1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has affected millions of people globally and caused substantial mortality and morbidity (1). SARS-CoV-2 spread rapidly throughout the world, leading to a health crisis. As of April 30, 2023, the Coronavirus Disease (COVID-19) infectious cases surpassed 750 million, with more than 6.5 million deaths reported by worldwometer data (2). SARS-CoV-2 is a contagious, large enveloped, single-stranded, non-segmented, positive-sense RNA virus belonging to the coronaviridae family and genus Beta coronavirus (3). The viruses of the coronaviridae family are further divided into two subfamilies; Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus (SARS-CoV). Like the previous zoonotic coronavirus outbreaks (SARS-CoV and MERS-CoV), the current SARS-CoV-2 virus causes lower respiratory tract infections and leads to respiratory diseases (4). International Committee on Taxonomy of Viruses, on February 11, 2020, recognized the outbreak of pneumonia that began in December 2019 in Wuhan, Hubei Province of China, as the SARS-CoV-2 based on its genetic similarity to SARS-CoV (5). The fatality rate due to SARS-CoV-2 (5%) was reported to be comparatively lower than SARS-CoV (10%) and MERS-CoV (30%) (6). Despite its lower fatality rate, it has severely affected people worldwide because of its high transmission rate and infectivity. Despite its large size, the SARS-CoV-2 genome encodes for four structural proteins spike (S), membrane (M), envelop (E), nucleocapsid (N), and 16-17 non-structural proteins (7). The structural proteins are the primary determinants of virulence and function. The S protein is highly immunogenic and contributes protective immunity compared to N, M, and E proteins (8). Studies have confirmed the entry of viruses into human cells after their fusion with the cell membrane of the target tissues. The entry of SARS-CoV2 is initiated by the binding of the Receptor-binding domain (RBD) of spike protein to the human host cell receptors present at the surface. RBD is a portion of spike protein at the S1sub-unit with a core structure and receptor binding motif (RBM) that binds to angiotensin-converting 2 (ACE2) during the entry process of SARS-CoV-2 (10). SARS-CoV-2 enters the host cell by direct fusion of the viral envelope within the host cell membrane or membrane fusion within the endosome after endocytosis. After fusion, the S protein undergoes proteolysis by a host protease, giving rise to S1 and S2 fragments. The host trans-membrane serine protease 2 (TMPRSS2) is important for proteolysis of spike protein for interaction with receptor and entry. After proteolytic cleavage, the S1 protein is ready to intact with ACE2 receptors widely expressed in the cells of the lungs, intestine, testis, liver, heart, and kidney (9).
The newly emerged variants of SARS-CoV-2 created a potential threat among societies and highlighted one of the significant concerns in facing the pandemic (11). Accumulation of mutations due to viral replication is a natural phenomenon. Generally, mutations do not alter the virus behaviors, but a few mutations that occur in the spike protein may give rise to novel, high-risk variants of the SARS-CoV-2 and change viral attributes of infectivity, transmission, severity, and/or its immunity-evading potential (12). Classifications of emerging variants of SARS-CoV-2 and appropriately naming them were challenging for the World health organization (WHO). WHO prompted the classification of novel SARS-CoV-2 strains as Variants of Concern (VOCs) and Variants of Interest (VOIs) in late 2020. VOCs include those variants whose mutations in the structural proteins, especially in the S protein, lead to a change in the virus attributes of transmissibility, an increase in disease severity, a notable reduction in neutralizing antibodies generated, and thus, the decreased response to vaccines and therapy (13). However, VOIs were defined as those strains of SARS-CoV-2 with mutations that result in changes in the affinity of the virus to the ACE-2 receptor, decreased neutralization by antibodies, reduced efficacy of treatments, and a potential increase in transmissibility and disease severity (14). Studies reported that spike protein of SARS-CoV-2, especially the S1 sub-unit of RBD, showed frequent mutations. The mutations reported in the spike protein, especially in the RBD, may reduce the immunogenicity and efficacy of vaccines (15). Therefore, the spike protein may be recognized as a primary target for vaccines and drugs. We have summarized the mutations that occur in the structural protein of SARS-CoV-2 variants and their Greek Alphabet names uniquely given by WHO (Figures 1 and 2). In this review, we discussed the impact of mutations that occurred in the spike protein of SARS-CoV-2, especially mutations that arise in RBD of S1 protein, on immunogenicity, immune evasion, and vaccine-induced immunity, which could potentially contribute to future studies focusing on vaccine design and immunotherapy. In this review, firstly, we will discuss the impact of the SARS-CoV-2 variants on the immune system and major immune components involved in COVID-19 infection. Further, we will also shed light on the impact of vaccination on different variants of SARS-CoV-2 and possible mechanisms evolved in the virus for immunogenicity and immune evasion.
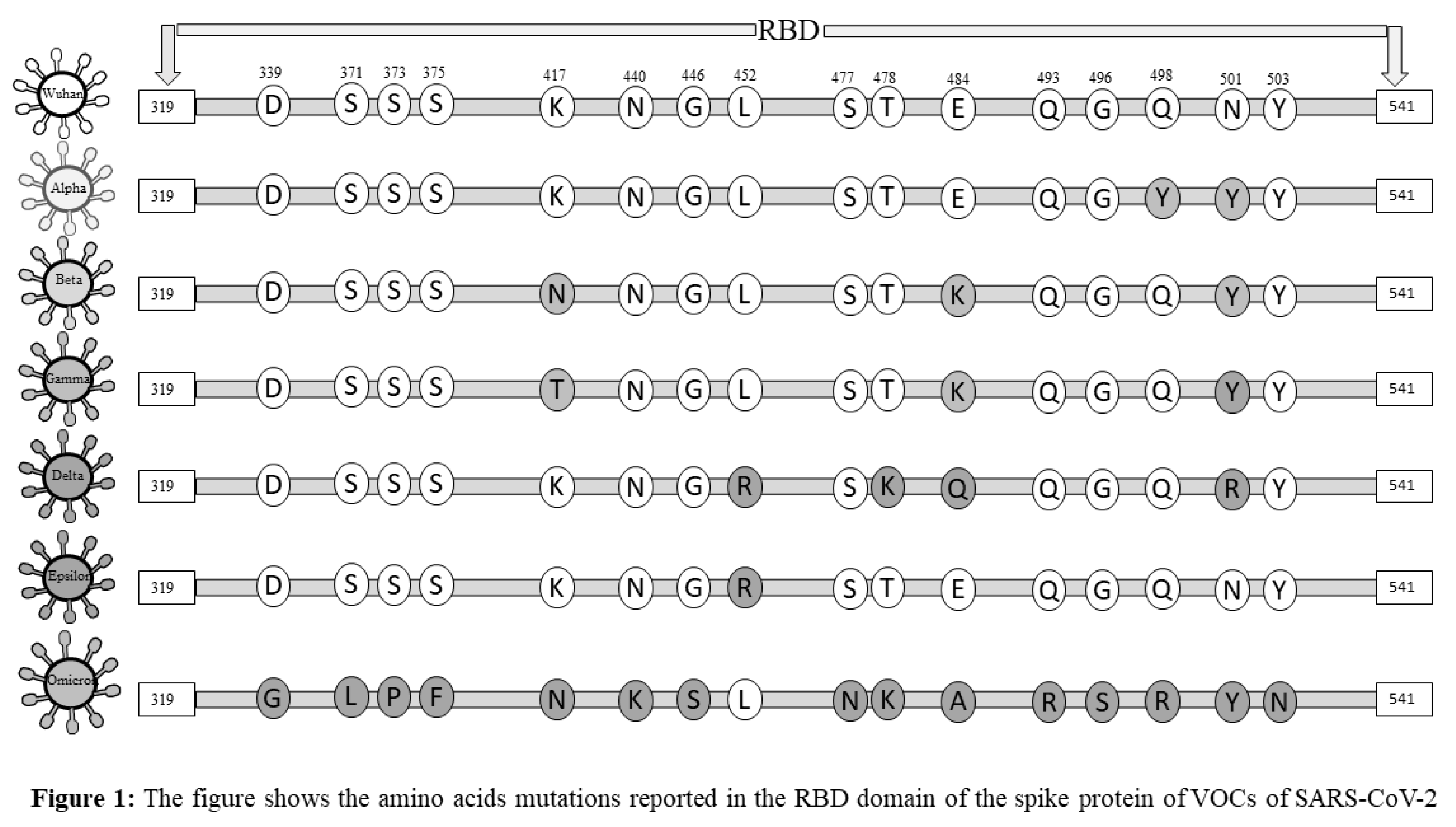
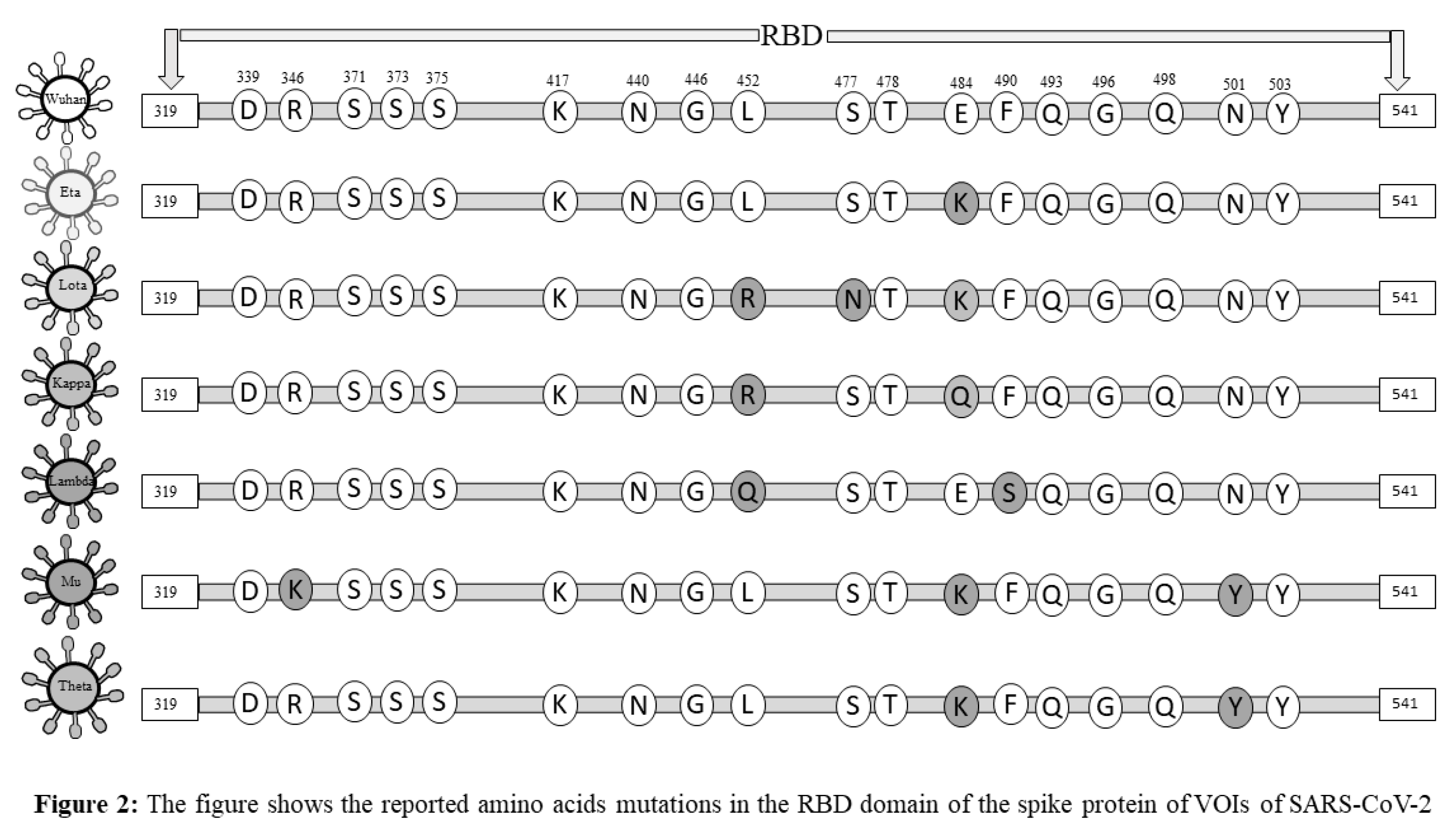
2. Immune Response to SARS-CoV-2 Infection
The viral infections activate both arms of immunity, innate and adaptive, to fight acute infection. Several studies confirmed that recovery from COVID-19 infection depends on the appropriate immune response (16). The disease severity and outcomes from infections with COVID-19 are linked to immune response (17). Persons with impaired immune function were more prone to develop severe complications and die due to COVID-19 disease. In this section, we will highlight the induced immune responses following COVID-19 infections and the impact of infections on disease severity and outcomes.
2.1. Innate Immunity
Innate immunity is the first-line response to protect host cells against early viral infection and turn off adaptive immune responses. Innate immune responses are very important to protect against zoonotic viruses, such as SARS-CoV-2, for which there is usually no-pre-existing adaptive immunity (18). SARS-CoV-2 elicits numerous key host immune responses, such as the release of inflammatory mediators, activation, and maturation of dendritic cells (DCs), and increasing the synthesis of type I interferons (IFNs) through which they limit the viral loads (19,20). Like other coronaviruses, SARS-CoV-2 causes upper and lower respiratory tract infections often associated with fever, cough, and loss of smell and taste. SARS-CoV-2 infection induces non-specific immune responses to combat infections (18). Mucus and other substances that help in defense like histamines, mucins, defensins, etc. are secreted by the mucosal epithelial cells. These substances afford protection against viral infections (21). When SARS-CoV-2 breaches this protection, the pathogenic associated molecular patterns (PAMPs) are recognized by innate immune sensors. These sensors are called pattern recognition receptors (PRRs). Within few hours of exposure to the virus, the PRRs release various kinds of innate immune proteins. Innate immunity players like granulocytes, monocytes, macrophages, and dendritic cells recognize the PAMPs, release the enzymes and present the antigens to T cells (22). Some recent studies have confirmed that endosomal Toll-like receptors (TLR-2, TLR-3 & TLR-7) or cytosolic retinoic acid-induced gene 1 (RIG-1) and melanoma differentiation-associated gene 5 (MDA5) detect the SARS-CoV-2 RNA. (23,24). Of these RIG-1 and MDA5 are the most studied and they provide the key regulation of the interferon pathway. Once the SARS-CoV-2 is recognized by the innate immune cells, they trigger the activation of transcription factors which causes concurrent release of both interferons and other inflammatory cytokines such as interleukins (IL-1, IL-2, IL-6), tumor necrosis factor-alpha (TNF-α), and chemokines (CXCL1, IP-10, CXCL5, CCL2/MCP1, CXCL10) (22). These cytokines and chemokines aid in clearing infections and maintaining cellular homeostasis. However, dysregulated release of these inflammatory mediators contributes to cytokine storm, define as a life-threatening condition caused by excessive production of cytokines. These inflammatory mediators stimulate the Natural killer (NK) cells that kill virus-infected cells, induce apoptosis, and trigger antibody-dependent cell-mediated cytotoxicity (ADCC) (25). The NK cells count was reduced in patients with severe COVID-19 cases (25). The activation of innate immune cells and the production of an adequate quantity of antiviral cytokines are required to stop the replication of SARS-CoV-2. The impaired production of antiviral cytokines, especially type I IFN, or excessive production of these inflammatory mediators may result in cytokine release syndrome or cytokine storm (26). The cytokines storms have been reported in patients with severe COVID-19 (27). Mice with severe COVID-19 infections developed a lethal shock syndrome that mirrored the cytokine storms seen in humans. In patients of COVID-19 with severe disease, phagocytic cells have been identified as the key contributors to the cytokine storm (28). SARS-CoV-2 infection induces cytokine storm, impairs type I IFN responses and suppresses antigen presentation to the T cells and thereby evades the host innate immunity. However, the ineffective IFN innate immunity is considered as a major factor responsible for early immune suppression. It is alsoassociated with failure to control a primary SARS-CoV-2 infection and a high risk of fatal COVID-19. Therefore, the poor early innate immune response to SARS-CoV-2 because of efficient evasion of the virus or defective innate immunity contributors to poor clinical outcomes in severe COVID-19 patients (26).
2.2. Adaptive Immunity
The immune response against SARS-CoV-2 has been reported mainly through innate rather than adaptive immune responses. The innate immune system rapidly recognizes SARS-CoV-2 infections, triggers the type I IFN response, and produces anti-viral proteins. The innate immune response limits viral entry, slows replication and assembly, identifies infections quickly, and removes infected cells. SARS-CoV-2 invades the host's innate immunity, possibly through inducing cytokine storm, impairing type I IFN responses, and suppressing antigens presentation to T cells. Therefore, a specific immune response is required to combat SARS-CoV-2 infection. The adaptive immune response is important for controlling and clearing SARS-CoV-2 infections that cause COVID-19. Understanding the adaptive immune response and memory for the success of all COVID-19 vaccines is critical. In patients who have recovered from COVID-19, spike protein-specific B-cell subsets and SARS-CoV-2 specific T-cell immune memory have been reported, thus suggesting a possible role of adaptive immunity in fighting the infection (29). By playing critical roles in identifying SARS-CoV-2 infections and clearance, antibodies, CD4+ T cells, and CD8+T cells help control SARS-CoV-2 infections. However, the importance of each component of adaptive immunity depends on the COVID-19 disease severity and viral loads. (30). To produce neutralizing antibodies, it is crucial that the viral antigen should recognize by antigen-presenting cells (APC) and thereby stimulates humoral immunity via virus-specific B and plasma cells (15). Several studies confirmed that spike protein is an important target for neutralizing antibodies and vaccine design (31). The RBD domain of the spike protein is the target of more than 90% of neutralizing antibodies in COVID-19 cases. In COVID-19 patients, increasing titers of the neutralizing anti-spike IgG antibodies and its association with disease severity were found (32). High titers of the neutralizing antibody are associated with severe COVID-19 disease and potentially extrafollicular B cell response. Adaptive immune response to SARS-CoV-2 infection reported to be highly heterogeneous. Compared to patients with mild/asymptomatic COVID-19 disease, patients with severe COVID-19 have different adaptive immune responses (33). Few studies reported an association between COVID-19 disease severity and adaptive immune response (33).
SARS-CoV-2 specific immunoglobulins (IgG and IgM) were found to have appeared 3-6 days after infections and remained in the circulation for 12-14 weeks, depending on viral load and severity of COVID-19 disease (34,35). Apart from viral neutralizing antibodies, secretary mucosal antibodies (IgA) are also essential to stop viral adherence to the epithelium thereby inhibiting the multiplication (21, 36). Recent study reported that deficiency of IgA antibodies in active infections of SARS-CoV-2 could exacerbate severe COVID-19 infection or result in delayed viral shedding (36). There is a lot of discrepancy about the titters of antibodies and their persistence in the circulations post-infection with SARS-CoV-2. A recent study highlighted that about 50% of antibody titters were reduced after 4-12 weeks of the start of SARS-COV-2 infections (37). Despite the importance of the antibody response, it is still optional. This is corroborated by reports of patients recovering from SARS-CoV-2 infection without functional effectors and memory B cells (38).
Much investigation has not been done on the T cell-mediated cellular immune response against SARS-CoV-2 infections, perhaps due to the complexity of T cell sub-populations and human leukocyte antigen (HLA) restricted epitope recognition. The cell-mediated immune response is important for recognizing and clearing intracellular pathogens. Evidence from previous infections of humans with SARS-CoV-1 and MERS indicates that T cells mediated cellular immune responses are critical in determining SARS-CoV-2 infections and disease severity (39). T-cell responses to MERS are also of attention and come into view to be more robust and sustained than humoral immunity (39). The extent of viral infections and the efficacy of the innate immune response, particularly type I IFN-mediated, are critical in determining subsequent adaptive immune response and the clinical outcome. Patients with asymptomatic/mild COVID-19 disease showed a balance production of inflammatory response and virus-specific T-cell activation (40). However, patients with severe COVID-19 were characterized by hyperinflammatory responses with robust production of inflammatory cytokines (39). The clinical association of T cells with viral load and disease severity in SARS-CoV-2 infection is not yet characterized. Still, systemic inflammation, virus-specific T-cell responses, and severe pneumonia have contributed to tissue damage in other respiratory infections (41). The absolute number of CD+4 and CD+8 T cells was decreased in COVID-19 patients, and the decline in T lymphocyte counts was correlated with disease severity. A study used ELISpot technology to assess the T cell immune response post-COVID-19 infections and found robust T cells mediated immune response in patients with more severe-clinical infection (42). Recent studies reported spike protein-specific T cell responses are dominated by CD4+ T helper cells and are likely to support immunoglobulin generation (43). CD4+ T helper cells are more significant than the CD8+Cytotoxic T cells pool and may increase frequency over time. Cytotoxic T-cell responses are crucial in controlling SARS-CoV-2 infections from the infected tissues and clearing them from circulation (30).
CD8+ cytotoxic T cells have been found to be abundant in the lung tissues of COVID-19 patients with asymptomatic/mild symptoms. However, perforin, and granzyme B production is reported only in patients with severe disease (44). Evidence of the role of CD8+ cytotoxic T cells in SARS-CoV-2 infections has been studied on non-human primates. Depletion of CD8+ cytotoxic T cells in macaques pre-immunized with a SARS-CoV-2 vaccine delayed the viral clearance in a later infection challenge. The T-cell response could be protective against SARS-CoV-2 infection for a long duration. However, the functional exhaustion of T cells or hyper-activation may increase the severity of the disease. The exact cause of T cell over-activation and its implication is not yet understood in SARS-CoV-2 infections.
3. Impact of COVID-19 Variants on Immune Evasion
Compared to DNA viruses, RNA viruses show higher mutation rates, where SARS-CoV-2 is not an exception. SARS-CoV-2 has continuously evolved through mutations due to its error prone RNA polymerase and anti-spike immune pressure, resulting in successive infections by these mutated strains. Continuous genetic variations in the genome and mutations in the spike proteins of SARS-CoV-2 have been reported (45). About 1-2 monthly mutations have been reported in the SARS-CoV-2 genome (46). Spreading SARS-CoV-2 variants share several mutations that empower them in rising population while expanding their replication wellness. The host immune responses against the virus have been primarily induced due to spike protein (47). The RBD domain of the spike protein is a primary target for neutralizing antibodies because it is a highly immunogenic protein of SARS-CoV-2. Monoclonal antibodies have shown a clinical benefit in preventing infection by acting on the SARS-CoV-2 spike protein. Mutations in SARS-CoV-2 spike protein cause significant alters in possible evasion of immunity and vaccine design. The adaptive immune response is extremely necessary to fight SARS-CoV-2 variants. The fact that antibodies are effective in preventing the binding of SARS-CoV-2 to the ACE-2 receptor of host cells and thereby prevent infections, is well known. However, the effectiveness of antibodies is reduced against spike protein variants. Various SARS-CoV-2 variants have been reported to successfully dodge the humoral immune response (48). Some of these spike protein mutations render antibodies elicited post vaccination with COVID-19 vaccines and against earlier virus strains. This allows the variants to escape the immune response following vaccination or infection. Recent studies confirmed the reduced efficacy of neutralizing antibodies against the SARS-CoV-2 variants in circulation. According to a study, sera of RBD nanoparticle-vaccinated rhesus macaques demonstrated moderate to low efficacy for different SARS-CoV-2 variants (49). While the B.1.1.7 pseudotype virus was efficiently neutralized the sera showed very weak inhibition against the 501Y.V2 variant (50). According to various studies, mutations that occur in the RBD domain of spike protein compromise the humoral immune response (31,51). The common mutations reported in the spike protein and their effect on immunity are summarized in
Table 1. More recently, a combination of mutations and deletions was reported in the RBD domain of spike protein. The mutation at 484 residues of the RBD domain in the spike protein was first time reported in South African variants and later in the Brazilian variant. Mutations E484 GK/Q/P present in highly contagious variants B.1.617 and B.1.1.7 that reduced antibody binding and neutralization titer with polyclonal serum antibodies obtained from people who have recovered from COVID-19disease and vaccinated with BNT162b2 vaccines (13,52). Therefore, the mutation at this residue E484Q eradicates the binding with the ACE-2 receptor and destabilizes the complex, favoring the virus's immune escape. Another important mutation that compromises humoral immune response and increased resistance to neutralizing monoclonal antibodies and COVID-19 sera includes N439K (53). The substitution of amino acid at N439K residue of RBD enhanced RBD affinity for ACE2 receptor, subsequently increasing viral load. The mutation at residue N501Y was first time reported in the United Kingdom and South Africa. This mutation enhances ACE2 proximity and replication of the virus. The variants having N501Y mutations showed about four-fold higher affinity to wild-type SARS-CoV-2, the ACE 2 receptor (54). The D614G mutation at residue 614 in the spike protein was observed in one of the first reported and subsequently in nearly all variants of the virus. This mutation is shown to shift the S protein conformation towards the ACE-2 binding fusion state that allows viral entry and replication, thus enhancing the viral infectivity (48). Therefore, D614G confers a moderate advantage for infectivity and transmissibility. Multiple mutations in RBD increases the affinity of spike protein binding with the ACE-2 receptor of host cells and increases resistance to neutralizing antibodies, thereby highly compromising the humoral immune response, resulting in immune escape. These multiple substitutions are observed in the RBD domain of spike protein of COVID-19 variants known to have higher infectivity and the ability to resist pre-existing antibodies or vaccine-induced immunity (15,53). The mutations that occurred in other structural proteins of the SARS-CoV-2 are also associated with immune escape. Recent studies reported immune escape associated with mutations in the NTD domain centered in loop N3 (140-156) and loop N5 (246-260) (46). Deletions in the NTD have been observed repeatedly in the evolution of SARS-CoV-2 and have been described as changing NTD antigenicity. These studies suggested that emerging SARS-CoV-2 RBD variants could reduce the vaccine's efficacy.
Like humoral immune response, the cell-mediated immune responses by T cells are important to resolve SARS-CoV-2 infections. The potential importance of amino acid substitutions in the structural protein in driving escape from the cellular immune response of T cells is a topic of considerable debate (55). Unlike B cells, T cells recognized the broad array of epitopes of SARS-CoV-2 viruses. T helper cells (CD4+) and cytotoxic T cells (CD8+) recognize more than ten epitopes of SARS-CoV-2. A study reported the cross-reactive T cells against omicron variants. The observed mutations in the omicron spike protein could not evade cellular immunity and may not abrogate antigen presentations. However, the large number of amino acids substitution within spike protein may inactivate the presentation or recognition of some epitopes to the MHC. Therefore, the possibility of escaping cell-mediated immunity by SARS-CoV-2 variants is rare.
Most studies focused on a potential immune escape by SARS-CoV-2 variants on the antibody level, little is known about immune escape at the T cell. A recent study reported that emerged mutations in the RBD domain at residue L452 R in B.1.427/429 and Y453F in B.1.298 can escape from the HLA-24-restricted cellular immune response (56). Few studies suggested the immune escape of T cells to SARS-CoV-2 variants by using CD8+ T cell epitope profiling (57). Mutations in the MHC-I-restricted epitopes of spike protein abolish MHC-I binding and thus escape in vitro CD8+ T cell response. As per recent data, the K417 mutations found in the few strains of SARS-CoV-2 variants (B.1.1.7, B.1.351 and P.1) and mutation at the residue Y155 reported in B.1.1.7, B.1.351, and B.1.525 variants abolished the capacity of the loading of the peptide to the relevant HLA-A class I molecule (58). These data provide insights that emerging SARS-CoV-2 variants can evade the host cellular immunity. Hence, in view of the impact of genetic variations on the immune evasion, infectivity, transmissibility and antigenicity of SARS-CoV-2, it is imperative to design and /or update vaccines and therapeutics after understanding the host immune response against spike protein variants of the virus.
4. Impact of COVID-19 Variants on Vaccine-Induced Immunity
Vaccines are considered the most potent weapons in the fight against COVID-19 disease. Countries with high rate of vaccination had significantly reduced number of infective cases, hospitalization, and death. Although, the emergence of SAR-CoV-2 variants has jeopardized vaccination's success. The vaccines currently used were based on prototype viruses. Still, it showed good neutralization with different variants of the SARS-CoV-2 viruses (59). However, these emerging variants pose a big concern about vaccine efficacy and are responsible for the pandemic. Since the mutations are primarily observed in structural proteins, especially in the RBD domain of the spike protein of the newly emerged viruses. These mutations confirmed moderate to poor neutralization in vaccinated individuals (59). In-vitro neutralization assays conducted by using the pseudo viruses possessing RBD mutations showed that the neutralizing activity of plasma from vaccinated individuals significantly decreased against E484K, N501, or the K417 N + E484K, + N501Y triple variant (32). Though alpha variants did not contribute much mortality, other variants, such as Delta, Beta, Gamma, and Lambda, showed more aggressiveness, leading to many deaths globally. Notable mutations observed in the RBD domain of the spike protein, N-terminal domain (NTD), and mutation near the furin cleavage site posing a major concern to the scientists that this variant could halt vaccines and monoclonal antibodies-based therapies (60) The beta variant (B.1.351) resisted neutralization by sera obtained from individuals receiving both Moderna-vaccine doses (59). In contrast, this variant did not escape the neutralization from Pfizer mRNA vaccine-elicited sera (61). However, neutralization of the B.1.351 variant was diminished by 7.6-fold with BTN162b2 vaccinated individuals.
In-vitro neutralization assay of pseudoviruses carrying the set of B.351 spike protein mutations showed moderate to poor neutralization with sera obtained from individuals who received the both doses of BNT162b2 vaccine or mRNA-1273 vaccine (32). The chAdOx1 nCOV-19 vaccine showed clinical efficacy against the B.1.1.7 variant but failed to protect mild to moderate disease caused by B.351. variants (60). A similar type of study done in other cohort using recombinant viruses carrying N501Y, Δ69-V70, E484K and D614G demonstrated that compared with Wuhan-Hu1 reference viruses, only moderate reduction in neutralization by post vaccination sera elicited by two BNT162b2 doses (61). The B.1.1.7 variant was effectively neutralized with sera obtained from Moderna and Pfizer mRNA vaccinated individuals, but this variant escaped neutralization with BTN162b2 vaccinated individuals (8,62). Post-vaccinated sera obtained from individuals who received both doses of AZD1222 and BNT162b2 could not neutralize the B.1.617.2 Delta variant (62). However, Pfizer mRNA vaccine-elicited sera efficiently neutralized this deadly delta variant (63). These findings suggest that mRNA-based vaccines have comparatively better responses in tolerating the VOCs to other types of vaccines. The mutation at the residue E484K in the RBD of the spike protein is regarded as a most potent mutation that evades the host immunity after vaccination and natural infections with SARS-CoV-2. In-vitro neutralization data of several variants of SARS-CoV-2 indicate that E484K is the primary determinant of the diereses in neutralization titers, which distinguish P.1, P.2, and the three B.1351 variants from the other pseudoviruses tested (63). Clinical trials conducted in South Africa found poor efficacy of Novavax and Johnson & Johnson vaccines. This was perhaps due to the country's high circulation of these SARS-CoV-2 variants (64,65). These variants also showed resistance to vaccine-elicited neutralizing and monoclonal antibodies induced after Pfizer mRNA-based BNT162b2 vaccination. Several studies based on protein modeling suggest that though vaccinations significantly reduced infections, they may not avoid a new wave. Therefore, most studies recommended updating the vaccines based on emerging variants and their efficacy against VOCs.
5. Conclusions
The increased number of COVID-19 cases across the globe provides the basis for SARS-CoV-2 to mutate and better evade host immunity. The mutations that occur in the structural proteins, especially in the RBD domain of spike protein, led to new variants of SARS-CoV-2, which showed higher transmission, virulence, and antigenicity. RBD mutants are mainly important to track and study due to the role of the RBD: ACE2 interaction in the virulence, transmission, and lethality of emerging variants. The RBD is immunodominant and accounts for 90% of serum-neutralizing activity. The amino acid substitution at RBD has been associated with a higher potential to escape the immune system. Therefore, it is crucial that the emergence of SARS-CoV-2 variants globally is monitored and their potential for immune escape is quickly recognized. Any future global immunization program will need to consider emerging variants and their impact on evading the host immunity to ensure the effectiveness of these vaccination programs. Knowing how much cross-protection is offered between different strains following vaccinations or infection is important. For providing comprehensive immune response against all variants by developing more effective vaccines, strategies such as finding the cross-neutralizing activities and screening key epitopes among variants may be fruitful. Further research must be conducted to investigate the impact of spike protein mutations on host immune response after vaccinations or natural infections and its association with the COVID-19 heterogeneous phenotypes. Vaccines directed at the ancestral spike protein and treatment strategies involving monoclonal antibodies need a re-look for offering reliable protection against the emerging variants.
Author Contributions
DL Gupta contributed to the conception and design of the study. He took the lead in writing the manuscript and comparative searched the articles on PubMed, Medline, and Global Health. DN Rao contributed to the manuscript's revision and read and approved the submitted version.
Funding
This review was commissioned as a part of a project the study the presence of cross-reactive antibodies against the spike protein variants (B.1.617) of SARS-CoV-2 in vaccinated people and non-vaccinated people who have recovered from natural infection funded by All India Institute of Medical Sciences, Raipur, Chhattisgarh, India (Reference Number: 22/41/2019/Admin/1346).
Conflicts of Interest
The authors do not have any conflict of interest.
References
- Zhang JJ, Dong X, Liu GH, Gao YD. Risk and Protective Factors for COVID-19 Morbidity, Severity, and Mortality. Clin Rev Allergy Immunol. 2023 Feb;64(1):90–107.
- Martín Sánchez FJ, Martínez-Sellés M, Molero García JM, Moreno Guillén S, Rodríguez-Artalejo FJ, Ruiz-Galiana J, et al. Insights for COVID-19 in 2023. Rev Espanola Quimioter Publicacion Of Soc Espanola Quimioter. 2023 Apr;36(2):114–24.
- Wu CR, Yin WC, Jiang Y, Xu HE. Structure genomics of SARS-CoV-2 and its Omicron variant: drug design templates for COVID-19. Acta Pharmacol Sin. 2022 Dec;43(12):3021–33.
- Hallek M, Adorjan K, Behrends U, Ertl G, Suttorp N, Lehmann C. Post-COVID Syndrome. Dtsch Arzteblatt Int. 2023 Jan 27;120(4):48–55.
- Kaur I, Behl T, Sehgal A, Singh S, Sharma N, Subramanian V, et al. A motley of possible therapies of the COVID-19: reminiscing the origin of the pandemic. Environ Sci Pollut Res Int. 2022 Sep;29(45):67685–703. [CrossRef]
- Alsafi RT. Lessons from SARS-CoV, MERS-CoV, and SARS-CoV-2 Infections: What We Know So Far. Can J Infect Dis Med Microbiol J Can Mal Infect Microbiol Medicale. 2022;2022:1156273.
- Rashid F, Xie Z, Suleman M, Shah A, Khan S, Luo S. Roles and functions of SARS-CoV-2 proteins in host immune evasion. Front Immunol. 2022;13:940756.
- Lim H, Kim SE, Lee YH, Hwang YH, Kim SH, Kim MY, et al. Immunogenicity of candidate SARS-CoV-2 DNA vaccines based on the spike protein. Virology. 2022 Aug;573:118–23.
- Baldari CT, Onnis A, Andreano E, Del Giudice G, Rappuoli R. Emerging roles of SARS-CoV-2 Spike-ACE2 in immune evasion and pathogenesis. Trends Immunol. 2023 Jun;44(6):424–34.
- Thakur S, Verma RK, Kepp KP, Mehra R. Modelling SARS-CoV-2 spike-protein mutation effects on ACE2 binding. J Mol Graph Model. 2023 Mar;119:108379.
- Fernandes Q, Inchakalody VP, Merhi M, Mestiri S, Taib N, Moustafa Abo El-Ella D, et al. Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann Med. 2022 Dec;54(1):524–40.
- Chen KWK, Tsung-Ning Huang D, Huang LM. SARS-CoV-2 variants - Evolution, spike protein, and vaccines. Biomed J. 2022 Aug;45(4):573–9.
- Shrestha LB, Foster C, Rawlinson W, Tedla N, Bull RA. Evolution of the SARS-CoV-2 omicron variants BA.1 to BA.5: Implications for immune escape and transmission. Rev Med Virol. 2022 Sep;32(5):e2381. [CrossRef]
- Scovino AM, Dahab EC, Vieira GF, Freire-de-Lima L, Freire-de-Lima CG, Morrot A. SARS-CoV-2’s Variants of Concern: A Brief Characterization. Front Immunol. 2022;13:834098.
- Focosi D, Maggi F. Neutralising antibody escape of SARS-CoV-2 spike protein: Risk assessment for antibody-based Covid-19 therapeutics and vaccines. Rev Med Virol. 2021 Nov;31(6):e2231.
- Xiong L, Li Q, Cao X, Xiong H, Huang M, Yang F, et al. Recovery of functional fitness, lung function, and immune function in healthcare workers with nonsevere and severe COVID-19 at 13 months after discharge from the hospital: a prospective cohort study. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2022 Oct;123:119–26. [CrossRef]
- Liechti T, Iftikhar Y, Mangino M, Beddall M, Goss CW, O’Halloran JA, et al. Immune phenotypes that are associated with subsequent COVID-19 severity inferred from post-recovery samples. Nat Commun. 2022 Nov 25;13(1):7255. [CrossRef]
- Müller S, Schultze JL. Systems analysis of human innate immunity in COVID-19. Semin Immunol. 2023 Jul;68:101778. [CrossRef]
- Karki R, Kanneganti TD. Innate immunity, cytokine storm, and inflammatory cell death in COVID-19. J Transl Med. 2022 Nov 22;20(1):542. [CrossRef]
- Kaur BP, Secord E. Innate Immunity. Pediatr Clin North Am. 2019 Oct;66(5):905–11.
- Russell MW, Mestecky J. Mucosal immunity: The missing link in comprehending SARS-CoV-2 infection and transmission. Front Immunol. 2022;13:957107.
- Diamond MS, Lambris JD, Ting JP, Tsang JS. Considering innate immune responses in SARS-CoV-2 infection and COVID-19. Nat Rev Immunol. 2022 Aug;22(8):465–70.
- Fitzgerald KA, Kagan JC. Toll-like Receptors and the Control of Immunity. Cell. 2020 Mar 19;180(6):1044–66. [CrossRef]
- Wallach T, Raden M, Hinkelmann L, Brehm M, Rabsch D, Weidling H, et al. Distinct SARS-CoV-2 RNA fragments activate Toll-like receptors 7 and 8 and induce cytokine release from human macrophages and microglia. Front Immunol. 2022;13:1066456.
- Malengier-Devlies B, Filtjens J, Ahmadzadeh K, Boeckx B, Vandenhaute J, De Visscher A, et al. Severe COVID-19 patients display hyper-activated NK cells and NK cell-platelet aggregates. Front Immunol. 2022;13:861251. [CrossRef]
- Park SH. An Impaired Inflammatory and Innate Immune Response in COVID-19. Mol Cells. 2021 Jun 30;44(6):384–91. [CrossRef]
- Murata K, Nakao N, Ishiuchi N, Fukui T, Katsuya N, Fukumoto W, et al. Four cases of cytokine storm after COVID-19 vaccination: Case report. Front Immunol. 2022;13:967226. [CrossRef]
- Cron RQ, Goyal G, Chatham WW. Cytokine Storm Syndrome. Annu Rev Med. 2023 Jan 27;74:321–37.
- Kim J, Seo H, Kim HW, Kim D, Kwon HJ, Kim YK. Effect of Previous COVID-19 Vaccination on Humoral Immunity 3 Months after SARS-CoV-2 Omicron Infection and Booster Effect of a Fourth COVID-19 Vaccination 2 Months after SARS-CoV-2 Omicron Infection. Viruses. 2022 Nov 6;14(11):2458.
- McCafferty S, Haque AKMA, Vandierendonck A, Weidensee B, Plovyt M, Stuchlíková M, et al. A dual-antigen self-amplifying RNA SARS-CoV-2 vaccine induces potent humoral and cellular immune responses and protects against SARS-CoV-2 variants through Tcell-mediated immunity. Mol Ther J Am Soc Gene Ther. 2022 Sep 7;30(9):2968–83.
- Chen LL, Chua GT, Lu L, Chan BPC, Wong JSC, Chow CCK, et al. Omicron variant susceptibility to neutralizing antibodies induced in children by natural SARS-CoV-2 infection or COVID-19 vaccine. Emerg Microbes Infect. 2022 Dec;11(1):543–7.
- Lyke KE, Atmar RL, Islas CD, Posavad CM, Szydlo D, Paul Chourdhury R, et al. Rapid decline in vaccine-boosted neutralizing antibodies against SARS-CoV-2 Omicron variant. Cell Rep Med. 2022 Jul 19;3(7):100679.
- Sapir T, Averch Z, Lerman B, Bodzin A, Fishman Y, Maitra R. COVID-19 and the Immune Response: A Multi-Phasic Approach to the Treatment of COVID-19. Int J Mol Sci. 2022 Aug 3;23(15):8606. [CrossRef]
- Gregory DJ, Vannier A, Duey AH, Roady TJ, Dzeng RK, Pavlovic MN, et al. Repertoires of SARS-CoV-2 epitopes targeted by antibodies vary according to severity of COVID-19. Virulence. 2022 Dec;13(1):890–902.
- Fonseca MHG, Silva MFS, Pinto ACMD, de Melo ACL, de Oliveira F de CE, Araújo FM de C, et al. Persistently positive SARS-CoV-2-specific IgM during 1-year follow-up. J Med Virol. 2022 Sep;94(9):4037–9.
- Liew F, Talwar S, Cross A, Willett BJ, Scott S, Logan N, et al. SARS-CoV-2-specific nasal IgA wanes 9 months after hospitalisation with COVID-19 and is not induced by subsequent vaccination. EBioMedicine. 2023 Jan;87:104402.
- Cheng ZJ, Huang H, Zheng P, Xue M, Ma J, Zhan Z, et al. Humoral immune response of BBIBP COVID-19 vaccination before and after the booster immunization. Allergy. 2022 Aug;77(8):2404–14. [CrossRef]
- Sette A, Crotty S. Immunological memory to SARS-CoV-2 infection and COVID-19 vaccines. Immunol Rev. 2022 Sep;310(1):27–46.
- Le Bert N, Tan AT, Kunasegaran K, Tham CYL, Hafezi M, Chia A, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020 Aug;584(7821):457–62.
- Mortezaee K, Majidpoor J. Cellular immune states in SARS-CoV-2-induced disease. Front Immunol. 2022;13:1016304.
- Heinen N, Marheinecke CS, Bessen C, Blazquez-Navarro A, Roch T, Stervbo U, et al. In-depth analysis of T cell immunity and antibody responses in heterologous prime-boost-boost vaccine regimens against SARS-CoV-2 and Omicron variant. Front Immunol. 2022;13:1062210.
- Wakui M, Uwamino Y, Yatabe Y, Nakagawa T, Sakai A, Kurafuji T, et al. Assessing anti-SARS-CoV-2 cellular immunity in 571 vaccines by using an IFN-γ release assay. Eur J Immunol. 2022 Dec;52(12):1961–71.
- Fries L, Formica N, Mallory RM, Zhou H, Plested JS, Kalkeri R, et al. Strong CD4+ T-Cell Responses to Ancestral and Variant Spike Proteins Are Established by NVX-CoV2373 SARS-CoV-2 Primary Vaccination. J Infect Dis. 2023 May 21;jiad163.
- Lu X, Yamasaki S. Current understanding of T cell immunity against SARS-CoV-2. Inflamm Regen. 2022 Nov 29;42(1):51.
- Starr TN, Greaney AJ, Hannon WW, Loes AN, Hauser K, Dillen JR, et al. Shifting mutational constraints in the SARS-CoV-2 receptor-binding domain during viral evolution. Science. 2022 Jul 22;377(6604):420–4.
- Abulsoud AI, El-Husseiny HM, El-Husseiny AA, El-Mahdy HA, Ismail A, Elkhawaga SY, et al. Mutations in SARS-CoV-2: Insights on structure, variants, vaccines, and biomedical interventions. Biomed Pharmacother Biomedecine Pharmacother. 2023 Jan;157:113977.
- Zhang Z, Zhang J, Wang J. Surface charge changes in spike RBD mutations of SARS-CoV-2 and its variant strains alter the virus evasiveness via HSPGs: A review and mechanistic hypothesis. Front Public Health. 2022;10:952916.
- Valério M, Borges-Araújo L, Melo MN, Lousa D, Soares CM. SARS-CoV-2 variants impact RBD conformational dynamics and ACE2 accessibility. Front Med Technol. 2022;4:1009451.
- Chang X, Liu X, Martina B, Zeltins A, Augusto G, Vogel M, et al. Vaccination using mutated receptor binding domains of SARS-CoV-2: Evidence for partial immune escape but not serotype formation. Front Immunol. 2023;14:1114396.
- Zhang L, Li Q, Liang Z, Li T, Liu S, Cui Q, et al. The significant immune escape of pseudotyped SARS-CoV-2 variant Omicron. Emerg Microbes Infect. 2022 Dec;11(1):1–5.
- Zhang L, Li Q, Wu J, Yu Y, Zhang Y, Nie J, et al. Analysis of SARS-CoV-2 variants B.1.617: host tropism, proteolytic activation, cell-cell fusion, and neutralization sensitivity. Emerg Microbes Infect. 2022 Dec;11(1):1024–36.
- Chen Y, Zhao X, Zhou H, Zhu H, Jiang S, Wang P. Broadly neutralizing antibodies to SARS-CoV-2 and other human coronaviruses. Nat Rev Immunol. 2023 Mar;23(3):189–99.
- Weisblum Y, Schmidt F, Zhang F, DaSilva J, Poston D, Lorenzi JC, et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. eLife. 2020 Oct 28;9:e61312.
- Bartsch YC, Cizmeci D, Kang J, Gao H, Shi W, Chandrashekar A, et al. Selective SARS-CoV2 BA.2 escape of antibody Fc/Fc-receptor interactions. iScience. 2023 May 19;26(5):106582. [CrossRef]
- Li F, Xu W, Zhang X, Wang W, Su S, Han P, et al. A spike-targeting bispecific T cell engager strategy provides dual layer protection against SARS-CoV-2 infection in vivo. Commun Biol. 2023 Jun 1;6(1):592.
- Dolton G, Rius C, Hasan MS, Wall A, Szomolay B, Behiry E, et al. Emergence of immune escape at dominant SARS-CoV-2 killer Tcell epitope. Cell. 2022 Aug 4;185(16):2936-2951.e19.
- Kombe Kombe AJ, Biteghe FAN, Ndoutoume ZN, Jin T. CD8+ T-cell immune escape by SARS-CoV-2 variants of concern. Front Immunol. 2022;13:962079.
- Emmelot ME, Vos M, Boer MC, Rots NY, van Els CACM, Kaaijk P. SARS-CoV-2 Omicron BA.4/BA.5 Mutations in Spike Leading to T Cell Escape in Recently Vaccinated Individuals. Viruses. 2022 Dec 29;15(1):101. [CrossRef]
- Li D, Martinez DR, Schäfer A, Chen H, Barr M, Sutherland LL, et al. Breadth of SARS-CoV-2 neutralization and protection induced by a nanoparticle vaccine. Nat Commun. 2022 Oct 23;13(1):6309.
- Jia Z, Gong W. Will Mutations in the Spike Protein of SARS-CoV-2 Lead to the Failure of COVID-19 Vaccines? J Korean Med Sci. 2021 May 10;36(18):e124.
- Kelly JD, Leonard S, Hoggatt KJ, Boscardin WJ, Lum EN, Moss-Vazquez TA, et al. Incidence of Severe COVID-19 Illness Following Vaccination and Booster With BNT162b2, mRNA-1273, and Ad26.COV2.S Vaccines. JAMA. 2022 Oct 11;328(14):1427–37. [CrossRef]
- Shaw RH, Greenland M, Stuart ASV, Aley PK, Andrews NJ, Cameron JC, et al. Persistence of immune response in heterologous COVID vaccination schedules in the Com-COV2 study - A single-blind, randomised trial incorporating mRNA, viral-vector and protein-adjuvant vaccines. J Infect. 2023 Jun;86(6):574–83.
- Bader G, Itan M, Edry-Botzer L, Cohen H, Haskin O, Mozer-Glassberg Y, et al. Adaptive immune response to BNT162b2 mRNA vaccine in immunocompromised adolescent patients. Front Immunol. 2023;14:1131965. [CrossRef]
- Hardt K, Vandebosch A, Sadoff J, Le Gars M, Truyers C, Lowson D, et al. Efficacy, safety, and immunogenicity of a booster regimen of Ad26.COV2.S vaccine against COVID-19 (ENSEMBLE2): results of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Infect Dis. 2022 Dec;22(12):1703–15. [CrossRef]
- Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, et al. Safety and Efficacy of the NVX-CoV2373 Coronavirus Disease 2019 Vaccine at Completion of the Placebo-Controlled Phase of a Randomized Controlled Trial. Clin Infect Dis Off Publ Infect Dis Soc Am. 2023 Feb 8;76(3):398–407.
Table 1.
SARS-CoV-2 variants and their impact on natural and vaccine-induced immunity.
Table 1.
SARS-CoV-2 variants and their impact on natural and vaccine-induced immunity.
| Variants name |
WHO label |
Country of first detection |
Notable mutations |
Location of mutation |
Impact on natural and vaccine-induced immunity |
| B.1.1.7 |
Alpha |
United Kingdom |
60-70del, 144Ydel, N501Y, A570D, D614G, P681H |
S protein |
Reduced neutralization by sera from preudotype virus RBD nanoparticle vaccinated macaques |
| B.1.351 B.1.351.2 B.1.351.3 |
Beta |
South Africa |
K417N, E484K, N501Y, D614G, A701V |
S protein |
Reduced neutralization to convalescent and post-vaccinated sera
Resisted neutralization by a cluster of III RBD specific monoclonal antibodies |
P.1
P.1.1
P.1.2 |
Gamma |
Brazil |
K417T, E484K, N501Y, D614G, H655Y |
S protein |
There is moderate change in neutralization to convalescent and post-vaccinated sera |
| B.1.617.2 |
Delta |
India |
K417N, L452R, T478K
E484Q, D614G, P681R, D950N |
S protein |
Significantly reduced the neutralization to post-vaccination sera
Enhanced RBD affinity to ACE-2 receptor
Escape from the HLA-24-restricted cellular immunity |
B.1.427
B.1.429 |
Epsilon |
United States |
W152C, L452R |
S protein |
Showed poor neutralization by RBD-specific monoclonal antibodies
Reduced neutralization to convalescent and post-vaccination sera |
| B.1.1.529 |
Omicron |
South Africa |
69-70 del, G339D, S371L, S373P, S375F, K417N, N440K, G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, Y505H, T547K, D614G, H655Y, N679K, P681H, N764K, D796Y, N856K, Q954H, N969K, L981F |
S protein |
Slight change in antigenicity |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).






