I. Introduction
For female patients, breast cancer is the most common malignancy worldwide. Almost 1.5 million women below the age of 45 every year are breast cancer patients. This is about 11% of total reported cancer cases per year [
1,
2,
3]. These tumors tend to begin from ductal hyperproliferation and can either grow into tumors of a benign nature or aggressive metastatic carcinomas. It is worth mentioning that young patients have a higher risk of developing more aggressive carcinomas [
4,
5]. Breast cancer has a multitude of risk factors that can increase the possibility of developing the disease; among them are age, sex, family history unhealthy lifestyle, gene mutations, or even hormone replacement therapy [
6].
For a patient diagnosed with breast cancer at a young age, fertility and pregnancy issues that might arise during treatment contribute to their emotional and psychological distress [
7]. Physicians should address these issues early and intervene promptly after the diagnosis, to positively affect the outcome of the treatment and the long-term quality of life for these women [
8]. If the disease is diagnosed early, it has a high survival rate and a good prognosis [
9] and therefore, guidelines internationally recommend that physicians should discuss in the early stages of the disease informing young patients of the potential risks that they might suffer from during treatment and discuss potential solutions for their fertility preservation [
10,
11,
12]. Cancer treatment can damage primordial follicles which can lead to early menopause or premature ovarian insufficiency.
The amount of primordial follicles, also known as ovarian reserve, plays a substantial role in a patient’s fertility status [
13]. To tackle the problem, physicians need to present sustainable resolutions to the problem. One of the most suitable treatments for fertility preservation in these patients, is the temporary suppression with luteinizing hormone-releasing analogs (LHRHa), while the patient undergoes chemotherapy and cryopreservation. For cryopreservation, the physicians might deem it necessary to either cryopreserve ovarian tissue taken from the patient before any treatment or cryopreserve embryos/oocytes [
14]. Something of great interest is that after young patients are diagnosed with breast cancer approximately 50% of them are interested in becoming pregnant right after completion of therapy, unfortunately, breast cancer survivors are amongst the lowest, possibility-wise, cancer survivors that can have a subsequent pregnancy. This is due to the gonado-toxic therapeutic approach and the prolonged period of treatment that physicians tend to follow [
15,
16].
Although there is a plethora of data available on the matter at hand, there still exist several obstacles that limit access to fertility preservation techniques and mechanisms [
14,
17], while it is also notable that there are very limited data on the number of patients that are willing to adopt any of these preservation techniques. The lack of this information also affects the public health sector and the health organization system to cater for both fertility and oncology units. The authors aim to comprehensively review published evidence on breast cancer patients and their fertility status but also identify specific treatment and non-treatment-related factors linked to the impaired infertility of these patients.
II. Methodology
II.a Search Strategy
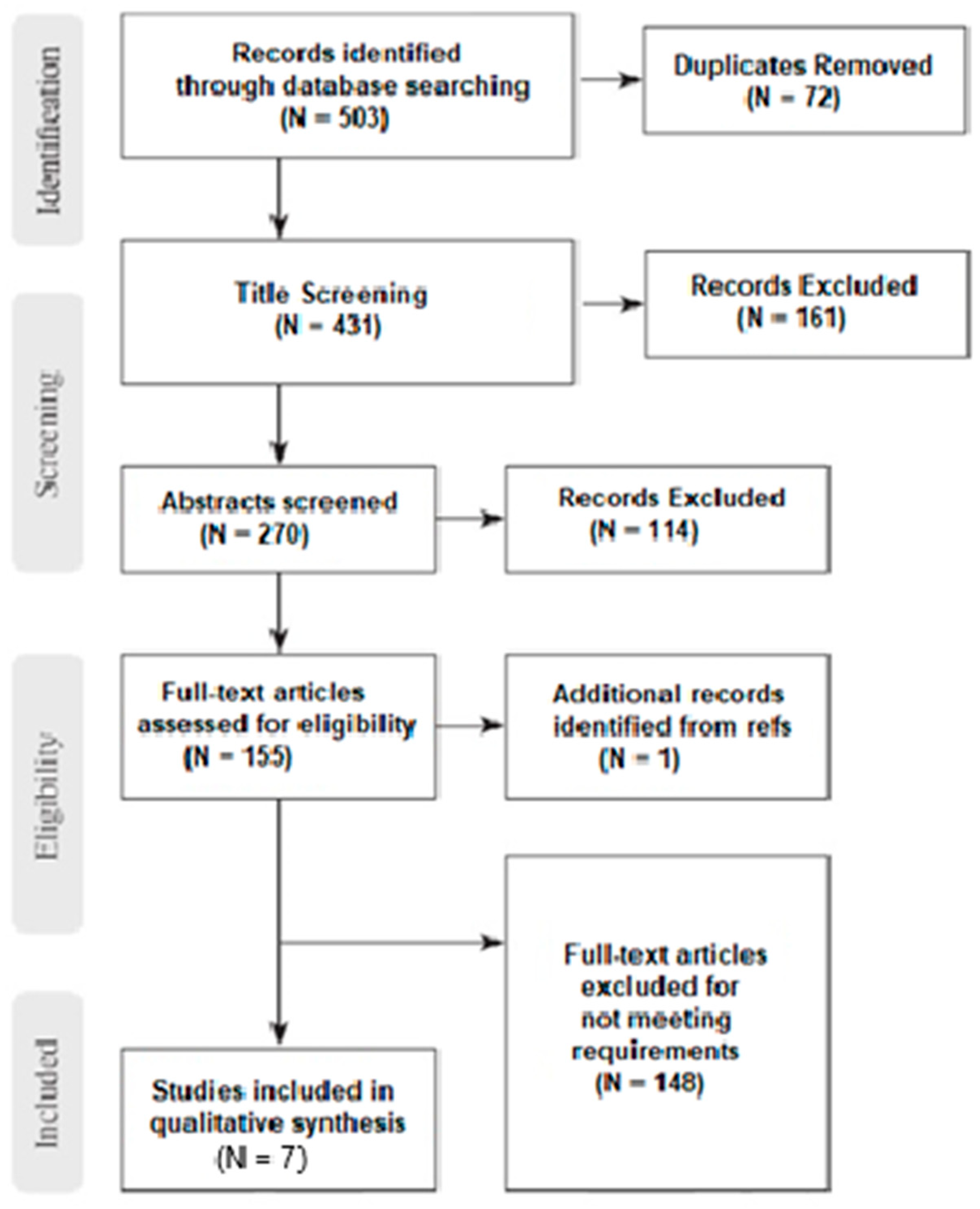
This review is following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The authors conducted an extensive search on various medical databases until the 31 of July 2023. The databases utilized for the gathering of potentially relevant studies were PubMed, Embase, and the Cochrane Library.
Only the studies from the last 15 years were considered relevant due to the different modalities that were used for treating breast cancer in the past few decades and to ensure consistency in evidence across the various relevant study groups. The following terms were used for searching: “ovaria”, “ovarian”, “fertility”, “reserve”, “reservation”, “preserve”, “preservation”, “female”, “woman”, “women”, “females”, “breast”, “breasts”, “mammary”, “mammary gland”, “lymphomas”, “lymphoma”, “malignancy”, “malignancies”, “cancer”, “cancers”, “survival, “survivor”, “survivors”, ((ovaria OR ovary OR ovarian OR fertility) AND (reserve OR reservation OR preserve OR preservation)) AND((women OR woman OR female OR females) AND ((breast OR breasts OR mammary gland OR lymphomas AND (malignancy OR malignancies OR cancer OR cancers)) AND (survival OR survivor OR survivors))). No other restrictions were applied to the query and articles from all languages were considered.
The results were independently assessed by three authors (IB, MK, KD) by reading their abstracts. If any results were considered relevant, the authors carried on reading the entire paper, and all inclusion and exclusion criteria were applied to narrow down the dataset to the relevant studies of interest. Any disagreements between authors were resolved following consensus. Furthermore, all selected papers were manually searched for relevant articles that could be of interest following the snowball procedure.
Figure 1.
Prisma flow diagram.
Figure 1.
Prisma flow diagram.
II.b Inclusion and Exclusion Criteria.
Inclusion Criteria
The eligible studies that the authors considered were case-control, cross-sectional and cohort studies, which examined the fertility or infertility status for all survivors of breast cancer through their achieving of pregnancy. Survivors were considered all patients that have achieved full remission after finishing treatment and were assessed for their fertility status post treatment.
Exclusion Criteria
All animal studies, cell culture studies, case reports and case series were not considered by the authors and were excluded from the result set. Any studies that assessed the fertility status of patients straight after their breast cancer treatment was concluded were also deemed as not suitable. The authors also stumbled upon some studies with overlapping populations, in all these cases the most up to date, relevant study was considered.
II.c Data Extraction and Quality Assessment
After applying all inclusion and exclusion criteria the result set was narrowed down to 7 relevant studies. Two authors (IB and MK) independently reviewed all articles and extracted all the data of interest using a customized data extraction form. The form included the following characteristics from all related studies: Author, Year of publication, Type of Study, Period of Study, Country where applicable, number of patients, number of control groups where applicable, outcome variables, exposure variables, and assessment of the outcome of the study where mentioned. Based on the extracted characteristics, each study was rated either poor, fair, or good, emphasizing the sample size and the appropriate reporting of the outcome variables.
III. Ovarian Suppression with Gonadotropin-Releasing Hormone Agonists
The use of Gonadotropin-releasing hormone agonists aims to lower both gonadotropins and sex hormone levels. They are commonly used to lower sex hormone levels in the treatment of hormone-sensitive cancers like breast and prostate cancers [
17]. The mechanism by which ovarian suppression during chemotherapy protects ovarian function is not clear. The first studies concerned patients who received combination chemotherapy for Hodgkin's disease and acute lymphocytic leukemia or cyclophosphamide therapy for renal diseases [
18,
19]. In this group, ovarian function was more disrupted in women of reproductive age than in children and young preadolescent girls.
Another proposed mechanism is the medically induced hypogonadotropic status which reduces the number of primordial follicles that are in a differentiation state and are more susceptible to alterations by chemotherapy [
20]. Accordingly, both the hypo-estrogenic environment and low inhibin may prevent the increase in FSH thus protecting the follicles from atresia [
21,
22].
GnRH agonists have a higher potency compared to the natural GnRH molecule due to their reduced susceptibility to enzymatic degradation and higher receptor affinity. A transient release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) is caused by GnRH agonists when they bind to GnRH receptors on pituitary gonadotropin-producing cells. It usually takes 1 week of therapy for the GnRH receptors to be downregulated along with a decline in the pituitary production of both LH and FSH [
23]. Several formulations of GnRH agonists are approved for parenteral administration and available on the market, including leuprolide, goserelin, triptorelin, buserelin, and histrelin.
In the last 20 years, numerous clinical studies have been conducted concerning the prophylactic administration of GnRH agonists alongside chemotherapy in women with breast cancer [
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40]. The authors of this article have chosen to refer exclusively to the studies that comment on the achievement of pregnancy for each of the fertility preservation techniques.
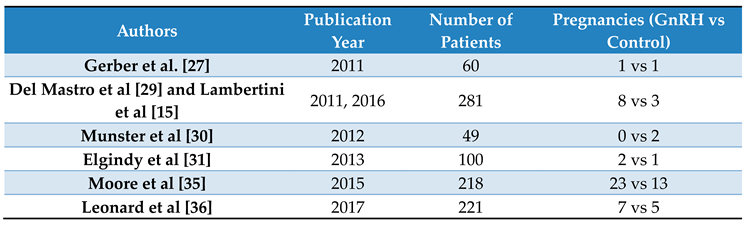
Table 1 lists the studies that report pregnancy and examines its achievement between the group of women who underwent ovarian suppression and those who did not receive prophylactic hormone therapy.
Among the 739 patients 66 achieved spontaneous pregnancy, of which 25 (37.87%) did not receive prophylactic agonist therapy and 41 (62.13%) did. The above results were obtained in the context of the clinical studies ZORO [
27], POEMS study [
35], MOFFITTs [
30], OPTION [
36], and PROMISE-GIM6 [
29] from 2011 to 2017. In these studies, Gosarelin 3.6 mg SC and Triptorelin 3.75 mg IM were administered 1 to 2 weeks before the start of chemotherapy and then for up to 4 weeks during chemotherapy.
There is controversy regarding the effectiveness of GnRH agonists in achieving pregnancy. The use of gonadotropin-releasing hormone agonists (GnRHa) is still considered investigational, by several authorities. Whereas previous publications have raised the fear of GnRHa's possible detrimental effects in patients with hormone receptor-positive breast cancers, recent randomized controlled trials have shown that it either improves or does not affect disease-free survival in such patients [
41].
IV. Oocyte Vetrifications and Embryo Cryopreservation
IV.a. Ovarian Stimulation Protocol
Cryopreservation of embryos or eggs is the most effective solution for preserving fertility in women with breast cancer [
42]. However, oocyte retrieval is preceded by ovarian stimulation through a hyperestrogenic environment [
43]. Short-term exposure to high levels of estrogens has raised concerns about the safety of conventional protocols and has led to the development of new ones that aim to counterbalance estrogen exposure in women with breast cancer undergoing ovarian stimulation for fertility preservation [
44,
45]. These alternative stimulation protocols consist of the addition of the selective estrogen receptor (ER) modulator tamoxifen or the aromatase-inhibitor letrozole, but their effectiveness has never been compared to standard ovarian stimulation in any randomized controlled trial (RCT) [
46].
Balkenende et al in 2022 were the first to compare the effectiveness of alternatives with traditional stimulation protocols and concluded that despite the noticeable reduction in estradiol peak, alternative ovarian stimulation protocols that included tamoxifen or letrozole did not affect the number of cumulus-oocyte complexes (COCs) retrieved at follicle aspiration. There was also no evidence of a difference in the number of oocytes or embryos banked and no difference in number of canceled cycles [
47]. And in the antipode 2 studies only comment that there may be a negative effect of letrozole or tamoxifen on fertilization and embryo quality, in fertility preservation cycles. Further studies are needed to confirm these findings [
48,
49].
The most widely used protocol to stimulate patients with breast cancer is the oral administration of letrozole 5 mg or 60mg tamoxifen from days 2–3 of the cycle. After 2 days of treatment with letrozole, a variable dose of recombinant FSH (rFSH) between 150 and 300 IU/day is added. When the concentration of serum estradiol exceeds 250 pg/ml or the follicles reach a size greater than 13 mm in diameter, administration of GnRH antagonists is started to avoid the premature peak of LH. Follicular growth is monitored until at least two of the follicles reach 20 mm in diameter and at that moment ovulation is triggered with the agonists of GnRH [
50,
51,
52,
53,
54,
55,
56,
57]. By comparing the use of GnRH agonists versus hCG trigger ovulation, it was found that the agonists achieved a greater and faster decline of the estradiol levels without reducing the number of mature oocytes collected or the fertilization rate [
52,
54,
57]. This protocol with letrozole, along with final rFSH and the induction of ovulation with GnRH agonists (triptorelin), has been implemented in an extended form, independent of the molecular phenotype of breast cancer [
57].
Apart from the addition of letrozole or tamoxifen to conventional stimulation protocols, alternative approaches have now been patented. A practical issue that arises in the management of these women is that in many cases the urgency of fertility preservation does not allow the time to initiate induction early in the follicular phase. Developments in the physiology of human reproduction and the investigation of the multiple waves theory contributed in this direction, which states that recruitment of a group of follicles is done multiple times throughout a single menstrual cycle, enabling the initiation of ovarian stimulation at any time during the menstrual cycle [
58]. The present study discusses the novel ovarian stimulation regimen, known as the random-start and luteal-phase protocol, which is distinct from the conventional stimulation approach. The development of this method represents a significant milestone in the field of fertility preservation technology, as it allows for the preservation of fertility in cancer patients without any delay in cancer therapy. It is noteworthy that the primary objective of the random-start method is cryopreservation, and hence, the challenge of utero-ovarian synchronization and preparation for embryo transfer, which is a potential pitfall of this approach, is not a concern for cancer patients. In comparison to conventional techniques, the random-start method exhibits a tendency towards a slightly elevated total dose of gonadotropin and a prolonged stimulation period during the cycle. Nevertheless, no discernible distinction in the total quantity of retrieved oocytes and mature oocytes was observed between the two approaches. [
59].
Finally, a promising technique that aims at obtaining a high number of oocytes in a limited time is done by performing two cycles of ovarian stimulation within one menstrual cycle, each at the follicular and luteal phases. Originally introduced by Kuang et al [
60] the initial oocyte retrieval is done following the first round of stimulation. The second round begins immediately on the following day of oocyte retrieval, followed by the second oocyte retrieval. In other trials examining the efficacy of this approach, variations of the double stimulation regimen utilizing different types and doses of gonadotropin are effective. The number of total oocytes, mature oocytes, and blastocysts in the first and second cycles was similar. More importantly, the number of total oocytes, mature oocytes, and embryos from the double stimulation method was greater than that from the conventional cycle with a single stimulation [
60,
61,
62].
IV.b Oocyte/Embryo Cryopreservation
Cryopreservation of oocytes and/or embryos is the first option for fertility preservation in women of reproductive age, who have sufficient ovarian reserve and are diagnosed with breast cancer, regardless of the histological type of the tumor [
63]. Achieving pregnancy is related to the number of mature oocytes retrieved, which is dependent on the age of the patient and her ovarian reserve at diagnosis [
64,
65]. A live birth rate of >40% can be estimated in women younger than 35 years, and <30% in older patients, with a very low success after the age of 40 years [
66].
Specifically for breast cancer, although there is no apparent negative influence of breast cancer diagnosis on the success of the procedure, some evidence suggests a potentially reduced performance of oocyte/embryo cryopreservation in breast cancer patients carrying germline BRCA pathogenic variants [
66]. However, oocyte/embryo cryopreservation remains the first option to be discussed also in BRCA-mutated breast cancer patients [
63,
65]. Importantly, this strategy allows access to preimplantation genetic testing that can be of importance for these women [
67].
There is a plethora of evidence in the literature regarding the technique of oocyte/embryo cryopreservation as a way of preserving fertility in young cancers [
68]. However, the data we have from the literature only targeted at breast cancer patients are extremely limited and the authors of this article chose to refer exclusively to them. More specifically, from the literature we have 9 clinical studies that investigate this particular technique in women of reproductive age [
68,
69,
70,
71,
72,
73,
74,
75,
76], and 5 of them report the achievement or not of pregnancy [
68,
69,
70,
71,
72]. More specifically, of the 72 women who underwent embryo transfer, 38 pregnancies were achieved (52.7%). All studies report no disease recurrence after embryo transfer. A comparison of the results between embryo cryopreservation and oocyte cryopreservation is not performed due to a lack of data from studies comparing breast cancer. Finally, it is worth noting that Alvarez et al [
70], compared pregnancy rates in women with different cancers (gynecological cancer, breast cancer, hematological malignancies), and the case of breast cancer recorded the highest pregnancy rates.
V. Ovarian Tissue Cryopreservation (OTC)
OTC is a method of preserving ovarian tissue with a cryopreservation technique without the ovarian stimulation process. This method is suitable for prepubertal girls or pre-menarchal adolescents diagnosed with malignancy and patients unable to undergo COS because of an urgent need for cancer therapy. It is also recommended for single women who do not wish to seek a sperm donor to freeze embryos. OTC is not considered an experimental method anymore, and it has been recognized since 2013 by the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology as one of the clinically established methods for fertility preservation [
77].
The utilization of OCT offers numerous benefits, including the ability to preserve fertility in emergency situations without the need for prior ovarian stimulation protocols, while also ensuring the maintenance of both fertility and hormonal production [
78]. Upon the decision to proceed with transplantation, the thawed ovarian tissue can be re-implanted either in its original location within the pelvic cavity (orthotopic transplantation) or in an alternative site (heterotopic transplantation), such as the abdominal wall or forearm. Recent studies have reported a pregnancy success rate of 26% following the transplantation of cryopreserved ovarian tissue, which encompasses both natural and IVF conceptions. [
79].
Again, regarding the case of breast cancer, the data we have from the literature is limited [
75]. 12 were found [
78,
79,
80,
81,
82,
83,
84,
85,
86,
87,
88,
89] which report a total of 24 pregnancies. Of these, 3 concerned case reports [
81,
89,
90], with one of the cases concerning ovarian tissue cryopreservation in breast cancer diagnosed during pregnancy [
81]. The rest of the data is derived from case series that generally concern the preservation of fertility with OCT in various malignancies. The cases were screened so that the data would have consistency exclusively for breast cancer.
VI. In-Vitro Maturation (IVM)
It is known that breast cancer cell proliferation can be induced by estrogen [
91]; therefore, it is recommended to avoid high concentrations of oestradiol in these patients [
92]. During IVM cycles, oestradiol concentrations are within the natural follicular phase range of up to 150 pmol/l [
93], far from the supra-high concentrations of oestradiol during ovarian stimulation. In this aspect, the advantage of IVM treatment for breast cancer patients is the reduced risk of stimulating estrogen-sensitive tumours, which can enhance malignant cell proliferation [
94].
Initially, IVM was proposed as a means to circumvent the need for ovarian stimulation, followed by oocyte and embryo freezing. However, there is a dearth of data on the application of this method. The first clinical study on breast cancer patients was conducted in 2010, which reported pregnancy rates of 3.8% and 8.1% for oocyte freezing and embryo freezing, respectively [
95]. A more recent study involving 9 breast cancer patients revealed that out of 22 oocytes subjected to IVM, 12 matured successfully [
96]. Furthermore, this study compared the number of mature oocytes obtained through IVM in groups of patients with different types of cancer and fertility problems. The results indicated that patients with breast cancer had a lower proportion of oocytes that matured in vitro compared to patients with other cancers and those with fertility problems (54.5% vs. 81.2% and 80.0%) [
96,
97]. In 2020, Malacarne et al[
98] arrived at the conclusion that there was no significant difference in the mean number of oocytes retrieved for each breast cancer patient following ovarian stimulation, as compared to healthy control women, including oocyte donors, women undergoing fertility preservation for non-medical reasons, and female partners of infertile men participating in an in vitro fertilization (IVF) program.
Consequently, the notion of integrating IVM after ovarian tissue oocyte retrieval has garnered significant interest in enhancing fertility preservation outcomes, owing to the successful results of IVM [
99]. This approach entails the maturation of immature oocytes obtained during ovarian tissue cryopreservation (OTC) through IVM, followed by their cryopreservation alongside ovarian tissue [
100,
101,
102]. Despite the growing body of evidence on OTO-IVM, its implementation remains in its nascent stages, and its efficacy is yet to be fully established [
99].
VII. Discussion
Infertility among young female breast cancer patients is still a big-impact, negative outcome from treatment of the disease. Most of these women will be advised to undergo adjuvant chemotherapy with the assistance of anti-hormonal therapy in some instances to minimize the risk of death or recurrence of the tumor. The gonadotoxicity of adjuvant chemotherapy, especially if it is performed with alkylating agents like cyclophosphamide and the fact that it can accelerate the rate of primordial follicles loss or decrease the reserve of primordial follicles for a patient can be catastrophic for them and cause infertility and thus any physiological or psychological problems related to it.
Multiple factors can affect ovarian failure following breast cancer treatment, namely the age of a patient, the type and dosage of chemotherapy, how many cycles of chemotherapy they underwent, their ovarian reserve before treatment, etc. These patients must have an assessment of their fertility status before undergoing therapy. In developed countries, this is already standard practice with hormonal evaluation tests or even descriptive statistics. Educating the patients accordingly and keeping family history data along with annual mammography screening and chemo-preventative drugs have led to higher survival rates, fewer deaths, and lower occurrence. Maintaining fertility after breast cancer treatment for these patients is very important. Scientists are looking into new ways of preserving a patient’s fertility by ovarian stimulation with different agents like Letrozole, FSH, and Tamoxifen in order to minimize the impact that elevated serum estrogen levels can have on the growth of the tumor.
Oncofertility counseling is now a concept that is catching on and allows patients to be informed on the options they have in order to be able to live a normal life and have their own offsprings after they are done with the cancer treatment. Be educated and counseled appropriately. This requires a multi-disciplinary approach to the planning of the treatment, with free access to resources and information to have a better chance against infertility. Establishing rapid fertility consultation links with programs related to the survival of breast cancer could help in ensuring that all these young women who would possibly lose their fertility due to the gonadotoxic chemotherapy treatment are counseled for the adherent effects of it and have an increased likelihood of childbearing after cancer treatment.
On the bright side though, these new techniques that scientists are looking into have very positive outcomes, with tests indicating that offsprings born by breast cancer survivors have no congenital abnormalities and can live a healthy life. Although currently we are passing through a period of change and uncertainty regarding the variety of fertility preservation mechanisms for young breast cancer survivors, the evolution and development of new techniques in the near future promise to be very exciting.
References
- National Cancer Institute - Surveillance, Epidemiology and End Results Program. http://seer.cancer.gov/statfacts/html/breast.html. Accessed 7 May 20.
- Stewart BW, Wild CP. World Cancer Report 2014. Geneva, Switzerland: WHO Press; 2014.
- WHO: Geneva, Switzerland. Breast cancer. http://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/17.
- Azim HA, Partridge AH. Biology of breast cancer in young women. Breast Cancer Res. 2014;16:427. [CrossRef]
- Lambertini M, Pinto AC, Ameye L, Jongen L, Del Mastro L, Puglisi F, et al. The prognostic performance of adjuvant! Online and Nottingham prognostic index in young breast cancer patients. Br J Cancer. 2016;115: 1471–8. [CrossRef]
- Majeed W, Aslam B, Javed I. et al. Breast cancer: major risk factors and recent developments in treatment. APJCP. 2014;15:3353–3358. [CrossRef]
- Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104:386–405. [CrossRef]
- Rosenberg SM, Newman LA, Partridge AH. Breast cancer in young women: rare disease or public health problem? JAMA Oncol. 2015;1:877–8.
- DeSantis CE, Fedewa SA, Goding Sauer A. et al. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66:31–42. [CrossRef]
- Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013; 31:2500–10. [CrossRef]
- Peccatori FA, Azim HA Jr, Orecchia R, Hoekstra HJ, Pavlidis N, Kesic V, et al. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi160–170. [CrossRef]
- Paluch-Shimon S, Pagani O, Partridge AH, Bar-Meir E, Fallowfield L, Fenlon D, et al. Second international consensus guidelines for breast cancer in young women (BCY2). Breast. 2016;26:87–99. [CrossRef]
- Coccia ME, Rizzello F. Ovarian reserve. Ann N Y Acad Sci. 2008;1127:27–30.
- Lambertini M, Goldrat O, Barragan-Carrillo R, Viglietti G, Demeestere I, Villarreal-Garza C. Viable options for fertility preservation in breast cancer patients: a focus on Latin America. Rev Investig Clin. 2017;69:103–13. [CrossRef]
- Letourneau JM, Ebbel EE, Katz PP, Katz A, Ai WZ, Chien AJ, et al. Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer. 2012;118: 1710–7. [CrossRef]
- Stensheim H, Cvancarova M, Møller B, Fosså SD. Pregnancy after adolescent and adult cancer: a population-based matched cohort study. Int J Cancer. 2011;129:1225–36. [CrossRef]
- Choi S, Lee AK. Efficacy and safety of gonadotropin-releasing hormone agonists used in the treatment of prostate cancer. Drug Healthc Patient Saf. 2011;3:107-19. Epub 2011 Dec 22. PMID: 22279415; PMCID: PMC3264425. [CrossRef]
- Rivkees SA, Crawford JD. The relationship of gonadal activity and chemotherapy-induced gonadal damage. JAMA. 1988;259(14):2123–2125. [CrossRef]
- Karalexi MA, Kontogeorgi A, Papaioannou G, Neofytou S, Messaropoulos P, Moschovi M, Kalantaridou SN. Fertility status in childhood cancer survivors of hematological malignancies: a systematic review. Hormones (Athens). 2023 Jun;22(2):211-221. Epub 2023 Mar 25. PMID: 36964890. [CrossRef]
- Lobo RA. Potential options for preservation of fertility in women. N Engl J Med. 2005;353:64–73. [CrossRef]
- Blumenfeld Z. How to preserve fertility in young women exposed to chemotherapy? The role of GnRH agonist cotreatment in addition to cryopreservation of embrya, oocytes, or ovaries. Oncologist. 2007;12(9):1044–1054. [CrossRef]
- Blumenfeld Z. Gonadotropin-releasing hormone agonist for preservation of ovarian function during (neo)adjuvant chemotherapy for breast cancer. J Clin Oncol. 2012 Sep 10;30(26):3310; author reply 3312-3. Epub 2012 May 29. PMID: 22649145. [CrossRef]
- Conn PM, Crowley WF Jr. Gonadotropin-releasing hormone and its analogues. N Engl J Med. 1991;324(2):93. [CrossRef]
- Li M, Huang H, Liang Y, Tan J, Lin D. Effect of Zoladex administered before chemotherapy on menstruation of patients with breast cancer. Chin J Clin Oncol. 2008;35:905–907.
- Badawy A, Elnashar A, El-Ashry M, Shahat M. Gonadotropin-releasing hormone agonists for prevention of chemotherapy-induced ovarian damage: prospective randomized study. Fertil Steril. 2009;91:694–697. [CrossRef]
- Sverrisdottir A, Nystedt M, Johansson H, Fornander T. Adjuvant goserelin and ovarian preservation in chemotherapy treated patients with early breast cancer: results from a randomized trial. Breast Cancer Res Treat. 2009;117:561–567. [CrossRef]
- Gerber B, von Minckwitz G, Stehle H, et al. Effect of luteinizing hormone-releasing hormone agonist on ovarian function after modern adjuvant breast cancer chemotherapy: the GBG 37 ZORO study. J Clin Oncol. 2011;29:2334–2341. [CrossRef]
- Sun J, Ren Y, Li W. Effect of Zoladex administered before chemotherapy on menstruation of patients with breast cancer. China Disabil Med. 2011;19:15–16.
- Del Mastro L, Boni L, Michelotti A, et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trial. JAMA. 2011;306:269–276.
- Munster PN, Moore AP, Ismail-Khan R, et al. Randomized trial using gonadotropin-releasing hormone agonist triptorelin for the preservation of ovarian function during (neo)adjuvant chemotherapy for breast cancer. J Clin Oncol. 2012;30:533–538. [CrossRef]
- Elgindy EA, El-Haieg DO, Khorshid OM, et al. Gonadatrophin suppression to prevent chemotherapy-induced ovarian damage: a randomized controlled trial. Obstet Gynecol. 2013;121:78–86.
- Song G, Gao H, Yuan Z. Effect of leuprolide acetate on ovarian function after cyclophosphamide-doxorubicin-based chemotherapy in premenopausal patients with breast cancer: results from a phase II randomized trial. Med Oncol. 2013;30:667. [CrossRef]
- Jiang FY, Zhang QQ, Zeng J. Protective effect of GnRHa on chemo-therapy induced ovarian damage in breast cancer patients. Shandong Med J. 2013;53:16–18.
- Karimi-Zarchi M, Forat-Yazdi M, Vafaeenasab MR, et al. Evaluation of the effect of GnRH agonist on menstrual reverse in breast cancer cases treated with cyclophosphamide. Eur J Gynaecol Oncol. 2014;35:59–61.
- Moore HCF, Unger JM, Phillips K-A, et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med. 2015;372:923–932. [CrossRef]
- Leonard RCF, Adamson DJA, Bertelli G, et al. GnRH agonist for protection against ovarian toxicity during chemotherapy for early breast cancer: the Anglo Celtic Group OPTION trial. Ann Oncol. 2017;28:1811–1816. [CrossRef]
- Zhang Y, Ji Y, Li J, et al. Sequential versus simultaneous use of chemotherapy and gonadotropin-releasing hormone agonist (GnRHa) among estrogen receptor (ER)-positive premenopausal breast cancer patients: effects on ovarian function, disease-free survival, and overall survival. Breast Cancer Res Treat. 2018;168:679–686. [CrossRef]
- Lee DY, Kim JY, Yu J, Kim SW. Prediction of Successful Ovarian Protection Using Gonadotropin-Releasing Hormone Agonists During Chemotherapy in Young Estrogen Receptor-Negative Breast Cancer Patients. Front Oncol. 2020 Jun 16;10:863. PMID: 32656076; PMCID: PMC7326007. [CrossRef]
- Zong X, Yu Y, Yang H, et al. Effects of Gonadotropin-Releasing Hormone Analogs on Ovarian Function Against Chemotherapy-Induced Gonadotoxic Effects in Premenopausal Women With Breast Cancer in China: A Randomized Clinical Trial. JAMA Oncol. 2022;8(2):252–258. [CrossRef]
- Blondeaux E, Massarotti C, Fontana V, Poggio F, Arecco L, Fregatti P, Bighin C, Giannubilo I, Ruelle T, Razeti MG, Boni L, Anserini P, Del Mastro L, Lambertini M. The PREgnancy and FERtility (PREFER) Study Investigating the Need for Ovarian Function and/or Fertility Preservation Strategies in Premenopausal Women With Early Breast Cancer. Front Oncol. 2021 Jun 3;11:690320. PMID: 34150661; PMCID: PMC8210666. [CrossRef]
- Blumenfeld Z. Fertility Preservation Using GnRH Agonists: Rationale, Possible Mechanisms, and Explanation of Controversy. Clin Med Insights Reprod Health. 2019 Aug 21;13:1179558119870163. PMID: 31488958; PMCID: PMC6710670. [CrossRef]
- Razeti MG, Soldato D, Arecco L, Levaggi A, Puglisi S, Solinas C, Agostinetto E, Spinaci S, Lapuchesky L, Genova C, Massarotti C, Lambertini M. Approaches to Fertility Preservation for Young Women With Breast Cancer. Clin Breast Cancer. 2023 Apr;23(3):241-248. PMID: 36710145. [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril 2019;112:1022–1033.
- Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z.. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol 2005;23:4347–4353. [CrossRef]
- Revelli A, Porcu E, Levi Setti PE, Delle Piane L, Merlo DF, Anserini P.. Is letrozole needed for controlled ovarian stimulation in patients with estrogen receptor-positive breast cancer? Gynecol Endocrinol 2013;29:993–996.
- Dahhan T, Balkenende E, van WM, Linn S, Goddijn M.. Tamoxifen or letrozole versus standard methods for women with estrogen-receptor positive breast cancer undergoing oocyte or embryo cryopreservation in assisted reproduction. Cochrane Database Syst Rev 2013;11:CD010240. [CrossRef]
- Balkenende EME, Dahhan T, Beerendonk CCM, Fleischer K, Stoop D, Bos AME, Lambalk CB, Schats R, Smeenk JMJ, Louwé LA, Cantineau AEP, de Bruin JP, Linn SC, van der Veen F, van Wely M, Goddijn M. Fertility preservation for women with breast cancer: a multicentre randomized controlled trial on various ovarian stimulation protocols. Hum Reprod. 2022 Jul 30;37(8):1786-1794. PMID: 35776109; PMCID: PMC9340107. [CrossRef]
- Shulman Y, Almog B, Kalma Y, Fouks Y, Azem F, Cohen Y. Effects of letrozole or tamoxifen coadministered with a standard stimulation protocol on fertility preservation among breast cancer patients. J Assist Reprod Genet. 2021 Mar;38(3):743-750. PMID: 33409757; PMCID: PMC7910385. [CrossRef]
- Sonigo C, Sermondade N, Calvo J, Benard J, Sifer C, Grynberg M. Impact of letrozole supplementation during ovarian stimulation for fertility preservation in breast cancer patients. Eur J Obstet Gynecol Reprod Biol X. 2019 May 11;4:100049. PMID: 31673686; PMCID: PMC6817658. [CrossRef]
- Sonmezer M, Oktay K. Fertility preservation in young women undergoing breast cancer therapy. Oncologist. 2006;11:422–36. [CrossRef]
- Oktay K, et al. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab. 2006;91:3885–90. [CrossRef]
- Oktay K, et al. GnRH agonist trigger for women with breast cancer undergoing fertility preservation by aromatase inhibitor/FSH stimulation. Reprod Biomed Online. 2010;20:783–8. [CrossRef]
- Oktay K, Buyuk E, Davis O. Fertility preservation in breast cancer patients: IVF and embryo cryopreservation afterovarian stimulation with tamoxifen. Hum Reprod. 2003;18:90–5. [CrossRef]
- Reddy J, Oktay K. Ovarian stimulation and fertility preservation with the use of aromatase inhibitors in women with breast cancer. Fertil Steril. 2012;98:1363–9. [CrossRef]
- Oktay K. Further evidence on the safety and success of ovarian stimulation with letrozole and tamoxifen in breast cancer patients undergoing in vitro fertilization to cryopreserve their embryos for fertility preservation. J Clin Oncol. 2005;23:3858–9. [CrossRef]
- Checa M, et al. The effects of letrozole on ovarian stimulation for fertility preservation in cancer-affected women. Reprod Biomed Online. 2012;24:606–10. [CrossRef]
- Lalami, I., Labrosse, J., Cedrin-Durnerin, I. et al. Is letrozole during ovarian stimulation useful in breast cancer patients undergoing fertility preservation to reduce early luteal progesterone levels following GnRH-agonist trigger?. Reprod Biol Endocrinol 20, 87 (2022). [CrossRef]
- Baerwald A, Pierson R. Ovarian follicular waves during the menstrual cycle: physiologic insights into novel approaches for ovarian stimulation. Fertil Steril. 2020;114:443–457. [CrossRef]
- Danis RB, Pereira N, Elias RT. Random start ovarian stimulation for oocyte or embryo cryopreservation in women desiring fertility preservation prior to gonadotoxic cancer therapy. Curr Pharm Biotechnol. 2017;18:609–613. [CrossRef]
- Kuang Y, Chen Q, Hong Q, Lyu Q, Ai A, Fu Y, et al. Double stimulations during the follicular and luteal phases of poor responders in IVF/ICSI programmes (Shanghai protocol) Reprod Biomed Online. 2014;29:684–691.
- Ubaldi FM, Capalbo A, Vaiarelli A, Cimadomo D, Colamaria S, Alviggi C, et al. Follicular versus luteal phase ovarian stimulation during the same menstrual cycle (DuoStim) in a reduced ovarian reserve population results in a similar euploid blastocyst formation rate: new insight in ovarian reserve exploitation. Fertil Steril. 2016;105:1488–1495.e1. [CrossRef]
- Sfakianoudis K, Pantos K, Grigoriadis S, Rapani A, Maziotis E, Tsioulou P, Giannelou P, Kontogeorgi A, Pantou A, Vlahos N, Koutsilieris M, Simopoulou M. What is the true place of a double stimulation and double oocyte retrieval in the same cycle for patients diagnosed with poor ovarian reserve? A systematic review including a meta-analytical approach. J Assist Reprod Genet. 2020 Jan;37(1):181-204. Epub 2019 Dec 3. PMID: 31797242; PMCID: PMC7000611. [CrossRef]
- Warner E, Glass K, Foong S, Sandwith E. Update on fertility preservation for younger women with breast cancer. CMAJ. 2020 Aug 31;192(35):E1003-E1009. PMID: 32868272; PMCID: PMC7458684. [CrossRef]
- Lambertini M, Peccatori FA, Demeestere I. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2020;31(12):1664–1678. [CrossRef]
- ESHRE Guideline Group on female fertility preservation. Anderson R.A., Amant F., Braat D. ESHRE guideline: female fertility preservation. Hum Reprod Open. 2020;(4):hoaa052.
- Cobo A., García-Velasco J., Domingo J. Elective and onco-fertility preservation: factors related to IVF outcomes. Hum Reprod. 2018;33(12):2222–2231. [CrossRef]
- Vuković P., Peccatori F.A., Massarotti C. Preimplantation genetic testing for carriers of BRCA1/2 pathogenic variants. Crit Rev Oncol Hematol. 2021;157:103201.
- Oktay K, Turan V, Bedoschi G, Pacheco FS, Moy F. Fertility preservation success subsequent to concurrent aromatase inhibitor treatment and ovarian stimulation in women with breast cancer. J Clin Oncol: Off J Am Soc Clin Oncol. 2015;33(22):2424–2429. [CrossRef]
- Hashimoto T, Nakamura Y, Obata R, Doshida M, Toya M, Takeuchi T, Kyono K. Effects of fertility preservation in patients with breast cancer: A retrospective two-centers study. Reprod Med Biol. 2017 Sep 10;16(4):374-379. PMID: 29259491; PMCID: PMC5715900. [CrossRef]
- Alvarez RM, Ramanathan P. Fertility preservation in female oncology patients: the influence of the type of cancer on ovarian stimulation response. Hum Reprod. 2018 Nov 1;33(11):2051-2059. PMID: 27370358. [CrossRef]
- Specchia C, Baggiani A, Immediata V, Ronchetti C, Cesana A, Smeraldi A, Scaravelli G, Levi-Setti PE. Oocyte Cryopreservation in Oncological Patients: Eighteen Years Experience of a Tertiary Care Referral Center. Front Endocrinol (Lausanne). 2019 Sep 3;10:600. PMID: 31551931; PMCID: PMC6733913. [CrossRef]
- Okutsu-Horage Y, Iwahata H, Suzuki-Takahashi Y, Sugishita Y, Takae S, Suzuki N. Clinical outcome of embryo cryopreservation in Japanese breast cancer patients: pregnancy rates after transfer of thawed embryos. J Assist Reprod Genet. 2022 Aug;39(8):1769-1777. Epub 2022 Aug 18. Erratum in: J Assist Reprod Genet. 2022 Sep;39(9):2201. PMID: 35980490; PMCID: PMC9428083. [CrossRef]
- Azim AA, Costantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol. 2008 Jun 1;26(16):2630-5. PMID: 18509175. [CrossRef]
- Pereira N, Hancock K, Cordeiro CN, Lekovich JP, Schattman GL, Rosenwaks Z. Comparison of ovarian stimulation response in patients with breast cancer undergoing ovarian stimulation with letrozole and gonadotropins to patients undergoing ovarian stimulation with gonadotropins alone for elective cryopreservation of oocytes†. Gynecol Endocrinol. 2016 Oct;32(10):823-826. Epub 2016 Apr 26. PMID: 27114051. [CrossRef]
- Chien AJ, Chambers J, Mcauley F, Kaplan T, Letourneau J, Hwang J, Kim MO, Melisko ME, Rugo HS, Esserman LJ, Rosen MP. Fertility preservation with ovarian stimulation and time to treatment in women with stage II-III breast cancer receiving neoadjuvant therapy. Breast Cancer Res Treat. 2017 Aug;165(1):151-159. Epub 2017 May 13. Erratum in: Breast Cancer Res Treat. 2017 Aug;165(1):161. PMID: 28503722. [CrossRef]
- Vriens IJH, Ter Welle-Butalid EM, de Boer M, de Die-Smulders CEM, Derhaag JG, Geurts SME, van Hellemond IEG, Luiten EJT, Dercksen MW, Lemaire BMD, van Haaren ERM, Vriens BEPJ, van de Wouw AJ, van Riel AMGH, Janssen-Engelen SLE, van de Poel MHW, Schepers-van der Sterren EEM, van Golde RJT, Tjan-Heijnen VCG. Preserving fertility in young women undergoing chemotherapy for early breast cancer; the Maastricht experience. Breast Cancer Res Treat. 2020 May;181(1):77-86. Epub 2020 Mar 31. PMID: 32236826; PMCID: PMC7182539. [CrossRef]
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99:37–43.
- Kristensen SG, Andersen CY. Cryopreservation of Ovarian Tissue: Opportunities Beyond Fertility Preservation and a Positive View Into the Future. Front Endocrinol (Lausanne). 2018 Jun 28;9:347. PMID: 30002647; PMCID: PMC6031740. [CrossRef]
- Dolmans MM, von Wolff M, Poirot C, Diaz-Garcia C, Cacciottola L, Boissel N, Liebenthron J, Pellicer A, Donnez J, Andersen CY. Transplantation of cryopreserved ovarian tissue in a series of 285 women: a review of five leading European centers. Fertil Steril. 2021 May;115(5):1102-1115. PMID: 33933173. [CrossRef]
- Fleury A, Pirrello O, Maugard C, Mathelin C, Linck C. Breast cancer and ovarian tissue cryopreservation: review of the literature. J Gynecol Obstet Hum Reprod. 2018;47:351–357. [CrossRef]
- Cheng J, Ruan X, Du J, Jin F, Li Y, Liu X, Wang H, Gu M, Mueck AO. Ovarian tissue cryopreservation in a patient with breast cancer during pregnancy: a case report. J Ovarian Res. 2021 Dec 12;14(1):176. PMID: 34895280; PMCID: PMC8667354. [CrossRef]
- Fabbri R, Vicenti R, Magnani V, Pasquinelli G, Macciocca M, Parazza I, Paradisi R, Battaglia C, Venturoli S. Cryopreservation of ovarian tissue in breast cancer patients: 10 years of experience. Future Oncol. 2012 Dec;8(12):1613-9. PMID: 23231523. [CrossRef]
- Jensen AK, Macklon KT, Fedder J, Ernst E, Humaidan P, Andersen CY. 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue: focus on birth and perinatal outcome in 40 of these children. J Assist Reprod Genet. 2017;34(3):325-36.
- Van der Ven H, Liebenthron J, Beckmann M, Toth B, Korell M, Krussel J, et al. Ninety-five orthotopic transplantations in 74 women of ovarian tissue after cytotoxic treatment in a fertility preservation network: tissue activity, pregnancy and delivery rates. Hum Reprod. 2016;31(9):2031- 41. [CrossRef]
- Schmidt KT, Rosendahl M, Ernst E, Loft A, Andersen AN, Dueholm M, et al. Autotransplantation of cryopreserved ovarian tissue in 12 women with chemotherapy-induced premature ovarian failure: the Danish experience. Fertil Steril. 2011;95(2):695-701. [CrossRef]
- Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, et al. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23(10):2266-72. [CrossRef]
- Fabbri R, Pasquinelli G, Magnani V, Macciocca M, Vicenti R, Parazza I, et al. Autotransplantation of cryopreserved ovarian tissue in oncological patients: recovery of ovarian function. Future Oncol. 2014;10(4):549-61. [CrossRef]
- Imbert R, Moffa F, Tsepelidis S, Simon P, Delbaere A, Devreker F, et al. Safety and usefulness of cryopreservation of ovarian tissue to preserve fertility: a 12-year retrospective analysis. Hum Reprod. 2014;29(9):1931-40. [CrossRef]
- Sanchez-Serrano M, Crespo J, Mirabet V, Cobo AC, Escriba MJ, Simon C, et al. Twins born after transplantation of ovarian cortical tissue and oocyte vitrification. Fertil Steril. 2010;93(1):268 e11-3. [CrossRef]
- Ernst EH, Offersen BV, Andersen CY, Ernst E. Legal termination of a pregnancy resulting from transplanted cryopreserved ovarian tissue due to cancer recurrence. J Assist Reprod Genet. 2013;30(7):975-978.
- Rosendahl M, Schmidt KT, Ernst E, Rasmussen PE, Loft A, Byskov AG, et al. Cryopreservation of ovarian tissue for a decade in Denmark: a view of the technique. Reprod Biomed Online. 2011;22(2):162-71. [CrossRef]
- Tian JM, Ran B, Zhang CL, Yan DM, Li XH. Estrogen and progesterone promote breast cancer cell proliferation by inducing cyclin G1 expression. Braz J Med Biol Res. 2018 Jan 23;51(3):1-7. PMID: 29513878; PMCID: PMC5912097. [CrossRef]
- Farhud DD, Zokaei S, Keykhaei M, Hedayati M, Zarif Yeganeh M. In-Vitro Fertilization Impact on the Risk of Breast Cancer: A Review Article. Iran J Public Health. 2021 Mar;50(3):438-447. PMID: 34178791; PMCID: PMC8214614. [CrossRef]
- Khalili MA, Shahedi A, Ashourzadeh S, Nottola SA, Macchiarelli G, Palmerini MG. Vitrification of human immature oocytes before and after in vitro maturation: a review. J Assist Reprod Genet. 2017;34:1413–26. [CrossRef]
- Ata B, Chian RC, Tan SL. Cryopreservation of oocytes and embryos for fertility preservation for female cancer patients. Best Pract Res Clin Obstet Gynaecol. 2010;24:101–12. [CrossRef]
- Shalom-Paz E, Almog B, Shehata F, Huang J, Holzer H, Chian RC, Son WY, Tan SL. Fertility preservation for breast-cancer patients using IVM followed by oocyte or embryo vitrification. Reprod Biomed Online. 2010 Oct;21(4):566-71. Epub 2010 May 13. PMID: 20822957. [CrossRef]
- Virant-Klun I, Bedenk J, Jancar N. In vitro maturation of immature oocytes for fertility preservation in cancer patients compared to control patients with fertility problems in an in vitro fertilization program. Radiol Oncol. 2021 Dec 22;56(1):119-128. PMID: 34957736; PMCID: PMC8884857. [CrossRef]
- Lefebvre T, Mirallié S, Leperlier F, Reignier A, Barrière P, Fréour T. Ovarian reserve and response to stimulation in women undergoing fertility preservation according to malignancy type. Reprod Biomed Online. 2018;37:201–7. [CrossRef]
- Malacarne E, Devesa M, Martinez F, Rodriguez I, Coroleu B. COH outcomes in breast cancer patients for fertility preservation: a comparison with the expected response by age. J Assist Reprod Genet. 2020;37:3069–76. [CrossRef]
- Mohd Faizal A, Sugishita Y, Suzuki-Takahashi Y, Iwahata H, Takae S, Horage-Okutsu Y, Suzuki N. Twenty-first century oocyte cryopreservation-in vitro maturation of immature oocytes from ovarian tissue cryopreservation in cancer patients: A systematic review. Womens Health (Lond). 2022 Jan-Dec;18:17455057221114269. PMID: 35983837; PMCID: PMC9393350. [CrossRef]
- De Roo C, Tilleman K. In vitro maturation of oocytes retrieved from ovarian tissue: outcomes from current approaches and future perspectives. J Clin Med 2021; 10: 4680. [CrossRef]
- Azem F, Shwartz T, Dina K, et al. Combining ovarian tissue cryobanking with retrieval of immature oocytes followed by in vitro maturation for fertility preservation. Fertil Steril 2008; 90: S420. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).