Submitted:
06 September 2023
Posted:
07 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ZnO NPs
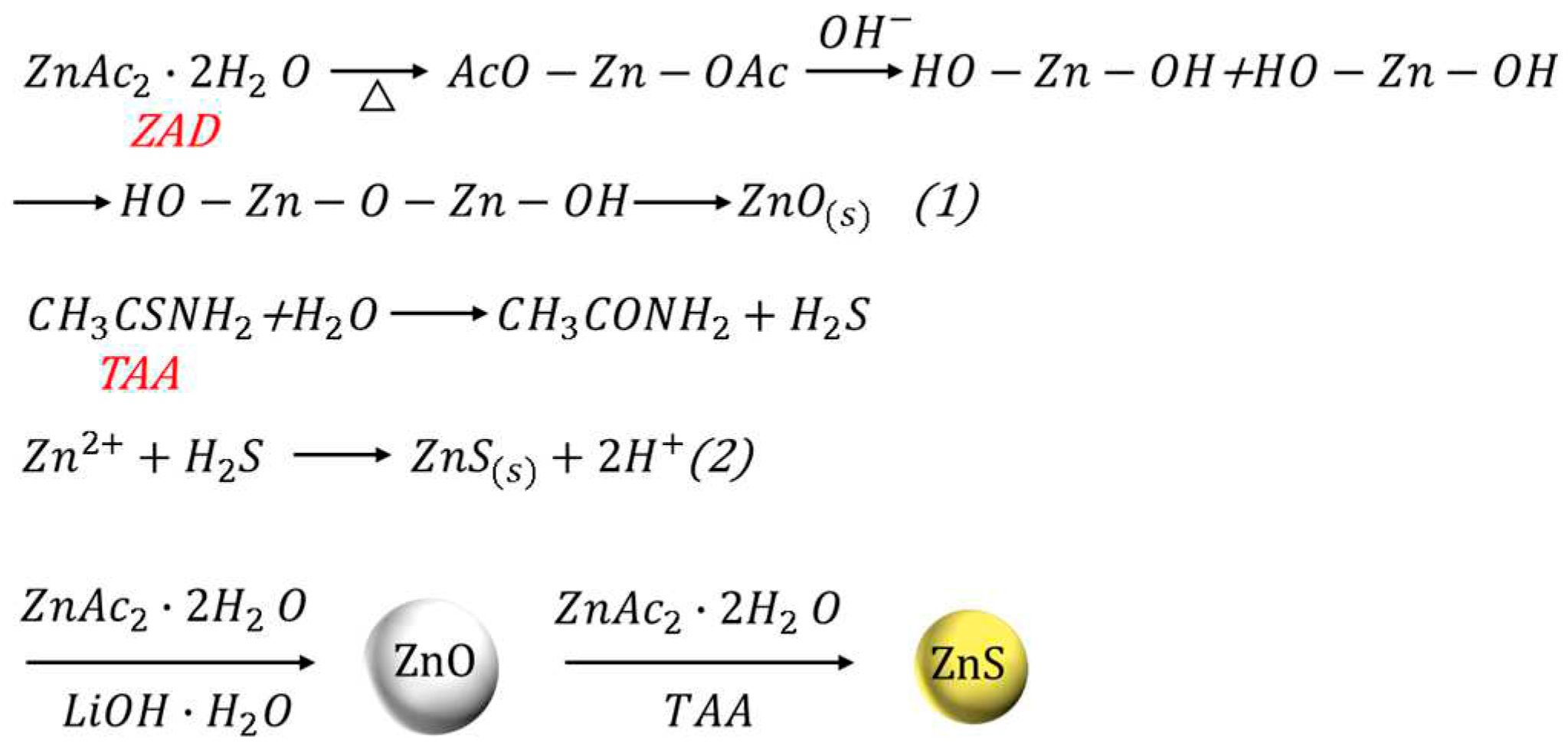
2.3. Synthesis of ZnO/ZnS nanocomposites
2.4. Characterization
3. Results and discussions
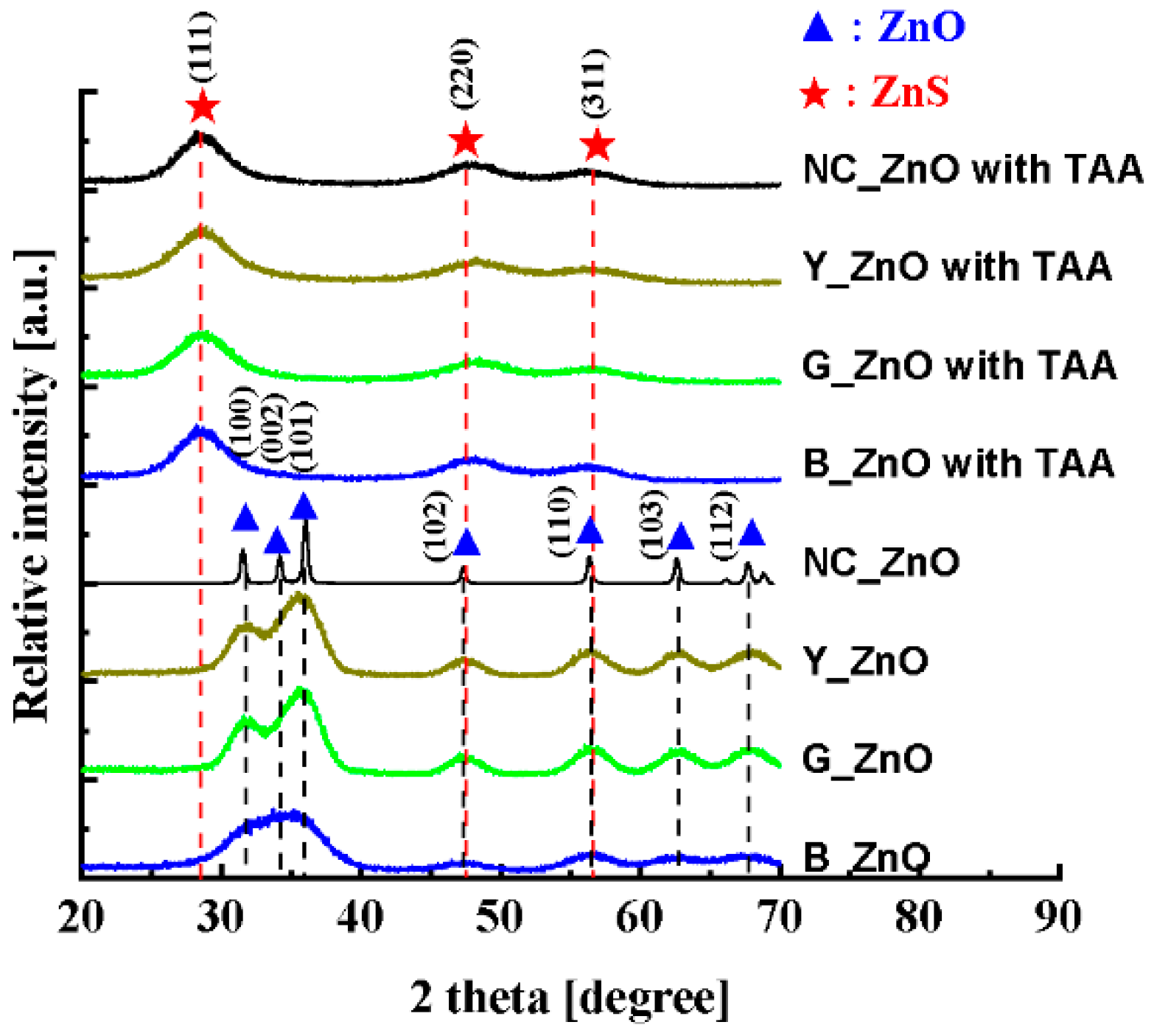
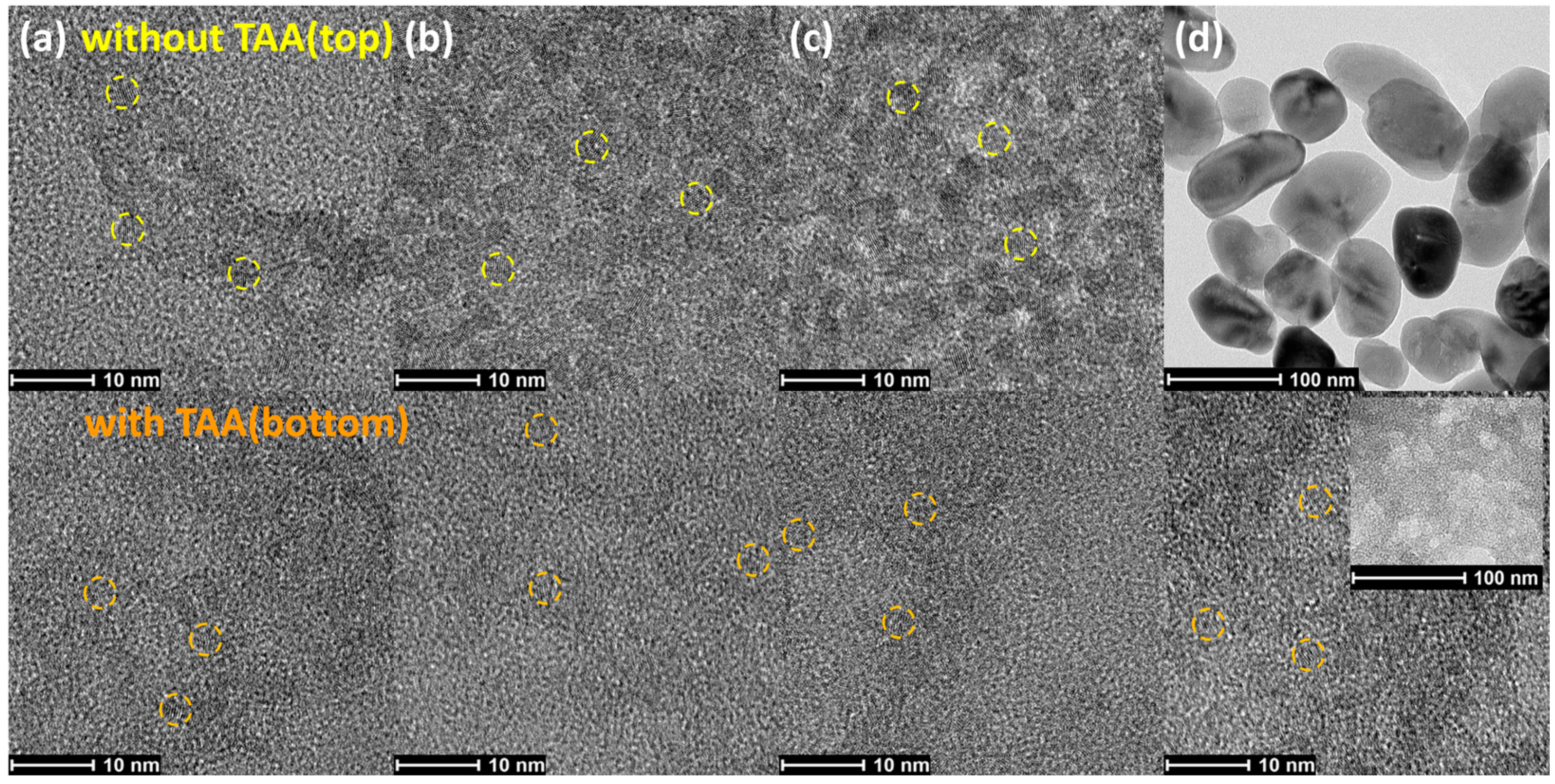
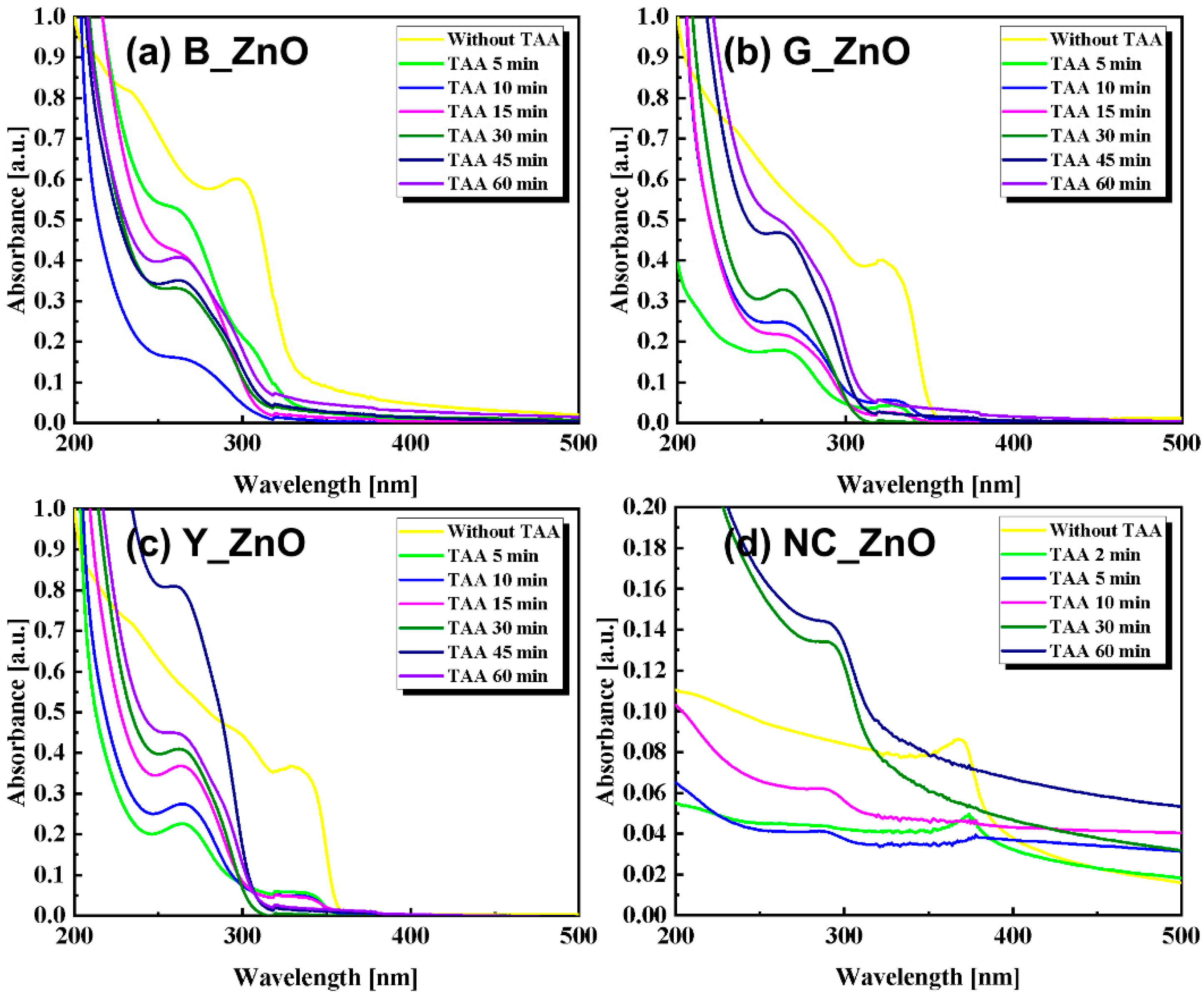

4. Conclusions
Author Contributions
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morkoç, H.; Özgür, Ü., Zinc Oxide. Wiley-VCH Velag GmbH & Co. KGaA, Weinheim 2009.
- Furno, E.; Bertazzi F.; Goano, Michele.; Ghione, G.; Bellotti, E., Hydrodynamic transport parameters of wurtzite ZnO from analytic- and full-band Monte Carlo simulation. Solid-State Electron. 2008, 52, 1796. [CrossRef]
- Park, J.-S.; Kyhm, J.; Kim, H. H.; Jeong, S.; Kang, J.; Lee, S.-e.; Lee, K.-T.; Park, K.; Barange, N.; Han, J.; Song, J. D.; Choi, W. K.; Han, I. K., Alternative Patterning Process for Realization of Large-Area, Full-Color, Active Quantum Dot Display. Nano Lett. 2016, 16, 6946.
- Chao, M.-R.; Chang, Y.-Z.; Chen, J.-L., Hydrophilic ionic liquid-passivated CdTe quantum dots for mercury iondetection. Biosens. Bioelectron. 2013, 42, 397–402.
- Tan, L; Kang, C; Xu, S; Tang, Y, Selective room temperature phosphorescence sensing of target protein using Mn-doped ZnS QDs-embedded molecularly imprinted polymer. Biosens. Bioelectron. 2013, 48, 216–223.
- Zou, W.-s.; Qiao, J.-q.; Hu, X.; Ge, X.; Lian, H.-z., Synthesis in aqueous solution and characterisation of a new cobalt-doped ZnS quantum dot as a hybrid ratiometric chemosensor. Anal. Chim. Acta 2011, 708, 134–140.
- Liu, J.; Wei, X.; Qu, Y.; Cao, J.; Chen, C.; Jiang, H., Aqueous synthesis and bio-imaging application of highly luminescent and low cytotoxicity Mn2+-doped ZnSe nanocrystals. Mater. Lett. 2011, 65, 2139–2141. [CrossRef]
- Subash, B.; Krishnakumar, B.; Pandiyan, V.; Swaminathan, M.; Shanthi, M., An efficient nanostructured Ag2S–ZnO for degradation of Acid Black 1 dye under day light illumination. Sep. Purif. Technol. 2012, 96, 204–213. [CrossRef]
- Liu, C.; Wang, Y.; Meng, D.; Yu, X.; Wang, Y.; Liu, J.; Lu, C.; Xu, K., Enhanced visible light photocatalytic performance of ZnO/ZnS/CuS ternary nanocomposites. Mater. Lett. 2014, 122, 197–200.
- Nguyen, H. T.; Nguyen, N. D.; Lee, S., Application of solution-processed metal oxide layers as charge transport layers for CdSe/ZnS quantum-dot LEDs. Nanotechnology 2013, 24, 115201. [CrossRef] [PubMed]
- Janotti, A.; Walle, C. G. Van de, Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501.
- Xiong, H.-M., ZnO Nanoparticles Applied to Bioimaging and Drug Delivery. Adv. Mater. 2013, 37, 5329–5335.
- Matsuyama, K.; Ihsan, N.; Irie, K.; Mishima, K.; Okuyamam, T.; Mutom, H., Bioimaging application of highly luminescent silica-coated ZnO-nanoparticle quantum dots with biotin. J. Colloid Interface Sci. 2013, 399, 19–25.
- Moussodia, R.-O.; Balan, L.; Merlin, C.; Mustin, C.; Schneider, R., Biocompatible and stable ZnO quantum dots generated by functionalization with siloxane-core PAMAM dendrons. J. Mater. Chem. 2010, 20, 1147–1155. [CrossRef]
- Manaia, E.B.; Kaminski, R.C.K.; Caetano, B.L.; Briois, V.; Chiavacci, L.A.; Bourgaux, C. Surface modified Mg-doped ZnO QDs for biological imaging. Eur. J. Nanomed. 2015, 7, 109–120. [Google Scholar]
- Zhao, H.; Lv, P.; Huo, D.; Zhang, C.; Ding, Y.; Xu, P.; Hu, Y. Doxorubicin loaded chitosan-ZnO hybrid nanospheres combining cell imaging and cancer therapy. RSC Adv. 2015, 5, 60549–60551. [Google Scholar]
- Ischenko, V.; Polarz, S.; Grote, D.; Stavarache, V.; Fink, K.; Driess, M., Zinc Oxide Nanoparticles with Defects, Adv. Funct. Mater. 2005, 15, 1945.
- Gong, Y.; Andelman, T.; Neumark, G. F.; O’Brien, S.; Kuskovsky, I. L., Origin of defect-related green emission from ZnO nanoparticles: effect of surface modification, Nanoscale Res. Lett. 2007, 2, 297.
- Asok, A.; Gandhia, M. N.; Kulkarni, A. R., Enhanced visible photoluminescence in ZnO quantum dots by promotion of oxygen vacancy formation, Nanoscale 2012, 4, 4943–4946. [CrossRef]
- Zhang, L.; Yin, L.; Wang, C.; lun, N.; Qi, Y.; Xiang, D., Origin of Visible Photoluminescence of ZnO Quantum Dots: Defect-Dependent and Size-Dependent, J. Phys. Chem. C, 2010, 114, 9651–9658.
- Kim, H. H.; Lee, H.; Kang, J. K.; Choi, W. K., Photoluminescence and Electron Paramagnetic Resonance Spectroscopy for Revealing Visible Emission of ZnO Quantum Dots. Ann. Phys. 2022, 534, 2100382. [CrossRef]
- Kim, H. H.; Park, S.; Lee, H.; Kang, J. K.; Choi, W. K., Blue-Light Emissive Type II ZnO@ 5-Amino-2-Naphthalene Sulfonic Acid Core–Shell Quantum Dots. Adv. Photonics Res. 2022, 3, 2100315.
- Kim, H. H.; Lee, Y.; Lee, Y. J.; Jeong, J.; Yi, Y.; Park, C.; Yim, S.-Y.; Angadi, B.; Ko, K.-J.; Kang, J.-W.; Choi, W. K., Realization of excitation wavelength independent blue emission of ZnO quantum dots with intrinsic defects. ACS Photonics 2020, 7, 723–734.
- Y. J. Lee,; H. H. Kim,; Y. J. Lee,; J. H. Kim,; H.-J. Choi,; Choi, W. K., Electron transport phenomena at the interface of Al electrode and heavily doped degenerate ZnO nanoparticles in quantum dot light emitting diode. Nanotechnology 2019, 30, 035207.
- Kim, H. H.; Kumi, D. O.; Kim, K.; Park, D.; Yi, Y.; Cho, S. H.; Park, C.; Ntwaeaborwa, O. M.; Choi, W. K., Optimization of the electron transport in quantum dot light-emitting diodes by codoping ZnO with gallium (Ga) and magnesium (Mg). RSC Adv., 2019, 9, 32066–32071.
- Srinatha, N.; Angadi, B.; Son, D. I.; Choi, W. K., Structural and optical studies on spin coated ZnO-graphene conjugated thin films. AIP Conf. Proc. 2018, 1953, 100042.
- Sharma, S.; Chawla, S. Enhanced UV emission in ZnO/ZnS core shell nanoparticles prepared by epitaxial growth in solution. Electron. Mater. Lett. 2013, 9, 267–271. [Google Scholar]
- Luo, J.; Zhao, S.; Wu, P.; Zhang, K.; Peng, C.; Zheng, S. Synthesis and characterization of new Cd-doped ZnO/ZnS core-shell quantum dots with tunable and highly visible photoluminescence. J. Mater. Chem. C 2015, 3, 3391–3398. [Google Scholar]
- Borgohain, R.; Das, R.; Mondal, B.; Yordsri, V.; Thanachayanont, C.; Baruah, S., ZnO/ZnS Core-Shell Nanostructures for Low-Concentration NO2 Sensing at Room Temperature, IEEE Sensors Journal 2018, 18, 7203–7208.
- Zhang, W.; Wang, S.; Wang, Y.; Zhu, Z.; Gao, X.; Yang, J.; Zhang, H. xin, ZnO@ZnS core/shell microrods with enhanced gas sensing properties, RSC Advances 2015, 5, 2620–2629.
- Mun, Y.; Park, S.; Ko, H.; Lee, C.; Lee, S., NO2 gas sensing properties of ZnO/ZnS core-shell nanowires, Journal of the Korean Physical Society 2013, 63, 1595–1600.
- Qi, G.; Zhang, L.; Yuan, Z., Improved H2S gas sensing properties of ZnO nanorods decorated by a several nm ZnS thin layer, Physical Chemistry Chemical Physics 2014, 16, 13434–13439. [CrossRef]
- Park, S.; Kim, S.; Ko, H.; Lee, C., Light Assisted Room Temperature Ethanol Gas Sensing of ZnO–ZnS Nanowires, Journal of nanoscience and nanotechnology 2014, 14, 9025–9028. [CrossRef]
- Park, S.; Kim, S.; Ko, H.; Lee, C., Light-enhanced gas sensing of ZnS-core/ZnO-shell nanowires at room temperature, Journal of Electroceramics 2014, 33, 75–81. [CrossRef]
- Gao, P.; Wang, L.; Wang, Y.; Chen, Y.; Wang, X.; Zhang, G., Onepot hydrothermal synthesis of heterostructured ZnO/ZnS nanorod arrays with high ethanol sensing properties. Chemistry–A European Journal 2012, 18, 4681–4686.
- Na, C. W.; Park, S.-Y.; Lee, J.-H., Punched ZnO nanobelt networks for highly sensitive gas sensors, Sensors and Actuators B: Chemical 2012, 174, 495–499. [CrossRef]
- Yu, X.-L.; Ji, H.-M.; Wang, H.-L.; Sun, J.; Du, X.-W., Synthesis and sensing properties of ZnO/ZnS nanocages, Nanoscale research letters 2010, 5, 644.
- Yu, X.; Zhang, G.; Cao, H.; An, X.; Wang, Y.; Shu, Z.; An, X.; Hua, F., ZnO@ZnS hollow dumbbells–graphene composites as high-performance photocatalysts and alcohol sensors, New Journal of Chemistry 2012, 36, 2593–2598. [CrossRef]
- Reiss, P.; Protière, M.; Li, L. Core/Shell Semiconductor Nanocrystals. Small 2009, 5, 154–168. [Google Scholar] [PubMed]
- Manaia, E. B.; Kaminski, R. C. K.; Caetano, B. L.; Magnani, M.; Meneau, F.; Rochet, A.; Santilli, C. V.; Briois, V.; Bourgaux, C.; Chiavacci L. A., The Critical Role of Thioacetamide Concentration in the Formation of ZnO/ZnS Heterostructures by Sol-Gel Process. Nanomaterials 2018, 8, 55.
- Kim, J.-S.; Kang, B.-H.; Jeong, H.-M.; Kim, S.-W.; Xu, B.; Kang, S.-W., Quantum dot light emitting diodes using size-controlled ZnO NPs. Curr. Appl. Phys. 2018, 18, 681–685. [CrossRef]
- Son, D., Kwon, B., Park, D. et al. Emissive ZnO–graphene quantum dots for white-light-emitting diodes. Nature Nanotech 2012, 7, 465–471. [CrossRef]
- Briois, V.; Giorgetti, Ch.; Baudelet, F.; Blanchandin, S.; Tokumoto, M. S.; Pulcinelli, S. H.; Santilli, C. V., Dynamical Study of ZnO Nanocrystal and Zn-HDS Layered Basic Zinc Acetate Formation from Sol-Gel Route. J. Phys. Chem. C 2007, 111, 3253. [CrossRef]
- Spanhel, L., Colloidal ZnO nanostructures and functional coatings: A survey. J Sol-Gel Sci Techn. 2006, 39, 7.
- Segets, D.; Marczak, R.; Schäfer, S.; Paula, C.; Gnichwitz, J.-F.; Hirsch, A.; Peukert, W., Experimental and Theoretical Studies of the Colloidal Stability of Nanoparticles−A General Interpretation Based on Stability Maps. ACS Nano 2011, 5, 4658–4669.
- Wang, F.; Liu, J.; Wang, Z.; Lin, A.-J.; Luo, H.; Yu, X., Interfacial Heterostructure Phenomena of Highly Luminescent ZnS ∕ ZnO Quantum Dots. J. Electrochem. Soc. 2011, 158, H30. [CrossRef]
- West, A. R., Solid State Chemistry and Its Applications, 2nd ed. John Wiley and Sons 1992, ISBN 978-1-119-94294-8.
- Meulenkamp, E. A., Synthesis and Growth of ZnO Nanoparticles. J. Phys. Chem. B 1998, 102, 5566–5572. [CrossRef]
- Mehta, S.K.; Kumar, S.; Chaudhary, S.; Bhasin, K.K.; Gradzielski, M., Evolution of ZnS Nanoparticles via Facile CTAB Aqueous Micellar Solution Route: A Study on Controlling Parameters. Nanoscale Res. Lett. 2009, 4, 17.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




