Submitted:
04 September 2023
Posted:
08 September 2023
You are already at the latest version
Abstract
Keywords:
Introduction
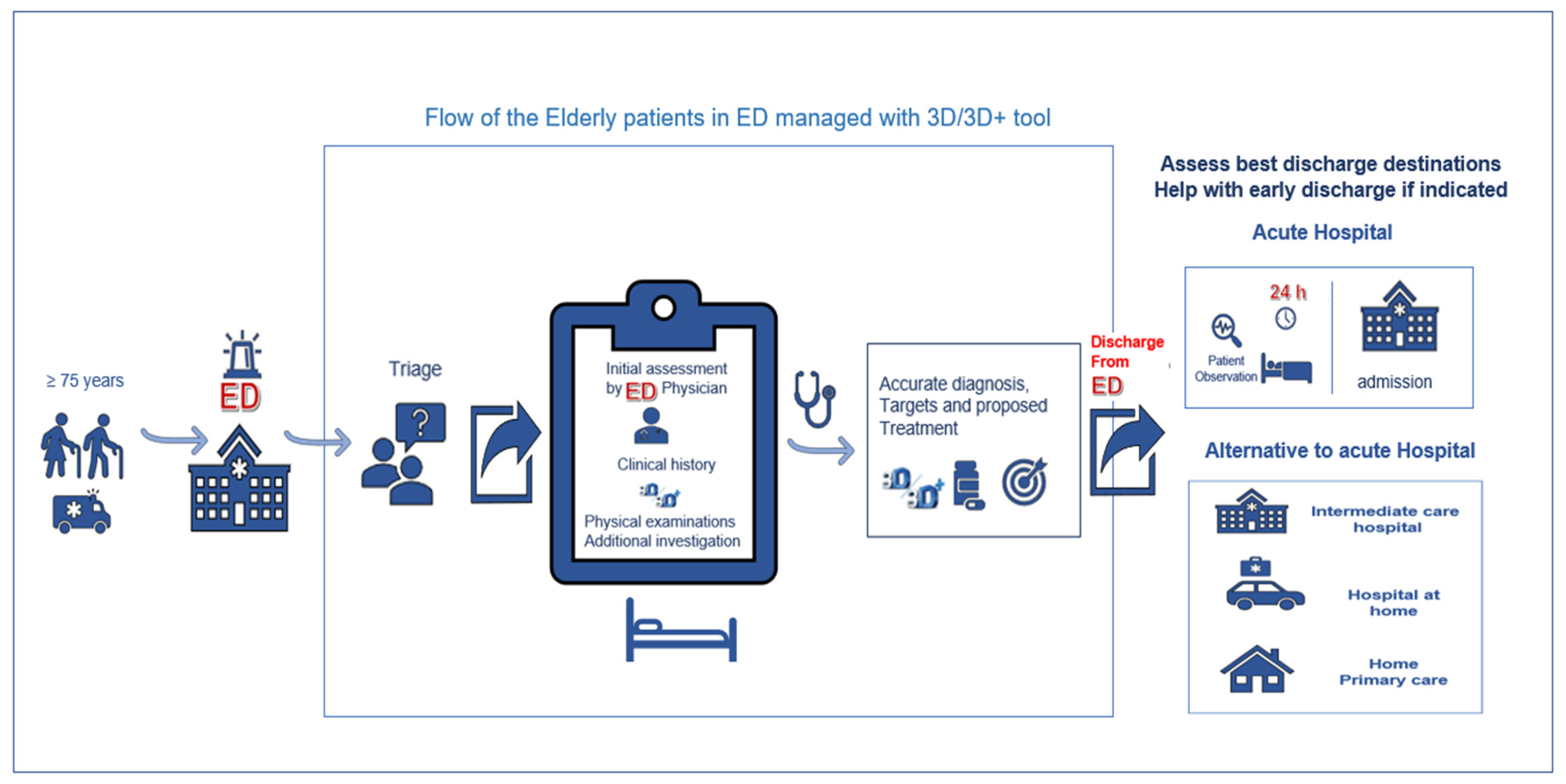
Materials and Methods
Results
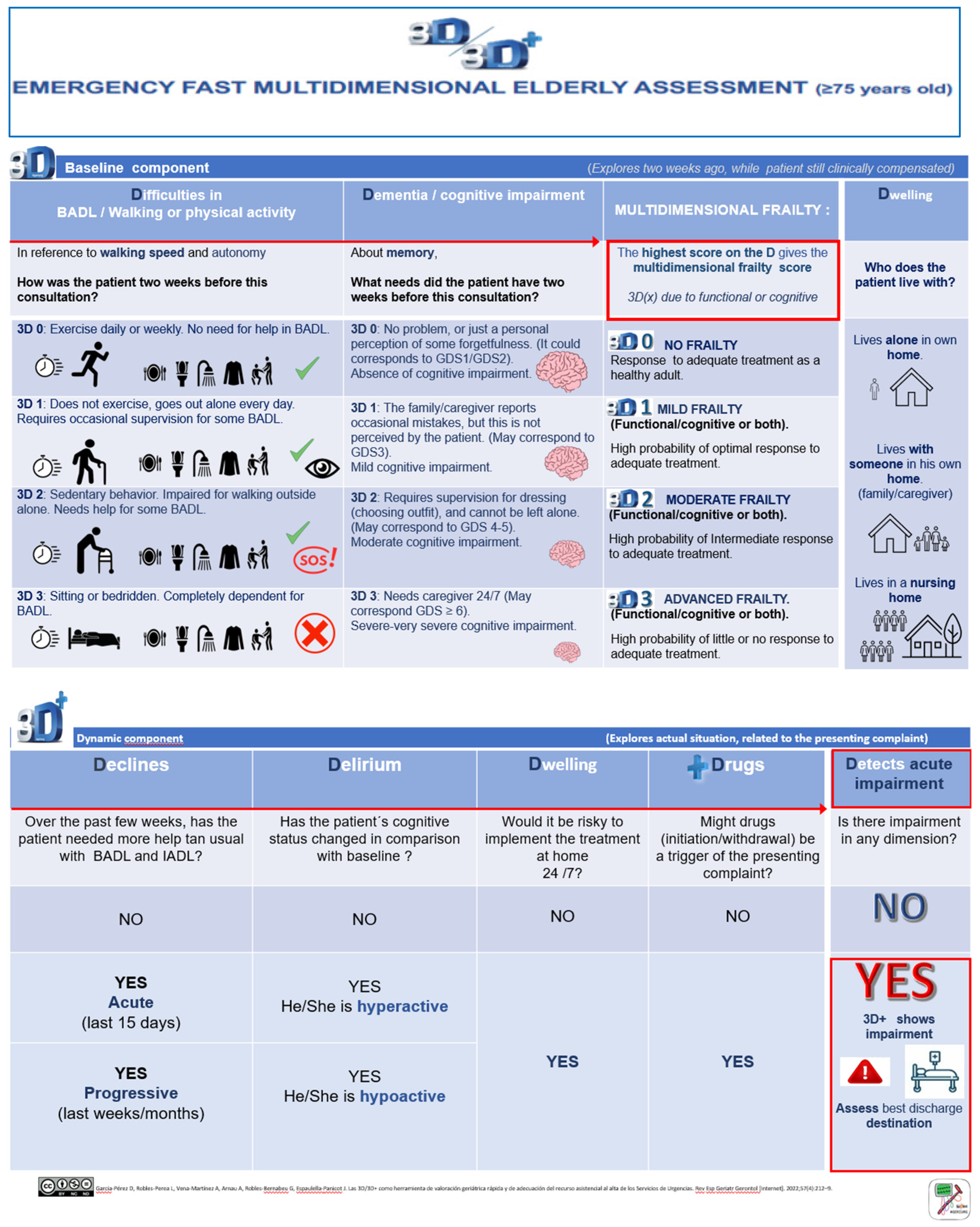
The 3D/3D+ tool
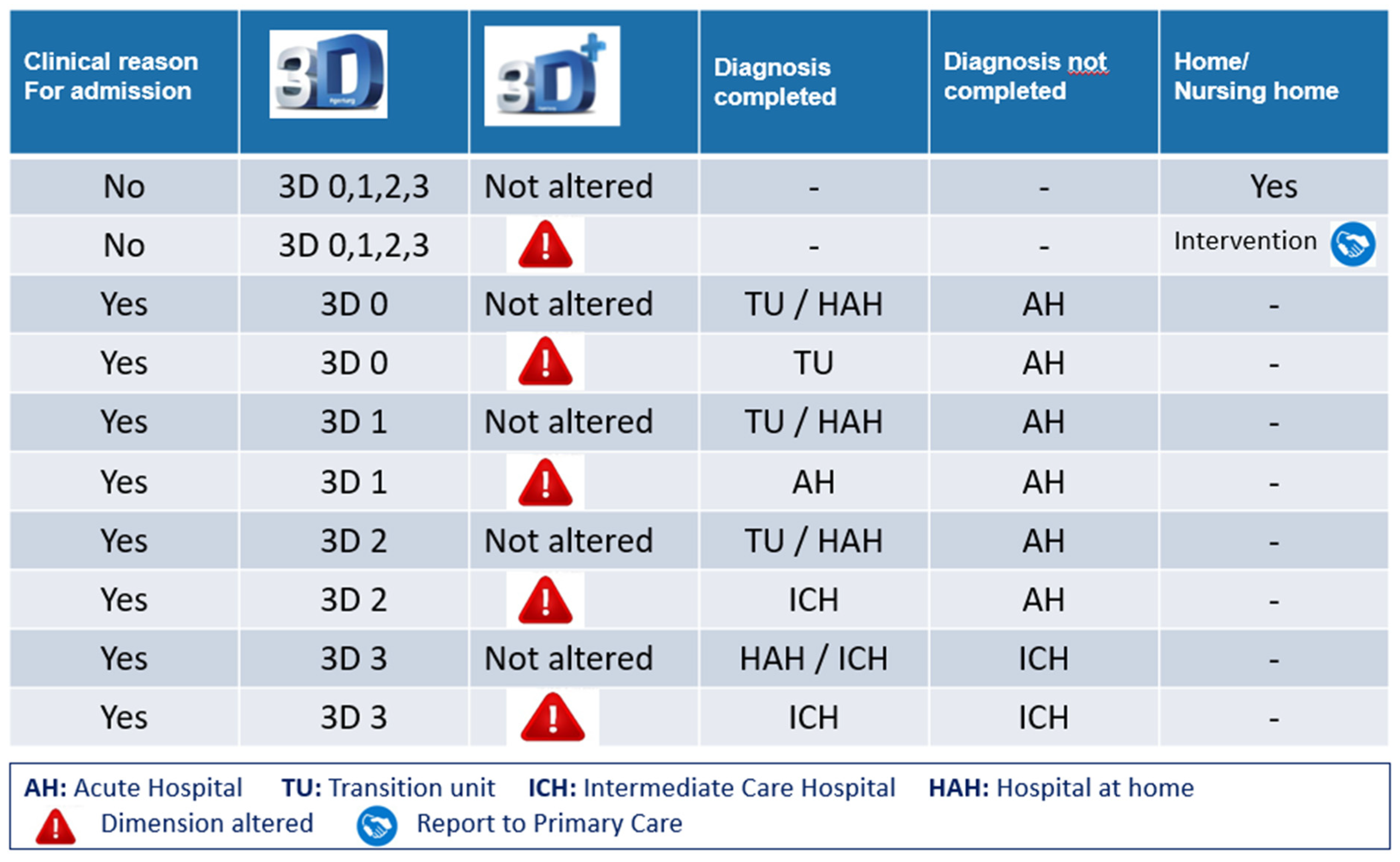
Destination upon discharge from the ED and its suitability
Prognostic accuracy of 3D/3D+ for predicting adverse outcomes
Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aminzadeh, F.; Dalziel, W.B. Older adults in the emergency department: a systematic review of patterns of use, adverse outcomes, and effectiveness of interventions. Ann Emerg Med. 2002, 39, 238–247. [CrossRef]
- Terrell, K.M.; Hustey, F.M.; Hwang, U.; Gerson, L.W.; Wenger, N.S.; Miller, D.K. Quality indicators for geriatric Emergency care. Acad Emerg Med May. 2009, 16, 441–449. [CrossRef]
- Puig-Campmany, M.; Ris-Romeu, J. Frail older patients in the emergency department: main challenges. Emergencias. 2022, 34, 415–417. PMID: 36625690.
- Martín-Sánchez, F.J.; Fernández-Alonso, C.; Merino, C. El paciente geriátrico en urgencias. An Sist Sanit. 2010, 33, 163–172. PMID: 20508687.
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001, 56, 146–156. http://www.ncbi.nlm.nih.gov/pubmed/11253156. [CrossRef]
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J Am Med Dir Assoc. 2013, 14, 392–397. http://www.ncbi.nlm.nih.gov/pubmed/23764209. PMID: 23764209. [CrossRef]
- Rodríguez-Mañas, L.; Féart, C.; Mann, G.; Viña, J.; Chatterji. S.; Chodzko-Zajko, W.; Gonzalez-Colaço Harmand, M.; Bergman, H.; Carcaillon, L.; Nicholson, C.; et al. Searching for an operational definition of frailty: a Delphi method based consensus statement. The frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci. 2013, 68, 62–67. PMID: 22511289. [CrossRef]
- Pulok, MH.; Theou O.; van der Valk AM.; Rockwood K. The role of illness acuity on the association between frailty and mortality in emergency department patients referred to internal medicine. Age Ageing. 2020, 49, 1071–1079. PMID: 32392289. [CrossRef]
- American College of Emergency Physicians; American Geriatrics Society, Emergency Nurses Association, Society for Academic Emergency Medicine, Geriatric Emergency Department Guidelines Task Force. Geriatric emergency department guidelines. Ann Emerg Med. 2014, 63, 7– 25. [CrossRef]
- Ellis, G.; Gardner, M.; Tsiachristas, A.; Langhorne, P.; Burke, O.; Harwood, R. H.; Conroy, S. P.; Kircher, T.; Somme, D.; Saltvedt, I.; et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2017, 9, CD006211. PMID: 28898390. [CrossRef]
- Pilotto, A.; Gallina, P.; Fontana, A.; Sancarlo, D.; Bazzano, S.; Copetti, M.; Maggi,S.; Paroni, G.; Marcato, F.; Pellegrini, F. et al. Development and validation of a multidimensional prognostic index for mortality based on a standardized Multidimensional Assessment Schedule (MPI-SVaMA) in community-dwelling older subjects. J AM Med Dir Assoc. 2013, 14, 287–292. PMID: 23402948. [CrossRef]
- McCusker, J.; Bellavance, F.; Cardin, S.; Trepanier, S.; Verdon, J.; Ardman, O. Detection of older people at increased risk of adverse health outcomes after an emergency visit: the ISAR screening tool. J Am Geriatr Soc. 1999, 47, 1229–1237. PMID: 10522957. [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, DB.; McDowell, I.; Mitnitski,A. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005, 173, 489–495. PMID: 16129869. [CrossRef]
- Serina, P.; Lo, AX.; Kocherginsky, M.; Gray, E.; Lindquist, LA.; Post, LA.; Heinemann, AW.; Cruz, D.; Dresden, SM. The clinical frailty scale and health services use for older adults in the emergency department. J Am Geriatr Soc. 2021, 69, 837– 839. PMID: 33205408. [CrossRef]
- Martín- Sánchez, FJ.; Fernández Alonso, C.; Gil Gregorio, P.; Puntos claves en la asistencia al anciano frágil en Urgencias. Med Clin (Barc). 2013, 140, 24–29. PMID: 22672966. [CrossRef]
- Church, S.; Rogers, E.; Rockwood, K.; Theou, O. A scoping review of the Clinical Frailty Scale. BMC Geriatr. 2020, 20, 393. PMID: 33028215. [CrossRef]
- Garcia-Pérez, D.; Robles-Perea, L.; Vena-Martínez, A.; Arnau, A.; Robles-Bernabeu, G.; Espaulella-Panicot, J. Las 3D/3D+ como herramienta de valoración geriátrica rápida y de adecuación del recurso asistencial al alta de los Servicios de Urgencias. Rev Esp Geriatr Gerontol. 2022, 57, 212–219. PMID: 35781176. [CrossRef]
- Rivero-Santana, A.; del Pino-Sedeño, T.; Ramallo-Fariña, Y.; Vergara, I.; Serrano-Aguilar, P. Valor de los instrumentos ISAR y TRST para predecir resultados adversos en población general geriátrica asistida en los servicios de urgencias: metaanálisis. Emergencias. 2017, 29, 49–60. PMID: 28825270.
- Bases conceptuals i model d’atenció per a les persones fràgils, amb cronicitat complexa (PCC) o avançada (MACA). (Consultado 04-03-2022). Departament de Salut. Generalitat de Catalunya. Disponible en: https://salutweb.gencat.cat/ca/ambits_actuacio/linies_dactuacio/estrategies_salut/cronicitat/documentacio-pla-de-salut-2016-2020/.
- Lasmarías, C.; Aradilla-Herrero, A.; Esquinas, C.; Santaeugènia, S.; Cegri, F.; Limón, E.; Subirana-Casacuberta, M. Primary care professionals' self-efficacy surrounding advance care planning and its link to sociodemographics, background and perceptions: A cross-sectional study. Int J Environ Res Public Health. 2021, 18, 9034. [CrossRef]
- Wylie, CM. Measuring end results of rehabilitation of patients with stroke. Public Health Rep. 1967, 82, 893–898. PMID: 4964119.
- Gresham, GE.; Philips, TF.; Labi, MLC. ADL status in stroke: relative merits of three standard indexes. Arch Phys Med Rehabil. 1980, 61, 355–358. PMID: 7406673.
- Pfeiffer, E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975, 23, 433–441. PMID: 1159263. [CrossRef]
- Sánchez Bermejo, R.; Cortés Fadrique, C.; Rincón Fraile, B.; Fernández Centeno, E.; Peña Cueva, S.; de las Heras Castro, EM. El triaje en urgencias en los hospitales españoles. Emergencias. 2013, 25, 66–70.
- Harris, PA.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, JG. Research electronic data capture (REDCap) a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009, 42, 377–381. PMID: 18929686. [CrossRef]
- Church, S.; Rogers, E.; Rockwood, K.; Theou, O. A scoping review of the Clinical Frailty Scale. BMC Geriatr. 2020, 20, 393. [CrossRef]
- COVID-19 rapid guideline: critical care in adults. London: National Institute for Health and Care Excellence (NICE); 2021. PMID: 33497153.
- Rockwood, K.; Theou, O. Using the clinical frailty scale in allocating scarce health care resources. Can Geriatr J. 2020, 23, 210–215. PMID: 32904824. [CrossRef]
- Hubbard, RE.; Peel, NM.; Samanta, M.; Gray, LC.; Mitnitski, A.; Rockwood, K. Frailty status at admission to hospital predicts multiple adverse outcomes. Age Ageing. 2017, 46, 801–806. PMID: 28531254. [CrossRef]
- Evans, SJ.; Sayers, M.; Mitnitski, A.; Rockwood K. The risk of adverse outcomes in hospitalized older patients in relation to a frailty index based on a comprehensive geriatric assessment. Age Ageing. 2014, 43, 127–32. PMID: 24171946. [CrossRef]
- Elliott, A.; Phelps, K.; Regen, E.; Conroy, SP. Identifying frailty in the emergency department-feasibility study. Age Ageing. 2017, 46, 840–845. PMID: 28541400. [CrossRef]
- Elliott, A.; Hull, L.; Conroy, SP. Frailty identification in the emergency department-a systematic review focussing on feasibility. Age Ageing 2017, 46, 509–513. PMID: 28200012. [CrossRef]
- Fehlmann, CA.; Nickel, CH.; Cino, E.; Al-Najjar, Z.; Langlois, N.; Eagles, D. Frailty assessment in emergency medicine using the Clinical Frailty Scale: a scoping review. Intern and Emerg. Med. 2022, 17, 2407–2418. PMID: 35864373. [CrossRef]
- Elliott, A.; Taub, N.; Banerjee, J.; Aijaz. F.; Jones, W.; Teece, L.; van Oppen, J.; Conroy, S. Does the Clinical Frailty Scale at Triage Predict Outcomes From Emergency Care for Older People? Ann Emerg Med. 2021, 77, 620–627. PMID: 33328147. [CrossRef]
- Nissen, SK.; Rueegg, M.; Carpenter, CR.; Kaeppeli, T.; Busch, JM.; Fournaise, A.; Nickel, CH. Prognosis for older people at presentation to emergency department based on frailty and aggregated vital signs. J Am Geriatr Soc. 2023, 71, 1250–1258. PMID: 36511431. [CrossRef]
- Rueegg, M.; Nissen, SK.; Brabrand, M.; Kaeppeli, T.; Dreher, T.; Carpenter, CR.; Bingisser, R.; Nickel, CH. The clinical frailty scale predicts 1-year mortality in emergency department patients aged 65 years and older. Acad Emerg Med. 2022, 29, 572– 580. PMID: 35138670. [CrossRef]
- Carpenter, CR.; Shelton, E.; Fowler, S.; Suffoletto, B.; Platts-Mills, TF.; Rothman, RE.; Hogan, TM. Risk factors and screening instrument to predict adverse outcome for undifferentiated older emergency department patients: a systematic review and meta-analysis. Acad Emerg Med. 2015, 22, 1–21. PMID: 25565487. [CrossRef]
- Francis, J.; Kapoor, WN. Prognosis after hospital discharge of older medical patients with delirium. J Am Geriatr Soc. 1992, 40, 601–606. PMID: 1587979. [CrossRef]
- Marcantonio, ER. Delirium in hospitalized older adults. N Engl J Med. 2017,15,1456-1466. PMID: 29020579. [CrossRef]
- Rockwood, K.; Cosway, S.; Carver, D.; Jarrett, P.; stadnyk, K.; FisK, J. The risk of dementia and death after delirium. Age Ageing. 1999, 28, 551–556. PMID: 10604507. [CrossRef]
- J. McCusker, M.; Cole, M.; Abrahamowicz, M; Primeau, F.; Belzile, E. Delirium predicts 12-month mortality Arch Intern Med. 2002, 162, 457–463. PMID: 11863480. [CrossRef]
- Leslie, DL.; Zhang, Y.; Holford, TR.; Bogardus, ST.; Leo-Summers, LS.; Inouye, SK. Premature death associated with delirium at 1-year follow-up. Arch Intern Med. 2005, 165, 1657–1662. [CrossRef]
- Cano-Escalera, G.; Graña, M.; Irazusta, J.; Labayen, I.; Besga, A. Survival of frail elderly with delirium. Int J Environ Res Public Health. 2022, 19, 2247. PMID: 35206439. [CrossRef]
- Umegaki, H.; Nagae, M.; Komiya, H.; Watanabe, K.; Yamada, Y.; Sakai, T. Association between changes in frailty during hospitalization in older adults and 3-month mortality after discharge. Eur Geriatr Med. 2022, 13, 1403–1406. PMID: 36260280. [CrossRef]
- Amblàs-Novellas, J.; Torné, A.; Oller, R.; Martori, JC.; Espaulella, J.; Romero-Ortuno, R. Transitions between degrees of multidimensional frailty among older people admitted to intermediate care: a multicentre prospective study. BMC Geriatr. 2022, 22, 722. PMID: 36050635. [CrossRef]
- Megalla, M.; Avula, R.; Manners, C.; Chinnery, P.; Perrella, L.; Finefrock, D. Using the 4M Model to Screen Geriatric Patients in the Emergency Department. JGEM. 2021, 2, 9, Article 1. [CrossRef]
- Mate, K.; Fulmer, T.; Pelton, L.; Berman, A.; Bonner, A.; Huang, W.; Zhang, J. Evidence for the 4Ms: interactions and outcomes across the care continuum. J. Aging Health. 2021,33, 469-481. PMID: 33555233. [CrossRef]
- Puig Campmany, M.; Ris Romeu, J.; Blázquez Andión, M.; Benito Vales, S. Development of a comprehensive, multidisciplinary program of care for frailty in an emergency department. Eur Geriatr Med. 2019, 10, 37–46. PMID: 32720288. [CrossRef]
| Total | Women | Men | p-value | |
|---|---|---|---|---|
| N=278 | n=166 | n=112 | ||
| Age [median (p25-p75)] | 86.0 [83.0-90.0] | 87.0 [83.0-91.8] | 85.0 [81.8-89.0] | 0.021 |
| Gender (female) | 166 (59.7) | |||
| Time frame | 0.523 | |||
| 07:01-14:00 | 120 (43.2) | 71 (42.8) | 49 (43.8) | |
| 14:01:22:00 | 106 (38.1) | 67 (40.4) | 39 (34.8) | |
| 22:01-07:00 | 52 (18.7) | 28 (16.9) | 24 (21.4) | |
| Triage level | 0.222 | |||
| II | 47 (16.9) | 24 (14.5) | 23 (20.5) | |
| III | 175 (62.9) | 104 (62.7) | 71 (63.4) | |
| IV | 56 (20.1) | 38 (22.9) | 18 (16.1) | |
| Prior medical care at home (Yes) | 119 (42.8) | 76 (45.8) | 43 (38.4) | 0.222 |
| Arrival at ED by ambulance (Yes) | 223 (80.2) | 141 (84.9) | 82 (73.2) | 0.016 |
| PCC (Yes) | 186 (66.9) | 117 (70.5) | 69 (61.6) | 0.123 |
| MACA (Yes) | 36 (12.9) | 18 (10.8) | 18 (16.1) | 0.203 |
| Readmission (≥2 or more times/last year) | 107 (38.9) | 62 (37.6) | 45 (40.9) | 0.579 |
| Reason for consultation | ||||
| Dyspnea | 140 (50.4) | 78 (47.0) | 62 (55.4) | 0.171 |
| General malaise | 40 (14.4) | 30 (18.1) | 10 (8.93) | 0.033 |
| Anemia | 6 (2.16) | 4 (2.41) | 2 (1.79) | 0.726 |
| Fever | 34 (12.2) | 20 (12.0) | 14 (12.5) | 0.910 |
| Chest pain | 9 (3.24) | 5 (3.01) | 4 (3.57) | 0.796 |
| Abdominal pain | 20 (7.19) | 16 (9.64) | 4 (3.57) | 0.055 |
| Locomotor pain | 1 (0.36) | 1 (0.60) | 0 (0.00) | 0.410 |
| Falls | 23 (8.27) | 15 (9.04) | 8 (7.14) | 0.574 |
| Neurological symptoms | 38 (13.7) | 22 (13.3) | 16 (14.3) | 0.806 |
| Others | 16 (5.76) | 7 (4.22) | 9 (8.04) | 0.180 |
| Barthel Index1 | 0.041 | |||
| Independent (90-100) | 60 (23.9) | 27 (18.1) | 33 (32.3) | |
| Mild dependency (61-89) | 51 (20.3) | 29 (19.5) | 22 (21.6) | |
| Moderate dependency (45-60) | 63 (25.1) | 41 (27.5) | 22 (21.6) | |
| Severe dependency (<45) | 77 (30.7) | 52 (34.9) | 25 (24.5) | |
| Pfeiffer Questionnaire2 | 0.094 | |||
| No cognitive impairment | 128 (52.9) | 67 (47.2) | 61 (61.0) | |
| Mild cognitive impairment | 67 (27.7) | 43 (30.3) | 24 (24.0) | |
| Moderate cognitive impairment | 10 (4.1) | 5 (3.5) | 5 (5.0) | |
| Severe cognitive impairment | 37 (15.3) | 27 (19.0) | 10 (10.0) | |
| Rockwood Clinical Frailty Scale | 0.008 | |||
| Fit (CFS 1) | 3 (1.08) | 0 (0.00) | 3 (2.68) | |
| Well (CFS 2) | 15 (5.40) | 4 (2.41) | 11 (9.82) | |
| Managing well (CFS 3) | 28 (10.1) | 13 (7.83) | 15 (13.4) | |
| Vulnerable (CFS 4) | 44 (15.8) | 26 (15.7) | 18 (16.1) | |
| Slightly frail (CFS 5) | 32 (11.5) | 18 (10.8) | 14 (12.5) | |
| Moderately frail (CFS 6) | 56 (20.1) | 38 (22.9) | 18 (16.1) | |
| Severely frail (CFS 7) | 66 (23.7) | 47 (28.3) | 19 (17.0) | |
| Very severely frail (CFS 8) | 30 (10.8) | 19 (11.4) | 11 (9.82) | |
| Terminally ill (CFS 9) | 4 (1.4) | 1 (0.60) | 3 (2.68) | |
| ISAR scale | ||||
| ≥2 | 253 (91.0) | 157 (94.6) | 96 (85.7) | 0.011 |
| ≥3 | 209 (75.2) | 137 (82.5) | 72 (64.3) | <0.001 |
| ≥4 | 141 (50.7) | 92 (55.4) | 49 (43.8) | 0.056 |
| Diagnosis at ED discharge | 0.524 | |||
| Cardiac respiratory failure | 32 (11.5) | 21 (12.7) | 11 (9.82) | |
| Lung respiratory failure | 65 (23.4) | 36 (21.7) | 29 (25.9) | |
| Mixed respiratory failure | 12 (4.3) | 7 (4.22) | 5 (4.46) | |
| Lung infection | 36 (12.9) | 18 (10.8) | 18 (16.1) | |
| Abdominal infection | 8 (2.9) | 7 (4.22) | 1 (0.89) | |
| Urinary infection | 26 (9.4) | 18 (10.8) | 8 (7.14) | |
| Skin infection | 2 (0.7) | 1 (0.60) | 1 (0.89) | |
| Fractures | 3 (1.1) | 3 (1.81) | 0 (0.00) | |
| Stroke | 12 (4.3) | 7 (4.22) | 5 (4.46) | |
| Others | 82 (29.5) | 48 (28.9) | 34 (30.4) | |
| The patient or family prioritizes treatment at home (Yes) | 121 (43.5) | 73 (44.0) | 48 (42.9) | 0.854 |
| Total N=278 |
Women n=166 | Men n=112 |
p-value |
|
|---|---|---|---|---|
| 3D Baseline component | ||||
| Difficulties with BADL, walking, or physical activity | <0.001 | |||
| No (D0) | 52 (18.7) | 18 (10.8) | 34 (30.4) | |
| Mild (D1) | 53 (19.1) | 28 (16.9) | 25 (22.3) | |
| Moderate (D2) | 96 (34.5) | 66 (39.8) | 30 (26.8) | |
| Severe (D3) | 77 (27.7) | 54 (32.5) | 23 (20.5) | |
| Dementia/Cognitive impairment | 0.142 | |||
| No (D0) | 139 (50.0) | 75 (45.2) | 64 (57.1) | |
| Mild (D1) | 55 (19.8) | 33 (19.9) | 22 (19.6) | |
| Moderate (D2) | 47 (16.9) | 34 (20.5) | 13 (11.6) | |
| Severe (D3) | 37 (13.3) | 24 (14.5) | 13 (11.6) | |
| Dwelling | 0.030 | |||
| Lives in own home alone | 30 (10.8) | 18 (10.8) | 12 (10.7) | |
| Lives in own home with family or caregiver | 178 (64.0) | 97 (58.4) | 81 (72.3) | |
| Nursing home | 70 (25.2) | 51 (30.7) | 19 (17.0) | |
| 3D Baseline component | <0.001 | |||
| No frailty (3D 0) | 47 (16.9) | 16 (9.6) | 31 (27.7) | |
| Mild frailty (3D 1) | 52 (18.7) | 28 (16.9) | 24 (21.4) | |
| Moderate frailty (3D 2) | 94 (33.8) | 65 (39.2) | 29 (25.9) | |
| Advanced frailty (3D 3) | 85 (30.6) | 57 (34.3) | 28 (25.0) | |
| 3D+ Dynamic component | ||||
| Decline in BADL-IADL | 0.530 | |||
| No | 116 (41.7) | 65 (39.2) | 51 (45.5) | |
| Yes, acute | 104 (37.4) | 66 (39.8) | 38 (33.9) | |
| Yes, progressive | 58 (20.9) | 35 (21.1) | 23 (20.5) | |
| Delirium | 0.130 | |||
| No | 212 (76.3) | 122 (73.5) | 90 (80.4) | |
| Yes, hyperactive | 17 (6.1) | 14 (8.4) | 3 (2.7) | |
| Yes, hypoactive | 49 (17.6) | 30 (18.1) | 19 (17.0) | |
| Dwelling | 0.572 | |||
| Is 24-hour treatment at home feasible? (No) | 55 (19.8) | 31 (18.7) | 24 (21.4) | |
| Drugs | 0.090 | |||
| Might drugs (start/withdrawal) be a trigger of the presenting complaint? | 39 (14.0) | 29 (17.5) | 10 (8.93) | |
| 3D+ Dynamic component (impact of acute illness) | 0.187 | |||
| Impairment | 167 (60.1) | 105 (63.3) | 62 (55.4) | |
| No impairment | 111 (39.9) | 61 (36.7) | 50 (44.6) | |
| 3D+ (delirium) | 3D+ (delirium and functional decline) |
||||||
|---|---|---|---|---|---|---|---|
| N=278 | No alteration n=212 |
Alteration n=66 |
p-value | No alteration n=220 |
Alteration n=58 |
p-value |
|
| 72-hour ED returns | 5 (1.8) | 4 (1.9) | 1 (1.5) | 0.843 | 4 (1.8) | 1 (1.7) | 0.962 |
| 72-hour hospital readmission | 3 (1.1) | 3 (1.4) | 0 (0) | 0.331 | 3 (1.6) | 0 (0) | 0.371 |
| 30-day ED returns | 50 (18.0) | 42 (19.8) | 8 (12.1) | 0.155 | 43 (19.6) | 7 (12.1) | 0.187 |
| 30-day hospital readmission | 28 (10.1) | 23 (10.9) | 5 (7.6) | 0.440 | 23 (10.5) | 5 (8.6) | 0.680 |
| 30-day mortality | 54 (19.4) | 21 (9.9) | 33 (50.0) | <0.001 | 23 (10.5) | 31 (53.5) | <0.001 |
| 30-day any adverse outcome | 100 (36.0) | 60 (28.3) | 40 (60.6) | <0.001 | 63 (28.6) | 37 (63.8) | <0.001 |
| 6-month mortality | 86 (30.9) | 45 (21.2) | 41 (62.1) | <0.001 | 49 (22.2) | 37 (63.8) | <0.001 |
| 12-month mortality | 107 (38.5) | 64 (30.2) | 43 (65.2) | <0.001 | 69 (31.4) | 38 (65.5) | <0.001 |
| Sensitivity (95%CI) |
Specificity (95%CI) |
PPV (95%CI) |
NPV (95%CI) |
|
|---|---|---|---|---|
| 72-hour ED returns | ||||
| 3D+ (delirium) | 0.20 (0.15-0.25) | 0.76 (0.71-0.81) | 0.02 (0.00-0.03) | 0.98 (0.97-1.00) |
| 3D+ (delirium and functional decline) | 0.20 (0.15-0.25) | 0.79 (0.74-0.84) | 0.02 (0.00-0.04) | 0.98 (0.97-1.00) |
| CFS (≥5) | 1.00 (0.48-1.00) | 0.33 (0.27-0.39) | 0.03 (0.01-0.06) | 1.00 (0.96-1.00) |
| CFS (≥7) | 0.80 (0.28-0.99) | 0.65 (0.59-0.70) | 0.04 (0.01-0.10) | 0.99 (0.97-1.00) |
| ISAR (≥2) | 1.00 (0.48-1.00) | 0.09 (0.06-0.13) | 0.02 (0.01-0.05) | 1.00 (0.86-1.00) |
| ISAR (≥3) | 1.00 (0.48-1.00) | 0.25 (0.20-0.31) | 0.02 (0.01-0.05) | 1.00 (0.95-1.00) |
| 30-day ED returns | ||||
| 3D+ (delirium) | 0.16 (0.12-0.20) | 0.75 (0.69-0.80) | 0.12 (0.08-0.16) | 0.80 (0.76-0.85) |
| 3D+ (delirium and functional decline) | 0.14 (0.10-0.18) | 0.78 (0.73-0.83) | 0.12 (0.08-0.16) | 0.80 (0.76-0.85) |
| CFS (≥5) | 0.66 (0.51-0.79) | 0.32 (0.26-0.38) | 0.18 (0.12-0.24) | 0.81 (0.71-0.89) |
| CFS (≥7) | 0.28 (0.16-0.42) | 0.62 (0.56-0.69) | 0.14 (0.08-0.22) | 0.80 (0.73-0.85) |
| ISAR (≥2) | 0.88 (0.76-0.95) | 0.08 (0.05-0.13) | 0.17 (0.13-0.23) | 0.76 (0.55-0.91) |
| ISAR (≥3) | 0.70 (0.55-0.82) | 0.24 (0.18-0.30) | 0.17 (0.12-0.23) | 0.78 (0.67-0.87) |
| 30-day mortality | ||||
| 3D+ (delirium) | 0.61 (0.55-0.67) | 0.85 (0.81-0.89) | 0.50 (0.44-0.56) | 0.90 (0.87-0.94) |
| 3D+ (delirium and functional decline) | 0.57 (0.52-0.63) | 0.88 (0.84-0.92) | 0.53 (0.48-0.59) | 0.90 (0.86-0.93) |
| CFS (≥5) | 0.81 (0.69-0.91) | 0.36 (0.29-0.42) | 0.23 (0.18-0.30) | 0.89 (0.81-0.95) |
| CFS (≥7) | 0.54 (0.40-0.67) | 0.68 (0.62-0.74) | 0.29 (0.20-0.39) | 0.86 (0.80-0.91) |
| ISAR (≥2) | 0.94 (0.85-0.99) | 0.10 (0.06-0.14) | 0.20 (0.15-0.26) | 0.88 (0.69-0.97) |
| ISAR (≥3) | 0.83 (0.71-0.92) | 0.27 (0.21-0.33) | 0.22 (0.16-0.28) | 0.87 (0.77-0.94) |
| 30-day any adverse outcome | ||||
| 3D+ (delirium) | 0.40 (0.34-0.46) | 0.85 (0.81-0.90) | 0.61 (0.55-0.66) | 0.72 (0.66-0.77) |
| 3D+ (delirium and functional decline) | 0.37 (0.31-0.43) | 0.88 (0.84-0.92) | 0.64 (0.58-0.69) | 0.71 (0.66-0.77) |
| CFS (≥5) | 0.74 (0.64-0.82) | 0.36 (0.29-0.43) | 0.39 (0.32-0.47) | 0.71 (0.61-0.80) |
| CFS (≥7) | 0.41 (0.31-0.51) | 0.67 (0.59-0.74) | 0.41 (0.31-0.51) | 0.67 (0.59-0.74) |
| ISAR (≥2) | 0.92 (0.85-0.96) | 0.10 (0.06-0.15) | 0.36 (0.30-0.43) | 0.68 (0.46-0.85) |
| ISAR (≥3) | 0.78 (0.69-0.86) | 0.26 (0.20-0.34) | 0.37 (0.31-0.44) | 0.68 (0.56-0.79) |
| 6-month mortality | ||||
| 3D+ (delirium) | 0.48 (0.42-0.54) | 0.87 (0.83-0.91) | 0.62 (0.56-0.68) | 0.79 (0.74-0.84) |
| 3D+ (delirium and functional decline) | 0.43 (0.37-0.49) | 0.89 (0.85-0.93) | 0.64 (0.58-0.69) | 0.78 (0.73-0.83) |
| CFS (≥5) | 0.81 (0.72-0.89) | 0.39 (0.32-0.46) | 0.37 (0.30-0.45) | 0.82 (0.73-0.89) |
| CFS (≥7) | 0.52 (0.41-0.63) | 0.71 (0.64-0.78) | 0.45 (0.35-0.55) | 0.77 (0.70-0.83) |
| ISAR (≥2) | 0.95 (0.89-0.99) | 0.11 (0.07-0.16) | 0.32 (0.27-0.39) | 0.84 (0.64-0.95) |
| ISAR (≥3) | 0.83 (0.73-0.90) | 0.28 (0.22-0.35) | 0.34 (0.28-0.41) | 0.78 (0.67-0.87) |
| 12-month mortality | ||||
| 3D+ (delirium) | 0.40 (0.34-0.46) | 0.87 (0.83-0.91) | 0.65 (0.60-0.71) | 0.70 (0.64-0.75) |
| 3D+ (delirium and functional decline) | 0.36 (0.30-0.41) | 0.88 (0.85-0.92) | 0.66 (0.60-0.71) | 0.69 (0.63-0.74) |
| CFS (≥5) | 0.80 (0.72-0.87) | 0.40 (0.33-0.48) | 0.46 (0.38-0.53) | 0.77 (0.67-0.85) |
| CFS (≥7) | 0.51 (0.42-0.61) | 0.74 (0.66-0.80) | 0.55 (0.45-0.65) | 0.71 (0.64-0.77) |
| ISAR (≥2) | 0.93 (0.86-0.97) | 0.10 (0.06-0.15) | 0.39 (0.33-0.45) | 0.68 (0.46-0.85) |
| ISAR (≥3) | 0.79 (0.71-0.87) | 0.27 (0.21-0.35) | 0.41 (0.34-0.48) | 0.68 (0.56-0.79) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




