Google Scholar] [CrossRef]
- Katsuya, H.; Yamanaka, T.; Ishitsuka, K.; Utsunomiya, A.; Sasaki, H.; Hanada, S.; Eto, T.; Moriuchi, Y.; Saburi, Y.; Miyahara, M.; et al. Prognostic Index for Acute- and Lymphoma-Type Adult T-Cell Leukemia/Lymphoma. J. Clin. Oncol. 2012, 30, 1635–1640. [Google Scholar] [CrossRef]
- Fuji, S.; Yamaguchi, T.; Inoue, Y.; Utsunomiya, A.; Moriuchi, Y.; Uchimaru, K.; Owatari, S.; Miyagi, T.; Taguchi, J.; Choi, I.; et al. Development of a modified prognostic index for patients with aggressive adult T-cell leukemia-lymphoma aged 70 years or younger: possible risk-adapted management strategies including allogeneic transplantation. Haematologica 2017, 102, 1258–1265. [Google Scholar] [CrossRef]
- Ishida, T.; Hishizawa, M.; Kato, K.; Tanosaki, R.; Fukuda, T.; Taniguchi, S.; Eto, T.; Takatsuka, Y.; Miyazaki, Y.; Moriuchi, Y.; et al. Allogeneic hematopoietic stem cell transplantation for adult T-cell leukemia-lymphoma with special emphasis on preconditioning regimen: a nationwide retrospective study. Blood 2012, 120, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Paijens, S.T.; Vledder, A.; de Bruyn, M.; Nijman, H.W. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell. Mol. Immunol. 2020, 18, 842–859. [Google Scholar] [CrossRef]
- Jo, T.; Noguchi, K.; Sakai, T.; Kubota-Koketsu, R.; Irie, S.; Matsuo, M.; Taguchi, J.; Abe, K.; Shigematsu, K. HTLV-1 Tax-specific memory cytotoxic T lymphocytes in long-term survivors of aggressive-type adult T-cell leukemia/lymphoma. Cancer Med. 2022, 11, 3238–3250. [Google Scholar] [CrossRef]
- Harashima, N.; Kurihara, K.; Utsunomiya, A.; Tanosaki, R.; Hanabuchi, S.; Masuda, M.; Ohashi, T.; Fukui, F.; Hasegawa, A.; Masuda, T.; et al. Graft-versus-Tax Response in Adult T-Cell Leukemia Patients after Hematopoietic Stem Cell Transplantation. Cancer Res 2004, 64, 391–399. [Google Scholar] [CrossRef]
- Jo, T.; Kubota-Koketsu, R.; Kaneko, Y.; Sakai, T.; Noguchi, K.; Irie, S.; Matsuo, M.; Taguchi, J.; Abe, K.; Shigematsu, K. Live attenuated VZV vaccination induces antitumor immunity in ATL patients. Cancer Immunol Immunother. 2023, 72, 929–944. [Google Scholar] [CrossRef] [PubMed]
- Masaki, A.; Ishida, T.; Suzuki, S.; Ito, A.; Narita, T.; Kinoshita, S.; Ri, M.; Kusumoto, S.; Komatsu, H.; Inagaki, H.; et al. Human T-cell lymphotropic/leukemia virus type 1 (HTLV-1) Tax-specific T-cell exhaustion in HTLV-1-infected individuals. Cancer Sci. 2018, 109, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Narita, T.; Ishida, T.; Masaki, A.; Suzuki, S.; Ito, A.; Mori, F.; Yamada, T.; Ri, M.; Kusumoto, S.; Komatsu, H.; et al. HTLV-1 bZIP Factor–Specific CD4 T Cell Responses in Adult T Cell Leukemia/Lymphoma Patients after Allogeneic Hematopoietic Stem Cell Transplantation. J. Immunol. 2014, 192, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Suemori, K.; Fujiwara, H.; Ochi, T.; Ogawa, T.; Matsuoka, M.; Matsumoto, T.; Mesnard, J.-M.; Yasukawa, M. HBZ is an immunogenic protein, but not a target antigen for human T-cell leukemia virus type 1-specific cytotoxic T lymphocytes. J. Gen. Virol. 2009, 90, 1806–1811. [Google Scholar] [CrossRef] [PubMed]
- Macnamara, A.; Rowan, A.; Hilburn, S.; Kadolsky, U.; Fujiwara, H.; Suemori, K.; Yasukawa, M.; Taylor, G.; Bangham, C.R.; Asquith, B. HLA class I binding of HBZ determines outcome in HTLV-1 infection. PLoS Pathog. 2010, 6, e1001117. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Ishida, T.; Utsunomiya, A.; Inagaki, A.; Yano, H.; Komatsu, H.; Iida, S.; Imada, K.; Uchiyama, T.; Akinaga, S.; et al. Defucosylated Humanized Anti-CCR4 Monoclonal Antibody KW-0761 as a Novel Immunotherapeutic Agent for Adult T-cell Leukemia/Lymphoma. Clin. Cancer Res. 2010, 16, 1520–1531. [Google Scholar] [CrossRef]
- Saito, M.; Ishii, T.; Urakawa, I.; Matsumoto, A.; Masaki, A.; Ito, A.; Kusumoto, S.; Suzuki, S.; Takahashi, T.; Morita, A.; et al. Robust CD8+ T-cell proliferation and diversification after mogamulizumab in patients with adult T-cell leukemia-lymphoma. Blood Adv. 2020, 4, 2180–2191. [Google Scholar] [CrossRef]
- Ishida, T.; Jo, T.; Takemoto, S.; Suzushima, H.; Suehiro, Y.; Choi, I.; Yoshimitsu, M.; Saburi, Y.; Nosaka, K.; Utsunomiya, A.; et al. Follow-up of a randomised phase II study of chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive adult T-cell leukaemia-lymphoma: impact on allogeneic haematopoietic stem cell transplantation. Br. J. Haematol. 2019, 184, 479–483. [Google Scholar] [CrossRef]
- Tanaka, T.; Inamoto, Y.; Ito, A.; Watanabe, M.; Takeda, W.; Aoki, J.; Kim, S.; Fukuda, T. Lenalidomide treatment for recurrent adult T-cell leukemia/lymphoma after allogeneic hematopoietic cell transplantation. Hematol. Oncol. 2022, 41, 389–395. [Google Scholar] [CrossRef]
- Shichijo, T.; Nosaka, K.; Tatetsu, H.; Higuchi, Y.; Endo, S.; Inoue, Y.; Toyoda, K.; Kikukawa, Y.; Kawakita, T. ; Yasunaga, J-I. ; et al. Beneficial impact of first-line mogamulizumab-containing chemotherapy in adult T-cell leukaemia-lymphoma. Br J Haematol. 2022, 198, 983–987. [Google Scholar]
- Kawano, N.; Kuriyama, T.; Yoshida, S.; Kawano, S.; Yamano, Y.; Marutsuka, K.; Minato, S.; Yamashita, K.; Ochiai, H.; Shimoda, K.; et al. The Impact of a Humanized CCR4 Antibody (Mogamulizumab) on Patients with Aggressive-Type Adult T-Cell Leukemia-Lymphoma Treated with Allogeneic Hematopoietic Stem Cell Transplantation. J. Clin. Exp. Hematop. 2017, 56, 135–144. [Google Scholar] [CrossRef]
- Kamada, Y.; Arima, N.; Hayashida, M.; Nakamura, D.; Yoshimitsu, M.; Ishitsuka, K. Prediction of the risk for graft versus host disease after allogeneic hematopoietic stem cell transplantation in patients treated with mogamulizumab. Leuk. Lymphoma 2022, 63, 1701–1707. [Google Scholar] [CrossRef]
- Iwai, Y.; Hamanishi, J.; Chamoto, K.; Honjo, T. Cancer immunotherapies targeting the PD-1 signaling pathway. J. Biomed. Sci. 2017, 24, 26. [Google Scholar] [CrossRef]
- Ratner, L.; Waldmann, T.A.; Janakiram, M.; Brammer, J.E. Rapid Progression of Adult T-Cell Leukemia–Lymphoma after PD-1 Inhibitor Therapy. New Engl. J. Med. 2018, 378, 1947–1948. [Google Scholar] [CrossRef]
- Rauch, D.A.; Conlon, K.C.; Janakiram, M.; Brammer, J.E.; Harding, J.C.; Ye, B.H.; Zang, X.; Ren, X.; Olson, S.; Cheng, X.; et al. Rapid progression of adult T-cell leukemia/lymphoma as tumor-infiltrating Tregs after PD-1 blockade. Blood 2019, 134, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Armoiry, X.; Aulagner, G.; Facon, T. Lenalidomide in the treatment of multiple myeloma: a review. J. Clin. Pharm. Ther. 2008, 33, 219–226. [Google Scholar] [CrossRef]
- Kondo, N.; Nagano, Y.; Hasegawa, A.; Ishizawa, M.; Katagiri, K.; Yoneda, T.; Masuda, T.; Kannagi, M. Involvement of EZH2 inhibition in lenalidomide and pomalidomide-mediated growth suppression in HTLV-1-infected cells. Biochem. Biophys. Res. Commun. 2021, 574, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Fujiwara, H.; Nosaka, K.; Taira, N.; Abe, Y.; Imaizumi, Y.; Moriuchi, Y.; Jo, T.; Ishizawa, K.; Tobinai, K.; et al. Multicenter Phase II Study of Lenalidomide in Relapsed or Recurrent Adult T-Cell Leukemia/Lymphoma: ATL-002. J Clin Oncol. 2016, 34, 4086–4093. [Google Scholar] [CrossRef] [PubMed]
- Palucka, K.; Banchereau, J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012, 12, 265–77. [Google Scholar] [CrossRef] [PubMed]
- Suehiro, Y.; Hasegawa, A.; Iino, T.; Sasada, A.; Watanabe, N.; Matsuoka, M.; Takamori, A.; Tanosaki, R.; Utsunomiya, A.; Choi, I.; et al. Clinical outcomes of a novel therapeutic vaccine with Tax peptide-pulsed dendritic cells for adult T cell leukaemia/lymphoma in a pilot study. Br. J. Haematol. 2015, 169, 356–367. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




