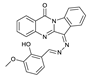
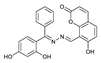
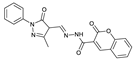
1. Introduction
Copper (II) ion is one of the essential transition metal ions that plays a crucial role in many key physiological processes in living organisms, including electron transport oxidoreductases, the produce of hemocyanin, blue copper protein, cytochrome C oxidase, lactase, ascorbate oxidase, superoxide dismutase, and skin pigmentation and connective tissue repair [
1,
2,
3]. However, excessive amounts of Cu
2+ can lead to a variety of diseases, such as induction of cell death, Parkinson’s, Alzheimer’s and prion diseases [
4,
5,
6]. In addition, the widespread use of Cu
2+ in the electronic and electrical industries makes it an environmental pollutant [
7,
8]. Consequently, the development of a highly sensitive and selective Cu
2+ assay for the efficient detection of Cu
2+ in aqueous solutions or biological system is of great importance for life and environmental sciences.
Nowadays, various detection technologies have been developed to test Cu
2+, such as chem-iluminescence [
9], electro-chemistry [
10], colorimetry [
11], atomic absorption spectrometry [
12], inductively coupled plasma-mass spectrometry [
13]. However, these detection strategies have drawbacks of low sensitivity and cumbersome operation. In recent years, fluorescent molecular probes are rapidly developing in molecular recognition because of their excellent recognition properties, high selectivity and sensitivity, being accessible in site detection, and real-time imaging [
14,
15,
16,
17]. Actually, most fluorescent probes that identify Cu
2+ are restricted in their applications for the poor selectivity, short wavelength emission, interference from autofluorescence, the need for a high organic phase detection environment, and the presence of many possible interfering agents.
Coumarin (2
H-1-benzopyran-2-one), a fluorophore used extensively, possesses high fluorescence quantum yields, good photostability, large Stokes shifts, and easy structural modifications. Coumarin derivatives have been found in various plants, which widely used in aqueous environmental monitoring, antibacterial, antitumor and others [
18]. Currently, coumarin-based fluorescent probes consist essentially of Schiff bases [
19,
20,
21,
22] and biological thiols [
23,
24]. In particular, few studies on the coumarin-based pyrazolone fluorescent probes have been reported [
23,
24,
25].
Thus, a fluorescent probe based on coumarin and pyrazole Schiff base, N′-((3-methyl-5-oxo-1-phenyl-4,5-dihydro-1H-pyrazol-4-yl)methylene)-2-oxo-2H-chromene-3-carbohy-drazide (MPMC), was designed and synthesized in this paper. The coumarin moiety belongs to MPMC served as fluorophore, besides that the acylhydrazone structure belongs to MPMC served as recognition receptor and bursting part. The results demonstrated that MPMC probe was selective and sensitive for the detection of Cu2+.
2. Results and discussion
2.1. Fluorescence spectra of probe MPMC for selectivity and anti-interference detection
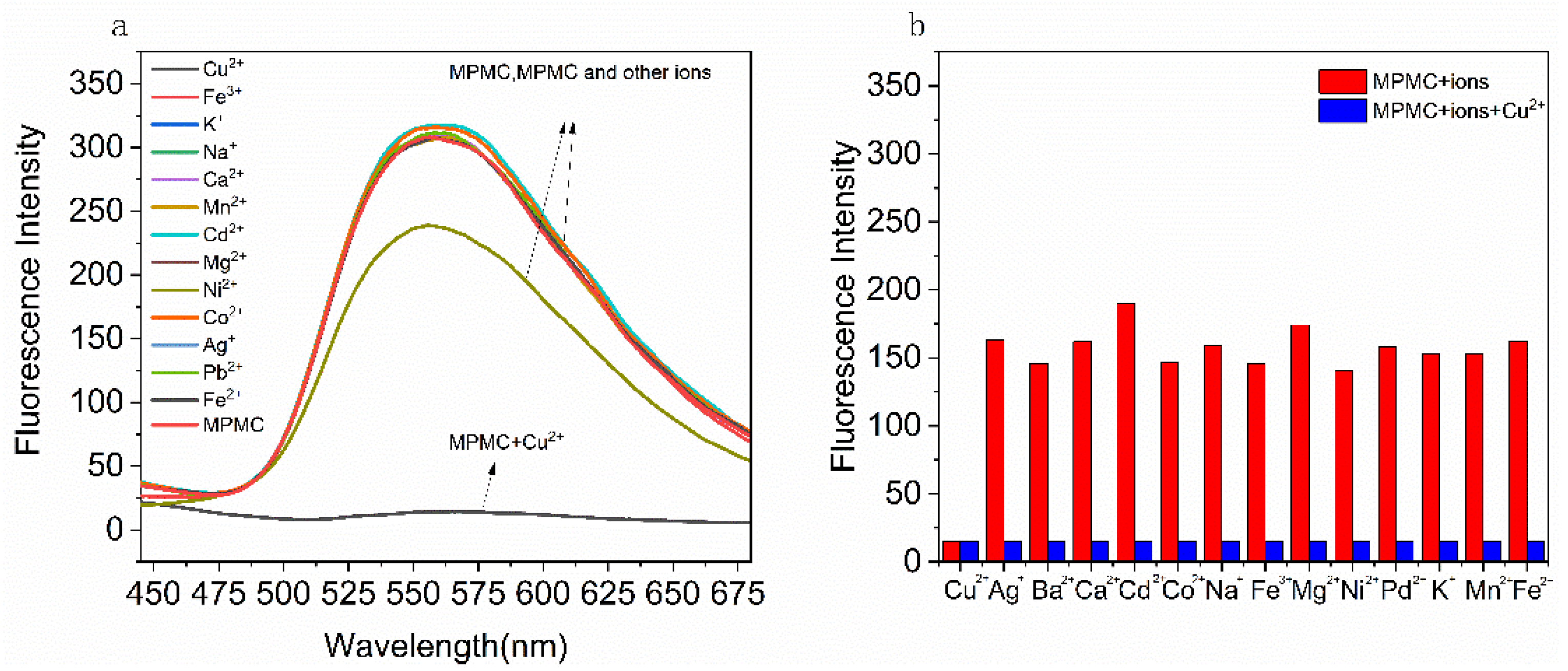
The fluorescence emission behaviors of probe MPMC towards diverse metal ions were detected in ethanol (EtOH)/H
2O (v/v = 1/1) solution, respectively. Fluorescence intensities of diverse metal ions added to MPMC were detected as the wavelength variation. The fluorescence emission spectrums are illustrated in
Figure 1a. As shown in
Figure 1a, when excitation wavelength 358 nm was given to probe MPMC, a fluorescence emission band at 548 nm generated. Then fluorescence quenching followed along with the insertion of Cu
2+ (1.0 equiv), as the emission minimum being at 548 nm. Conversely, the fluorescence emission spectrums of MPMC were affected little by introducing other metal ions, in addition to a weak quenching of nickel ions.
Furthermore, in view of potential interference of other metal ions in practical applications, sorts of metal ions were added successively into the MPMC-Cu2+ system, and the influences on fluorescence selectivity were investigated.
100 μM of competitive metal ions (Pb
2+, Fe
3+, Fe
2+, Mg
2+, Ni
2+, Cd
2+, Mn
2+, Ca
2+, Ba
2+, Na
+, K
+, Ag
+) along with 10 μM MPMC in EtOH-H
2O (v/v = 1/1) were prepared and determined to access the fluorescence intensities. As
Figure 1b illustrates, a fluorescence emission band at 548 nm generated again. Thereafter, with the addition of 10 μM Cu
2+, the fluorescence quenching took place. The existence of other metal actions made no difference on the fluorescence intensity apparently. In general, probe MPMC has the characteristics of selectively recognizing Cu
2+, displaying a good anti-interference ability.
2.2. Titration experiment of probe MPMC to Cu2+
Colorimetric experiments were conducted to study the specificity of probe MPMC towards Cu
2+. As
Figure 2a shows, while adding Cu
2+ to MPMC dissolved in EtOH/H
2O (v/v = 1/1), the color of solution passed from colorless to yellow in seconds. The result shows that probe MPMC can realize colorimetric detection of Cu
2+ with a detection limit 10 μM. In the same way, the fluorescence color turned from colorless to yellow under 365 nm ultraviolet (UV) light, shown in
Figure 2b. The applied results clearly demonstrate that probe MPMC could make application for qualitative and quantitative detection of Cu
2+, in the forms of color change and spectrum signals multiplying.
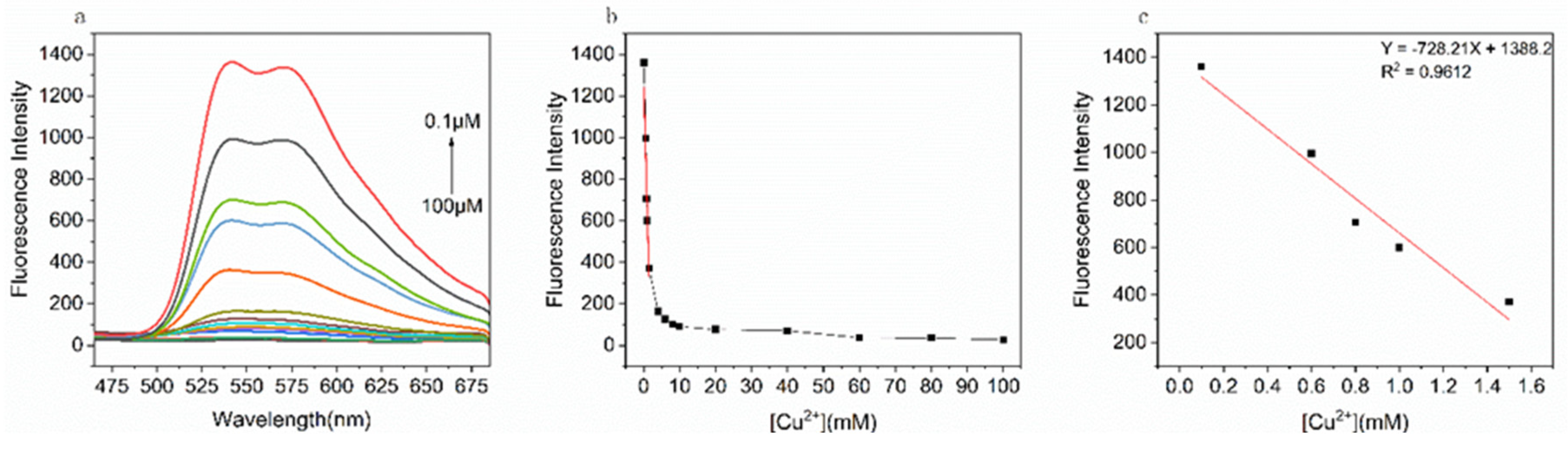
As the concentration variation of Cu
2+, fluorescence titration experiments were performed to explore the sensitivity of probe MPMC to Cu
2+. As illustrated in
Figure 3, with Cu
2+ concentration rising, the fluorescence intensity of probe MPMC reflected a continued decrease. The linear equation for Copper (II) ions is Y = -728.21X+1388.2, and the correlated coefficient is 0.9612. Based on formula L = 3σ/Ka, the minimum detectable concentration of the probe for copper (II) ions was calculated as 15.16 nM [
26]. Compared with other fluorescent probes for the detection of Cu
2+ listed in
Table 1, the probe MPMC prepared in this paper had a lower detection limit.
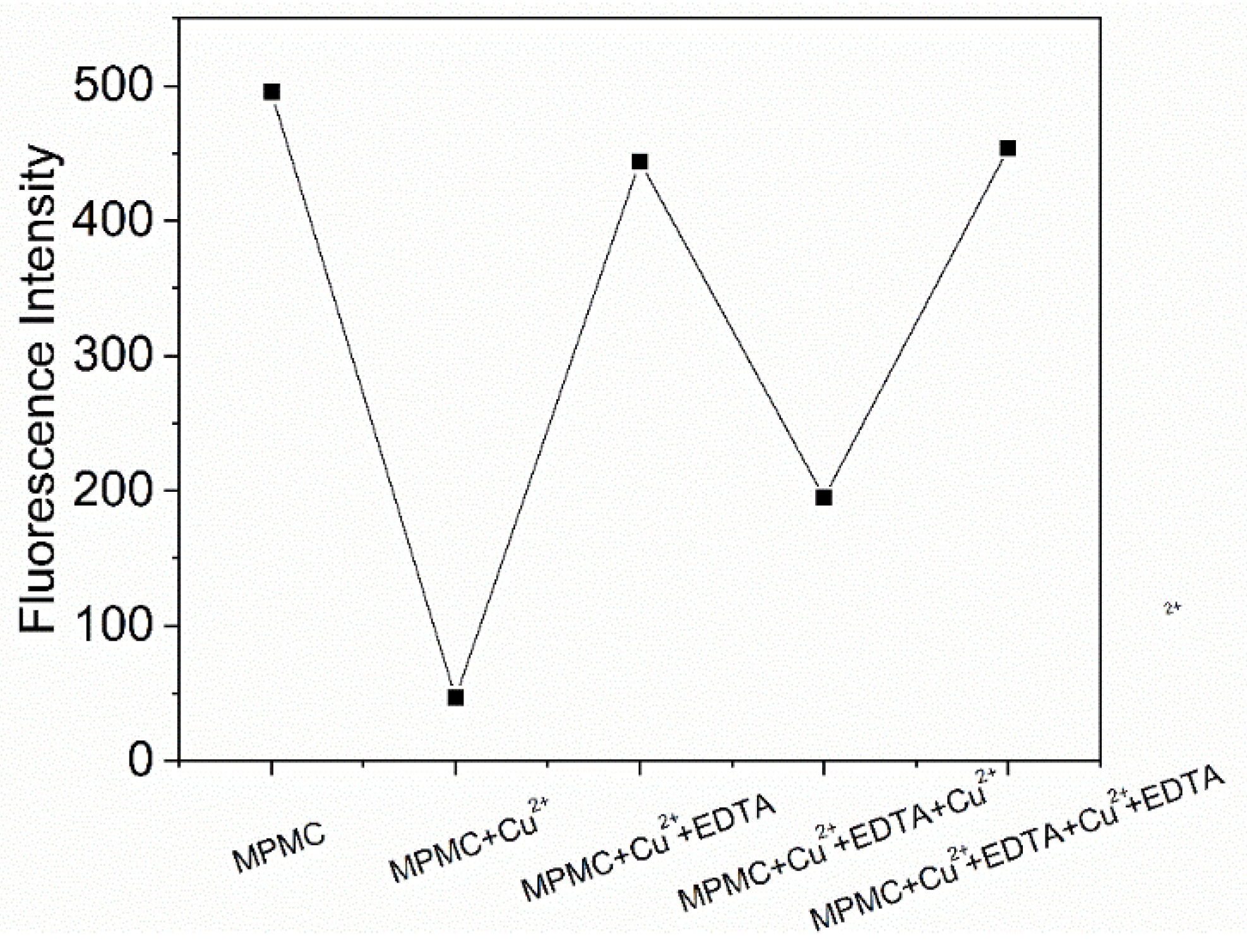
2.3. Study of EDTA effect of probe MPMC to Cu2+
To fully investigate the response between probe MPMC and Cu
2+, the procedures involved addition of ethylene diamine tetraacetate acid (EDTA) to MPMC-Cu
2+ complex and detection of fluorescence intensity were performed. As shown in
Figure 4, the fluorescence intensity was recovered with the increase of addition amount of EDTA into the MPMC-Cu
2+ complex. It suggests that the complexation ability of EDTA to Cu
2+ is stronger than that of the MPMC probe. EDTA seized the Cu
2+ bound to the MPMC probe, leading to the recovery of fluorescence. Namely, the fluorescence recognition between probe MPMC and Cu
2+ is reversible [
30]. Then the fluorescence quenching still occurred after the sustainable addition of Cu
2+. Similarly, the fluorescence intensity recovered again after the addition of EDTA. The results indicate that MPMC probe is reusable.
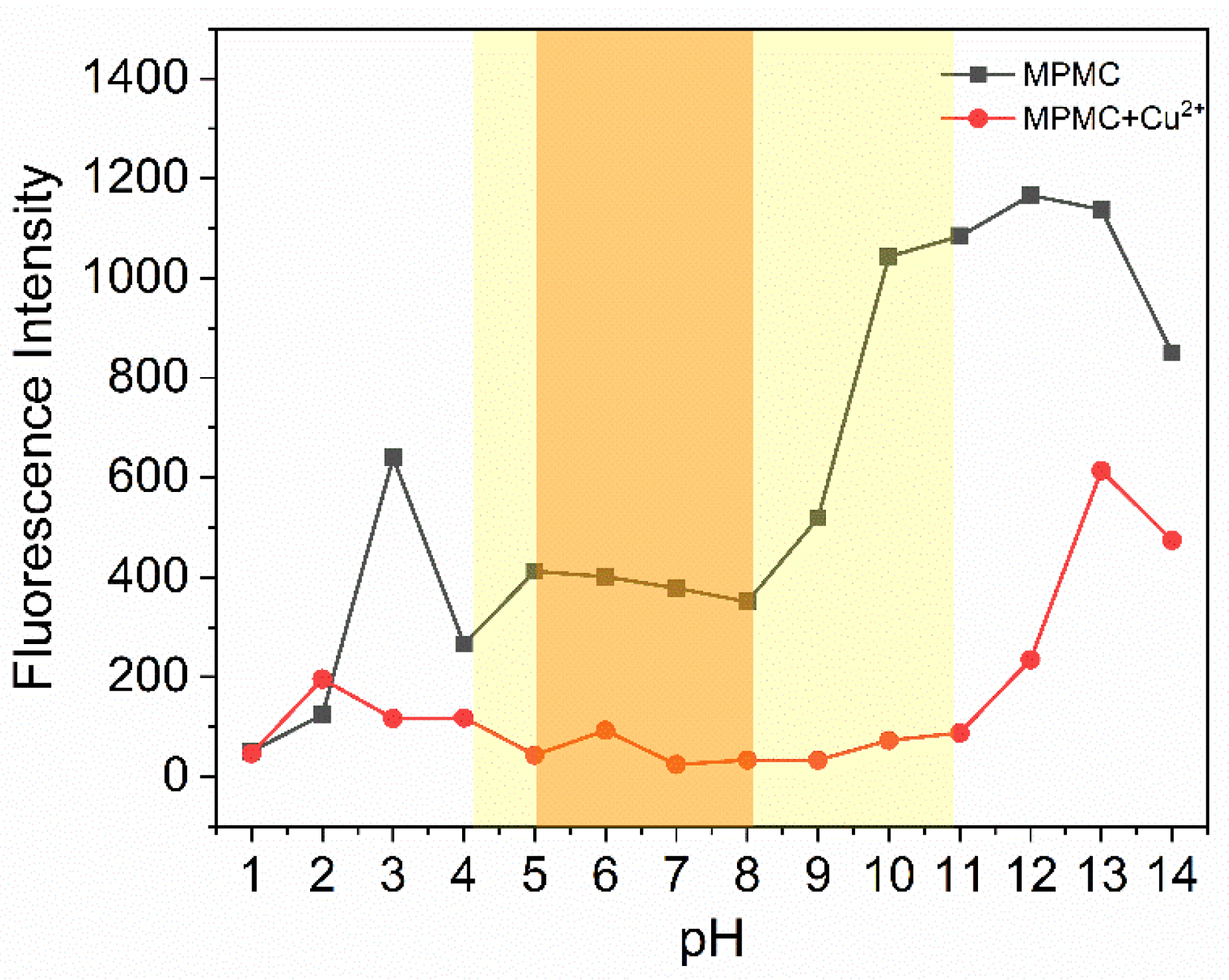
2.4. Study of pH effect of probe MPMC to Cu2+
Probe MPMC is required not only for highly sensitive and selective performance, but also for good sensing ability of the probe MPMC at different acidities in practical applications. The sensing ability was detected by adjusting the pH of EtOH-PBS (phosphate buffer solution) from 1.0 to 14.0. As
Figure 5 illustrates, with a range 4.0-11.0 of pH variation, the fluorescence intensity of the probe MPMC maintained constantly. Then dropped dramatically in a range 5.0-8.0 of pH variation while adding Cu
2+ to MPMC solution. At low pH range, the fluorescence intensity trended no variance. It is probably caused by hydrolysis of Cu
2+ under acidic conditions, which inhibited the formation of the MPMC-Cu
2+ complex
30. Thus, the prepared probe MPMC acted as a fluorescent pH sensor, suitable for a broad range of pH detection (4.0 to 11.0), especially from 5.0 to 8.0.
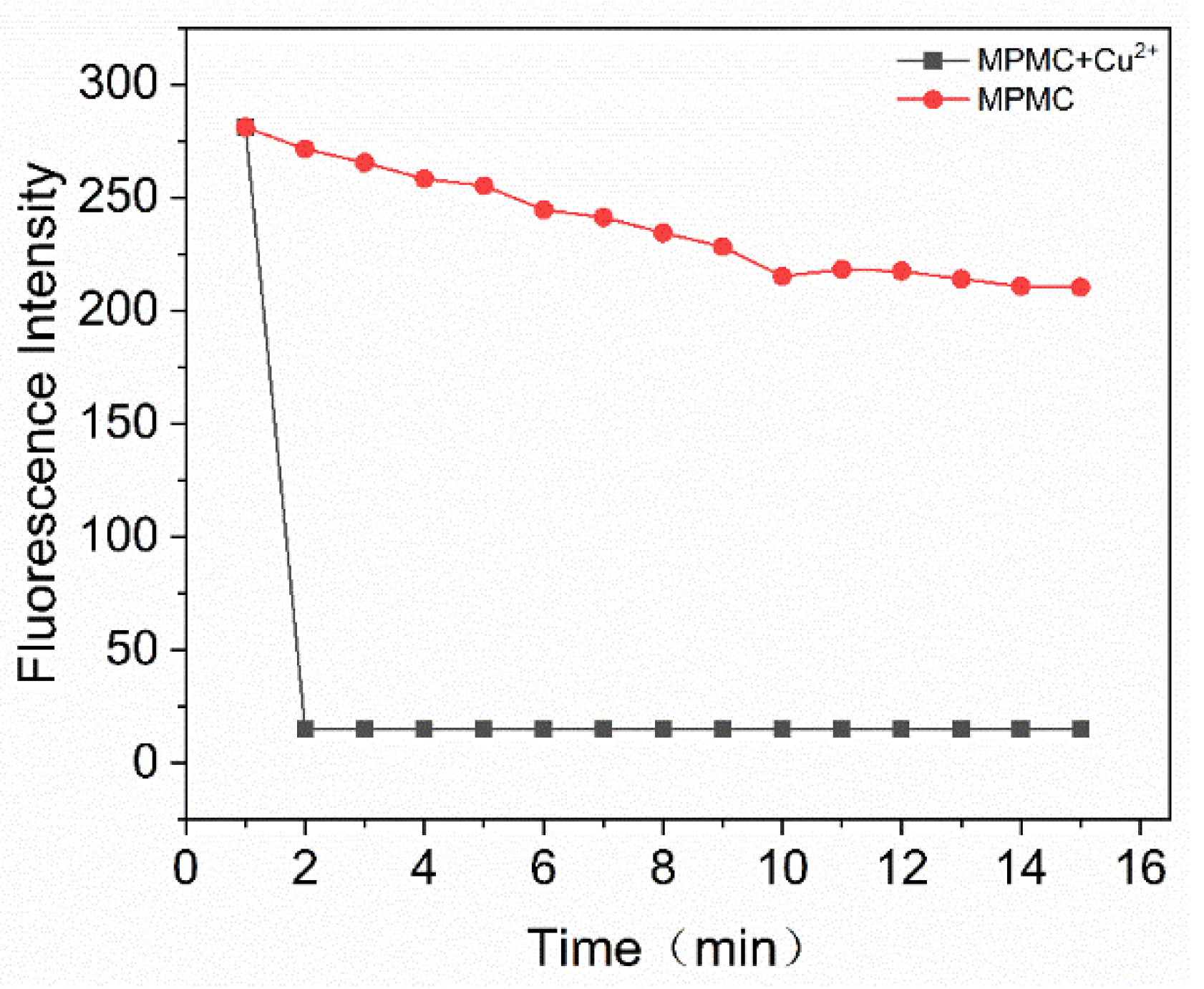
2.5. Study of the response time of probe MPMC to Cu2+
The response time of probe MPMC for determining Copper (II) ions was detected. The variation of the fluorescence intensity at 548 nm as the reaction time of the probe MPMC along with Cu
2+ is shown in
Figure 6. After the addition of Cu (II) ions, the fluorescence intensity of the probe MPMC declined markedly within 1 min, then achieved an equilibrium after 2 mins.
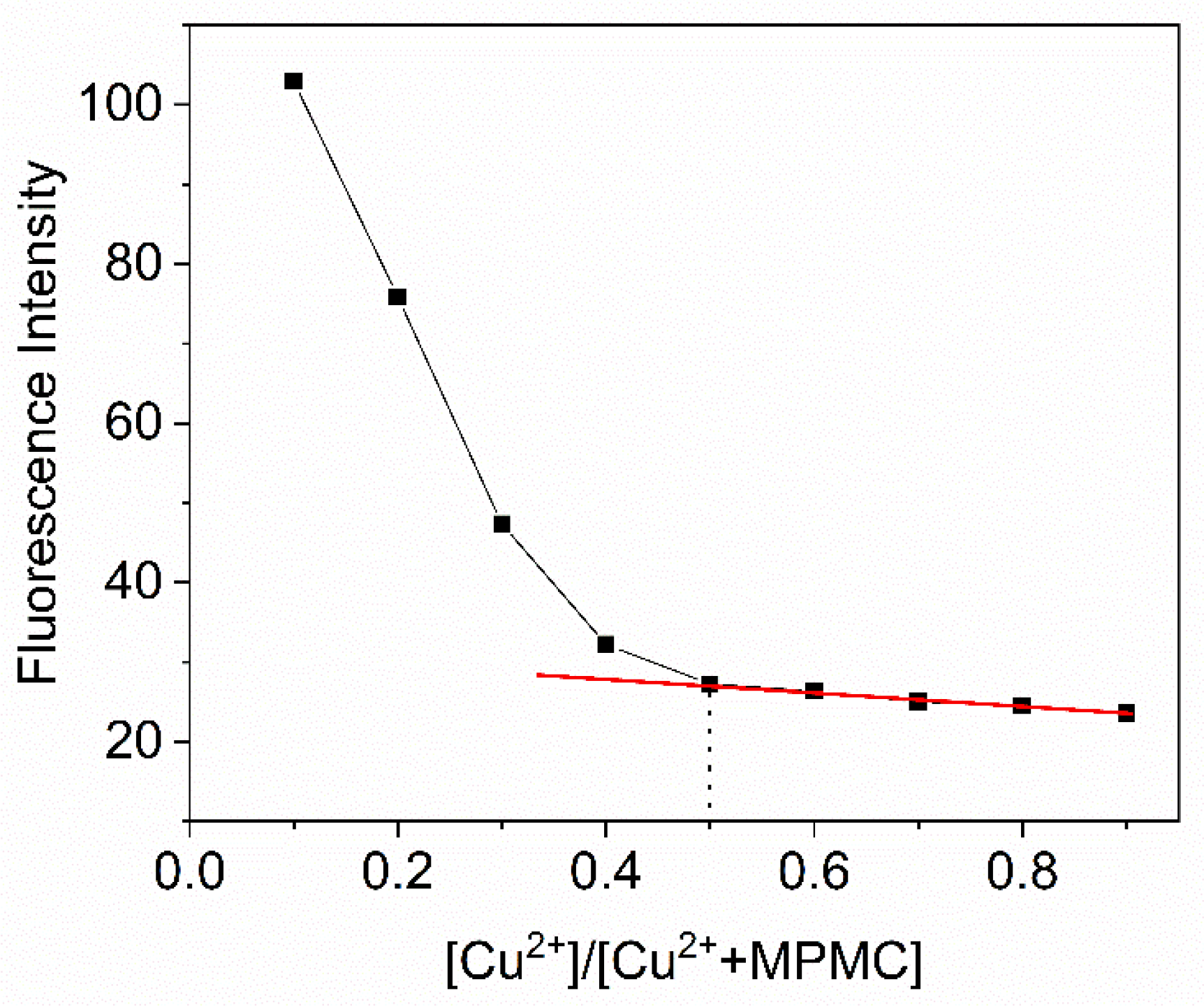
2.6. Contact mode detection between probe MPMC and Cu2+
To explore binding ratio of probe MPMC to Cu
2+, different ratios of probe MPMC to Cu
2+ (1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, 9:1) were prepared. As the molar fraction changed, the fluorescence characteristics were analysed and Job’s plot curve was illustrated in
Figure 7. With the molar fraction varying from 1:9 to 5:5, the fluorescence intensity decreased firstly, while the fluorescence intensity was almost unchanged in the range from 5:5 to 9:1. The molar fraction corresponding to the minimum fluorescence intensity of the probe MPMC appeared at 0.5, indicating that MPMC-Cu
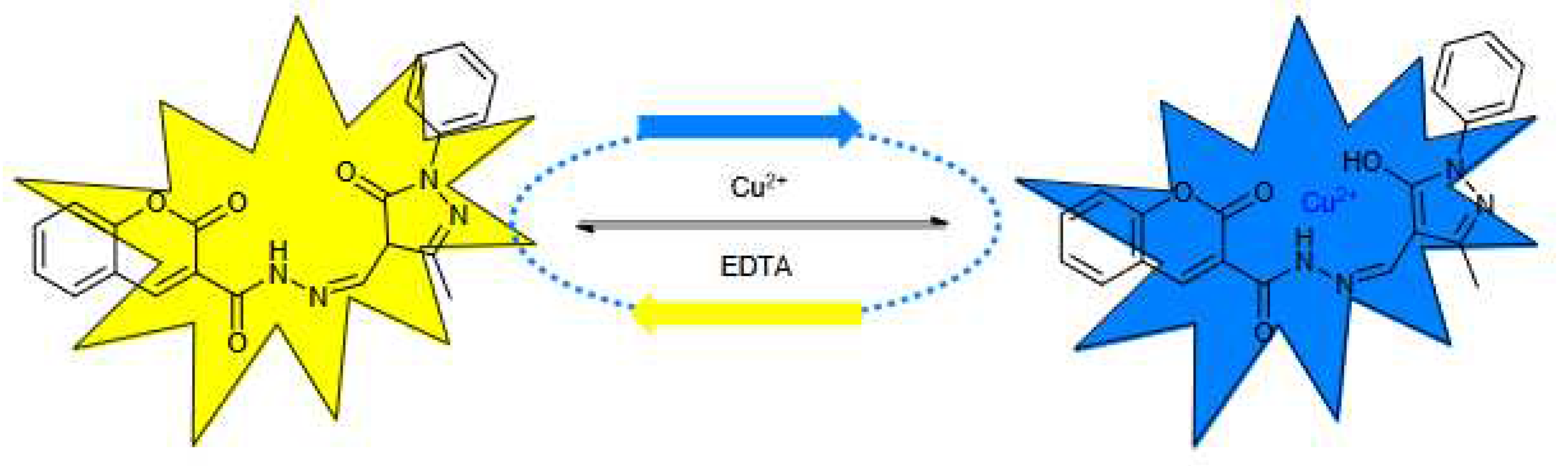
2+ complex was formed by 1:1. The possible binding mechanism of MPMC to Cu
2+ induced fluorescence changes is shown in
Figure 8.
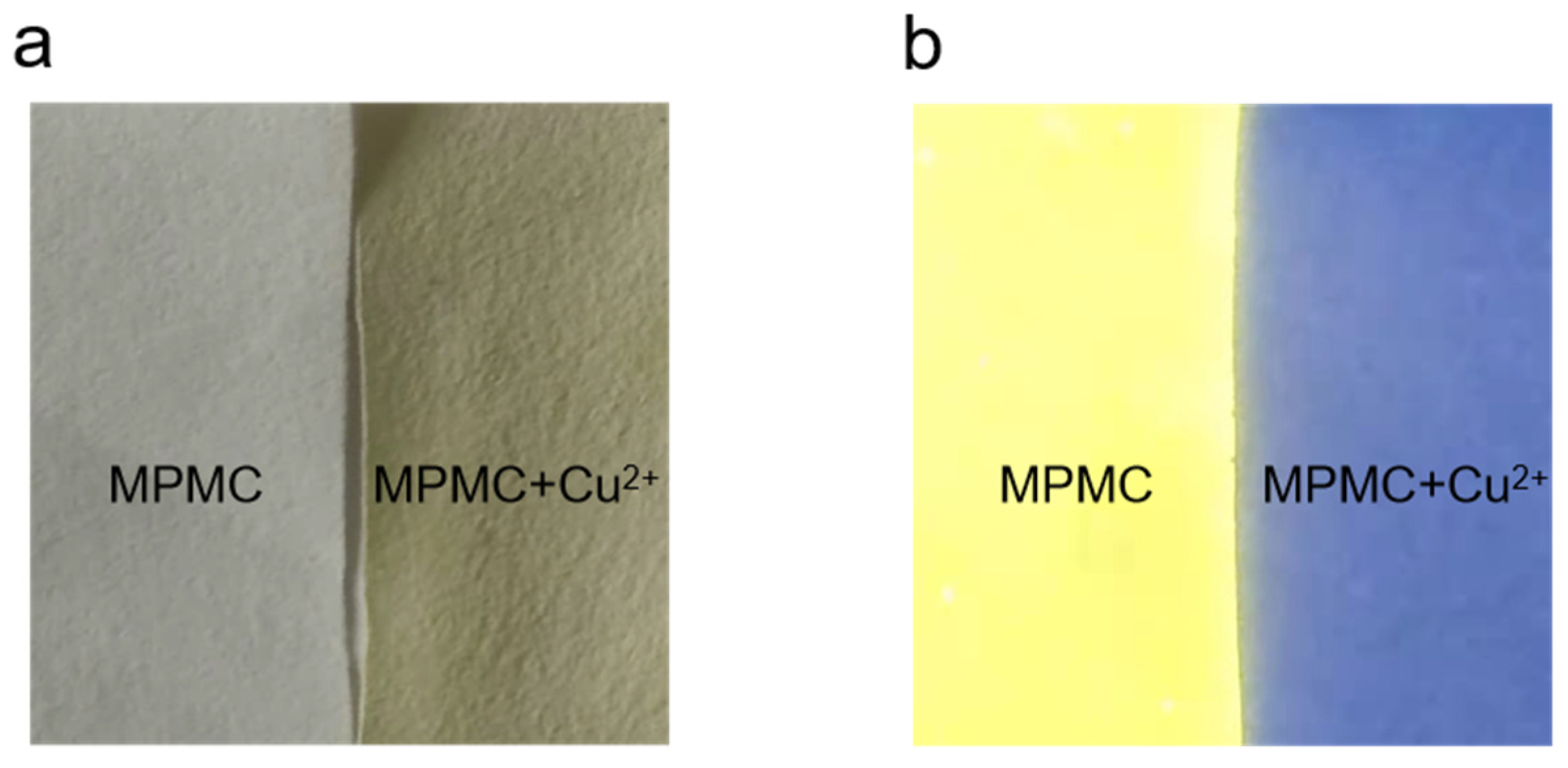
2.7. The test paper for Cu2+ ions
To study the multifunction applications of solid-state probe MPMC for its high efficiency and simplicity, the recognition of Cu
2+ by MPMC was performed on pre-treated paper. Filter paper were steeped in MPMC probe dissolved in an ethanol-saturated solution (1.0 × 10
−3 mol/L) for a few seconds to obtain test strips. Afterward, solid-state experiments were carried out by drying test strips in air and treating with an aqueous solution of Cu
2+ (1.0 × 10
−3 mol/L). Under sunlight, test strips before and after solid-state experiments presented white and yellow color respectively, seen in
Figure 9. In the same way, the color became light yellow and blue under 365 nm UV light, respectively [
31]. Thus, the solid-state method for detecting Cu
2+ with the naked-eye, was both economical and convenient.
3. Experiments
3.1. Reagents and chemicals
All raw chemicals were purchased from commercial sources without further purification. A stock solution of MPMC was prepared in EtOH at a concentration of 1.0 × 10−3 mol/L. The stock perchlorate solutions (including the perchlorate of Cu2+, Pb2+, Fe3+, Fe2+, Mg2+, Ni2+, Cd2+, Mn2+, Ca2+, Ba2+, Na+, K+ and Ag+) were freshly prepared in deionized water at a concentration of 1.0 × 10−3 mol/L.
1H and 13C NMR spectra were recorded on a Bruker AVANCE III 400 MHz NMR spectrometer in DMSO-d6 with TMS as an internal standard. Mass spectrum was recorded on the Thermo Q-Exactive mass spectrometer. The melting point was measured on the XRC-1 melting point instrument. The ultraviolet absorption was recorded on Cary50. The fluorescence test was recorded on the RF-6000 luminescence spectrophotometer. ESI-MS data was recorded with Mariner System 5304 mass spectrometer. Elemental analyses (C, H, and N) were gathered on a CHN-O-Rapid instrument within 0.4% of the theoretical values.
3.2. Synthesis of MPMC
The synthetic route of the target probe MPMC is shown in
Scheme 1. Compound
2 and
4 were easily synthesized in reference to the methods reported in the literatures [
32,
33,
34,
35]. Compound
2 (1.0 g, 4.95 mmol) and compound
4 (1.0 g, 4.95 mmol) were dissolved in 40 mL of ethanol. With the addition of catalyst acetic acid, the reaction proceeded at reacting temperature 78 °C, heating reflux for 2 h. After restored at room temperature, a light yellow precipitate generated and was obtained by filtering, washing with ethanol for several times. Finally, the precipitate was dried to give probe MPMC (0.902 g, yield 46.96%). m.p. >280 °C.
1H NMR and
13C NMR spectrum of MPMC are shown in Figures 11 and 12.
1H NMR (400 MHz, DMSO-
d6) δ 11.14 (s, 1H), 9.01 (s, 1H), 8.77 (s, 1H), 7.93 (d, J = 7.6 Hz, 1H), 7.76 (d, J = 8.0 Hz, 1H), 7.73–7.68 (m, 1H), 7.45 (d, J = 8.6 Hz, 1H), 7.44–7.37 (m, 2H), 7.00 (s, 1H), 6.97 (d, J = 7.4 Hz, 1H), 4.30 (q, J = 7.0 Hz, 2H), 1.32 (t, J = 7.1 Hz, 3H).
13C NMR (100 MHz, DMSO-
d6) δ 163.26, 163.07, 159.11, 156.48, 155.01, 149.19, 134.97, 133.72, 131.28, 130.76, 125.33, 120.08, 118.66, 118.29, 118.14, 117.00, 116.63, 61.71, 14.54. MS (ESI): 389.38 (M+H)+. Anal.Calcd for C
21H
16N
4O
4: C, 64.94; H, 4.15; N, 14.43. Found: C, 64.92; H, 4.13; N, 14.41.
3.3. General procedure for the spectrum measurement
Stock solutions of MPMC (1 mM) were prepared in EtOH. The metal ions stock solutions (1 mM) were prepared with the nitrate or chloride salts (Cu2+, Pb2+, Fe3+, Fe2+, Mg2+, Ni2+, Cd2+, Mn2+, Ca2+, Ba2+, Na+, K+ and Ag+) in deionized water. Absorption and emission spectra were obtained at room temperature using PBS solution (pH 7.5) in MPMC (10 μM) with different concentrations of analyzer. Fluorescence spectra were recorded at an excitation wavelength of 358 nm. The total concentration of Cu2+ and MPMC was kept constantly (2.0 mM). The fluorescence intensity of MPMC was then recorded by varying the molar ratio of MPMC to Cu2+. The selectivity of MPMC towards Cu2+ was tested by comparing other metal ions. MPMC (1 mM) was treated with Cu2+ (1 mM) and other metal ions (10 mM) for 10 mins and the fluorescence intensity of the mixtures was recorded. For reproducibility testing, Cu2+ (1.0 mM) was incubated with MPMC aqueous solution (1.0 mM), and the fluorescence of MPMC was quenched. The fluorescence intensity of MPMC (1.0 mM), MPMC-Cu2+ ensemble (1.0 mM) was determined in a series of buffers of pH 1.0 to 14.0.
4. Conclusions
A fluorescence probe with highly selective and sensitive performance based on coumarin and pyrazole Schiff base has been synthesized. The fluorescence emission behaviors of probe MPMC towards diverse metal ions were detected and the probe exhibited high sensitivity and selectivity towards Cu2+ over other metal ions via quenching of its fluorescence. Furthermore, the existence of other metal actions made no difference on the fluorescence intensity of the MPMC-Cu2+ system apparently, that is, MPMC displayed a good anti-interference ability. Job’s plot of MPMC and copper ions indicated the detection limit was 15.16 nM (R2 = 0.9612) for the assayed actions, with a stoichiometric ratio of 1:1 for MPMC and Cu2+. Additionally, the color of MPMC probe solution changed from nearly colorless to yellow in the presence of Cu2+ in visible light, which the color change could be observed by the naked eye. Similarly, the color resolved bright yellow into blue in ultraviolet light. Also MPMC probe was reusable. The pH effect of probe MPMC to Cu2+ had a broad range of pH detection 4.0 to 11.0, especially from 5.0 to 8.0. The response time of MPMC probe for determining Cu2+ was within 1 min. The recognition of Cu2+ by MPMC performed on pre-treated paper under sunlight and UV light both had a distinct colour change. In conclusion, MPMC probe could be applied to recognize Cu2+ in the environment, also the applications in biological system may be achieved in future.
Author Contributions
Conceptualization, H.-L.L., J.L., P.-Y.C., and X.W.; Data curation, H.-L.L., J.L., P.-Y.C., X.W., Q.W., S.C., M.W., K.W., Y.L., Y.-Y.C., X.-Y.L., and X.Z.; Formal Analysis, P.-Y.C., Q.W., S.C., M.W., K.W., Y.L., Y.-Y.C., X.-Y.L., and X.Z.; Funding acquisition, H.-L.L. and J.L.; Investigation, J.L., P.-Y.C., Q.W., S.C., M.W., K.W., Y.L., Y.-Y.C., X.-Y.L., and X.Z.; Methodology, H.-L.L. and P.-Y.C.; Project administration, H.-L.L., J.L., P.-Y.C., and X.W.; Resources, H.-L.L., J.L. and X.W.; Supervision, H.-L.L. and X.W.; Validation, J.L., P.-Y.C., Q.W., S.C., M.W., K.W., Y.L., Y.-Y.C., X.-Y.L., and X.Z.; Visualization, H.-L.L., J.L., P.-Y.C., X.W., Q.W., S.C., M.W., K.W., Y.L., Y.-Y.C., X.-Y.L., and X.Z.; Writing-original draft, H.-L.L., J.L., P.-Y.C., and X.W.; Writing-review & editing, H.-L.L., J.L., P.-Y.C., X.W., Q.W., S.C., M.W., K.W., Y.L., Y.-Y.C., X.-Y.L., and X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Jiangsu Key Laboratory of Marine Pharmaceutical Compound Screening (No. HY201602), Scientific research personnel training program of Kangda College of Nanjing Medical University (No. KD2022KYRC007), Science & Technology Funds of Nanjing Medical University (No. NMUB20210365), Social Development Funds of Lianyungang (No. SF2107), Science & Technology Funds of Kangda College of Nanjing Medical University (No. KD2021KYJJZD009&KD2022KYJJZD002).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Peers, G.; Price, N.M. Copper-containing plastocyanin used for electron transport by an oceanic diatom. Nature 2006, 441, 341–344. [Google Scholar] [CrossRef]
- Uauy, R.; Olivares, M.; Gonzalez, M. Essentiality of copper in humans. Am J Clin Nutr 1998, 67, 952s–959s. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.C.; HazeghAzam, M. Copper biochemistry and molecular biology. Am J Clin Nutr 1996, 63, 797–811. [Google Scholar]
- Gaggelli, E.; Kozlowski, H.; Valensin, D.; Valensin, G. Copper homeostasis and neurodegenerative disorders (Alzheimer’s, prion, and Parkinson’s diseases and amyotrophic lateral sclerosis). Chem Rev 2006, 106, 1995–2044. [Google Scholar] [CrossRef] [PubMed]
- Strausak, D.; Mercer, J.F.B.; Dieter, H.H.; Stremmel, W.; Multhaup, G. Copper in disorders with neurological symptoms: Alzheimer’s, Menkes, and Wilson diseases. Brain Res Bull 2001, 55, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef]
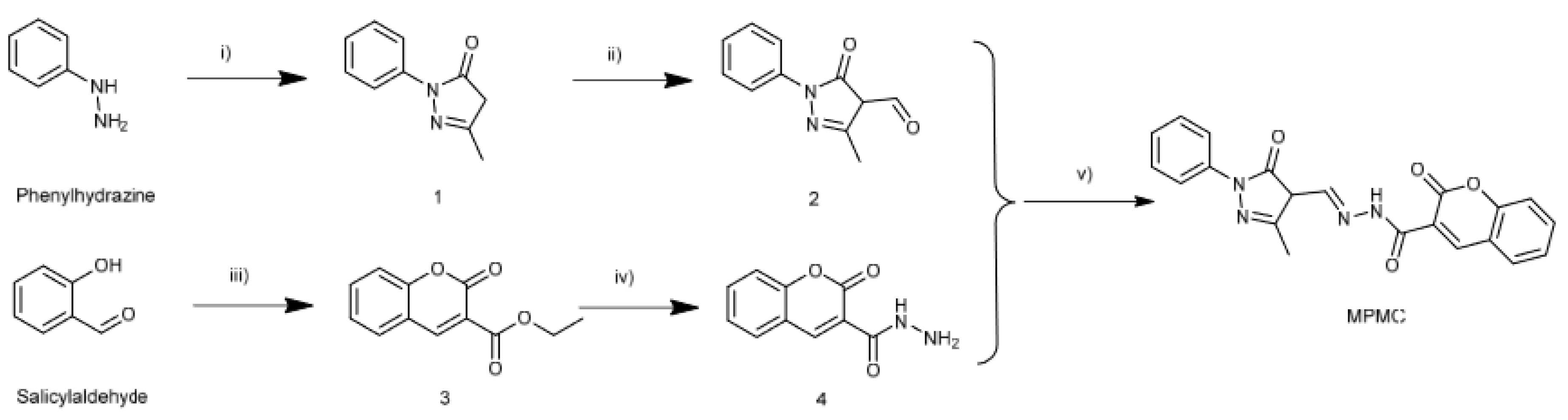
- Sun, J.Z.; Xu, X.; Yu, G.H.; Li, W.N.; Shi, J.S. Coumarin-based tripodal chemosensor for selective detection of Cu(II) ion and resultant complex as anion probe through a Cu(II) displacement approach. Tetrahedron 2018, 74, 987–991. [Google Scholar] [CrossRef]
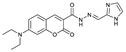
- Meng, X.J.; Cao, D.L.; Hu, Z.Y.; Han, X.H.; Li, Z.C.; Liang, D.; Ma, W.B. A coumarin based highly selective fluorescent chemosensor for sequential recognition of Cu2+ and PPi. Tetrahedron Lett 2018, 59, 4299–4304. [Google Scholar] [CrossRef]
- Amjadi, M.; Abolghasemi-Fakhri, Z. Gold nanostar-enhanced chemiluminescence probe for highly sensitive detection of Cu(II) ions. Sensor Actuat B-Chem 2018, 257, 629–634. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Yu, C.; Yin, T.X.; Ou, S.S.; Sun, X.Y.; Wen, X.R.; Zhang, L.; Tang, D.Q.; Yin, X.X. Functionalized poly (ionic liquid) as the support to construct a ratiometric electrochemical biosensor for the selective determination of copper ions in AD rats. Biosens Bioelectron 2017, 87, 278–284. [Google Scholar] [CrossRef]
- Al-Nidawi, M.; Alshana, U. Reversed-phase switchable-hydrophilicity solvent liquid-liquid microextraction of copper prior to its determination by smartphone digital image colorimetry. J Food Compos Anal 2021, 104, 104140. [Google Scholar] [CrossRef]
- Gamela, R.R.; Costa, V.C.; Pereira, E.R. Multivariate Optimization of Ultrasound-Assisted Extraction Procedure for the Determination of Ca, Fe, K, Mg, Mn, P, and Zn in Pepper Samples by ICP OES. Food Anal Method 2020, 13, 69–77. [Google Scholar] [CrossRef]
- Bonin, M.; Lariviere, D.; Povinec, P.P. Detection of radium at the attogram per gram level in copper by inductively coupled plasma mass spectrometry after cation-exchange chromatography. Anal Methods-Uk 2020, 12, 2272–2278. [Google Scholar] [CrossRef]
- Zhou, Z.L.; Tang, H.H.; Chen, S.Y.; Huang, Y.H.; Zhu, X.H.; Li, H.T.; Zhang, Y.Y.; Yao, S.Z. A turn-on red-emitting fluorescent probe for determination of copper(II) ions in food samples and living zebrafish. Food Chem 2021, 343, 128513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.D.; Cai, Y.H.; He, Y.H.; Lin, Q.L.; Ren, J.L.; Cao, D.S.; Zhang, L. A label-free fluorescent peptide probe for sensitive and selective determination of copper and sulfide ions in aqueous systems. Rsc Adv 2021, 11, 7426–7435. [Google Scholar] [CrossRef]
- Zeng, X.D.; Gao, S.; Jiang, C.; Duan, Q.X.; Ma, M.S.; Liu, Z.G.; Chen, J. Rhodol-derived turn-on fluorescent probe for copper ions with high selectivity and sensitivity. Luminescence 2021, 36, 1761–1766. [Google Scholar] [CrossRef]
- Liu, J.Q.; Liu, Z.C.; Wang, W.K.; Tian, Y. Real-time Tracking and Sensing of Cu+ and Cu2+ with a Single SERS Probe in the Live Brain: Toward Understanding Why Copper Ions Were Increased upon Ischemia. Angew Chem Int Edit 2021, 60, 21351–21359. [Google Scholar] [CrossRef]
- Sun, X.Y.; Liu, T.; Sun, J.; Wang, X.J. Synthesis and application of coumarin fluorescence probes. Rsc Adv 2020, 10, 10826–10847. [Google Scholar] [CrossRef]
- Wang, K.N.; Sun, P.Z.; Chao, X.J.; Cao, D.X.; Mao, Z.W.; Liu, Z.Q. A coumarin Schiff’s base two-photon fluorescent probe for hypochlorite in living cells and zebrafish. Rsc Adv 2018, 8, 6904–6909. [Google Scholar] [CrossRef]
- He, G.J.; Hua, X.B.; Yang, N.; Li, L.L.; Xu, J.H.; Yang, L.L.; Wang, Q.Z.; Ji, L.G. Synthesis and application of a “turn on” fluorescent probe for glutathione based on a copper complex of coumarin hydrazide Schiff base derivative. Bioorg Chem 2019, 91, 103176. [Google Scholar] [CrossRef]
- Yan, L.Q.; Hu, C.J.; Li, J.P. A fluorescence turn-on probe for rapid monitoring of hypochlorite based on coumarin Schiff base. Anal Bioanal Chem 2018, 410, 7457–7464. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hao, X.H.; Liang, L.X.; Gao, L.Y.; Ren, X.M.; Wu, Y.G.; Zhao, H.C. A coumarin-containing Schiff base fluorescent probe with AIE effect for the copper(ii) ion. Rsc Adv 2020, 10, 6109–6113. [Google Scholar] [CrossRef] [PubMed]
- Mani, K.S.; Rajamanikandan, R.; Ravikumar, G.; Pandiyan, B.V.; Kolandaivel, P.; Ilanchelian, M.; Rajendran, S.P. Highly Sensitive Coumarin-Pyrazolone Probe for the Detection of Cr3+ and the Application in Living Cells. Acs Omega 2018, 3, 17212–17219. [Google Scholar] [CrossRef]
- Babur, B.; Seferoglu, N.; Seferoglu, Z. A coumarin-pyrazolone based fluorescent probe for selective colorimetric and fluorimetric fluoride detection: Synthesis, spectroscopic properties and DFT calculations. J Mol Struct 2018, 1161, 218–225. [Google Scholar] [CrossRef]
- Babur, B.; Seferoglu, N.; Ocal, M.; Sonugur, G.; Akbulut, H.; Seferoglu, Z. A novel fluorescence turn-on coumarin-pyrazolone based monomethine probe for biothiol detection. Tetrahedron 2016, 72, 4498–4502. [Google Scholar] [CrossRef]
- Xia, X.C.; Fu, Y.; Tang, H.; Li, Y.; Wang, F.Y.; Ren, J. A self-assembled micellar nanoprobe for specific recognition of hydrazine in vitro and in vivo. Sensor Actuat B-Chem 2018, 272, 479–484. [Google Scholar] [CrossRef]
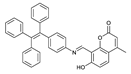
- Yin, J.; Wang, Z.L.; Zhao, F.; Yang, H.Y.; Li, M.X.; Yang, Y.Q. A novel dual functional pyrene-based turn-on fluorescent probe for hypochlorite and copper (II) ion detection and bioimaging applications. Spectrochim Acta A 2020, 239, 118470. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Ai, Y.; Zhang, Y.L.; Ren, Y.Y.; Wang, J.L.; Yao, F.Y.; Li, W.Y.; Zhou, Y.; Sun, Y.N.; Liu, J.L. A new probe with high selectivity and sensitivity for detecting copper ions in traditional Chinese medicine and water sample. Inorg Chem Commun 2021, 128, 108563. [Google Scholar] [CrossRef]
- Wang, M.M.; Wang, C.; Wang, M.; Sun, T.M.; Huang, Y.; Tang, Y.F.; Ju, J.F.; Shen, L.J.; Hu, Y.Y.; Zhu, J.L. A Dual-Functional “On-Off-On” Relay Fluorescent Probe for the Highly Sensitive Detection of Copper(II) and Phosphate Ions. Chemistryselect 2020, 5, 1331–1334. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Teng, Q.; Yang, Y.S.; Guo, H.C.; Xue, J.J. A novel coumarin-based pyrazoline fluorescent probe for detection of Fe3+and its application in cells. Inorg Chim Acta 2021, 525, 120469. [Google Scholar] [CrossRef]
- Zong, L.Y.; Wang, C.; Song, Y.C.; Hu, J.; Li, Q.Q.; Li, Z. A fluorescent and colorimetric probe based on naphthalene diimide and its high sensitivity towards copper ions when used as test strips. Rsc Adv 2019, 9, 12675–12680. [Google Scholar] [CrossRef] [PubMed]
- Vijay, K.; Nandi, C.; Samant, S.D. Synthesis of a dihydroquinoline based merocyanine as a ‘naked eye’ and ‘fluorogenic’ sensor for hydrazine hydrate in aqueous medium and hydrazine gas. Rsc Adv 2014, 4, 30712–30717. [Google Scholar] [CrossRef]
- Surati, K.R.; Thaker, B.T. Synthesis, spectroscopic and thermal investigation of Schiff-base complexes of Cu(II) derived from heterocyclic beta-diketone with various primary amines. J Coord Chem 2006, 59, 1191–1202. [Google Scholar] [CrossRef]
- Naik, M.D.; Bodke, Y.D.; Kumar, V.M.; Revanasiddappa, B.C. An efficient one-pot synthesis of coumarin-amino acid derivatives as potential anti-inflammatory and antioxidant agents. Synthetic Commun 2020, 50, 1210–1216. [Google Scholar] [CrossRef]
- Taha, M.; Shah, S.A.A.; Afifi, M.; Imran, S.; Sultan, S.; Rahim, F.; Khan, K.M. Synthesis, alpha-glucosidase inhibition and molecular docking study of coumarin based derivatives. Bioorg Chem 2018, 77, 586–592. [Google Scholar] [CrossRef]
Figure 1.
(a) Fluorescence emission spectra of probe MPMC (10 μM) without or with metal ions (including Cu2+, Pb2+, Fe3+, Fe2+, Mg2+, Ni2+, Cd2+, Mn2+, Ca2+, Ba2+, Na+, K+, Ag+) (10 μM) in ethanol with excitation wave length of 358 nm; (b) Fluorescence response of MPMC (10 μM) to Cu2+ in the presence of various metal ions (including Pb2+, Fe3+, Fe2+, Mg2+, Ni2+, Cd2+, Mn2+, Ca2+, Ba2+, Na+, K+, Ag+) (100 μM) in EtOH/H2O (v/v = 1/1) (λex = 358 nm, λem = 548 nm).
Figure 1.
(a) Fluorescence emission spectra of probe MPMC (10 μM) without or with metal ions (including Cu2+, Pb2+, Fe3+, Fe2+, Mg2+, Ni2+, Cd2+, Mn2+, Ca2+, Ba2+, Na+, K+, Ag+) (10 μM) in ethanol with excitation wave length of 358 nm; (b) Fluorescence response of MPMC (10 μM) to Cu2+ in the presence of various metal ions (including Pb2+, Fe3+, Fe2+, Mg2+, Ni2+, Cd2+, Mn2+, Ca2+, Ba2+, Na+, K+, Ag+) (100 μM) in EtOH/H2O (v/v = 1/1) (λex = 358 nm, λem = 548 nm).
Figure 2.
(a) Colorimetric performance of sensor MPMC (1 mM) upon addition of different metal ions (including Cu2+, Pb2+, Fe3+, Fe2+, Mg2+, Ni2+, Cd2+, Mn2+, Ca2+, Ba2+, Na+, K+, Ag+) (1 mM) in EtOH/H2O (v/v = 1/1) solution; (b) color change induced upon addition of Cu2+ under 365 nm UV lamp.
Figure 2.
(a) Colorimetric performance of sensor MPMC (1 mM) upon addition of different metal ions (including Cu2+, Pb2+, Fe3+, Fe2+, Mg2+, Ni2+, Cd2+, Mn2+, Ca2+, Ba2+, Na+, K+, Ag+) (1 mM) in EtOH/H2O (v/v = 1/1) solution; (b) color change induced upon addition of Cu2+ under 365 nm UV lamp.
Figure 3.
(a) The fluorescence spectra of MPMC (10μM) with the increasing concentration of Cu2+ ion (0.01-10.0 equiv.) in EtOH/H2O (v/v = 1/1); (b) The changes of fluorescence signal with different concentrations of copper ions; (c) The linear fit between MPMC and Cu2+ ion.
Figure 3.
(a) The fluorescence spectra of MPMC (10μM) with the increasing concentration of Cu2+ ion (0.01-10.0 equiv.) in EtOH/H2O (v/v = 1/1); (b) The changes of fluorescence signal with different concentrations of copper ions; (c) The linear fit between MPMC and Cu2+ ion.
Figure 4.
Changes in emission spectra of MPMC in the presence of Cu2+ and EDTA in EtOH/H2O (v/v = 1/1) (λex = 358 nm, λem = 548 nm).
Figure 4.
Changes in emission spectra of MPMC in the presence of Cu2+ and EDTA in EtOH/H2O (v/v = 1/1) (λex = 358 nm, λem = 548 nm).
Figure 5.
Fluorescence intensity changes of probe MPMC (black line) and MPMC-Cu2+ complexes (red line) under different pH values in phosphate buffer system (λex = 358 nm, λem = 548 nm).
Figure 5.
Fluorescence intensity changes of probe MPMC (black line) and MPMC-Cu2+ complexes (red line) under different pH values in phosphate buffer system (λex = 358 nm, λem = 548 nm).
Figure 6.
Time-dependent changes of MPMC (10 μM) (black line) with the addition of Cu2+ (10 μM) (red line) in EtOH/H2O (v/v = 1/1) (λex = 358 nm, λem = 548 nm).
Figure 6.
Time-dependent changes of MPMC (10 μM) (black line) with the addition of Cu2+ (10 μM) (red line) in EtOH/H2O (v/v = 1/1) (λex = 358 nm, λem = 548 nm).
Figure 7.
Job’s plot of MPMC and copper ions ([MPMC] + [Cu2+] = 20 μM) in EtOH/H2O (v/v = 1/1) by fluorescence spectra, where the fluorescence intensity at 548 nm was plotted against the mole fraction of [[Cu2+]/[[MPMC] + [Cu2+]]].
Figure 7.
Job’s plot of MPMC and copper ions ([MPMC] + [Cu2+] = 20 μM) in EtOH/H2O (v/v = 1/1) by fluorescence spectra, where the fluorescence intensity at 548 nm was plotted against the mole fraction of [[Cu2+]/[[MPMC] + [Cu2+]]].
Figure 8.
The proposed sensing mechanism of MPMC to Cu2+ in the system.
Figure 8.
The proposed sensing mechanism of MPMC to Cu2+ in the system.
Figure 9.
Photographs showing the color changes of probe MPMC (1.0 mM) before and after addition of Cu2+ (1.0 mM) under (a) sunlight and (b) 365 nm UV light.
Figure 9.
Photographs showing the color changes of probe MPMC (1.0 mM) before and after addition of Cu2+ (1.0 mM) under (a) sunlight and (b) 365 nm UV light.
Scheme 1.
Reagents and conditions: i) Ethyl acetoacetate, 60% EtOH solution, 45 °C, 2 h; ii) DMF, POCl3, 100 °C, 15 h; iii) Diethyl malonate, Piperidine, EtOH, 25 °C, overnight; iv) EtOH, 85% Hydrazine hydrate, 20 h; v) EtOH, CH3COOH, reflux, 2 h.
Scheme 1.
Reagents and conditions: i) Ethyl acetoacetate, 60% EtOH solution, 45 °C, 2 h; ii) DMF, POCl3, 100 °C, 15 h; iii) Diethyl malonate, Piperidine, EtOH, 25 °C, overnight; iv) EtOH, 85% Hydrazine hydrate, 20 h; v) EtOH, CH3COOH, reflux, 2 h.
Table 1.
Performance compared with available Cu2+ probes.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).