1. Introduction
The shifting landscape of parasitic infections affecting the central nervous system (CNS) is challenging the established paradigms in Europe. While traditionally confined to low- and middle-income countries, these infections are now encroaching upon non-endemic regions, propelled by escalating international travel, immunosuppression trends, and climatic variations [
1,
2]. The augmentation of risk via prolonged immunosuppression and medications such as prednisone accentuates the gravity of the situation [
3]. However, the limited availability of empirical data underscores the exigency for a prompt and comprehensive comprehension of this evolving scenario [
4].
In the European context, the evolving dynamics of CNS parasitic infections have catalyzed a compelling public health concern. Transformative variables including global tourism, migratory fluxes, and the HIV/AIDS pandemic have expedited the geographical dissemination of these infections, necessitating recalibration of the epidemiological fabric [
1,
5,
6]. Diverse parasites, encompassing protozoa and helminths of the cestode, nematode, and trematode classes, collectively contribute to intricate clinical manifestations spanning subacute meningitis, encephalitis, cerebral lesions, vascular events, and myelopathy [
3]. Diagnostic endeavors are obfuscated by the nebulous symptomatology and the unreliability of conventional serological assays, while therapeutic interventions confront the absence of standardized regimens [
7].
In this intricate scenario, the importance of heightened clinician awareness is crucial [
7]. Clinicians, as primary healthcare providers, play a key role in vigilantly monitoring both common and uncommon cases. It’s essential for them to be alert to subtle and unusual symptoms while considering factors like international travel and weakened immune systems as potential risks [
3]. In this context, this article aims to emphasize the pressing nature of the situation. It provides insights to improve clinical understanding and healthcare practices, effectively addressing the increasing threat posed by documented and potential CNS parasitic infections in Europe.
2. Parasitic Infections of the Central Nervous System:
2.1. Protozoal Infections of CNS:
2.1.1. Amebiasis (Entamoeba histolytica):
Amebiasis, caused by Entamoeba histolytica, presents a global health challenge, particularly in regions with inadequate sanitation. Endemic areas in Africa, Asia, and Latin America report an estimated annual burden of around 50 million symptomatic cases, accompanied by a considerable number of asymptomatic carriers [
8].GeoSentinel Surveillance Network data underscores E. histolytica as the third most common pathogen among travelers returning with infectious gastrointestinal disease, accounting for 12.5% of confirmed cases and an estimated incidence of 14 cases per 1000 returning traveler [
9].In parts of Asia, Europe, North America, and Australia, specific populations, including gay, bisexual, and other men who have sex with men (MSM), are identified as being at higher risk of acquiring amebiasis [
10].In Europe, while relatively infrequent, instances of amebiasis are often linked to travel, immigration, or localized transmission, necessitating vigilant surveillance and strategic intervention strategies [
10]. There have been reports of brain abscesses caused by E. histolytica from Turkey and Spain in Europe [
11,
12] (
Table 1).
The pathogenesis of amoebic brain abscess intricately involves E. histolytica’s interactions with the host’s immune system and neural brain tissue [
13]. Trophozoites originating from the intestines, following hematogenous spread, reach distant locations, including the brain. By adhering to endothelial cells and overcoming the blood-brain barrier, these trophozoites infiltrate brain tissue [
14]. Subsequent immune responses elicit inflammation and tissue damage [
15]. The culmination of these events leads to the formation of pus-filled abscesses within the brain . The resultant abscess formation underscores severe clinical manifestations, including severe headaches, fever, seizures, and neurological deficits [
13].Timely diagnosis is pivotal in curbing brain tissue damage and potential complications associated with this life-threatening condition.
Diagnosing amoebic brain abscess involves a multi-faceted approach, including clinical evaluation, imaging techniques, and laboratory confirmation [
14]. Identification of E. histolytica trophozoites in brain tissue samples, obtained via biopsy or surgical drainage, confirms the diagnosis [
10]. Efficient management entails a combined pharmacological approach and surgical intervention, if needed. Metronidazole, effective against E. histolytica, is coupled with other antibiotics to address potential bacterial infections [
14]. Surgical drainage alleviates pressure and removes infected tissue, warranting neurosurgical consultation and close monitoring.
2.1.2. Free Living Amebiasis:
Free-living amoebae (FLA), including Naegleria fowleri, Acanthamoeba spp., Balamuthia mandrillaris, and Sappinia, cause severe central nervous system diseases and infections in humans and animals [
16]. FLA are found in water and soil worldwide, with infections often linked to water exposure or contact lenses. FLA’s complex pathogenesis involves trophozoites and cyst stages, cytopathic effects, invasion, and immune responses [
17]. The most severe FLA infections involve the central nervous system (CNS). Granulomatous amoebic encephalitis (GAE) and primary amoebic meningoencephalitis (PAM) are the main categories [
16]. GAE is characterized by subacute to chronic neurological symptoms, including headache, visual disturbances, neurological deficits, and coma, with a high mortality rate. PAM, caused by N. fowleri, progresses rapidly with flu-like symptoms, followed by severe neurological signs and death within days[
16]. Differential diagnoses vary based on the site of infection and risk factors. Although rare, cases of GAE, PAM, and keratitis associated with all the FLA have been reported in Europe [
18,
19,
20].
Diagnosis of FLA infections relies on microscopy, histopathology, and molecular tests. Treatment effectiveness is highest when administered early in the course of the disease. Combination antimicrobial therapies are often used, with miltefosine showing promise in treatment [
21]. In Europe, FLA infections are less common than in some other regions, and there’s a focus on awareness, prevention, and prompt diagnosis. Surveillance, awareness campaigns, and education on proper contact lens hygiene are important in managing these rare but serious infections.
2.1.3. Cerebral Malaria:
Cerebral malaria is a severe complication of Plasmodium falciparum infection and is characterized by the sequestration of infected red blood cells (iRBCs) in the cerebral microvasculature, leading to endothelial activation, inflammation, and disruption of the blood-brain barrier [
22]. The pathogenesis of cerebral malaria involves a complex interplay between parasite-related factors, host immune responses, and endothelial dysfunction. One of the key mechanisms in the development of cerebral malaria is the sequestration of iRBCs in the cerebral microvasculature. This sequestration leads to the activation of endothelial cells, triggering the release of pro-inflammatory cytokines, chemokines, and adhesion molecules [
23]. This inflammatory response contributes to the recruitment and activation of immune cells, including monocytes, T cells, and platelets. These cells release additional inflammatory mediators, amplifying the immune response and exacerbating endothelial dysfunction [
24]. Endothelial dysfunction and disruption of the blood-brain barrier further contribute to the pathogenesis of cerebral malaria. Impaired endothelial function results in increased vascular permeability, allowing the leakage of fluid and proteins into the brain parenchyma, leading to cerebral edema and increased intracranial pressure [
25]
According to a Eurosurveillance report, malaria emerged as the predominant arthropod-borne ailment among travelers from Africa, with 34,235 reported cases (with an incidence rate of 28.8 per 100,000 travelers) between 2015 and 2019, exhibiting a steady rise in the number of cases during this period except for a decline in 2016, while the case fatality ratio consistently remained below 1%.[
26]. According to a meta-analysis, Europe had a pooled prevalence of 13.2% for severe imported malaria, with a 6.3% prevalence of deaths among severe malaria patients. Asia, on the other hand, had the highest proportion of deaths from severe imported malaria, followed by Europe [
27]. Between 2006 and 2014, a multicenter study conducted by The European Network for Tropical Medicine and Travel Health (TropNet) analyzed 185 patients with severe malaria treated in 12 European countries, reporting that 46 (25%) of these patients presented with cerebral malaria, which was found to be associated with older age [
28].
A retrospective study conducted at the Hospital for Tropical Diseases in London, UK, included 124 patients with severe falciparum malaria admitted to the ICU, revealing that cerebral malaria was the most prevalent condition, and both cerebral malaria and acute kidney injury were observed earlier (median day 1) compared to acute respiratory distress syndrome (median day 3) [
29]. According to a recent report from Switzerland, malaria cases in Switzerland primarily originate from West Africa, especially among travelers, indicating the need for focused travel medicine efforts in that region, while potential future waves of migrants from Afghanistan and climate change could contribute to the establishment of P. vivax malaria transmission in Switzerland, and post-pandemic travel trends may increase malaria cases [
30].
The debated post-malaria neurological syndrome (PMNS), which was defined in 1997 by Nguyen et al., is another manifestation of P. falciparum in the central nervous system. This syndrome is characterized by the emergence of encephalitic signs in patients after a symptom-free period (with a median time of 96 hours) following malaria cure, and it is notable that these patients have negative blood smears for Plasmodium falciparum and negative results in investigations for other potential causes [
31]. Despite its association with mefloquine treatment by Nguyen et al., several reports from Europe challenge the notion of an association or causative effect between mefloquine and the condition, instead highlighting numerous cases treated with quinine and artemisinin [
32,
33].
2.1.4. Toxoplasmosis:
Toxoplasma gondii is a parasite that causes zoonotic infections in humans and infects a wide range of animals. While it usually only results in mild illness in healthy individuals, toxoplasmosis can be a common opportunistic infection with high mortality in immunocompromised individuals, often due to reactivation of infection in the central nervous system [
34]. During the acute phase of infection, interferon-dependent immune responses control rapid parasite expansion and mitigate acute disease symptoms. However, after dissemination, the parasite differentiates into semi-dormant cysts within muscle cells and neurons, where they persist for life in the infected host [
34] (
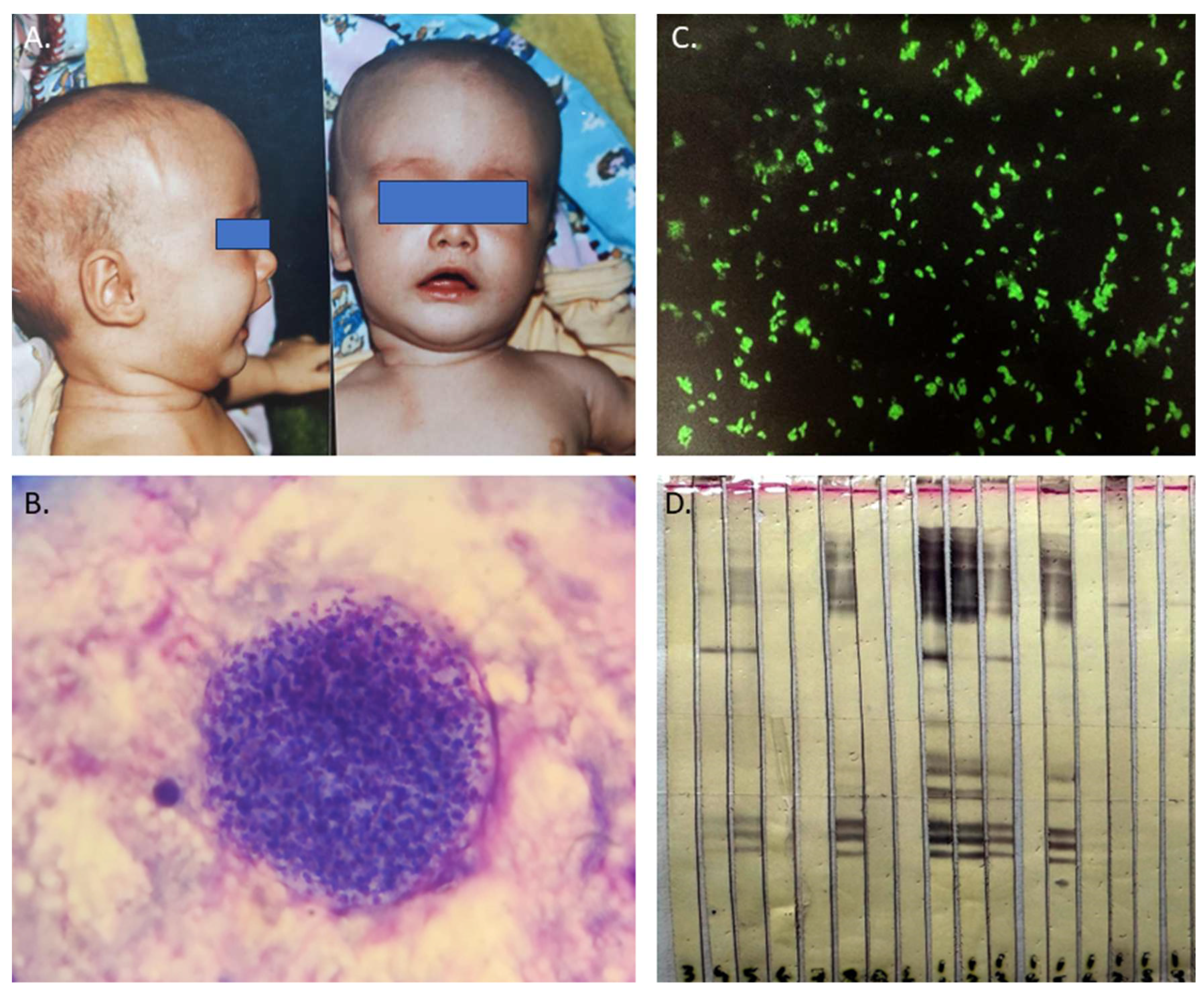
Figure 1). The mechanisms of reactivation are not fully understood but it is thought that it may be due to a decline in cell-mediated immunity. Recent studies suggest that cellular stress is a key factor not only in prompting development of bradyzoites but also in maintaining the encysted form. In immunocompromised patients, reactivation of Toxoplasma infection is typically seen after the CD4 + T-cell count drops below 100–200 cells/mm3 [
35].
Currently, it is reported that 30% of the world’s population has antibodies against T. gondii and the estimated pooled prevalence of T. gondii infection in people with HIV infection is 35.8% overall, with specific rates of 30.1% in Western and Central Europe [
36]. Toxoplasma encephalitis and Toxoplasma retinitis, present in approximately 30% of this risk group, were considered AIDS-defining opportunistic infections before the introduction of highly active antiretroviral therapy (HAART). Even today, toxoplasmosis remains the leading cause of neurological disease in HIV-positive patients, often leading to severe pathology or fatal outcomes [
37,
38]. Patients with CNS toxoplasmosis commonly experience one or more CNS mass lesions, resulting in symptoms such as headache, confusion, lethargy, changes in consciousness, convulsions, paralysis, emotional dysregulations, poor coordination, muscle weakness, seizures, and alterations in alertness [
36].
In addition, several studies have suggested a potential association between toxoplasmosis and mental health disorders, particularly schizophrenia and suicidal behavior. Torrey et al. found a higher prevalence of Toxoplasma gondii infection among individuals with schizophrenia, indicating a possible link between the parasite and schizophrenia symptoms [
39]. Additionally, Sutterland et al. conducted a systematic review that provided evidence of an association between toxoplasmosis and suicide attempts [
40].
CNS toxoplasmosis, caused by the Toxoplasma gondii parasite, affects immunocompromised individuals and requires swift diagnosis and treatment to prevent severe neurological complications. Common in those with weakened immune systems, the infection presents with non-specific symptoms like headaches, confusion, and seizures. Diagnosis involves neuroimaging, revealing characteristic ring-enhancing lesions on MRI, alongside serological tests and CSF analysis for antibodies and DNA detection [
41] (
Figure 1).
Treatment combines antimicrobial therapy and immune restoration. Pyrimethamine and sulfadiazine form the core of antimicrobial treatment, supplemented by leucovorin to mitigate side effects. Corticosteroids might be used to control inflammation. Maintaining lower doses of antimicrobial drugs prevents relapse. Immune function restoration, often through antiretroviral therapy for HIV/AIDS patients, addresses the underlying immune deficiency. Regular monitoring is essential, and early intervention coupled with immune management generally leads to a favorable prognosis [
42].
2.1.5. Trypanosomiasis:
The pathophysiology of trypanosomiasis in the central nervous system (CNS) differs depending on the type and stage of the infection. In Human African trypanosomiasis (HAT), caused by Trypanosoma brucei species, the parasites undergo a complex life cycle in the tsetse fly vector, where they multiply and develop into epimastigotes in the salivary glands. These forms are injected into the human bloodstream during a tsetse fly bite, where they differentiate into trypomastigotes and rapidly divide by binary fission [
43]. The parasites evade the host immune system by changing their variant surface glycoproteins (VSGs), which are exposed on their plasma membrane and elicit a strong antibody response. The parasites disseminate through the bloodstream and lymphatics, reaching various organs and tissues, including the skin, spleen, liver, heart, kidneys, and eyes. Eventually, the parasites cross the blood-brain barrier into the CNS, causing the meningo-encephalitic or second stage [
44]. The mechanisms by which the parasites invade the CNS are not fully understood but may involve direct transcytosis across endothelial cells, paracellular migration through tight junctions, or Trojan horse mechanisms involving infected immune cells [
45]. Once in the CNS, the parasites induce a progressive neuroinflammation that involves the activation of microglia and astrocytes, the production of pro-inflammatory cytokines and chemokines, the recruitment of peripheral immune cells, and the disruption of the blood-brain barrier integrity. These changes underlie the altered behavior in the late or secondary disease stages, prevalent in the chronic Gambian form, characterized by hypersomnia leading, if untreated or if treatment is followed by reactive changes, to coma and death[
44].
In American trypanosomiasis (Chagas disease), caused by Trypanosoma cruzi, the infection can be divided into three stages: acute, intermediate, and chronic. The parasites are transmitted by triatomine bugs that defecate on the skin after biting and depositing metacyclic trypomastigotes on it [
46]. The parasites enter through mucous membranes or skin abrasions and invade various cell types, including muscle cells, macrophages, and neurons. The parasites differentiate into amastigotes and multiply intracellularly until they lyse the host cell and release trypomastigotes that can infect new cells or enter the bloodstream[
46]. In the acute stage, which lasts for 4 to 8 weeks after infection, the parasite produces direct destructive and inflammatory changes in various organs and tissues, including the CNS. The CNS involvement can manifest as meningoencephalitis or chagomas (granulomatous lesions) that can be life-threatening, but which normally resolve spontaneously or with treatment [
44]. The intermediate stage is a prolonged asymptomatic period that can last for years or decades, during which most parasites are suppressed by the host immune system and only low levels of parasitemia are detected. However, some parasites may persist in tissues such as cardiac muscle or neurons and cause chronic damage [
47]. Characterized by alterations in progressive peripheral neuroimmunopathology, the chronic stage involves autoimmune destruction of various nerve components, particularly the autonomic innervation of the heart and gut. This results in cardiomyopathy or mega syndromes (such as esophagus or colon dilation), affecting approximately 30% of individuals with chronic infections. [
48].
Chagas disease (CD) and HAT have surfaced in Europe primarily due to migration from endemic regions. Countries like Spain, Portugal, Italy, France, the United Kingdom, and Switzerland, which host substantial migrant populations from CD-endemic areas, have encountered the impact of CD [
49]. Spain, in particular, has documented CD cases among diverse patient groups, including those with HIV infection, rheumatologic disorders, transplant recipients, and cancer patients [
50]. Europe has also experienced imported HAT cases, with historical data dating back to 1904-1963 and recent reports in countries like France, Italy, Spain, the United Kingdom, Germany, the Netherlands, Belgium, Norway, Sweden, Switzerland, and Poland [
51].
2.2. Helminth Infections of CNS:
2.2.1. Angiostrongyliasis:
Neural angiostrongyliasis, an emerging zoonotic disease caused by the rat lungworm Angiostrongylus cantonensis, poses a growing concern due to its potential to cause eosinophilic meningitis and severe central nervous system disorders in humans. Initially considered nonendemic in Europe, the paradigm shifted in 2018 when A. cantonensis worms were discovered on the Mediterranean island of Mallorca, Spain, a popular tourist destination [
52]. This discovery marked the incursion of the rat lungworm into continental Europe and prompted heightened surveillance efforts.
The transmission of A. cantonensis occurs through a complex life cycle involving definitive hosts, rats, and intermediate hosts, snails, and slugs. Over the past several decades, A. cantonensis has rapidly expanded its geographical distribution beyond its original Asian range, mainly facilitated by rats that serve as its definitive hosts [
53]. These rats often accompany ships and human activities, effectively aiding the dispersion of the parasite to tropical and subtropical regions worldwide [
54].
The initial finding of A. cantonensis in the sewer system of Valencia, Spain, marked its presence in Continental Europe [
55]. Subsequent investigations revealed the parasite’s presence in both Rattus norvegicus and Rattus rattus, with higher prevalence noted in rats residing in orchards surrounding the city [
55]. These orchards contribute to the global distribution of A. cantonensis by exporting vegetables to various regions, potentially exposing populations elsewhere to the parasite [
55].
Angiostrongyliasis primarily affects the CNS and can lead to neurological symptoms due to the migration of larvae within the brain and spinal cord. The larvae can cause inflammation, tissue damage, and immune responses, resulting in various neurological manifestations [
56].
Neurological manifestations of Angiostrongylus cantonensis infection include eosinophilic meningitis, encephalitis/encephalomyelitis, radiculitis, cranial nerve abnormalities, and ataxia. Adults with CNS symptoms commonly present severe headaches, neck stiffness, and paraesthesias, while children exhibit nausea, vomiting, somnolence, fever, and muscle twitching [
57]. Encephalitis is associated with mental status changes, focal neurological signs, and abdominal pain, often evolving into sensory and motor disturbances, and is more prevalent in children and the elderly. The incubation period varies, and the severity of the disease can depend on the worm burden and the intermediate host [
57,
58].
Diagnosing angiostrongyliasis often involves a combination of clinical evaluation, examination of cerebrospinal fluid, and imaging studies such as MRI . The presence of eosinophilia and evidence of inflammation in the CNS can support the diagnosis [
59]. Treatment may include supportive care to manage symptoms and reduce inflammation [
57]. In severe cases, corticosteroids may be used to control the immune response [
60]. There is no specific antiparasitic drug for angiostrongyliasis, but the infection is typically self-limiting in many cases.
The epidemiology of Angiostrongylosis in Europe has been demonstrated through reported cases in various countries including France, Germany, Netherlands, Switzerland, Belgium, Croatia, Italy, Spain, and the United Kingdom [
56] (
Table 2). In New Caledonia, a retrospective study of eosinophilic meningitis cases revealed that 17 out of 92 cases were confirmed as angiostrongyliasis between 2004 and 2019, predominantly affecting young adults and non-walking infants [
59]. Concerning the Angiostrongylus spp. distribution in Europe, A. vasorum’s expansion across the continent is evidenced by increasing prevalence in foxes and disease reports in dogs, while A. cantonensis, although detected on European islands, has limited zoonotic evidence [
58].
2.2.2. Echinococcosis:
Echinococcosis, caused by Echinococcus granulosus and Echinococcus multilocularis, presents diverse epidemiological dynamics in Europe, intertwining human and animal health. These parasitic infections underscore the complex interplay between host populations, transmission patterns, and pathogenesis mechanisms within the continent.
Cystic echinococcosis (CE), primarily caused by E. granulosus, exhibits varying prevalence across Europe. Endemic regions encompass Mediterranean countries like Greece, Italy, and Turkey, as well as Eastern European nations such as Romania and Bulgaria [
61]. Human infections stem from the accidental ingestion of eggs shed by canids, notably dogs. Autochthonous occurrences of E. multilocularis, responsible for alveolar echinococcosis (AE), are confirmed in regions including the Baltic states, Belgium, the Netherlands, Italy, Austria, Hungary, and Slovenia [
61,
62]. High-risk areas often align with environments where intermediate hosts thrive.
E. granulosus infects humans through the oral route, with ingested eggs releasing oncospheres that penetrate the intestinal wall and reach various organs, primarily the liver and lungs. In rare cases, oncospheres may reach the CNS via the bloodstream, leading to cyst formation in the spine or surrounding tissues [
63]. The host’s immune response to the developing cysts often leads to fibrous encapsulation, attempting to contain the infection [
64].
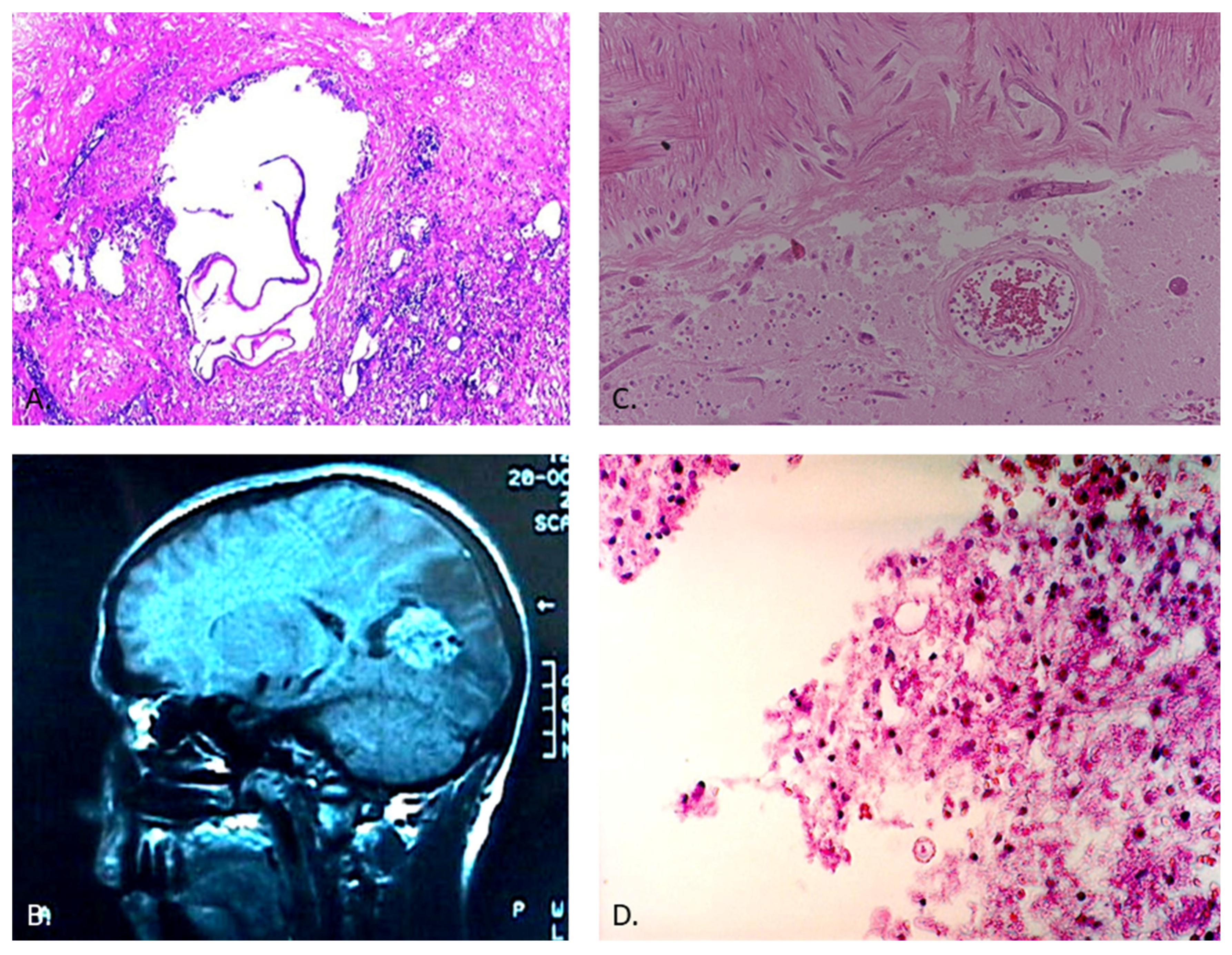
For E. multilocularis, the larval stage infiltrates the intermediate host’s organs, forming invasive, multi-vesicular lesions. Upon ingestion of eggs, humans become accidental hosts. E. multilocularis can disseminate hematogenously, with the potential to develop metastatic lesions in various organs, including the CNS [
65]. In the CNS, these parasites trigger host immune responses, leading to granulomatous inflammation and potential neurological symptoms (
Figure 2). CNS involvement is often associated with hematogenous dissemination or direct extension from nearby structures [
66].
In both species, disease severity is determined by factors including the parasite’s biology, host immune responses, and lesion location [
63]. Early diagnosis through imaging (
Figure 2), serological tests, and molecular techniques is paramount, as CNS involvement can result in neurological complications. A multidisciplinary approach is essential for accurate diagnosis, effective management, and the potential prevention of transmission [
66]. Ongoing surveillance and awareness efforts remain crucial to understanding the evolving epidemiology of echinococcosis and its impact on human and animal health in Europe[
67].
2.2.3. Schistosomiasis:
Neuroschistosomiasis represents a significant subset of helminthic infections within the CNS. Schistosomiasis, second only to malaria in its global impact, is a paramount concern in the realm of public health and disease burden [
68]. Prevalent across 74 endemic countries, schistosomiasis affects over 230 million individuals, with approximately 120 million presenting with symptomatic expressions [
68,
69].
The etiological agents of neuroschistosomiasis are diverse Schistosoma species, prominently encompassing S. mansoni, S. haematobium, and S. japonicum [
70]. These parasites, intricately entwined in their life cycles involving both freshwater snails and human hosts, contribute to the persistent dissemination of the disease. The excretion of parasite eggs in urine and feces by infected individuals perpetuates the cycle of transmission[
70].
Neuroschistosomiasis encompasses cerebral and spinal forms, characterized by the invasion of the CNS by Schistosoma parasites [
68]. Clinical manifestations encompass a spectrum of neurological anomalies, including encephalopathy, myelopathy, seizures, and focal deficits [
70]. The immune retort incited by the presence of schistosome eggs within the CNS culminates in granuloma formation, inflammatory processes, and consequential tissue damage [
71].
Diagnostic methodologies entail the identification of schistosome eggs in urine or stool specimens, complemented by serological assays to detect specific antibodies. Cerebrospinal fluid analysis often reveals elevated protein levels and the presence of eosinophils, providing additional insights [
72]. Advanced imaging techniques, notably MRI, serve as pivotal tools in visualizing granulomas and structural aberrations within the cerebral and spinal domains [
71,
73].
Therapeutic approaches predominantly center on praziquantel, a schistosomicidal agent effective against adult worms, supplemented by adjunctive administration of steroids to mitigate inflammatory responses [
70].
The epidemiology of neuroschistosomiasis in Europe reveals a diverse range of cases. In France, a 28-year-old woman experienced stroke and cerebral vasculitis six months after returning from Burkina Faso, occurring alongside a diagnosed S. mansoni disseminated infection [
74]. Similarly, cases in Paris showcased encephalitis and focal neurologic deficits during acute schistosomiasis. Cerebral imaging unveiled arterial junctional territory cerebral vasculitis, potentially mediated by eosinophil-induced toxicity [
75]. In the UK, a retrospective review identified four neuroschistosomiasis cases, all exhibiting symptoms of transverse myelitis. These cases were linked to freshwater exposure in Uganda, Malawi, and Nigeria [
73]. Meanwhile, a patient from São Tomé and Príncipe, Portugal, displayed a successful clinical response to praziquantel therapy, marking the first reported neuroschistosomiasis case associated with that region [
76]. In Spain, a male presenting myelopathy and multifocal neuritis tested positive for Schistosoma haematobium serology. This positivity supported the diagnosis, which was based on exposure history, clinical presentation, and radiological findings [
72]. These instances collectively emphasize the diverse epidemiological facets of neuroschistosomiasis across Europe.
2.2.4. Strongyloidiasis:
Strongyloidiasis is a parasitic infection caused by the roundworm Strongyloides stercoralis. This infection primarily affects the intestines but can also lead to systemic effects and potential neurological complications [
77].
Infection occurs when individuals come into contact with soil contaminated with Strongyloides larvae. The larvae penetrate the skin and migrate to the lungs, where they are coughed up and swallowed, eventually reaching the intestines. In some cases, the larvae can complete their life cycle within the body, leading to chronic infection.
Central nervous system (CNS) strongyloidiasis represents a recognized yet uncommon form of disseminated infection. Its initial description dates back to 1973 when it was identified postmortem [
78]. Nevertheless, the occurrence of CNS involvement in strongyloidiasis is exceedingly rare, particularly in non-endemic countries. A mere eight antemortem cases have been documented in such regions, reflecting the exceptional nature of this presentation [
79]. Notably, the majority of these cases involve individuals with compromised immune systems, often linked to factors like corticosteroid therapy, malignancy, or chemotherapy. The prognosis for disseminated strongyloidiasis with CNS engagement remains bleak, marked by elevated mortality rates [
79]. Alarmingly, the diagnosis of CNS-associated strongyloidiasis (
Figure 2) is frequently postmortem, underlining the diagnostic challenges and the advanced disease state often encountered [
80].
Diagnosing strongyloidiasis involves identifying larvae in stool samples. Serologic testing, while broadly available and sensitive, lacks specificity and can be influenced by infections with certain parasites; reduced sensitivity is observed in cases of HTLV-1 infection and hematologic malignancies [
77]. The treatment approach for S. stercoralis infection is a matter of debate and evidence scarcity. Recommendations vary from a two-dose ivermectin regimen to single or multiple doses, particularly in uncomplicated cases, while hyper-infection requires prolonged ivermectin use with a possible antibiotic combination [
79]. In CNS-involved cases, evidence remains limited, with treatments including albendazole, ivermectin, and their combination showing mixed outcomes [
77].
In a case series and review conducted in France, neurological symptoms were reported in 25.6% of all cases reviewed, with a higher prevalence of 72.6% observed in the reported case series, compared to 21.2% reported in the broader literature [
80]. Strongyloides stercoralis meningitis was documented in an immunocompromised patient of Belgian origin, illustrating the potential for infection even in temperate regions like Europe, possibly related to occupational exposure[
81]. A case of Strongyloides infection involving a Brazilian man living in Portugal, who had a history of previous bacterial meningitis treated with various medications, underscores the potential complexity of CNS infections caused by Strongyloides stercoralis in Europe [
82].
2.2.5. Taeniasis (Neurocysticercosis):
Neurocysticercosis is a parasitic infection caused by Taenia solium larvae in the central nervous system. The pathogenesis involves interactions between the parasite, host immune response and cysticerci location. After ingestion, larvae invade the bloodstream, reaching the central nervous system where they develop into cysticerci. These bladder-like structures lodge in the brain, triggering an inflammatory response with immune cell recruitment [
83]. Lymphocytes, macrophages, and eosinophils release pro-inflammatory cytokines, such as TNF-α and IL-1β, leading to the formation of an inflammatory granuloma around the cysticerci. The cysticerci’s location within the brain affects disease progression, with critical areas like the brainstem or ventricles causing more severe symptoms and complications due to disruption of neurological function [
84].
Neurocysticercosis exhibits varying clinical features influenced by factors such as cysticerci characteristics and the individual’s immune response[
85]. The most common symptom is seizures, occurring in about 70% of cases and ranging in type and intensity. Headaches are frequent and result from increased pressure or inflammation. Neurological deficits such as weakness, sensory changes, visual disturbances, aphasia, or cranial nerve palsies can manifest based on the location of cysticerci [
83]. Cognitive impairment, memory difficulties, personality changes, depression, or psychosis are possible. Obstruction of cerebrospinal fluid flow by cysticerci can lead to hydrocephalus, characterized by headaches, vomiting, papilledema, and altered mental status. The presentation varies among individuals, and the symptoms can overlap with other neurological conditions, making diagnosis challenging [
86]. Recognizing the diverse clinical features is crucial for the timely management of neurocysticercosis.
Even though cysticercosis is a non-notifiable disease in most European countries, human cysticercosis cases were reported in 17 Western and 15 Eastern European countries [
87,
88] Most cases of human cysticercosis diagnosed in Western Europe are associated with immigration or travel to endemic countries, particularly Latin America and the Caribbean, with a recent increase in cases from Africa and Eastern Europe; however, autochthonously acquired cases in the region are rare [
87]. The highest number of diagnosed human cysticercosis cases in Eastern Europe was reported in Romania and Serbia, indicating potential differences in reporting and epidemiology among countries, and factors such as underreporting, underdiagnosis, and lack of additional information hinder the understanding of parasite transmission in the region [
88]. According to a study conducted in Spain, there was an initial increase in hospitalizations for neurocysticercosis from 1998 to 2008, followed by a decrease, which coincided with a decline in external migration. The most commonly associated diagnoses were epilepsy and convulsions (49.5%), hydrocephalus (11.8%), and encephalitis/myelitis/meningitis (11.6%) [
89].
Recent literature highlights the diagnostic challenge posed by a limited number of laboratories in Europe that have the required tools to identify cysticercosis. These laboratories are primarily found in regions where the disease was previously endemic or where there are significant connections to endemic areas [
90].
2.2.6. Toxocariasis:
Toxocariasis is a parasitic infection caused by the larvae of the roundworms Toxocara canis (from dogs) and Toxocara cati (from cats). While the primary effects of toxocariasis are on the visceral organs, the infection can also lead to systemic and potential neurological complications [
91].
Toxocara larvae are found in the intestines of infected dogs and cats. Eggs from the worms are shed in the animals’ feces and can contaminate the environment. Humans can become infected by ingesting soil or objects contaminated with infective eggs. Once ingested, the larvae can migrate to various organs, including the liver, lungs, eyes, and CNS [
92].
In some cases, toxocariasis can lead to neurological symptoms due to the migration of larvae to the CNS (
Figure 2).
Neurotoxocariasis: Larvae migrating to the brain can cause neurotoxocariasis, resulting in symptoms such as headache, seizures, altered mental status, and focal neurological deficits [
93].
Ocular Larva Migrans: Larvae migrating to the eyes can lead to ocular larva migrans, causing visual disturbances and inflammation of the eyes [
91,
92].
Diagnosing toxocariasis involves clinical evaluation, serological tests to detect antibodies (
Figure 1), and imaging studies such as MRI [
92]. Treatment may involve anthelminthic drugs such as albendazole or mebendazole. Management of ocular and neurological complications may require specialized care .
Epidemiological data on toxocariasis in Europe is limited, but case reports and seroprevalence studies provide insights into its occurrence. A study in Spain reported a seroprevalence of 6.3% among healthy individuals, indicating exposure to Toxocara species [
94]. Case reports from France have highlighted the potential for ocular and neurological complications [
95]. The seroprevalence rates have been reported as 2.4% in Denmark, and 7% in Sweden [
92]. These findings suggest a varying burden of toxocariasis across different European regions.
2.2.7. Trichinellosis (Neurotrichinellosis):
Trichinella spp. is the causative agent of trichinellosis, a zoonotic infection primarily associated with the consumption of undercooked meat from infected animals. The infection is mainly contracted through the ingestion of raw or undercooked pork, game meat, or horse meat, in which Trichinella larvae encyst within the muscle tissues [
96]. The consumption of these larvae initiates the infection in humans. Although Trichinella infection primarily presents with muscle-related symptoms, it has also been associated with cases of CNS involvement, referred to as neurotrichinellosis, which account for approximately 10–15% of reported trichinellosis cases [
97,
98]. This CNS involvement is often overshadowed by the more prevalent muscular symptoms [
99].
Neurotrichinellosis presents a complex spectrum of symptoms including headache, myelitis, and cranial nerve palsies, and causes meningitis or encephalitis [
98]. These neurological symptoms can accompany or follow the more common muscular symptoms of trichinellosis, such as muscle pain, fever, and edema [
96]. Pathogenetic mechanisms include cerebral artery obstruction by larvae, cysts, granulomas, toxic vasculitis with secondary thrombosis, bleeding, granulomatous cerebrum inflammation, or allergic reactions[
97,
98,
100]. Initial CNS manifestations include diffuse encephalopathy symptoms like disorientation and somnolence, progressing to focal neurological deficits such as hemiparesis [
101]. Rarely, sinus venous thrombosis can occur [
102].
The diagnosis of CNS involvement due to Trichinella spp. infection relies on a combination of factors. Clinical presentation, including neurological symptoms accompanied by severe muscle pain, should raise suspicion, particularly in regions where the infection is not endemic [
97,
99]. Hypereosinophilia often accompanies the infection[
100]. Serological tests, such as enzyme-linked immunosorbent assay (ELISA), and western blot (WB) are employed to detect specific antibodies against Trichinella antigens[
96]. Additionally, larval identification through imaging studies can contribute to the diagnosis. Imaging commonly reveals multifocal small lesions in the cortex and white matter, which may indicate multifocal ischemic rather than inflammatory brain infiltrations [
103]. In severe cases presenting with neurological symptoms, cerebrospinal fluid analysis may reveal pleocytosis and elevated protein levels, aiding in the diagnostic process [
98].
In France, nine patients displayed neurological signs such as encephalopathy and focal deficits alongside small hypodensities in the cortex and white matter on brain CT scans, with eight of them also experiencing cardiovascular events [
100]. Another French case exhibited marked hypereosinophilia and subacute cortical infarcts with Gd-DTPA enhancement on MRI [
103]. Romania reported a case with eosinophilia and multiple bilateral brain lesions, particularly in border zones [
104]. In Germany, a patient manifested sudden-onset blindness, paralysis of the legs, and progressive arm weakness [
105]. Turkish documentation identified multifocal brain lesions via MRI and diffusion-weighted MRI [
106]. Lastly, in Belgrade, Serbia, a 51-year-old female with trichinellosis developed neurological symptoms, including confusion and limb weakness, with imaging revealing multiple hypodense changes in the brain corresponding to parasitic infection-induced vasculitis [
107].
2.2.8. Other Helminthiases of CNS:
While CNS involvement by the following parasites has not been reported in Europe so far, it’s important to note that they also rarely cause CNS infections even in their endemic regions and on a global scale. This highlights the relatively infrequent occurrence of CNS manifestations associated with these parasites, underscoring the need for heightened vigilance and consideration of alternative etiologies in cases presenting with neurological symptoms.
2.2.8.1. Filariidae (Wuchereria bancrofti, Brugia malayi, Brugia timori, Onchocerca volvulus):
Filariidae infections, caused by various species including Wuchereria bancrofti, Brugia malayi, Brugia timori, and Onchocerca volvulus, are transmitted through the bites of infected mosquitoes (W. bancrofti, B. malayi, B. timori) or blackflies (O. volvulus). The mosquitoes or blackflies transfer the infective larvae (microfilariae) to humans during blood meals. These larvae then develop into adult worms, primarily residing in the lymphatic system (W. bancrofti, B. malayi, B. timori) or subcutaneous tissues (O. volvulus) [
108].
While these filariidae infections primarily affect the lymphatic system (W. bancrofti, B. malayi, B. timori) or the skin and eyes (O. volvulus), CNS involvement is not a characteristic feature of these infections [
109]. The pathogenesis of Onchocerca volvulus infection in the CNS is associated with various neurological manifestations, such as epilepsy and nodding syndrome [
110]. Adult worms residing in subcutaneous nodules and the release of microfilariae can trigger inflammatory responses in the CNS. The presence of O. volvulus antigens and immune reactions are believed to contribute to the development of epileptic seizures [
111]. Nodding syndrome, a severe neurological disorder affecting children, is also found in areas with onchocerciasis. The relationship between onchocerciasis and nodding syndrome is intricate, involving potential neuroinflammation and interactions between O. volvulus antigens and the immune system, which could be implicated in the syndrome’s pathogenesis [
112].
The diagnosis of Filariidae infections involves identifying microfilariae in blood samples. Treatment often includes antiparasitic drugs such as diethylcarbamazine (DEC) or ivermectin, along with managing symptoms [
109,
113].
2.2.8.2. Paragonimus spp.:
Paragonimus spp. are lung flukes transmitted to humans through the consumption of undercooked or raw freshwater crustaceans containing infective metacercariae. Once ingested, the parasites migrate to various organs, including the lungs, where they cause pulmonary symptoms [
114].
Paragonimus spp. can occasionally migrate to the CNS, causing cerebral paragonimiasis. However, such cases are typically more common in regions where paragonimiasis is endemic [
115].
Diagnosing cerebral paragonimiasis involves clinical evaluation, imaging studies, and serological tests. Treatment often includes antiparasitic drugs such as praziquantel, along with corticosteroids to manage inflammation and alleviate symptoms [
116].
2.2.8.3. Soil-Transmitted Helminths (STHs):
Soil-transmitted helminths, including Ascaris lumbricoides, Trichuris trichiura, Necator americanus, and Ancylostoma duodenale, are transmitted through ingestion of contaminated food or water (A. lumbricoides, T. trichiura) or skin penetration (N. americanus, A. duodenale) [
117]. Soil-transmitted helminths primarily affect the intestines and other organs, with CNS involvement not being a typical feature of these infections [
69].
Diagnosing STH infections involves identifying eggs or larvae in stool samples. Treatment often includes anthelminthic drugs such as albendazole or mebendazole [
69].
3. Discussion
Parasitic infections impacting the CNS are rare but impose significant morbidity and mortality [
2]. The complexity of these infections and their severe consequences highlight the need for comprehensive strategies. The absence of precise diagnostic tools is a major hurdle, as these infections often present with nonspecific symptoms, causing delays in diagnosis and treatment [
3,
7,
118]. Their insidious nature results in missed chances for intervention, compounded by limited research and diagnostic progress [
119].
Migration, climate change, and an aging population with increasing immunosuppression have reshaped the landscape of CNS parasitic infections [
6,
120]. Population movement introduces infections to new areas, sparking localized outbreaks, and altered climate patterns expand vector ranges, facilitating infection spread [
120]. Immunocompromised individuals are more susceptible, as these infections exploit weakened immunity [
15,
77,
84].
The zoonotic nature of these diseases necessitates a “one health” approach, understanding human-animal-environment interactions [
4,
8,
111]. Grasping parasite life cycles and reservoir hosts is key to curbing transmission. Collaborative medical-veterinary efforts provide comprehensive insights and holistic strategies [
85,
94,
121].
Active surveillance is crucial for identifying unusual clusters promptly, enabling swift responses [
4,
62]. Equally important is the education of healthcare professionals and the public. Clinicians must be able to recognize the array of clinical presentations and risk factors, while public awareness empowers individuals to take preventive measures and seek timely care [
6,
7]. Vital policy adjustments are needed to tackle evolving challenges, including the development of customized diagnostic tools, healthcare personnel training, and integration into existing control programs. Adapting policies to this changing landscape enhances overall healthcare responses [
3].
4. Conclusions
In conclusion, while CNS parasitic infections are rare, their impact on morbidity and mortality is considerable. The complex interplay of factors such as diagnostic limitations, lack of awareness, changing demographics, and zoonotic potential underscores the urgency of adopting a multidimensional approach. By fostering collaboration between medical, veterinary, and environmental sectors, enhancing surveillance and awareness, and revising policies, we can collectively address the challenges posed by these infections and minimize their devastating effects on public health.
Author Contributions
Conceptualization, V.T.; methodology, V.T.; software, M.K.; validation, V.T. and M.K.; formal analysis, V.T.; investigation, V.T.; resources, V.T. and M.K.; data curation, V.T.; writing—original draft preparation, V.T.; writing—review and editing, V.T. and M.K; visualization, V.T., and M.K; supervision, M.K.; project administration, V.T.; funding acquisition, N/A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data sets generated or analyzed during the current review are available from the corresponding author upon reasonable request. The reviewed articles, studies, and references cited in this review are publicly available through their respective journals, databases, and sources. Any additional information required to support the findings of this review can be obtained by contacting the corresponding author.
Acknowledgments
The authors would like to thank Prof. Dr. Ahmet Özbilgin for his supervision in the drafting of the article and Dr. Sıla Selin Tunalı for her continued support in data analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Deksne G, Davidson RK, Buchmann K, Kärssin A, Kirjušina M, Gavarāne I, et al. Parasites in the changing world - Ten timely examples from the Nordic-Baltic region. Parasite Epidemiol Control [Internet]. 2020 [cited 2023 Aug 28];10. Available from: https://pubmed.ncbi.nlm.nih.gov/32435705/. [CrossRef]
- Deigendesch N, Schlüter D, Siebert E, Stenzel W. [Infections of the central nervous system by protozoa, helminths and fungi]. Nervenarzt [Internet]. 2019 [cited 2023 Aug 28];90:623–41. Available from: https://pubmed.ncbi.nlm.nih.gov/31073673/.
- Carpio A, Romo ML, Parkhouse RME, Short B, Dua T. Parasitic diseases of the central nervous system: lessons for clinicians and policy makers. Expert Rev Neurother [Internet]. 2016 [cited 2023 Aug 28];16:401. Available from: /pmc/articles/PMC4926779/. [CrossRef]
- van der Giessen J, Deksne G, Gómez-Morales MA, Troell K, Gomes J, Sotiraki S, et al. Surveillance of foodborne parasitic diseases in Europe in a One Health approach. Parasite Epidemiol Control. 2021;13:e00205. [CrossRef]
- Trevisan C, Torgerson PR, Robertson LJ. Foodborne Parasites in Europe: Present Status and Future Trends. Trends Parasitol [Internet]. 2019 [cited 2023 Aug 28];35:695–703. Available from: http://www.cell.com/article/S1471492219301679/fulltext. [CrossRef]
- Semenza JC, Paz S. Climate change and infectious disease in Europe: Impact, projection and adaptation. The Lancet Regional Health - Europe. 2021;9:100230. [CrossRef]
- Kenfak A, Eperon G, Schibler M, Lamoth F, Vargas MI, Stahl JP. Diagnostic approach to encephalitis and meningoencephalitis in adult returning travellers. Clin Microbiol Infect [Internet]. 2019 [cited 2023 Aug 28];25:415–21. Available from: https://pubmed.ncbi.nlm.nih.gov/30708123/. [CrossRef]
- Li J, Cui Z, Li X, Zhang L. Review of zoonotic amebiasis: Epidemiology, clinical signs, diagnosis, treatment, prevention and control. Res Vet Sci. 2021;136:174–81. [CrossRef]
- Swaminathan A, Torresi J, Schlagenhauf P, Thursky K, Wilder-Smith A, Connor BA, et al. A global study of pathogens and host risk factors associated with infectious gastrointestinal disease in returned international travellers. J Infect [Internet]. 2009 [cited 2023 Aug 20];59:19–27. Available from: https://pubmed.ncbi.nlm.nih.gov/19552961/. [CrossRef]
- Shirley DAT, Farr L, Watanabe K, Moonah S. A Review of the Global Burden, New Diagnostics, and Current Therapeutics for Amebiasis. Open Forum Infect Dis [Internet]. 2018 [cited 2023 Aug 20];5. Available from: https://dx.doi.org/10.1093/ofid/ofy161. [CrossRef]
- Zamora PS, Gallotti AC, Ramos R, López JL, González Y, Mejía RA, et al. An Unexpected Case of Disseminated Amebiasis with Cerebral Involvement and Successful Recovery in a Non-Endemic Context. Am J Case Rep [Internet]. 2021 [cited 2023 Aug 20];22:e934188-1. Available from: /pmc/articles/PMC8672918/. [CrossRef]
- Tamer GS, Öncel S, Gökbulut S, Arisoy ES. A rare case of multilocus brain abscess due to Entamoeba histolytica infection in a child. Saudi Med J [Internet]. 2015 [cited 2023 Aug 20];36:356. Available from: /pmc/articles/PMC4381022/. [CrossRef]
- Haque R, Huston CD, Hughes M, Houpt E, Petri WA. Amebiasis. N Engl J Med [Internet]. 2003 [cited 2023 Aug 20];348:1565–73. Available from: https://pubmed.ncbi.nlm.nih.gov/12700377/.
- Morán P, Serrano-Vázquez A, Rojas-Velázquez L, González E, Pérez-Juárez H, Hernández EG, et al. Amoebiasis: Advances in Diagnosis, Treatment, Immunology Features and the Interaction with the Intestinal Ecosystem. Int J Mol Sci [Internet]. 2023 [cited 2023 Aug 20];24. Available from: http://www.ncbi.nlm.nih.gov/pubmed/37511519. [CrossRef]
- Verkerke HP, Petri WA, Marie CS. The Dynamic Interdependence of Amebiasis, Innate Immunity, and Undernutrition. Semin Immunopathol [Internet]. 2012 [cited 2023 Aug 20];34:771. Available from: /pmc/articles/PMC3510265/.
- Kofman A, Guarner J. Infections Caused by Free-Living Amoebae. J Clin Microbiol [Internet]. 2022 [cited 2023 Aug 23];60. Available from: https://journals.asm.org/doi/10.1128/JCM.00228-21. [CrossRef]
- Zhang Y, Xu X, Wei Z, Cao K, Zhang Z, Liang Q. The global epidemiology and clinical diagnosis of Acanthamoeba keratitis. J Infect Public Health. 2023;16:841–52. [CrossRef]
- Cogo PE, Scaglia M, Gatti S, Rossetti F, Alaggio R, Laverda AM, et al. Fatal Naegleria fowleri meningoencephalitis, Italy. Emerg Infect Dis [Internet]. 2004 [cited 2023 Aug 23];10:1835–7. Available from: https://pubmed.ncbi.nlm.nih.gov/15504272/.
- van der Beek NAME, van Tienen C, de Haan JE, Roelfsema J, Wismans PJ, van Genderen PJJ, et al. Fatal Balamuthia mandrillaris Meningoencephalitis in the Netherlands after Travel to The Gambia. Emerg Infect Dis [Internet]. 2015 [cited 2023 Aug 23];21:896–8. Available from: https://pubmed.ncbi.nlm.nih.gov/25897644/. [CrossRef]
- Modica S, Miracco C, Cusi MG, Tordini G, Muzii VF, Iacoangeli F, et al. Non-granulomatous cerebellar infection by Acanthamoeba spp. in an immunocompetent host. Infection [Internet]. 2018 [cited 2023 Aug 23];46:885–9. Available from: https://pubmed.ncbi.nlm.nih.gov/30288678/. [CrossRef]
- Capewell LG, Harris AM, Yoder JS, Cope JR, Eddy BA, Roy SL, et al. Diagnosis, Clinical Course, and Treatment of Primary Amoebic Meningoencephalitis in the United States, 1937-2013. J Pediatric Infect Dis Soc [Internet]. 2015 [cited 2023 Aug 23];4:e68–75. Available from: https://pubmed.ncbi.nlm.nih.gov/26582886/. [CrossRef]
- Schiess N, Villabona-Rueda A, Cottier KE, Huether K, Chipeta J, Stins MF. Pathophysiology and neurologic sequelae of cerebral malaria. Malar J [Internet]. 2020 [cited 2023 May 21];19:1–12. Available from: https://malariajournal.biomedcentral.com/articles/10.1186/s12936-020-03336-z. [CrossRef]
- Turner L, Lavstsen T, Berger SS, Wang CW, Petersen JEV, Avril M, et al. Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature [Internet]. 2013 [cited 2023 May 21];498:502–5. Available from: https://pubmed.ncbi.nlm.nih.gov/23739325/. [CrossRef]
- Hunt NH, Grau GE. Cytokines: Accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol [Internet]. 2003 [cited 2023 May 21];24:491–9. Available from: http://www.cell.com/article/S1471490603002291/fulltext. [CrossRef]
- Dondorp AM, Ince C, Charunwatthana P, Hanson J, Van Kuijen A, Faiz MA, et al. Direct In Vivo Assessment of Microcirculatory Dysfunction in Severe Falciparum Malaria. J Infect Dis [Internet]. 2008 [cited 2023 May 21];197:79–84. Available from: https://academic.oup.com/jid/article/197/1/79/799258. [CrossRef]
- Gossner CM, Hallmaier-Wacker L, Briet O, Haussig JM, de Valk H, Wijermans A, et al. Arthropod-borne diseases among travellers arriving in Europe from Africa, 2015 to 2019. Euro Surveill [Internet]. 2023 [cited 2023 May 21];28:2200270. Available from: https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2023.28.7.2200270. [CrossRef]
- Mahittikorn A, Mala W, Wilairatana P, Siri S, Masangkay FR, Kotepui KU, et al. Prevalence, anti-malarial chemoprophylaxis and causes of deaths for severe imported malaria: A systematic review and meta-analysis. Travel Med Infect Dis. 2022;49:102408. [CrossRef]
- Kurth F, Develoux M, Mechain M, Malvy D, Clerinx J, Antinori S, et al. Severe malaria in Europe: an 8-year multi-centre observational study. Malar J [Internet]. 2017 [cited 2023 May 23];16:1–11. Available from: https://malariajournal.biomedcentral.com/articles/10.1186/s12936-016-1673-z. [CrossRef]
- Marks ME, Armstrong M, Suvari MM, Batson S, Whitty CJM, Chiodini PL, et al. Severe imported falciparum malaria among adults requiring intensive care: A retrospective study at the hospital for tropical diseases, London. BMC Infect Dis [Internet]. 2013 [cited 2023 May 23];13:1–8. Available from: https://bmcinfectdis.biomedcentral.com/articles/10.1186/1471-2334-13-118. [CrossRef]
- Giannone B, Hedrich N, Schlagenhauf P. Imported malaria in Switzerland, (1990–2019): A retrospective analysis. Travel Med Infect Dis. 2022;45:102251.
- Mai NTH, Day NPJ, Chuong L Van, Waller D, Phu NH, Bethell DB, et al. Post-malaria neurological syndrome. Lancet [Internet]. 1996 [cited 2023 May 23];348:917–21. Available from: http://www.thelancet.com/article/S0140673696014092/fulltext.
- Poulet A, Bou Ali H, Savini H, Kaphan E, Parola P. Post-malaria neurological syndrome: Imported case series and literature review to unscramble the auto-immune hypothesis. Travel Med Infect Dis. 2019;29:16–20. [CrossRef]
- Tamzali Y, Demeret S, Haddad E, Guillot H, Caumes E, Jauréguiberry S. Post-malaria neurological syndrome: Four cases, review of the literature and clarification of the nosological framework. Malar J [Internet]. 2018 [cited 2023 May 21];17:1–12. Available from: https://malariajournal.biomedcentral.com/articles/10.1186/s12936-018-2542-8. [CrossRef]
- Matta SK, Rinkenberger N, Dunay IR, Sibley LD. Toxoplasma gondii infection and its implications within the central nervous system. Nature Reviews Microbiology 2021 19:7 [Internet]. 2021 [cited 2023 May 21];19:467–80. Available from: https://www.nature.com/articles/s41579-021-00518-7. [CrossRef]
- Matta SK, Rinkenberger N, Dunay IR, Sibley LD. Toxoplasma gondii infection and its implications within the central nervous system. Nat Rev Microbiol [Internet]. 2021 [cited 2023 Aug 23];19:467–80. Available from: https://pubmed.ncbi.nlm.nih.gov/33627834/. [CrossRef]
- Wang ZD, Wang SC, Liu HH, Ma HY, Li ZY, Wei F, et al. Prevalence and burden of Toxoplasma gondii infection in HIV-infected people: a systematic review and meta-analysis. Lancet HIV [Internet]. 2017 [cited 2023 May 21];4:e177–88. Available from: http://www.thelancet.com/article/S235230181730005X/fulltext. [CrossRef]
- Pleyer U, Groß U, Schlüter D, Wilking H, Seeber F. Toxoplasmosis in Germany: Epidemiology, Diagnosis, Risk factors, and Treatment. Dtsch Arztebl Int [Internet]. 2019 [cited 2023 May 21];116:435. Available from: /pmc/articles/PMC6706837/.
- Ford N, Meintjes G, Calmy A, Bygrave H, Migone C, Vitoria M, et al. Managing Advanced HIV Disease in a Public Health Approach. Clin Infect Dis [Internet]. 2018 [cited 2023 May 21];66:S106. Available from: /pmc/articles/PMC5850613/. [CrossRef]
- Torrey EF, Bartko JJ, Yolken RH. Toxoplasma gondii and Other Risk Factors for Schizophrenia: An Update. Schizophr Bull [Internet]. 2012 [cited 2023 May 21];38:642–7. Available from: https://academic.oup.com/schizophreniabulletin/article/38/3/642/1867672. [CrossRef]
- Sutterland AL, Fond G, Kuin A, Koeter MWJ, Lutter R, van Gool T, et al. Beyond the association. Toxoplasma gondii in schizophrenia, bipolar disorder, and addiction: systematic review and meta-analysis. Acta Psychiatr Scand [Internet]. 2015 [cited 2023 May 21];132:161–79. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/acps.12423. [CrossRef]
- Liu Q, Wang ZD, Huang SY, Zhu XQ. Diagnosis of toxoplasmosis and typing of Toxoplasma gondii. Parasit Vectors [Internet]. 2015 [cited 2023 Aug 23];8. Available from: https://pubmed.ncbi.nlm.nih.gov/26017718/. [CrossRef]
- Dunay IR, Gajurel K, Dhakal R, Liesenfeld O, Montoya JG. Treatment of toxoplasmosis: Historical perspective, animal models, and current clinical practice. Clin Microbiol Rev [Internet]. 2018 [cited 2023 Aug 23];31. Available from: https://journals.asm.org/doi/10.1128/cmr.00057-17. [CrossRef]
- Papagni R, Novara R, Minardi ML, Frallonardo L, Panico GG, Pallara E, et al. Human African Trypanosomiasis (sleeping sickness): Current knowledge and future challenges. Frontiers in Tropical Diseases. 2023;4:1087003. [CrossRef]
- Rodgers J. Trypanosomiasis and the brain. Parasitology [Internet]. 2010 [cited 2023 May 24];137:1995–2006. Available from: https://www.cambridge.org/core/journals/parasitology/article/trypanosomiasis-and-the-brain/D624AD68A2095BA0870BDE3E6B06E48C.
- Cain MD, Salimi H, Diamond MS, Klein RS. Mechanisms of Pathogen Invasion into the Central Nervous System. Neuron [Internet]. 2019 [cited 2023 May 24];103:771–83. Available from: http://www.cell.com/article/S0896627319306415/fulltext. [CrossRef]
- Lidani KCF, Andrade FA, Bavia L, Damasceno FS, Beltrame MH, Messias-Reason IJ, et al. Chagas disease: From discovery to a worldwide health problem. J Phys Oceanogr. 2019;49:458711. [CrossRef]
- Le Govic Y, Demey B, Cassereau J, Bahn YS, Papon N. Pathogens infecting the central nervous system. PLoS Pathog [Internet]. 2022 [cited 2023 May 24];18:e1010234. Available from: https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1010234. [CrossRef]
- Malik LH, Singh GD, Amsterdam EA. The Epidemiology, Clinical Manifestations, and Management of Chagas Heart Disease. Clin Cardiol [Internet]. 2015 [cited 2023 Aug 23];38:565. Available from: /pmc/articles/PMC6490782/.
- Antinori S, Galimberti L, Bianco R, Grande R, Galli M, Corbellino M. Chagas disease in Europe: A review for the internist in the globalized world. Eur J Intern Med. 2017;43:6–15. [CrossRef]
- Pinazo MJ, Espinosa G, Cortes-Lletget C, de Posada EJ, Aldasoro E, Oliveira I, et al. Immunosuppression and Chagas Disease: A Management Challenge. PLoS Negl Trop Dis [Internet]. 2013 [cited 2023 Sep 1];7:e1965. Available from: https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0001965. [CrossRef]
- Gautret P, Clerinx J, Caumes E, Simon F, Jensenius M, Loutan L, et al. Imported human African trypanosomiasis in Europe, 2005-2009. Euro Surveill [Internet]. 2009 [cited 2023 Sep 1];14:19327. Available from: https://www.eurosurveillance.org/content/10.2807/ese.14.36.19327-en. [CrossRef]
- Paredes-Esquivel C, Sola J, Delgado-Serra S, Riera MP, Negre N, Miranda MÁ, et al. Angiostrongylus cantonensis in North African hedgehogs as vertebrate hosts, Mallorca, Spain, October 2018. Euro Surveill [Internet]. 2019 [cited 2023 Aug 26];24. Available from: https://pubmed.ncbi.nlm.nih.gov/31431209/. [CrossRef]
- Martín-Carrillo N, Feliu C, Abreu-Acosta N, Izquierdo-Rodriguez E, Dorta-Guerra R, Miquel J, et al. A Peculiar Distribution of the Emerging Nematode Angiostrongylus cantonensis in the Canary Islands (Spain): Recent Introduction or Isolation Effect? Animals (Basel) [Internet]. 2021 [cited 2023 Aug 26];11. Available from: https://pubmed.ncbi.nlm.nih.gov/33924825/.
- Delgado-Serra S, Sola J, Negre N, Paredes-Esquivel C. Angiostrongylus cantonensis Nematode Invasion Pathway, Mallorca, Spain. Emerg Infect Dis [Internet]. 2022 [cited 2023 Aug 26];28:1163–9. Available from: https://pubmed.ncbi.nlm.nih.gov/35608603/.
- Galán-Puchades MT, Gómez-Samblás M, Osuna A, Sáez-Durán S, Bueno-Marí R, Fuentes M V. Update on the First Finding of the Rat Lungworm, Angiostrongylus cantonensis, in Rattus spp. in Continental Europe, Valencia, Spain, 2022. Pathogens [Internet]. 2023 [cited 2023 Aug 26];12. Available from: https://pubmed.ncbi.nlm.nih.gov/37111453/. [CrossRef]
- Federspiel F, Skovmand S, Skarphedinsson S. Eosinophilic meningitis due to Angiostrongylus cantonensis in Europe. Int J Infect Dis [Internet]. 2020 [cited 2023 Aug 26];93:28–39. Available from: https://pubmed.ncbi.nlm.nih.gov/31972289/. [CrossRef]
- Martins YC, Tanowitz HB, Kazacos KR. Central nervous system manifestations of Angiostrongylus cantonensis infection. Acta Trop [Internet]. 2015 [cited 2023 Aug 26];141:46–53. Available from: https://pubmed.ncbi.nlm.nih.gov/25312338/. [CrossRef]
- Morgan ER, Modry D, Paredes-Esquivel C, Foronda P, Traversa D. Angiostrongylosis in Animals and Humans in Europe. Pathogens 2021, Vol 10, Page 1236 [Internet]. 2021 [cited 2023 Aug 26];10:1236. Available from: https://www.mdpi.com/2076-0817/10/10/1236/htm. [CrossRef]
- Melot B, Delvallez G, Gourinat AC, Molko N, Goarant C, Ducrot YM, et al. Eosinophilic meningitis in New Caledonia: The role of Angiostrongylus cantonensis? PLoS One [Internet]. 2021 [cited 2023 Aug 26];16. Available from: https://pubmed.ncbi.nlm.nih.gov/34383759/.
- Nguyen Y, Rossi B, Argy N, Baker C, Nickel B, Marti H, et al. Autochthonous Case of Eosinophilic Meningitis Caused by Angiostrongylus cantonensis, France, 2016. Emerg Infect Dis [Internet]. 2017 [cited 2023 Aug 26];23:1045–6. Available from: https://pubmed.ncbi.nlm.nih.gov/28518042/.
- Echinococcosis - Annual Epidemiological Report for 2020 [Internet]. [cited 2023 Aug 26]. Available from: https://www.ecdc.europa.eu/en/publications-data/echinococcosis-annual-epidemiological-report-2020.
- Food E, Authority S, Zancanaro G, Oksanen A, Boufana B, Simpson C, et al. Annual assessment of Echinococcus multilocularis surveillance reports submitted in 2020 in the context of Commission Delegated Regulation (EU) 2018/772. EFSA Journal [Internet]. 2021 [cited 2023 Aug 26];19:e06382. Available from: https://onlinelibrary.wiley.com/doi/full/10.2903/j.efsa.2021.6382. [CrossRef]
- Jacquier M, Piroth L. Vertebral Hydatidosis. N Engl J Med [Internet]. 2018 [cited 2023 Aug 26];379:e5. Available from: https://pubmed.ncbi.nlm.nih.gov/29996078/.
- Imperato A, Consales A, Ravegnani M, Castagnola E, Bandettini R, Rossi A. Primary Hydatid Cyst of the Brain in a Child: A Case Report. Pol J Radiol [Internet]. 2016 [cited 2023 Aug 26];81:578–82. Available from: https://pubmed.ncbi.nlm.nih.gov/27994696/. [CrossRef]
- Kantzanou M, Karalexi MA, Vassalos CM, Kostare G, Vrioni G, Tsakris A. Central nervous system cystic echinococcosis: a systematic review. Germs [Internet]. 2022 [cited 2023 Aug 26];12:283–91. Available from: https://pubmed.ncbi.nlm.nih.gov/36504616/. [CrossRef]
- Algros MP, Majo F, Bresson-Hadni S, Koch S, Godard J, Cattin F, et al. Intracerebral alveolar echinococcosis. Infection [Internet]. 2003 [cited 2023 Aug 26];31:63–5. Available from: https://pubmed.ncbi.nlm.nih.gov/12590338/. [CrossRef]
- Cenni L, Simoncini A, Massetti L, Rizzoli A, Hauffe HC, Massolo A. Current and future distribution of a parasite with complex life cycle under global change scenarios: Echinococcus multilocularis in Europe. Glob Chang Biol [Internet]. 2023 [cited 2023 Aug 26];29:2436–49. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/gcb.16616. [CrossRef]
- Carod Artal FJ. Cerebral and spinal schistosomiasis. Curr Neurol Neurosci Rep [Internet]. 2012 [cited 2023 Aug 26];12:666–74. Available from: https://pubmed.ncbi.nlm.nih.gov/22903225/.
- John CC, Carabin H, Montano SM, Bangirana P, Zunt JR, Peterson PK. Global research priorities for infections that affect the nervous system. Nature [Internet]. 2015 [cited 2023 Aug 26];527:S178–86. Available from: https://pubmed.ncbi.nlm.nih.gov/26580325/. [CrossRef]
- Ross AG, McManus DP, Farrar J, Hunstman RJ, Gray DJ, Li YS. Neuroschistosomiasis. J Neurol [Internet]. 2012 [cited 2023 Aug 26];259:22–32. Available from: https://pubmed.ncbi.nlm.nih.gov/21674195/.
- Devine MJ, Wilkinson PA, Doherty JF, Jarman PR. Neuroschistosomiasis presenting as brainstem encephalitis. Neurology [Internet]. 2008 [cited 2023 Aug 26];70:2262–4. Available from: https://pubmed.ncbi.nlm.nih.gov/18519877/. [CrossRef]
- Tarabini-Castellani P, González-Chinchón G, Aldamiz-Echebarría M, Portu-Zapirain J, Apraiz-Garmendia L, Álvarez De Arcaya A. Neuroschistosomiasis: A challenging diagnosis. Rev Neurol. 2007;44:154–6.
- de Wilton A, Aggarwal D, Jäger HR, Manji H, Chiodini PL. Delayed diagnosis of spinal cord schistosomiasis in a non-endemic country: A tertiary referral centre experience. PLoS Negl Trop Dis [Internet]. 2021 [cited 2023 Aug 26];15. Available from: https://pubmed.ncbi.nlm.nih.gov/33571228/. [CrossRef]
- Camuset G, Wolff V, Marescaux C, Abou-Bacar A, Candolfi E, Lefebvre N, et al. Cerebral vasculitis associated with Schistosoma mansoni infection. BMC Infect Dis [Internet]. 2012 [cited 2023 Aug 26];12. Available from: https://pubmed.ncbi.nlm.nih.gov/22978371/.
- Jauréguiberry S, Ansart S, Perez L, Danis M, Bricaire F, Caumes E. ACUTE NEUROSCHISTOSOMIASIS: TWO CASES ASSOCIATED WITH CEREBRAL VASCULITIS. Am J Trop Med Hyg [Internet]. 2007 [cited 2023 Aug 26];76:964–6. Available from: https://www.ajtmh.org/view/journals/tpmd/76/5/article-p964.xml. [CrossRef]
- Duarte Armindo R, Costa S, Almeida V, Barroso C. Cerebral schistosomiasis in a patient travelling from São Tomé and Príncipe. BJR Case Rep [Internet]. 2020 [cited 2023 Aug 26];6:20190055. Available from: https://pubmed.ncbi.nlm.nih.gov/32201606/.
- Keiser PB, Nutman TB. Strongyloides stercoralis in the Immunocompromised Population. Clin Microbiol Rev [Internet]. 2004 [cited 2023 Aug 26];17:208. Available from: /pmc/articles/PMC321465/.
- Tam J, Schwartz KL, Keystone J, Dimitrakoudis D, Downing M, Krajden S. Case Report: Central Nervous System Strongyloidiasis: Two Cases Diagnosed Antemortem. Am J Trop Med Hyg [Internet]. 2019 [cited 2023 Aug 26];100:130. Available from: /pmc/articles/PMC6335887/. [CrossRef]
- Pedersen AA, Hartmeyer GN, Stensvold CR, Martin-Iguacel R. Strongyloides stercoralis hyperinfection syndrome with cerebral involvement. BMJ Case Reports CP [Internet]. 2022 [cited 2023 Aug 26];15:e247032. Available from: https://casereports.bmj.com/content/15/9/e247032. [CrossRef]
- Geri G, Rabbat A, Mayaux J, Zafrani L, Chalumeau-Lemoine L, Guidet B, et al. Strongyloides stercoralis hyperinfection syndrome: a case series and a review of the literature. Infection [Internet]. 2015 [cited 2023 Aug 26];43:691–8. Available from: https://link.springer.com/article/10.1007/s15010-015-0799-1. [CrossRef]
- Pypen Y, Oris E, Meeuwissen J, Vander Laenen M, Van Gompel F, Coppens G. Late onset of Strongyloides stercoralis meningitis in a retired Belgian miner. Acta Clin Belg [Internet]. 2015 [cited 2023 Aug 26];70:447–50. Available from: https://pubmed.ncbi.nlm.nih.gov/26790558/. [CrossRef]
- Pintado Maury I, Neves D, Pereira A. Recurrent meningitis associated to Strongyloides hyperinfection. Enfermedades infecciosas y microbiologia clinica (English ed) [Internet]. 2019 [cited 2023 Aug 26];37:683–4. Available from: https://pubmed.ncbi.nlm.nih.gov/30772102/.
- Garcia HH, Nash TE, Del Brutto OH. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol [Internet]. 2014 [cited 2023 May 21];13:1202. Available from: /pmc/articles/PMC6108081/.
- Prodjinotho UF, Lema J, Lacorcia M, Schmidt V, Vejzagic N, Sikasunge C, et al. Host immune responses during Taenia solium Neurocysticercosis infection and treatment. PLoS Negl Trop Dis [Internet]. 2020 [cited 2023 May 21];14:e0008005. Available from: https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0008005. [CrossRef]
- Butala C, Brook TM, Majekodunmi AO, Welburn SC. Neurocysticercosis: Current Perspectives on Diagnosis and Management. Front Vet Sci. 2021;8:256. [CrossRef]
- Zammarchi L, Bonati M, Strohmeyer M, Albonico M, Requena-Méndez A, Bisoffi Z, et al. Screening, diagnosis and management of human cysticercosis and Taenia solium taeniasis: technical recommendations by the COHEMI project study group. Trop Med Int Health [Internet]. 2017 [cited 2023 May 21];22:881–94. Available from: https://pubmed.ncbi.nlm.nih.gov/28449318/. [CrossRef]
- Laranjo-González M, Devleesschauwer B, Trevisan C, Allepuz A, Sotiraki S, Abraham A, et al. Epidemiology of taeniosis/cysticercosis in Europe, a systematic review: Western Europe. Parasit Vectors [Internet]. 2017 [cited 2023 May 21];10:1–14. Available from: https://parasitesandvectors.biomedcentral.com/articles/10.1186/s13071-017-2280-8. [CrossRef]
- Trevisan C, Sotiraki S, Laranjo-González M, Dermauw V, Wang Z, Kärssin A, et al. Epidemiology of taeniosis/cysticercosis in Europe, a systematic review: Eastern Europe. Parasit Vectors [Internet]. 2018 [cited 2023 May 21];11:1–11. Available from: https://parasitesandvectors.biomedcentral.com/articles/10.1186/s13071-018-3153-5. [CrossRef]
- Herrador Z, Fernandez-Martinez A, Benito A, Lopez-Velez R. Clinical Cysticercosis epidemiology in Spain based on the hospital discharge database: What’s new? PLoS Negl Trop Dis [Internet]. 2018 [cited 2023 May 21];12. Available from: https://pubmed.ncbi.nlm.nih.gov/29621234/.
- Gómez-Morales MA, Gárate T, Blocher J, Devleesschauwer B, Smit GSA, Schmidt V, et al. Present status of laboratory diagnosis of human taeniosis/cysticercosis in Europe. European Journal of Clinical Microbiology and Infectious Diseases [Internet]. 2017 [cited 2023 May 21];36:2029–40. Available from: https://link.springer.com/article/10.1007/s10096-017-3029-1. [CrossRef]
- Deshayes S, Bonhomme J, de La Blanchardière A. Neurotoxocariasis: a systematic literature review. Infection [Internet]. 2016 [cited 2023 Aug 26];44:565–74. Available from: https://pubmed.ncbi.nlm.nih.gov/27084369/. [CrossRef]
- Ma G, Holland C V., Wang T, Hofmann A, Fan CK, Maizels RM, et al. Human toxocariasis. Lancet Infect Dis [Internet]. 2018 [cited 2023 Aug 26];18:e14–24. Available from: https://pubmed.ncbi.nlm.nih.gov/28781085/.
- Luna J, Cicero CE, Rateau G, Quattrocchi G, Marin B, Bruno E, et al. Updated evidence of the association between toxocariasis and epilepsy: Systematic review and meta-analysis. PLoS Negl Trop Dis [Internet]. 2018 [cited 2023 Aug 26];12. Available from: https://pubmed.ncbi.nlm.nih.gov/30028858/. [CrossRef]
- Martínez-Moreno FJ, Hernández S, López-Cobos E, Becerra C, Acosta I, Martínez-Moreno A. Estimation of canine intestinal parasites in Córdoba (Spain) and their risk to public health. Vet Parasitol [Internet]. 2007 [cited 2023 Aug 26];143:7–13. Available from: https://pubmed.ncbi.nlm.nih.gov/16971046/. [CrossRef]
- Bourgoin G, Callait-Cardinal MP, Bouhsira E, Polack B, Bourdeau P, Roussel Ariza C, et al. Prevalence of major digestive and respiratory helminths in dogs and cats in France: results of a multicenter study. Parasit Vectors [Internet]. 2022 [cited 2023 Aug 26];15. Available from: https://pubmed.ncbi.nlm.nih.gov/36068597/. [CrossRef]
- Neghina R, Neghina AM, Marincu I, Iacobiciu I. Trichinellosis, another helminthiasis affecting the central nervous system. Parasitol Int [Internet]. 2011 [cited 2023 Aug 29];60:230. Available from: https://pubmed.ncbi.nlm.nih.gov/21292025/. [CrossRef]
- Rosca EC, Tudor R, Cornea A, Simu M. Central Nervous System Involvement in Trichinellosis: A Systematic Review. Diagnostics (Basel) [Internet]. 2021 [cited 2023 Aug 29];11. Available from: https://pubmed.ncbi.nlm.nih.gov/34070586/. [CrossRef]
- Bruschi F, Brunetti E, Pozio E. Neurotrichinellosis. Handb Clin Neurol [Internet]. 2013 [cited 2023 Aug 29];114:243–9. Available from: https://pubmed.ncbi.nlm.nih.gov/23829915/.
- Neghina R, Neghina AM, Marincu I, Iacobiciu I. Reviews on trichinellosis (II): neurological involvement. Foodborne Pathog Dis [Internet]. 2011 [cited 2023 Aug 29];8:579–85. Available from: https://pubmed.ncbi.nlm.nih.gov/21186993/. [CrossRef]
- Knezević K, Turkulov V, Canak G, Lalosević V, Tomić S. Neurotrichinosis. A cerebrovascular disease associated with myocardial injury and hypereosinophilia. Brain [Internet]. 1993 [cited 2023 Aug 29];116 ( Pt 3):483–5. Available from: https://pubmed.ncbi.nlm.nih.gov/8513394/.
- Dzikowiec M, Góralska K, Błaszkowska J. Neuroinvasions caused by parasites. Ann Parasitol [Internet]. 2017 [cited 2023 Aug 29];63. Available from: https://pubmed.ncbi.nlm.nih.gov/29385325/.
- Dalcin D, Zarlenga DS, Larter NC, Hoberg E, Boucher DA, Merrifield S, et al. Trichinella Nativa Outbreak With Rare Thrombotic Complications Associated With Meat From a Black Bear Hunted in Northern Ontario. Clinical Infectious Diseases [Internet]. 2017 [cited 2023 Sep 1];64:1367–73. Available from: https://dx.doi.org/10.1093/cid/cix165. [CrossRef]
- Feydy A, Touze E, Miaux Y, Bolgert F, Martin-Duverneuil N, Laplane D, et al. MRI in a case of neurotrichinosis. Neuroradiology [Internet]. 1996 [cited 2023 Aug 29];38 Suppl 1:S80–2. Available from: https://pubmed.ncbi.nlm.nih.gov/8811688/.
- Rosca EC, Simu M. Border zone brain lesions due to neurotrichinosis. Int J Infect Dis [Internet]. 2018 [cited 2023 Sep 1];67:43–5. Available from: https://pubmed.ncbi.nlm.nih.gov/29253712/. [CrossRef]
- Langner S, Kirsch M, Khaw A V., Stein T, Vogelgesang S, Hosten N. Diffusion-weighted imaging proves watershed infarction in neurotrichinosis. European Journal of Radiology Extra. 2007;64:45–8. [CrossRef]
- Gelal F, Kumral E, Dirim Vidinli B, Erdogan D, Yucel K, Erdogan N. Diffusion-weighted and conventional MR imaging in neurotrichinosis. http://dx.doi.org/101080/02841850510020969 [Internet]. 2005 [cited 2023 Sep 1];46:196–9. Available from: https://journals.sagepub.com/doi/10.1080/02841850510020969?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0pubmed. [CrossRef]
- Mitrović N, Milošević B, Urošević A, Nikolić N, Dakić Z, Nikolić I, et al. Severe Trichinellosis with Neurological Involvement - Neurotrichinellosis: A Case Report. Vet Glas [Internet]. 2019 [cited 2023 Sep 1];73:178–86. Available from: https://www.veterinarskiglasnik.rs/index.php/vg/article/view/114. [CrossRef]
- Walker MD, Zunt JR. Neuroparasitic infections: cestodes, trematodes, and protozoans. Semin Neurol [Internet]. 2005 [cited 2023 Aug 26];25:262–77. Available from: https://pubmed.ncbi.nlm.nih.gov/16170739/. [CrossRef]
- Bhalla D, Dumas M, Preux PM. Neurological manifestations of filarial infections. Handb Clin Neurol [Internet]. 2013 [cited 2023 Aug 26];114:235–42. Available from: https://pubmed.ncbi.nlm.nih.gov/23829914/. [CrossRef]
- Hadermann A, Amaral LJ, Van Cutsem G, Siewe Fodjo JN, Colebunders R. Onchocerciasis-associated epilepsy: an update and future perspectives. Trends Parasitol [Internet]. 2023 [cited 2023 Aug 26];39:126–38. Available from: https://pubmed.ncbi.nlm.nih.gov/36528471/. [CrossRef]
- Singh G, Angwafor SA, Njamnshi AK, Fraimow H, Sander JW. Zoonotic and vector-borne parasites and epilepsy in low-income and middle-income countries. Nat Rev Neurol [Internet]. 2020 [cited 2023 Aug 26];16:333–45. Available from: https://pubmed.ncbi.nlm.nih.gov/32427939/. [CrossRef]
- Arndts K, Kegele J, Massarani AS, Ritter M, Wagner T, Pfarr K, et al. Epilepsy and nodding syndrome in association with an Onchocerca volvulus infection drive distinct immune profile patterns. PLoS Negl Trop Dis [Internet]. 2023 [cited 2023 Aug 26];17:e0011503. Available from: https://pubmed.ncbi.nlm.nih.gov/37535695/. [CrossRef]
- Finsterer J, Auer H. Parasitoses of the human central nervous system. J Helminthol [Internet]. 2013 [cited 2023 Aug 26];87:257–70. Available from: https://pubmed.ncbi.nlm.nih.gov/23046708/. [CrossRef]
- Chai JY. Paragonimiasis. Handb Clin Neurol [Internet]. 2013 [cited 2023 Aug 26];114:283–96. Available from: https://pubmed.ncbi.nlm.nih.gov/23829919/.
- Blair D. Paragonimiasis. Adv Exp Med Biol [Internet]. 2019 [cited 2023 Aug 26];1154:105–38. Available from: https://pubmed.ncbi.nlm.nih.gov/31297761/.
- Chai JY, Jung BK. General overview of the current status of human foodborne trematodiasis. Parasitology [Internet]. 2022 [cited 2023 Aug 26];149:1262. Available from: /pmc/articles/PMC10090779/. [CrossRef]
- Gildner TE, Cepon-Robins TJ, Urlacher SS. Cumulative host energetic costs of soil-transmitted helminth infection. Trends Parasitol [Internet]. 2022 [cited 2023 Aug 26];38:629–41. Available from: https://pubmed.ncbi.nlm.nih.gov/35599133/. [CrossRef]
- Hotez PJ, Gurwith M. Europe’s neglected infections of poverty. Int J Infect Dis [Internet]. 2011 [cited 2023 Aug 28];15. Available from: https://pubmed.ncbi.nlm.nih.gov/21763173/. [CrossRef]
- Momčilović S, Cantacessi C, Arsić-Arsenijević V, Otranto D, Tasić-Otašević S. Rapid diagnosis of parasitic diseases: current scenario and future needs. Clinical Microbiology and Infection. 2019;25:290–309. [CrossRef]
- Short EE, Caminade C, Thomas BN. Climate Change Contribution to the Emergence or Re-Emergence of Parasitic Diseases. Infect Dis [Internet]. 2017 [cited 2023 Aug 29];10:117863361773229. Available from: /pmc/articles/PMC5755797/. [CrossRef]
- Romig T. Echinococcus multilocularis in Europe--state of the art. Vet Res Commun [Internet]. 2009 [cited 2023 Aug 26];33 Suppl 1. Available from: https://pubmed.ncbi.nlm.nih.gov/19578966/. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).