1. Introduction
SARS-CoV-2 is the coronavirus responsible for the COVID-19 pandemic, which has caused at least 690 million cases and 6.9 million deaths worldwide [
1]. In contrast with other RNA viruses, this viral family harbors a proof-reading capacity, which limits the error induced by the RNA polymerase during replication. However, due to the enormous replication cycles that this virus has experienced during the pandemic, in addition to a high frequency of recombination and the effect of host editing enzymes, the mutation rate in the SARS-CoV-2 genome has been estimated at around 9.9 × 10−4 to 2.2 × 10−3 [
2,
3]. The high frequency of mutations has allowed the selection of variants with higher fitness, transmission capacity, and particularly evasion to the immunity present in the human host, during the successive waves of infection [
4].
The Receptor Binding Domain (RBD) of SARS-CoV-2, is the functional region of the viral Spike protein (S) involved in cell attachment to target cells, through the ACE2 receptor. S can be divided into two regions: S1, which contains the RBD and S2, which harbors a furin-cleavage site and a hydrophobic domain. Cleavage of the protease-sensitive site leads to exposure of the hydrophobic domain, allowing the fusion of the viral and the endosomal cellular membrane [
5]. S has been the main target of vaccines non involving the whole inactivated virus, but RBD has also been tested for the design of some prototype vaccines [
6]. Neutralizing antibodies operate mainly by preventing the interaction of the RBD with the ACE2 receptor. Mutations on the spike protein may be associated with changes in the RBD affinity to cellular receptor [
7], as well as with cellular tropism. The accumulation of mutations in S, and particularly RBD, can also affect the binding of the neutralizing antibodies produced during immunization (by natural infection or vaccination), allowing new variants to be less sensitive to neutralization, but not totally resistant [
4]. The emergence of variants had a great impact on the efficacy of the vaccines, allowing the variant viruses to infect the host despite the pre-existing immunity, vaccination retaining, however, the ability to prevent the severe clinical presentation of the disease [
8].
Five VOCs of SARS-CoV-2 were recognized since the end of 2020 [
9]. The first VOC described was the Alpha one (original lineage B.1.1.7), which emerged in the UK [
10]. The second VOC identified was the Beta one (original lineage B.1.351), which emerged in South Africa [
11]. The Gamma VOC (original lineage B.1.1.28.2, P.1) was the third designated VOC, originated in Brazil [
12]. In April 2021, emerged in India the Delta VOC (original lineage B.1.617.2), [
13,
14]. The last and only at present VOC is the Omicron one (original lineage B.1.1.529), which emerged in South Africa, and harbors a huge number of mutations [
15]. In addition to VOCs, the WHO also classified some lineages as Variant of Interest (VOIs), variants similar to VOC but for which the enhanced transmissibility or immune evasion, were not necessarily confirmed [
9]. Among these VOIs, the two most important ones emerged in South America: Lambda and Mu. The Lambda VOI (original lineage C.37) emerged probably in Peru [
16] and the Mu VOI in Colombia (original lineage B.1.621) [
17].
Monoclonal antibodies are used as passive therapy against COVID-19 [
18]. The production of equine sera against venoms of snakes and scorpions laid the foundations for producing an anti-SARS-CoV-2 equine serum [19-21]. A living systematic review of randomized controlled trials suggests that RBD-specific polyclonal F(ab´)2 fragments of equine antibodies may reduce mortality and serious adverse events, and may reduce clinical worsening, at least before widespread vaccination against this virus [
22]. Repeated immunizations were undertaken to produce hyperimmune FAb2 preparations with high titers of antibodies against RBD [
19]. The aim of this study was to evaluate neutralizing activity of this hyperimmune serum against different variants of SARS-CoV-2.
2. Materials and Methods
2.1. Equine anti-RBD hyperimmune preparation
The production and purification of a F(ab’)2 preparation anti-RBD serum was previously described [
19]. Briefly, 3 horses were immunized several times with RBD antigen (Acro Biosystem, South Croydon, United Kingdom). Three different F(ab’)2 preparations, product of the mixture of the 3 horse sera, corresponding to different time points, were evaluated at different dilutions (
Table 1).
2.2. Viral strains and cells
Vero-E6 cells were kindly donated by the “Instituto Nacional de Higiene Rafael Rangel”, Caracas, Venezuela, adapted and maintained in RPMI 1640 medium supplemented with 10% FBS and antibiotic/antimycotic. Cells were passage every 3-4 days based on the monolayer confluence. The viruses were isolated in a BSL3 facility, from nasopharyngeal swabs of patients positive (Ct below 25?) between July 2020 and January 2022. For infection, the Vero-E6 cells were incubated with 0.22 μm filtered transport medium of positive samples for one hour. All the cultures were daily monitored and the new virus production was confirmed by cytopathic effect, then the supernatant was tested by RTq-PCR (Sansure Biotech Inc., Changsha, Hunan Province, China). For variant assignment, the complete genome sequence of each strain was performed by NGS, as previously described [
23]. The different strains were titrated by serial dilution, in order to use the same virus titer in each assay.
2.3. Neutralization assay
Plates of 24 wells were seeded with 200,000 cells/well and incubated overnight. The cells were infected with a mixture of each virus previously incubated at 37°C for 30 minutes with different dilutions of anti-RBD F(ab’)2 preparation. Then a volume of this mix, calculated to produce approximately 80 plaques, was inoculated to the cells in triplicate wells. The infection process was performed for one hour at 37°C in a 5% CO2 atmosphere, and the cells were washed twice with PBS to retire all the non-internalized viruses Then the cells were overlayed with 0.5% carboxymethyl cellulose in culture medium [
24]. The plates were incubated for 72h, fixed with 4% formaldehyde and stained with crystal violet to count the number of lytic plaques under each condition. Neutralization titers were determined by logistic regression as the final dilution producing a 50% reduction of the average number of plaques of the controls (GraphPad Software Inc., La Jolla, CA, USA).
3. Results
In order to test the neutralization ability of the F(ab’)2 preparation obtained from equine hyperimmune sera, several lineages of SARS-CoV-2 were successfully cultivated and used for neutralization assays (
Table 2). The different isolated viruses were: B.1.1.33 (equivalent to the ancestral virus), P.1 (Gamma VOC), AY122 (Delta VOC), B.1.621 (Mu VOC) and BA.1.1 (Omicron VOC). It is interesting to note that for all the lineages except Omicron, only one isolate was necessary to succeed in obtaining the viral culture used for the assays. For the Omicron lineage, 3 isolates were tested to finally obtain the viral culture. The isolated successfully cultivated was from a non-vaccinated patient.
Figure 1 shows the amino acid sequence of each of the lineages used in this study, together with the sequence used for producing the RBD immunogen. Three variants exhibit mutations in amino acid 484, associated with an important reduction of antibody binding to the RBD: The P1, Mu (E484K) and Omicron (E484A). In addition, this last variant harbors several other mutations involved, at different intensity, in the reduction of antibody binding: of note the Y505H mutations, which has also been associated with an important reduction of antibody binding (
Figure 2). The amino acid sequences of P1 and Mu variants are very similar: the main differences are the presence of the mutation K417T in the P1 variant and of R346K in the Mu one. Both mutations have been associated with a low intensity reduction of polyclonal antibody binding (
Figure 1).
F(ab’)2 preparations from sera sampled at different time points (
Table 1), were evaluated.
Table 3 shows the titer of each preparation able to reduce in 50% the number of lytic plaques against each of the lineages tested (IC50). No neutralization was observed at 1/50 dilution for an unrelated anti-ophidic F(ab’)2 preparation. No neutralization was observed at 1/50 dilution for F(ab’)2 preparation 001: this dilution was able to reduce only between 30 and 45% the number of lytic plaques of the different strains, not reaching 50% reduction for any of them. The 1/200 dilution of the F(ab’)2 preparation 002 led to a reduction in lytic plaques between 52 and 76% for the different variants. The enrichment in neutralizing antibodies was notorious for the F(ab’)2 preparation 003 (
Table 3). This F(ab’)2 preparation exhibited a high titer of neutralizing antibodies against the ancestral-like strain (1/18,528). A reduction in the titer of the F(ab’)2 preparation was observed against the different variants tested. The highest reduction was observed for the Omicron VOC (4.7 fold), followed by the Mu VOI (2.6), Delta VOC (1.8 fold) and Gamma VOC (1.5). Even if a progressive reduction in the neutralizing antibodies titer against the different variants evaluated was observed, the serum still exhibited a significant neutralizing titer, against the Mu VOI and the Omicron VOC, the strains evaluated most resistant to neutralization.
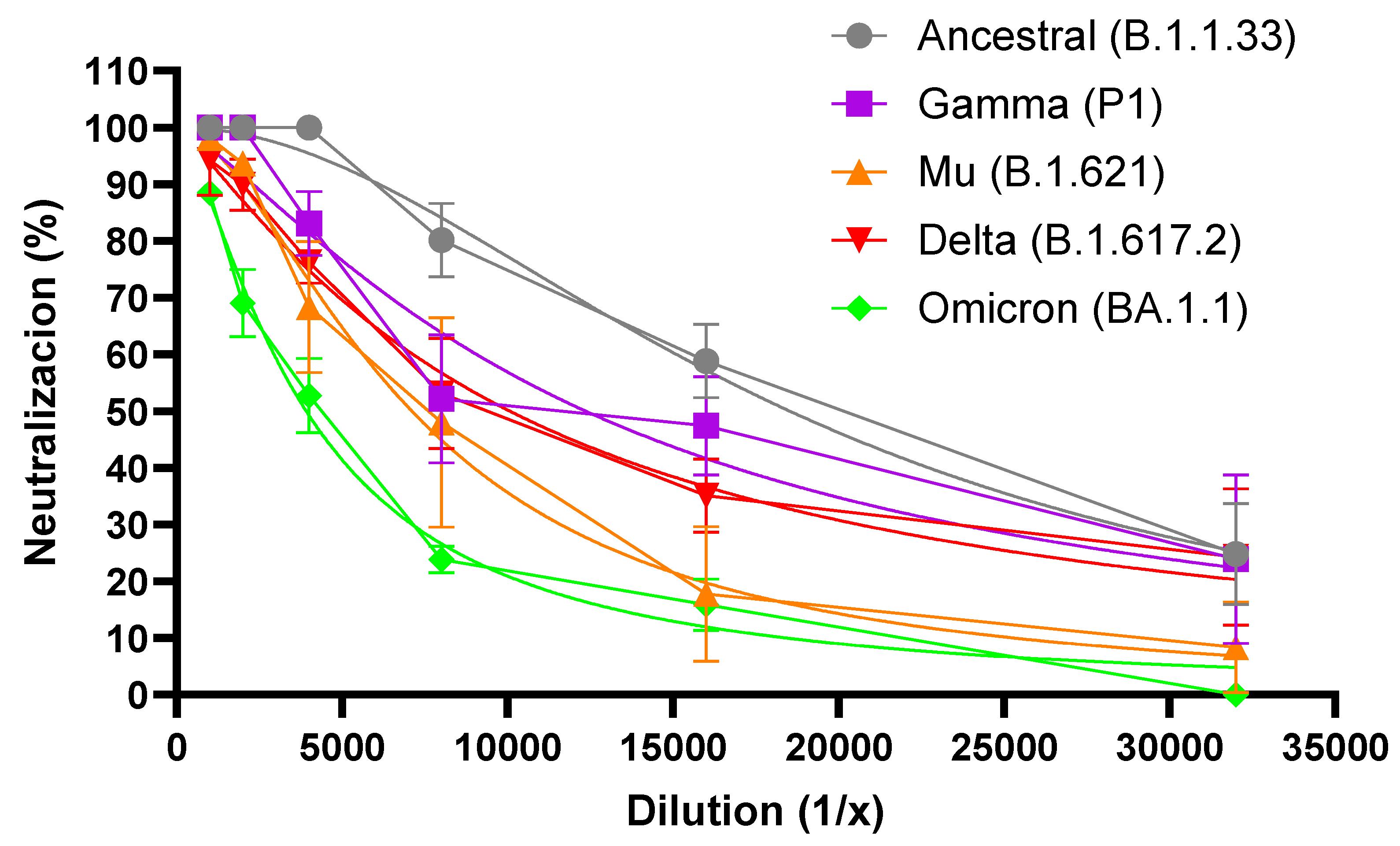
Figure 2 shows the percentage of neutralization for each dilution of the F(ab’)2 preparation 003 against each variant. A reduction in the neutralizing capacity of the F(ab’)2 preparation 003 was observed at every dilution for each variant, although not at the same intensity. The highest reduction in the percentage of neutralization was observed for the Omicron variant, followed by the Mu, Delta, and P1 ones (
Figure 2).
4. Discussion
The knowledge accumulated since 2003 with the emergence of SARS-CoV, stressed the importance of the RBD in triggering the production of strong neutralizing antibodies [
26]. A study conducted on 647 patients infected with SARS-CoV-2 indicated that around 90% of the neutralizing antibodies target the RBD region. The immunodominance of the RBD could be associated with its low level of glycosylation and its higher accessibility compared to the rest of S [
27]. Nevertheless, it is known that other regions of S contribute to induce a protective immune response against SARS-CoV-2, as the N-terminal region of S1 [
28,
29].
The equine F(ab’)2 preparations analyzed in this study were obtained with immunization with the RBD of SARS-CoV-2. Although the animals might have been exposed previously to equine coronavirus [
19], the only source of the neutralizing antibodies found in the F(ab’)2 preparation should be the commercial RBD used as immunogen. This allows for evaluating the immunogenicity of this region in inducing neutralizing antibodies against SARS-CoV-2, out of the context of the whole S protein, and eliminating the participation of other regions of this protein. Although a high titer of antibodies against RBD was detected by ELISA after 3-5 immunizations, more than 1/24,000 [
19], this high titer was not accompanied by a high titer of neutralizing antibodies in the first F(ab’)2 preparations. A great number of immunizations, and a higher dose of the immunogen, were needed to induce a significant level of neutralizing antibodies, as shown by the absence or a minimal neutralizing activity of F(ab’)2 preparations 001 and 002, respectively. In contrast, after 15 immunizations with higher doses of RBD (
Table 1), the neutralization titer of the F(ab’)2 preparation 003, was quite important.
Since the end of 2020 until now, the waves of the SARS-Cov2 epidemic have been characterized by the emergence of VOCs, generally disseminating all around the world. Each VOC showed different mutation patterns in different regions of the genome: many of them occurred in the RBD and precisely these mutations are the ones that can confer increased infectivity to the viruses, increase in the receptor binding process and evasion of neutralizing antibodies [
30,
31]. In this study, we tested the neutralizing ability of the F(ab’)2 preparations against 4 VOCs and one VOI. As expected, a significant reduction in the titer of neutralizing antibodies was found for the F(ab’)2 preparation 003 (the one exhibiting a significant neutralizing activity) against all the variants, when compared to the activity against the ancestral strain. The highest reduction in neutralizing activity was observed against the Omicron variant, in agreement with previously reported characteristic of this VOC to exhibit a great number of mutations associated with immune escape [
15,
25].
The second variant exhibiting the highest reduction in neutralizing activity of the equine preparation was indeed the VOI Mu. It is interesting to note that the RBD sequence of the Mu VOI is very similar to the one of the P1 VOC. Both variants harbor the mutation E484K, which has been associated with a strong reduction in the binding of neutralizing antibodies: the Mu VOI lacks the mutation K417T characteristic of the P1 VOC, but harbors instead the R346K mutation. K417T and R346K mutations have been graded similarly in their contribution to immune evasion (
Figure 1) [
4,
25]. A study of free energy perturbation predicts that the reduction of antibody binding caused by the R346K mutation (Mu) might be even lower than the one caused by the K417N one (present in the Beta VOC, not tested in our study) [
32]. However, the R346K mutation is also present in the strain used in our study, sub-lineage BA.1.1 of the Omicron VOC: this mutation has been associated with immune evasion.
Therapeutic equine polyclonal antibodies from Costa Rica were assessed against several variants, including P1 and Delta VOCs. The authors found similar IC50 concentrations in their preparations, and a similar increase in IC50 when testing P1 and delta VOCs. In their case, the animals were immunized with the S1 region of the S protein, which includes RBD, or with all the structural viral proteins. The authors did not test the Mu VOI, nor the Omicron VOC, the later not yet emerged when this study was conducted [
33]. In a study from Japan, the neutralization titer of sera from vaccinated and convalescent patients was tested against 7 variants: Alpha, Beta, Gamma, Delta, Epsilon, Lambda and Mu, but not Omicron, which emerged after the time of their study. The Mu VOI was the one that elicited the highest reduction in neutralization titer [34], in agreement with the observation of our study.
5. Conclusions
The immunization with several boosters of high doses of SARS-CoV-2 RBD led to the production of a F(ab’)2 preparation with high titers of neutralizing antibodies against the ancestral viral strain. As expected, this titer was reduced against some of the variants, particularly the Omicron VOC and interestingly against the Mu VOI, although the preparation retained significant levels of neutralizing antibodies with all the strains tested.
Author Contributions
Conceptualization, supervision, and project administration, H.R.R.; investigation, M.R., R.C.J., F.H.P., Y.S., C.L.L.; resources, H.R.R., E.M., F.B., M.C., C.B.; writing—original draft preparation, F.H.P., H.R.R.; writing—review and editing, M.R., R.C.J., F.L..; funding acquisition, F.H.P., H.R.R., M.A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministerio del Poder Popular de Ciencia, Tecnología e Innovación of Venezuela.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Bioethical Committee of Instituto Venezolano de Investigaciones Cientificas (IVIC) (CBioEtIVIC-2022-001). This research was also approved by the Bioethics Committee of Hospital Universitario de Caracas, in an Ordinary Meeting via online N° 06 dated December 22, 2020, following the norms obtained from the ARRIVE guidelines and was carried out in accordance with the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines, EU Directive 2010/63/EU for animal experiments. Trained staff organized all the experimental methods relating to the use of live animals.
Informed Consent Statement
Patient consent was waived due to samples, from nasopharyngeal or nasal swabs and positive by qRT-PCR during the routine COVID-19 diagnosis in Venezuela. The identity of the patients was maintained anonymous.
Data Availability Statement
The complete genome sequences have been deposited in the GISAID database.
Acknowledgments
This study was supported by Ministerio del Poder Popular de Ciencia, Tecnología e Innovación of Venezuela. We are also indebted to the Pan American Health Organization (PAHO) for the generous gift of reagents for NGS sequencing.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- COVID - Coronavirus Statistics – Worldometer. Available online: https://www.worldometers.info/coronavirus/ (accessed on 31 July 2023).
- Domingo, E.; García-Crespo, C.; Lobo-Vega, R.; Perales, C. Mutation Rates, Mutation Frequencies, and Proofreading-Repair Activities in RNA Virus Genetics. Viruses 2021, 13, 1882. [Google Scholar] [CrossRef] [PubMed]
- Wei, L. Retrospect of the Two-Year Debate: What Fuels the Evolution of SARS-CoV-2: RNA Editing or Replication Error? Curr Microbiol. 2023, 80, 151. [Google Scholar] [CrossRef] [PubMed]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes J; COVID-19 Genomics UK Consortium; Peacock SJ, Barclay WS; de Silva TI; Towers GJ; et al. SARS-CoV-2 variant biology: immune escape, transmission and fitness. Nat Rev Microbiol. 2023, 21, 162–177.
- Ortega, J.T.; Zambrano, J.L. , Jastrzebska, B.; Liprandi, F.; Rangel, H.R.; Pujol, F.H. Understanding Severe Acute Respiratory Syndrome Coronavirus 2 Replication to Design Efficient Drug Combination Therapies. Intervirology 2020, 63, 2–9. [Google Scholar] [CrossRef]
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat Rev Immunol. 2021, 21, 73–82. [Google Scholar]
- Ortega, J.T.; Pujol, F.H.; Jastrzebska, B.; Rangel, H.R. Mutations in the SARS-CoV-2 spike protein modulate the virus affinity to the human ACE2 receptor, an in silico analysis. EXCLI J. 2021, 20, 585–600. [Google Scholar]
- Miteva, D.; Kitanova, M.; Batselova, H.; Lazova, S.; Chervenkov, L.; Peshevska-Sekulovska, M.; Sekulovski, M.; Gulinac, M.; Vasilev, G.V.; Tomov, L.; et al. The End or a New Era of Development of SARS-CoV-2 Virus: Genetic Variants Responsible for Severe COVID-19 and Clinical Efficacy of the Most Commonly Used Vaccines in Clinical Practice. Vaccines (Basel) 2023, 11, 1181. [Google Scholar] [CrossRef]
- Davies, N.G.; Abbott, S.; Barnard, R.C.; Jarvis, C.I.; Kucharski, A.J.; Munday, J.D.; Pearson, C.A.B.; Russell, T.W.; Tully, D.C.; Washburne, A.D.; et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science 2021, 372, eabg3055. [Google Scholar] [CrossRef]
- Tegally, H.; Wilkinson, E.; Giovanetti, M. , Iranzadeh, A.; Fonseca, V.; Giandhari, J.; Doolabh, D.; Pillay, S.; San, E.J.; Msomi, N.; et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature 2021, 592, 438–443. [Google Scholar] [CrossRef]
- Faria, N.R.; Mellan, T.A.; Whittaker, C.; Claro, I.M.; Candido, D.D.S.; Mishra, S.; Crispim, M.A.E.; Sales, F.C.S.; Hawryluk, I.; McCrone, J.T.; et al. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science 2021, 372, 815–821. [Google Scholar] [CrossRef]
- Dhar, M.S.; Marwal, R.; Vs, R. , Ponnusamy, K.; Jolly, B.; Bhoyar, R.C.; Sardana, V.; Naushin, S.; Rophina, M.; Mellan, T.A.; Mishra, S.; et al. Genomic characterization and epidemiology of an emerging SARS-CoV-2 variant in Delhi, India. Science 2021, 374, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Novelli, G.; Colona, V.L.; Pandolfi, P.P. A focus on the spread of the delta variant of SARS-CoV-2 in India. Indian J Med Res. 2021, 153, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Viana, R.; Moyo, S.; Amoako, D.; Tegally, H.; Scheepers, C.; Althaus, C.L.; Anyaneji, U.J.; Bester, P.A.; Boni, M.F.; Chand, M.; et al. Rapid epidemic expansion of the SARSCoV-2 Omicron variant in Southern Africa. Nature 2022, 603, 679–686. [Google Scholar] [CrossRef] [PubMed]
- WHO. “Tracking SARS-CoV-2 variants.”. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed on 26 June 2022).
- Romero, P.E.; Dávila-Barclay, A.; Salvatierra, G.; González, L.; Cuicapuza, D.; Solís, L.; Marcos-Carbajal, P.; Huancachoque, J.; Maturrano, L.; Tsukayama, P. The Emergence of Sars-CoV-2 Variant Lambda (C.37) in South America. Microbiol Spectr. 2021, 9, e0078921. [Google Scholar] [CrossRef]
- Laiton-Donato, K.; Franco-Muñoz, C.; Álvarez-Díaz, D.A.; Ruiz-Moreno, H.A.; Usme-Ciro, J.A.; Prada, D.A.; Reales-González, J.; Corchuelo, S.; Herrera-Sepúlveda, M.T.; Naizaque, J.; et al. Characterization of the emerging B.1.621 variant of interest of SARS-CoV-2. Infect Genet Evol. 2021, 95, 105038. [Google Scholar] [CrossRef]
- Griffin, D.; McNeil, C.; Okusa, J.; Berrent, D.; Guo, Y.; Daugherty, S.E. Does monoclonal antibody treatment for COVID-19 impact short and long-term outcomes in a large generalisable population? A retrospective cohort study in the USA. BMJ Open 2023, 13, e069247. [Google Scholar] [CrossRef]
- Cepeda, M.V.; Jiménez, J.C.; Pujol, F.H.; Rangel, H.R.; Bello, C.; Cubillan, J.; Serrano, M.L.; Chacón, T.; Saba, A.; López, M.A.; et al. Production of equine sera as a potential immunotherapy against COVID-19. Invest Clin 2021, 62, 3–17. [Google Scholar] [CrossRef]
- Zylberman, V.; Sanguineti, S.; Pontoriero, A.V.; Higa, S.; Cerutti, M.L.; Morrone Seijo, S.M.; Pardo, R. , Muñoz, L.; Acuña Intrieri, M.E.; Alzogaray, V.A.; et al. Development of a hyperimmune equine serum therapy for COVID-19 in Argentina. Medicina (B Aires) 2020, 80, 1–6. [Google Scholar]
- Barbier, M.; Lee, K.S.; Vikharankar, M.S.; Rajpathak, S.N.; Kadam, N.; Wong, T.Y.; Russ, B.P.; Cyphert, H.A.; Miller, O.A.; Rader, N.A.; et al. Passive immunization with equine RBD-specific Fab protects K18-hACE2-mice against Alpha or Beta variants of SARS-CoV-2. Front Immunol. 2022, 13, 948431. [Google Scholar] [CrossRef]
- Kimber, C.; Valk, S.J.; Chai, K.L.; Piechotta, V.; Iannizzi, C.; Monsef, I.; Wood, E.M.; Lamikanra, A.A.; Roberts, D.J.; McQuilten, Z.; et al. Hyperimmune immunoglobulin for people with COVID-19. Cochrane Database Syst Rev. 2023, 1, CD015167. [Google Scholar]
- Jaspe, R.C.; Loureiro, C.L.; Sulbaran, Y.; Moros, Z.C.; D'Angelo, P.; Hidalgo, M.; Rodríguez, L.; Alarcón, V.; Aguilar, M.; Sánchez, D.; et al. Description of a One-Year Succession of Variants of Interest and Concern of SARS-CoV-2 in Venezuela. Viruses 2022, 14, 1378. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.T.; Serrano, M.L.; Suárez, A.; Baptista, J.; Pujol, F.H.; Cavallaro, L.V.; Campos, H.R.; Rangel, H.R. Antiviral activity of flavonoids present in aerial parts of Marcetia taxifolia against Hepatitis B virus, Poliovirus, and Herpes Simplex Virus in vitro. EXCLI J. 2019, 18, 1037–1048. [Google Scholar] [PubMed]
- Wang, W.B.; Ma, Y.B.; Lei, Z.H.; Zhang, X.F.; Li, J.; Li, S.S.; Dong, Z.Y.; Liang, Y.; Li, Q.M.; Su, J.G. Identification of key mutations responsible for the enhancement of receptor-binding affinity and immune escape of SARS-CoV-2 Omicron variant. J Mol Graph Model. 2023, 124, 108540. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhou, Y.; Liu, S.; Kou, Z.; Li, W.; Farzan, M.; Jiang, S. Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: implication for developing subunit vaccine. Biochem Biophys Res Commun. 2004, 324, 773–781. [Google Scholar] [CrossRef]
- Piccoli, L.; Park, Y.J.; Tortorici, M.A.; Czudnochowski, N.; Walls, A.C.; Beltramello, M.; Silacci-Fregni, C.; Pinto, D.; Rosen, L.E.; Bowen, J.E.; et al. Mapping Neutralizing and Immunodominant Sites on the SARS-CoV-2 Spike Receptor-Binding Domain by Structure-Guided High-Resolution Serology. Cell 2020, 183, 1024–1042. [Google Scholar] [CrossRef]
- Chi, X.; Yan, R.; Zhang, J.; Zhang, G.; Zhang, Y.; Hao, M.; Zhang, Z.; Fan, P.; Dong, Y.; Yang, Y.; et al. N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2. Cell. 2021, 184, 2332–2347. [Google Scholar]
- VanBlargan, L.A.; Errico, J.M.; Halfmann, P.J.; Zost, S.J.; Crowe, J.E.Jr.; Purcell, L.A.; Kawaoka, Y.; Corti, D.; Fremont, D.H.; Diamond, M.S. An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat Med. 2022, 28, 490–495. [Google Scholar] [CrossRef]
- Willett, B.J.; Grove, J.; MacLean, O.A.; Wilkie, C.; De Lorenzo, G.; Furnon, W.; Cantoni, D.; Scott, S.; Logan, N.; Ashraf, S.; et al. SARS-CoV-2 Omicron is an immune escape variant with an altered cell entry pathway. Nat Microbiol. 2022, 7, 1161–1179. [Google Scholar]
- Fratev, F. R346K Mutation in the Mu Variant of SARS-CoV-2 Alters the Interactions with Monoclonal Antibodies from Class 2: A Free Energy Perturbation Study. J Chem Inf Model. 2022, 62, 627–631. [Google Scholar] [CrossRef]
- Moreira-Soto, A.; Arguedas, M.; Brenes, H.; Buján, W.; Corrales-Aguilar, E.; Díaz, C.; Echeverri, A.; Flores-Díaz, M.; Gómez, A.; Hernández, A.; et al. High Efficacy of Therapeutic Equine Hyperimmune Antibodies Against SARS-CoV-2 Variants of Concern. Front Med (Lausanne) 2021, 8, 735853. [Google Scholar] [CrossRef]
- Uriu, K.; Kimura, I.; Shirakawa, K.; Takaori-Kondo, A.; Nakada, T.A.; Kaneda, A.; Nakagawa, S.; Sato, K. Genotype to Phenotype Japan (G2P-Japan) Consortium. Neutralization of the SARS-CoV-2 Mu Variant by Convalescent and Vaccine Serum. N Engl J Med. 2021, 385, 2397–2399. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).