Submitted:
05 September 2023
Posted:
07 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
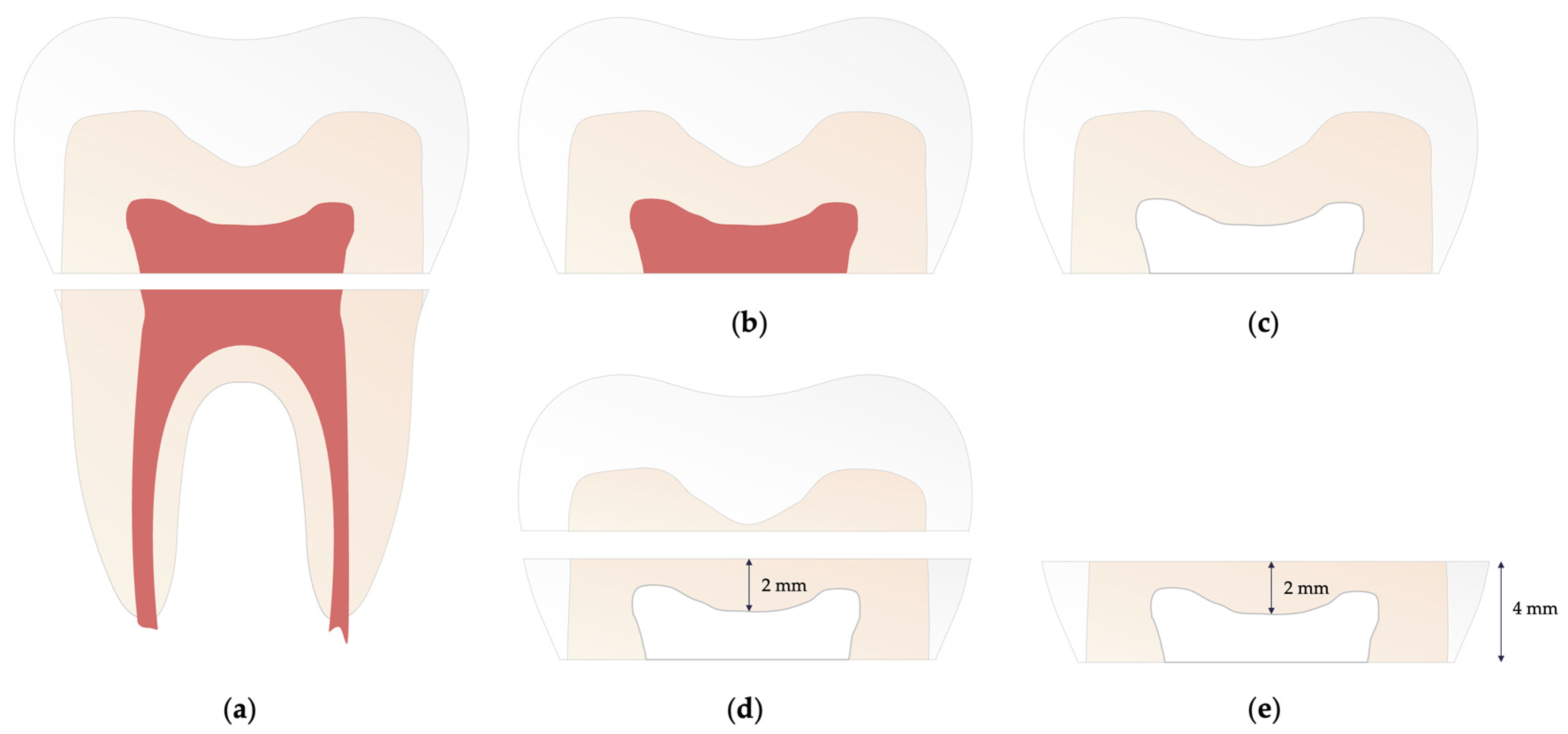
2.1. Preparation of specimens
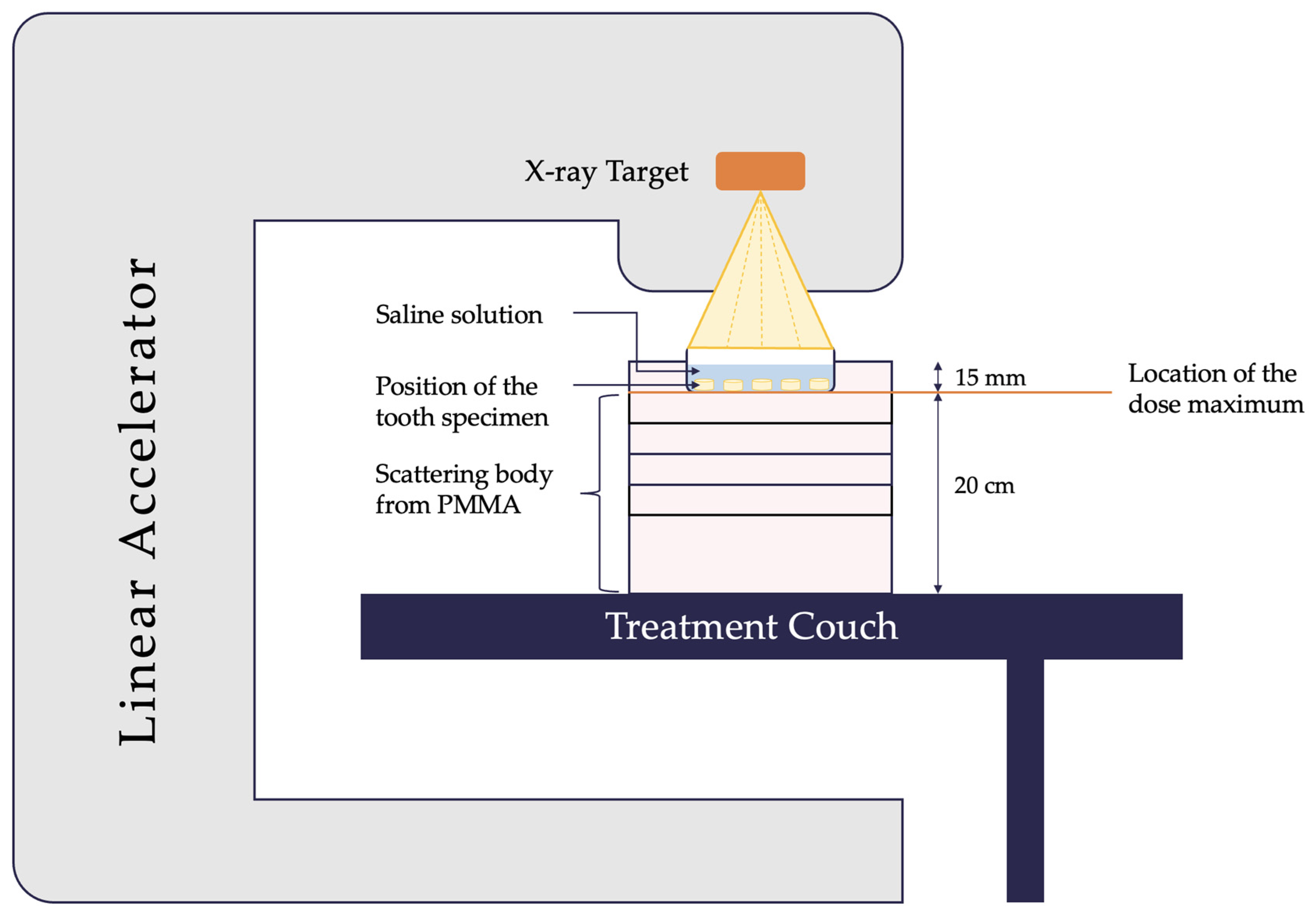
2.2. Irradiation of specimens
2.3. Materials used to perform the experimental procedure
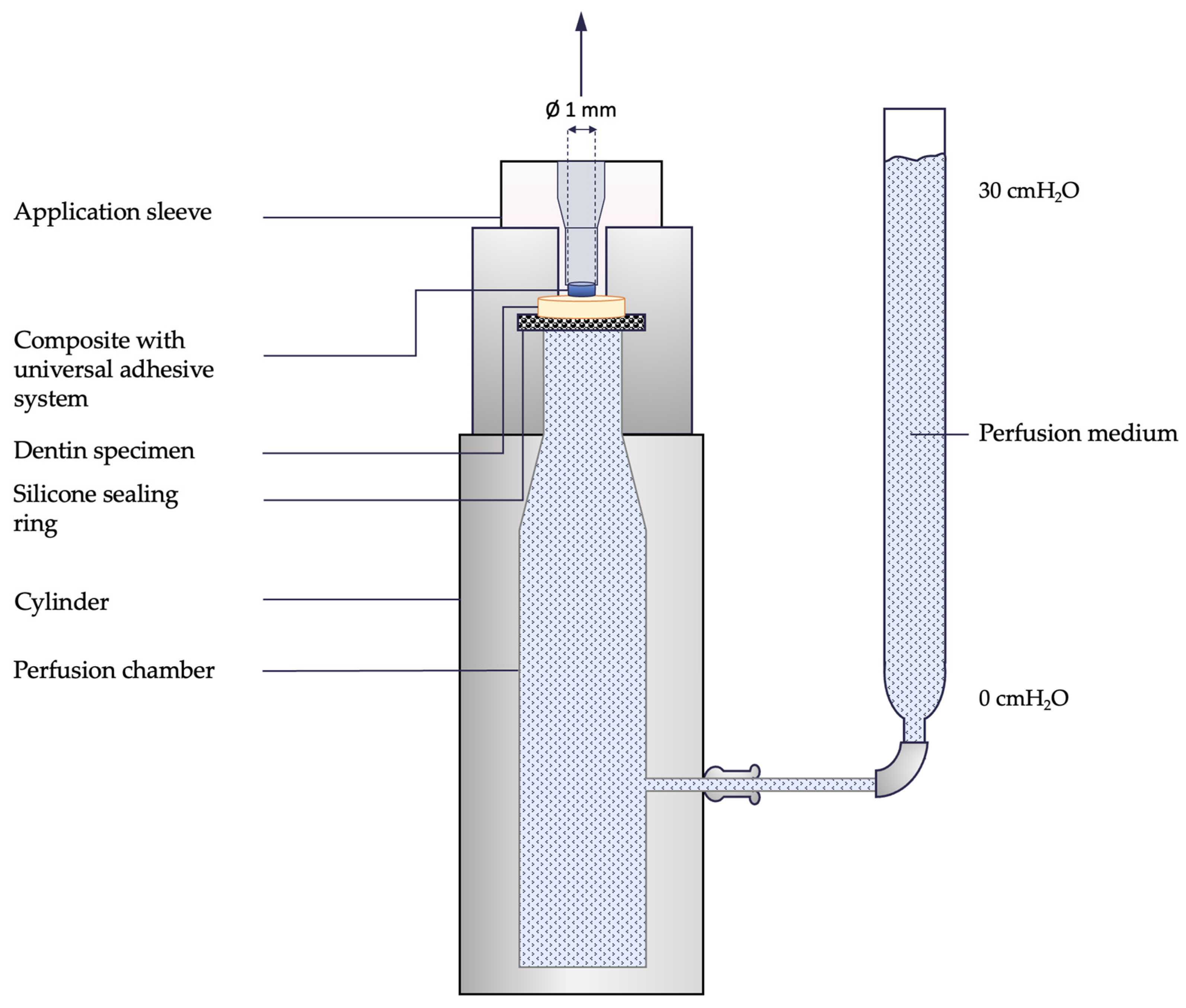
2.4. Experimental procedure
2.5. Data analysis and statistical evaluation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sofan, E.; Sofan, A.; Palaia, G.; Tenore, G.; Romeo, U.; Migliau, G. Classification review of dental adhesive systems: from the IV generation to the universal type. Ann Stomatol (Roma) 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Hardan, L.; Bourgi, R.; Kharouf, N.; Mancino, D.; Zarow, M.; Jakubowicz, N.; Haikel, Y.; Cuevas-Suárez, C.E. Bond Strength of Universal Adhesives to Dentin: A Systematic Review and Meta-Analysis. Polymers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Diniz, A.C.; Bandeca, M.C.; Pinheiro, L.M.; Dos Santosh Almeida, L.J., Jr.; Torres, C.R.; Borges, A.H.; Pinto, S.C.; Tonetto, M.R.; De Jesus Tavarez, R.R.; Firoozmand, L.M. Influence of Different Etching Modes on Bond Strength to Enamel using Universal Adhesive Systems. J Contemp Dent Pract 2016, 17, 820–825. [Google Scholar] [CrossRef]
- Follak, A.C.; Ilha, B.D.; Oling, J.; Savian, T.; Rocha, R.O.; Soares, F.Z.M. Clinical behavior of universal adhesives in non-carious cervical lesions: A randomized clinical trial. J Dent 2021, 113, 103747. [Google Scholar] [CrossRef]
- Josic, U.; Maravic, T.; Mazzitelli, C.; Radovic, I.; Jacimovic, J.; Del Bianco, F.; Florenzano, F.; Breschi, L.; Mazzoni, A. Is clinical behavior of composite restorations placed in non-carious cervical lesions influenced by the application mode of universal adhesives? A systematic review and meta-analysis. Dent Mater 2021, 37, e503–e521. [Google Scholar] [CrossRef]
- Josic, U.; Mazzitelli, C.; Maravic, T.; Radovic, I.; Jacimovic, J.; Mancuso, E.; Florenzano, F.; Breschi, L.; Mazzoni, A. The influence of selective enamel etch and self-etch mode of universal adhesives’ application on clinical behavior of composite restorations placed on non-carious cervical lesions: A systematic review and meta-analysis. Dent Mater 2022, 38, 472–488. [Google Scholar] [CrossRef]
- Gernhardt, C.R.; Nguyen, A.D.; Michaelis, M.; Pütz, N. Clinical Outcome of Class I and II Restorations with and without an Intermediary Layer of a Flowable Composite after 24 Months: A Prospective, Randomized, Split-Mouth-Designed, Controlled and Single-Blinded Clinical Trial. Applied Sciences 2023, 13, 4224. [Google Scholar] [CrossRef]
- Wiegand, A.; Lechte, C.; Kanzow, P. Adhesion to eroded enamel and dentin: systematic review and meta-analysis. Dent Mater 2021, 37, 1845–1853. [Google Scholar] [CrossRef]
- Cruz, J.; Sousa, B.; Coito, C.; Lopes, M.; Vargas, M.; Cavalheiro, A. Microtensile bond strength to dentin and enamel of self-etch vs. etch-and-rinse modes of universal adhesives. Am J Dent 2019, 32, 174–182. [Google Scholar]
- Jacker-Guhr, S.; Sander, J.; Luehrs, A.K. How “Universal” is Adhesion? Shear Bond Strength of Multi-mode Adhesives to Enamel and Dentin. J Adhes Dent 2019, 21, 87–95. [Google Scholar] [CrossRef]
- Jayasudha; Baswaraj; H, K.N.; K, B.P. Enamel regeneration - current progress and challenges. J Clin Diagn Res 2014, 8, Ze06–09. [Google Scholar] [CrossRef]
- Devarasa, G.M.; Subba Reddy, V.V.; Chaitra, N.L.; Swarna, Y.M. Self-etching adhesive on intact enamel, with and without pre-etching. Microsc Res Tech 2012, 75, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh, H.; Nemati-Karimooy, A.; Nasseh, A.; Rahmanpour, N. Evaluating the shear bond strength of enamel and dentin with or without etching: A comparative study between dimethacrylate-based and silorane-based adhesives. J Clin Exp Dent 2015, 7, e563–568. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Al-Bayatee, S.; Khurshid, Z.; Shavandi, A.; Brunton, P.; Ratnayake, J. Hydroxyapatite in Oral Care Products-A Review. Materials (Basel) 2021, 14. [Google Scholar] [CrossRef]
- Goldberg, M.; Kulkarni, A.B.; Young, M.; Boskey, A. Dentin: structure, composition and mineralization. Front Biosci (Elite Ed) 2011, 3, 711–735. [Google Scholar] [CrossRef]
- Siriporananon, C.; Senawongse, P.; Sattabanasuk, V.; Srimaneekarn, N.; Sano, H.; Saikaew, P. Effects of dentin surface preparations on bonding of self-etching adhesives under simulated pulpal pressure. Restor Dent Endod 2022, 47, e4. [Google Scholar] [CrossRef]
- Alshaikh, K.H.; Hamama, H.H.H.; Mahmoud, S.H. Effect of smear layer deproteinization on bonding of self-etch adhesives to dentin: a systematic review and meta-analysis. Restor Dent Endod 2018, 43, e14. [Google Scholar] [CrossRef]
- Scheffel, D.L.; Tenuta, L.M.; Cury, J.A.; Hebling, J. Effect of acid etching time on demineralization of primary and permanent coronal dentin. Am J Dent 2012, 25, 235–238. [Google Scholar]
- Perdigão, J. Dentin bonding-variables related to the clinical situation and the substrate treatment. Dent Mater 2010, 26, e24–37. [Google Scholar] [CrossRef]
- Katsuki, S.; Takamizawa, T.; Yokoyama, M.; Sai, K.; Tamura, T.; Ishii, R.; Kamimoto, A.; Miyazaki, M. Influence of bonding agent application method on the dentin bond durability of a two-step adhesive utilizing a universal-adhesive-derived primer. Eur J Oral Sci 2022, 130, e12868. [Google Scholar] [CrossRef]
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjäderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the art etch-and-rinse adhesives. Dent Mater 2011, 27, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Masarwa, N.; Mohamed, A.; Abou-Rabii, I.; Abu Zaghlan, R.; Steier, L. Longevity of Self-etch Dentin Bonding Adhesives Compared to Etch-and-rinse Dentin Bonding Adhesives: A Systematic Review. J Evid Based Dent Pract 2016, 16, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Breschi, L.; Maravic, T.; Cunha, S.R.; Comba, A.; Cadenaro, M.; Tjäderhane, L.; Pashley, D.H.; Tay, F.R.; Mazzoni, A. Dentin bonding systems: From dentin collagen structure to bond preservation and clinical applications. Dental Materials 2018, 34, 78–96. [Google Scholar] [PubMed]
- Frassetto, A.; Breschi, L.; Turco, G.; Marchesi, G.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H.; Cadenaro, M. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability—A literature review. Dental Materials 2016, 32, e41–e53. [Google Scholar] [CrossRef]
- Stape, T.H.S.; Mutluay, M.M.; Tjäderhane, L.; Uurasjärvi, E.; Koistinen, A.; Tezvergil-Mutluay, A. The pursuit of resin-dentin bond durability: Simultaneous enhancement of collagen structure and polymer network formation in hybrid layers. Dental Materials 2021, 37, 1083–1095. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Yoshihara, K.; Van Landuyt, K.; Yoshida, Y.; Peumans, M. From Buonocore’s Pioneering Acid-Etch Technique to Self-Adhering Restoratives. A Status Perspective of Rapidly Advancing Dental Adhesive Technology. J Adhes Dent 2020, 22, 7–34. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Yoshihara, K.; Yoshida, Y.; Mine, A.; De Munck, J.; Van Landuyt, K.L. State of the art of self-etch adhesives. Dent Mater 2011, 27, 17–28. [Google Scholar] [CrossRef]
- Carrilho, E.; Cardoso, M.; Marques Ferreira, M.; Marto, C.M.; Paula, A.; Coelho, A.S. 10-MDP Based Dental Adhesives: Adhesive Interface Characterization and Adhesive Stability-A Systematic Review. Materials (Basel) 2019, 12. [Google Scholar] [CrossRef]
- Fehrenbach, J.; Isolan, C.P.; Münchow, E.A. Is the presence of 10-MDP associated to higher bonding performance for self-etching adhesive systems? A meta-analysis of in vitro studies. Dent Mater 2021, 37, 1463–1485. [Google Scholar] [CrossRef]
- Alterio, D.; Marvaso, G.; Ferrari, A.; Volpe, S.; Orecchia, R.; Jereczek-Fossa, B.A. Modern radiotherapy for head and neck cancer. Semin Oncol 2019, 46, 233–245. [Google Scholar] [CrossRef]
- Caudell, J.J.; Torres-Roca, J.F.; Gillies, R.J.; Enderling, H.; Kim, S.; Rishi, A.; Moros, E.G.; Harrison, L.B. The future of personalised radiotherapy for head and neck cancer. Lancet Oncol 2017, 18, e266–e273. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.A.; Bradley, K.L.; Brands, M. Intensity-modulated radiotherapy in head and neck cancer - an update for oral and maxillofacial surgeons. Br J Oral Maxillofac Surg 2017, 55, 770–774. [Google Scholar] [CrossRef]
- Sonis, S.T. Oral mucositis in head and neck cancer: risk, biology, and management. Am Soc Clin Oncol Educ Book 2013. [Google Scholar] [CrossRef]
- Brook, I. Early side effects of radiation treatment for head and neck cancer. Cancer Radiother 2021, 25, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.E.; Bonzanini, L.I.L.; Ortigara, G.B.; Soldera, E.B.; Danesi, C.C.; Antoniazzi, R.P.; Ferrazzo, K.L. Prevalence of hyposalivation and associated factors in survivors of head and neck cancer treated with radiotherapy. J Appl Oral Sci 2021, 29, e20200854. [Google Scholar] [CrossRef]
- Moore, C.; McLister, C.; Cardwell, C.; O’Neill, C.; Donnelly, M.; McKenna, G. Dental caries following radiotherapy for head and neck cancer: A systematic review. Oral Oncol 2020, 100, 104484. [Google Scholar] [CrossRef] [PubMed]
- Sroussi, H.Y.; Epstein, J.B.; Bensadoun, R.J.; Saunders, D.P.; Lalla, R.V.; Migliorati, C.A.; Heaivilin, N.; Zumsteg, Z.S. Common oral complications of head and neck cancer radiation therapy: mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med 2017, 6, 2918–2931. [Google Scholar] [CrossRef]
- Kuhnt, T.; Jirsak, N.; Müller, A.C.; Pelz, T.; Gernhardt, C.; Schaller, H.G.; Janich, M.; Gerlach, R.; Dunst, J. [Quantitative and qualitative investigations of salivary gland function in dependence on irradiation dose and volume for reduction of xerostomia in patients with head-and-neck cancer]. Strahlenther Onkol 2005, 181, 520–528. [Google Scholar] [CrossRef]
- Corvò, R. Evidence-based radiation oncology in head and neck squamous cell carcinoma. Radiother Oncol 2007, 85, 156–170. [Google Scholar] [CrossRef]
- Hey, J.; Seidel, J.; Schweyen, R.; Paelecke-Habermann, Y.; Vordermark, D.; Gernhardt, C.; Kuhnt, T. The influence of parotid gland sparing on radiation damages of dental hard tissues. Clin Oral Investig 2013, 17, 1619–1625. [Google Scholar] [CrossRef]
- Gonçalves, L.M.; Palma-Dibb, R.G.; Paula-Silva, F.W.; Oliveira, H.F.; Nelson-Filho, P.; Silva, L.A.; Queiroz, A.M. Radiation therapy alters microhardness and microstructure of enamel and dentin of permanent human teeth. J Dent 2014, 42, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Douchy, L.; Gauthier, R.; Abouelleil-Sayed, H.; Colon, P.; Grosgogeat, B.; Bosco, J. The effect of therapeutic radiation on dental enamel and dentin: A systematic review. Dental Materials 2022, 38, e181–e201. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.-Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Pioch, T.; Golfels, D.; Staehle, H.J. An experimental study of the stability of irradiated teeth in the region of the dentinoenamel junction. Endod Dent Traumatol 1992, 8, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Mellara, T.d.S.; Paula-Silva, F.W.G.; Arid, J.; de Oliveira, H.F.; Nelson-Filho, P.; Bezerra Silva, R.A.; Torres, F.M.; Faraoni, J.J.; Palma-Dibb, R.G.; de Queiroz, A.M. Radiotherapy impairs adhesive bonding in primary teeth: An in vitro study. Journal of Dentistry for Children 2020, 87, 69–76. [Google Scholar]
- Oglakci, B. How does radiotherapy affect the adhesion of universal adhesive to enamel and dentin? A qualitative and quantitative analysis?. Odovtos [online]. 2022, vol. 24, n. 3. 2022, 24, 2215–3411. [Google Scholar] [CrossRef]
- Broscheit, S.; Vordermark, D.; Gerlach, R.; Gernhardt, C.R. Impact of Preceded Tumor Therapeutic Irradiation on the Microtensile Bond Strength of Universal Adhesives Applied in Self-Etch Mode to Human Dentin In Vitro. Applied Sciences 2023, 13, 7873. [Google Scholar] [CrossRef]
- Eggmann, F.; Hwang, J.D.; Ayub, J.M.; Mante, F.K. Impact of Irradiation on the Adhesive Performance of Resin-Based Dental Biomaterials: A Systematic Review of Laboratory Studies. Materials (Basel) 2023, 16. [Google Scholar] [CrossRef]
- Gernhardt, C.R.; Schaller, H.G.; Kielbassa, A.M. The influence of human plasma used for dentin perfusion on tensile bond strength of different light-curing materials. Am J Dent 2005, 18, 318–322. [Google Scholar]
- Kielbassa, A.M.; Beetz, I.; Schendera, A.; Hellwig, E. Irradiation effects on microhardness of fluoridated and non-fluoridated bovine dentin. Eur J Oral Sci 1997, 105, 444–447. [Google Scholar] [CrossRef]
- Sano, H.; Shono, T.; Sonoda, H.; Takatsu, T.; Ciucchi, B.; Carvalho, R.; Pashley, D.H. Relationship between surface area for adhesion and tensile bond strength--evaluation of a micro-tensile bond test. Dent Mater 1994, 10, 236–240. [Google Scholar] [CrossRef]
- Brkanović, S.; Sever, E.K.; Vukelja, J.; Ivica, A.; Miletić, I.; Krmek, S.J. Comparison of Different Universal Adhesive Systems on Dentin Bond Strength. Materials (Basel) 2023, 16. [Google Scholar] [CrossRef]
- Rosa, W.L.d.O.d.; Piva, E.; Silva, A.F.d. Bond strength of universal adhesives: A systematic review and meta-analysis. Journal of Dentistry 2015, 43, 765–776. [Google Scholar] [CrossRef]
- Takamizawa, T.; Barkmeier, W.W.; Tsujimoto, A.; Berry, T.P.; Watanabe, H.; Erickson, R.L.; Latta, M.A.; Miyazaki, M. Influence of different etching modes on bond strength and fatigue strength to dentin using universal adhesive systems. Dent Mater 2016, 32, e9–21. [Google Scholar] [CrossRef]
- Yamauchi, K.; Tsujimoto, A.; Jurado, C.A.; Shimatani, Y.; Nagura, Y.; Takamizawa, T.; Barkmeier, W.W.; Latta, M.A.; Miyazaki, M. Etch-and-rinse vs self-etch mode for dentin bonding effectiveness of universal adhesives. J Oral Sci 2019, 61, 549–553. [Google Scholar] [CrossRef]
- Chowdhury, A.; Islam, R.; Alam, A.; Matsumoto, M.; Yamauti, M.; Carvalho, R.M.; Sano, H. Variable Smear Layer and Adhesive Application: The Pursuit of Clinical Relevance in Bond Strength Testing. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef]
- Kambara, K.; Nakajima, M.; Hosaka, K.; Takahashi, M.; Thanatvarakorn, O.; Ichinose, S.; Foxton, R.M.; Tagami, J. Effect of smear layer treatment on dentin bond of self-adhesive cements. Dent Mater J 2012, 31, 980–987. [Google Scholar] [CrossRef]
- Saikaew, P.; Sattabanasuk, V.; Harnirattisai, C.; Chowdhury, A.; Carvalho, R.; Sano, H. Role of the smear layer in adhesive dentistry and the clinical applications to improve bonding performance. Jpn Dent Sci Rev 2022, 58, 59–66. [Google Scholar] [CrossRef]
- Yoshida, Y.; Nagakane, K.; Fukuda, R.; Nakayama, Y.; Okazaki, M.; Shintani, H.; Inoue, S.; Tagawa, Y.; Suzuki, K.; De Munck, J.; et al. Comparative study on adhesive performance of functional monomers. J Dent Res 2004, 83, 454–458. [Google Scholar] [CrossRef]
- Jin, X.; Han, F.; Wang, Q.; Yuan, X.; Zhou, Q.; Xie, H.; Niu, L.; Chen, C. The roles of 10-methacryloyloxydecyl dihydrogen phosphate and its calcium salt in preserving the adhesive–dentin hybrid layer. Dental Materials 2022, 38, 1194–1205. [Google Scholar] [CrossRef]
- Fronza, B.M.; Braga, R.R.; Cadenaro, M. Dental Adhesives-Surface Modifications of Dentin Structure for Stable Bonding. Dent Clin North Am 2022, 66, 503–515. [Google Scholar] [CrossRef]
- Kiuru, O.; Sinervo, J.; Vähänikkilä, H.; Anttonen, V.; Tjäderhane, L. MMP Inhibitors and Dentin Bonding: Systematic Review and Meta-Analysis. Int J Dent 2021, 2021, 9949699. [Google Scholar] [CrossRef]
- Montagner, A.F.; Sarkis-Onofre, R.; Pereira-Cenci, T.; Cenci, M.S. MMP Inhibitors on Dentin Stability: A Systematic Review and Meta-analysis. J Dent Res 2014, 93, 733–743. [Google Scholar] [CrossRef]
- Tian, F.; Zhou, L.; Zhang, Z.; Niu, L.; Zhang, L.; Chen, C.; Zhou, J.; Yang, H.; Wang, X.; Fu, B.; et al. Paucity of Nanolayering in Resin-Dentin Interfaces of MDP-based Adhesives. J Dent Res 2016, 95, 380–387. [Google Scholar] [CrossRef]
- Torres, C.R.; Zanatta, R.F.; Silva, T.J.; Huhtala, M.F.; Borges, A.B. Influence of previous acid etching on bond strength of universal adhesives to enamel and dentin. Gen Dent 2017, 65, e17–e21. [Google Scholar]
- Grötz, K.A.; Duschner, H.; Kutzner, J.; Thelen, M.; Wagner, W. [Histotomography studies of direct radiogenic dental enamel changes]. Mund Kiefer Gesichtschir 1998, 2, 85–90. [Google Scholar] [CrossRef]
- Kielbassa, A.M.; Hinkelbein, W.; Hellwig, E.; Meyer-Lückel, H. Radiation-related damage to dentition. The Lancet Oncology 2006, 7, 326–335. [Google Scholar] [CrossRef]
- Lieshout, H.F.J.; Bots, C.P. The effect of radiotherapy on dental hard tissue—a systematic review. Clinical Oral Investigations 2014, 18, 17–24. [Google Scholar] [CrossRef]
- Martini, G.R.; Bortoluzzi, E.A.; Minamisako, M.C.; Bordignon, N.C.T.; Rodrigues, P.M.; Gondak, R. Impact of radiotherapy on the morphological and compositional structure of intra-radicular dentin. Braz Dent J 2023, 34, 45–51. [Google Scholar] [CrossRef]
- Al-Nawas, B.; Grötz, K.; Rose, E.; Duschner, H.; Kann, P.; Wagner, W. Using ultrasound transmission velocity to analyse the mechanical properties of teeth after in vitro, in situ, and in vivo irradiation. Clinical oral investigations 2000, 4, 168–172. [Google Scholar] [CrossRef]
- Silva, A.; Alves, F.; Antunes, A.; Goes, M.; Lopes, M. Patterns of demineralization and dentin reactions in radiation-related caries. Caries research 2009, 43, 43–49. [Google Scholar] [CrossRef]
- Siripamitdul, P.; Sivavong, P.; Osathanon, T.; Pianmee, C.; Sangsawatpong, W.; Bunsong, C.; Nantanapiboon, D. The Effects of Radiotherapy on Microhardness and Mineral Composition of Tooth Structures. Eur J Dent 2023, 17, 357–364. [Google Scholar] [CrossRef] [PubMed]
- McGuire, J.D.; Gorski, J.P.; Dusevich, V.; Wang, Y.; Walker, M.P. Type IV collagen is a novel DEJ biomarker that is reduced by radiotherapy. J Dent Res 2014, 93, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- McGuire, J.D.; Mousa, A.A.; Zhang, B.J.; Todoki, L.S.; Huffman, N.T.; Chandrababu, K.B.; Moradian-Oldak, J.; Keightley, A.; Wang, Y.; Walker, M.P.; et al. Extracts of irradiated mature human tooth crowns contain MMP-20 protein and activity. J Dent 2014, 42, 626–635. [Google Scholar] [CrossRef]
- Lu, Y.; Papagerakis, P.; Yamakoshi, Y.; Hu, J.C.; Bartlett, J.D.; Simmer, J.P. Functions of KLK4 and MMP-20 in dental enamel formation. Biol Chem 2008, 389, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Arid, J.; Palma-Dibb, R.G.; de Oliveira, H.F.; Nelson-Filho, P.; de Carvalho, F.K.; da Silva, L.A.B.; de Siqueira Mellara, T.; da Silva, R.A.B.; Faraoni, J.J.; de Queiroz, A.M. Radiotherapy impairs adhesive bonding in permanent teeth. Support Care Cancer 2020, 28, 239–247. [Google Scholar] [CrossRef]
- Galetti, R.; Santos-Silva, A.R.; Nogueira da Gama Antunes, A.; de Abreu Alves, F.; Lopes, M.A.; de Goes, M.F. Radiotherapy does not impair dentin adhesive properties in head and neck cancer patients. Clinical Oral Investigations 2014, 18, 1771–1778. [Google Scholar] [CrossRef]
- de Barros da Cunha, S.R.; Minorin Mendes Ramos, P.A.; Kalil Haddad, C.M.; Fernandes da Silva, J.L.; Fregnani, E.R.; Corrêa Aranha, A.C. Effects of Different Radiation Doses on the Bond Strengths of Two Different Adhesive Systems to Enamel and Dentin. Journal of Adhesive Dentistry 2016, 18. [Google Scholar] [CrossRef]
- Gernhardt, C.; Kielbassa, A.; Hahn, P.; Schaller, H.G. Tensile bond strengths of four different dentin adhesives on irradiated and non-irradiated human dentin in vitro. Journal of Oral Rehabilitation 2001, 28, 814–820. [Google Scholar] [CrossRef]
- Bernard, C.; Villat, C.; Abouelleil, H.; Gustin, M.P.; Grosgogeat, B. Tensile Bond Strengths of Two Adhesives on Irradiated and Nonirradiated Human Dentin. Biomed Res Int 2015, 2015, 798972. [Google Scholar] [CrossRef]
- Rodrigues, R.B.; Soares, C.J.; Junior, P.C.S.; Lara, V.C.; Arana-Chavez, V.E.; Novais, V.R. Influence of radiotherapy on the dentin properties and bond strength. Clin Oral Investig 2018, 22, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.A.; Garin-Correa, C.; Gonzalez-Arriagada, W.; Quintela Davila, X.; Häberle, P.; Bedran-Russo, A.; Luque-Martinez, I. The adverse effects of radiotherapy on the structure of dental hard tissues and longevity of dental restoration. International journal of radiation biology 2020, 96, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Naves, L.Z.; Novais, V.R.; Armstrong, S.R.; Correr-Sobrinho, L.; Soares, C.J. Effect of gamma radiation on bonding to human enamel and dentin. Supportive Care in Cancer 2012, 20, 2873–2878. [Google Scholar] [CrossRef] [PubMed]
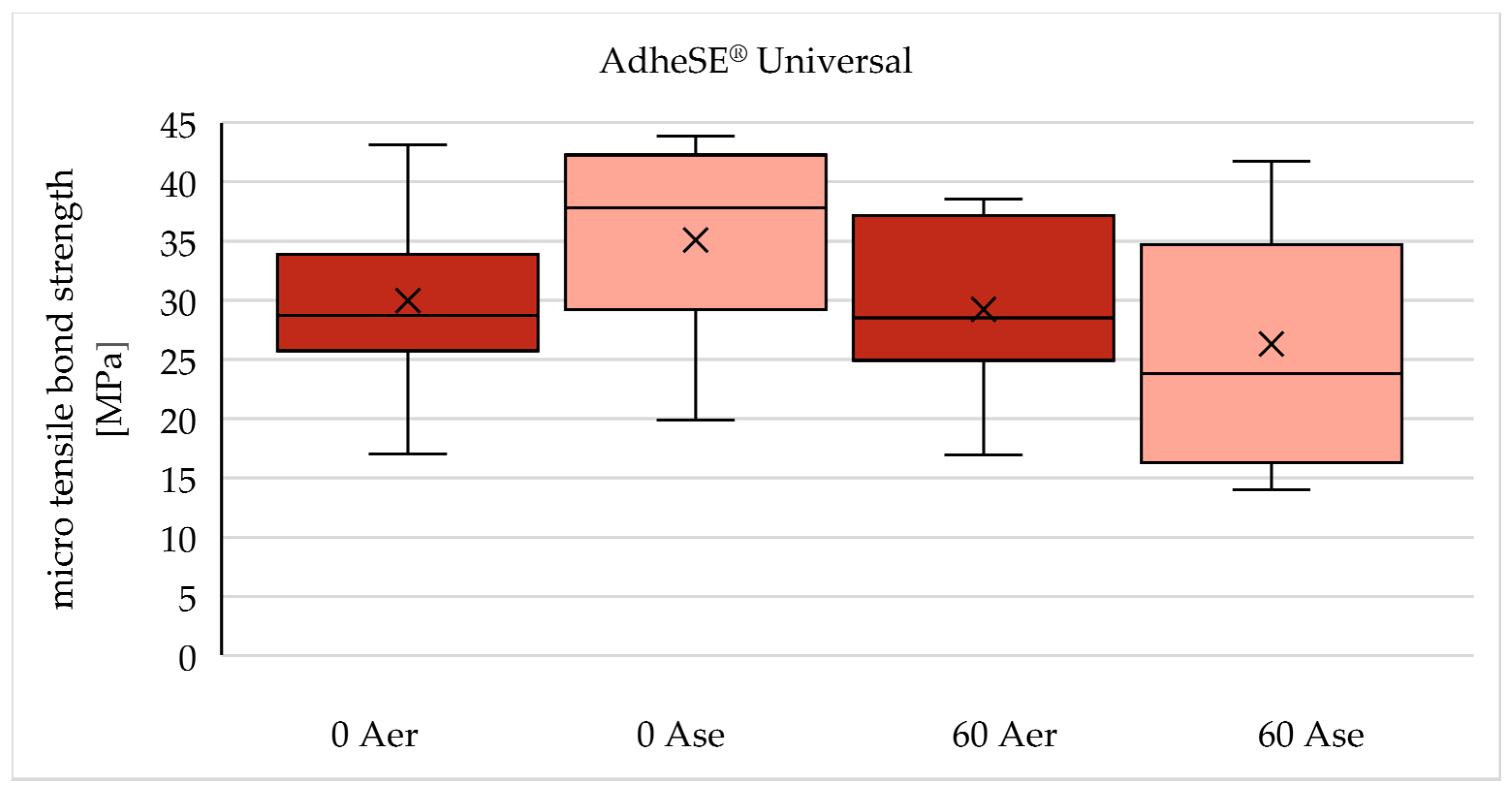
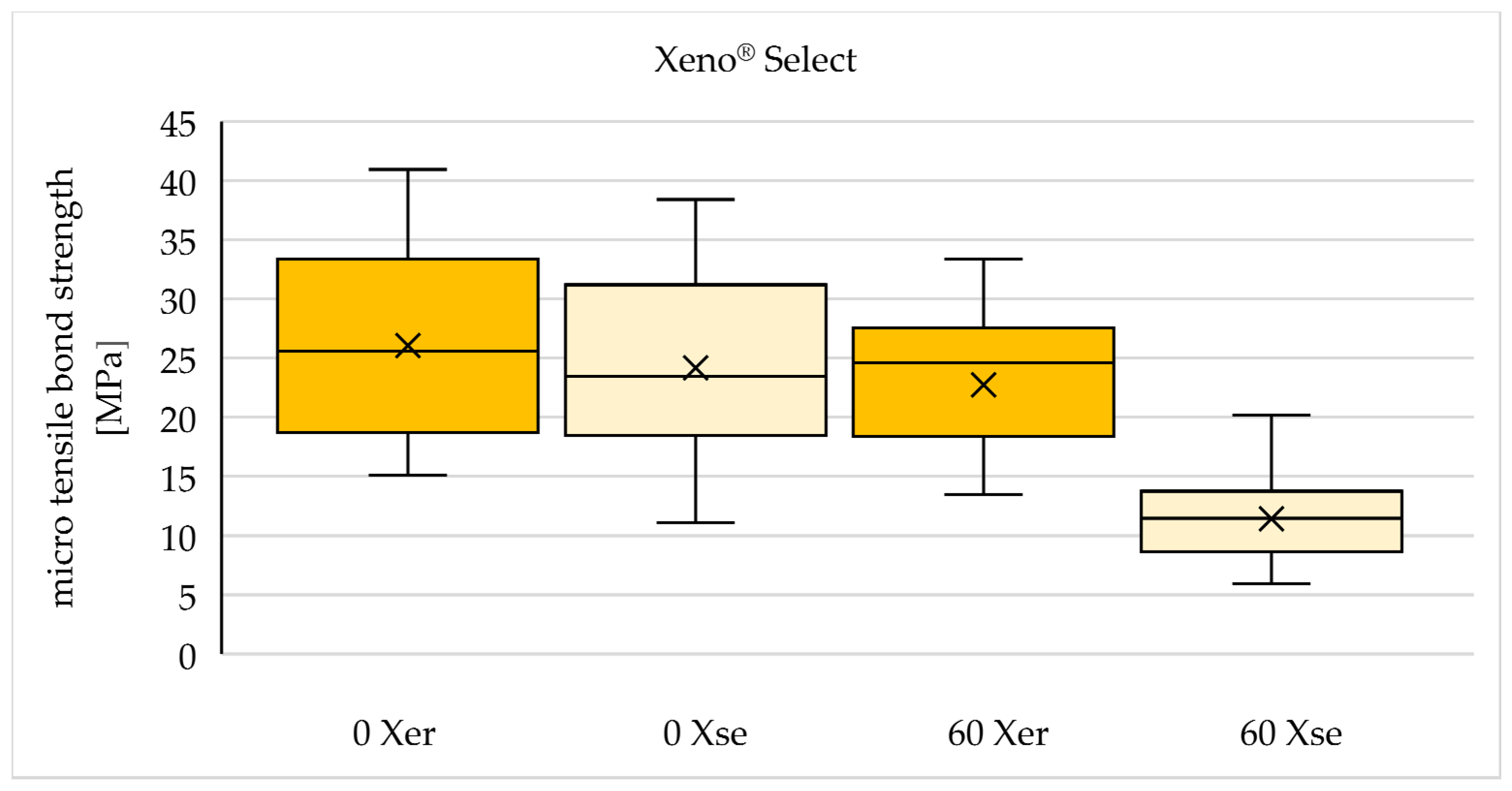
| 0 Gy (n=90) | 60 Gy (n=90) | |
|---|---|---|
| Futurabond® U | 0 Fer | 60 Fer |
| 0 Fse | 60 Fse | |
| AdheSE® Universal | 0 Aer | 60 Aer |
| 0 Ase | 60 Ase | |
| Xeno® Select | 0 Xer | 60 Xer |
| 0 Xse | 60 Xse |
| Material | pH | Main components* | Manufacturer |
| Futurabond® U | 2,3 | BIS-GMA1, HEMA2, HEDMA3, acidic adhesive monomer, UDMA4, 10-MDP5, catalyst | VOCO GmbH, Cuxhaven, Liechtenstein, Germany |
| AdheSE® Universal | 2,5-3 | BIS-GMA1, HEMA2, ethanol, 10-MDP5, D3MA6 , MCAP7, camphorquinone | Ivoclar Vivadent AG, Schaan, Liechtenstein |
| Xeno® Select | < 2 | bifunctional acrylates, tert-butyl alcohol, functionalized phosphoric acid ester (ethyl 2-[5-dihydrogen phosphoryl-5,2-dioxapentyl]acrylate), acidic acrylates, 4-dimethylaminobenzonitrile | Dentsply Sirona, Konstanz, Germany |
| Total Etch | 37% Phosphoric Acid | Ivoclar Vivadent AG, Schaan, Liechtenstein | |
| GrandioSO®, Color A2 | Monomers: BIS-GMA1, BIS-EMA8, TEGDMA9Fillers: glass ceramic, functionalized SiO210 nanoparticles | VOCO GmbH, Cuxhaven, Germany |
| Test group | 0 Gy | 60 Gy | Decrease of tensile bond strength after irradiation |
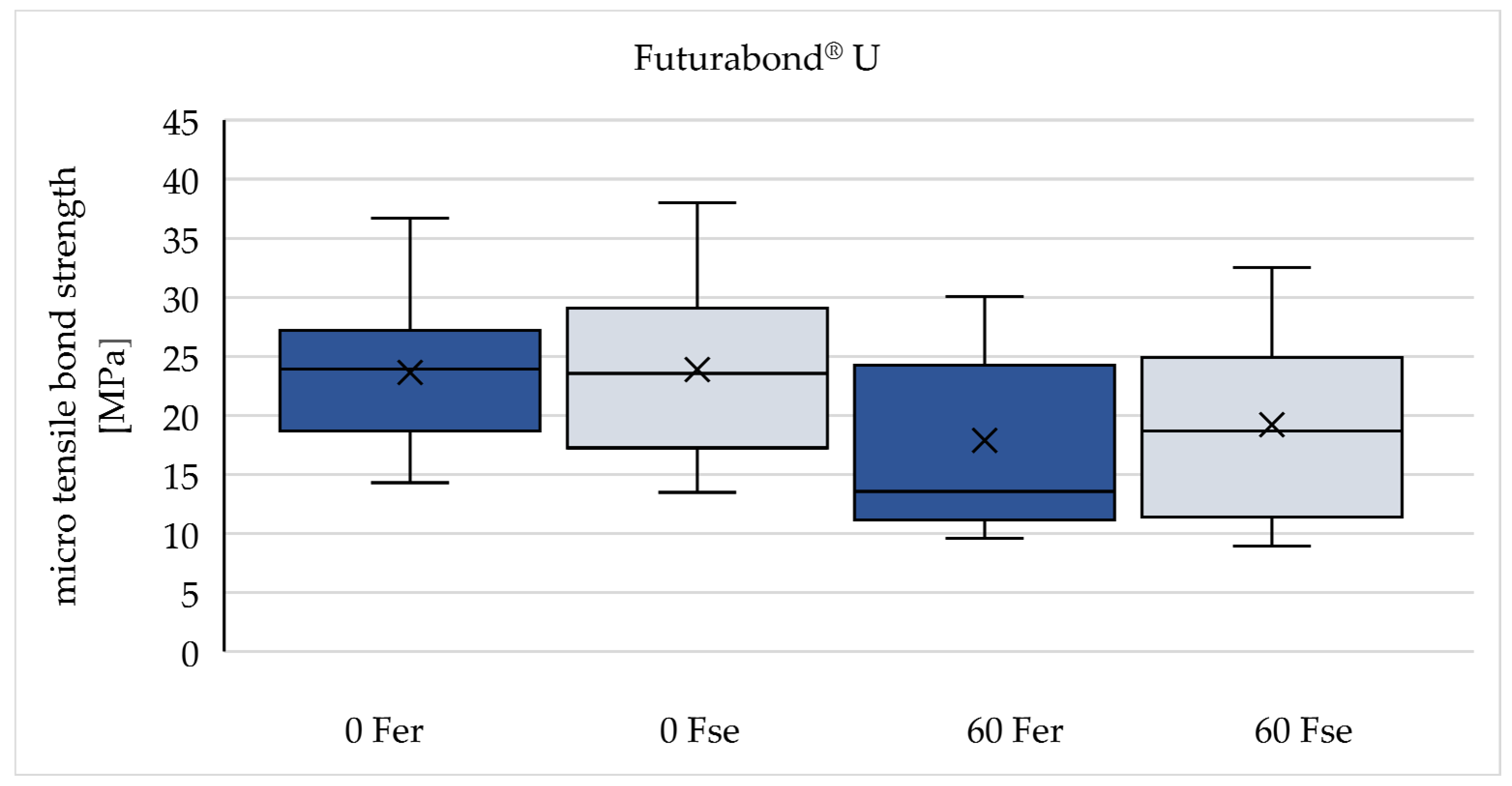
| F er | 23,64 (± 6,76) | 17,88 (± 7,54) | -24,4 % |
| F se | 23,87 (± 7,49) | 19,21 (± 7,34) | -19,5 % |
| A er | 29,97 (± 7,18) | 29,24 (± 7,28) | -2,4 % |
| A se | 35,10 (± 8,41) | 26,30 (± 10,07) | -25,1 % |
| X er | 26,06 (± 8,20) | 22,74 (± 6,22) | -12,7 % |
| X se | 24,17 (± 8,36) | 11,42 (± 3,86) | -52,8 % |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





