Submitted:
06 September 2023
Posted:
08 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Epidemiology
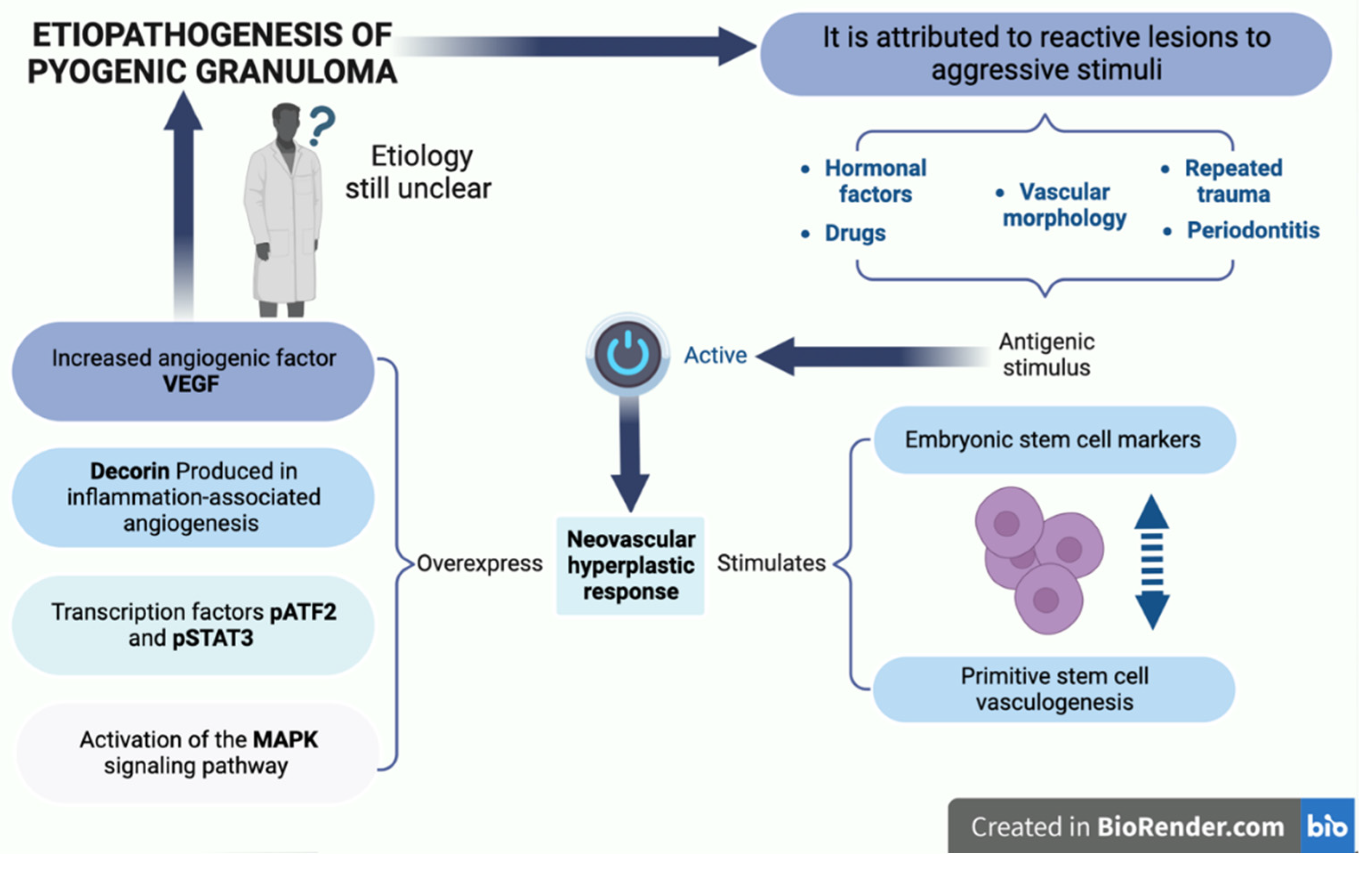
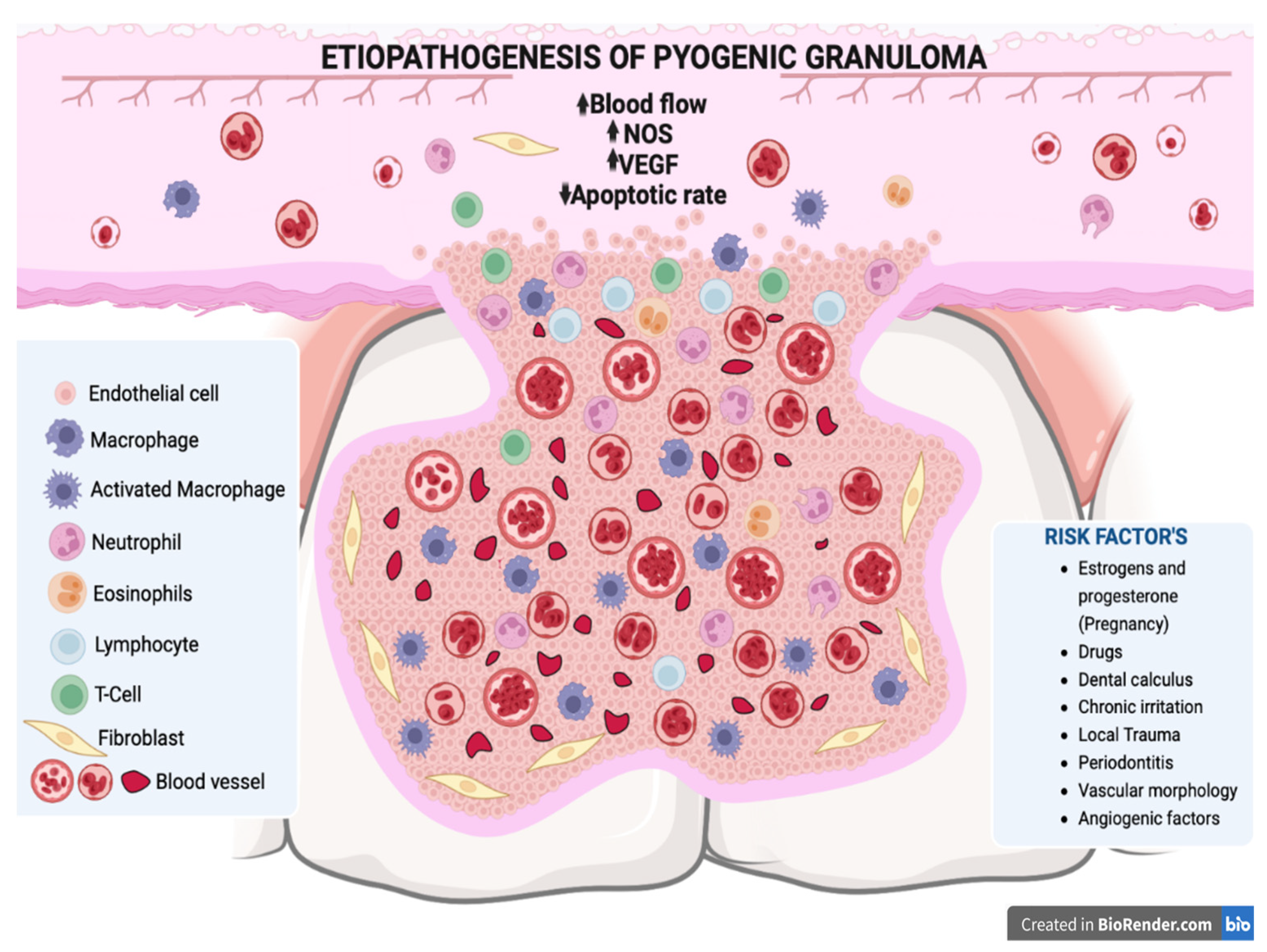
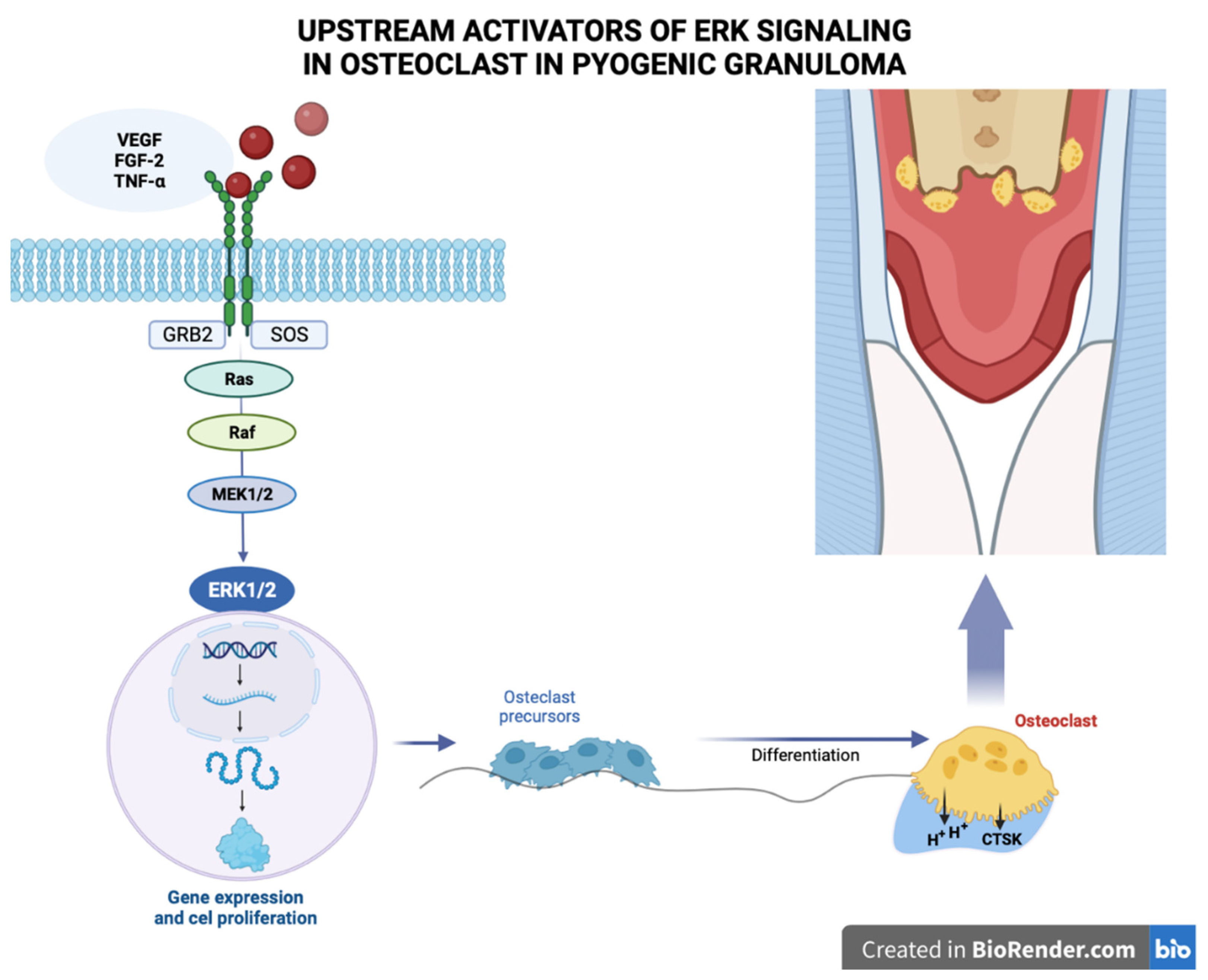
3. Etiopathogenesis
4. Clinical features
5. Radiographic features
6. Microscopic features
7. Differential diagnosis of oral PG
| Year | Author | Country | Age (Years) |
Gender | Location | Consistency | Comorbidity | Radiographic | Lesion size | Diagnosis | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Features | |||||||||||
| 2001 | Akyol et al., 2001 [46] | Turkey | 4 m | B | Tongue | Soft | No | NA | 1 x 0.8 x 0.8 cm | H | Surgical excision |
| 2002 | Aguilo L. 2002 [56] | Spain | 19 m | B | Gingiva | Soft | No | Fracture of the crown of 61 | NA | H | Surgical excision |
| 2006 | Parisi E et al., (2006) [73] | USA | 33 | F | Gingiva | NA | NA | NA | 0.5 x 0.5 cm | H | Intralesional corticosteroids |
| 2006 | Patil et al., 2006 [84] | India | 50 | F | Lower Lip | NA | No | NA | 1 x 0.5 cm | H | Surgical excision |
| 2006 | Shenoy S. 2006 [64] | India | 8 | G | Gingiva | NA | NA | loss of alveolar crestal bone | 2.0 x 1.0 x 1.0 cm | H | Surgical excision |
| 2008 | Amirchaghmaghi et al., 2008 [85] | Iran | 16 | M | Hard palate | Firm | No | NA | 0.7 cm | H | Surgical excision |
| 2009 | Goncalves 2009 [101] | Brazil | 12 | F | Upper lip | NA | NA | NA | 1.0 cm | H | Surgical excision |
| 2009 | Olmedo D et al., (2009) [86] | Argentina | 75 | F | Peri-implant mucosa | NA | No | No abnormalities | 1.0 x 1.0 x 0.6 cm | H | Surgical excision |
| Argentina | 64 | F | Peri-implant mucosa | Firm | No | Bone loss | 0.6 x 0.5 x 0.4 cm | H | Surgical excision | ||
| 2010 | Gondivkar et al., 2010 [61] | India | 25 | F | Gingiva | Soft | Pregnancy | Alveolar bone loss | 3 x 7 cm | H | Surgical excision |
| 2010 | Rizwanulla et al., 2010 [87] | Nepal | 13 | F | Gingiva | Firm | No | NA | 2.0 x 1.0 x 1.0 cm | H | Surgical excision |
| 2011 | Behl et al., 2011 [60] | India | 60 | F | Gingiva | Firm | No | Vertical bone loss | 3.2 x 3.4 cm | H | Surgical excision |
| 2011 | Mubben et al., 2011 [102] | India | 63 | F | Gingiva | Soft | No | NA | 5.0 x 3.5 cm | H | Surgical excision |
| 2011 | Penseriya et al. 2011 [88] | India | 30 | M | Gingiva | NA | No | Interdental bone loss | 2.0 x 1.5 cm | H | Surgical excision |
| 2011 | Shivaswamy et al., 2011 [65] | India | 19 | M | Gingiva and palate | Soft | NA | Horizontal bone loss | 4.0 x 5.0 mm | H | Surgical excision |
| 2012 | Chandrashekar 2012 [69] | India | 28 | F | Gingiva | Soft | No | NA | NA | H | Surgical excision |
| 2012 | Panjwani et al., 2012 [50] | India | 69 | M | Tongue | Firm | No | NA | 2.0 x 3.0 cm | H | Surgical excision |
| 2012 | Ravi et al., 2012 [89] | India | 33 | M | Lower lip | Firm | NA | NA | 3.0 x 2.0 cm | H | Surgical excision |
| 2012 | Piscoya et al., 2012 [90] | Brazil | 44 | M | Lower lip | NA | NA | NA | NA | H | Surgical excision |
| 2012 | Verma et al., 2012 [62] | India | 30 | F | Gingiva | NA | No | Alveolar bone loss | 1.5 x 1.0 cm | H | Surgical excision |
| 2013 | Adusumilli et al., 2013 [91] | India | 24 | F | Gingiva | Firm | NA | NA | 2 x 3.5 cm | H | Surgical excision |
| 27 | F | Gingiva | Firm | NA | NA | 2 x 3.5 cm | Surgical excision | ||||
| 27 32 |
F F |
Gingiva Gingiva |
Firm Firm |
NA NA |
NA NA |
2 x 3.5 cm 1.5 x 2.5 cm |
H | Surgical excision Surgical excision |
|||
| H | |||||||||||
| 23 | F | Gingiva | Soft | NA | NA | 1.5 x 1.75 cm | H | Surgical excision | |||
| 26 | F | Gingiva | Soft | NA | NA | 2.0 x 2.5 cm | H | Surgical excision | |||
| 28 | F | Gingiva | Soft | NA | NA | 0.5 x 0.8 cm | H | Surgical excision | |||
| 2013 | Deshmukh et al., 2013 [58] | India | 9 | M | Gingiva and mucogingival junction | Soft | No | Horizontal bone loss | NA | H | Surgical excision |
| 2013 | Gomes et al., 2013 [2] | India | 22 | M | Gingiva | NA | NA | No bone loss | 2.1 x 4.4 cm | H | Surgical excision |
| 2013 | Kamala 2013 [3] | India | 30 | F | Upper lip | Firm | No | NA | 0.8 cm | H | Surgical excision |
| 2013 | Mahabob et al., 2013 [92] | India | 22 | F | Palate | Firm | Pregnancy | NA | 2 x 2 cm | H | Surgical excision |
| 2013 | Moraes, et al., 2013 [93] | Brazil | 65 | M | Gingiva | NA | Diabetes and high blood pressure | No abnormalities | NA | H | Surgical excision |
| 2013 | Sangamesh et al., 2013 [94] | India | 40 | F | Buccal mucosa | Firm | NA | NA | 1.5 x 1.5 cm | H | Surgical excision |
| 2014 | Asha et al., 2014 [59] | India | 54 | M | Lower lip | Firm | No | NA | 3.0 x 3.0 cm | H | Surgical excision |
| 2014 | Fekrazad R et al., (2014) [54] | Iran | 24 | F | Gingiva | Soft | NA | No abnormalities | 1.4 x 0.8 mm | H | Laser excision |
| 2014 | Ghalayani et al., 2014 [83] | Iran | 45 | M | Tongue | NA | Epilepsy beginning | NA | 4.0 x 3.0 x 1.0 cm | H | Surgical excision |
| 2014 | Kejriwal et al., 2014 [81] | India | 59 | M | Gingiva | Firm | No | No abnormalities | 1.5 x 2.0 x 1.0 cm | H | Surgical excision |
| 2014 | Mastammanavar et al., 2014 [52] | India | 44 | F | Gingiva | Soft | NA | NA | 1.5 x 3.0 cm | H | Surgical excision |
| 2014 | Sun et al., 2014 [78] | China | 22 | F | Gingiva | NA | Pregnancy | NA | 3 cm | C | No |
| 2015 | Asnaashari M et al., (2015) [70] | Iran | 6 | M | Gingiva | Soft | No | No abnormalities | 1.1 x 1.3 cm | H | Laser excision |
| 2015 | Bugshan a., et al., (2015) [74] | USA | 51 | F | Gingiva and palatal | NA | NA | NA | 0.9x0.6 cm buccal and 0.8x0.7 cm palatal. | H | Intralesional inyections |
| 2015 | De Carvalho et al., 2015 [95] | Brazil | 11 | B | Upper Lip | NA | No | NA | 4.5 cm | H | Surgical |
| 2015 | Ganesan A. 2015 [55] | India | 49 | F | Gingiva | Firm | No | No bone loss | 4.0 x 3.0 cm | H | Surgical excision |
| 2015 | Sachdeva 2015 [96] | India | 45 | F | Buccal mucosa | Firm | No | NA | 2 x 1 cm | H | Surgical excision |
| 2015 | Tripathi et al., 2015 [63] | India | 55 | M | Gingiva | NA | No | Alveolar bone loss | 3 x 3 cm | H | Surgical excision |
| 2016 | Agarwal N et al., 2016 [97] | India | 8 d | B | Gingiva | Soft | No | NA | 0.5 x 0.8 cm | H | Surgical excision |
| 2016 | Al-Mohaya et al., 2016 [23] | Saudí arabia | 51 | F | Gingiva | Firm | Uncontrolled type II diabetes mellitus | NA | 2.0 x 1.5 cm | H | Laser excision |
| 2016 | Marla et al., 2016 [98] | Nepal | 40 | F | Gingiva | NA | NA | NA | 1.0 – 2.0 cm | H | Surgical excision |
| Nepal | 40 | F | Buccal mucosa | NA | NA | NA | 1.0 – 2.0 cm | H | Surgical excision | ||
| Nepal | 9 | M | Buccal mucosa | NA | NA | NA | <1.0 cm | H | Surgical excision | ||
| Nepal | 23 | F | Gingiva | NA | NA | NA | 1.0 – 2.0 cm | H | Surgical excision | ||
| 2016 | Cheney et al., 2016 [22] | USA | 16 | M | Tongue, Buccal mucosa, | Soft | Acute lymphoblastic leukemia | N/A | N/A | Surgical excision | |
| USA | 14 | G | Tongue | N/A | Fanconi anemia | No | 0.5 x 0.2cm 1.0 x 0.3 cm 0.3 x 0.2 |
H | Surgical excision | ||
| USA | 11 | G | Tongue | N/A | Fanconi anemia | No | 2.0 x 2.5 cm | H | Surgical excision | ||
| USA | 15 | Buccal mucosa | Stage IIIB nodular sclerosing Hodgkin lymphoma. | No | 1 cm 2.0 x 4.0 cm | H | CO2 laser | ||||
| USA | 6 | B | Tongue | N/A | Junctional epidermolysis bullosa | No | 1.0 x 0.5 cm | H | Surgical excision | ||
| 2017 | Rosa et al., 2017 [48] | México | 34 | F | Gingiva | Firm | No | Absence of interproximal contact | 1.5 x 0.9 cm | H | Surgical excision |
| 2018 | Canivell et al [77] | Spain | 32 | F | Lower lip | NA | Pregnancy | NA | 0.5 x 1.0 cm | H | Surgical excision |
| 2018 | Parajuli et al., 2018 [49] | Nepal | 26 | F | Tongue | Soft | No | NA | 2.5 x 2.0 cm | H | Surgical excision |
| Nepal | 15 | G | Upper lip | NA | NA | NA | 0.5 x 0.5 cm | C | Surgical excision | ||
| 2019 | Poudel et al., 2019 [99] | Nepal | 49 | M | Upper lip | Firm | NA | NA | 0.6 x 0.8 cm | H | Surgical excision |
| 2020 | Gutierrez 2020 [25] | Perú | 51 | F | Alveolar ridge | NA | Hypothyroidism erythrodermic psoriasis | Alveolar bone loss | 2.5 x 2.5 cm | H | Surgical excision |
| 2020 | Banjar et al., 2020 [100] | Saudí arabia | 15 | B | Lower lip | Soft | No | NA | 1.2 × 0.8 × 0.6 cm | H | Surgical excision |
| 2021 | Aragaki et al 2021 [28] | Japan | 55 | M | Tongue | Soft | Gastric Cancer | No abnormalities | 0.6cm | H | Surgical excision |
| 67 | M | Upper lip | Soft | Gastric Cancer | No abnormalities | 0.5 mm | H | Surgical excision | |||
| 2021 | Pisano et al., 2021 [72] | Italy | 11 | G | Lower lip | Soft | No | N/A | 1.5 cm | H | Diode Laser |
| 2023 | Lomelí et al., | México | 32 | F | Hard palate | Soft | No | Alveolar bone loss | 25 x 12 mm | H | Surgical excision |
| 2023 | [66] | México | 42 | F | Gingiva | Soft | No | Alveolar bone loss | 16 x 10 mm | H | Surgical excision |
| 38 | F | Gingiva | 20 x 15 | ||||||||
8. Treatment of oral PG
9. Conclusion
References
- Sarwal P, Lapumnuaypol K. Pyogenic Granuloma. 2021 Nov 21. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. PMID: 32310537.
- Gomes SR, Shakir QJ, Thaker PV, Tavadia JK. Pyogenic granuloma of the gingiva: A misnomer?—A case report and review of literature. J Indian Soc Periodontol. 2013 Jul;17(4):514-9. PMID: 24174735; PMCID: PMC3800418. [CrossRef]
- Kamal R, Dahiya P, Puri A. Oral pyogenic granuloma: Various concepts of etiopathogenesis. J Oral Maxillofac Pathol. 2012 Jan;16(1):79-82. PMID: 22434943; PMCID: PMC3303528. [CrossRef]
- Sharma S, Chandra S, Gupta S, Srivastava S. Heterogeneous conceptualization of etiopathogenesis: Oral pyogenic granulo-ma. Natl J Maxillofac Surg. 2019 Jan-Jun;10(1):3-7. PMID: 31205381; PMCID: PMC6563641. [CrossRef]
- Ghalayani P, Hajisadeghi S, Babadi F. Extragingival pyogenic granuloma associated with medication: Report of an unusual case. Dent Res J (Isfahan). 2014 May;11(3):400-4. PMID: 25097653; PMCID: PMC4119376.
- Parisi E, Glick PH, Glick M. Recurrent intraoral pyogenic granuloma with satellitosis treated with corticosteroids. Oral Dis. 2006 Jan;12(1):70-2. PMID: 16390473. [CrossRef]
- Al-Noaman AS. Pyogenic granuloma: Clinicopathological and treatment scenario. J Indian Soc Periodontol. 2020 May-Jun;24(3):233-236. Epub 2020 May 4. PMID: 32773973; PMCID: PMC7307466. [CrossRef]
- Poncet A, Dor L (1897) Botyromycose humaine. Rev Chir 18: 996.
- Greenberger S, Boscolo E, Adini I, Mulliken JB, Bischoff J: Corticosteroid suppression of VEGF-A in infantile hemangio-ma-derived stem cells. N Engl J Med 2010;362:1005–1013. [CrossRef]
- Jafarzadeh H, Sanatkhani M, Mohtasham N. Oral pyogenic granuloma: A review. J Oral Sci. 2006 Dec;48(4):167-75. PMID: 17220613. [CrossRef]
- Wiener RC, Wiener-Pla R. Literacy, pregnancy and potential oral health changes: The Internet and readability levels. Matern Child Health J. 2014 Apr;18(3):657-62. PMID: 23784613; PMCID: PMC4919661. [CrossRef]
- Montazer Lotf-Elahi MS, Farzinnia G, Jaafari-Ashkavandi Z. Clinicopathological study of 1000 biopsied gingival lesions among dental outpatients: A 22-year retrospective study. BMC Oral Health. 2022 Apr 29;22(1):154. PMID: 35488268; PMCID: PMC9052626. [CrossRef]
- Krishnapillai, R., Punnoose, K., Angadi, P. V., & Koneru, A. (2012). Oral pyogenic granuloma—A review of 215 cases in a South Indian Teaching Hospital, Karnataka, over a period of 20 years. Oral and Maxillofacial Surgery, 16(3), 305–309. [CrossRef]
- Shamim T, Varghese VI, Shameena PM, Sudha S. A retrospective analysis of gingival biopsied lesions in South Indian pop-ulation: 2001-2006. Med Oral Patol Oral Cir Bucal. 2008 Jul 1;13(7):E414-8. PMID: 18587304.
- Nejad ES, BigomTaheri J, Azimi S. Frequency of gingival pregnancy tumor in iran (confirmed by biopsy). J Int Oral Health. 2014 Nov-Dec;6(6):72-6. PMID: 25628488; PMCID: PMC4295460.
- Chamani G,NavvabiN,AbdollahzadehSH.Thefrequency of pregancny tumor in pregnant mothers. Dent J Shiraz Med Sci Univ 2009;10:79-82. [CrossRef]
- Tabatabaei et al., (2014) Frequency of Gingival Pregnancy Tumor in Iran (confirmed by biopsy) Journal of international Oral Health, 6(6):72-76.
- Lee J, Lynde C. Pyogenic granuloma: Pyogenic again? Association between pyogenic granuloma and Bartonella. J Cutan Med Surg. 2001 Nov-Dec;5(6):467-70. PMID: 11907853. [CrossRef]
- KERR DA. Granuloma pyogenicum. Oral Surg Oral Med Oral Pathol. 1951 Feb;4(2):158-76. PMID: 14807485. [CrossRef]
- Bhaskar SN, Jacoway JR. Pyogenic granuloma – Clinical features, incidence, histology, and result of treatment: Report of 242 cases. J Oral Surg 1966;24:391-8.
- Piraccini BM, Bellavista S, Misciali C, Tosti A, de Berker D, Richert B. Periungual and subungual pyogenic granuloma. Br J Dermatol. 2010 Nov;163(5):941-53. PMID: 20545691. [CrossRef]
- Cheney-Peters D, Lund TC. Oral Pyogenic Granuloma After Bone Marrow Transplant in the Pediatric/Adolescent Population: Report of 5 Cases. J Pediatr Hematol Oncol. 2016 Oct;38(7):570-3. PMID: 27271813. [CrossRef]
- Al-Mohaya M, Treister N, Al-Khadra O; et al. Calcineurin inhibitor-associated oral inflammatory polyps after trans- planta-tion. J Oral Pathol Med. 2007;36:570–574. [CrossRef]
- Palmero ML, Pope E. Eruptive pyogenic granulomas developing after drug hypersensitivity reaction. J Am Acad Dermatol. 2009 May;60(5):855-7. Epub 2009 Feb 10. PMID: 19211171. [CrossRef]
- Gutierrez PA. An unusual case of multiple oral pyogenic granuloma, ¿associated with treatment with levothyroxin?. Rev Estomatol Herediana 2020 Oct-Dic;30(4):294-301.
- Tosti A, Piraccini BM, D’Antuono A, Marzaduri S, Bettoli V. Paronychia associated with antiretroviral therapy. Br J Derma-tol. 1999 Jun;140(6):1165-8. PMID: 10354091. [CrossRef]
- Leung AKC, Barankin B, Hon KL (2014) Pyogenic Granuloma. Clinics Mother Child Health 11: e106. [CrossRef]
- Aragaki T, Tomomatsu N, Michi Y, Hosaka H, Fukai Y, Iijima M, Yoda T. Ramucirumab-related Oral Pyogenic Granuloma: A Report of Two Cases. Intern Med. 2021 Aug 15;60(16):2601-2605. Epub 2021 Mar 8. PMID: 33678742; PMCID: PMC8429295. [CrossRef]
- Uzel MI, Kantarci A, Hong HH; et al. Connective tissue growth factor in drug-induced gingival overgrowth. J Perio- dontol. 2001;72:921–931. [CrossRef]
- Baran R. Etretinate and the nails (study of 130 cases) possible mechanisms of some side-effects. Clin Exp Dermatol 1986; 11:148– 52. [CrossRef]
- Williams LH, Fleckman P. Painless periungual pyogenic granulo- mata associated with reverse transcriptase inhibitor therapy in a patient with human immunodeficiency virus infection. Br J Dermatol 2007; 156:163–4. [CrossRef]
- Silva de Araujo Figueiredo C, Gonçalves Carvalho Rosalem C, Costa Cantanhede AL, Abreu Fonseca Thomaz ÉB, Fontoura Nogueira da Cruz MC. Systemic alterations and their oral manifestations in pregnant women. J Obstet Gynaecol Res. 2017 Jan;43(1):16-22. PMID: 28074549. [CrossRef]
- Figuero-Ruiz E, Prieto Prieto I, Bascones-Martínez A. Cambios hormonales asociados al embarazo. ón gin-givo-periodontal. Av Periodon Implantol. 2006; 18, 2: 101-113. First Published: 09 June 2006. Cryotherapy in the treatment of pyogenic granuloma.
- Cardoso JA, Spanemberg JC, Cherubini K, Figueiredo MA, Salum FG. Oral granuloma gravidarum: A retrospective study of 41 cases in Southern Brazil. J Appl Oral Sci. 2013;21(3):215-8. PMID: 23857656; PMCID: PMC3881906. [CrossRef]
- Jang H, Patoine A, Wu TT, Castillo DA, Xiao J. Oral microflora and pregnancy: A systematic review and meta-analysis. Sci Rep. 2021 Aug 19;11(1):16870. PMID: 34413437; PMCID: PMC8377136. [CrossRef]
- Borgo PV, Rodrigues VA, Feitosa AC, Xavier KC, Avila-Campos MJ. Association between periodontal condition and sub-gingival microbiota in women during pregnancy: A longitudinal study. J Appl Oral Sci. 2014 Nov-Dec;22(6):528-33. PMID: 25591021; PMCID: PMC4307767. [CrossRef]
- Ojanotko-Harri AO, Harri MP, Hurttia HM, Sewon LA. Altered tissue metabolism of progesterone in pregnancy gingivitis and granuloma. J Clin Periodontol 1991;18:262-6. [CrossRef]
- Yuan K, Jin YT, Lin MT. Expression of Tie-2, angiopoietin-1, angiopoietin-2, ephrinB2 and EphB4 in pyogenic granuloma of human gingiva implicates their roles in inflammatory angiogenesis. J Periodontal Res. 2000 Jun;35(3):165-71. PMID: 10929871. [CrossRef]
- Yuan K, Jin YT, Lin MT. The detection and comparison of angiogenesis-associated factors in pyogenic granuloma by im-munohistochemistry. J Periodontol. 2000 May;71(5):701-9. PMID: 10872949.Author 1, A.B. Title of Thesis. Level of Thesis, Degree-Granting University, Location of University, Date of Completion. [CrossRef]
- Shetty SJ, Hallikeri K, Anehosur V, Desai A. An aggressive pyogenic granuloma masquerading as a vascular neoplasm. J Indian Soc Periodontol. 2020 May-Jun;24(3):276-279. Epub 2020 Jan 27. PMID: 32773980; PMCID: PMC7307463. [CrossRef]
- Nelimarkka L, Salminen H, Kuopio T, Nikkari S, Ekfors T, Laine J, Pelliniemi L, Järveläinen H. Decorin is produced by capillary endothelial cells in inflammation-associated angiogenesis. Am J Pathol. 2001 Feb;158(2):345-53. PMID: 11159170; PMCID: PMC1850307. [CrossRef]
- Blackwell MG, Itinteang T, Chibnall AM, Davis PF, Tan ST. Expression of embryonic stem cell markers in pyogenic granu-loma. J Cutan Pathol. 2016 Dec;43(12):1096-1101. Epub 2016 Sep 15. PMID: 27509392. [CrossRef]
- Pereira TDSF, de Amorim LSD, Pereira NB, Vitório JG, Duarte-Andrade FF, Guimarães LM, Diniz MG, Gomes CC, Gomez RS. Oral pyogenic granulomas show MAPK/ERK signaling pathway activation, which occurs independently of BRAF, KRAS, HRAS, NRAS, GNA11, and GNA14 mutations. J Oral Pathol Med. 2019 Nov;48(10):906-910. Epub 2019 Aug 4. PMID: 31310691. [CrossRef]
- Yao T, Nagai E, Utsunomiya T, Tsuneyoshi M. An intestinal.
- Sharma, S., Chandra, S., Gupta, S., & Srivastava, S. (2019). Heterogeneous conceptualization of etiopathogenesis: Oral pyo-genic granuloma. National journal of maxillofacial surgery, 10(1), 3–7. [CrossRef]
- Akyol MU, Yalçiner EG, Doğan AI. Pyogenic granuloma (lobular capillary hemangioma) of the tongue. Int J Pediatr Otorhinolaryngol. 2001 May 11;58(3):239-41. PMID: 11335013. [CrossRef]
- Nejad ES, BigomTaheri J, Azimi S. Frequency of gingival pregnancy tumor in Iran (confirmed by biopsy). J Int Oral Health. 2014 Nov-Dec;6(6):72-6.
- Rosa G., Cartagena C., Andrea L, & La Torre C.,(2017). Oral pyogenic granuloma diagnosis and treatment: A series of cases. Revista odontológica mexicana, 21(4), 253-261. Recuperado en 04 de septiembre de 2023, de http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1870-199X2017000400253&lng=es&tlng=en.
- Parajuli R, Maharjan S. Unusual presentation of oral pyogenic granulomas: A review of two cases. Clin Case Rep. 2018 Feb 27;6(4):690-693. PMID: 29636941; PMCID: PMC5889261. [CrossRef]
- Panjwani S., Issrani R., Keluskar V., Naik Z. (2012). An unusual presentation of pyogenic granuloma, Indian Journal of Dentistry, Volume 3, Number 3; pp. 178e181. [CrossRef]
- Mubeen K., Vijayalakshmi K. R. and Abhishek R. P., (2011). Oral pyogenic granuloma with mandible involvement: An unusual presentation, Journal of Dentistry and Oral Hygiene Vol. 3(1), pp.6- 9, 2141-2472.
- Mastammanavar, D., Hunasgi, S., Koneru, A., Vanishree, M.R., Surekha, R., & Vardendra, M. (2014). Aggressive Pyogenic Granuloma: A Case Report. International Journal of Oral and Maxillofacial Pathology, 5, 29-32.
- Gonçales ES, Damante JH, Fischer Rubira CM, Taveira LA. Pyogenic granuloma on the upper lip: An unusual location. J Appl Oral Sci. 2010 Sep-Oct;18(5):538-41. PMID: 21085814; PMCID: PMC4246389. [CrossRef]
- Fekrazad R, Nokhbatolfoghahaei H, Khoei F, Kalhori KA. Pyogenic Granuloma: Surgical Treatment with Er:YAG Laser. J Lasers Med Sci. 2014 Fall;5(4):199-205. PMID: 25653822; PMCID: PMC4281984.
- Ganesan A, Kumar N G, Azariah E, Asokan G S. Oral pyogenic granuloma: A case report and a comprehensive review. SRM J Res Dent Sci [serial online] 2015 [cited 2023 Sep 3];6:257-60. Available from: https://www.srmjrds.in/text.asp?2015/6/4/257/170284.
- Aguilo L. Pyogenic granuloma subsequent to injury of a primary tooth. A case report. Int J Paediatr Dent. 2002 Nov;12(6):438-41. PMID: 12452987. [CrossRef]
- Panseriya BJ, Hungund S. Pyogenic granuloma associated with periodontal abscess and bone loss—A rare case report. Contemp Clin Dent. 2011 Jul;2(3):240-4. PMID: 22090773; PMCID: PMC3214537. [CrossRef]
- Deshmukh J. Kulkarni VK., Katti G., Deshpande S. (2013). Pyogenic Granuloma, An Unusual Presentation In Pediatric Patient—A Case Report.. Indian Journal of Dental Sciences. 5. 90-93.
- Asha V, Dhanya M, Patil BA, Revanna G. An unusual presentation of pyogenic granuloma of the lower lip. Contemp Clin Dent. 2014 Oct;5(4):524-6. PMID: 25395771; PMCID: PMC4229764. [CrossRef]
- Behl AB., Bali V., Bali R. Pyogenic Granuloma—A case report and review of literature, international journal of stomatology & occlusion medicine, 2011. 4:166–170. [CrossRef]
- Gondivkar SM, Gadbail A, Chole R. Oral pregnancy tumor. Contemp Clin Dent. 2010 Jul;1(3):190-2. PMID: 22114415; PMCID: PMC3220110. [CrossRef]
- Verma PK, Srivastava R, Baranwal HC, Chaturvedi TP, Gautam A, Singh A. “Pyogenic granuloma–Hyperplastic lesion of the gingiva: Case reports”. Open Dent J. 2012;6:153-6. Epub 2012 Oct 5. Erratum in: Open Dent J. 2012;6:188. PMID: 23091574; PMCID: PMC3474946. [CrossRef]
- Tripathi AK, Upadhaya V, Kumar V, Saimbi CS. Hyperactive lesions of gingiva associated with severe alveolar bone loss: A rare finding. Contemp Clin Dent. 2015 Apr-Jun;6(2):223-5. PMID: 26097359; PMCID: PMC4456746. [CrossRef]
- Shenoy SS, Dinkar AD. Pyogenic granuloma associated with bone loss in an eight year old child: A case report. J Indian Soc Pedod Prev Dent. 2006 Dec;24(4):201-3. PMID: 17183185. [CrossRef]
- Shivaswamy S, Siddiqui N, Jain SA, Koshy A, Tambwekar S, Shankar A. A rare case of generalized pyogenic granuloma: A case report. Quintessence Int. 2011 Jun;42(6):493-9. PMID: 21519587.
- Lomelí Martínez SM, Bocanegra Morando D, Mercado González AE, Gómez Sandoval JR. Unusual clinical presentation of oral pyogenic granuloma with severe alveolar bone loss: A case report and review of literature. World J Clin Cases. 2023 Jun 6;11(16):3907-3914. PMID: 37383141; PMCID: PMC10294161. [CrossRef]
- Ma Y, Nicolet J. Specificity models in MAPK cascade signaling. FEBS Open Bio. 2023 Jul;13(7):1177-1192. Epub 2023 Jun 11. PMID: 37157227; PMCID: PMC10315774. [CrossRef]
- Rodríguez-Carballo E, Gámez B, Ventura F. p38 MAPK Signaling in Osteoblast Differentiation. Front Cell Dev Biol. 2016 May 6;4:40. PMID: 27200351; PMCID: PMC4858538. [CrossRef]
- Chandrashekar B. Minimally invasive approach to eliminate pyogenic granuloma: A case report. Case Rep Dent. 2012;2012:909780. Epub 2012 Jan 26. PMID: 22567459; PMCID: PMC3335552. [CrossRef]
- Asnaashari M, Mehdipour M, MoradiAbbasabadi F, Azari-Marhabi S. Expedited removal of pyogenic granuloma by diode laser in a pediatric patient. J Lasers Med Sci. 2015 Winter;6(1):40-4. PMID: 25699167; PMCID: PMC4329141.
- Al-Mohaya MA, Al-Malik AM. Excision of oral pyogenic granuloma in a diabetic patient with 940nm diode laser. Saudi Med J. 2016 Dec;37(12):1395-1400. PMID: 27874157; PMCID: PMC5303780. [CrossRef]
- Pisano M, Sammartino P, Di Vittorio L, Iandolo A, Caggiano M, Roghi M, Bizzoca ME, Lo Muzio L. Use of Diode Laser for Surgical Removal of Pyogenic Granuloma of the Lower Lip in a Pediatric Patient: A Case Report. Am J Case Rep. 2021 Jun 19;22:e929690. PMID: 34146391; PMCID: PMC8218884. [CrossRef]
- Parisi E, Glick PH, Glick M: Recurrent intraoral pyogenic granuloma with satellitosis treated with corticosteroids. Oral Dis 2006;12:70–72. [CrossRef]
- Bugshan A, Patel H, Garber K, Meiller TF. Alternative Therapeutic Approach in the Treatment of Oral Pyogenic Granuloma. Case Rep Oncol. 2015 Nov 14;8(3):493-7. PMID: 26668570; PMCID: PMC4677718. [CrossRef]
- Mirshams M, Daneshpazhooh M, Mirshekari A, Taheri A, Mansoori P, Hekmat S. Cryotherapy in the treatment of pyogen-ic granuloma. J Eur Acad Dermatol Venereol. 2006 Aug;20(7):788-90. PMID: 16898898. [CrossRef]
- Moon SE, Hwang EJ, Cho KH. Treatment of pyogenic granuloma by sodium tetradecyl sulfate sclerotherapy. Arch Derma-tol. 2005 May;141(5):644-6. PMID: 15897398. [CrossRef]
- Canivell-Zabaleta M, Martin-Lozano G, Olmos-Juarez E, Fontillon-Alberdi M, Infante-Cossio P. Extragingival Pregnancy Pyogenic Granuloma on the Lip. J Craniofac Surg. 2018 Jan;29(1):e49-e50. PMID: 29040143. [CrossRef]
- Sun WL., Lei L., Zhong L., Yu S., Zhou JW., Multiple gingival pregnancy tumors with rapid growth, Journal of Dental Sciences. 2013, 9 (3), 289-293, ISSN 1991-7902. [CrossRef]
- Román-Quesada, N., González-Navarro, B., Izquierdo-Gómez, K. et al. An analysis of the prevalence of peripheral giant cell granuloma and pyogenic granuloma in relation to a dental implant. BMC Oral Health 21, 204 (2021). [CrossRef]
- Baesso RCP, de Lima Jacy Monteiro Barki MC, de Souza Azevedo R, da Costa Fontes KBF, Pereira DL, Tucci R, Pires FR, Picciani BLS. Peripheral giant cell granuloma associated with a dental implant. BMC Oral Health. 2019;19:283. [CrossRef]
- Kejriwal S, Bhandary R, Thomas B. Oral pyogenic granuloma: A case report, Nitte University Journal of Health Science, Vol. 4, No.1, March 2014, ISSN 2249-7110.
- Ghalayani P, Hajisadeghi S, Babadi F. Extragingival pyogenic granuloma associated with medication: Report of an unusual case. Dent Res J 2014; 11(3): 400e404.
- Frumkin N, Nashef R, Shapira L, Wilensky A. Nonsurgical treatment of recurrent gingival pyogenic granuloma: A case report. Quintessence Int 2015; 46(6): 539e544. [CrossRef]
- Patil K, Mahima VG, Lahari K. Extragingival pyogenic granuloma. Indian J Dent Res. 2006 Oct-Dec;17(4):199-202. Erratum in: Indian J Dent Res. 2007 Jan-Mar;18(1):45. Ambika, L [corrected to Lahari, K]. PMID: 17217217. [CrossRef]
- Amirchaghmaghi M, Falaki F, Mohtasham N, Mozafari PM. Extragingival pyogenic granuloma: A case report. Cases J. 2008 Dec 3;1(1):371. PMID: 19055747; PMCID: PMC2614954. [CrossRef]
- Olmedo DG, Paparella ML, Brandizzi D, Cabrini RL. Reactive lesions of peri-implant mucosa associated with titanium dental implants: A report of 2 cases. Int J Oral Maxillofac Surg. 2010 May;39(5):503-7. Epub 2009 Dec 11. PMID: 20005076. [CrossRef]
- Rizwanulla MD, Koirala B., Sharma S., Adhikari L., Pradhan A. (2011). Pyogenic Granuloma: A Case Report. Health Renaissance. 8. [CrossRef]
- Penseriya et al. (2011). Pyogenic granuloma associated with periodontal abscess and bone loss—A rare case report, Con-temporary Clinical Dentistry, 2(3): 240–244. [CrossRef]
- Ravi V, Jacob M, Sivakumar A, Saravanan S, Priya K. Pyogenic granuloma of labial mucosa: A misnomer in an anomolous site. J Pharm Bioallied Sci. 2012 Aug;4(Suppl 2):S194-6. PMID: 23066251; PMCID: PMC3467924. [CrossRef]
- Piscoya MDB de V., Ximenes, RA de A., Silva GM., Jamelli SR., Coutinho SB. (2012). Periodontitis-associated risk factors in pregnant women. Clinics, 67(1), 27–33. [CrossRef]
- Adusumilli S, Yalamanchili PS, Manthena S. Pyogenic granuloma near the midline of the oral cavity: A series of case reports. J Indian Soc Periodontol. 2014 Mar;18(2):236-9. PMID: 24872636; PMCID: PMC4033894. [CrossRef]
- Mahabob N, Kumar S, Raja S. Palatal pyogenic granulomaa. J Pharm Bioallied Sci. 2013 Jul;5(Suppl 2):S179-81. PMID: 23956603; PMCID: PMC3740672. [CrossRef]
- Moraes SH., Moraes GF., Durski J., Viero FL., Da Silva Meira DD. And Caron ME. (2013) GRANULOMA PIOGÊNICO: RELATO DE CASO CLÍNICO. Revista Gestão & Saúde, Curitiba, v. 9, n. 2, p.12-19.
- Sangamesh NC., Poornima B., Vidya KCh., Sakri SB. (2013). Extragingival pyogenic granuloma: A rare case report. J Sci Soc, 2013;40:49-51. [CrossRef]
- de Carvalho FK, Pinheiro TN, Arid J, de Queiroz AM, de Rossi A, Nelson-Filho P. Trauma-Induced Giant Pyogenic Granuloma in the Upper Lip. J Dent Child (Chic). 2015 Sep-Dec;82(3):168-70. PMID: 26731254.
- Sachdeva SK. Extragingival Pyogenic Granuloma: An Unusual Clinical Presentation. J Dent (Shiraz). 2015 Sep;16(3 Suppl):282-5. PMID: 26535410; PMCID: PMC4623838.
- Agarwal N, Kumar D, Vaish A, Anand A. A Rare Case of Pyogenic Granuloma with a Natal Tooth. J Clin Diagn Res. 2016 Oct;10(10):ZD28-ZD29. Epub 2016 Oct 1. PMID: 27891487; PMCID: PMC5121825. [CrossRef]
- Marla V, Shrestha A, Goel K, Shrestha S. The Histopathological Spectrum of Pyogenic Granuloma: A Case Series. Case Rep Dent. 2016;2016:1323798. Epub 2016 Jun 12. PMID: 27382492; PMCID: PMC4921146. [CrossRef]
- Poudel P, Chaurasia N, Marla V, Srii R. Pyogenic granuloma of the upper lip: A common lesion in an uncommon location. J Taibah Univ Med Sci. 2018 Dec 4;14(1):95-98. PMCID: PMC6694911. PMID: 31435396. [CrossRef]
- Banjar A, Abdrabuh A, Al-Habshi M, Parambil M, Bastos P, Abed H. Labial pyogenic granuloma related to trauma: A case report and mini-review. Dent Traumatol. 2020 Aug;36(4):446-451. Epub 2020 Jan 9. PMID: 31869498. [CrossRef]
- Gonçales ES, Damante JH, Fischer Rubira CM, Taveira LA. Pyogenic granuloma on the upper lip: An unusual location. J Appl Oral Sci. 2010 Sep-Oct;18(5):538-41. PMID: 21085814; PMCID: PMC4246389. [CrossRef]
- Mubeen K., Vijayalakshmi K. R. and Abhishek R. P. (2011). Oral pyogenic granuloma with mandible involvement: An unusual presentation, Journal of Dentistry and Oral Hygiene Vol. 3(1), pp.6- 9, 2141-2472.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





