Submitted:
07 September 2023
Posted:
11 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
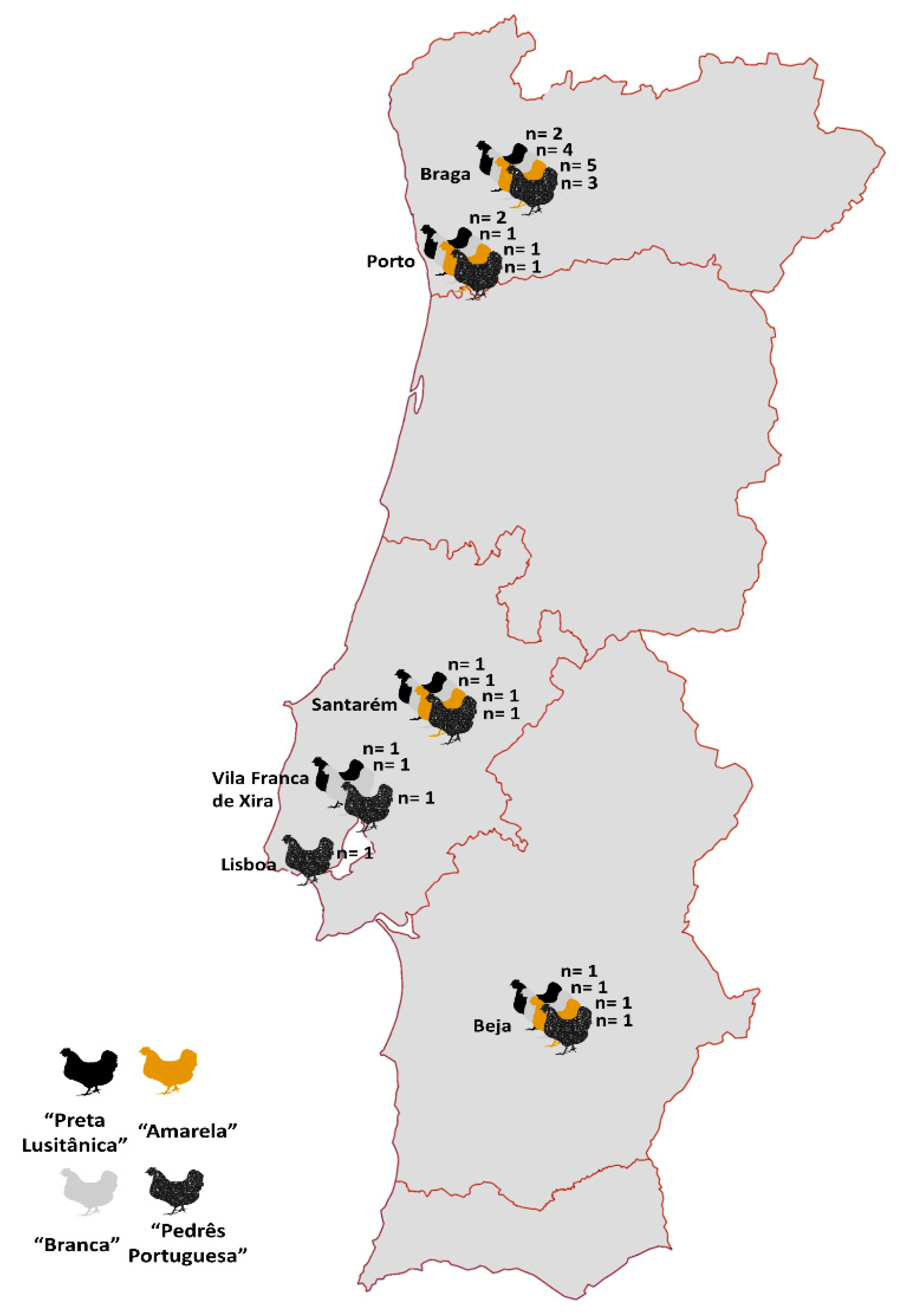
2.1. Sample Size and Distribution
2.2. Sample Collection
2.3. Isolation of Salmonella spp.
2.4. Data Analysis
3. Results
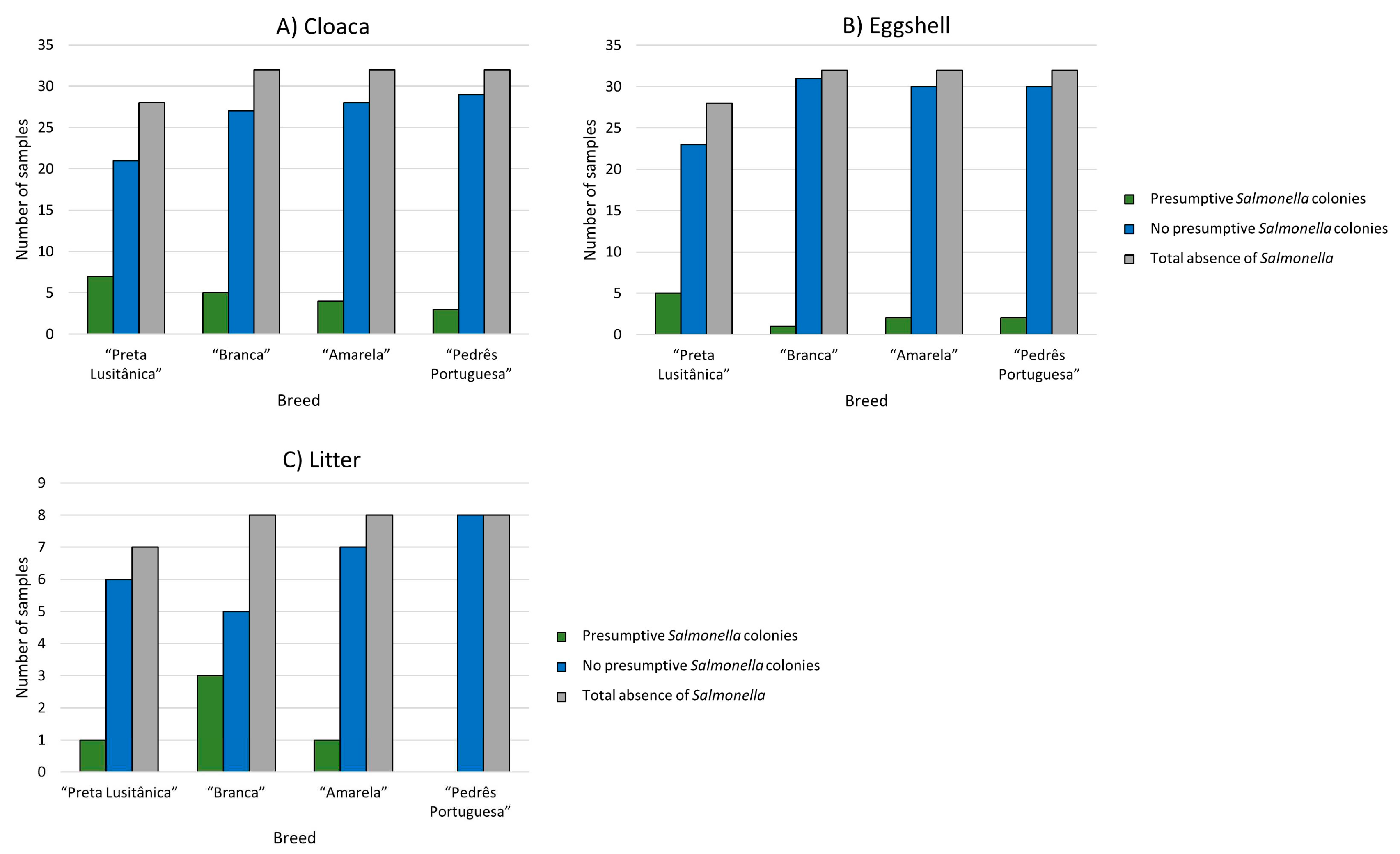
Occurrence of Salmonella spp.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ehuwa, O.; Jaiswal, A.K.; Jaiswal, S. Salmonella, Food Safety and Food Handling Practices. Foods 2021, 10, 907. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.; Ribeiro, V.; Dantas, R.; Brito, N. V. Salmonella in Portuguese autochthonous hens breeds: a One Health concern. Sci. Lett. 2023, 1. [Google Scholar] [CrossRef]
- Brown, E.W.; Bell, R.; Zhang, G.; Timme, R.; Zheng, J.; Hammack, T.S.; Allard, M.W. Salmonella Genomics in Public Health and Food Safety. EcoSal Plus 2021, 9. [Google Scholar] [CrossRef]
- Jibril, A.H.; Okeke, I.N.; Dalsgaard, A.; Kudirkiene, E.; Akinlabi, O.C.; Bello, M.B.; Olsen, J.E. Prevalence and risk factors of Salmonella in commercial poultry farms in Nigeria. PLOS ONE 2020, 15, e0238190. [Google Scholar] [CrossRef]
- Louvau, H.; Harris, L.J. Levels and Distribution of Salmonella in Naturally Contaminated Cashews. J. Food Prot. 2023, 86, 100109. [Google Scholar] [CrossRef] [PubMed]
- Munck, N.; Smith, J.; Bates, J.; Glass, K.; Hald, T.; Kirk, M.D. Source Attribution of Salmonella in Macadamia Nuts to Animal and Environmental Reservoirs in Queensland, Australia. Foodborne Pathog. Dis. 2020, 17, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, C.; Stephan, R. Spices and herbs as source of Salmonella-related foodborne diseases. Food Res. Int. 2012, 45, 765–769. [Google Scholar] [CrossRef]
- Humphrey, T. Contamination of egg shell and contents with Salmonella enteritidis: a review. Int. J. Food Microbiol. 1994, 21, 31–40. [Google Scholar] [CrossRef]
- EFSA. Salmonella. Available online: https://www.efsa.europa.eu/en/topics/topic/Salmonella (accessed on August 3, 2023).
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, e07666. [Google Scholar] [CrossRef]
- Buzala, M.; Janicki, B. Review: Effects of different growth rates in broiler breeder and layer hens on some productive traits. Poult. Sci. 2016, 95, 2151–2159. [Google Scholar] [CrossRef]
- Di Rosa, A.R.; Chiofalo, B.; Presti, V.L.; Chiofalo, V.; Liotta, L. Egg Quality from Siciliana and Livorno Italian Autochthonous Chicken Breeds Reared in Organic System. Animals 2020, 10, 864. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.L.; Brewer, M.T.; Rasmussen, M.A.; Carlson, S.A. Identification of heritage chicken breeds with diminished susceptibility to intestinal colonization by multiple antibiotic-resistant Salmonella spp. Livest. Sci. 2015, 182, 34–37. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Fifteenth Regular Session of the Commission on Genetic Resources for Food and Agriculture; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2019; pp. 1–131. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Eleventh Session of the Intergovernmental Technical Working Group on Animal Genetic Resources for Food and Agriculture; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2021; Volume 6. [Google Scholar]
- Carolino, N.; Afonso, F.; Calção, S. Avaliação do estatuto de risco de extinção das raças autóctones portuguesas-PDR2020. Gab. Planeam. Políticas Adm. Geral. 2013, 1, 9. [Google Scholar]
- Gama, L.; Martinez, A.; Ginja, C.; Cañon, J.; Martin-Burriel, I.; Revidatti, M.; Ribeiro, M.; Jordana, J.; Cortes, O.; Sevane, N. (2020). Genetic diversity and structure of Iberoamerican livestock breeds. Advances in Animal Health, Medicine and Production: A Research Portrait of the Centre for Interdisciplinary Research in Animal Health (CIISA), University of Lisbon, Portugal, 2020.
- DGAV and CAP, Raças Autóctones Portuguesas; Direção Geral da Agricultura e Veterinária, D.G.d.A.e.V.a.C.d.A.d. Portugal, Editor. 2021: Lisboa.
- Brito, N. V.; Lopes, J. C.; Ribeiro, V.; Dantas, R.; Leite, J.V. Biometric Characterization of the Portuguese autochthonous hens breeds. Animals, 2021; 11, 498. [Google Scholar] [CrossRef]
- Brito, N.V.; Lopes, J.C.; Ribeiro, V.; Dantas, R.; Leite, J.V. Small Scale Egg Production: The Challenge of Portuguese Autochthonous Chicken Breeds. Agriculture 2021, 11, 818. [Google Scholar] [CrossRef]
- Vaarst, M.; Steenfeldt, S.; Horsted, K. Sustainable development perspectives of poultry production. World’s Poult. Sci. J. 2015, 71, 609–620. [Google Scholar] [CrossRef]
- Zanetti, E.; De Marchi, M.; Dalvit, C.; Cassandro, M. Genetic characterization of local Italian breeds of chickens undergoing in situ conservation. Poult. Sci. 2010, 89, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Franzoni, A.; Gariglio, M.; Castillo, A.; Soglia, D.; Sartore, S.; Buccioni, A.; Mannelli, F.; Cassandro, M.; Cendron, F.; Castellini, C.; et al. Overview of Native Chicken Breeds in Italy: Small Scale Production and Marketing. Animals 2021, 11, 629. [Google Scholar] [CrossRef]
- Ferreira, V.; Cardoso, M.; Magalhães, R.; Maia, R.; Neagu, C.; Dumitraşcu, L.; Nicolau, A.; Teixeira, P. Occurrence of Salmonella spp. in eggs from backyard chicken flocks in Portugal and Romania - Results of a preliminary study. Food Control. 2020, 113, 107180. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Outbreak of Salmonella infections linked to backyard poultry, 2020. Available online: https://www.cdc.gov/salmonella/backyardpoultry-05-20/index.html (accessed on 14 July 2023).
- Costa, L.; Leite, J.V.; Lopes, J.C.; Soares, L.; Arranz, J.J.; Brito, N.V. Genetic characterization of Portuguese autochthonous chicken breeds. In Proceedings of the 8th World Congress on Genetics Applied to Livestock Production, Belo Horizonte, Minas Gerais, Brazil, 13–18 August 2006; pp. 8–10. [Google Scholar]
- ISO 6579-1:2017—Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. [WWW Document], n.d. Available online: https://www.iso.org/standard/56712.html.
- Rama, E.N.; Bailey, M.; Kumar, S.; Leone, C.; Bakker, H.C.D.; Thippareddi, H.; Singh, M. Prevalence and antimicrobial resistance of Salmonella in conventional and no antibiotics ever broiler farms in the United States. Food Control. 2022, 135. [Google Scholar] [CrossRef]
- WHO. WHO estimates of the global burden of foodborne diseases: Foodborne disease burden epidemiology reference group 2007-2015 (No. 9789241565165). WHO Library Cataloguing-in-Publication Data. 2015. Available online: https://www.who.int/publications/i/item/9789241565165.
- Rondoni, A.; Asioli, D.; Millan, E. Consumer behaviour, perceptions, and preferences towards eggs: A review of the literature and discussion of industry implications. Trends Food Sci. Technol. 2020, 106, 391–401. [Google Scholar] [CrossRef]
- Lordelo, M.; Cid, J.; Cordovil, C.M.S.; Alves, S.P.; Bessa, R.J.; Carolino, I. A comparison between the quality of eggs from indigenous chicken breeds and that from commercial layers. Poult. Sci. 2020, 99, 1768–1776. [Google Scholar] [CrossRef] [PubMed]
- Soares, L.C.; Lopes, J.C.; Brito, N.V.; Carvalheira, J. Growth and Carcass Traits of Three Portuguese Autochthonous Chicken Breeds:Amarela, Preta LusitânicaandPedrês Portuguesa. Ital. J. Anim. Sci. 2015, 14. [Google Scholar] [CrossRef]
- Trampel, D.W.; Holder, T.G.; Gast, R.K. Integrated farm management to prevent Salmonella Enteritidis contamination of eggs. J. Appl. Poult. Res. 2014, 23, 353–365. [Google Scholar] [CrossRef]
- Lawrence, J.R.P.; Cudnik, D.; Oladeinde, A. Bacterial Detection and Recovery From Poultry Litter. Front. Microbiol. 2022, 12, 803150. [Google Scholar] [CrossRef]
- Fenollar, A.; Doménech, E.; Ferrús, M.A.; Jiménez-Belenguer, A. Risk Characterization of Antibiotic Resistance in Bacteria Isolated from Backyard, Organic, and Regular Commercial Eggs. J. Food Prot. 2019, 82, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.; Nasef, S.; Erfan, A. Multidrug resistant bacterial pathogens in eggs collected from backyard chickens. Assiut Veter- Med J. 2015, 61, 87–103. [Google Scholar] [CrossRef]
- Samanta, I.; Joardar, S.N.; Das, P.K.; Sar, T.K.; Bandyopadhyay, S.; Dutta, T.K.; Sarkar, U. Prevalence and antibiotic resistance profiles ofSalmonella serotypes isolated from backyard poultry flocks in West Bengal, India. J. Appl. Poult. Res. 2014, 23, 536–545. [Google Scholar] [CrossRef]
- Schoeni, J.L.; Glass, K.A.; McDermott, J.L.; Wong, A.C. Growth and penetration of Salmonella enteritidis, Salmonella heidelberg and Salmonella typhimurium in eggs. Int. J. Food Microbiol. 1995, 24, 385–396. [Google Scholar] [CrossRef]
- Rayan, G.; Galal, A.; Fathi, M.; El-Attar, A. Impact of Layer Breeder Flock Age and Strain on Mechanical and Ultrastructural Properties of Eggshell in Chicken. Int. J. Poult. Sci. 2010, 9, 139–147. [Google Scholar] [CrossRef]
- Samiullah, S.; Roberts, J.; Chousalkar, K. Eggshell color in brown-egg laying hens — a review. Poult. Sci. 2015, 94, 2566–2575. [Google Scholar] [CrossRef]
- Sekeroglu, A.; Sarica, M.; Demir, E.; Ulutas, Z.; Saatci, M.; Omed, H. Effects of Different Housing Systems on Some Performance Traits and Egg Qualities of Laying Hens. J. Anim. Veter- Adv. 2010, 9, 1739–1744. [Google Scholar] [CrossRef]
- Dominguez-Gasca, N.; Muñoz, A.; Rodriguez-Navarro, A.B. Quality assessment of chicken eggshell cuticle by infrared spectroscopy and staining techniques: a comparative study. Br. Poult. Sci. 2017, 58, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shallo Thesmar, H.; Kerr, W. L. Outgrowth of Salmonellae and the physical property of albumen and vitelline membrane as influenced by egg storage conditions. J. Food Prot. 2005, 68, 2553–2558. [Google Scholar] [CrossRef] [PubMed]
- Messens, W.; Grijspeerdt, K.; De Reu, K.; Mertens, K.; Bamelis, F.; Kemps, B.; De Baerdemaeker, J.; Decuypere, E.; Herman, L. Eggshell penetration of various types of hens’ eggs by Salmonella enterica Serovar Enteritidis. J. Food Prot. 2007, 70, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Leleu, S.; Messens, W.; De Reu, K.; De Preter, S.; Herman, L.; Heyndrickx, M.; De Baerdemaeker, J.; Michiels, C.; Bain, M. Effect of Egg Washing on the Cuticle Quality of Brown and White Table Eggs. J. Food Prot. 2011, 74, 1649–1654. [Google Scholar] [CrossRef]
- Ishikawa, S.-I.; Suzuki, K.; Fukuda, E.; Arihara, K.; Yamamoto, Y.; Mukai, T.; Itoh, M. Photodynamic antimicrobial activity of avian eggshell pigments. FEBS Lett. 2009, 584, 770–774. [Google Scholar] [CrossRef]
- Dearborn, D.C.; Page, S.M.; Dainson, M.; Hauber, M.E.; Hanley, D. Eggshells as hosts of bacterial communities: An experimental test of the antimicrobial egg coloration hypothesis. Ecol. Evol. 2017, 7, 9711–9719. [Google Scholar] [CrossRef]
- Mori, H.; Takaya, M.; Nishimura, K.; Goto, T. Breed and feed affect amino acid contents of egg yolk and eggshell color in chickens. Poult. Sci. 2020, 99, 172–178. [Google Scholar] [CrossRef]
- Cardoso, M.J.; Nicolau, A.I.; Borda, D.; Nielsen, L.; Maia, R.L.; Møretrø, T.; Ferreira, V.; Knøchel, S.; Langsrud, S.; Teixeira, P. Salmonella in eggs: From shopping to consumption—A review providing an evidence-based analysis of risk factors. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2716–2741. [Google Scholar] [CrossRef]
- Gast, R.K.; Dittoe, D.K.; Ricke, S.C. Salmonella in eggs and egg-laying chickens: pathways to effective control. Crit. Rev. Microbiol. 2022, 1–25. [Google Scholar] [CrossRef]
- Holt, P.S. Centennial Review: A revisiting of hen welfare and egg safety consequences of mandatory outdoor access for organic egg production. Poult. Sci. 2021, 100, 101436. [Google Scholar] [CrossRef] [PubMed]
- Denagamage, T.; Jayarao, B.; Patterson, P.; Wallner-Pendleton, E.; Kariyawasam, S. Risk Factors Associated WithSalmonellain Laying Hen Farms: Systematic Review of Observational Studies. Avian Dis. 2015, 59, 291–302. [Google Scholar] [CrossRef] [PubMed]
| Breed/Farm | Region | Total number of animals (male, female) | Conditions | ||
|---|---|---|---|---|---|
| Presence of Roosts | Presence of Bedding | Type of bedding | |||
| “Preta Lusitânica” | |||||
| 1 | Porto | 16 (2, 14) | Yes. | Yes | Straw |
| 2 | Braga | 13 (2, 11) | Yes | No | |
| 3 | Porto | 7 (1, 6) | Yes | Yes | Wood |
| 4 | Braga | 16 (1, 15) | Yes | No | |
| 5 | Santarém | 17 (3, 14) | Yes | Yes | Wood |
| 6 | Beja | 11 (1, 10) | No | Yes | Wood |
| 7 | Vila Franca de Xira | 18 (2, 16) | Yes | Yes | Straw |
| “Branca” | |||||
| 1 | Porto | 16 (2, 14) | Yes | Yes | Straw |
| 4 | Braga | 14 (1, 13) | Yes | No | |
| 5 | Santarém | 11 (2, 9) | Yes | Yes | Wood |
| 6 | Beja | 10 (1, 9) | No | Yes | Wood |
| 7 | Vila Franca de Xira | 21 (4, 17) | Yes | Yes | Straw |
| 8 | Braga | 10 (1, 9) | Yes | No | |
| 9 | Braga | 14 (1, 13) | Yes | No | |
| 10 | Braga | 16 (1, 15) | Yes | No | |
| “Amarela” | |||||
| 1 | Porto | 17 (2, 15) | Yes | No | |
| 2 | Braga | 16 (2, 14) | Yes | No | |
| 4 | Braga | 16 (1, 15) | Yes | No | |
| 5 | Santarém | 13 (2, 11) | Yes | Yes | Wood |
| 6 | Beja | 11 (1, 10) | No | Yes | Wood |
| 8 | Braga | 14 (1, 13) | Yes | No | |
| 9 | Braga | 15 (1, 14) | Yes | No | |
| 11 | Braga | 46 (6, 40) | No | Yes | Wood |
| “Pedrês Portuguesa” | |||||
| 1 | Porto | 17 (2, 15) | Yes | Yes | Straw |
| 2 | Braga | 15 (2, 13) | Yes | Yes | Straw |
| 4 | Braga | 20 (2, 18) | Yes | No | |
| 5 | Santarém | 46 (6, 40) | Yes | Yes | Wood |
| 6 | Beja | 11 (1, 10) | No | Yes | Wood |
| 7 | Vila Franca de Xira | 25 (3, 22) | Yes | Yes | Wood |
| 8 | Braga | 16 (1, 15) | Yes | No | |
| 12 | Lisboa | 50 (3, 47) | Yes | Yes | Wood |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





