Submitted:
06 September 2023
Posted:
07 September 2023
You are already at the latest version
Abstract
Keywords:
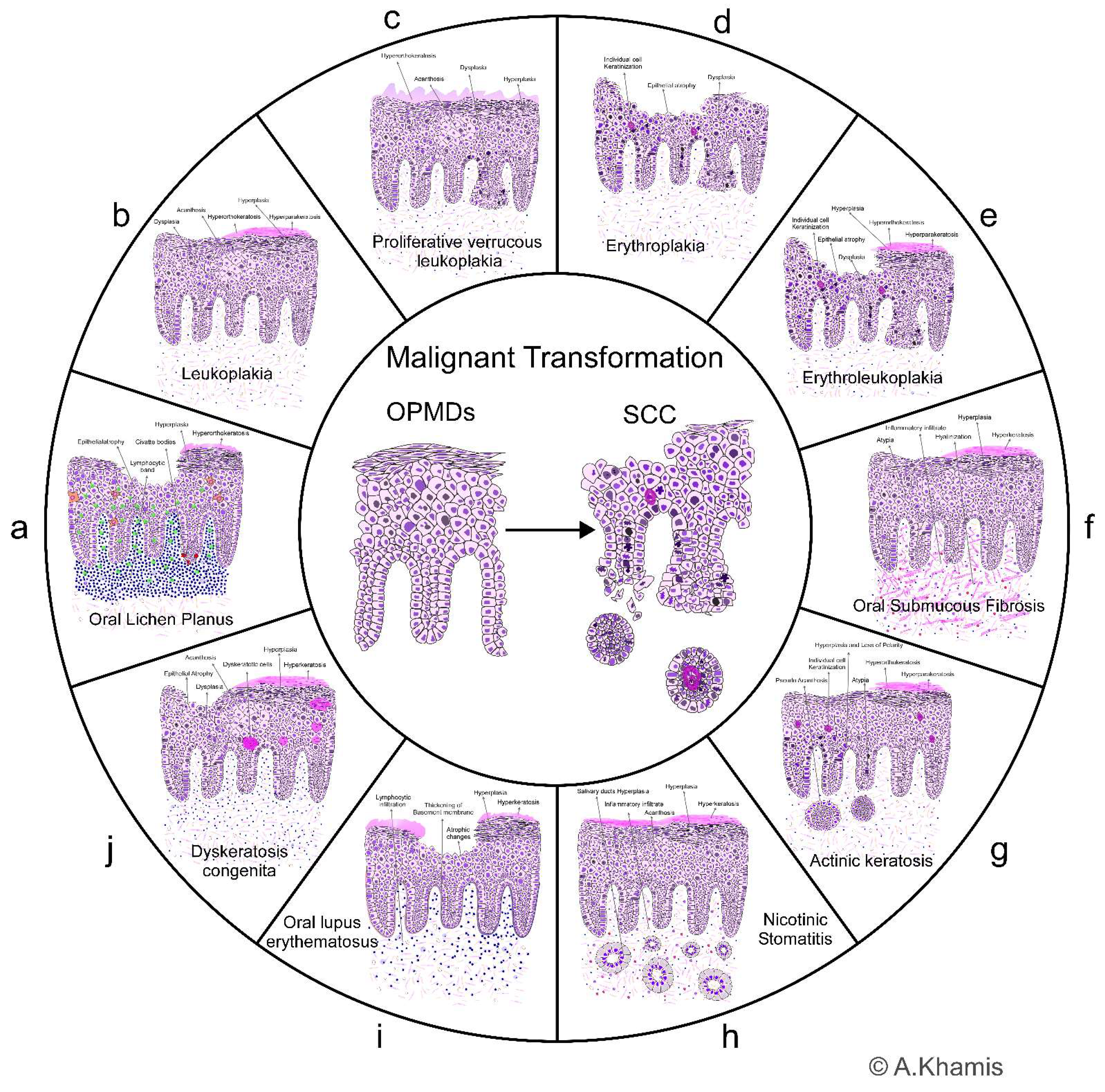
1. Oral potentially malignant disorders
1.1. Oral Lichen Planus (OLP)
1.2. Leukoplakia
- Non-scrapable, primarily white patch/plaque.
- Homogenous Leukoplakia mainly presents itself with well-defined borders (in very few cases, it may present itself with diffuse, ill-defined borders.
- Non-homogeneous Leukoplakia has more diffuse boundaries and may contain red or nodular components in some incidences.
- Excluding the presence of any chronic traumatic irritation to the area (for example, sharp tooth edge, sharp prosthesis, wrong tooth brushing technique or habits).
- Doesn't disappear or improve after elimination of apparent traumatic causes.
- Doesn't disappear or fade away on tissue retraction or stretching (buccal mucous).
- Excluding all other red/white lesions (no other signs and symptoms fitting the presentation of other red/white lesions).
1.3. Proliferative verrucous Leukoplakia
1.4. Erythroplakia
1.5. Erythroleukoplakia
1.6. Oral Submucous Fibrosis (OSF)
1.7. Actinic keratosis (AK)
1.8. Nicotinic Stomatitis (Palatal lesion in reverse smokers)
1.9. Oral lupus erythematosus (OLE)
1.10. Dyskeratosis congenita (DKC)
- 1.
- Preventive measures and treatment options for OPMDs
- 2.
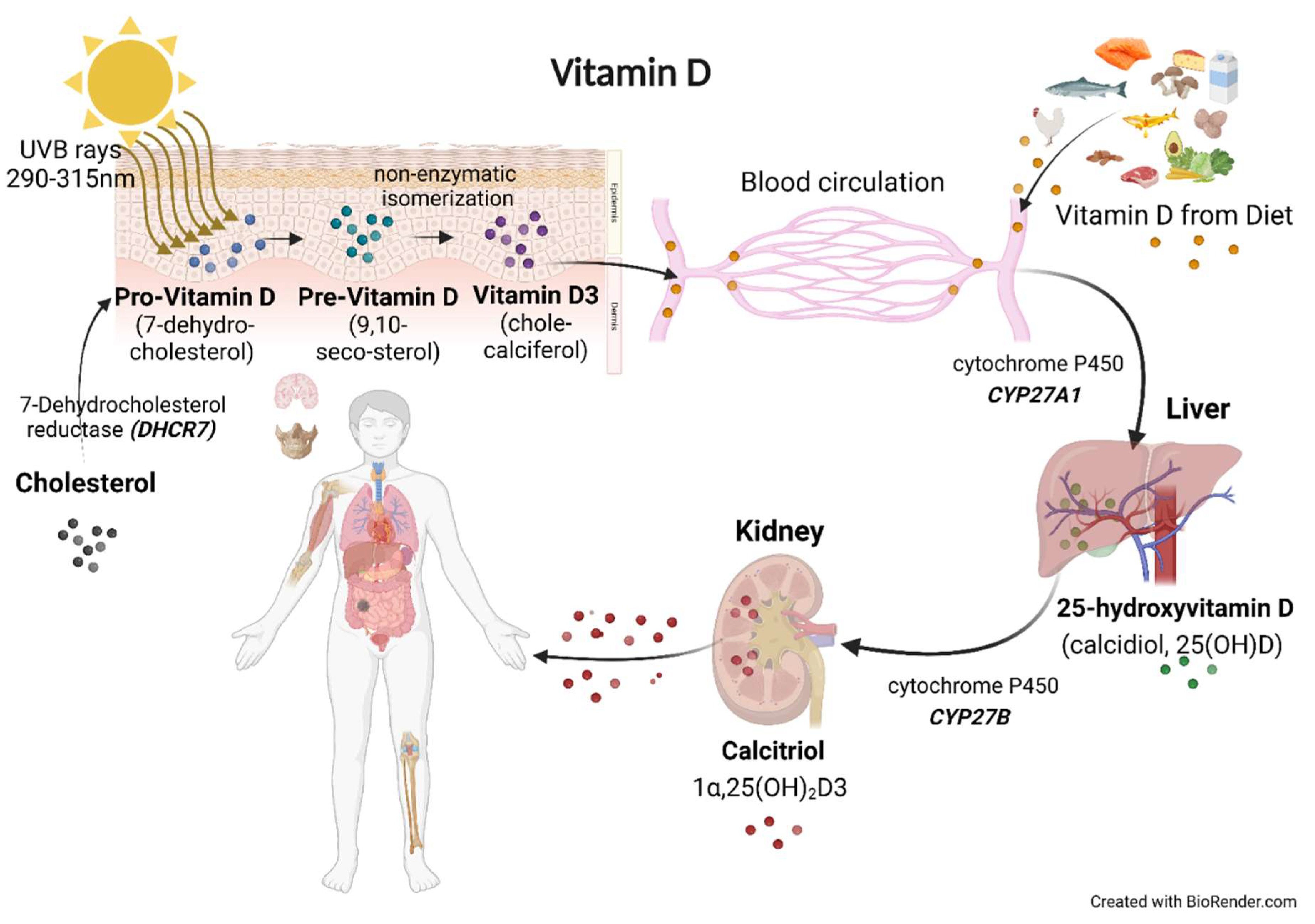
- Physiological Roles of Vitamin D and Vitamin D Receptor
- 3.
- Vitamin D and Head and Neck Cancer
- 4.
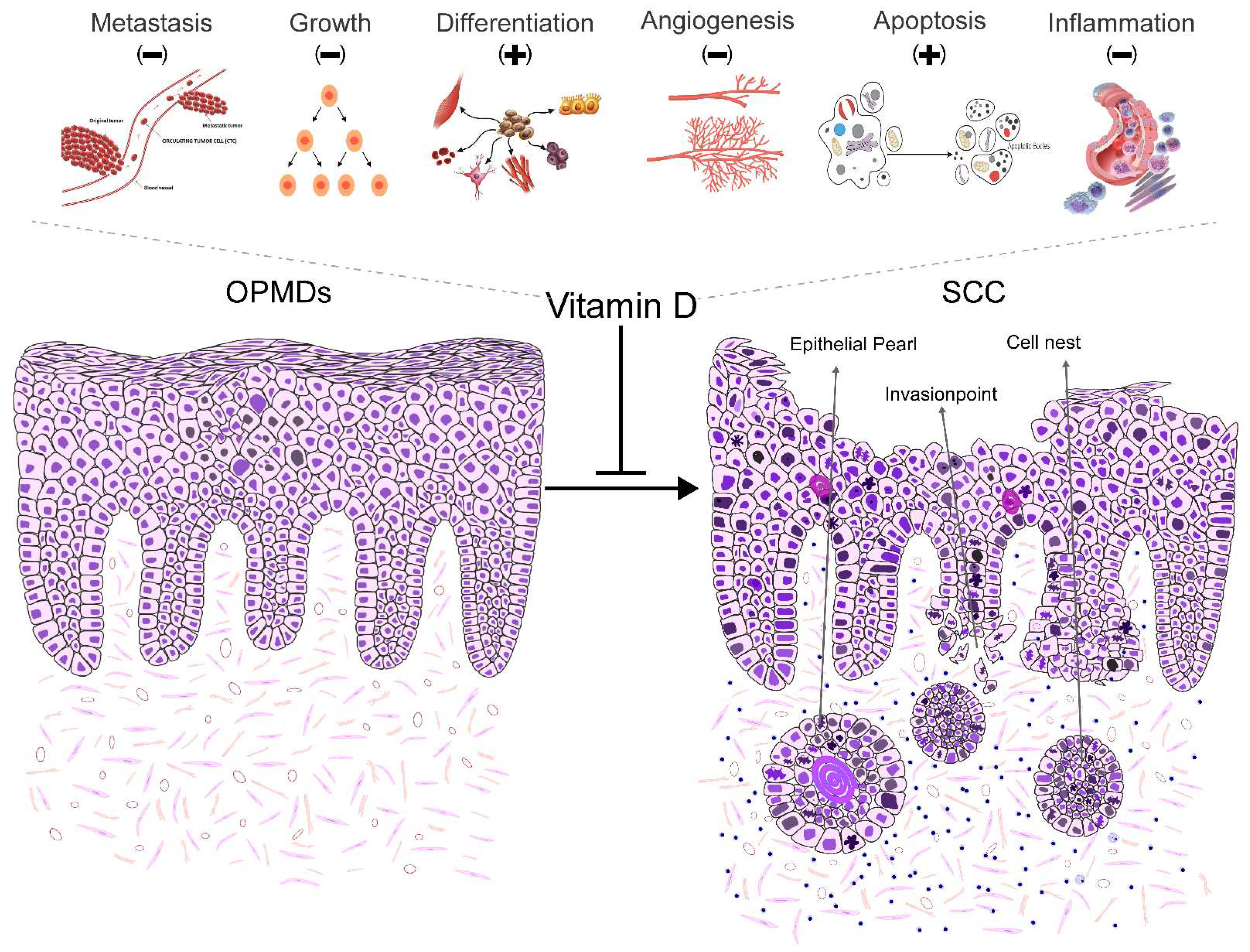
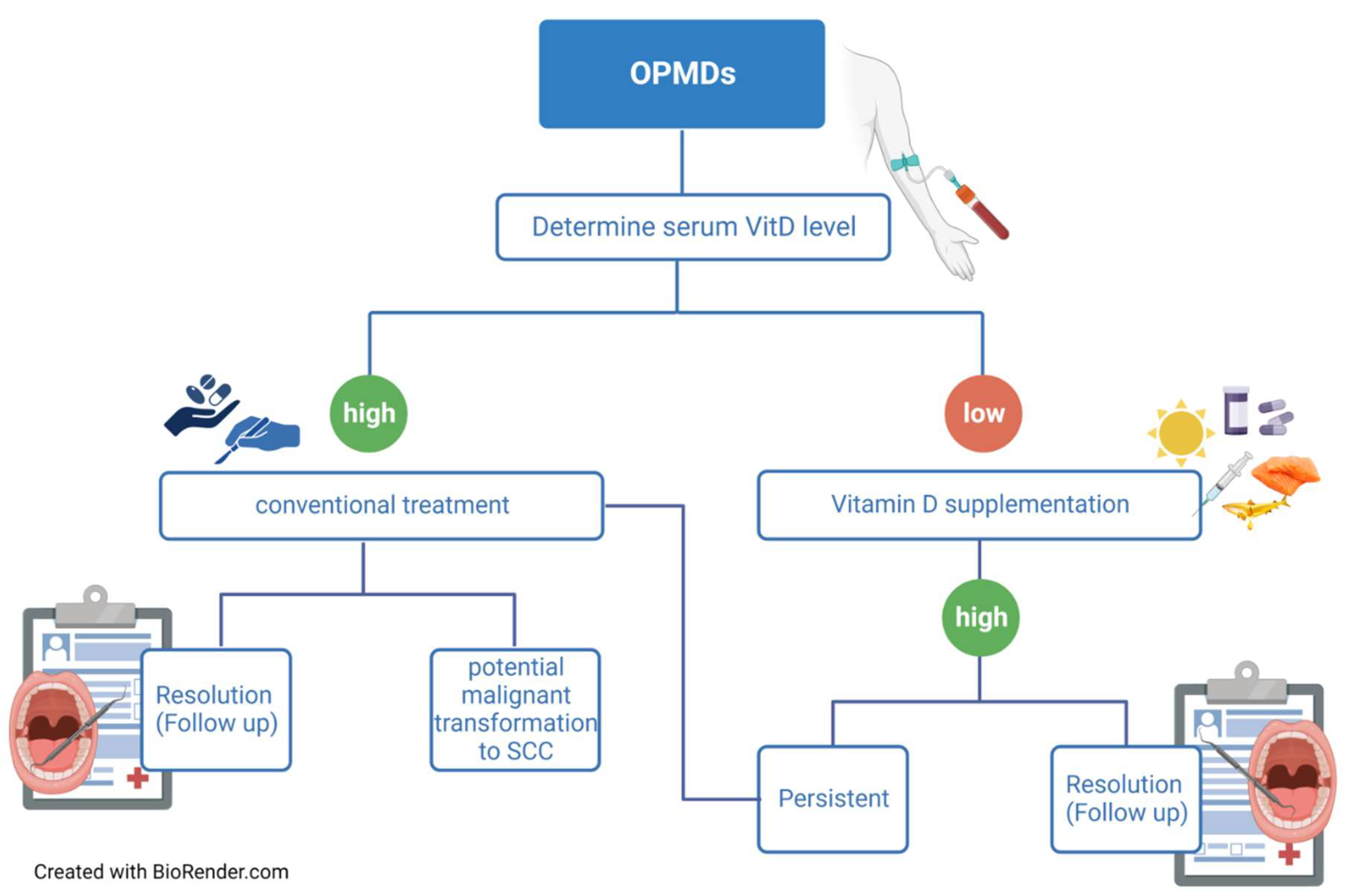
- Vitamin D and OPMDs
- 5.
- Discussion and Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Lorini L, Bescós Atín C, Thavaraj S, Müller-Richter U, Alberola Ferranti M, Pamias Romero J, et al. Overview of oral potentially malignant disorders: from risk factors to specific therapies. Cancers. 2021;13(15):3696. [CrossRef]
- Warnakulasuriya S, Kujan O, Aguirre-Urizar JM, Bagan JV, González-Moles M, Kerr AR, et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral diseases. 2021 Nov;27(8):1862-80. PubMed PMID: 33128420. Epub 2020/11/01. eng. [CrossRef]
- Tilakaratne W, Jayasinghe RD. Oral Potentially Malignant Disorders (OPMDs). Atlas of dermatoses in pigmented skin. 2021:879-902. [CrossRef]
- Rad M, Hashemipoor MA, Mojtahedi A, Zarei MR, Chamani G, Kakoei S, et al. Correlation between clinical and histopathologic diagnoses of oral lichen planus based on modified WHO diagnostic criteria. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2009;107(6):796-800. [CrossRef]
- Alaraik AF, Sollecito TP, Stoopler ET, Akintoye SO, Bindakhil MA. A Retrospective Pilot Study of the Clinical and Histopathological Features of Oral Lichen Planus: Comparison of the Diagnostic Criteria. 2022.
- Khamis AK, Aboushelib MN, Helal MH. Clinical Management Protocol for Dental Implants Inserted in Patients with Active Lichen Planus. Part II 4-Year Follow-Up. Journal of Prosthodontics. 2019;28(5):519-25. [CrossRef]
- Tovaru S, Costache M, Perlea P, Caramida M, Totan C, Warnakulasuriya S, et al. Oral Leukoplakia: A clinicopathological study and malignant transformation. Oral diseases. 2023;29(4):1454-63. [CrossRef]
- Evren I, Brouns ER, Poell JB, Wils LJ, Brakenhoff RH, Bloemena E, et al. Associations between clinical and histopathological characteristics in oral Leukoplakia. Oral diseases. 2023;29(2):696-706.
- Farah CS. Molecular, genomic and mutational landscape of oral Leukoplakia. Oral diseases. 2021;27(4):803-12. [CrossRef]
- Thompson LD, Fitzpatrick SG, Müller S, Eisenberg E, Upadhyaya JD, Lingen MW, et al. Proliferative verrucous Leukoplakia: an expert consensus guideline for standardized assessment and reporting. Head and neck pathology. 2021;15:572-87. [CrossRef]
- Upadhyaya JD, Fitzpatrick SG, Cohen DM, Bilodeau EA, Bhattacharyya I, Lewis JS, et al. Inter-observer variability in the diagnosis of proliferative verrucous Leukoplakia: clinical implications for oral and maxillofacial surgeon understanding: a collaborative pilot study. Head and Neck Pathology. 2020;14:156-65. [CrossRef]
- Parasuraman L, Bal M, Pai PS. Erythroplakia and erythroleucoplakia. Premalignant Conditions of the Oral Cavity. 2019:87-95.
- Lorenzo-Pouso AI, Lafuente-Ibáñez de Mendoza I, Pérez-Sayáns M, Pérez-Jardón A, Chamorro-Petronacci CM, Blanco-Carrión A, et al. Critical update, systematic review, and meta-analysis of oral erythroplakia as an oral potentially malignant disorder. Journal of Oral Pathology & Medicine. 2022;51(7):585-93.
- Yuwanati M, Ramadoss R, Kudo Y, Ramani P, Senthil Murugan M. Prevalence of oral submucous fibrosis among areca nut chewers: A systematic review and meta-analysis. Oral diseases. 2022. [CrossRef]
- Sarode SC, Gondivkar S, Gadbail A, Sarode GS, Yuwanati M. Oral submucous fibrosis and heterogeneity in outcome measures: a critical viewpoint. Future Oncology. 2021;17(17):2123-6. [CrossRef]
- Motgi AA, Shete MV, Chavan MS, Diwaan NN, Sapkal R, Channe P. Assessment of correlation between clinical staging, functional staging, and histopathological grading of oral submucous fibrosis. Journal of Carcinogenesis. 2021;20. [CrossRef]
- Reinehr CPH, Bakos RM. Actinic keratoses: review of clinical, dermoscopic, and therapeutic aspects. Anais Brasileiros de Dermatologia. 2020;94:637-57. [CrossRef]
- Balcere A, Konrāde-Jilmaza L, Pauliņa LA, Čēma I, Krūmiņa A. Clinical characteristics of actinic keratosis associated with the risk of progression to invasive squamous cell carcinoma: a systematic review. Journal of clinical medicine. 2022;11(19):5899. [CrossRef]
- Heerfordt IM, Poulsen T, Wulf HC. Actinic keratoses contiguous with squamous cell carcinomas are mostly non-hyperkeratotic and with severe dysplasia. Journal of Clinical Pathology. 2022;75(8):560-3. [CrossRef]
- Won J, Clark H. Palatal keratosis associated with reverse (or" backwards") smoking (PKARS). The New Zealand Medical Journal (Online). 2022;135(1562):104-7.
- Slootweg PJ, Merkx TA. Premalignant lesions of the oral cavity. Surgical pathology of the head and neck: CRC Press; 2019. p. 277-94.
- García-Ríos P, Pecci-Lloret MP, Oñate-Sánchez RE. Oral Manifestations of Systemic Lupus Erythematosus: A Systematic Review. International Journal of Environmental Research and Public Health. 2022;19(19):11910. [CrossRef]
- Cardoso IL, Leal F, Regis RC. Systemic lupus erythematosus and implications for the oral cavity. Journal of Medical Care Research and Review. 2020;3(9):444-53. [CrossRef]
- Noto Z, Tomihara K, Furukawa K, Noguchi M. Dyskeratosis congenita associated with Leukoplakia of the tongue. International Journal of Oral and Maxillofacial Surgery. 2016;45(6):760-3. [CrossRef]
- Sinha S, Trivedi V, Krishna A, Rao N. Dyskeratosis congenita-management and review of complications: a case report. Oman Medical Journal. 2013;28(4):281. [CrossRef]
- Moretti S, Spallanzani A, Chiarugi A, Muscarella G, Battini M. Oral carcinoma in a young man: a case of dyskeratosis congenita. Journal of the European Academy of Dermatology and Venereology. 2000;14(2):123-5. [CrossRef]
- Mohan P. Assessment of the feasibility of opportunistic screening for oral potentially malignant disorders and oral cancer at dental colleges in India: a public health initiative from Bengaluru. 2022.
- Kumari P, Debta P, Dixit A. Oral potentially malignant disorders: etiology, pathogenesis, and transformation into oral cancer. Frontiers in pharmacology. 2022;13:825266. [CrossRef]
- Iocca O, Sollecito TP, Alawi F, Weinstein GS, Newman JG, De Virgilio A, et al. Potentially malignant disorders of the oral cavity and oral dysplasia: A systematic review and meta-analysis of malignant transformation rate by subtype. Head & neck. 2020;42(3):539-55. [CrossRef]
- Chen J, Tang Z, Slominski AT, Li W, Żmijewski MA, Liu Y, et al. Vitamin D and its analogs as anticancer and anti-inflammatory agents. European Journal of Medicinal Chemistry. 2020 2020/12/01/;207:112738.
- Janoušek J, Pilařová V, Macáková K, Nomura A, Veiga-Matos J, Silva DDd, et al. Vitamin D: sources, physiological role, biokinetics, deficiency, therapeutic use, toxicity, and overview of analytical methods for detection of vitamin D and its metabolites. Critical Reviews in Clinical Laboratory Sciences. 2022:1-38. [CrossRef]
- Starczak Y, Reinke DC, Barratt KR, Ryan JW, Russell PK, Clarke MV, et al. Absence of vitamin D receptor in mature osteoclasts results in altered osteoclastic activity and bone loss. J Steroid Biochem Mol Biol. 2018 Mar;177:77-82. PubMed PMID: 29107736. [CrossRef]
- Vincent-Chong VK, DeJong H, Attwood K, Hershberger PA, Seshadri M. Pre-clinical Prevention Trial of Calcitriol: Impact of Stage of Intervention and Duration of Treatment on Oral Carcinogenesis. Neoplasia. 2019 Apr;21(4):376-88. PubMed PMID: 30875566. Pubmed Central PMCID: PMC6416727.
- Schweitzer A, Knauer SK, Stauber RH. Nuclear receptors in head and neck cancer: current knowledge and perspectives. International Journal of Cancer. 2010;126(4):801-9. [CrossRef]
- Warwick T, Schulz MH, Gilsbach R, Brandes RP, Seuter S. Nuclear receptor activation shapes spatial genome organization essential for gene expression control: lessons learned from the vitamin D receptor. Nucleic Acids Research. 2022;50(7):3745-63. [CrossRef]
- Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)-mediated actions of 1α, 25 (OH) 2vitamin D3: genomic and non-genomic mechanisms. Best practice & research Clinical endocrinology & metabolism. 2011;25(4):543-59.
- Carlberg C, Muñoz A. An update on vitamin D signaling and cancer. Seminars in cancer biology. 2020 May 30. PubMed PMID: 32485310. Epub 2020/06/03. eng. [CrossRef]
- Bakke D, Sun J. Ancient nuclear receptor VDR with new functions: microbiome and inflammation. Inflammatory bowel diseases. 2018;24(6):1149-54. [CrossRef]
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology & metabolism. 2011;96(7):1911-30. [CrossRef]
- Khamis A, Gül D, Wandrey M, Lu Q, Knauer SK, Reinhardt C, et al. The Vitamin D Receptor–BIM Axis Overcomes Cisplatin Resistance in Head and Neck Cancer. Cancers. 2022;14(20):5131. [CrossRef]
- Bikle D, Christakos S. New aspects of vitamin D metabolism and action—Addressing the skin as source and target. Nature Reviews Endocrinology. 2020;16(4):234-52. [CrossRef]
- Saponaro F, Saba A, Zucchi R. An update on vitamin D metabolism. International journal of molecular sciences. 2020;21(18):6573.
- Minisola S, Ferrone F, Danese V, Cecchetti V, Pepe J, Cipriani C, et al. Controversies Surrounding Vitamin D: Focus on Supplementation and Cancer. Int J Environ Res Public Health. 2019 Jan 11;16(2). PubMed PMID: 30641860. Pubmed Central PMCID: PMC6352116. [CrossRef]
- Lehmann B, Querings K, Reichrath J. Vitamin D and skin: new aspects for dermatology. Experimental dermatology. 2004;13:11-5. [CrossRef]
- Holick MF. Vitamin D 2, editor: Humana Press; 2010.
- Carlberg C. Nutrigenomics of Vitamin D. Nutrients. 2019 Mar 21;11(3). PubMed PMID: 30901909.
- Deb S, Reeves AA, Lafortune S. Simulation of Physicochemical and Pharmacokinetic Properties of Vitamin D3 and Its Natural Derivatives. Pharmaceuticals (Basel). 2020 Jul 23;13(8). PubMed PMID: 32717896. [CrossRef]
- Lehmann B, Meurer M. Vitamin D metabolism. Dermatologic therapy. 2010;23(1):2-12.
- Bikle DD. Vitamin D: production, metabolism and mechanisms of action. Endotext [Internet]. 2021.
- Niedermaier T, Gredner T, Kuznia S, Schöttker B, Mons U, Lakerveld J, et al. Vitamin D food fortification in European countries: the underused potential to prevent cancer deaths. European Journal of Epidemiology. 2022;37(4):309-20. [CrossRef]
- Dunlop E, Kiely M, James AP, Singh T, Black LJ. The efficacy of vitamin D food fortification and biofortification in children and adults: a systematic review protocol. JBI Evidence Synthesis. 2020;Online First. PubMed PMID: 02174543-900000000-99831. [CrossRef]
- Moslemi E, Musazadeh V, Kavyani Z, Naghsh N, Shoura SMS, Dehghan P. Efficacy of vitamin D supplementation as an adjunct therapy for improving inflammatory and oxidative stress biomarkers: An umbrella meta-analysis. Pharmacological Research. 2022:106484. [CrossRef]
- Bouillon R, Gomez JMQ. Comparison of calcifediol with vitamin D for prevention or cure of vitamin D deficiency. The Journal of Steroid Biochemistry and Molecular Biology. 2023:106248. [CrossRef]
- Giustina A, Bouillon R, Dawson-Hughes B, Ebeling PR, Lazaretti-Castro M, Lips P, et al. Vitamin D in the older population: a consensus statement. Endocrine. 2023;79(1):31-44. [CrossRef]
- Gandini S, Boniol M, Haukka J, Byrnes G, Cox B, Sneyd MJ, et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011 Mar 15;128(6):1414-24. PubMed PMID: 20473927. Epub 2010/05/18. eng. [CrossRef]
- Billington EO, Burt LA, Rose MS, Davison EM, Gaudet S, Kan M, et al. Safety of high-dose vitamin D supplementation: secondary analysis of a randomized controlled trial. The Journal of Clinical Endocrinology & Metabolism. 2020;105(4):1261-73. [CrossRef]
- Farrell C-J, Herrmann M. Determination of vitamin D and its metabolites. Best practice & research Clinical endocrinology & metabolism. 2013;27(5):675-88.
- Bouillon R, Marcocci C, Carmeliet G, Bikle D, White JH, Dawson-Hughes B, et al. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocrine reviews. 2019;40(4):1109-51. [CrossRef]
- Heath AK, Kim IY, Hodge AM, English DR, Muller DC. Vitamin D Status and Mortality: A Systematic Review of Observational Studies. Int J Environ Res Public Health. 2019 Jan 29;16(3). PubMed PMID: 30700025. Pubmed Central PMCID: PMC6388383. [CrossRef]
- Migliaccio S, Di Nisio A, Magno S, Romano F, Barrea L, Colao AM, et al. Vitamin D deficiency: a potential risk factor for cancer in obesity? International Journal of Obesity. 2022 2022/04/01;46(4):707-17.
- Sîrbe C, Rednic S, Grama A, Pop TL. An Update on the Effects of Vitamin D on the Immune System and Autoimmune Diseases. International Journal of Molecular Sciences. 2022;23(17):9784. PubMed PMID: doi:10.3390/ijms23179784. [CrossRef]
- Filipe Rosa L, Petersen PP, Görtz LF, Stolzer I, Kaden-Volynets V, Günther C, et al. Vitamin A-and D-Deficient Diets Disrupt Intestinal Antimicrobial Peptide Defense Involving Wnt and STAT5 Signaling Pathways in Mice. Nutrients. 2023;15(2):376. [CrossRef]
- Shahrajabian MH, Cheng Q, Sun W. Vitamin C and D Supplements to Prevent the Risk of COVID-19. The Natural Products Journal. 2023;13(1):47-59. [CrossRef]
- Koll L, Gül D, Elnouaem MI, Raslan H, Ramadan OR, Knauer SK, et al. Exploiting Vitamin D Receptor and Its Ligands to Target Squamous Cell Carcinomas of the Head and Neck. International Journal of Molecular Sciences. 2023;24(5):4675. [CrossRef]
- Cashman KD, Dowling KG, Škrabáková Z, Gonzalez-Gross M, Valtueña J, De Henauw S, et al. Vitamin D deficiency in Europe: pandemic? The American journal of clinical nutrition. 2016;103(4):1033-44.
- Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nature reviews cancer. 2014;14(5):342-57. [CrossRef]
- Bouillon R, Manousaki D, Rosen C, Trajanoska K, Rivadeneira F, Richards JB. The health effects of vitamin D supplementation: evidence from human studies. Nature Reviews Endocrinology. 2022 2022/02/01;18(2):96-110. [CrossRef]
- Tuna S, Aydin MA, Aydin MF. The Four Horsemen of the Apocalypse: Cancer, Depression, Vitamin D Deficiency, and Obesity: An Observational Study. Disease Markers. 2023;2023.
- Ginde AA, Liu MC, Camargo CA. Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Archives of internal medicine. 2009;169(6):626-32. [CrossRef]
- Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT. Vitamin D status: United states, 2001–2006. NCHS data brief. 2011;59(59):1-8.
- Hagenau T, Vest R, Gissel T, Poulsen C, Erlandsen M, Mosekilde L, et al. Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: an ecologic meta-regression analysis. Osteoporosis international. 2009;20:133-40. [CrossRef]
- Bischoff-Ferrari HA, Conzelmann M, Stähelin H, Dick W, Carpenter M, Adkin AL, et al. Is fall prevention by vitamin D mediated by a change in postural or dynamic balance? Osteoporosis international. 2006;17:656-63.
- Hilger J, Friedel A, Herr R, Rausch T, Roos F, Wahl DA, et al. A systematic review of vitamin D status in populations worldwide. British journal of nutrition. 2014;111(1):23-45. [CrossRef]
- Holick MF. Vitamin D deficiency. New England journal of medicine. 2007;357(3):266-81.
- Wietrzyk J, Milczarek M, Kutner A. The effect of combined treatment on head and neck human cancer cell lines with novel analogs of Calcitriol and cytostatics. Oncology research. 2007;16(11):517-25. PubMed PMID: 18306931. Epub 2008/03/01. eng. [CrossRef]
- Martinez-Reza I, Diaz L, Barrera D, Segovia-Mendoza M, Pedraza-Sanchez S, Soca-Chafre G, et al. Calcitriol Inhibits the Proliferation of Triple-Negative Breast Cancer Cells through a Mechanism Involving the Pro-inflammatory Cytokines IL-1beta and TNF-alpha. Journal of immunology research. 2019;2019:6384278. PubMed PMID: 31093512. Pubmed Central PMCID: PMC6481021. Epub 2019/05/17. eng.
- Garland CF, Gorham ED, Mohr SB, Garland FC. Vitamin D for cancer prevention: global perspective. Annals of epidemiology. 2009 Jul;19(7):468-83. PubMed PMID: 19523595. Epub 2009/06/16. eng. [CrossRef]
- Muñoz A, Grant WB. Vitamin D and cancer: an historical overview of the epidemiology and mechanisms. Nutrients. 2022;14(7):1448. [CrossRef]
- Hernández-Alonso P, Boughanem H, Canudas S, Becerra-Tomás N, Fernández de la Puente M, Babio N, et al. Circulating vitamin D levels and colorectal cancer risk: A meta-analysis and systematic review of case-control and prospective cohort studies. Critical Reviews in Food Science and Nutrition. 2023;63(1):1-17.
- Brozyna AA, Hoffman RM, Slominski AT. Relevance of Vitamin D in Melanoma Development, Progression and Therapy. Anticancer Res. 2020 Jan;40(1):473-89. PubMed PMID: 31892603. Pubmed Central PMCID: PMC6948187. [CrossRef]
- Carlberg C, Velleuer E. Vitamin D and the risk for cancer: A molecular analysis. Biochemical pharmacology. 2022;196:114735. [CrossRef]
- Lo CS-C, Kiang KM-Y, Leung GK-K. Anti-tumor effects of vitamin D in glioblastoma: Mechanism and therapeutic implications. Laboratory Investigation. 2022;102(2):118-25. [CrossRef]
- Virtanen JK, Nurmi T, Aro A, Bertone-Johnson ER, Hyppönen E, Kröger H, et al. Vitamin D supplementation and prevention of cardiovascular disease and cancer in the Finnish Vitamin D Trial: a randomized controlled trial. The American journal of clinical nutrition. 2022;115(5):1300-10. [CrossRef]
- Valdés-López JF, Velilla P, Urcuqui-Inchima S. Vitamin D modulates the expression of Toll-like receptors and pro-inflammatory cytokines without affecting Chikungunya virus replication, in monocytes and macrophages. Acta Tropica. 2022;232:106497. [CrossRef]
- Ali AJM, Hamoud MJM. Estimation the Relationship Between Vitamin D and Some Immune Parameters Among Patients with Graves' Disease. Pakistan Journal of Medical & Health Sciences. 2022;16(05):854-. [CrossRef]
- Henn M, Martin-Gorgojo V, Martin-Moreno JM. Vitamin D in Cancer Prevention: Gaps in Current Knowledge and Room for Hope. Nutrients. 2022;14(21):4512. [CrossRef]
- Gugatschka M, Kiesler K, Obermayer-Pietsch B, Groselj-Strele A, Griesbacher A, Friedrich G. Vitamin D status is associated with disease-free survival and overall survival time in patients with squamous cell carcinoma of the upper aerodigestive tract. European archives of oto-rhino-laryngology. 2011;268:1201-4. [CrossRef]
- Orell–Kotikangas H, Schwab U, Österlund P, Saarilahti K, Mäkitie O, Mäkitie AA. High prevalence of vitamin D insufficiency in patients with head and neck cancer at diagnosis. Head & neck. 2012;34(10):1450-5. [CrossRef]
- Afzal S, Bojesen SE, Nordestgaard BG. Low plasma 25-hydroxyvitamin D and risk of tobacco-related cancer. Clinical chemistry. 2013;59(5):771-80. [CrossRef]
- Fanidi A, Muller DC, Midttun Ø, Ueland PM, Vollset SE, Relton C, et al. Circulating vitamin D in relation to cancer incidence and survival of the head and neck and oesophagus in the EPIC cohort. Scientific reports. 2016;6(1):36017. [CrossRef]
- Mostafa BE-D, Abdelmageed HM, El-Begermy MM, Taha MS, Hamdy TA-E, Omran A, et al. Value of vitamin D assessment in patients with head and neck squamous cell cancer before treatment. The Egyptian Journal of Otolaryngology. 2016;32:279-86. [CrossRef]
- Bochen F, Balensiefer B, Körner S, Bittenbring JT, Neumann F, Koch A, et al. Vitamin D deficiency in head and neck cancer patients–prevalence, prognostic value and impact on immune function. Oncoimmunology. 2018;7(9):e1476817.
- Nejatinamini S, Debenham BJ, Clugston RD, Mawani A, Parliament M, Wismer WV, et al. Poor Vitamin Status is Associated with Skeletal Muscle Loss and Mucositis in Head and Neck Cancer Patients. Nutrients. 2018 Sep 5;10(9). PubMed PMID: 30189611. Pubmed Central PMCID: PMC6165496. Epub 2018/09/08. eng. [CrossRef]
- Arem H, Weinstein SJ, Horst RL, Virtamo J, Yu K, Albanes D, et al. Serum 25-Hydroxyvitamin D and Risk of Oropharynx and Larynx Cancers in Finnish MenVitamin D and Head and Neck Cancers. Cancer epidemiology, biomarkers & prevention. 2011;20(6):1178-84.
- Skaaby T, Husemoen LLN, Thuesen BH, Pisinger C, Jørgensen T, Roswall N, et al. Prospective Population-Based Study of the Association between Serum 25-Hydroxyvitamin-D Levels and the Incidence of Specific Types of CancerVitamin D and Cancer Incidence. Cancer epidemiology, biomarkers & prevention. 2014;23(7):1220-9.
- Akutsu T, Kitamura H, Himeiwa S, Kitada S, Akasu T, Urashima M. Vitamin D and Cancer Survival: Does Vitamin D Supplementation Improve the Survival of Patients with Cancer? Current oncology reports. 2020 2020/06/04;22(6):62.
- Lathers DM, Clark JI, Achille NJ, Young MRI. Phase IB study of 25-hydroxyvitamin D3 treatment to diminish suppressor cells in head and neck cancer patients. Human immunology. 2001;62(11):1282-93. [CrossRef]
- Grimm M, Cetindis M, Biegner T, Lehman M, Munz A, Teriete P, et al. Serum vitamin D levels of patients with oral squamous cell carcinoma (OSCC) and expression of vitamin D receptor in oral precancerous lesions and OSCC. Medicina oral, patologia oral y cirugia bucal. 2015;20(2):e188. [CrossRef]
- Jolliffe DA, Holt H, Greenig M, Talaei M, Perdek N, Pfeffer P, et al. Effect of a test-and-treat approach to vitamin D supplementation on risk of all cause acute respiratory tract infection and covid-19: phase 3 randomised controlled trial (CORONAVIT). BMJ. 2022;378:e071230. [CrossRef]
- Maturana-Ramírez A, Aitken-Saavedra J, Guevara-Benítez AL, Espinoza-Santander I. Hypovitaminosis D, oral potentially malignant disorders, and oral squamous cell carcinoma: a systematic review. Med Oral Patol Oral Cir Bucal. 2022 Mar 1;27(2):e135-e41. PubMed PMID: 35218642. Pubmed Central PMCID: PMC8898584. Epub 2022/02/27. eng.
- Shukla A, Mehrotra D. Oral Potentially Malignant Disorders. Fundamentals of Oral and Maxillofacial Surgery-E-Book. 2020:313.
- Gupta J, Aggarwal A, Asadullah M, Khan MH, Agrawal N, Khwaja KJ. Vitamin D in the treatment of oral lichen planus: A pilot clinical study. Journal of Indian Academy of Oral Medicine and Radiology. 2019;31(3):222-7. [CrossRef]
- Grimm M, Cetindis M, Biegner T, Lehman M, Munz A, Teriete P, et al. Serum vitamin D levels of patients with oral squamous cell carcinoma (OSCC) and expression of vitamin D receptor in oral precancerous lesions and OSCC. Med Oral Patol Oral Cir Bucal. 2015 Mar 1;20(2):e188-95. PubMed PMID: 25662556. Pubmed Central PMCID: PMC4393981 interest exist. Epub 2015/02/11. eng. [CrossRef]
- McMahon L, Schwartz K, Yilmaz O, Brown E, Ryan LK, Diamond G. Vitamin D-mediated induction of innate immunity in gingival epithelial cells. Infection and immunity. 2011;79(6):2250-6. [CrossRef]
- Walsh JE, Clark A-M, Day TA, Gillespie MB, Young MRI. Use of α, 25-dihydroxyvitamin D3 treatment to stimulate immune infiltration into head and neck squamous cell carcinoma. Human immunology. 2010;71(7):659-65. [CrossRef]
- Sakyi SA, Owusu-Yeboah M, Obirikorang C, Dadzie Ephraim RK, Kwarteng A, Opoku S, et al. Profiling vitamin D, its mediators and pro-inflammatory cytokines in rheumatoid arthritis: A case–control study. Immunity, Inflammation and Disease. 2022;10(8):e676.
- Halim C, Mirza AF, Sari MI. The Association between TNF-α, IL-6, and Vitamin D Levels and COVID-19 Severity and Mortality: A Systematic Review and Meta-Analysis. Pathogens (Basel, Switzerland). 2022 Feb 1;11(2). PubMed PMID: 35215138. Pubmed Central PMCID: PMC8879207. Epub 2022/02/27. eng. [CrossRef]
- Bayraktar N, Turan H, Bayraktar M, Ozturk A, Erdoğdu H. Analysis of serum cytokine and protective vitamin D levels in severe cases of COVID-19. Journal of medical virology. 2022 Jan;94(1):154-60. PubMed PMID: 34427934. Pubmed Central PMCID: PMC8661791. Epub 2021/08/25. eng. [CrossRef]
- Giulietti A, van Etten E, Overbergh L, Stoffels K, Bouillon R, Mathieu C. Monocytes from type 2 diabetic patients have a pro-inflammatory profile: 1, 25-Dihydroxyvitamin D3 works as anti-inflammatory. Diabetes research and clinical practice. 2007;77(1):47-57. [CrossRef]
- Gwenzi T, Zhu A, Schrotz-King P, Schöttker B, Hoffmeister M, Brenner H. Effects of vitamin D supplementation on inflammatory response in patients with cancer and precancerous lesions: Systematic review and meta-analysis of randomized trials. Clinical nutrition (Edinburgh, Scotland). 2023 Jul;42(7):1142-50. PubMed PMID: 37244755. Epub 2023/05/28. eng. https://doi.org/10.1016/j.clnu.2023.05.009. [CrossRef]
- McLachlan CS. The angiotensin-converting enzyme 2 (ACE2) receptor in the prevention and treatment of COVID-19 are distinctly different paradigms. Clin Hypertens. 2020;26:14. PubMed PMID: 32685191. Pubmed Central PMCID: PMC7360378. https://doi.org/10.1186/s40885-020-00147-x. [CrossRef]
- Zhang YG, Lu R, Xia Y, Zhou D, Petrof E, Claud EC, et al. Lack of Vitamin D Receptor Leads to Hyperfunction of Claudin-2 in Intestinal Inflammatory Responses. Inflamm Bowel Dis. 2019 Jan 1;25(1):97-110. PubMed PMID: 30289450. Pubmed Central PMCID: PMC6290786. https://doi.org/10.1093/ibd/izy292. [CrossRef]
- Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annual review of pharmacology and toxicology. 2011;51:311-36. PubMed PMID: 20936945. Epub 2010/10/13. eng. [CrossRef]
- Fernández-Barral A, Bustamante-Madrid P, Ferrer-Mayorga G, Barbáchano A, Larriba MJ, Muñoz A. Vitamin D Effects on Cell Differentiation and Stemness in Cancer. Cancers (Basel). 2020 Aug 25;12(9). PubMed PMID: 32854355. Pubmed Central PMCID: PMC7563562. Epub 2020/08/29. eng. [CrossRef]
- Samuel S, Sitrin MD. Vitamin D's role in cell proliferation and differentiation. Nutrition Reviews. 2008;66(suppl_2):S116-S24.
- Hendi NN, Nemer G. Epigenetic regulation of vitamin D deficiency. Future Medicine; 2023. [CrossRef]
- Snegarova V, Naydenova D. Vitamin D: a Review of its Effects on Epigenetics and Gene Regulation. Folia Med (Plovdiv). 2020 Dec 31;62(4):662-7. PubMed PMID: 33415918. Epub 2021/01/09. eng. [CrossRef]
- Fetahu IS, Höbaus J, Kállay E. Vitamin D and the epigenome. Frontiers in physiology. 2014;5:164. [CrossRef]
- Skrajnowska D, Bobrowska-Korczak B. Potential Molecular Mechanisms of the Anti-cancer Activity of Vitamin D. Anticancer Res. 2019 Jul;39(7):3353-63. PubMed PMID: 31262856. Epub 2019/07/03. eng. [CrossRef]
- Diaz L, Diaz-Munoz M, Garcia-Gaytan AC, Mendez I. Mechanistic Effects of Calcitriol in Cancer Biology. Nutrients. 2015 Jun 19;7(6):5020-50. PubMed PMID: 26102214. Pubmed Central PMCID: PMC4488829. [CrossRef]
- Vuolo L, Di Somma C, Faggiano A, Colao A. Vitamin D and cancer. Front Endocrinol (Lausanne). 2012;3:58. PubMed PMID: 22649423. Pubmed Central PMCID: PMC3355893.
- Zhang J, Yang S, Xu B, Wang T, Zheng Y, Liu F, et al. p62 functions as an oncogene in colorectal cancer through inhibiting apoptosis and promoting cell proliferation by interacting with the vitamin D receptor. Cell Prolif. 2019 May;52(3):e12585. PubMed PMID: 30793399. Pubmed Central PMCID: PMC6536406. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




