1. Introduction
The idiopathic inflammatory myopathies (IIM) represent a heterogeneous group of distinct autoimmune conditions jointly termed myositis. Subclassification of patients is a necessary effort to develop appropriate disease management and for disease prognosis. Methodologies have been developed for accurate classification, yet they continue to evolve and debate persists over definitions and validation of diagnostic criteria. Since the subgroups of polymyositis (PM) and dermatomyositis (DM) were first described based upon clinical and myopathological criteria [
1,
2], deepened understanding of IIM pathophysiology and heterogeneity led to further inclusion of in-depth diagnostic imaging and laboratory testing. Autoantibody profiles and muscle magnetic resonance imaging (MRI) have been incorporated successfully in the diagnostic arsenal [
3]. The distinct subgroup of sporadic inclusion body myositis (IBM) was recognized, characterized by specific clinical features and presence of endomysial auto-aggressive inflammation and muscle fiber vacuoles and amyloid deposits [
4], and frequent presence of anti-cytosolic 5’–nucleotidase 1A (CN1A) autoantibodies [
5]. The subgroup of immune-mediated necrotizing myopathy (IMNM) has also been recognized and is characterized by muscle necrosis predominating over inflammation in the diagnostic biopsies [
6], and association with anti-signal recognition particle (SRP) or anti-3-hydroxy-3-methylglutaryl-coA reductase (HMGCR) autoantibodies in part of the patients [
7]. Autoantibodies directed against aminoacyl tRNA synthetases reveal myositis as part of the antisynthetase syndrome (ASS), a subgroup of patients who frequently suffer from interstitial lung disease (ILD) [
8]. Myositis may also occur in overlap with other connective tissue diseases.
Conclusive diagnosis of the IIM may require specialized and elaborate clinical, genetic, histological and biochemical evaluation, and for many patients means taking a diagnostic muscle biopsy as a necessary yet invasive and time-consuming effort for which standardized diagnostic procedures have been proposed [
9]. Further implementation of blood-based disease biomarkers therefore represents a convenient alternative approach with the potential to further reduce the need for diagnostic muscle biopsies in the myositis patient population. This is a very plausible approach, as a blood sample is routinely taken from patients for measurement of skeletal muscle markers (including the inevitable creatine kinase) and autoantibody typing, the latter already an established part of the diagnostic process. This study focusses on two stress-related proteins and their biomarker potential for identifying and subtyping the IIM. C-X-C chemokine ligand 10 (CXCL10), also known as interferon γ-induced protein 10 (IP-10) is a chemokine with a pathogenic role in autoimmune diseases that features among the main myokines involved in the pathogenesis and progression of myositis [
10]. Damaged muscle expresses higher levels of CXCL10, yet the chemokine is dispensable for effective muscle regeneration [
11]. A strong association of CXCL10 with the IIM has been known for two decades, with documented expression in skeletal muscle [
12,
13,
14] and elevation of circulating levels in the blood [
15,
16,
17,
18]. Growth differentiation factor 15 (GDF15) is a transforming growth factor β superfamily cytokine implicated in age-related disorders, inflammation and cognitive decline [
19]. Elevated GDF15 was only recently described in IIM [
20,
21], with GDF15 levels associated to an increased risk of myocardial injury [
22].
In this study, we explore the potential of CXCL10 and GDF15 evaluation in patient sera for diagnosing and subdividing the IIM.
2. Materials and Methods
2.1. Subjects and materials
This retrospective study included sera and muscle biopsies from an established cohort of 45 adult IIM patients with confirmed clinical and myopathological diagnosis of IMNM (n=21), IBM (n=18), PM in overlap with other autoimmune diseases (n=3), DM (n=2) and ASS (n=1) (
Table 1).
Control materials were commercially obtained samples from healthy subjects (Zenbio, Durham, NC) and sera from patients with hereditary muscle disease that were diagnosed in our hospital (supplementary
Table S1). Sampling adhered to ethical and privacy regulations.
2.2. Quantification of serum CXCL10 and GDF15 levels
Enzyme-linked immunosorbent assays were performed with human GDF15 (DGD150) and CXCL10 (DIP100) Quantikine ELISA kits from R&D Systems (Bio-Techne, Abingdon, UK) according to the manufacturer’s specifications. Based upon preliminary experiments, optimal dilutions were determined (1/10 and 1/20 for control, 1/10 and 1/50 for patient sera). Sera were loaded onto 96-well plates in duplicate. Values were calculated as the mean of duplicates and the two dilutions tested, and reported as mean±SD. Shapiro-Wilk test determined that variables were not normally distributed, hence the Kruskal Wallis test by ranks for multiple groups of independent values was used, comparing values pairwise between groups. Asymptotic significance values in 2-sided tests were adjusted by Bonferroni correction for multiple tests, with mean differences considered significant from the 0.05 level. Bivariate Pearson’s correlation tests were performed to evaluate correlations between variables. Receiver operating characteristics (ROC) analysis was used to compare diagnostic performances, and graphic representation with area under the curve (AUC) measured separability. All analyses were done with SPSS software version 28 (IBM, New York, NY).
2.3. Immunofluorescence, immunohistochemistry and histochemistry
Immunostaining was performed on six µm frozen muscle sections, first treated with blocking solution containing 5% donkey serum, 10% heat-inactivated human serum and 2% bovine serum albumin in phosphate buffered saline. Immunofluorescent immunolocalization of GDF15 was carried out with 4µg/ml of mouse monoclonal IgG2a anti-GDF15 (clone H-2; Santa Cruz Biotechnology, Santa Cruz, CA), combined with 0.7µg/ml rabbit polyclonal anti-CD68 (H-255; Agilent, Santa Clara, CA) or 1µg/ml rabbit polyclonal anti-CD56 (Fisher Scientific, Waltham, MA) or 1.25 µg/ml rabbit polyclonal anti-LC3B (ab48394, Abcam, Cambridge, United Kingdom), and incubated for 2h at room temperature. Secondary antibodies were used labeled with CY3 (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and AlexaFluor488 (Invitrogen, Carlsbad, CA) and slides were mounted with Fluoromount (Southern Biotech, Birmingham, AL). Serial sections were immunostained with mouse monoclonal IgG2a anti-CXCL10 (4D5; Biorad Laboratories, Temse, Belgium), 4µg/ml mouse monoclonal IgG1 anti-CD68 (KP1, Abcam, Trumpington Cambridge, United Kingdom), and 1.3µg/ml mouse monoclonal IgG1 anti-SQSTM1 (BD Biosciences, San Jose, CA) for 1h (or 2h for anti-CXCL10) at room temperature. Sections were stained with Envision anti-mouse and DAB substrate (Agilent) according to the manufacturer’s specifications, and mounted with aquatex (Merck Life Science, Hoeilaart, Belgium). Muscle tissues were imaged and recorded with a light/fluorescence microscope (Zeiss, Goettingen, Germany) and analyzed with CellF software (Olympus, Antwerp, Belgium). In a selection of patient biopsies, muscle histology and inflammation was evaluated in hematoxylin and eosin (H&E)-stained sections using standard histological procedures, and scored absent (0), intermediate (1) to severe (2) by an experienced myopathologist.
3. Results
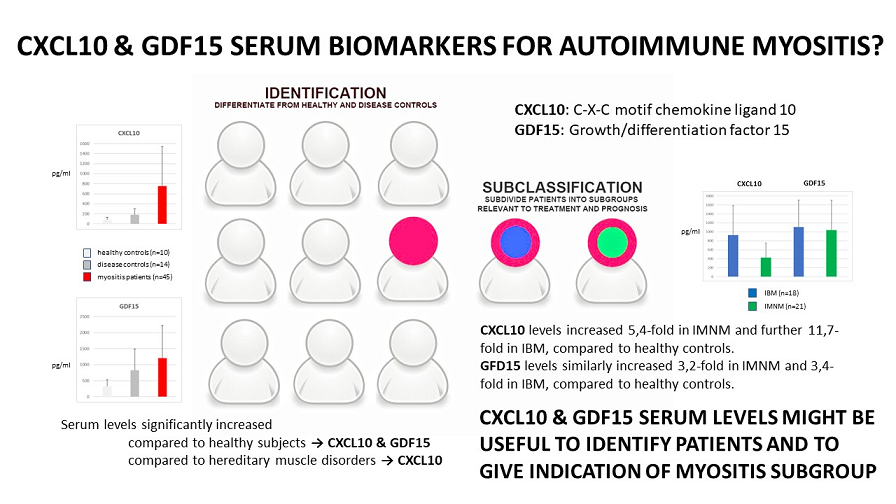
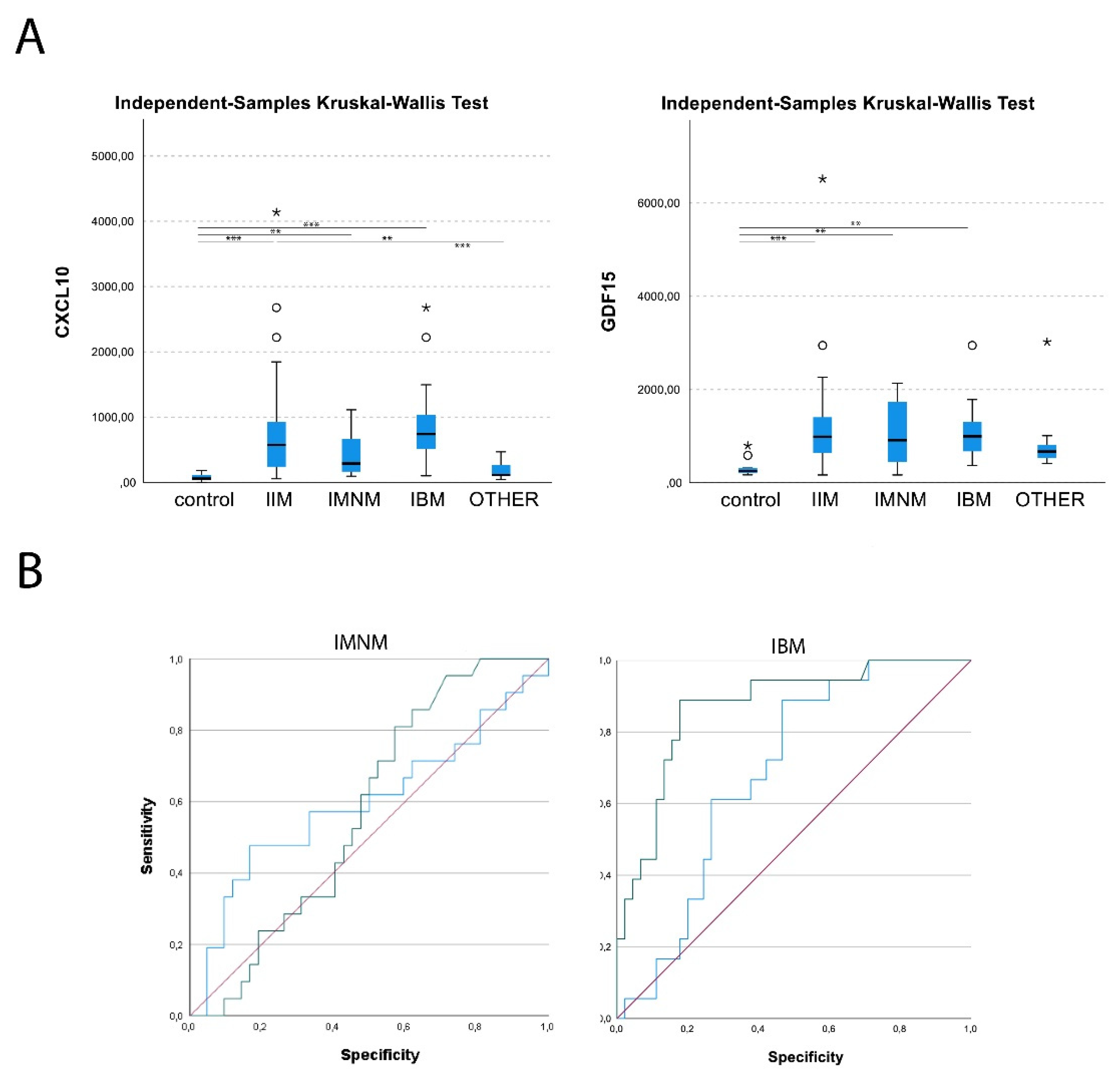
3.1. Increased CXCL10 and GDF15 levels in IIM sera
In individual patients and controls, levels of CXCL10 and GDF15 were determined in the same serum sample (supplementary
Table S2). Statistical analysis was done with Kruskal Wallis one-way analysis of variance with Bonferroni correction for multiple tests (
Figure 1A), and ROC analysis compared diagnostic performance (
Figure 1B).
Mean circulating levels of CXCL10 were 79±53 pg/ml for healthy controls, 180±123 pg/ml for patients with hereditary muscle disorders and 755±783 pg/ml for IIM patients. In IMNM, values were increased 5.4-fold compared to healthy controls, and 2.4-fold compared to disease controls. In IBM, CXCL10 levels were increased further 11.7-fold compared to healthy and 5.2-fold compared to disease controls. Only weak correlations between CXCL10 serum levels and clinical characteristics could be observed (supplementary
Table S3), yet at times in different directions. Weak negative correlation with BMI was observed in IIM, while in hereditary muscle disorders weak positive correlation was found (r=0.22). Weak positive correlation with cardiac disease was observed in hereditary muscle disorders (r=0.20), while weak negative correlation was present in IIM and IBM (r=-0.24).
Mean circulating GDF15 levels were 326±204 pg/ml for healthy controls, 831±656 pg/ml for patients with hereditary muscle disorders and 1201±1017 pg/ml for IIM patients. Values were comparably increased in subgroups to 3.2-fold (IMNM) and 3.4-fold (IBM) compared to healthy controls, and 1.3-fold compared to disease controls. GDF15 levels were moderately correlated with age at sampling in IMNM and OTHER (r=0.53) (supplementary
Table S3). When the IIM were combined, the correlation with age was only weak (r=0.26). In IMNM, a weak correlation of GDF15 with blood CK values was noted (r=0.22). Weak correlation was observed with cardiac disease in the IIM (r=0.27) and its subgroup of IBM (r=0.36).
Levels of CXCL10 and GDF15 were not correlated in any of the sera from all diagnostic groups. ROC analysis found AUCs for CXCL10 were 0.573 for IMNM and 0.870 for IBM, and 0.879 for the whole group of IIM. With the threshold set to 180 pg/ml of CXCL10, myositis patients could be differentiated from healthy and disease controls with a sensitivity of 0.80 and a specificity of 0.71. For GDF15, AUC were 0.596 for IMNM and 0.688 for IBM, and 0.772 in IIM combined.
3.2. Localization of CXCL10 to muscle fibers and actively invading inflammatory cells
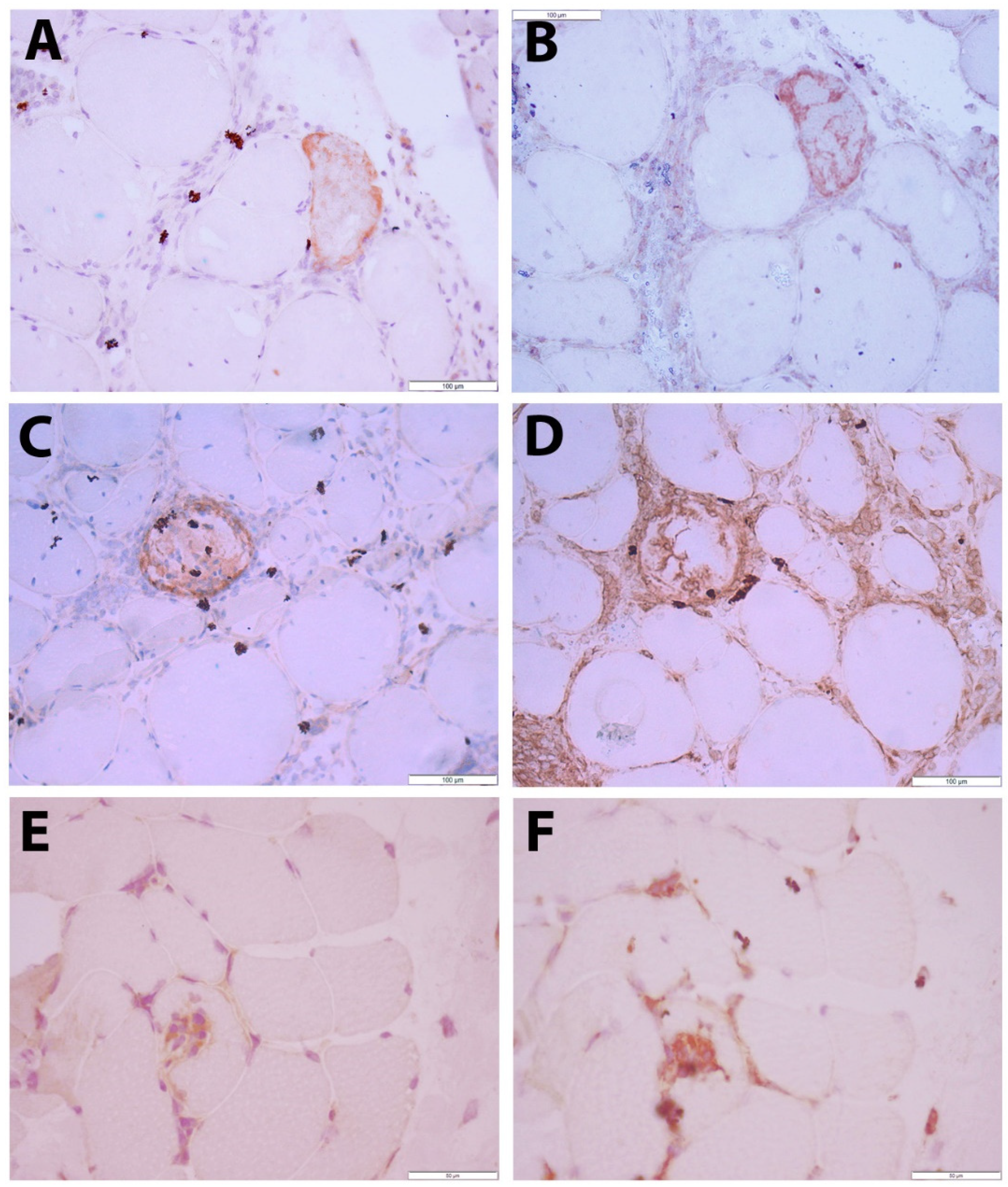
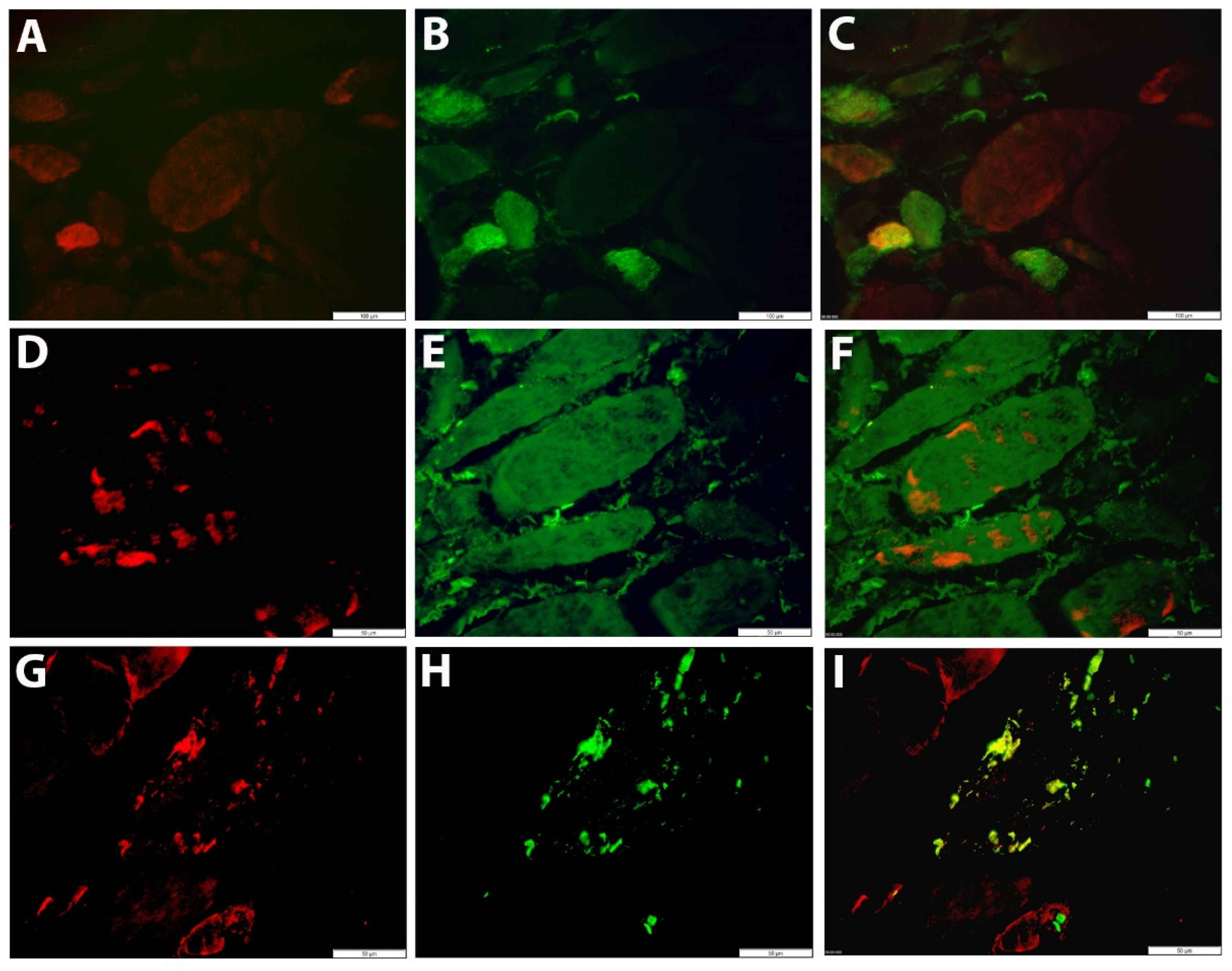
To allow evaluation of CXCL10 expression alongside pathological changes to the muscle tissue, immunohistochemical staining was performed in sequential muscle sections. Muscle biopsies with normal histology were largely CXCL10 negative. In contrast, subsets of small muscle fibers in IIM tissues displayed granular staining pattern in necrotic muscle fibers and in SQSTM1 positive muscle fibers (
Figure 2A–D).
The pattern of myopathological changes differed between IMNM and IBM patients (supplementary
Table S4). IMNM was associated with muscle fiber necrosis and less severe inflammatory damage, while IBM was strongly associated with endomysial buildup of inflammation and active invasion of non-necrotic muscle fibers by auto-aggressive immune cells. In IBM tissues, a subset of inflammatory cells was CXCL10 positive, notably immune cells invading non-necrotic muscle fibers of which most were CD68 positive (
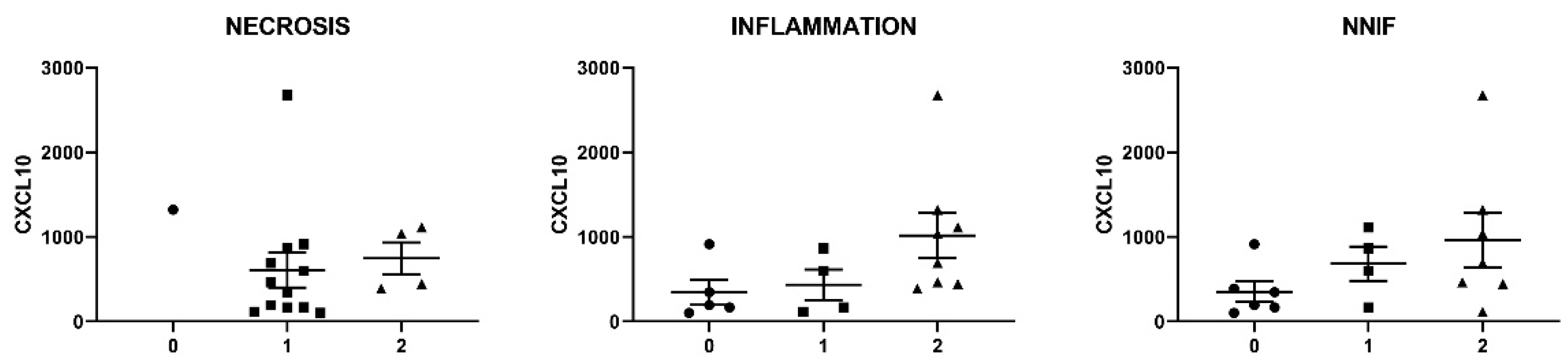
Figure 2E,F). Severity of inflammatory changes in individual IIM patients tended to associate with circulating levels of CXCL10 (
Figure 3), though no significance was shown in this smaller patient sample.
3.3. Co-localization of GDF15 with markers of autophagy and regeneration in muscle fibers
The low constitutive sarcoplasmic GDF15 staining observed in healthy controls was notably increased in IIM muscle biopsies, mostly in small regenerating muscle fibers (
Figure 4A–C). A granular staining pattern was observed in other subsets of muscle fibers, co-localizing with autophagic markers (
Figure 4D–I). The vast majority of inflammatory cells were GDF15 negative (data not shown).
4. Discussion
Subtyping of IIM is a necessary effort to design treatment strategies suited to the individual patient. While subgroups of patients react well to standard immunosuppressive therapies, others might require alternative immunomodulatory strategies. In IMNM, autoantibody status aids as an indicator whether the response to different treatment regimen would be favorable [
23]. IBM is largely unresponsive to current immunomodulatory treatment. In addition to subclassification, it is imperative to differentiate IIM from muscular dystrophies to avoid inappropriate treatment with glucocorticoids in the latter. Circulating biomarkers have been in use for diagnosing myositis for decades, with blood samples routinely taken to evaluate CK and other muscle enzymes. However, this strategy has certainly not yet been developed to its full potential. In this respect, implementing the analysis of the expression of key pathogenic factors in patient sera is an attractive prospect. A good choice would be to analyze myokines, i.e. cytokines and other proteins produced and released by muscle cells which enable the skeletal muscle tissue to communicate with the body’s other organs, as indicators of muscle dysfunction [
24].
Circulating CXCL10 has already been described a reliable and sensitive biomarker for IIM subgroups. In a study of 125 patients diagnosed with juvenile DM, serum CXCL10 levels displayed 0.87 sensitivity and 1.00 specificity for active disease [
17]. Our current study confirmed the association with IIM, and indicates higher levels in the subgroup of IBM in comparison to IMNM. Though CXCL10 is present in muscle fibers and subset of inflammatory cells, it remains enigmatic if the muscle tissue is an important source of the chemokine, or if intramuscular inflammation is more a consequence of systemic CXCL10 expression. CXCL10 elevation as an indicator of muscle disease severity goes beyond the IIM. In systemic sclerosis also, serum CXCL10 levels strongly correlate with clinical severity of muscle involvement and with CK serum concentration, suggesting a potential mechanistic involvement in muscle damage [
25].
No single diagnostic feature can differentiate IIM, let alone reliably subtype the different subgroups. A threshold of 180 pg/ml of CXCL10 differentiates myositis patients from healthy and disease controls with a sensitivity of 0.80 and a specificity of 0.71. Importantly, we showed that CXCL10 levels aid to differentiate IIM from hereditary muscle disorders, the latter often display secondary inflammatory changes that can be confused with myositis. We found CXCL10 levels in hereditary muscle disorders to be no different than in healthy controls, however, another study has reported CXCL10 to be significantly elevated in serum and muscle samples of DMD patients, relative to age-matched healthy controls [
26]. We speculate that adding CXCL10 to the diagnostic toolkit might be useful, but might not be able to boost diagnostic performance sufficiently. We propose circulating CXCL10 could, however, be part of a bigger strategy for evaluating clever combinations of biomarkers. In this respect, our results appoint GDF15 consideration as an additional, more general marker for muscle disorders [
27]. GDF15 is currently explored as a biomarker in many disorders including cardiovascular disease [
28], cancer [
29] and mitochondrial myopathy [
30].
When considering novel circulating biomarkers, it is imperative to determine normal value variations in the healthy population. Many factor may influence serum levels, including gender, age and physical activity. It is known that the complex mixture of myokines secreted into the bloodstream varies during muscle contraction [
31]. In this respect, GDF15 and CXCL10 seem to be somewhat opposite poles. While GDF15 gene expression is induced in muscle tissues of mice when exercised [
32] and in response to oxidative stress [
33], in contrast, treadmill running significantly reduced CXCL10 gene expression in mice soleus muscle [
34]. Either way, circulating GDF15 and CXCL10 both appear regulated by physical activity. Nonetheless, CXCL10 levels have been observed to remain stable among healthy controls [
26], while GDF15 values appear more prone to changes in humans. In pregnant women, blood levels rise rapidly and stay high during the whole pregnancy[
35]. In addition, GDF15 levels associate with aging and tend to increase across the lifespan. Elevated GDF15 has been observed to correlate with reduced muscle strength and extremity function in older patients with cardiometabolic disease [
36] and to associate with lower muscle mass in men specifically [
37], the latter a further indication of sex differences. A limitation of our study is the age variation between diagnostic groups, with average ages of healthy controls (34±12) and patients with hereditary muscle disorders (45±13) substantially lower than of IMNM (65±9) and IBM (71±7) patients. In IMNM patients and the group of patients with hereditary muscle disorders we found a moderate correlation of GDF15 serum levels with age at sampling. An effort to determine values that can be used as reference ranges has been published recently [
38], with most notable increases in the aging population associated with heart disease and diabetes. Another characteristic described to associate with elevated circulating GDF15 levels is obesity [
39,
40]. In our IIM cohort, 51% of patients were overweight of which 13.3% were obese (defined by a BMI over 30), yet we did not find a correlation between BMI and serum GDF15 levels.
We propose our study may contribute to patient-friendly diagnostic innovation. Further minimization and multiplex immunoassays could allow expansion and analysis of combinations of biomakers. In this respect, blood spot analysis could be put forward as a convenient approach, as sampling can be done by nontrained persons and the material can be stored and transported at ambient temperature Studies evaluating spotted TNFα confirmed this methodology can detect cytokine concentrations commonly observed in patient samples, which range from 5 to 27pg/ml [
41]. For CXCL10, high correlation of blood spot analysis with serum levels (r=0.96) have already been described [
17]. Another innovation could be to attempt the least invasive sample collection available, which is to analyze a urine sample. The urine proteome as a possible source of biomarkers has been explored for the juvenile form of DM [
42]. In chronic kidney disease, urine GDF15 levels have already been shown predictors of mortality with an AUC of 0.95 [
43].
In addition to the diagnostic purposes of biomarker studies, serum biomarkers can be useful as follow-up therapeutic markers in clinical trials, with comparison of levels pre- and post-treatment as exploratory outcome measures in individual patients. Additionally, biomarker studies advance our understanding of pathogenic changes in IIM patients and may identify novel therapeutic targets. Targeted modulation of myokines involved in the immunopathological processes triggered by the immune system, aggravating or ameliorating inflammatory muscle disease, may become important therapeutic targets in their own right as an appropriate personalized therapeutic strategy [
10]. Myokines evolving from biomarkers to therapeutic targets have been proposed for cancer cachexia [
44].
5. Conclusions
Our study found significant elevation of serum CXCL10 and GDF15 levels in myositis patients. The skeletal muscle tissue is one of the possible sources, with localization to subsets of affected muscle fibers and inflammatory cells. CXCL10 expression was notably high in immune cells invading non-necrotic muscle fibers and correlated with muscle tissue inflammation grade. We propose circulating CXCL10 and GDF15 levels could be of aid to diagnose myositis. If our findings were to be confirmed, GDF15 could be developed into a more general biomarker for muscle disease and CXCL10 levels as an indicator toward IIM subtypes characterized by severe muscle inflammation and active invasion of muscle fibers by auto-aggressive immune cells. Further implementation of circulating biomarkers might reduce the need for taking a diagnostic muscle biopsy further, at least in part of the patients.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org, Table S1: Healthy and disease controls; Table S2: Quantification of circulating CXCL10 and GDF15 in human sera using enzyme-linked immuno sorbent assays; Table S3: Pearson’s correlation coefficients between variables; Table S4: Scoring myopathological changes in muscle biopsies from a selection of patients.
Author Contributions
Conceptualization, B.D.P.; methodology, B.D.P, K.B., J.D.B.; validation, B.D.P.; formal analysis, B.D.P.; investigation, B.D.P., J.D.B.; resources, K.B., J.D.B.; data curation, J.D.B.; writing—original draft preparation, B.D.P.; writing—review and editing, B.D.P., K.B., J.D.B.; visualization, B.D.P.; supervision, J.D.B.; project administration, B.D.P; funding acquisition, B.D.P., K.B., J.D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CSL Behring N.V., ‘Biomarker discovery for myositis’ grant number A23/TT/0156.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Ghent University Hospital, protocol codes B670201836756 (2018-0820) and B670201938779 (2019-0046), dates of approval November 16th 2018 and April 1st 2019.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Acknowledgments
The authors thank Katleen De Saedeleer and Sophie D’hose for expert technical assistance. Jan L. De Bleecker participates in Euro-NMD.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Bohan, A.; Peter, J.B. Polymyositis and dermatomyositis (first of two parts) N Engl J Med 1975, 292, 344-347. [CrossRef]
- Bohan, A.; Peter, J.B. Polymyositis and dermatomyositis (second of two parts) N Engl J Med 1975, 292, 403-407. [CrossRef]
- Targoff, I.N.; Miller, F.W.; Medsger, T.A. Jr; Oddis, C.V. Classification criteria for the idiopathic inflammatory myopathies. Curr Opin Rheumatol 1997, 9, 527-35. [CrossRef]
- Griggs, R.C.; Askanas, V.; DiMauro, S.; Engel, A.; Karpati, G.; Mendell, J.R.; Rowland, L.P. Inclusion body myositis and myopathies. Ann Neurol 1995, 38, 705-713. [CrossRef]
- Amlani, A.; Choi M.Y.; Tarnopolsky, M.; Brady L.; Clarke, A.E.; Garcia-De La Torre, I.; Mahler, M.; Schmeling, H.; Barber, C.E.; Jung M.; Fritzler, M.J. Anti-NT5c1A Autoantibodies as Biomarkers in Inclusion Body Myositis. Front Immunol 2019, 10, e745. [CrossRef]
- Argov, Z.; de Visser, M. Dysphagia in adult myopathies, Neuromuscul Disord 2021, 31, 1, 5-20. [CrossRef]
- Liu, M.; Lin, Y.; Qiao, L. Chen, J.; Shi, Q. Characteristics of cardiac involvement in immune-mediated necrotizing myopathy. Front Immunol 2023, 14, e1094611. [CrossRef]
- Wells, M.; Alawi, S.; Thin, K.Y.M.; Gunawardena, H.; Brown, A.R.; Edey, A.; Pauling; J.D.; Barratt, S.L.; Adamali, H.I. A multidisciplinary approach to the diagnosis of antisynthetase syndrome. Front Med 2022, 9, 959653. [CrossRef]
- De Bleecker, J.L.; De Paepe, B.; Aronica, E.; de Visser, M.; ENMC Myositis Muscle Biopsy Study Group; Amato. 205th ENMC International Workshop: Pathology diagnosis of idiopathic inflammatory myopathies part II 28-30 March 2014, Naarden, The Netherlands. Neuromuscul Disord 2015, 25, 268-272. [CrossRef]
- Wells, M;. Alawi, S.; Thin, K.Y.M.; Gunawardena, H.; Brown, A.R.; Edey, A.; Pauling, J.D.; Barratt, S.L.; Adamali, H.I. A multidisciplinary approach to the diagnosis of antisynthetase syndrome. Front Med 2022, 9, 959653. [CrossRef]
- Deyhle, M.R.; Hafen, P.S.; Parmley, J.; Preece, C.N.; Robison, M.; Sorensen, J.R.; Jackson, B.; Eggett, D.L.; Hancock, C.R.; Hyldahl, R.D. CXCL10 increases in human skeletal muscle following damage but is not necessary for muscle regeneration. Physiol Rep 2018, 6, e13689. [CrossRef]
- Raju, R.; Vasconcelos, O.; Granger, R.; Dalakas, M.C. Expression of IFN-γ-inducible chemokines in inclusion body myositis. J Neuroimmunol 2003, 141, 125-131. [CrossRef]
- De Paepe, B.; De Keyzer, K.; Martin, J.J.; De Bleecker, J.L. Alpha-chemokine receptors CXCR1–3 and their ligands in idiopathic inflammatory myopathies. Acta Neuropathol 2005, 109, 576–582. [CrossRef]
- Limongi, F. The CXCR3 chemokines in inflammatory myopathies. Clin Ter 2015, 166, e56-61. [CrossRef]
- Szodoray, P.; Philip Alex, P.; Nicholas Knowlton, N.; Centola, M.; Dozmorov, I.; Csipo, I.; Nagy, A.T.; Constantin, T.; Ponyi, A.; Nakken, B.; Danko, K. Idiopathic inflammatory myopathies, signified by distinctive peripheral cytokines, chemokines and the TNF family members B-cell activating factor and a proliferation inducing ligand, Rheumatology 2010, 49, 1867–1877. [CrossRef]
- Uruha, A.; Noguchi, S.; Sato, W.; Nishimura, H.; Mitsuhashi, S.; Yamamura, T.; Nishino, I. Plasma IP-10 level distinguishes inflammatory myopathy. Neurology 2015, 85, 293-294. [CrossRef]
- Wienke, J.; Bellutti Enders, F.; Lim, J.; Mertens, J.S.; van den Hoogen, L.L.; Wijngaarde, C.A.; Yeo, J.G.; Meyer, A.; Otten, H.G.; Fritsch-Stork, R.D.E.; Kamphuis, S.S.M.; Hoppenreijs, E.P.A.H.; Armbrust, W.; van den Berg, J.M.; Hissink Muller, P.C.E.; Tekstra, J.; Hoogendijk, J.E.; Deakin, C.T.; de Jager, W.; van Roon, J.A.G.; van der Pol, W.L.; Nistala, K.; Pilkington, C.; de Visser, M;, Arkachaisri, T.; Radstake, T.R.D.J.; van der Kooi, A.J.; Nierkens, S.; Wedderburn, L.R.; van Royen-Kerkhof, A.; van Wijk, F. Galectin-9 and CXCL10 as Biomarkers for Disease Activity in Juvenile Dermatomyositis: A Longitudinal Cohort Study and Multicohort Validation. Arthritis Rheumatol, 2019, 71, 1377-1390. [CrossRef]
- Zhou, J.; Zhao, L.; Xiao, Y.; Xie, S.; Long, Y.; Wei, Y.; Meng, Q.; Li, X.; Luo, H.; Zhu, H. The Expression of Cytokine Profiles and Related Receptors in Idiopathic Inflammatory Myopathies. Front Pharmacol 2022, 13, e852055. [CrossRef]
- Fuchs, T.; Trollor, J.N.; Crawford, J.; Baune, B.T.; Samaras, K.; Campbell, L.; Breit, S.N.; Brodaty, H.; Sachdev, P.; Smith, E.; Brown, D.A. Macrophage inhibitory cytokine-1 is associated with cognitive impairment and predicts cognitive decline – The Sydney Memory and Aging Study. Neurol Psychiatr Brain Res 2014, 20, 9. [CrossRef]
- De Paepe, B.; Verhamme, F.; De Bleecker, .JL. The myokine GDF-15 is a potential biomarker for myositis and associates with the protein aggregates of sporadic inclusion body myositis. Cytokine 2020, 127, e154966. [CrossRef]
- Oikawa Y, Izumi R, Koide M, Hagiwara Y, Kanzaki M, Suzuki N, Kikuchi, K; Matsuhashi, T.; Akiyama, Y.; Ichijo, M.; Watanabe, S.; Toyohara, T.; Suzuki, T.; Mishima, E.; Akiyama, Y.; Ogata, Y.; Suzuki, C.; Hayashi, H.; Kodama, E.N.; Hayashi, K.I.; Itoi, E.; Aoki, M.; Kure, S.; Abe, T. (2020) Mitochondrial dysfunction underlying sporadic inclusion body myositis is ameliorated by the mitochondrial homing drug MA-5. PLoS ONE 2020, 15, e0231064. [CrossRef]
- Qiu, M.; Sun, X.; Qi, X.; Liu, X.; Zhang, Y.; Zhang, N.; Lu, F.; Liu, W.; Changjing, F.; Wang, Q.; Zhou, L. The diagnostic value of GDF-15 for myocardial involvement in idiopathic inflammatory myopathy. Rheumatology 2021, 60, 2826-2833. [CrossRef]
- Weeding, E.; Tiniakou, E. Therapeutic Management of Immune-Mediated Necrotizing Myositis. Curr Treat Options in Rheum 2021, 7, 150–160. [CrossRef]
- Coelho-Junior, H.J.; Picca, A.; Calvani, R.; Uchida, M.C.; Marzetti, E. If my muscle could talk: Myokines as a biomarker of frailty. Exp Gerontol 2019, 127, e110715. [CrossRef]
- Corinaldesi, C.; Ross, R.L.; Abignano, G.; Antinozzi, C.; Marampon, F.; di Luigi, L.; Buch, M.H.; Riccieri, V.; Lenzi, A.; Crescioli, C.; et al. Muscle Damage in Systemic Sclerosis and CXCL10: The Potential Therapeutic Role of PDE5 Inhibition. Int J Mol Sci 2021, 22, 2894. [CrossRef]
- Ogundele, M.; Zhang, J.S.; Goswami, M.V.; Barbieri, M.L.; Dang, U.J.; Novak, J.S.; Hoffman, E.P.; Nagaraju, K.; CINRG-DNHS Investigators; Hathout, Y. Validation of Chemokine Biomarkers in Duchenne Muscular Dystrophy. Life 2021, 11, e827. [CrossRef]
- De Paepe, B. The Cytokine Growth Differentiation Factor-15 and Skeletal Muscle Health: Portrait of an Emerging Widely Applicable Disease Biomarker. Int J Mol Sci 2022, 23, 13180. [CrossRef]
- May, B.M.; Pimentel, M.; Zimerman, L.I.; Rohde, L.E. GDF-15 as a Biomarker in Cardiovascular Disease. Arq Bras Cardiol 2021, 116, 494-500. [CrossRef]
- Wang, Y.; Jiang, T.; Jiang, M.; Gu, S. Appraising growth differentiation factor 15 as a promising biomarker in digestive system tumors: A meta-analysis. BMC Cancer 2019, 19, e177. [CrossRef]
- Li, Y.; Li, S.;Qiu, Y.; Zhou, M.; Chen, M.; Hu, Y.; Hong, S.; Jiang, L.; Guo, Y. Circulating FGF21 and GDF15 as Biomarkers for Screening, Diagnosis, and Severity Assessment of Primary Mitochondrial Disorders in Children. Front Ped 2022, 10, e851534. [CrossRef]
- Pedersen, B.; Febbraio, M. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat Rev Endocrinol 2012, 8, 457–465. [CrossRef]
- Gil, C.I.; Ost, M.; Kasch, J.; Schumann, S.; Heider, S.; Klaus, S. Role of GDF15 in active lifestyle induced metabolic adaptations and acute exercise response in mice. Sci Rep 2019, 9, e20120. [CrossRef]
- Morrow, R.M.; Picard, M.; Derbeneva, O.; Leipzig, J.; McManus, M.J.; Gouspillou, G.; Barbat-Artigas, S.; Dos Santos, C.; Hepple, R.T.; Murdock, D.G.; Wallace, D.C. Mitochondrial energy deficiency leads to hyperproliferation of skeletal muscle mitochondria and enhanced insulin sensitivity. PNAS 2017, 114, 2705-27. [CrossRef]
- Ishiuchi, Y.; Sato, H.; Tsujimura, K.; Kawaguchi, H.; Matsuwaki, T.; Yamanouchi, K.; Nishihara, M.; Nedachi, T. Skeletal muscle cell contraction reduces a novel myokine, chemokine (C-X-C motif) ligand 10 (CXCL10): Potential roles in exercise-regulated angiogenesis. Biosci Biotech Biochem 2018, 82, 97–105. [CrossRef]
- Moore, A.G.; Brown, D.A.; Fairlie, W.D.; Bauskin, A.R.; Brown, P.K.; Munier, M.L.C.; Russell, P.K.; Salamonsen, L.A.; Wallace, E.M.; Breit, S.N. The Transforming Growth Factor-β Superfamily Cytokine Macrophage Inhibitory Cytokine-1 Is Present in High Concentrations in the Serum of Pregnant Women. J Clin Endocrinol Meta 2000, 85, 4781–4788. [CrossRef]
- Oba, K, Ishikawa, J, Tamura, Y, Fujita, Y.; Ito, M.; Iizuka, A.; Fujiwara, Y.; Kodera, R.; Toba, A.; Toyoshima, K.; Chiba, Y.; Mori, S.; Tanaka, M.; Ito, H.; Harada, K.; Araki, A. Serum growth differentiation factor 15 level is associated with muscle strength and lower extremity function in older patients with cardiometabolic disease. Geriatr.Gerontol. Int 2020, 20, 980–987. [CrossRef]
- Herpich, C.; Franz, K.; Ost, M.; Otten, L.; Coleman, V.; Klaus, S.; Müller-Werdan, U.; Norman, K. Associations Between Serum GDF15 Concentrations, Muscle Mass, and Strength Show Sex-Specific Differences in Older Hospital Patients. Rejuvenation Res 2021, 24, 14-19. [CrossRef]
- Welsh, P.; Kimenai, D.M.; Marioni, R.E.; Hayward, C.; Campbell, A.; Porteous, D.; Mills, N.L.; O’Rahilly, S.; Sattar, N. Reference ranges for GDF-15, and risk factors associated with GDF-15, in a large general population cohort. Clin Chem Lab Med 2022; 60, 1820-1829. [CrossRef]
- Vila, G.; Riedl, M.; Anderwald, C.; Resl, M.; Handisurya, A.; Clodi, M.; Prager, G.; Ludvik, B.; Krebs, M.; Luger, A. The relationship between insulin resistance and the cardiovascular biomarker growth differentiation factor-15 in obese patients. Clin Chem 2011, 57, 309-16. [CrossRef]
- Carballo-Casla, A.; García-Esquinas, E.; Buño-Soto, A.; Struijk, E.A.; López-García, E.; Rodríguez-Artalejo, F.; Ortolá, R. Metabolic syndrome and Growth Differentiation Factor 15 in older adults. Geroscience. 2022, 44, 867-880. [CrossRef]
- Badowski, M.; Darbouze, L.; Harris, D.T. Evaluation of biobanked blood spot cards to detect cytokines in blood, Cytother 2020, 22, S139-S140. [CrossRef]
- Morales, M.; Alayi, T.D.; Tawalbeh, S.M.; Sydenstricker, A.V.; Spathis, R.; Kim, H.; Nagaraju, K.; Hathout, Y.; Rider, L.G, Urine proteomics by mass spectrometry identifies proteins involved in key pathogenic pathways in patients with juvenile dermatomyositis, Rheumatology 2023, kead033. [CrossRef]
- Perez-Gomez, M.V.; Pizarro-Sanchez, S.; Gracia-Iguacel, C.; Cano, S.; Cannata-Ortiz, P.; Sanchez-Rodriguez, J.; Sanz, A.B.; Sanchez-Niño, M.D.; Ortiz, A. Urinary Growth Differentiation Factor-15 (GDF15) levels as a biomarker of adverse outcomes and biopsy findings in chronic kidney disease. J Nephrol 2021, 34, 1819-1832. [CrossRef]
- Manole, E.; Ceafalan, L.C.; Popescu, B.O.; Dumitru, C.; Bastian, A.E. Myokines as Possible Therapeutic Targets in Cancer Cachexia. J Immunol Res 2018, 22, e8260742. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).