2. Materials and Methods
Clinical trial and dietary intervention
Data from the MEDGI-Carb trial was used in the present study because participants were at risk of developing T2DM and the intervention tested the effect of a high vs. low GI diet within the context of a healthy eating pattern, i.e., Mediterranean diet. By including individuals with elevated risk of T2D and using data from a dietary intervention with large contrasts in GI we had the chance to evaluate the possibility of identifying glycemic response clusters across a wide range of likely postprandial glucose responses and to assess their stability during the intervention.,
The MEDGI-Carb trial was an international multi-center randomized, controlled, parallel-group, 15-week dietary trial, including a 3-week baseline period followed by a 12-week controlled dietary intervention in adults at elevated risk of developing type 2 diabetes (give their age range, BMI range OR provide a small table with their features (also the other risk factors, up to you). During the 12-week intervention period, participants consumed a Mediterranean-style, controlled, isocaloric, weight-maintenance diet. Furthermore, the participants were instructed to consume either a low-GI or high-GI diet with intervention-specific foods. All participants were instructed to consume the same amount of digestible carbohydrates (270 g/d) and dietary fiber (35 g/d). Modulation of daily energy intake was achieved by adjusting intakes of proteins and fat. Half of the daily carbohydrate intake was identical in the two groups, including vegetables and fruit. The other half consisted of carbohydrates with GI < 55 and > 70 in the low and high GI groups, respectively. The intervention-specific carbohydrates were distributed throughout the day with 26% at breakfast, 30% at lunch, and 44% at dinner. Markers of glucose homeostasis were obtained during standardized testing days by completion of an eight-hour MMTT, an OGTT, and 6 days of 24-hour CGM at baseline and post-testing. Furthermore, blood samples were drawn to measure HbA1c, insulin, glucose, high-density lipoprotein, triglycerides, blood pressure, and anthropometrics. Insulin sensitivity indices such as quantitative insulin sensitivity check index (QUICKI), Stumvoll, and Matsuda were calculated using data from the OGTTs [
14]. The study was conducted at three centers: (i) Federico II University, Naples, Italy, (ii) Chalmers University of Technology, Gothenburg, Sweden, and (iii) Purdue University, West Lafayette, IN, USA. The study was initiated in January 2018 and the last participant finished the trial in December 2019. The trial was registered in the public trial registry clinicaltrials.gov as NCT03410719 prior to initiating participant recruitment. The study protocol was approved by the intuitional review boards at Purdue University and Federico II University and by the Swedish Ethical Review Authority. The study protocol with detailed descriptions of the trial such as randomization, blood sampling, anthropometric measurements, and intervention diet has previously been published [
12] as well as the results of the primary analysis [
13].
Mixed meal tolerance tests
Breakfast and lunch MMTT were performed at baseline, at mid-testing (USA only), and post-intervention. Prior to all testing days, participants were instructed not to eat or drink anything (except a small amount of water) from 10:00 p.m. the evening before the visit. Fasting blood samples were collected at the time point (TP) -15 min and TP -5 following 15 min of rest. The test meal was consumed at TP 0 in two parts, the participants had 7.5 minutes to consume the first part of the meal and 7.5 minutes to consume the last part, to control the pace of the meal consumption. The participants were allowed to drink 8 ounces of water (approx. 2.4 dL) during the meal. The test meals were strictly standardized across all three centers. All participants were served the same portion size i.e., kilocalories regardless of energy requirement for practical reasons. The food composition and nutrients of the standardized meals are provided in
Table 1.
Blood samples were collected at TP 15 after the test meal and then at TP 30, TP 45, TP 60, TP 90, TP 120, TP 180, and TP 240. A standardized lunch meal was served at TP 240, again with 7.5 minutes to consume the first half of the meal and 7.5 min to consume the second part. The blood sampling continued by the same pattern as after the breakfast meal (
Supplementary Figure S1). For the present analysis, only glucose data from testing minutes TP -15 to TP 240 (i.e., the breakfast meal) were included to accommodate the fit of a simple kinetic model of the postprandial glucose response.
Oral glucose tolerance test
Participants completed OGTT at baseline, at mid-testing (USA only), and post-intervention. Fasting blood samples were collected at TP -15 after 15 minutes of rest and at TP -5. At TP 0, participants were instructed to consume a test beverage containing 75 g glucose dissolved in water within 5 minutes. No additional fluids were permitted during the test. Blood samples were collected at TP 60 and TP 120 (
Supplementary Figure S1).
Fecal microbiota
During pre- and post-intervention study days, participants were asked to collect fecal samples using a stool sampling collection kit. Samples were taken using an EasySampler Stool Collector and a sample tube with a spoon-lid. The sample was protected by being placed in yet another tube and stored immediately in the freezer (-20 ℃). The samples were transported in a cooling box with an ice pack to the clinic within 72 hours after the sample was collected. At the clinic, the sample is transferred to -80℃ within 24 hours. The samples were analyzed with the 16S rRNA gene amplicon sequencing method where DNA was extracted and purified from fecal samples using the
QIAamp FAST DNA Stool mini kit from Qiagen. The DNA extraction followed the protocol from the manufacturer with one exception, where lysis of the bacterial cell walls used a mechanical lysis step (bead beating 2x1 minutes in a Precellys Evolution using 0.1 mm zirconium/silica beads). Once the DNA was extracted and purified from the sample, polymerase chain reaction (PCR) was used to amplify the V3-V4 region of the gene encoding 16S rRNA. This gene exists in all bacteria and is normally used for the taxonomic classification of bacteria since parts of the 16S gene vary in sequence composition between different bacteria. Sample-specific barcodes and Illumina adapters were then attached to the PCR amplicons to enable the pooling of samples. The library of PCR amplicons was then sequenced on the Illumina NovaSeq 6000 platform at Novogene. The bioinformatics analysis to handle the generated sequence data used QIIME2 via the dada2 pipeline. The sequences were first demultiplexed, i.e., separated according to the sample-specific barcode in the specific sample. Then, a quality control and filtration of the sequence data was performed to remove sequences with poor quality. Finally, a taxonomic classification of the sequences was performed. The gut microbiota analysis and subsequent data processing and analysis has been described in detail by Iversen, Dicksved [
15]. The gut microbiota was analyzed to investigate if it could explain inter-individual differences in glycemic postprandial response and provide some mechanistic insights into potential differential response profiles between subjects. The microbiota was aggregated to genus level prior to downstream analysis.
Species that were known from literature to associate with glucose regulation were selected and association with clusters was investigated using one-way ANOVA. Selected species were "
Bifidobacterium", "
Bacteroides", "
Faecalibacterium", "
Akkermansia", "
Roseburia", "
Fusobacterium", "
Blautia", "
Haemophilus", "
Ruminococcus", "
Clostridium", and "
Dorea" [
16,
17,
18,
19,
20,
21,
22].
Mechanistic model of glucose regulation
A modified version of the minimal glucose model [
23] proposed by Bolie [
24] was used to describe the glucose response to the MMTT from breakfast at baseline and after 12 wk in order to identify interpretable parameters that could be used as the basis for grouping individuals according to plasma glucose-time profiles [
25]. The model was initially developed for plasma glucose data following an OGTT and consists of two coupled differential equations describing the feedback loop of glucose and insulin blood concentrations in response to glucose intake [
25]. In the present study, the MMTT consisted of a carbohydrate-rich meal which was hypothesized to give a similar response to that of an OGTT, when consumed at a fasted state, although it is acknowledged that the model is oversimplifying the complex glucose-insulin system in response to foods that also contain other nutrients and non-nutrients. The idea was not to provide a model that explains all biological processes related to the postprandial response to a mixed meal, rather to provide a simple model that fits the data and could be used to group responders into clusters that are differently related to T2D risk factors.
The dynamics of the model are described using compartments that represent mechanisms in the glucose-insulin system and the exchange rates between compartments are described using rate constants. The model assumes that the ingested glucose is delayed by the digestive system and transferred to the bloodstream, where insulin acts to let the glucose be absorbed by the muscle tissue or the liver and converted to glycogen. Furthermore, the model assumes that the glucose can be discarded through the urine via the kidneys and that the pancreas produces insulin at a given rate in response to the current glucose concentration. Notably, the model assumes a linear relationship between insulin secretion and glucose which is often not the case due to the effects of incretin hormones for example, but the rationale is to obtain a more parsimonious model with easily grouped parameters. A particularly simple solution for the model glucose concentration can be formulated if the gastrointestinal absorption is assumed to rise very quickly and fall slowly and the ingested breakfast meal is modeled as a momentary impulse at the first measurement in time[
25]. This solution takes the shape of a damped sinusoidal wave (Equation 1), which is used widely in mechanics [
26]. Thus, the parameters governing the glucose dynamics were reduced to a glucose baseline level (
), sinusoidal amplitude (
) involved in the resulting amplitude of the glucose concentration, sinusoidal frequency (
) relating to the velocity of glucose oscillations, and damping coefficient (
) determining the rate of glucose decay.
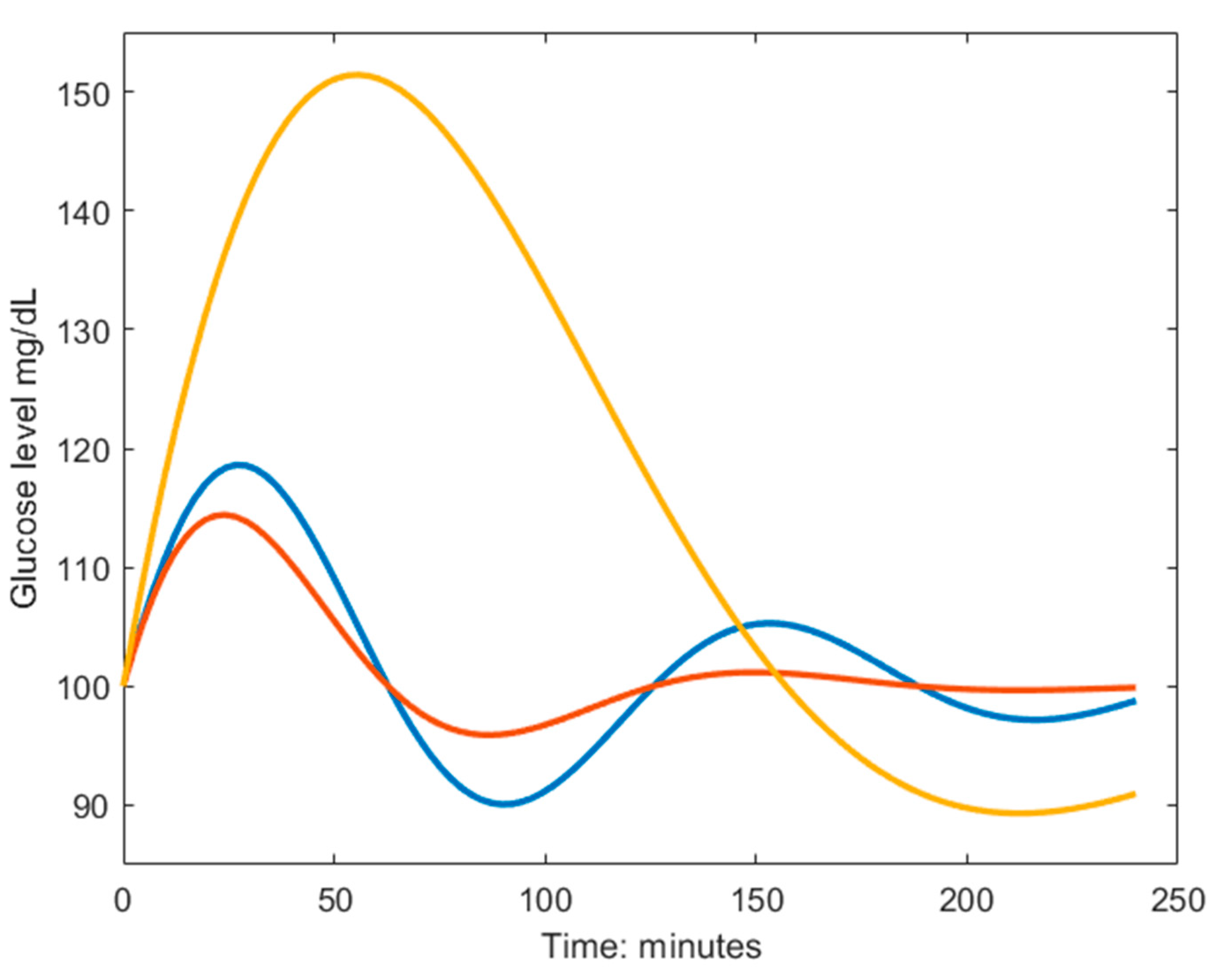
Figure 1.
Example dynamics generated from the model in Eq.1. The blue curve is characterized by a fast biphasic response to the MMTT, thus having high frequency () and low amplitude (). The red curve has a larger damping coefficient () which yields a faster monophasic return to baseline. The yellow curve is characterized by a slow response to the MMTT, meaning poor glucose regulation, and is described by the inverse parameter relationship as the blue line but sharing the same damping coefficient.
Figure 1.
Example dynamics generated from the model in Eq.1. The blue curve is characterized by a fast biphasic response to the MMTT, thus having high frequency () and low amplitude (). The red curve has a larger damping coefficient () which yields a faster monophasic return to baseline. The yellow curve is characterized by a slow response to the MMTT, meaning poor glucose regulation, and is described by the inverse parameter relationship as the blue line but sharing the same damping coefficient.
Although the parameters of the reduced model have no one-to-one correspondence to specific mechanisms in the body, they convey the general quality of the glucose control.
The sinusoidal frequency (
) relates to the rates of removal of glucose and insulin where a high frequency describes a fast response of the regulatory system meaning that the first glucose peak appears early, as seen in the blue and red line in Fig.1. The amplitude (
) of the undamped sinusoidal depends on the body’s tolerance of the ingested glucose and relates to the height of the glucose peak together with the damping coefficient (
). The yellow dynamic has a larger amplitude than the blue and red ones, and the larger damping coefficient in the red dynamics avoids the under- and overshoots seen in the blue dynamic Fig.1. It should be noted that insulin is not part of the solution in Eq.1 since it has been eliminated in the derivation and described in terms of glucose and the estimated parameters. This makes the model very attractive to use in a setting when insulin cannot be measured and CGM could be used to measure glucose, such as when performing measurements at home. Since no parameter directly describes the maximum postprandial glucose concentration, an expression of this was derived (Eq.2).
The model was originally shown to fit OGTT data well, despite mechanisms such as the role of adrenal cortical and medullary function in glucose economy were not accounted for [
27]. In the present study, the model was used to describe the postprandial glucose concentration for an MMTT where the subjects were fasted prior to ingesting the meal.
Statistical analyses
The parameters of the model were estimated within the nonlinear mixed effects model framework [
28]. Here, we refer to our nonlinear regression model (Eq. 1) as
which depends on the individual model parameters
and regresses to the glucose measurements
with some measurement or process error
with an assumed constant variance across observations (Eqs. 3 and 4). Here
represents the subject index.
The individual parameters are described by the fixed effects (shared among all individuals) , and the random effects . Additionally, they are affected by covariates via the covariate matrix . Here is a vector with four elements, representing the random effects (modeling the variation within the test population) on each of the four parameters in . The mean vector () of the multivariate normal distribution is dictated by what group individual is most likely to belong via the indicator function , effectively making a mixture of multivariate normal distributions and allowing identification of subgroups (clusters) within the data. The covariance matrix is denoted by , which we assume to be diagonal, i.e., no correlation between parameters. Associations between clusters and clinical parameters were investigated using one-way ANOVA and Chi-squared tests for continuous and categorical data, respectively.
The study effect on gut microbiota composition was investigated using log fold change of baseline and 12 wk. on all species using random forest analysis within a repeated double cross-validation framework [
29].
The parameter estimation software Monolix was used to simultaneously estimate the random and fixed effects (Monolix 2021R2, Lixoft SAS, a Simulations Plus company). Covariates were imposed to reduce the variance not reflecting blood glucose control. Age and site were imposed as covariates on the baseline (
). Since the breakfast meals given to the two treatment groups had similar nutritional composition (
Table 1), treatment (high or low GI) was imposed as a covariate on the amplitude (
), thus accounting for differences in MMTTs between treatments. Parameters were estimated per individual and occasion (pre- and post-trial). The relative standard error (RSE) was used as an estimate of the uncertainty in the estimated parameters:
Here, describe the standard error and the estimate. We consider an RSE value below 50% percent a valid estimate using the Monolix software.
3. Results
In total, 155 individuals completed the two MMTTs and OGTTs (baseline and wk. 12) and were included in the analyses. Calculations on fecal microbiota were based on 130 individuals who provided two fecal samples within the participants that performed the two MMTT and OGTTs (baseline and wk. 12) (
Table 2).
Postprandial MMTT glucose responses
Individual parameters of the kinetic model (baseline, amplitude, damping, and frequency) from Eq.1 were estimated using the postprandial MMTT glucose response at baseline and wk. 12. The parameters were successfully estimated with RSE < 43% in all cases, which indicated certainty in the estimates. Variation among individuals resulted only in the parameters amplitude
, frequency
, and baseline
since random effects on the damping parameter were not estimated with enough certainty. Clusters were therefore not based on the damping parameter
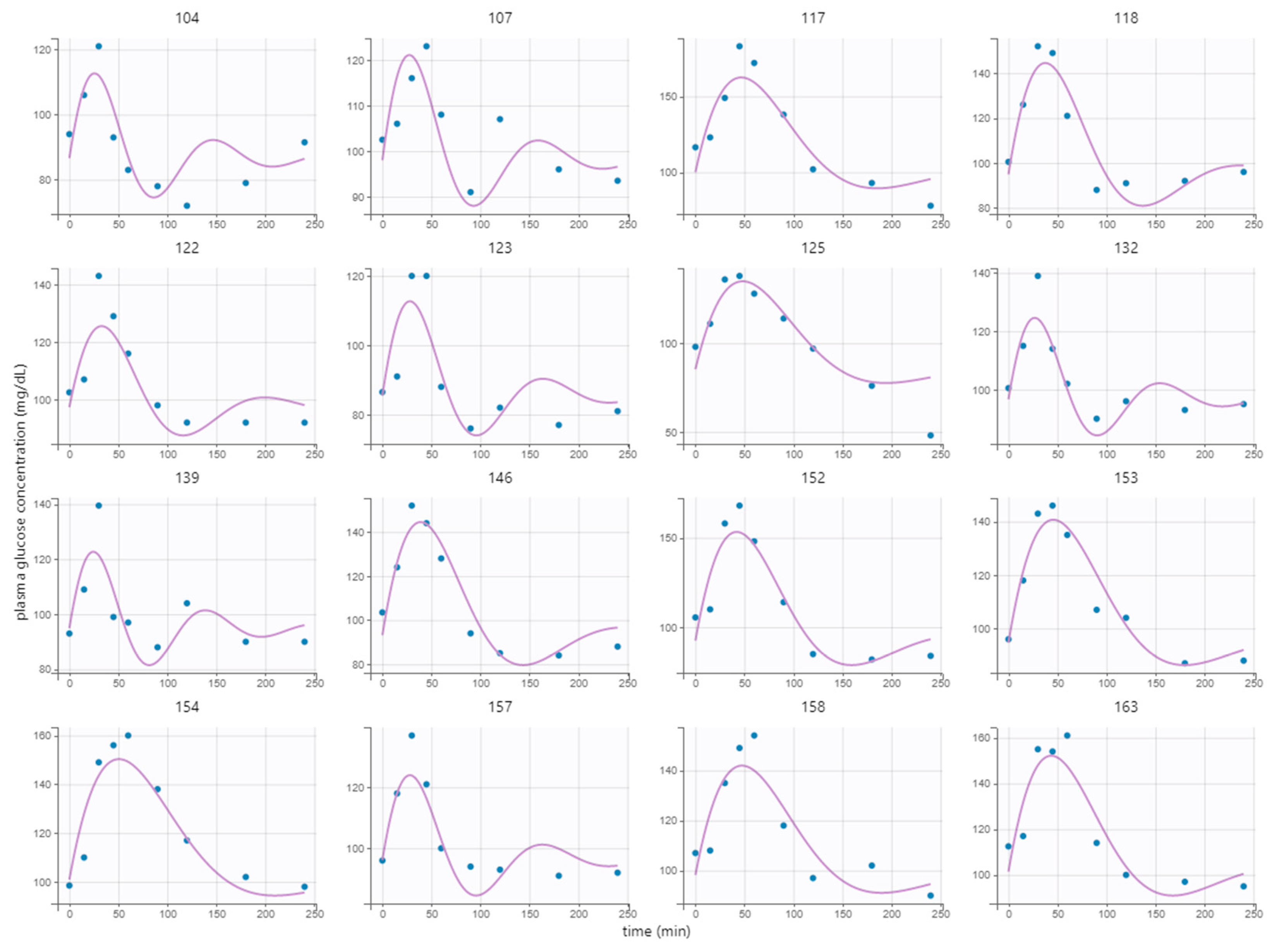
. Nor the covariate group membership (high-GI or low-GI) could be estimated with enough precision meaning that there was no effective difference in the response of the two meals. The model fitted well to the response of the breakfast MMTT as given by the low RSE, although some systematic phenomena could not be captured e.g., the slow undershoot in subject 104 (
Figure 2).
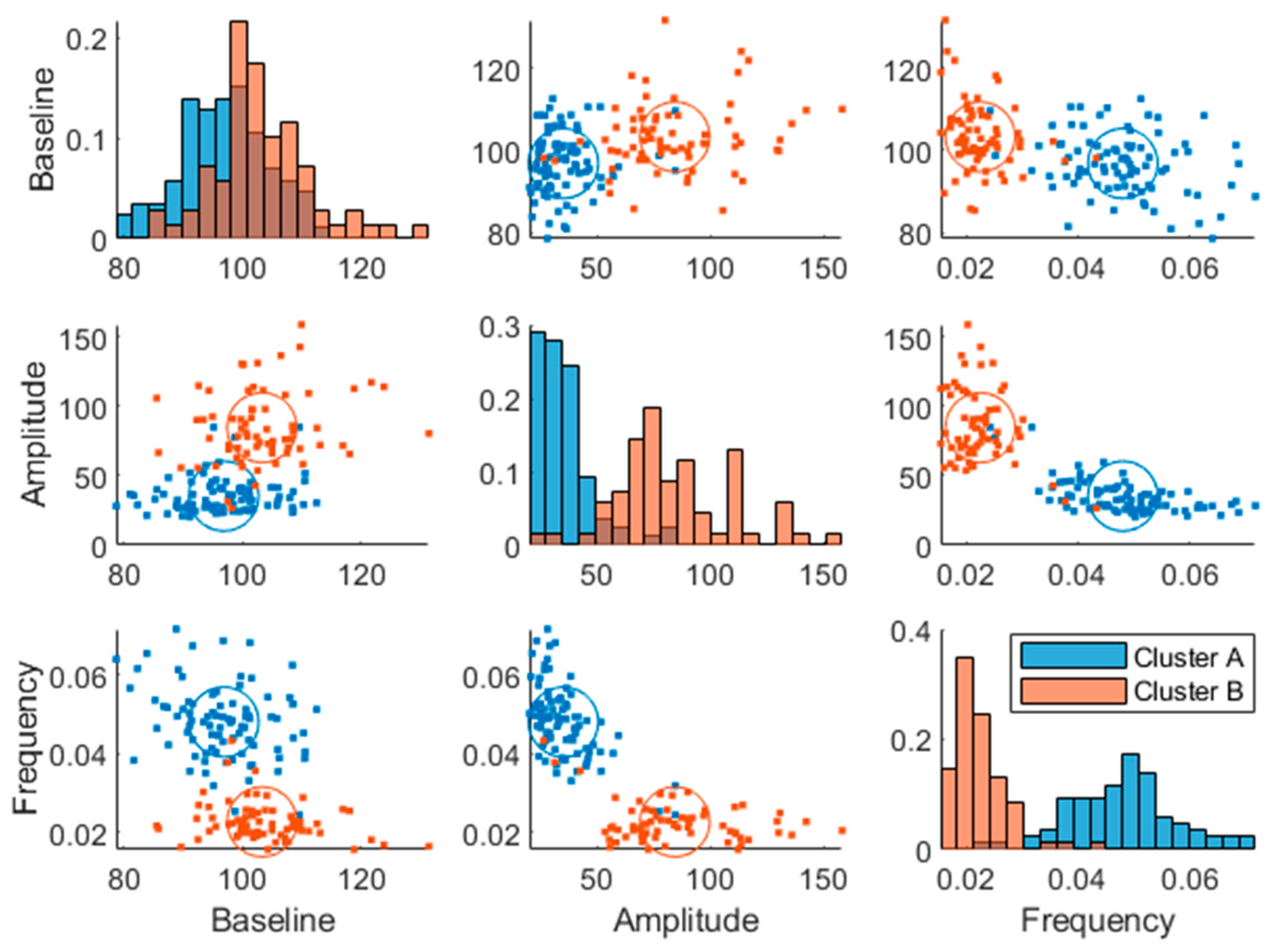
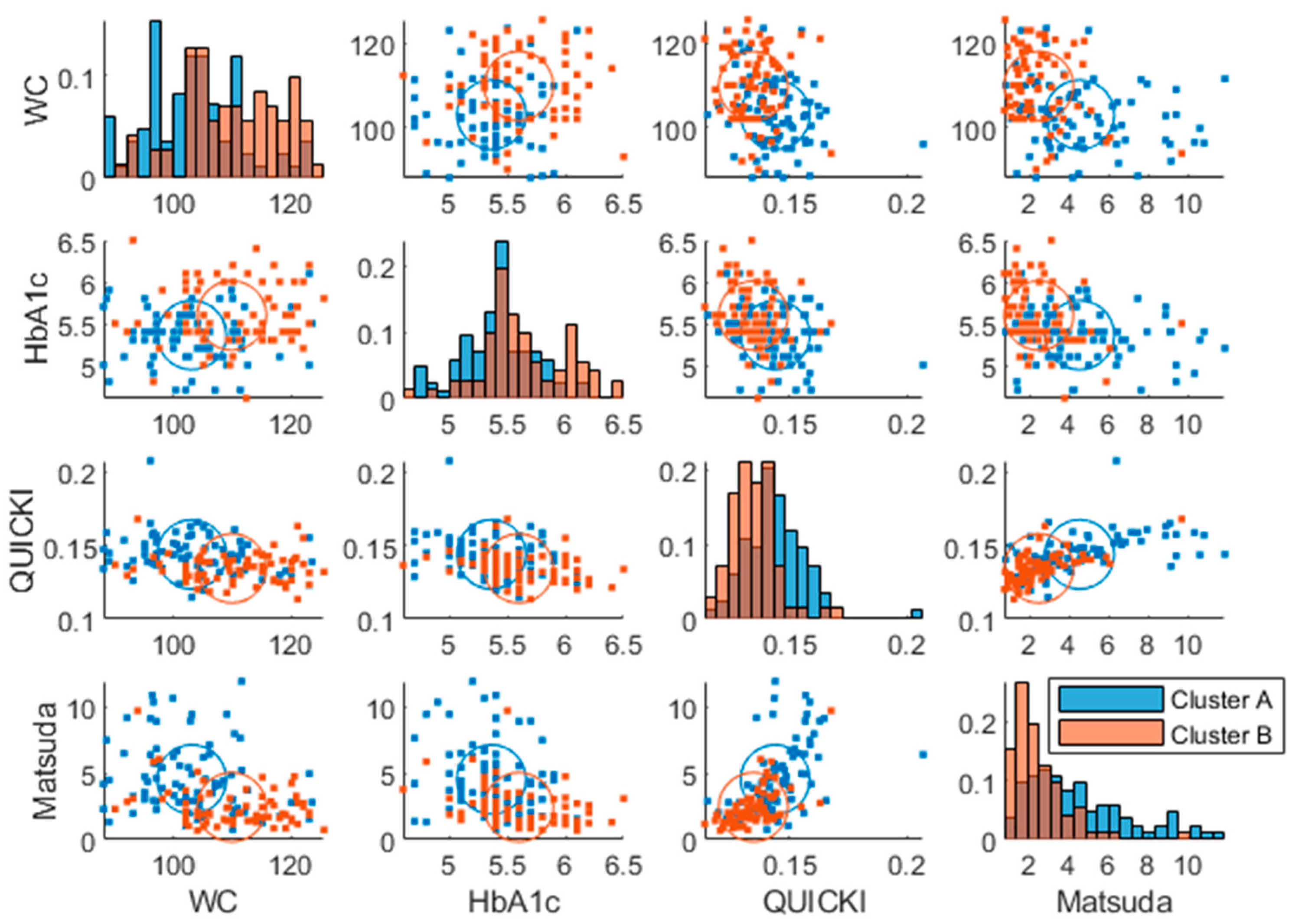
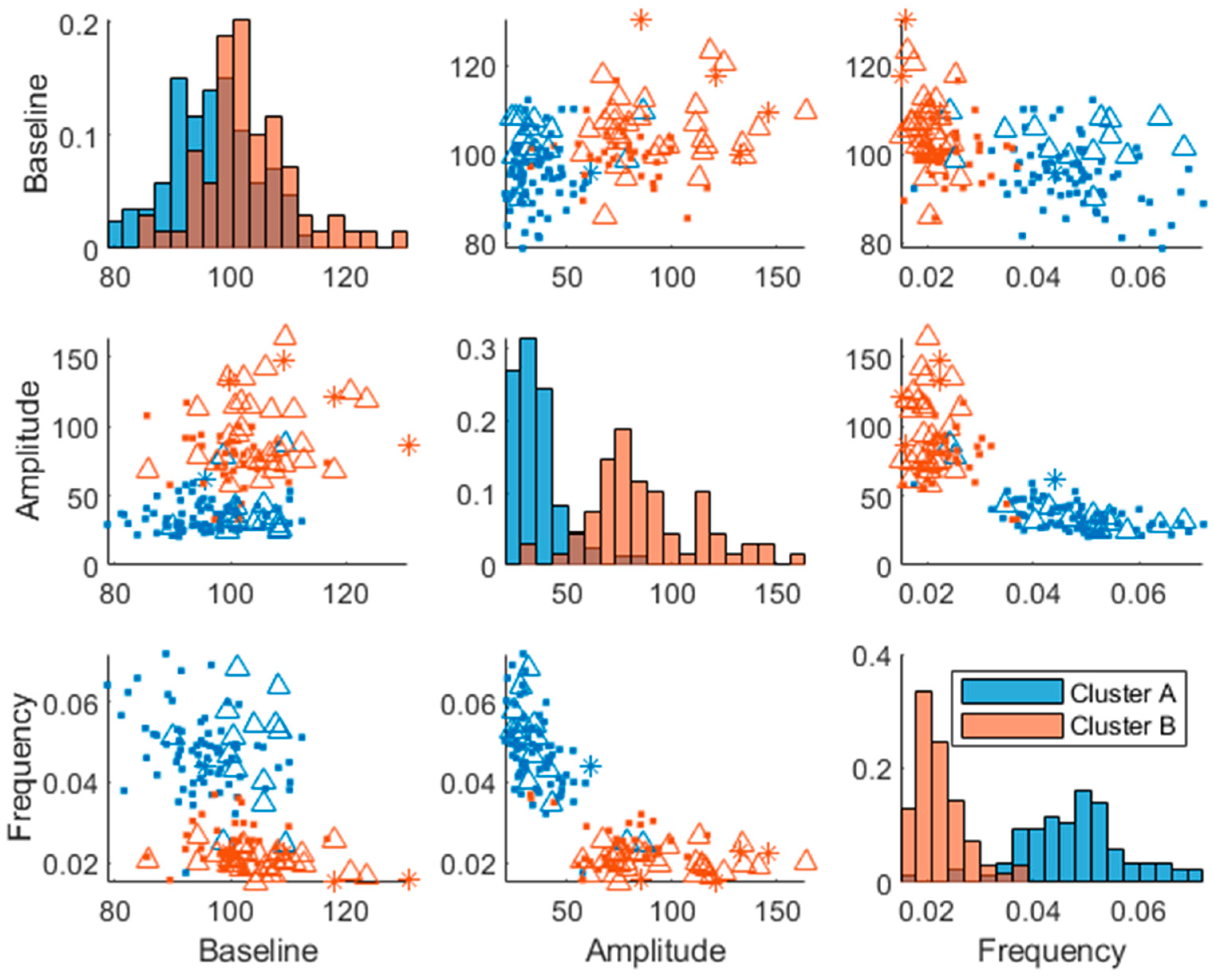
Two plasma glucose concentration profile clusters (A and B) were successfully identified (RSE < 33%), which were well separated in the amplitude and frequency parameters but not in the baseline parameter, although the cluster membership was estimated in the baseline parameter as well (
Figure 3). Estimating more than two clusters rendered the cluster membership parameter unidentifiable and a clear separation of individuals was visible when using a single lognormal distribution instead of a mixture of lognormal distributions (data not shown). This led to the choice of estimating the likelihood of cluster membership using two lognormal distribution modes (clusters). The distribution of the clusters in the covariates is shown in
Supplementary Figure S2.
The individuals in cluster A had in general a higher frequency
and a lower amplitude
(
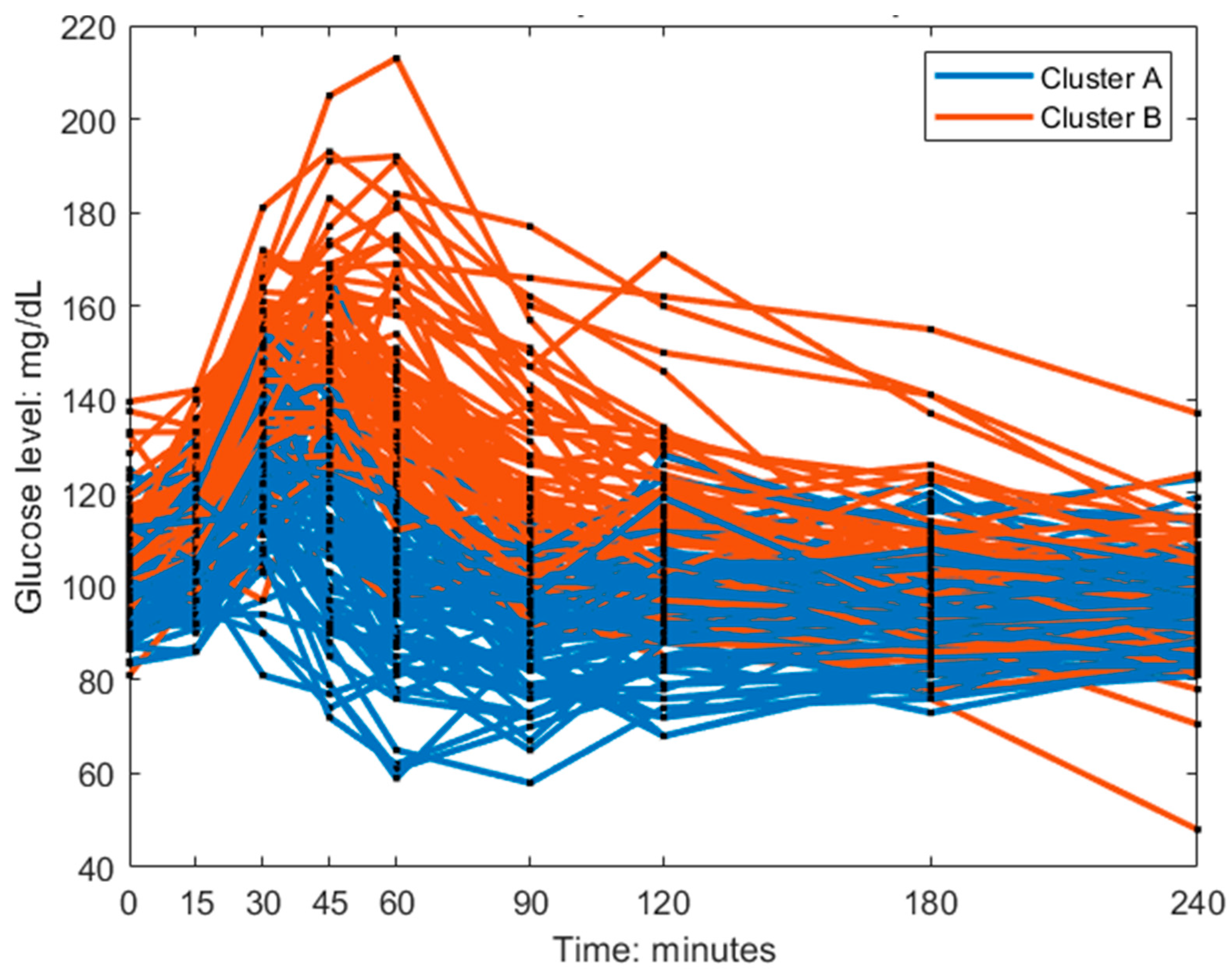
Figure 3). This is also visible in the postprandial glucose profiles of the MMTT data when participants were color-coded by cluster membership (
Figure 4). Individuals in cluster A, had in general a lower peak in plasma glucose response. The peak also appeared later for cluster B (which equates to a lower frequency
of the sinusoidal function in Equation 1). The clusters consisted of approximately 46% of cluster A and 54% of cluster B.
The clusters at baseline were associated with known diabetic risk markers such as HbA1c (p=
), insulin sensitivity indices (QUICKI (p=
), Stumvoll (p=
), Matsuda (p=
)) and waist circumference (p=
) using one-way ANOVA (
Figure 5). Although the clusters mostly involved the amplitude and frequency, all the parameters correlated with the risk factors (
Supplementary Figure S2).
Importantly, the clusters also associated differently with conditions reflecting clinical cut-offs for differential glucose control, i.e., prediabetes (fasting HbA1c
% and fasting blood glucose>100mg/dl, p=
[
30], insulin resistance (p=
), (Matsuda index
), glucose control (p=
) (normal, impaired, or diabetic [
30,
31]) using a Chi-squared test.
Most of the subjects classified as normoglycemic also belonged to cluster A and most of the subjects classified as “impaired” or “diabetic” belonged to cluster B (
Figure 6). The frequencies of glycemic control classes in each cluster are indicated in
Supplementary Figure S4.
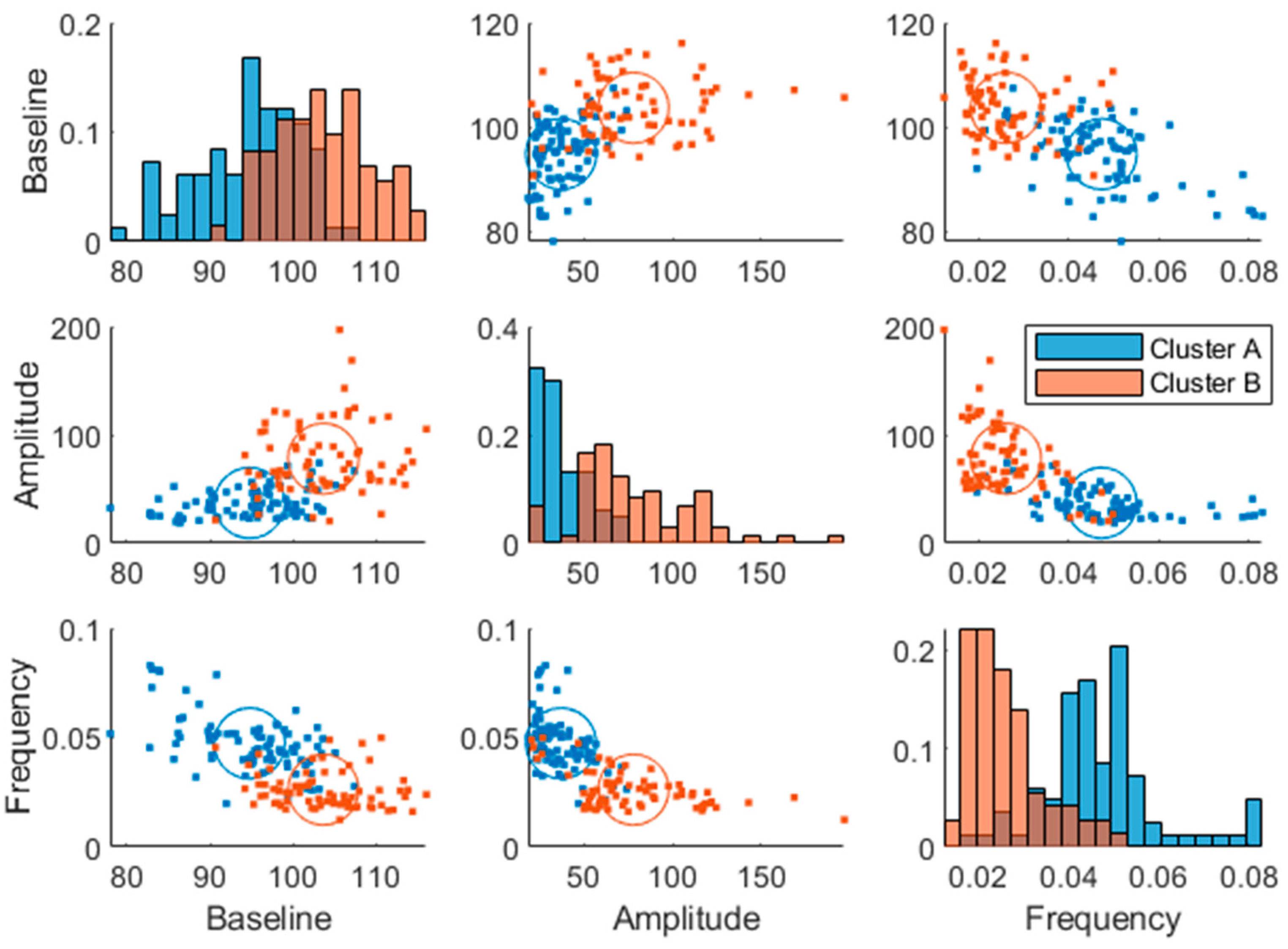
The same analysis as described using the breakfast MMTT response before the intervention (at baseline) was made using the breakfast MMTT response post-intervention where similar cluster memberships were identified (
Figure 7). However, the average Euclidean silhouette measure decreased from 0.58 to 0.36, indicating that the clusters were more distinct using baseline data. Moreover, it was observed that 60% of the subjects in cluster B improved in their glucose regulation by a decreased amplitude and increased frequency parameter value when comparing baseline values with those after 12 weeks of intervention. Additionally, in the low GI group, 58% decreased their baseline parameter, 61% decreased their amplitude parameter and 59% increased their frequency parameter after the intervention compared to baseline, which all relate to improved glycemic control. In comparison, in the high GI group, 49% decreased their baseline parameter, 46% decreased their amplitude parameter and 57% increased their frequency parameter compared to baseline. However, there was only a statistically significant change between the two time points in the low GI group in the amplitude parameter (Wilcoxon signed-rank test p=0.001). Furthermore, cluster A had a minor enrichment of women (63 and 64% pre- and post-intervention respectively) compared to the entire study population (53%).
Some of the individuals changed cluster from pre- to post-trial (~26% change in each cluster), but there was no significant difference between clusters (Cohen's kappa = 0.42, moderate stability between clusters (95%CI 0.27-0.56)). The change in parameters from baseline to wk. 12 (change = baseline – wk.12) did not correlate significantly with the change in risk markers.
Interestingly, the identified plasma glucose response clusters at baseline were associated with the gut microbiota genera
Clostridium sensu stricto 1 (ANOVA p = 0.007) and
Blautia (ANOVA p = 0.024). However, these genera correlated weakly with the estimated amplitude (
= -0.2, p = 0.02 and
= 0.2, p = 0.02 respectively) and frequency (
= 0.14, p = 0.08 and
= -0.19, p = 0.02 respectively) parameters that separated the clusters. Here,
denotes the Pearson correlation coefficient and p denotes the probability that the correlation is zero, using a t-test. As expected, we found no differences in gut microbiota composition between the two intervention groups (log fold change to predict low vs. high GI, balanced error rate 0.5) However, there was a clear difference attributed to the site (balanced error rate 0.09) and a difference in the Shannon diversity index (ANOVA p = 0.0134) between study centers was also noted (
Supplementary Figure S5).
4. Discussion
To dissect glucose data into features representing postprandial events, we used a model with only four parameters to identify clusters from standardized breakfast meal tolerance test responses that strongly related to T2DM risk factors. Although the model did not capture all systematic variation in the data, it was flexible enough to allow the identification of differential glycemic response clusters after mixed meal tests that were differentially associated with risk factors of T2D and gut microbiota. The results suggest that a standardized breakfast meal could provide meaningful data to predict risk factors of T2DM from dynamic glucose response measurements.
Plasma glucose response clusters were mostly separated in the amplitude and frequency parameters and not in the baseline glucose parameter (Figs. 3&7) which confirms that a dynamical model captures more information than a fasting plasma measurement and can more effectively be used for prediction of glycemic regulatory status. Individuals in cluster A were deemed to have better glucose control since they were characterized by a more favorable T2DM risk marker profile: a lower glycemic response, lower amplitude, and higher frequency parameters, whereas cluster B had the opposite traits and therefore more likely to develop health issues related to their glycemic control such as T2DM. However, the individuals classified as “diabetic” (
Figure 6) may be on the borderline to be classified as diabetics since this classification was using data from one OGTT when all participants were classified as non-diabetics at screening. Predictive tests of glycemic control classifications from estimated parameters were not analyzed due to the imbalance among classes, i.e., lack of diabetic patients in the data. Furthermore, some patients in the healthier cluster A had impaired glycemic regulation indicating that the estimated parameters give more information about the patient's glycemic regulation than solely a classification based on OGTTs.
The breakfast MMTT clusters did not associate with the study site nor with treatment, which suggests that using these as covariates successfully captured their variance in the data. However, the low GI group improved more than the high GI group in their estimated parameters (decreased average baseline, decreased average amplitude, and increased average frequency) after a 12-week intervention, which suggests that the low GI diet aided in improving the glycemic control of the participants [
32]. This could also explain why the cluster separation reduced from an average silhouette value of 0.58 to 0.36 from baseline to post-intervention, although there was no statistical difference overall between the two time points (Wilcoxon signed-rank test).
Our data suggests that a standardized breakfast MMTT based on regular foods may be an alternative to an OGTT, especially among patients with a high risk of nausea, such as pregnant women or bariatric surgery patients [
33,
34]. In contrast, an MMTT does not cause these side effects and is therefore an alternative to OGTT. Furthermore, our results based on a standardized breakfast meal including commonly consumed foods are in line with conclusions from a recent review that compared an OGTT to an MMTT and found a strong or very strong correlation (
= 0.9-0.97) between an OGTT and MMTT, which further supports that it may be used as an alternative to the OGTT [
11]. Additionally, the metabolic feedback from an MMTT that includes all macronutrients (carbohydrates, fat, and proteins) provides more comprehensive information on glucose homeostasis compared to a single macronutrient [
35,
36]. However, the MMTT should be standardized and preferably provided as a breakfast to avoid complications of the glucose dynamics with lingering metabolic effects of other meals. If standardized, cluster membership could potentially be estimated at home using the MMTT and a CGM connected to a cellular device, as CGM data has been shown to capture clusters of individuals based on glucose variability [
7].
Previous studies have shown that gut microbiota is associated with postprandial glucose response, but no studies have investigated associations with response clusters. In our study, we found that glucose response clusters were associated with the bacterial genera
Clostridium sensu stricto 1 and
Blautia. Cluster A had a higher proportion of
Clostridium sensu stricto 1 than cluster B and vice versa for
Blautia, which is consistent with previously reported associations of these genera with glucose control [
16,
17,
18]. Future studies should test how dietary interventions may affect these genera and to reveal their mechanistic links with postprandial glucose response. As expected, there were no differences in microbiota composition between groups after intervention, since the diets were similar except for low/high GI. In accordance with other studies, large differences between study centers were found, probably due to differences in dietary and lifestyle patterns as observed [
32].
Our study has several strengths including the large sample size with participants from three countries (Italy, USA, and Sweden), which reduces the chances that treatment effect or found clusters would be confounded by the cohort. In addition, the MMTT was robustly designed with participants carefully monitored during the test days and strictly standardized meal composition across the three centers to reduce the risk that the differences in response would be due to differences in intake. Furthermore, the mechanistic model gave interpretable clusters using only four identifiable parameters, which were estimated using a mixture of lognormal distributions. Although a mixture of distributions is rarely used, it proved useful in estimating the likelihood of cluster membership. However, although the method enabled investigating glucose control from the dynamical response, it should be noted that all descriptive variance was not captured using the model (e.g., slow undershoot).
Limitations included the fact that all participants were at risk of developing T2DM. Hence, although OGTT and MMTT responses have been described using the same model to estimate insulin sensitivity [
37], our method remains to be validated in a broader population, including patients with manifested T2DM and gestational diabetes. Also, the response to the lunch was not applied using this model since a more complex dynamic would be needed to account for the lingering response of the breakfast.
The MMTT used in the present trial was based on a Mediterranean diet. However, different diets and foods may have different effects on gastric emptying time and blood glucose response which should be taken into consideration when designing future studies [
38,
39]. Validating our method in a cohort with a balanced set of individuals with normoglycemic, impaired glucose control and diabetics may allow for the development of an algorithm to classify these states from the estimated parameters using our model on the response of MMTTs. Furthermore, this algorithm could potentially be used with a cellular device and a CGM in a home setting and to be done periodically to facilitate tailored preventative treatment of prediabetes and T2DM.
Author Contributions
Conceptualization, R.L. and V.S.; methodology, V.S., and M.W; software, V.S..; validation, V.S.; formal analysis, V.S.; investigation, V.S., and T.H.; resources, R.L, G.R, and W.W.C..; data curation, R.L., G.R., and W.C..; writing—original draft preparation, T.H., and V.S..; writing—review and editing, T.H. and V.S..; visualization, V.S., and T.H..; supervision, R.L, M.W., M.J., C.B., W.W.C., and, G.R..; project administration, R.L..; funding acquisition, G.R., R.L., and W.W.C All authors have read and agreed to the published version of the manuscript.