Submitted:
07 September 2023
Posted:
08 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
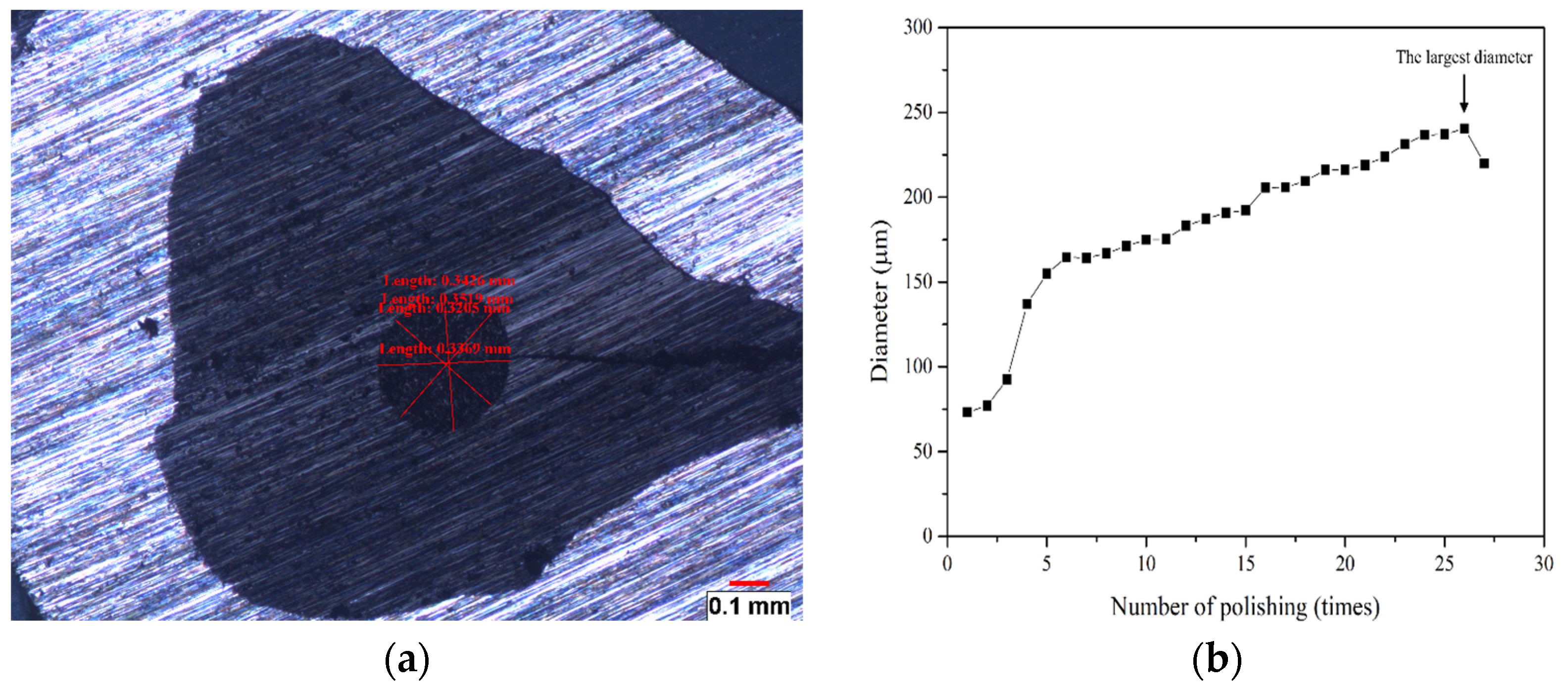
2.1. Sample Preparation
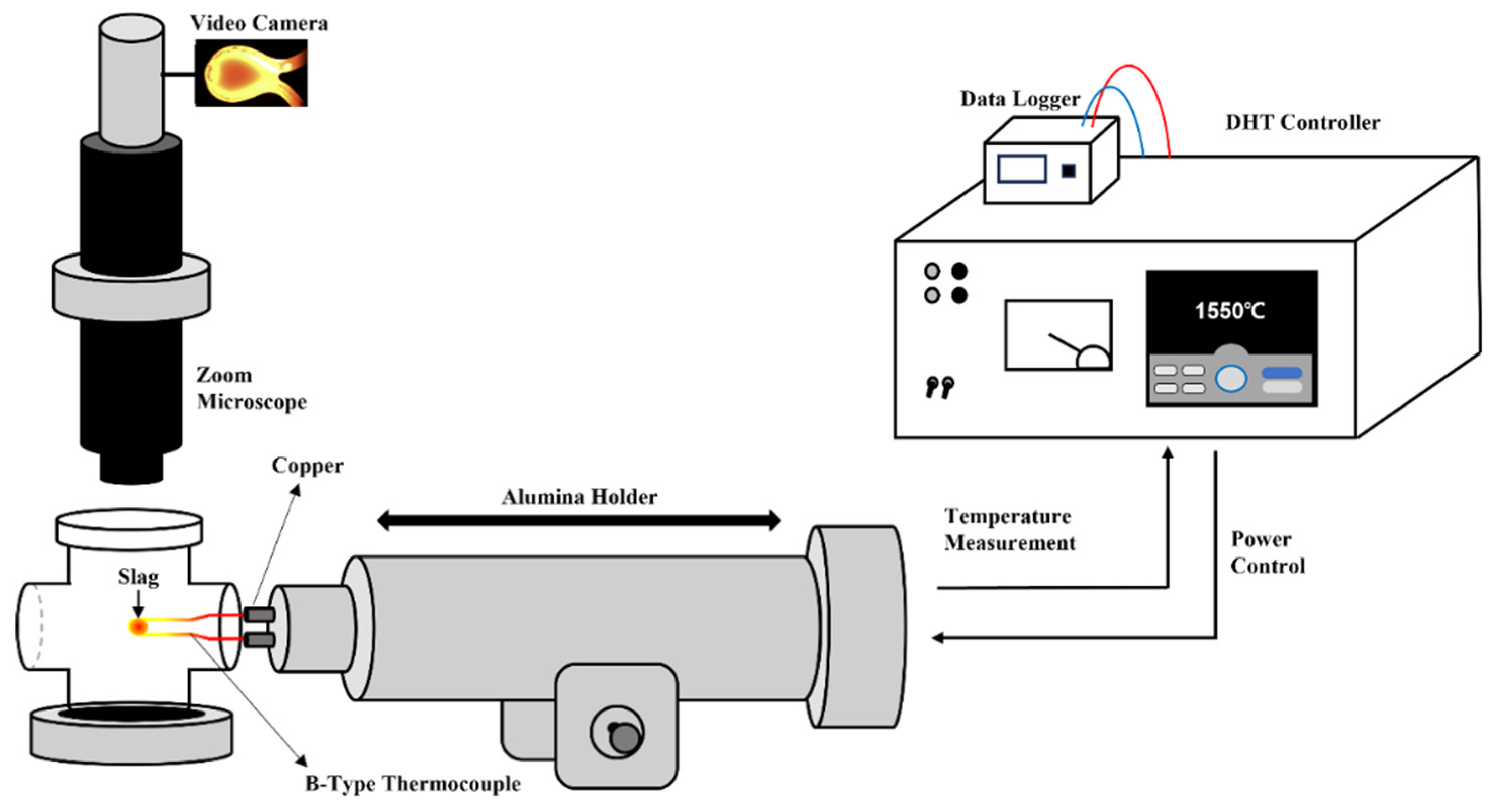
2.2. Single Hot Thermocouple Apparatus (SHT Apparatus)
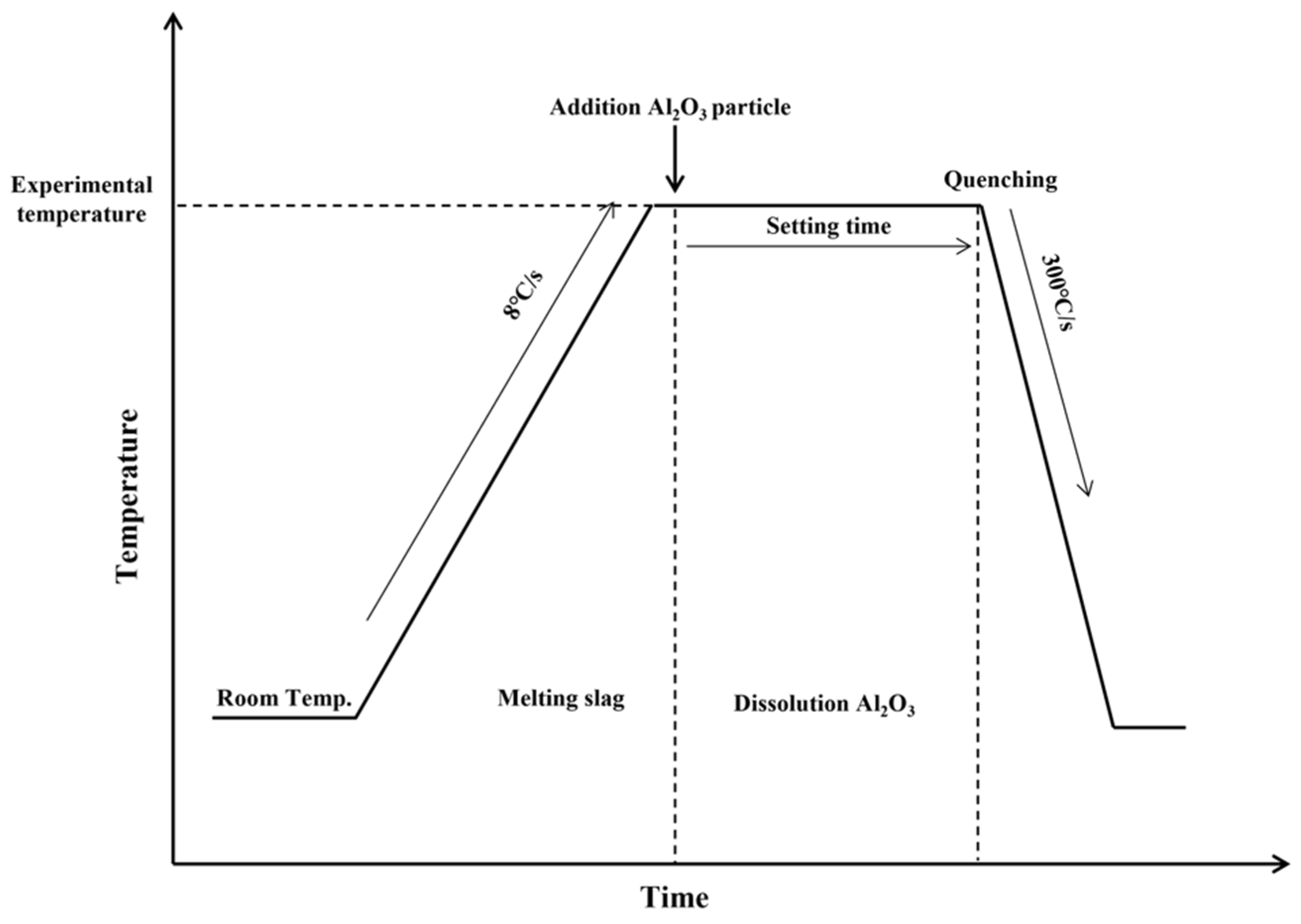
2.3. Experimental Conditions
3. Results and Discussion
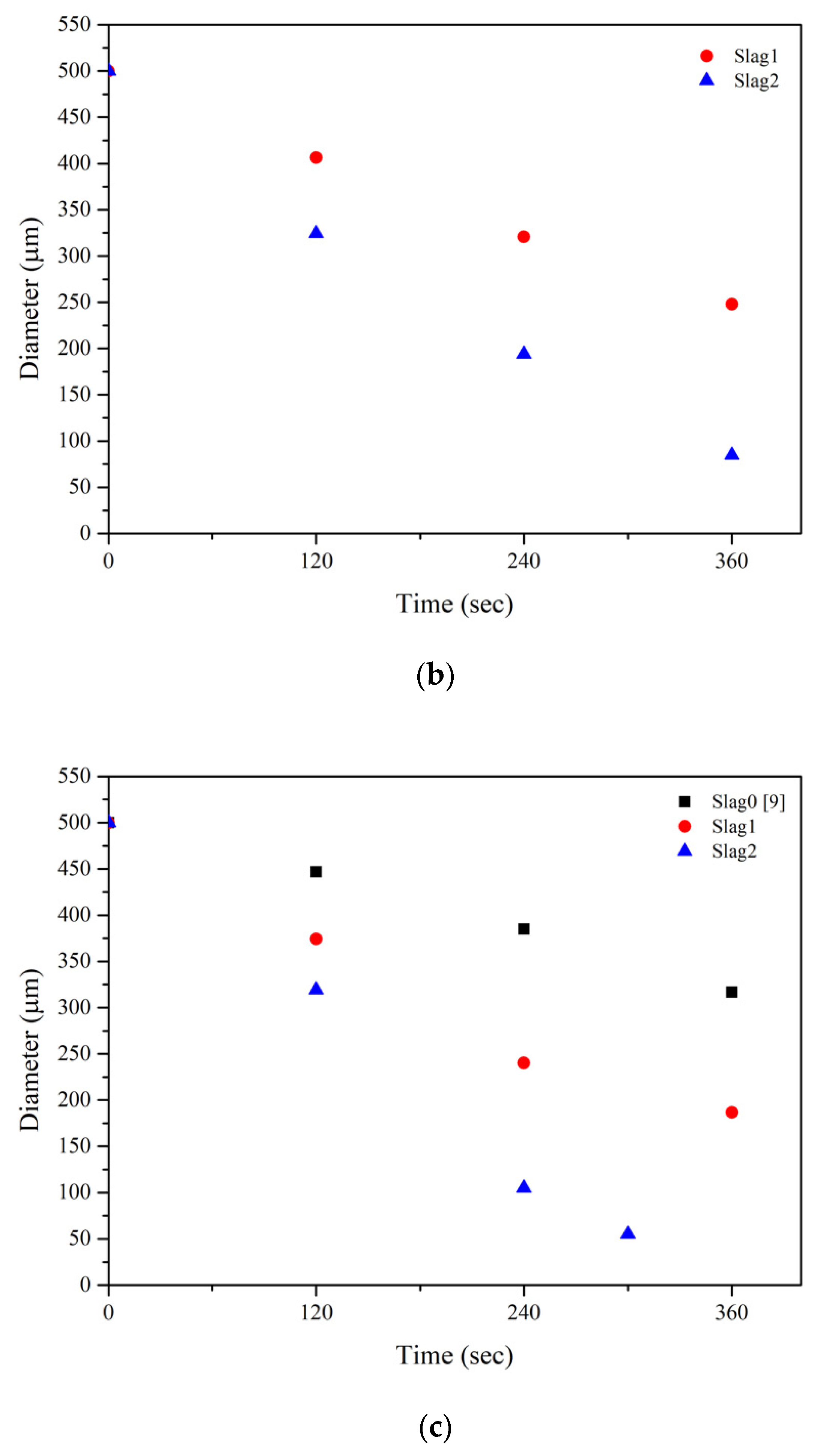
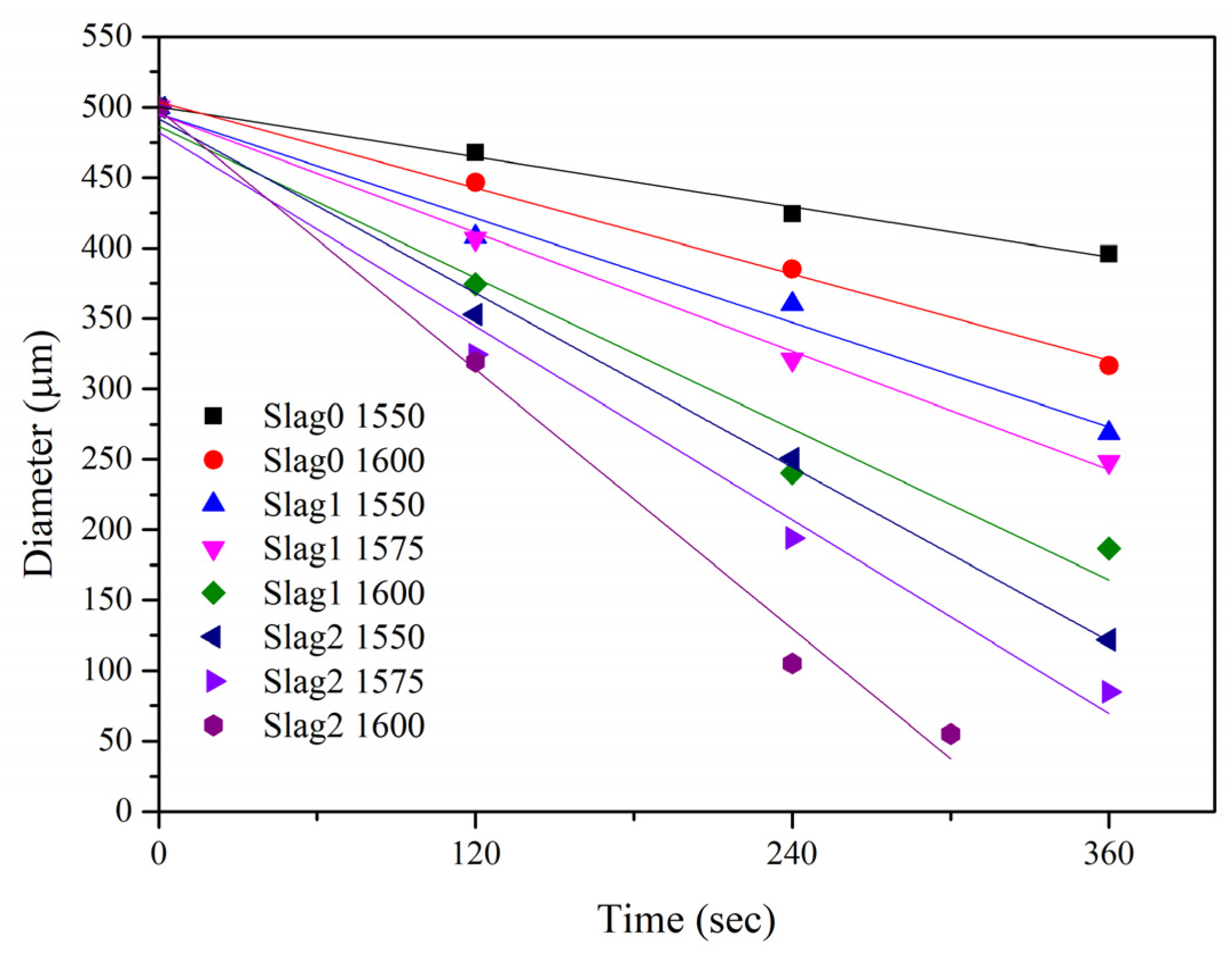
3.1. Dissolution Behavior of Al2O3 Particles according to Temperature and FexO content in Slag
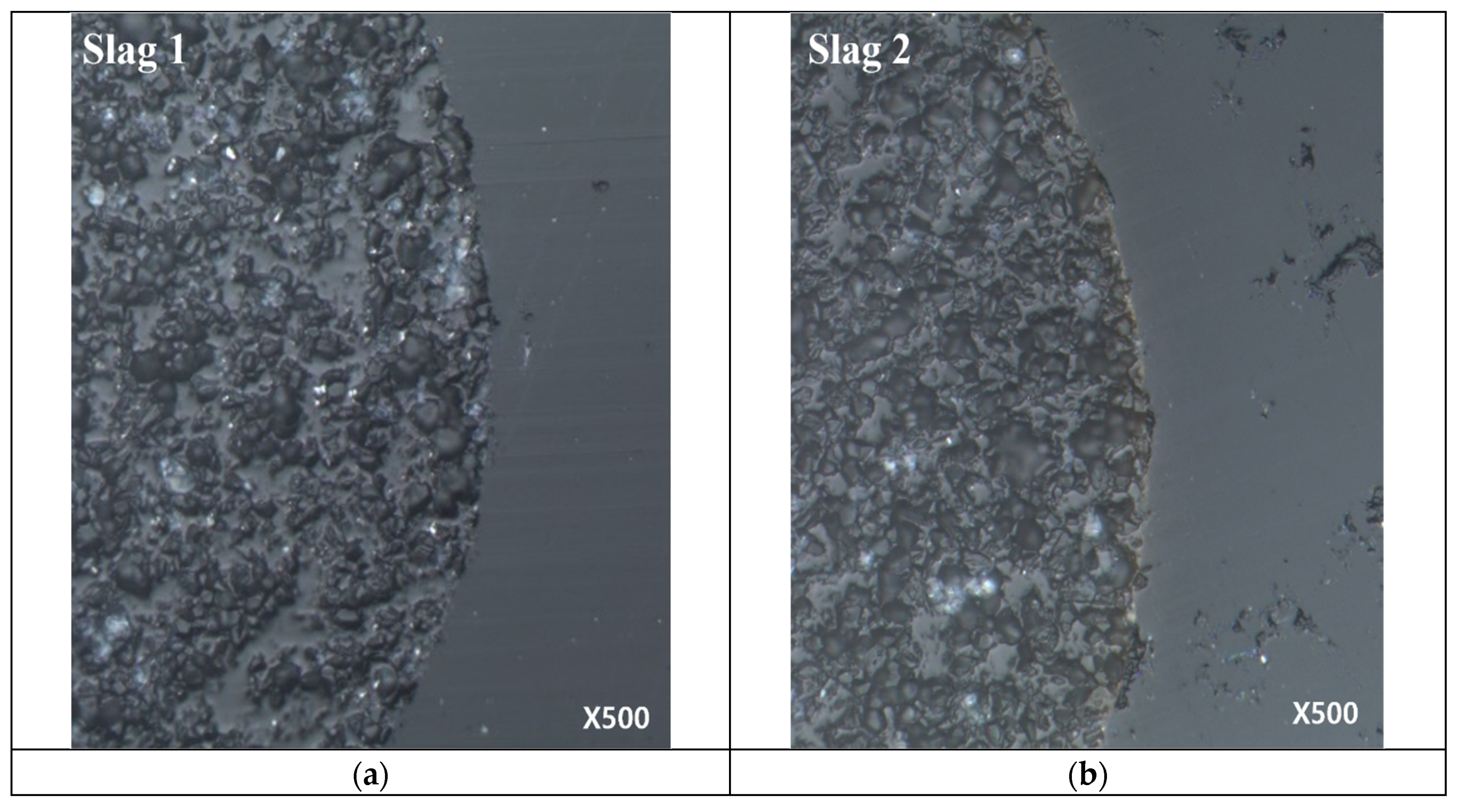
3.2. Analysis of Slag/Al2O3 Particle Interface by SEM
3.3. Dissolution Mechanism of Al2O3
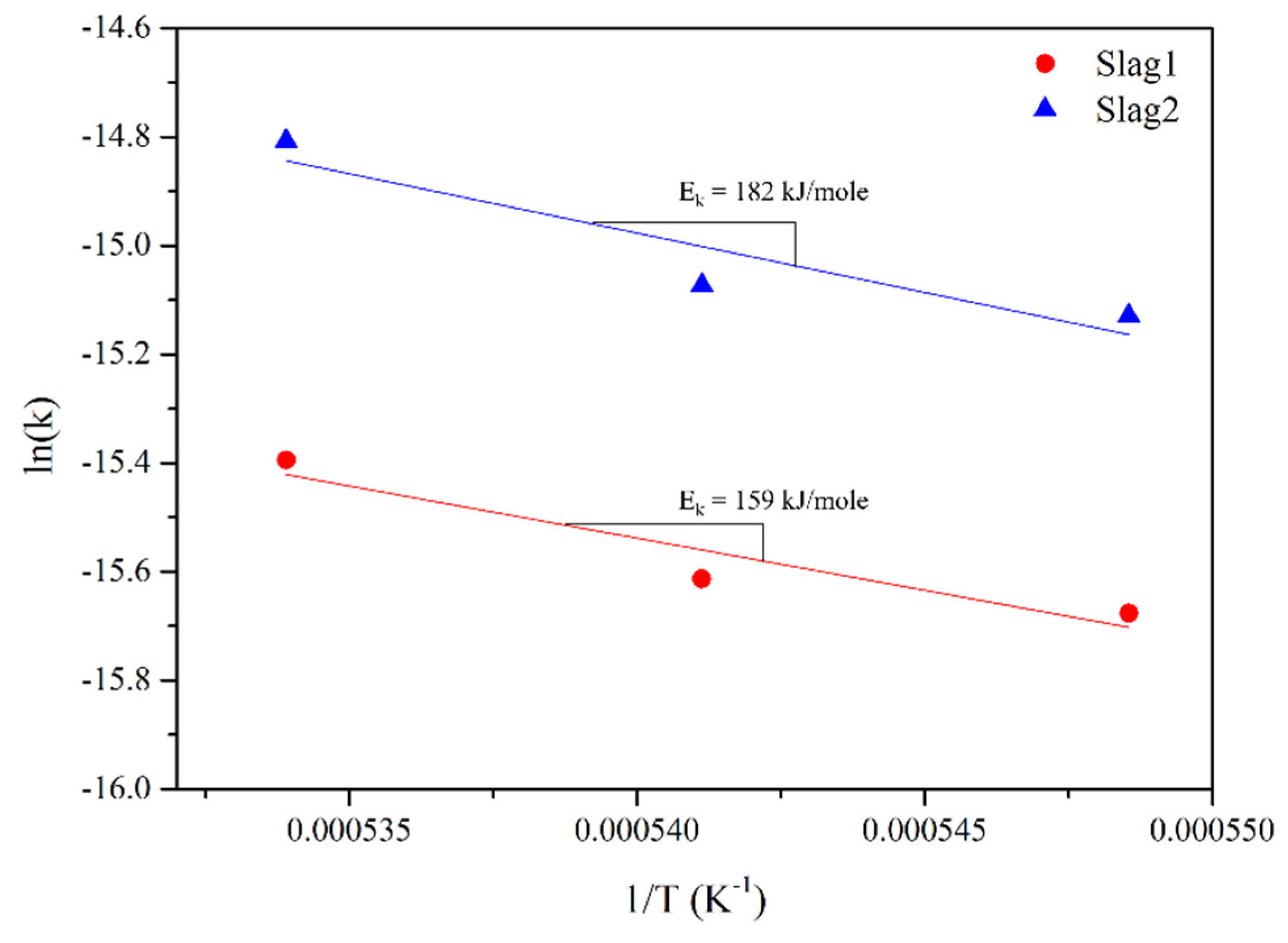
3.4. Activation Energy
4. Conclusions
- (1)
- The dissolution rate increased linearly as the FexO content of the slag increased from 0 to 20 wt% and the dissolution temperature increased from 1550 to 1600 °C.
- (2)
- Through a SEM analysis it was observed that no compound was formed at the interface of Al2O3 particles and slag. Therefore, the rate step of Al2O3 particle dissolution is interpreted as liquid phase mass transfer.
- (3)
- The mass transfer coefficient was obtained using the dissolution rate equation. The mass transfer coefficient increased with increasing FexO content in the slag and increasing dissolution temperature.
- (4)
- The mass transfer coefficient is plotted in a graph as a function of temperature and the Ek values of slag1 and slag2 (159 and 182 kJ/mole, respectively) are found using the Arrhenius equation.
- (5)
- The Ek of Al2O3 mass transfer in slag containing FexO in this study was lower than the Ek of slag without FexO.
Funding
Data Availability Statement
Conflicts of Interest
References
- Fan, Z., Friedmann, S.J., 2021. Low-carbon production of iron and steel: Technology options, economic assessment, and policy. Joule 5, 829–862. [CrossRef]
- Lee, B., Sohn, I., 2014. Review of Innovative Energy Savings Technology for the Electric Arc Furnace. JOM 66, 1581–1594. [CrossRef]
- Park, J.H., Todoroki, H., 2010. Control of MgO. Al2O3 spinel inclusions in stainless steels. ISIJ Int. 50, 1333–1346. [CrossRef]
- Dimitrov, S., Weyl, A., Janke, D., 1995. Control of the aluminium-oxygen reaction in pure iron melts. Steel Res. 66, 3–7. [CrossRef]
- Um, H., Yeo, S., Kang, Y.-B., Chung, Y., 2022. The effect of FexO content on dissolution behavior of an alumina inclusion in CaO–Al2O3–SiO2–FexO slag by a single hot thermocouple technique. Ceram. Int. 48, 35301–35309. [CrossRef]
- Jung, I.-H., Decterov, S.A., Pelton, A.D., 2004. Computer applications of thermodynamic databases to inclusion engineering. ISIJ Int. 44, 527–536. [CrossRef]
- Holappa, L., Hämäläinen, M., Liukkonen, M., Lind, M., 2003. Thermodynamic examination of inclusion modification and precipitation from calcium treatment to solidified steel. Ironmak. Steelmak. 30, 111–115. [CrossRef]
- Park, J.-H., Jung, I.-H., Hae-Geon, L.E.E., 2006. Dissolution behavior of Al2O3 and MgO inclusions in the CaO-Al2O3-SiO2 slags: Formation of ring-like structure of MgAl2O4 and Ca2SiO4 around MgO inclusions. ISIJ Int. 46, 1626–1634. [CrossRef]
- Sridhar, S., Cramb, A.W., 2000. Kinetics of Al2O3 dissolution in CaO-MgO-SiO2-Al2O3 slags: In situ observations and analysis. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 31, 406–410. [CrossRef]
- Shu, Q., Zhang, X., Wang, Y., Li, J., Chou, K., 2015. Effect of Na2O on dissolution rate of alumina in CaO-Al2O3-MgO-SiO2 slag. Presented at the Proceedings of the 6th International Congress on the Science and Technology of Steelmaking, ICS 2015, pp. 606–609.
- Yi, K.W., Tse, C., Park, J.-H., Valdez, M., Cramb, A.W., Sridhar, S., 2003. Determination of dissolution time of Al2O3 and MgO inclusions in synthetic Al2O3-CaO-MgO slags. Scand. J. Metall. 32, 177–184. [CrossRef]
- Ren, C., Zhang, L., Zhang, J., Wu, S., Zhu, P., Ren, Y., 2021. In Situ Observation of the Dissolution of Al2O3 Particles in CaO-Al2O3-SiO2 Slags. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 52, 3288–3301. [CrossRef]
- Holappa, L., Kekkonen, M., Louhenkilpi, S., Hagemann, R., Schröder, C., Scheller, P., 2013. Active tundish slag. Steel Res. Int. 84, 638–648. [CrossRef]
- Park, Y.-J., Cho, Y.-M., Cha, W.-Y., Kang, Y.-B., 2020. Dissolution kinetics of alumina in molten CaO–Al2O3–FetO–MgO–SiO2 oxide representing the RH slag in steelmaking process. J. Am. Ceram. Soc. 103, 2210–2224. [CrossRef]
- Yeo, S., Um, H., Chung, Y., 2021. The Effect of Alumina Activity on Dissolution Behavior of Alumina Particles in CaO–Al2O3–SiO2 Slags. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 52, 3938–3945. [CrossRef]
- Zhang, S., Rezaie, H.R., Sarpoolaky, H., Lee, W.E., 2000. Alumina dissolution into silicate slag. J. Am. Ceram. Soc. 83, 897–903. [CrossRef]
- Valdez, M., Prapakorn, K., Cramb, A.W., Seetharaman, S., 2001. A study of the dissolution of Al2O3, MgO and MgAl2O4 particles in a CaO-Al2O3-SiO2 slag. Steel Res. 72, 291–297. [CrossRef]
- Monaghan, B.J., Chen, L., Sorbe, J., 2005. Comparative study of oxide inclusion dissolution in CaO-SiO2-Al2O3 slag. Presented at the Ironmaking and Steelmaking, pp. 258–264. [CrossRef]
- Chen, G., He, S., Wang, Q., 2020. Dissolution behavior of Al2O3 into tundish slag for high-al steel. J. Mater. Res. Technol. 9, 11311–11318. [CrossRef]
- Shi, G.-Y., Zhang, T.-A., Dou, Z.-H., Niu, L.-P., 2020. Dissolution behavior of Al2O3 inclusions in CaO-Al2O3 based slag representing aluminothermic reduction slag. Crystals 10, 1–12. [CrossRef]
- Odenthal, H.-J., Kemminger, A., Krause, F., Sankowski, L., Uebber, N., Vogl, N., 2018. Review on Modeling and Simulation of the Electric Arc Furnace (EAF). Steel Res. Int. 89. [CrossRef]
- Lee, S., Chung, Y., 2022. The effect of C content in MgO–C on dissolution behavior in CaO–SiO2–Al2O3 slag. Ceram. Int. 48, 26984–26991. [CrossRef]
- Kim, Y., Kashiwaya, Y., Chung, Y., 2020. Effect of varying Al2O3 contents of CaO–Al2O3–SiO2 slags on lumped MgO dissolution. Ceram. Int. 46, 6205–6211. [CrossRef]
- Taira, S., Nakashima, K., Mori, K., 1993. Kinetic Behavior of Dissolution of Sintered Alumina Into CaO-SiO2&Al203 Slags. ISIJ Int. 33, 116–123. [CrossRef]
- Choi, J.-Y., Lee, H.-G., Kim, J.-S., 2002. Dissolution rate of Al2O3 into molten CaO-SiO2-Al2O3 slags. ISIJ Int. 42, 852–860. [CrossRef]
- SAMADDAR, B.N., KINGERY, W.D., COOPER, A.R., 1964. Dissolution in Ceramic Systems: 11, Dissolution of Aluminu, Mullite, Anorthite, and Silica in a Calcium-Aluminum-Silicate Slag. J. Am. Ceram. Soc. 47, 249–254. [CrossRef]
- OISHI, Y., COOPER, A.R., KINGERY, W.D., 1965. Dissolution in Ceramic Systems: III, Boundary Layer Concentration Gradients. J. Am. Ceram. Soc. 48, 88–95. [CrossRef]
- Bui, A.-H., Ha, H.-M., Kang, Y.-B., Chung, I.-S., Lee, H.-G., 2005. Dissolution behavior of alumina in mold fluxes for steel continuous casting. Met. Mater. Int. 11, 183–190. [CrossRef]
- Cho, W.D., Fan, P., 2004. Diffusional Dissolution of Alumina in Various Steelmaking Slags. ISIJ Int. 44, 229–234. [CrossRef]
- R. H. Petrucci., W. S. Harwood., 1993. General Chemistry, 6th ed., Macmillan, New York, 535.
- Liu, Y.Q., Wang, L.J., Chou, K.C., 2014. Dissolution behavior of Al2O3 in refining slags containing Ce2O3. ISIJ Int. 54, 728–733. [CrossRef]
| Type | Source | Diameter | Weight | Concentration | |
|---|---|---|---|---|---|
| Al2O3 (%) | Other (%) | ||||
| Alumina sphere | GoodFellow | 500 ± 2.5 μm | 0.25 ± 0.05 mg | 99.9 | 0.1 |
| CaO | SiO2 | Al2O3 | FexO | Basicity | References | |
|---|---|---|---|---|---|---|
| Slag0 | 47.5 | 47.5 | 5 | 0 | 1 | [9] |
| Slag1 | 42.5 | 42.5 | 5 | 10 | 1 | |
| Slag2 | 37.5 | 37.5 | 5 | 20 | 1 | |
| Slag3 | 32.5 | 32.5 | 5 | 30 | 1 | [11] |
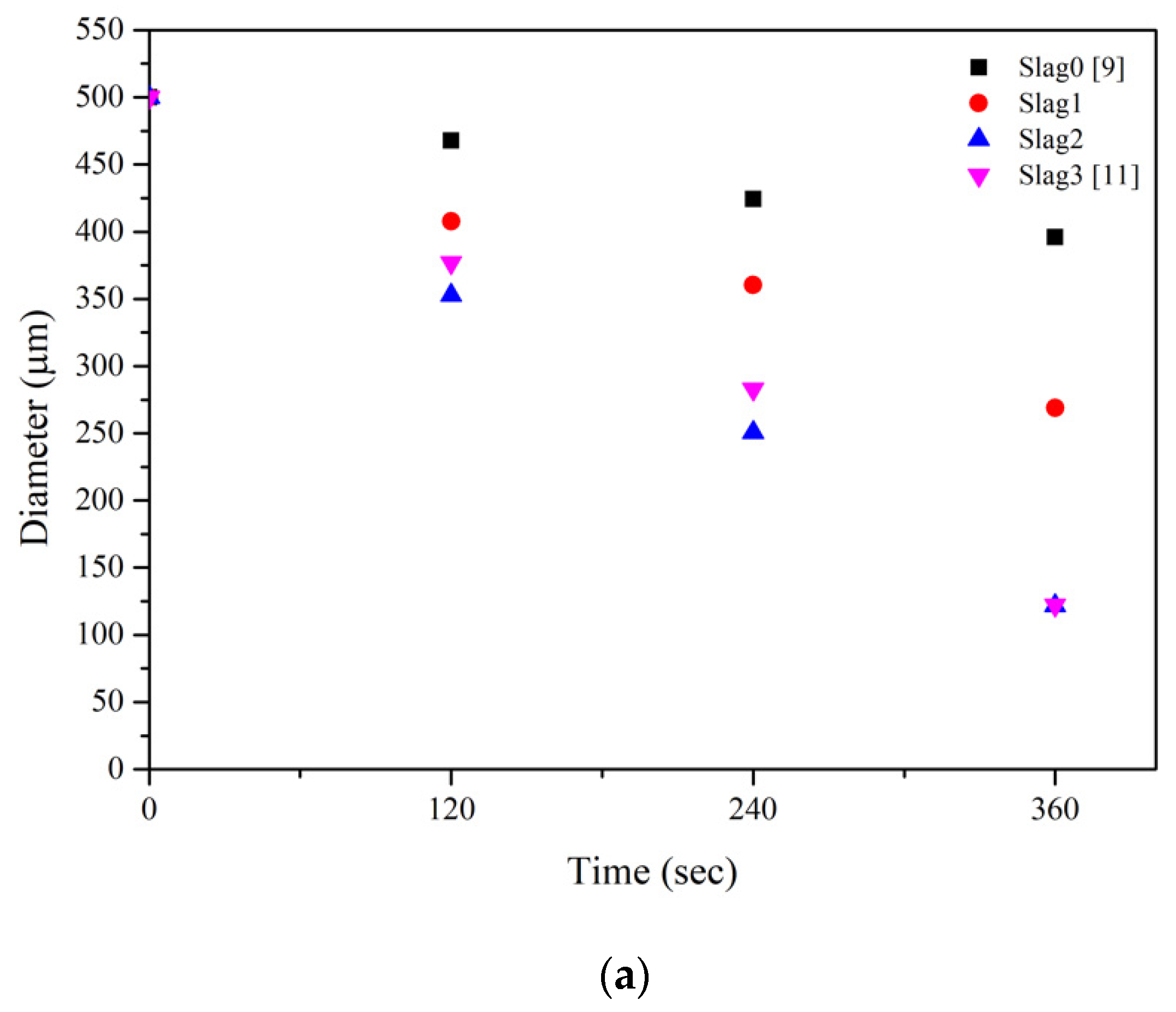
| Temperature (°C) | Slag | 120s | 240s | 360s | References |
|---|---|---|---|---|---|
| 1550 | 0 | 465 | 420 | 393 | [18] |
| 1 | 408 | 360 | 269 | ||
| 2 | 353 | 251 | 122 | ||
| 3 | 377 | 283 | 122 | [5] | |
| 1575 | 1 | 407 | 321 | 248 | |
| 2 | 324 | 194 | 85 | ||
| 1600 | 0 | 447 | 385 | 317 | [18] |
| 1 | 374 | 240 | 187 | ||
| 2 | 319 | 105 | 55 (300s) |
| (mole/m3) *Factsage7.3TM |
(kg/m3) |
(cm/s) |
(cm/s) |
References | |
|---|---|---|---|---|---|
| Slag0 1550℃ | 10,745 | 2660 | 2.96×10-5 | 7.19×10-8 | [18] |
| Slag0 1600℃ | 11,399 | 2647 | 5.10×10-5 | 1.16×10-7 | |
| Slag1 1550℃ | 10,885 | 2796 | 6.18×10-5 | 1.56×10-7 | Present study |
| Slag1 1575℃ | 11,581 | 2789 | 7.01×10-5 | 1.66×10-7 | |
| Slag1 1600℃ | 11,846 | 2782 | 8.95×10-5 | 2.06×10-7 | |
| Slag2 1550℃ | 11,050 | 2943 | 1.03×10-4 | 2.69×10-7 | Present study |
| Slag2 1575℃ | 11,600 | 2935 | 1.15×10-4 | 2.84×10-7 | |
| Slag2 1600℃ | 11,925 | 2928 | 1.65×10-4 | 3.71×10-7 |
| Slag | Chemical composition (wt%) | Ek (kJ/mole) | References | |||||
|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | MgO | Ce2O3 | FexO | |||
| 0 | 47.5 | 47.5 | 5.0 | 0 | 0 | 0 | 304 | [15] |
| 1 | 42.5 | 42.5 | 5.0 | 0 | 0 | 10 | 159 | Present study |
| 2 | 37.5 | 37.5 | 5.0 | 0 | 0 | 20 | 182 | |
| 4 | 45.0 | 10.0 | 45.0 | 0 | 0 | 0 | 445 | [29] |
| 5 | 35.0 | 30.0 | 35.0 | 0 | 0 | 0 | 334 | |
| 6 | 45.0 | 4.5 | 37.5 | 10.0 | 3 | 0 | 292 | [31] 1 |
| 7 | 45.0 | 4.5 | 35.5 | 10.0 | 5 | 0 | 347 | |
| 8 | 45.0 | 4.5 | 32.5 | 10.0 | 8 | 0 | 249 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




