1. Introduction
Chronic wasting disease (CWD) is a transmissible spongiform encephalopathy (TSE) that affects cervids, such as deer, elk, and moose [
1]. As of July 2023, it has been detected in at least 31 states in the United States, 4 provinces in Canada, Norway, Finland, Sweden, and South Korea [
2]. The majority of natural, horizontal CWD transmission occurs via mucosal exposure to infectious or misfolded prion proteins (PrP
CWD), either by contact with infected animals or by indirect environmental exposure associated with foraging and rutting [
3]. PrP
CWD may initiate the conversion of the host’s normal cellular prion protein to its misfolded form, which then induces further amplification of PrP
CWD in the local lymphoid tissues, followed by rapid dissemination via blood or lymphoid cells to systemic lymphoid tissues [
3]. PrP
CWD may also accumulate in peripheral nerves and then transport to the central nervous system. During disease progression, PrP
CWD has also been found in body fluids including blood, saliva, urine, skin, muscle, and feces [
4,
5,
6,
7]. Thus, PrP
CWD is widely used as a diagnostic marker for infected animals. In current CWD diagnostic schemes, enzyme-linked immunosorbent assay (ELISA) has been used as the primary screening assay, followed by detection of PrP
CWD in obex and/or retropharyngeal lymph node tissue specimens using immunohistochemistry (IHC) for confirmation [
8].
Within the last decade, the real-time quaking-induced conversion (RT-QuIC) assay has become common and has shown the potential to be used for the routine detection of CWD in cervids [
9]. Many test protocols based on RT-QuIC have been developed and used for the detection of PrP
CWD in a range of biological tissues and fluids and environmental samples [
5,
6,
7,
10,
11,
12]. The RT-QuIC assay exploits the ability of infectious or misfolded prion proteins to seed the conversion of monomeric prion substrates to form larger amyloid fibrils, which are then detected by an amyloid-sensitive fluorescent dye, such as thioflavin T (ThT, [
13]). As such, the RT-QuIC assay is extremely sensitive and able to detect sub-femtograms of misfolded prion protein provided sufficient assay time or cycles for target amplification [
14,
15]. However, as the assay time prolongs or the cycle number increases, the monomeric prion substrates tend to self-aggregate and the RT-QuIC reactions may produce false positive ThT signals [
16,
17]. The pre-determined, wide range of assay times, from 24 to 62.5 hours, used in the above RT-QuIC protocols [
5,
6,
7,
10,
11,
12] suggests a need for methods to determine the appropriate length or duration for the RT-QuIC assay.
In the above RT-QuIC protocols, to determine CWD positivity, a fluorescent threshold (T
stdev) was defined as a few (e.g. 10, [
5,
6,
7]) standard deviations above the average background fluorescence of all reactions. A specimen was determined CWD positive based on the probability test [
16], which considers a specimen positive when a certain number (e.g. ≥ 4 out of 8, [
5,
8]) of replicates crossed T
stdev. Additionally, the Mann–Whitney U-test was used to determine CWD positivity by comparing the cycle thresholds or rates (the reciprocals of cycle thresholds) of replicate reactions of a specimen that crossed T
stdev with those of the negative controls [
6]. Recently, the use of max-point ratios (maximum fluorescence/background fluorescence, MPR) has been proposed to improve the consistency of RT-QuIC analysis [
9]. CWD positivity was determined using the Welch’s analysis of variance (ANOVA) by comparing the MPR values of unknown specimens against those of a known negative control. In addition, a constant threshold, T
MPR, was proposed to replace assigning independent T
stdev per reaction plate. Building on the above applications of T
stdev or T
MPR in the determination of CWD positivity, this study proposed and demonstrated the application of receiver operating characteristic (ROC) analysis [
18] for optimizing the RT-QuIC assay duration, followed by evaluating the optimized assay duration for screening of PrP
CWD in white-tailed deer from a CWD-affected farm against the widely used screening tool, ELISA.
2. Materials and Methods
2.1. Sample Preparation
Obex and RLN tissue specimens from white-tailed testing positive or negative for CWD (CWD+ or CWD-) using IHC as described by [
19] were used as controls in this study for the determination of optimal assay durations. These tissue specimens were homogenized in grinding tubes containing quarter-inch grinding beads using a Precellys 24 Tissue Homogenizer (Bertin, France) to form 10% w/v homogenates in 0.05% sodium dodecyl sulfate (SDS). The homogenates were 10-fold serially diluted in 0.05% SDS to generate a gradient concentration ranging from 1.0 × 10
-2 to 1.0 × 10
-11 w/v, Homogenate of CWD+ tissue specimen was serially diluted in 0.05% SDS with at least 6 replicates for each concentration. Obex and RLN tissue specimens collected from white-tailed deer with unknown CWD status were homogenized the same way as described above and diluted in 0.05% SDS to form homogenates for RT-QuIC and ELISA tests as described below.
2.2. Production of recombinant prion protein
Recombinant Syrian hamster prion protein (PrP
rec), amino acids 90-231, was prepared as described by [
20]. In brief, protein expression in
Escherichia coli Rosetta
TM (DE3) culture was induced using the Overnight Express Autoinduction System 1 – Novagen kit (EMD Millipore, Germany). Inclusion bodies were harvested using the BugBuster® Master Mix (EMD Millipore) following the manufacturer’s protocol. The inclusion bodies were solubilized in denaturation buffer (8M guanidine hydrochloride, 0.1M sodium phosphate monobasic, 0.01M Tris, pH 8.0) for 1 hour at room temperature. The solubilized protein was bound to Ni-NTA Superflow resin (Qiagen, Netherlands) and refolded with a linear gradient from 100% denaturation buffer to 100% refolding buffer (0.1M sodium phosphate monobasic, 0.01M Tris, pH 8.0) flowing at 1.5 mL min
-1 over 3 hours. The protein was eluted with a linear gradient from 100% refolding buffer to 100% elution buffer (0.5M imidazole, 0.1M sodium phosphate monobasic, 0.01M Tris, pH 5.6) at 2.0 mL min
-1 over 40 minutes. The eluted protein was dialysed (0.05M sodium phosphate monobasic/dibasic buffer, pH 7.3) overnight and the following day twice over 2-hour periods in fresh dialysis buffer. The prepared PrP
rec was stored at -80°C until use.
2.3. RT-QuIC methods
RT-QuIC assays were performed as previously described [
21], with slight modifications. Briefly, 95 µL of the reaction master mix and 5 µL of diluted obex or RLN tissue homogenate were added to each well of a 96-well plate. Wells contained 300 mM NaCl, 1 mM EDTA, 10 µM Thioflavin-T (ThT), 0.1 mg/mL PrP
rec, and 50 mM Na
3PO4 (pH 7.2-7.4). The reactions were run using BMG FluoStar® plate readers (BMG Labtech, Germany). Assays were performed at 42 °C for 65 hours for the determination of optimal assay duration or using the optimized assay durations. Each cycle lasted approximately 15 min including 7 repeats of a 1 min shake at 700 rpm (double orbital) and a 1 min rest, followed by a 1 min reading. ThT fluorescence measurements were taken every 15 min at a gain of 1200, excitation of 450 nm, and emission of 480 nm. Data were processed using Mars Analytical Software (BMG Labtech).
2.4. Determination of optimal assay duration for Tstdev
The approach described by Gray et al. [
16] was followed to determine the optimal assay duration. In brief, distributions of RT-QuIC cycle thresholds were generated using reactions that were seeded with the control CWD+ and CWD- obex and RLN tissue homogenates in 10-fold serial dilutions. The cycle threshold was defined as the time when the ThT signal of a reaction crossed T
stdev, which was calculated using the average baseline or first cycle reading of all the reactions in relative fluorescent units (RFU) plus 10 sample standard deviations. A cycle threshold of 65 hours was assigned for reactions where the ThT signal did not cross the threshold within the 65-hour assay. To minimize false-positive ThT signals (type I errors), cycle threshold was used as a binary classifier for CWD positivity. Using the cumulative distributions of both CWD+ and CWD- cycle thresholds, ROC curve analyses were conducted comparing cycle threshold against the known CWD status of the tissue homogenates. The optimal assay durations were defined at the highest specificity with the highest sensitivity, or the point closest to (0,1) on the ROC curves [
22]. ROC curve calculations were performed with the RStudio ROCR package [
23].
2.5. Determination of optimal assay duration time for TMPR
Determination of optimal assay duration time for T
MPR was based on the same ThT data from the above RT-QuIC reactions that were seeded with the control CWD+ and CWD- brain and RLN tissue homogenates. MPR was defined as the ratio of maximum RFU to background (4
th cycle) RFU. In this study, one MPR was calculated for each addition of the cumulative cycles from the 4th cycle (52 minutes of the assay) to the 224th cycle (65 hours), and thus, 221 MPRs were calculated from each reaction. For each addition of the cumulative cycles, based on the distributions of MPRs of all reactions, a ROC curve using MPR as a binary classifier for CWD was constructed against the known CWD status of tissue homogenates. Therefore, 221 ROC curves were generated. The area under the ROC curve (AUC) was plotted against the assay duration of the corresponding cumulative assay cycles. The time corresponding to the ROC curve with the highest AUC was determined as the optimal assay duration, as it leads to the highest classification power of MPR [
18]. The T
MPR was then determined by finding the point closest to (0,1) on that particular ROC curve.
2.6. Evaluation of the optimized assay durations
The optimized assay durations were evaluated with obex and RLN tissue specimens that were collected from 104 white-tailed deer in a Canadian cervid farm affected by CWD. The tissue specimens were homogenized the same way as described above to form 1.0 × 10
-4 w/v homogenates for RT-QuIC and 20% obex and 12-15% RLN homogenates for ELISA. ELISA tests were performed following [
24]. RT-QuIC tests were performed as previously described using the optimal assay durations. Obex and RLN tissue specimens with any sample replicates that tested positive for CWD by RT-QuIC or ELISA were tested by IHC for confirmation.
The classification of specimens tested by RT-QuIC were carried out using the Mann-Whitney U test and the probability test based on T
stdev [
16], and the Welch’s t-test and the probability test based on T
MPR [
9]. Cycle threshold or MPR from the quadruplicate RT-QuIC reactions on each tissue specimens were analyzed with the Mann-Whitney U test and Welch’s t-test using R. For the probability test, tissue specimens were classified negative if no replicates crossed the threshold, positive if all 4 replicates crossed the threshold, and suspect if at least 1 out of 4 replicates crossed the threshold. Suspect specimens were re-tested in quadruplicate, and then classified positive if at least 4 out of 8 replicates crossed the threshold. Kappa analysis was used to test agreement between RT-QuIC and ELISA in R following the method in [
25].
To further evaluate the optimal assay durations, the quadruplicate RT-QuIC reactions performed on the 104 obex and RLN tissue specimens were extended to a 40 h assay duration, which was used for detection of CWD in white-tailed deer obex and lymph nodes [
8] and compared to their ELISA results. The ideal assay durations and 40 h assay duration were further compared at a replicate-level with a McNemar’s test, using the ideal assay duration classification as the expected results and the 40 h assay duration classification as the observed results.
3. Results
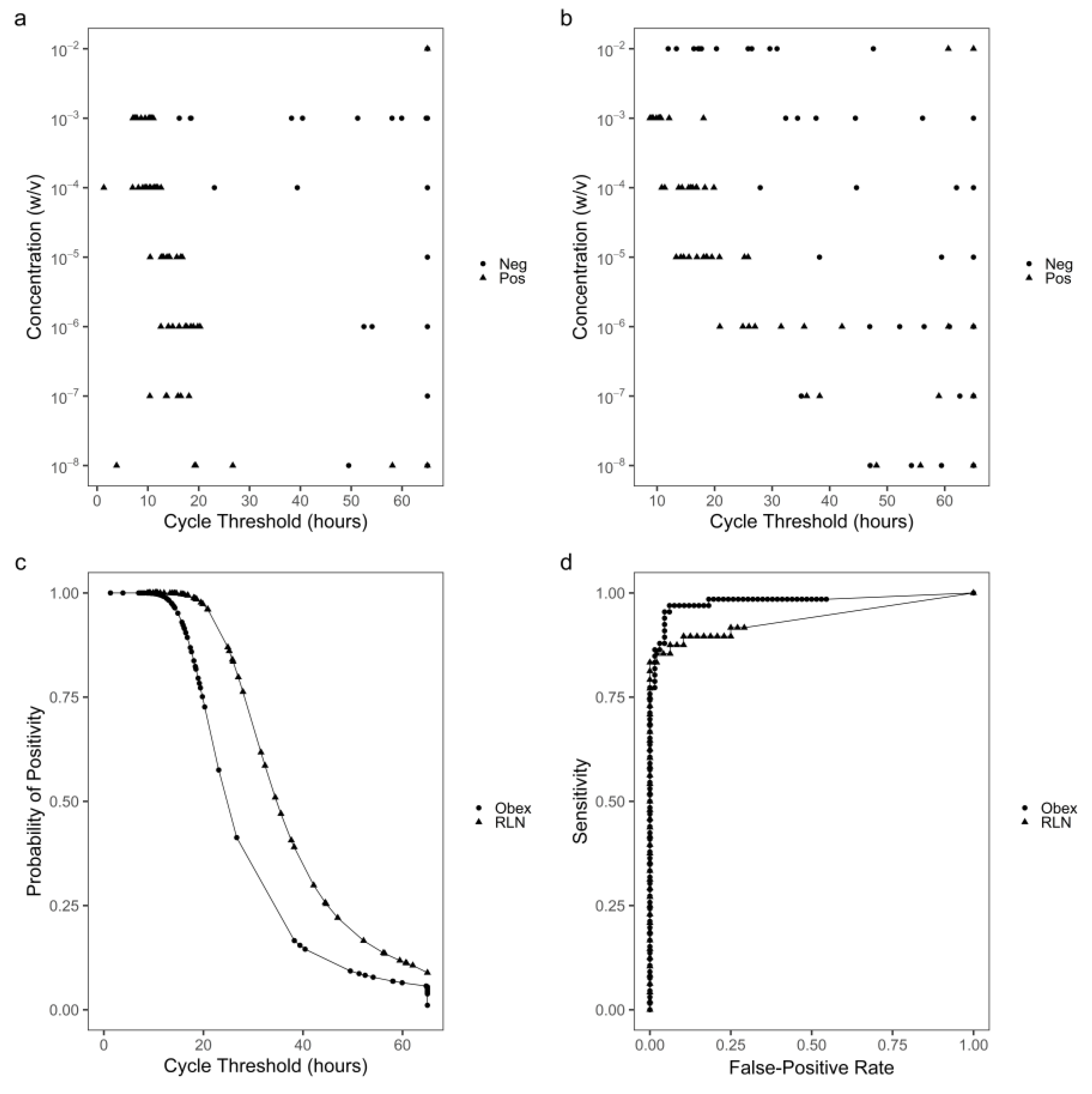
3.1. Optimization of RT-QuIC assay durations based on Tstdev
Obex and RLN tissue specimens with known CWD status were serially diluted to simulate specimens containing various concentrations of PrP
CWD. At 10
-2 w/v, the obex tissue homogenate inhibited the RT-QuIC reactions and prevented production of cycle thresholds based on T
stdev within the 65-hour assay (
Figure 1a). For RLN, at 10
-2 w/v, non-specific amyloid formation occurred as early as 12 hours (
Figure 1b). From 10
-3 to 10
-8 w/v for obex and 10
-3 to 10
-6 w/v for RLN, cycle thresholds for CWD+ tissue homogenates occurred earlier than those for CWD- homogenates (
Figure 1a, 1b). At 10
-9 w/v and lower for obex and 10
-7 w/v and lower for RLN, the cycle thresholds of most reactions seeded with either CWD+ or CWD- homogenates were close to 65 hours, suggesting an analytical sensitivity or limit of for detection of CWD in obex and RLN tissue specimens at 10
-8 and 10
-6 w/v, respectively. Thus, cycle threshold data generated from reactions seeded with 10
-3 to 10
-8 w/v of obex and 10
-3 to 10
-6 w/v of RLN tissue homogenates were used for the construction of logistic regression models and ROC curve analysis (
Figure 1c, 1d). The observed AUC was 0.979 and 0.939 for obex and RLN, respectively, indicating that cycle threshold has very strong classifying power for both types of tissues. Based on these ROC curves, 27 hours was determined as the cycle threshold cut-off or optimal assay duration for detection of CWD in both obex and RLN tissues.
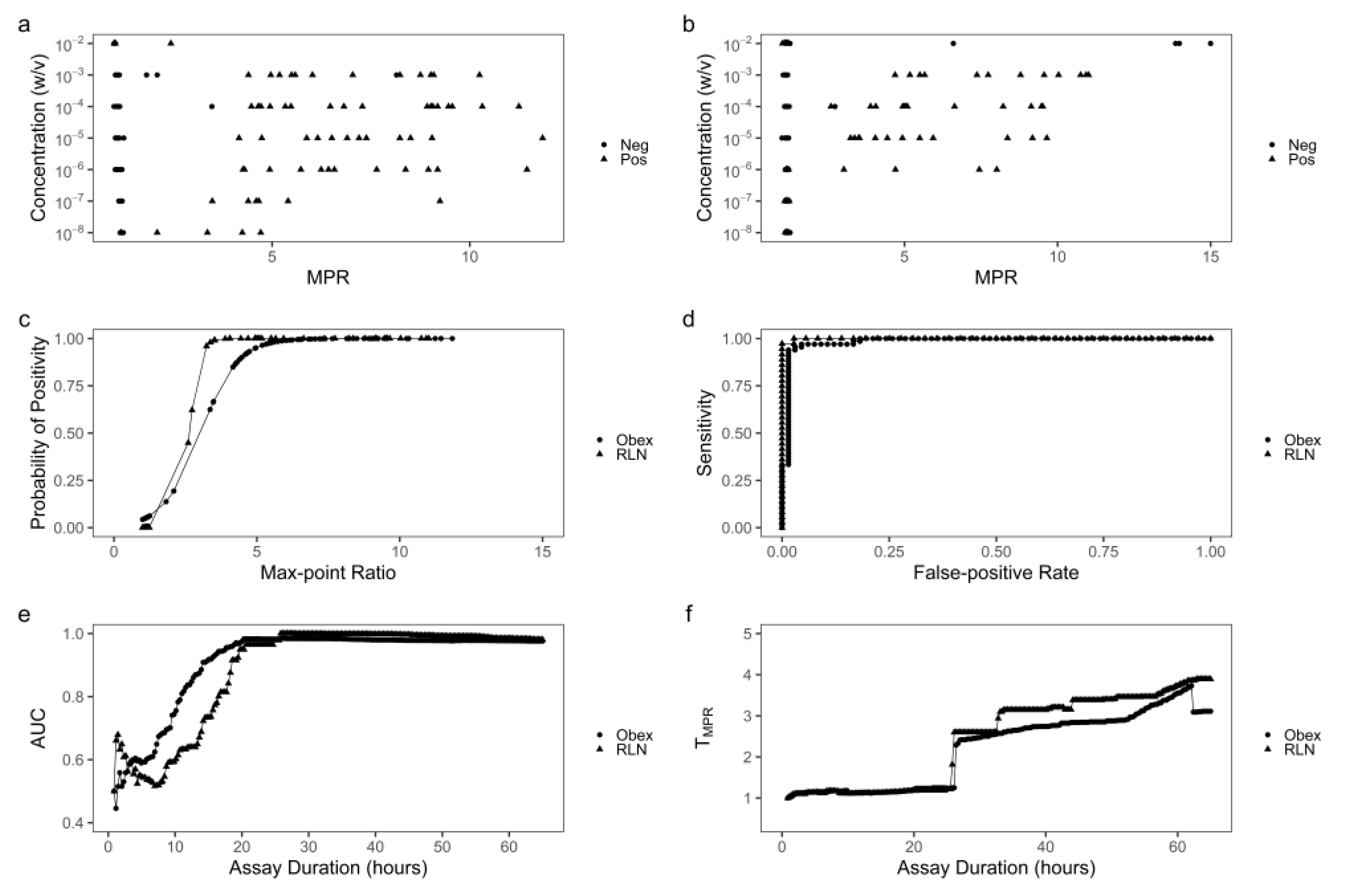
3.2. Optimization of RT-QuIC assay durations based on TMPR
The same ThT data from the above RT-QuIC reactions were used to calculate MPR values for each reaction along with the increasing assay durations. MPR values for CWD+ obex or RLN tissue homogenates at 10
-2 w/v were indistinguishable from those for CWD- tissue homogenates at various assay duration (
Figure 2a-c, 3a-c), reflecting the inhibited or non-specific amyloid formations as described above. Thus, MPR values at this concentration were not used for determination of optimal assay duration. For obex from 10
-3 to 10
-8 w/v and RLN from 10
-3 to 10
-5 w/v, MPR values from reactions seeded with CWD+ homogenates were greater than those with CWD- homogenates at assay durations of 28 or 46 hours (
Figure S1b-c, S2b-c). As such, ThT data generated from these concentrations were used to construct MPR distributions. Based on the MPR distribution corresponding to each increasing assay cycle from the 4th to 224th cycle, 221 ROC curves were constructed for obex or RLN tissue homogenates. Figures S1d and S2d showed ROC curves corresponding to the 35th, 97th and 159th cycle, or assay durations of 10, 28, or 46 hours. These ROC curves indicated that MPR has very strong classifying power when assay duration is 28 hours or longer. Such findings were confirmed by plotting AUC values of the 221 ROC curves against the corresponding assay durations for obex or RLN (
Figure 2e). The assay durations of 27 and 28 hours, corresponding to the ROC curves with the highest AUC values, were the optimum for detection of CWD in obex and RLN, respectively. Also, based on individual ROC curves, MPR threshold (T
MPR) was determined and plotted against assay duration (
Figure 2f). T
MPR corresponding to the optimal assay duration was 2.3 and 2.6 for obex and RLN tissue homogenates, respectively. As assay duration extended beyond 27 or 28 hours, the AUC values remained relatively stable for obex and RLN (
Figure 2e). However, the T
MPR values increased as assay duration prolonged (
Figure 2D) to overcome the increasing amount of non-specific amyloid formation in the late cycles of reactions seeded with CWD- homogenates. At an assay duration of 40 hours, the ideal T
MPR was 2.7 and 3.1 for obex and RLN, respectively.
3.3. Evaluation of the optimized RT-QuIC assay durations
To evaluate the optimized RT-QuIC assay durations, obex and RLN tissue specimens from 104 white-tailed deer in a CWD-affected farm were tested by RT-QuIC and ELISA. Specimens with any replicates that tested CWD+ by RT-QuIC (
Table S1 and S2) or ELISA were further tested by IHC for confirmation, and only the ELISA positive specimens were positive by IHC. When using cycle threshold as the classifier for RT-QuIC, the CWD positivity determined using the Mann-Whitney U test was in 100% agreement with ELISA results for both obex and RLN tissue specimens (
Table 1). In comparison, using the probability test to determine the CWD positivity, there were 11 and 15 obex and RLN tissue specimens with replicates that tested positive by RT-QuIC, which were considered “suspect” and retested (
Table S1). Upon retesting, all suspect specimens were determined negative for CWD by RT-QuIC, since less than 50% (4/8) of their replicates tested CWD+ (
Table 1). When using MPR as the classifier for RT-QuIC, the CWD positivity determined using the Welch’s t-test was in 98.1 % (κ = 0.823, 95% CI: 0.581- 1) and 92.3% (κ = 0.522, 95% CI: 0.204-0.840) agreement with that by ELISA for the obex and RLN tissue specimens, respectively (
Table 1 and S2).
Obex and RLN specimens were homogenized, diluted to 10-4 (w/v) and then tested by RT-QuIC using a 27 h and 28 h assay duration, respectively. The cycle thresholds of quadruplicate reactions on each specimen were compared with those from a negative control specimen using a Mann-Whitney U test, and a specimen was classified positive when p < 0.05. Cycle threshold was the time when the ThT signal of a reaction crossed Tstdev, which was the average baseline (the 1st cycle) reading of all the reactions in relative fluorescence units (RFU) plus 10 standard deviations.
Tissue specimens were classified negative if none of the 4 replicates crossed Tstdev, positive if all 4 replicates crossed Tstdev, and suspect if at least 1 out of 4 replicates crossed the threshold. Suspect specimens were re-tested in quadruplicate, and then classified positive if at least 4 out of 8 replicates crossed Tstdev.
The max-point ratios (MPRs) of quadruplicate reactions on each specimen were compared with those from a negative control specimen using a Welch’s t-test, and a specimen was classified positive when p < 0.05. MPR was defined as the ratio of maximum RFU to background (the 4th cycle) RFU within the 27 and 28 h assay duration for obex and RLN specimens, respectively.
Tissue specimens were classified negative if none of the 4 replicates crossed TMPR, positive if all 4 replicates crossed TMPR, and suspect if at least 1 out of 4 replicates crossed TMPR. Suspect specimens were re-tested in quadruplicate, and then classified positive if at least 4 out of 8 replicates crossed TMPR. TMPR was 2.3 and 2.6 for obex and RLN tissue homogenates, respectively.
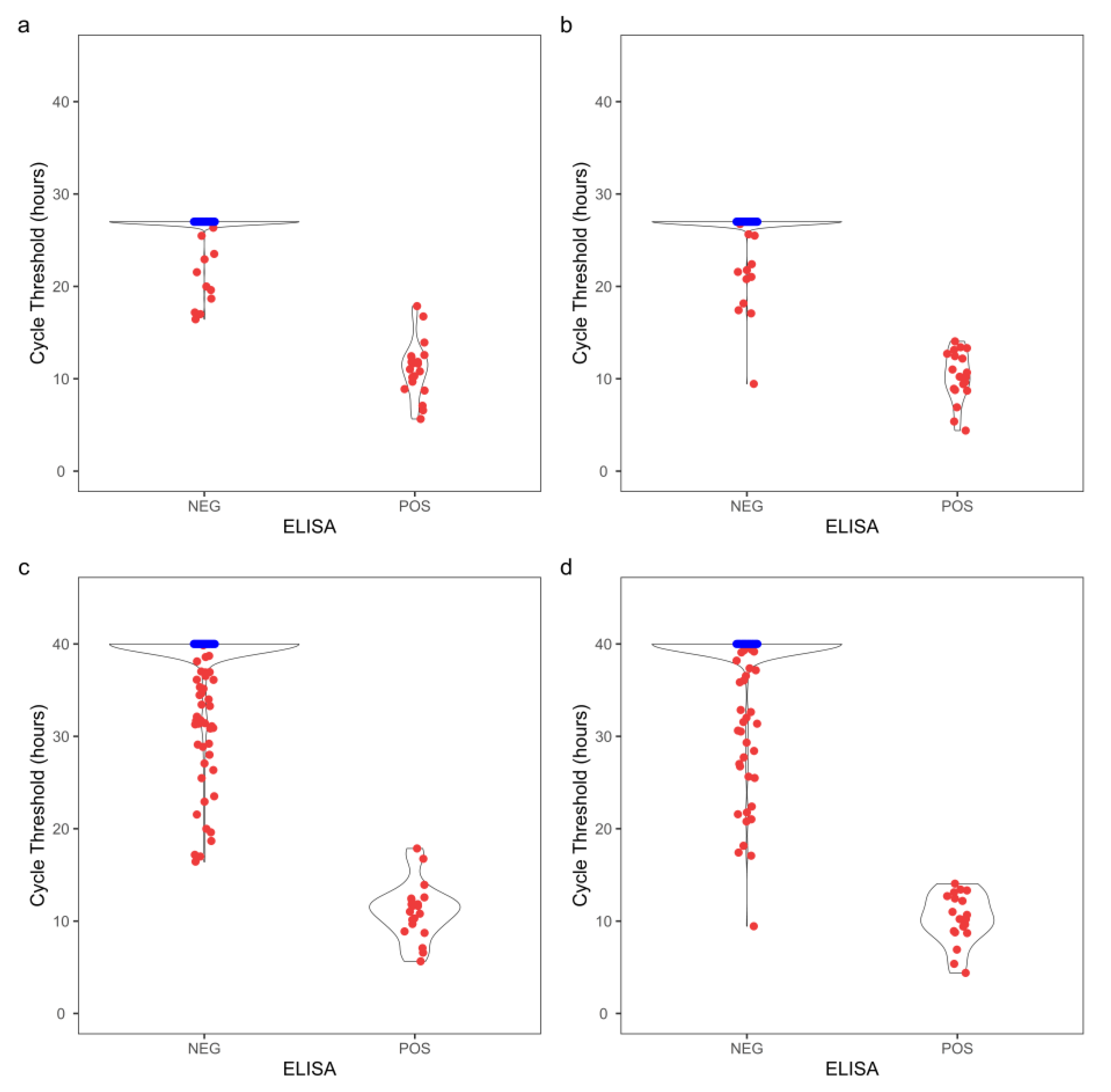
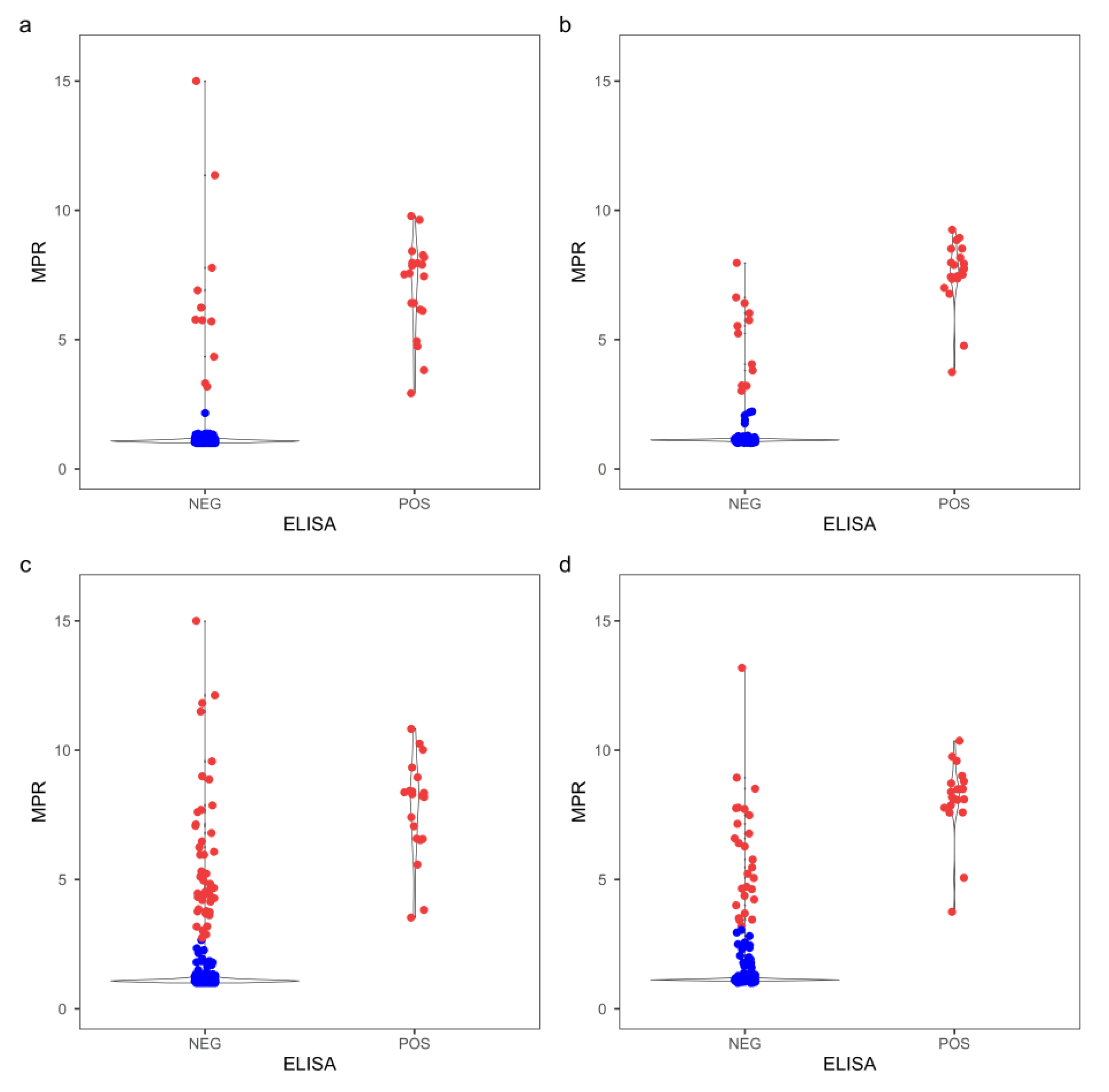
In comparison, CWD classification using the probability test was in 100% agreement with that by ELSA for both the obex and RLN tissue specimens (
Table 1 and S2). To further evaluate the optimized assay durations, the RT-QuIC assay was extended to 40 h for comparison. The distribution of cycle threshold and MPR for both obex and RLN clearly showed that more ELISA-negative replicates became CWD+ by RT-QuIC when assay duration prolonged to 40 h compared to the optimized duration (
Figure 3 and 4). Contingency tables were constructed at a replicate-level comparing the optimized assay durations with a 40 h assay duration (
Table S3), and the RT-QuIC results were significant different with both T
Stdev (
pobex = 2.54 × 10
-8,
pRLN = 4.49 × 10
-6) and T
MPR (
pobex = 1.52 × 10
-8,
pRLN = 3.01 × 10
-4) using a McNemar’s test.
4. Discussion
The RT-QuIC assay is extremely sensitive and able to detect sub-femtograms of infectious or misfolded prion proteins, which seed the conversion or aggregation of monomeric prion substrates to form larger amyloid fibrils [
14,
15]. The kinetics of amyloid fibril formation are affected by the types and concentrations of salts [
26] or solvents [
27] in the assay solutions, and also by the assay temperatures and shaking arrangements [
27]. As such, a wide range of assay durations have been used in RT-QuIC for detection of CWD [
5,
6,
7,
10,
11,
12]. In general, longer assay duration supports higher sensitivity but lower specificity due to self-aggregation of the prion substrates or spontaneous amyloid fibril formation [
16,
17]. To obtain the best combination of sensitivity and specificity, this study proposed and demonstrated the use of the ROC analysis for optimizing RT-QuIC assay duration to screen CWD in cervids.
The optimization of RT-QuIC assay duration was performed for both T
stdev and T
MPR, classifiers currently used in determination of CWD positivity. Considering specimens contain various concentrations of infectious prion protein, ThT signals generated from serial dilutions of the control specimens were used in ROC analysis for determining optimal assay duration based on T
stdev, instead of identifying the individual cut off values of assay duration with signals from each dilution as described by [
16]. Our study only used one CWD positive and one CWD negative specimen as control, and certainly, more control specimens with known CWD status would increase the power of the ROC analysis. Results by RT-QuIC using the optimized assay duration based on T
stdev were in 100% agreement with those by the widely used ELISA for screening CWD in obex and RLN tissue specimens collected from the affected white-tailed deer farm. In comparison, more replicates from specimens that were tested CWD- by ELISA became CWD+ by RT-QuIC when the assay duration extended to 40 h, which was used for detection of CWD in white-tailed deer obex and lymph nodes [
8]. Based on the IHC confirmation, RT-QuIC using the optimized assay durations produced significantly fewer false positive replicates compared to using 40 h. Again, when using the 40 h assay duration, RT-QuIC produced more false positive replicates than using optimized assay duration according to the IHC confirmation. It is possible that RT-QuIC might detect minute amounts of infectious prion protein that was missed by IHC, and mouse-bioassay could be the option for further confirmation [
16]. Nonetheless, our findings demonstrated the effectiveness of using ROC and AUC analysis for optimizing RT-QuIC assay durations in screening CWD as an alternative to ELISA. As many factors may affect RT-QuIC performance [
9,
26,
27], it is expected that optimal assay duration may vary for different sample matrixes, with different substrates and reagents, and using different fluorometers. Hence, tools like ROC and AUC analysis for optimizing assay duration would be helpful to enhance RT-QuIC in the detection of CWD.
In the determination of CWD positivity, the Mann-Whitney U-test and the probability test have been used based on T
stdev, and the Welch’s t-test and the probability test based on T
MPR [
9,
16]. In this study, based on T
stdev, the Mann-Whitney U-test and the probability test produced consistent results for screening CWD in the obex and RLN specimens from the affected farm. However, based on T
MPR, the probability test provided better performance than the Welch’s t-test for screening both the obex and RLN specimens. Self-aggregation of the protein substrates occurred at low levels in reactions on the false positive specimens. Although the ThT signals from these self-aggregations were not high enough to produce MPRs that crossed T
MPR, they were significantly higher than the signals from the negative controls. Our findings suggested that the application of T
MPR and the probability test might help prevent false positive results derived from low levels of self-aggregation of the prion substrates.
Overall, this study proposed and demonstrated the use of ROC analysis to optimize RT-QuIC assay duration based on both Tstdev and TMPR. The optimized assay durations were evaluated and proved to be effective in RT-QuIC applications for screening CWD in obex and RLN tissue specimens of white-tailed deer when compared to the widely used screening assay, ELISA. The findings suggest the potential of optimizing RT-QuIC assay duration for enhancing CWD detection in various animal specimens and environmental samples.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1: Distributions of obex RT-QuIC MPR at various assay durations; Figure S2: Distributions of RLN RT-QuIC MPR at various assay durations; Table S1: Distributions of RLN RT-QuIC MPR at various assay durations; Table S2: Distributions of RLN RT-QuIC MPR at various assay durations.
Author Contributions
Conceptualization, G.Y., J.G.; data curation: G.Y, T.M., G.M., methodology, G.Y, T.M, H.D, M.L, C.W, writing – review – original draft preparation, G.Y., J.G., supervision of ELISA, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Canadian Food Inspection Agency for the optimization of the RT-QuIC assay for detection of chronic wasting disease (Grant No. N-000271).
Institutional Review Board Statement
Ethical approval is not applicable for this study.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author. RStudio scripts to conduct the methodology can be accessed at github.com/gyilm039/Optimizing-RT-QuIC-Sample-Classification
Acknowledgments
We would like to thank the officers at the Canadian Food Inspection Agency - Operations for supplying us deer samples.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyzes, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- United States Department of Agriculture USDA APHIS | Chronic Wasting Disease (CWD). Available online: https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/nvap/NVAP-Reference-Guide/Control-and-Eradication/Chronic-Wasting-Disease (accessed on 26 May 2023).
- Expanding Distribution of Chronic Wasting Disease |, U.S. Geological Survey. Available online: https://www.usgs.gov/centers/nwhc/science/expanding-distribution-chronic-wasting-disease (accessed on 7 July 2023).
- Hoover, C.E.; Davenport, K.A.; Henderson, D.M.; Denkers, N.D.; Mathiason, C.K.; Soto, C.; Zabel, M.D.; Hoover, E.A. Pathways of Prion Spread during Early Chronic Wasting Disease in Deer. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Elder, A.M.; Henderson, D.M.; Nalls, A. V.; Hoover, E.A.; Kincaid, A.E.; Bartz, J.C.; Mathiason, C.K. Immediate and Ongoing Detection of Prions in the Blood of Hamsters and Deer Following Oral, Nasal, or Blood Inoculations. J. Virol. 2015, 89, 7421. [Google Scholar] [CrossRef]
- Burgener, K.R.; Lichtenberg, S.S.; Lomax, A.; Storm, D.J.; Walsh, D.P.; Pedersen, J.A. Diagnostic Testing of Chronic Wasting Disease in White-Tailed Deer (Odocoileus Virginianus) by RT-QuIC Using Multiple Tissues. PLoS One 2022, 17, e0274531. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Schwabenlander, M.D.; Rowden, G.R.; Schefers, J.M.; Jennelle, C.S.; Carstensen, M.; Seelig, D.; Larsen, P.A. RT-QuIC Detection of CWD Prion Seeding Activity in White-Tailed Deer Muscle Tissues. Sci. Reports 2021 111 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Greenlee, J.J.; Nicholson, E.M. Real-Time Quaking-Induced Conversion Detection of PrPSc in Fecal Samples From Chronic Wasting Disease Infected White-Tailed Deer Using Bank Vole Substrate. Front. Vet. Sci. 2021, 8, 643754. [Google Scholar] [CrossRef] [PubMed]
- Holz, C.L.; Darish, J.R.; Straka, K.; Grosjean, N.; Bolin, S.; Kiupel, M.; Sreevatsan, S. Evaluation of Real-Time Quaking-Induced Conversion, ELISA, and Immunohistochemistry for Chronic Wasting Disease Diagnosis. Front. Vet. Sci. 2021, 8, 824815. [Google Scholar] [CrossRef] [PubMed]
- Rowden, G.R.; Picasso-risso, C.; Li, M.; Marc, D. Standardization of Data Analysis for RT-QuIC-Based Detection of Chronic Wasting Disease. Pathogens 2023. [Google Scholar] [CrossRef]
- Haley, N.J.; Carver, S.; Hoon-Hanks, L.L.; Henderson, D.M.; Davenport, K.A.; Bunting, E.; Gray, S.; Trindle, B.; Galeota, J.; LeVan, I.; et al. Detection of Chronic Wasting Disease in the Lymph Nodes of Free-Ranging Cervids by Real-Time Quaking-Induced Conversion. J. Clin. Microbiol. 2014, 52, 3237. [Google Scholar] [CrossRef]
- Haley, N.J.; Siepker, C.; Hoon-Hanks, L.L.; Mitchell, G.; Walter, W.D.; Manca, M.; Monello, R.J.; Powers, J.G.; Wild, M.A.; Hoover, E.A.; et al. Seeded Amplification of Chronic Wasting Disease Prions in Nasal Brushings and Recto-Anal Mucosa-Associated Lymphoid Tissues from Elk by Real-Time Quaking-Induced Conversion. J. Clin. Microbiol. 2016, 54, 1117–1126. [Google Scholar] [CrossRef]
- Yuan, Q.; Rowden, G.; Wolf, T.M.; Schwabenlander, M.D.; Larsen, P.A.; Bartelt-Hunt, S.L.; Bartz, J.C. Sensitive Detection of Chronic Wasting Disease Prions Recovered from Environmentally Relevant Surfaces. Environ. Int. 2022, 166, 107347. [Google Scholar] [CrossRef]
- John, T.R.; Schätzl, H.M.; Gilch, S. Early Detection of Chronic Wasting Disease Prions in Urine of Pre-Symptomatic Deer by Real-Time Quaking-Induced Conversion Assay. Prion 2013, 7, 253. [Google Scholar] [CrossRef]
- Wilham, J.M.; Orrú, C.D.; Bessen, R.A.; Atarashi, R.; Sano, K.; Race, B.; Meade-White, K.D.; Taubner, L.M.; Timmes, A.; Caughey, B. Rapid End-Point Quantitation of Prion Seeding Activity with Sensitivity Comparable to Bioassays. PLoS Pathog. 2010, 6. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, R.; Satoh, K.; Sano, K.; Fuse, T.; Yamaguchi, N.; Ishibashi, D.; Matsubara, T.; Nakagaki, T.; Yamanaka, H.; Shirabe, S.; et al. Ultrasensitive Human Prion Detection in Cerebrospinal Fluid by Real-Time Quaking-Induced Conversion. Nat. Med. 2011, 17, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.G.; Graham, C.; Dudas, S.; Paxman, E.; Vuong, B.; Czub, S. Defining and Assessing Analytical Performance Criteria for Transmissible Spongiform Encephalopathy-Detecting Amyloid Seeding Assays. J. Mol. Diagnostics 2016, 18, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Sano, K.; Atarashi, R.; Nishida, N. Structural Conservation of Prion Strain Specificities in Recombinant Prion Protein Fibrils in Real-Time Quaking-Induced Conversion. Prion 2015, 9, 237. [Google Scholar] [CrossRef]
- Hajian-Tilaki, K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Casp. J Intern Med 2013, 4, 627–635. [Google Scholar]
- Sohn, H.J.; Mitchell, G.; Lee, Y.H.; Kim, H.J.; Park, K.J.; Staskevicus, A.; Walther, I.; Soutyrine, A.; Balachandran, A. Experimental Oral Transmission of Chronic Wasting Disease to Sika Deer (Cervus Nippon). Prion 2020, 14, 271–277. [Google Scholar] [CrossRef]
- Henderson, D.M.; Tennant, J.M.; Haley, N.J.; Denkers, N.D.; Mathiason, C.K.; Hoover, E.A. Detection of Chronic Wasting Disease Prion Seeding Activity in Deer and Elk Feces by Real-Time Quaking-Induced Conversion. J. Gen. Virol. 2017, 98, 1953–1962. [Google Scholar] [CrossRef]
- Haley, N. Amplification Techniques for the Detection of Misfolded Prion Proteins in Experimental and Clinical Samples. Curr. Protoc. Mol. Biol. 2020, 130. [Google Scholar] [CrossRef]
- Unal, I. Defining an Optimal Cut-Point Value in ROC Analysis: An Alternative Approach. Comput. Math. Methods Med. 2017, 2017. [Google Scholar] [CrossRef]
- Sing, T.; Sander, O.; Beerenwinkel, N.; Lengauer, T. ROCR: Visualizing Classifier Performance in R. Bioinformatics 2005, 21, 3940–3941. [Google Scholar] [CrossRef] [PubMed]
- Everest, S.J.; Thorne, L.; Barnicle, D.A.; Edwards, J.C.; Elliott, H.; Jackman, R.; Hope, J. Atypical Prion Protein in Sheep Brain Collected during the British Scrapie-Surveillance Programme. J. Gen. Virol. 2006, 87, 471–477. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Interrater Reliability: The Kappa Statistic. Biochem. Medica 2012, 22, 276. [Google Scholar] [CrossRef]
- Hwang, S.; Beckley, D.; Alekseev, K.P.; Nicholson, E.M. Hofmeister Effect in RT-QuIC Seeding Activity of Chronic Wasting Disease Prions. Front. Bioeng. Biotechnol. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Orrú, C.D.; Hughson, A.G.; Groveman, B.R.; Campbell, K.J.; Anson, K.J.; Manca, M.; Kraus, A.; Caughey, B. Factors That Improve RT-QuIC Detection of Prion Seeding Activity. Viruses 2016. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).