Submitted:
05 September 2023
Posted:
07 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Inflammation in the Central Nervous System
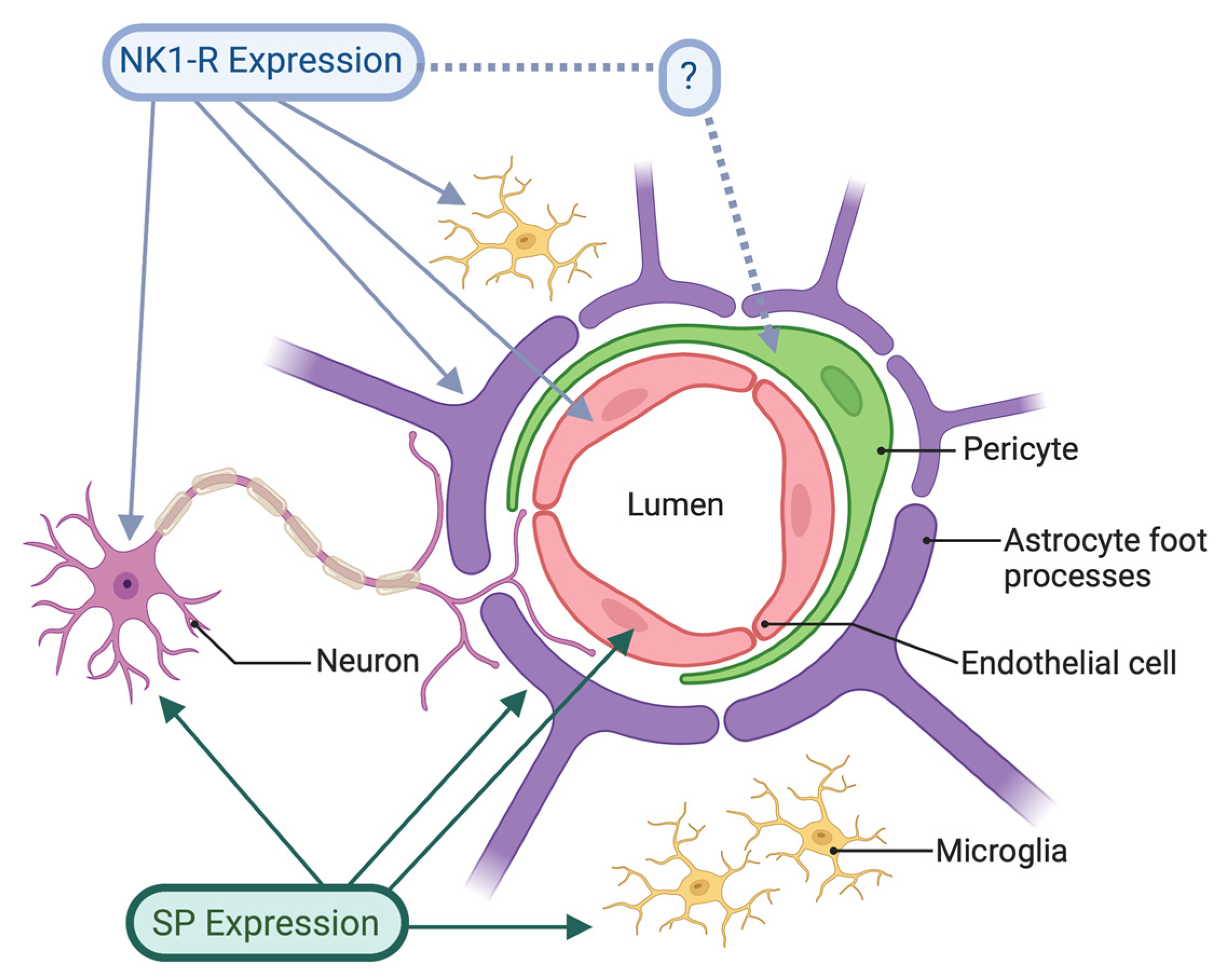
3. Perivascular Innervation and Neurogenic Inflammation
4. Distribution of SP and the NK1 receptor in the CNS
5. Non-neuronal NK1 receptor activity in the CNS
6. Evidence for the Involvement of the NK1-R in Acute CNS Inflammation
Stroke
Traumatic Brain Injury
Spinal Cord Injury
Bacterial Infections
Viral Infections
Parasitic Infections
7. The double-edged sword of inflammation
8. Concluding Remarks
Acknowledgments
Conflicts of Interest
References
- Beauchamp, K.; Mutlak, H.; Smith, W.R.; Shohami, E.; Stahel, P.F. Pharmacology of traumatic brain injury: where is the "golden bullet"? Mol Med 2008, 14, 731–740. [Google Scholar] [CrossRef]
- Cook, A.M.; Morgan Jones, G.; Hawryluk, G.W.J.; Mailloux, P.; McLaughlin, D.; Papangelou, A.; Samuel, S.; Tokumaru, S.; Venkatasubramanian, C.; Zacko, C.; et al. Guidelines for the Acute Treatment of Cerebral Edema in Neurocritical Care Patients. Neurocrit Care 2020, 32, 647–666. [Google Scholar] [CrossRef] [PubMed]
- Cardiovascular and cerebrovascular events in the randomized, controlled Alzheimer's Disease Anti-Inflammatory Prevention Trial (ADAPT). PLoS Clin Trials 2006, 1, e33. [CrossRef]
- Vink, R.; Nimmo, A.J. Multifunctional drugs for head injury. Neurotherapeutics 2009, 6, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, F.; Vink, R.; Turner, R.J. Inflammation in acute CNS injury: a focus on the role of substance P. Br J Pharmacol 2016, 173, 703–715. [Google Scholar] [CrossRef]
- Gordon, S. Phagocytosis: An Immunobiologic Process. Immunity 2016, 44, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. The Journal of Clinical Investigation 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Liu, C.H.; Abrams, N.D.; Carrick, D.M.; Chander, P.; Dwyer, J.; Hamlet, M.R.J.; Macchiarini, F.; PrabhuDas, M.; Shen, G.L.; Tandon, P.; et al. Biomarkers of chronic inflammation in disease development and prevention: challenges and opportunities. Nature Immunology 2017, 18, 1175–1180. [Google Scholar] [CrossRef]
- Lucas, S.M.; Rothwell, N.J.; Gibson, R.M. The role of inflammation in CNS injury and disease. Br J Pharmacol 2006, 147 Suppl 1, S232–240. [Google Scholar] [CrossRef]
- Kinoshita, K. Traumatic brain injury: pathophysiology for neurocritical care. J Intensive Care 2016, 4, 29. [Google Scholar] [CrossRef]
- Heiss, W.D. Malignant MCA Infarction: Pathophysiology and Imaging for Early Diagnosis and Management Decisions. Cerebrovasc Dis 2016, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bellaver, B.; Dos Santos, J.P.; Leffa, D.T.; Bobermin, L.D.; Roppa, P.H.A.; da Silva Torres, I.L.; Goncalves, C.A.; Souza, D.O.; Quincozes-Santos, A. Systemic Inflammation as a Driver of Brain Injury: the Astrocyte as an Emerging Player. Mol Neurobiol 2018, 55, 2685–2695. [Google Scholar] [CrossRef]
- Lapenna, A.; De Palma, M.; Lewis, C.E. Perivascular macrophages in health and disease. Nat Rev Immunol 2018, 18, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, S.; Iadecola, C. Revisiting the neurovascular unit. Nat Neurosci 2021, 24, 1198–1209. [Google Scholar] [CrossRef] [PubMed]
- Rustenhoven, J.; Jansson, D.; Smyth, L.C.; Dragunow, M. Brain Pericytes As Mediators of Neuroinflammation. Trends Pharmacol Sci 2017, 38, 291–304. [Google Scholar] [CrossRef]
- Annunziata, P.; Cioni, C.; Santonini, R.; Paccagnini, E. Substance P antagonist blocks leakage and reduces activation of cytokine-stimulated rat brain endothelium. J Neuroimmunol 2002, 131, 41–49. [Google Scholar] [CrossRef]
- Pober, J.S.; Sessa, W.C. Inflammation and the blood microvascular system. Cold Spring Harb Perspect Biol 2014, 7, a016345. [Google Scholar] [CrossRef]
- Hickey, W.F. Leukocyte traffic in the central nervous system: the participants and their roles. Semin Immunol 1999, 11, 125–137. [Google Scholar] [CrossRef]
- Jansson, D.; Rustenhoven, J.; Feng, S.; Hurley, D.; Oldfield, R.L.; Bergin, P.S.; Mee, E.W.; Faull, R.L.; Dragunow, M. A role for human brain pericytes in neuroinflammation. J Neuroinflammation 2014, 11, 104. [Google Scholar] [CrossRef]
- Koizumi, T.; Kerkhofs, D.; Mizuno, T.; Steinbusch, H.W.M.; Foulquier, S. Vessel-Associated Immune Cells in Cerebrovascular Diseases: From Perivascular Macrophages to Vessel-Associated Microglia. Front Neurosci 2019, 13, 1291. [Google Scholar] [CrossRef]
- da Fonseca, A.C.; Matias, D.; Garcia, C.; Amaral, R.; Geraldo, L.H.; Freitas, C.; Lima, F.R. The impact of microglial activation on blood-brain barrier in brain diseases. Front Cell Neurosci 2014, 8, 362. [Google Scholar] [CrossRef] [PubMed]
- Neuwelt, E.A.; Bauer, B.; Fahlke, C.; Fricker, G.; Iadecola, C.; Janigro, D.; Leybaert, L.; Molnar, Z.; O'Donnell, M.E.; Povlishock, J.T.; et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci 2011, 12, 169–182. [Google Scholar] [CrossRef]
- Sousa-Valente, J.; Brain, S.D. A historical perspective on the role of sensory nerves in neurogenic inflammation. Semin Immunopathol 2018, 40, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Chiu, I.M.; von Hehn, C.A.; Woolf, C.J. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci 2012, 15, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Nimmo, A.J.; Cernak, I.; Heath, D.L.; Hu, X.; Bennett, C.J.; Vink, R. Neurogenic inflammation is associated with development of edema and functional deficits following traumatic brain injury in rats. Neuropeptides 2004, 38, 40–47. [Google Scholar] [CrossRef]
- Sorby-Adams, A.J.; Marcoionni, A.M.; Dempsey, E.R.; Woenig, J.A.; Turner, R.J. The Role of Neurogenic Inflammation in Blood-Brain Barrier Disruption and Development of Cerebral Oedema Following Acute Central Nervous System (CNS) Injury. Int J Mol Sci 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.E.; Easton, A.S.; Fraser, P.A. TRPV1 activation results in disruption of the blood-brain barrier in the rat. Br J Pharmacol 2005, 146, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Kargbo, R.B. TRPV1 Modulators for the Treatment of Pain and Inflammation. ACS Medicinal Chemistry Letters 2019, 10, 143–144. [Google Scholar] [CrossRef]
- Harmar, A.; Keen, P. Synthesis, and central and peripheral axonal transport of substance P in a dorsal root ganglion-nerve preparation in vitro. Brain Res 1982, 231, 379–385. [Google Scholar] [CrossRef]
- Aalkjær, C.; Nilsson, H.; De Mey, J.G.R. Sympathetic and Sensory-Motor Nerves in Peripheral Small Arteries. Physiol Rev 2021, 101, 495–544. [Google Scholar] [CrossRef]
- Garret, C.; Carruette, A.; Fardin, V.; Moussaoui, S.; Peyronel, J.F.; Blanchard, J.C.; Laduron, P.M. Pharmacological properties of a potent and selective nonpeptide substance P antagonist. Proc Natl Acad Sci U S A 1991, 88, 10208–10212. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.V.; Helme, R.D.; Thomas, K.L. NK-1 receptor mediation of neurogenic plasma extravasation in rat skin. Br J Pharmacol 1989, 97, 1232–1238. [Google Scholar] [CrossRef]
- Lundberg, J.M.; Saria, A.; Brodin, E.; Rosell, S.; Folkers, K. A substance P antagonist inhibits vagally induced increase in vascular permeability and bronchial smooth muscle contraction in the guinea pig. Proc Natl Acad Sci U S A 1983, 80, 1120–1124. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.D.; Leeman, S.E. Neurokinin-1 receptor: functional significance in the immune system in reference to selected infections and inflammation. Ann N Y Acad Sci 2011, 1217, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P. Neurogenic vasodilatation and plasma leakage in the skin. Gen Pharmacol 1998, 30, 5–11. [Google Scholar] [CrossRef]
- Bekkemeyer, H.; Oehme, P.; Foreman, J.C. Accidental injection of substance P. J R Soc Med 1983, 76, 801–802. [Google Scholar] [CrossRef]
- van Hinsbergh, V.W.; van Nieuw Amerongen, G.P. Endothelial hyperpermeability in vascular leakage. Vascul Pharmacol 2002, 39, 171–172. [Google Scholar] [CrossRef]
- Baluk, P.; Hirata, A.; Thurston, G.; Fujiwara, T.; Neal, C.R.; Michel, C.C.; McDonald, D.M. Endothelial gaps: time course of formation and closure in inflamed venules of rats. American Journal of Physiology-Lung Cellular and Molecular Physiology 1997, 272, L155–L170. [Google Scholar] [CrossRef]
- Bowden, J.J.; Baluk, P.; Lefevre, P.M.; Vigna, S.R.; McDonald, D.M. Substance P (NK1) receptor immunoreactivity on endothelial cells of the rat tracheal mucosa. Am J Physiol 1996, 270, L404–414. [Google Scholar] [CrossRef]
- Mashaghi, A.; Marmalidou, A.; Tehrani, M.; Grace, P.M.; Pothoulakis, C.; Dana, R. Neuropeptide substance P and the immune response. Cell Mol Life Sci 2016, 73, 4249–4264. [Google Scholar] [CrossRef]
- Leroux, A.; Paiva Dos Santos, B.; Leng, J.; Oliveira, H.; Amedee, J. Sensory neurons from dorsal root ganglia regulate endothelial cell function in extracellular matrix remodelling. Cell Commun Signal 2020, 18, 162. [Google Scholar] [CrossRef]
- Nessler, S.; Stadelmann, C.; Bittner, A.; Schlegel, K.; Gronen, F.; Brueck, W.; Hemmer, B.; Sommer, N. Suppression of autoimmune encephalomyelitis by a neurokinin-1 receptor antagonist--a putative role for substance P in CNS inflammation. J Neuroimmunol 2006, 179, 1–8. [Google Scholar] [CrossRef]
- Foreman, J.C.; Jordan, C.C.; Oehme, P.; Renner, H. Structure-activity relationships for some substance P-related peptides that cause wheal and flare reactions in human skin. J Physiol 1983, 335, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, P. Mast Cells in Neuroimmune Interactions. Trends Neurosci 2019, 42, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Suvas, S. Role of Substance P Neuropeptide in Inflammation, Wound Healing, and Tissue Homeostasis. J Immunol 2017, 199, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Hamel, E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol (1985) 2006, 100, 1059–1064. [Google Scholar] [CrossRef]
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K.; Krause, D.N. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol 2018, 14, 338–350. [Google Scholar] [CrossRef]
- Markowitz, S.; Saito, K.; Moskowitz, M.A. Neurogenically mediated leakage of plasma protein occurs from blood vessels in dura mater but not brain. J Neurosci 1987, 7, 4129–4136. [Google Scholar] [CrossRef]
- Ashina, M.; Hansen, J.M.; Do, T.P.; Melo-Carrillo, A.; Burstein, R.; Moskowitz, M.A. Migraine and the trigeminovascular system-40 years and counting. Lancet Neurol 2019, 18, 795–804. [Google Scholar] [CrossRef]
- Saria, A.; Lundberg, J.M.; Skofitsch, G.; Lembeck, F. Vascular protein linkage in various tissue induced by substance P, capsaicin, bradykinin, serotonin, histamine and by antigen challenge. Naunyn Schmiedebergs Arch Pharmacol 1983, 324, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Hokfelt, T.; Kellerth, J.O.; Nilsson, G.; Pernow, B. Substance p: localization in the central nervous system and in some primary sensory neurons. Science 1975, 190, 889–890. [Google Scholar] [CrossRef] [PubMed]
- Mantyh, P.W.; Johnson, D.J.; Boehmer, C.G.; Catton, M.D.; Vinters, H.V.; Maggio, J.E.; Too, H.P.; Vigna, S.R. Substance P receptor binding sites are expressed by glia in vivo after neuronal injury. Proc Natl Acad Sci U S A 1989, 86, 5193–5197. [Google Scholar] [CrossRef]
- Johnson, M.B.; Young, A.D.; Marriott, I. The Therapeutic Potential of Targeting Substance P/NK-1R Interactions in Inflammatory CNS Disorders. Front Cell Neurosci 2016, 10, 296. [Google Scholar] [CrossRef]
- Mai, J.K.; Stephens, P.H.; Hopf, A.; Cuello, A.C. Substance P in the human brain. Neuroscience 1986, 17, 709–739. [Google Scholar] [CrossRef] [PubMed]
- Mantyh, P.W. Neurobiology of substance P and the NK1 receptor. J Clin Psychiatry 2002, 63 Suppl 11, 6–10. [Google Scholar]
- Dudas, B.; Merchenthaler, I. Substance P-Immunoreactive Fiber Varicosities Appear to Innervate Galaninergic Perikarya in the Human Hypothalamus. Brain Connect 2021, 11, 493–500. [Google Scholar] [CrossRef]
- Liu, H.; Brown, J.L.; Jasmin, L.; Maggio, J.E.; Vigna, S.R.; Mantyh, P.W.; Basbaum, A.I. Synaptic relationship between substance P and the substance P receptor: light and electron microscopic characterization of the mismatch between neuropeptides and their receptors. Proc Natl Acad Sci U S A 1994, 91, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, K.; Siddiq, A.; Baig, S.G.; Zehra, S. Substance P: A neuropeptide involved in the psychopathology of anxiety disorders. Neuropeptides 2020, 79, 101993. [Google Scholar] [CrossRef] [PubMed]
- Rupniak, N.M.J.; Kramer, M.S. NK1 receptor antagonists for depression: Why a validated concept was abandoned. J Affect Disord 2017, 223, 121–125. [Google Scholar] [CrossRef]
- Frick, A.; Ahs, F.; Linnman, C.; Jonasson, M.; Appel, L.; Lubberink, M.; Långström, B.; Fredrikson, M.; Furmark, T. Increased neurokinin-1 receptor availability in the amygdala in social anxiety disorder: a positron emission tomography study with [11C]GR205171. Translational Psychiatry 2015, 5, e597–e597. [Google Scholar] [CrossRef]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O'Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L.; et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med 2013, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Tattersall, F.D.; Rycroft, W.; Hill, R.G.; Hargreaves, R.J. Enantioselective inhibition of apomorphine-induced emesis in the ferret by the neurokinin1 receptor antagonist CP-99,994. Neuropharmacology 1994, 33, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Aapro, M.; Carides, A.; Rapoport, B.L.; Schmoll, H.J.; Zhang, L.; Warr, D. Aprepitant and fosaprepitant: a 10-year review of efficacy and safety. Oncologist 2015, 20, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Takano, Y.; Kamiya, H.O. Roles of substance P and NK(1) receptor in the brainstem in the development of emesis. J Pharmacol Sci 2003, 91, 87–94. [Google Scholar] [CrossRef]
- Burmeister, A.R.; Johnson, M.B.; Chauhan, V.S.; Moerdyk-Schauwecker, M.J.; Young, A.D.; Cooley, I.D.; Martinez, A.N.; Ramesh, G.; Philipp, M.T.; Marriott, I. Human microglia and astrocytes constitutively express the neurokinin-1 receptor and functionally respond to substance P. Journal of Neuroinflammation 2017, 14, 245. [Google Scholar] [CrossRef]
- Spitsin, S.; Pappa, V.; Douglas, S.D. Truncation of neurokinin-1 receptor-Negative regulation of substance P signaling. J Leukoc Biol 2018. [Google Scholar] [CrossRef]
- Lai, J.P.; Lai, S.; Tuluc, F.; Tansky, M.F.; Kilpatrick, L.E.; Leeman, S.E.; Douglas, S.D. Differences in the length of the carboxyl terminus mediate functional properties of neurokinin-1 receptor. Proc Natl Acad Sci U S A 2008, 105, 12605–12610. [Google Scholar] [CrossRef]
- Lai, J.P.; Cnaan, A.; Zhao, H.; Douglas, S.D. Detection of full-length and truncated neurokinin-1 receptor mRNA expression in human brain regions. J Neurosci Methods 2008, 168, 127–133. [Google Scholar] [CrossRef]
- Tuluc, F.; Lai, J.P.; Kilpatrick, L.E.; Evans, D.L.; Douglas, S.D. Neurokinin 1 receptor isoforms and the control of innate immunity. Trends Immunol 2009, 30, 271–276. [Google Scholar] [CrossRef]
- Stumm, R.; Culmsee, C.; Schafer, M.K.; Krieglstein, J.; Weihe, E. Adaptive plasticity in tachykinin and tachykinin receptor expression after focal cerebral ischemia is differentially linked to gabaergic and glutamatergic cerebrocortical circuits and cerebrovenular endothelium. J Neurosci 2001, 21, 798–811. [Google Scholar] [CrossRef]
- Cyrino, L.A.; Cardoso, R.C.; Hackl, L.P.; Nicolau, M. Effect of quercetin on plasma extravasation in rat CNS and dura mater by ACE and NEP inhibition. Phytother Res 2002, 16, 545–549. [Google Scholar] [CrossRef]
- Annunziata, P.; Cioni, C.; Toneatto, S.; Paccagnini, E. HIV-1 gp120 increases the permeability of rat brain endothelium cultures by a mechanism involving substance P. AIDS 1998, 12, 2377–2385. [Google Scholar] [CrossRef] [PubMed]
- Cioni, C.; Renzi, D.; Calabro, A.; Annunziata, P. Enhanced secretion of substance P by cytokine-stimulated rat brain endothelium cultures. J Neuroimmunol 1998, 84, 76–85. [Google Scholar] [CrossRef]
- Marriott, D.R.; Wilkin, G.P. Substance P receptors on O-2A progenitor cells and type-2 astrocytes in vitro. J Neurochem 1993, 61, 826–834. [Google Scholar] [CrossRef]
- Chauhan, V.S.; Sterka, D.G., Jr.; Gray, D.L.; Bost, K.L.; Marriott, I. Neurogenic exacerbation of microglial and astrocyte responses to Neisseria meningitidis and Borrelia burgdorferi. J Immunol 2008, 180, 8241–8249. [Google Scholar] [CrossRef] [PubMed]
- Lieb, K.; Fiebich, B.L.; Berger, M.; Bauer, J.; Schulze-Osthoff, K. The neuropeptide substance P activates transcription factor NF-kappa B and kappa B-dependent gene expression in human astrocytoma cells. J Immunol 1997, 159, 4952–4958. [Google Scholar] [CrossRef] [PubMed]
- Rasley, A.; Bost, K.L.; Olson, J.K.; Miller, S.D.; Marriott, I. Expression of functional NK-1 receptors in murine microglia. Glia 2002, 37, 258–267. [Google Scholar] [CrossRef]
- Vo, T.T.; Im, G.H.; Han, K.; Suh, M.; Drew, P.J.; Kim, S.G. Parvalbumin interneuron activity drives fast inhibition-induced vasoconstriction followed by slow substance P-mediated vasodilation. Proc Natl Acad Sci U S A 2023, 120, e2220777120. [Google Scholar] [CrossRef]
- Vruwink, M.; Schmidt, H.H.; Weinberg, R.J.; Burette, A. Substance P and nitric oxide signaling in cerebral cortex: anatomical evidence for reciprocal signaling between two classes of interneurons. J Comp Neurol 2001, 441, 288–301. [Google Scholar] [CrossRef]
- Guo, C.J.; Douglas, S.D.; Gao, Z.; Wolf, B.A.; Grinspan, J.; Lai, J.P.; Riedel, E.; Ho, W.Z. Interleukin-1beta upregulates functional expression of neurokinin-1 receptor (NK-1R) via NF-kappaB in astrocytes. Glia 2004, 48, 259–266. [Google Scholar] [CrossRef]
- Weinstock, J.V.; Blum, A.; Metwali, A.; Elliott, D.; Arsenescu, R. IL-18 and IL-12 signal through the NF-kappa B pathway to induce NK-1R expression on T cells. J Immunol 2003, 170, 5003–5007. [Google Scholar] [CrossRef]
- Yu, Z.; Cheng, G.; Huang, X.; Li, K.; Cao, X. Neurokinin-1 receptor antagonist SR140333: a novel type of drug to treat cerebral ischemia. Neuroreport 1997, 8, 2117–2119. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.J.; Helps, S.C.; Thornton, E.; Vink, R. A substance P antagonist improves outcome when administered 4 h after onset of ischaemic stroke. Brain Res 2011, 1393, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.J.; Vink, R. Combined tissue plasminogen activator and an NK1 tachykinin receptor antagonist: an effective treatment for reperfusion injury following acute ischemic stroke in rats. Neuroscience 2012, 220, 1–10. [Google Scholar] [CrossRef]
- Sorby-Adams, A.J.; Leonard, A.V.; Hoving, J.W.; Yassi, N.; Vink, R.; Wells, A.J.; Turner, R.J. NK1-r Antagonist Treatment Comparable to Decompressive Craniectomy in Reducing Intracranial Pressure Following Stroke. Front Neurosci 2019, 13, 681. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Sharma, S.; Sandhu, M.; Sharma, S. Neurokinin-1 receptor inhibition reverses ischaemic brain injury and dementia in bilateral common carotid artery occluded rats: possible mechanisms. Inflammopharmacology 2016, 24, 133–143. [Google Scholar] [CrossRef]
- Donkin, J.J.; Nimmo, A.J.; Cernak, I.; Blumbergs, P.C.; Vink, R. Substance P is associated with the development of brain edema and functional deficits after traumatic brain injury. J Cereb Blood Flow Metab 2009, 29, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Torsten Hoffmann, A.N., Andrew Sleight, Pierre Vankan, Robert Vink METHOD OF TREATMENT AND/OR PREVENTION OF BRAIN, SPINAL OR NERVE INJURY. 2003, Hoffmann La Roche Inc.
- Corrigan, F.; Cernak, I.; McAteer, K.; Hellewell, S.C.; Rosenfeld, J.V.; Turner, R.J.; Vink, R. NK1 antagonists attenuate tau phosphorylation after blast and repeated concussive injury. Sci Rep 2021, 11, 8861. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, X.; Yang, Y.; Zhang, Y.; He, F.; Xu, X.; Zhang, Z.; Tao, L.; Luo, C. Tachykinin NK1 receptor antagonist L-733,060 and substance P deletion exert neuroprotection through inhibiting oxidative stress and cell death after traumatic brain injury in mice. Int J Biochem Cell Biol 2019, 107, 154–165. [Google Scholar] [CrossRef]
- Vink, R.; Gabrielian, L.; Thornton, E. The Role of Substance P in Secondary Pathophysiology after Traumatic Brain Injury. Front Neurol 2017, 8, 304. [Google Scholar] [CrossRef]
- Zheng, G.; Harms, A.K.; Tail, M.; Zhang, H.; Nimmo, A.; Skutella, T.; Kiening, K.; Unterberg, A.; Zweckberger, K.; Younsi, A. Effects of a neurokinin-1 receptor antagonist in the acute phase after thoracic spinal cord injury in a rat model. Front Mol Neurosci 2023, 16, 1128545. [Google Scholar] [CrossRef]
- Chauhan, V.S.; Kluttz, J.M.; Bost, K.L.; Marriott, I. Prophylactic and therapeutic targeting of the neurokinin-1 receptor limits neuroinflammation in a murine model of pneumococcal meningitis. J Immunol 2011, 186, 7255–7263. [Google Scholar] [CrossRef]
- Rasley, A.; Marriott, I.; Halberstadt, C.R.; Bost, K.L.; Anguita, J. Substance P augments Borrelia burgdorferi-induced prostaglandin E2 production by murine microglia. J Immunol 2004, 172, 5707–5713. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.P.; Ho, W.Z.; Zhan, G.X.; Yi, Y.; Collman, R.G.; Douglas, S.D. Substance P antagonist (CP-96,345) inhibits HIV-1 replication in human mononuclear phagocytes. Proc Natl Acad Sci U S A 2001, 98, 3970–3975. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Douglas, S.D.; Song, L.; Wang, Y.J.; Ho, W.Z. Neurokinin-1 receptor antagonist (aprepitant) suppresses HIV-1 infection of microglia/macrophages. J Neuroimmune Pharmacol 2008, 3, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Manak, M.M.; Moshkoff, D.A.; Nguyen, L.T.; Meshki, J.; Tebas, P.; Tuluc, F.; Douglas, S.D. Anti-HIV-1 activity of the neurokinin-1 receptor antagonist aprepitant and synergistic interactions with other antiretrovirals. AIDS 2010, 24, 2789–2796. [Google Scholar] [CrossRef]
- Ronca, S.E.; Gunter, S.M.; Kairis, R.B.; Lino, A.; Romero, J.; Pautler, R.G.; Nimmo, A.; Murray, K.O. A Potential Role for Substance P in West Nile Virus Neuropathogenesis. Viruses 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.G.; Rodgers, J.; Jennings, F.W.; Murray, M.; Leeman, S.E.; Burke, J.M. A substance P antagonist, RP-67,580, ameliorates a mouse meningoencephalitic response to Trypanosoma brucei brucei. Proc Natl Acad Sci U S A 1997, 94, 4167–4170. [Google Scholar] [CrossRef]
- MacLeod, A.M.; Merchant, K.J.; Brookfield, F.; Kelleher, F.; Stevenson, G.; Owens, A.P.; Swain, C.J.; Casiceri, M.A.; Sadowski, S.; Ber, E.; et al. Identification of L-tryptophan derivatives with potent and selective antagonist activity at the NK1 receptor. J Med Chem 1994, 37, 1269–1274. [Google Scholar] [CrossRef]
- Matalinska, J.; Lipinski, P.F.J. Correcting a widespread error: Neuroprotectant N-acetyl-L-tryptophan does not bind to the neurokinin-1 receptor. Mol Cell Neurosci 2022, 120, 103728. [Google Scholar] [CrossRef]
- Satarker, S.; Maity, S.; Mudgal, J.; Nampoothiri, M. In silico screening of neurokinin receptor antagonists as a therapeutic strategy for neuroinflammation in Alzheimer's disease. Mol Divers 2022, 26, 443–466. [Google Scholar] [CrossRef] [PubMed]
- Nimmo, A.J.; Vink, R. Recent patents in CNS drug discovery: the management of inflammation in the central nervous system. Recent Pat CNS Drug Discov 2009, 4, 86–95. [Google Scholar] [CrossRef]
- Vinet-Oliphant, H.; Alvarez, X.; Buza, E.; Borda, J.T.; Mohan, M.; Aye, P.P.; Tuluc, F.; Douglas, S.D.; Lackner, A.A. Neurokinin-1 receptor (NK1-R) expression in the brains of SIV-infected rhesus macaques: implications for substance P in NK1-R immune cell trafficking into the CNS. Am J Pathol 2010, 177, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.; Tega, F.; Bruno, A.; Graf, U.; Corelli, F.; Molfetta, R.; Barucco, M. The role of substance P in cerebral ischemia. Int J Immunopathol Pharmacol 2003, 16, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.; Bös, M.; Stadler, H.; Schnider, P.; Hunkeler, W.; Godel, T.; Galley, G.; Ballard, T.M.; Higgins, G.A.; Poli, S.M.; et al. Design and synthesis of a novel, achiral class of highly potent and selective, orally active neurokinin-1 receptor antagonists. Bioorg Med Chem Lett 2006, 16, 1362–1365. [Google Scholar] [CrossRef]
- Visser, K.; Koggel, M.; Blaauw, J.; van der Horn, H.J.; Jacobs, B.; van der Naalt, J. Blood-based biomarkers of inflammation in mild traumatic brain injury: A systematic review. Neuroscience & Biobehavioral Reviews 2022, 132, 154–168. [Google Scholar]
- Sahuquillo, J.; Dennis, J.A. Decompressive craniectomy for the treatment of high intracranial pressure in closed traumatic brain injury. Cochrane Database Syst Rev 2019, 12, Cd003983. [Google Scholar] [CrossRef]
- Corrigan, F.; Mander, K.A.; Leonard, A.V.; Vink, R. Neurogenic inflammation after traumatic brain injury and its potentiation of classical inflammation. J Neuroinflammation 2016, 13, 264. [Google Scholar] [CrossRef] [PubMed]
- Hellenbrand, D.J.; Quinn, C.M.; Piper, Z.J.; Morehouse, C.N.; Fixel, J.A.; Hanna, A.S. Inflammation after spinal cord injury: a review of the critical timeline of signaling cues and cellular infiltration. J Neuroinflammation 2021, 18, 284. [Google Scholar] [CrossRef]
- Leonard, A.V.; Manavis, J.; Blumbergs, P.C.; Vink, R. Changes in substance P and NK1 receptor immunohistochemistry following human spinal cord injury. Spinal Cord 2014, 52, 17–23. [Google Scholar] [CrossRef]
- Leonard, A.V.; Thornton, E.; Vink, R. NK1 receptor blockade is ineffective in improving outcome following a balloon compression model of spinal cord injury. PLoS One 2014, 9, e98364. [Google Scholar] [CrossRef]
- Bystritsky, R.J.; Chow, F.C. Infectious Meningitis and Encephalitis. Neurol Clin 2022, 40, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.D.; Cnaan, A.; Lynch, K.G.; Benton, T.; Zhao, H.; Gettes, D.R.; Evans, D.L. Elevated substance P levels in HIV-infected women in comparison to HIV-negative women. AIDS Res Hum Retroviruses 2008, 24, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.D.; Ho, W.Z.; Gettes, D.R.; Cnaan, A.; Zhao, H.; Leserman, J.; Petitto, J.M.; Golden, R.N.; Evans, D.L. Elevated substance P levels in HIV-infected men. AIDS 2001, 15, 2043–2045. [Google Scholar] [CrossRef] [PubMed]
- Bost, K.L. Tachykinin-modulated anti-viral responses. Front Biosci 2004, 9, 1994–1998. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.; Spitsin, S.V.; Meshki, J.; Tuluc, F.; Douglas, S.D.; Wolfe, J.H. Substance P enhances HIV-1 infection in human fetal brain cell cultures expressing full-length neurokinin-1 receptor. J Neurovirol 2013, 19, 219–227. [Google Scholar] [CrossRef]
- Robinson, P.; White, A.C.; Lewis, D.E.; Thornby, J.; David, E.; Weinstock, J. Sequential expression of the neuropeptides substance P and somatostatin in granulomas associated with murine cysticercosis. Infect Immun 2002, 70, 4534–4538. [Google Scholar] [CrossRef]
- Garza, A.; Tweardy, D.J.; Weinstock, J.; Viswanathan, B.; Robinson, P. Substance P signaling contributes to granuloma formation in Taenia crassiceps infection, a murine model of cysticercosis. J Biomed Biotechnol 2010, 2010, 597086. [Google Scholar] [CrossRef]
- Garza, A.; Weinstock, J.; Robinson, P. Absence of the SP/SP receptor circuitry in the substance P-precursor knockout mice or SP receptor, neurokinin (NK)1 knockout mice leads to an inhibited cytokine response in granulomas associated with murine Taenia crassiceps infection. J Parasitol 2008, 94, 1253–1258. [Google Scholar] [CrossRef]
- Elsawa, S.F.; Taylor, W.; Petty, C.C.; Marriott, I.; Weinstock, J.V.; Bost, K.L. Reduced CTL response and increased viral burden in substance P receptor-deficient mice infected with murine gamma-herpesvirus 68. J Immunol 2003, 170, 2605–2612. [Google Scholar] [CrossRef]
- Blum, A.M.; Metwali, A.; Kim-Miller, M.; Li, J.; Qadir, K.; Elliott, D.E.; Lu, B.; Fabry, Z.; Gerard, N.; Weinstock, J.V. The substance P receptor is necessary for a normal granulomatous response in murine schistosomiasis mansoni. J Immunol 1999, 162, 6080–6085. [Google Scholar] [CrossRef]
- Blum, A.M.; Elliott, D.E.; Metwali, A.; Li, J.; Qadir, K.; Weinstock, J.V. Substance P regulates somatostatin expression in inflammation. J Immunol 1998, 161, 6316–6322. [Google Scholar] [CrossRef]
- Kincy-Cain, T.; Bost, K.L. Increased susceptibility of mice to Salmonella infection following in vivo treatment with the substance P antagonist, spantide II. J Immunol 1996, 157, 255–264. [Google Scholar] [CrossRef]
- de Oliveira, A.C.; Candelario-Jalil, E.; Fiebich, B.L.; Santos Mda, S.; Palotas, A.; dos Reis, H.J. Neuroinflammation and Neurodegeneration: Pinpointing Pathological and Pharmacological Targets. Biomed Res Int 2015, 2015, 487241. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.M.; Moore, Z.; Minter, M.R.; Crack, P.J. Type-I interferon pathway in neuroinflammation and neurodegeneration: focus on Alzheimer's disease. J Neural Transm (Vienna) 2018, 125, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Arena, G.; Sharma, K.; Agyeah, G.; Kruger, R.; Grunewald, A.; Fitzgerald, J.C. Neurodegeneration and Neuroinflammation in Parkinson's Disease: a Self-Sustained Loop. Curr Neurol Neurosci Rep 2022, 22, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Ellwardt, E.; Zipp, F. Molecular mechanisms linking neuroinflammation and neurodegeneration in MS. Exp Neurol 2014, 262 Pt A, 8–17. [Google Scholar] [CrossRef]
- Campolongo, P.; Ratano, P.; Ciotti, M.T.; Florenzano, F.; Nori, S.L.; Marolda, R.; Palmery, M.; Rinaldi, A.M.; Zona, C.; Possenti, R.; et al. Systemic administration of substance P recovers beta amyloid-induced cognitive deficits in rat: involvement of Kv potassium channels. PLoS One 2013, 8, e78036. [Google Scholar] [CrossRef]
- Benarroch, E.E.; Schmeichel, A.M.; Low, P.A.; Parisi, J.E. Depletion of ventromedullary NK-1 receptor-immunoreactive neurons in multiple system atrophy. Brain 2003, 126, 2183–2190. [Google Scholar] [CrossRef]
- Reinke, E.K.; Johnson, M.J.; Ling, C.; Karman, J.; Lee, J.; Weinstock, J.V.; Sandor, M.; Fabry, Z. Substance P receptor mediated maintenance of chronic inflammation in EAE. J Neuroimmunol 2006, 180, 117–125. [Google Scholar] [CrossRef]
- Stuckey, S.M.; Ong, L.K.; Collins-Praino, L.E.; Turner, R.J. Neuroinflammation as a Key Driver of Secondary Neurodegeneration Following Stroke? Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
| CNS Pathology | Model | Drug | Reference |
|
Ischaemic Stroke |
Rat – internal carotid A Rat – transient middle cerebral (tMC) Rat – t MC + tPA Sheep – t MC Rat – bilateral common carotid |
SR140333 NAT NAT EU-C-001 Aprepitant |
Yu et al [82] Turner et al [83] Turner et al [84] Sorby-Adams et al [85] Kaur et al [86] |
|
Traumatic Brain Injury |
Rat – diffuse axonal injury Rat – diffuse axonal injury Rat – diffuse axonal injury Rat – Blast injury and CTE Mouse – Cortical impact Sheep – Diffuse axonal injury |
Capsaicin NAT EU-C-001 EU-C-001 L-733,060 EU-C-001 |
Nimmo et al [25] Donkin et al [87] Hoffmann et al [88] Corrigan et al [89] Li et al [90] Vink et al [91] |
|
Spinal Cord Injury |
Rat - contusion/compression SCI | EU-C-001 | Zheng et al [92] |
|
Bacterial Infection |
Mouse – N meningitidis & B burgdorferi Mouse – S pneumoniae Primary mouse microglia - B burgdorferi |
L 703,606 L 703,606 L 703,606 |
Chauhan et al [75] Chauhan et al [93] Rasley et al [94] |
|
Viral Infection |
Human mononuclear phagocytes – HIV Microglia/macrophages - HIV Peripheral blood monocytes – HIV Mouse – WNV neuroinvasive disease |
CP-96,345 Aprepitant Aprepitant Ro 67,5930 |
Lai et al [95] Wang et al [96] Manak et al [97] Ronca et al [98] |
|
Parasitic Infection |
Mouse – Trypanosoma brucei brucei | RP 67,580 | Kennedy et al [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





