Submitted:
07 September 2023
Posted:
08 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
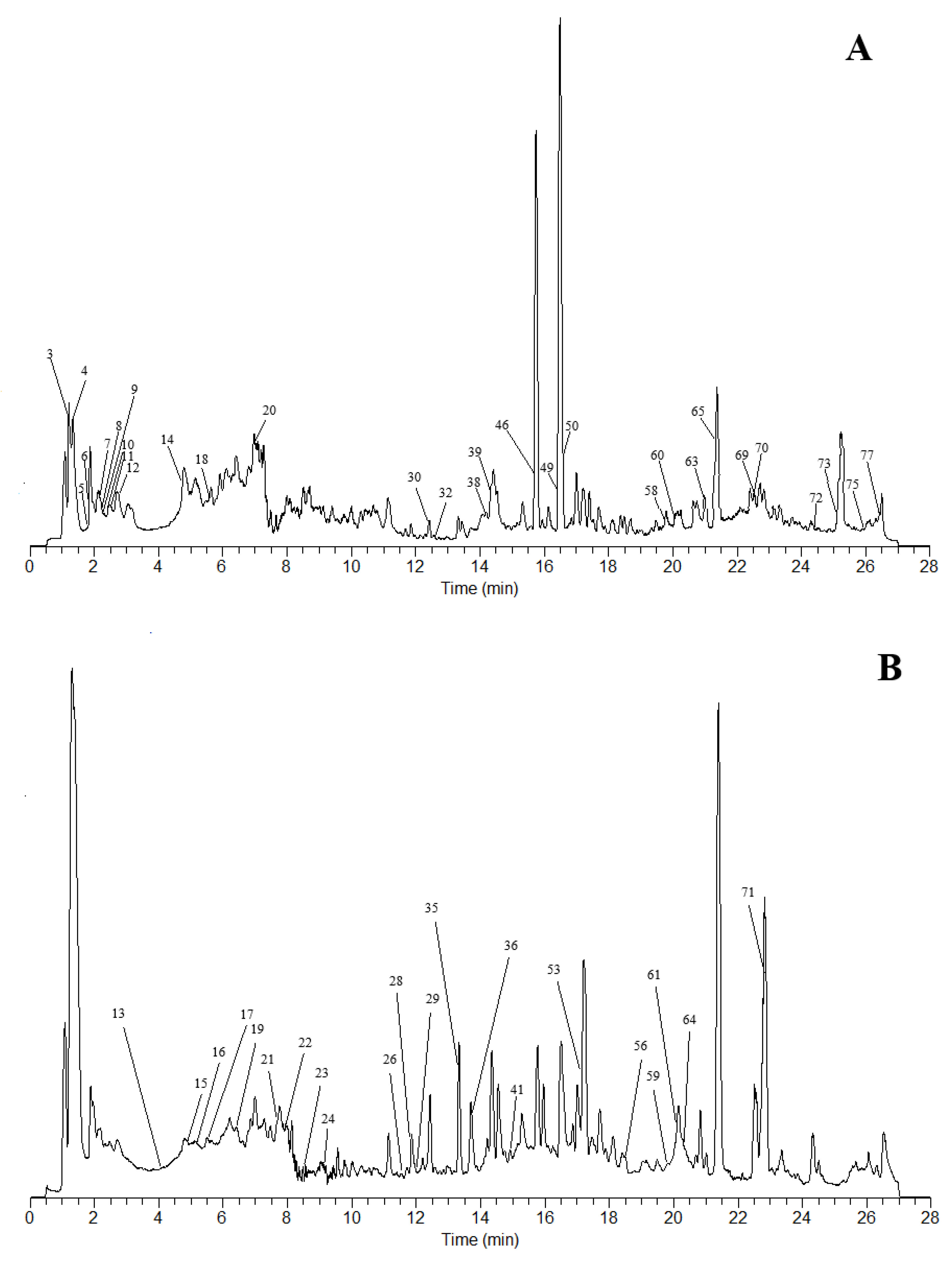
2.1. Chemical composition analysis of Gastrodia Tuder Halimasch Powder
2.2. Analysis of main components of Gastrodia Tuder Halimasch Powder
2.2.1. Identification of terpenoids
2.2.2. Identification of organic acid compounds
2.2.3. Identification of flavonoids
2.2.4. Identification of nucleoside compounds and amide compounds
2.2.5. Identification of amino acid compounds and pyrrolidone derivatives
2.2.6. Identification of steroids and alkaloids
2.2.7. Other classes
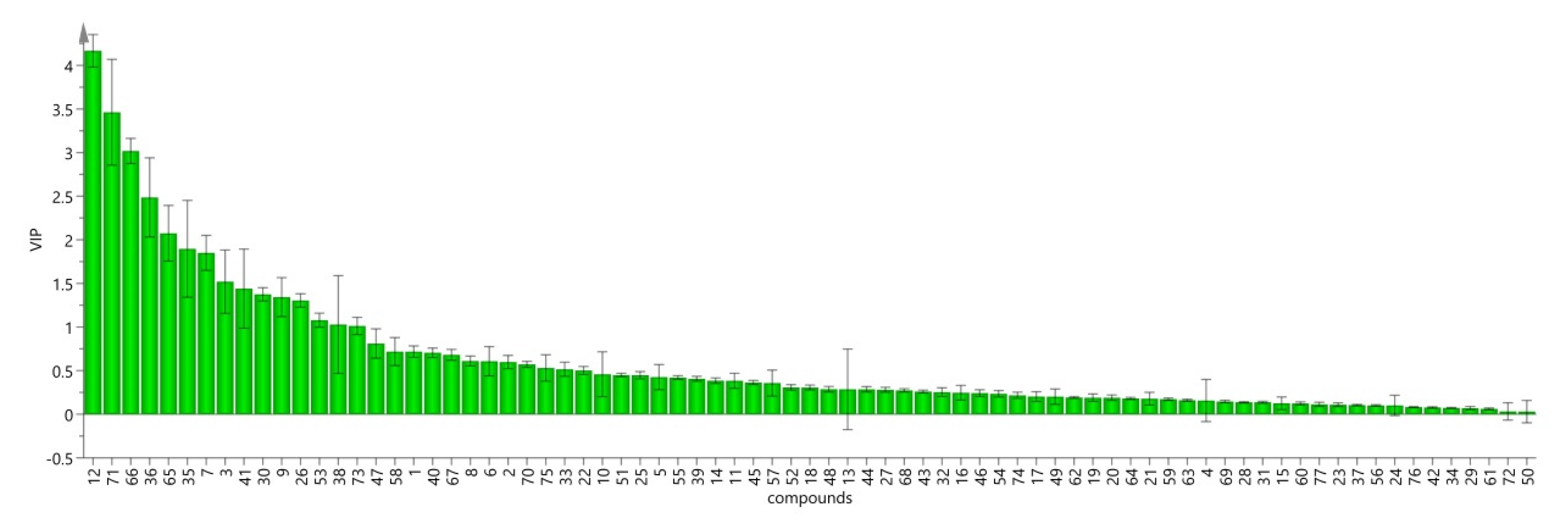
2.2.8. Orthogonal Partial least squares-Discriminant Analysis (OPLS-DA)
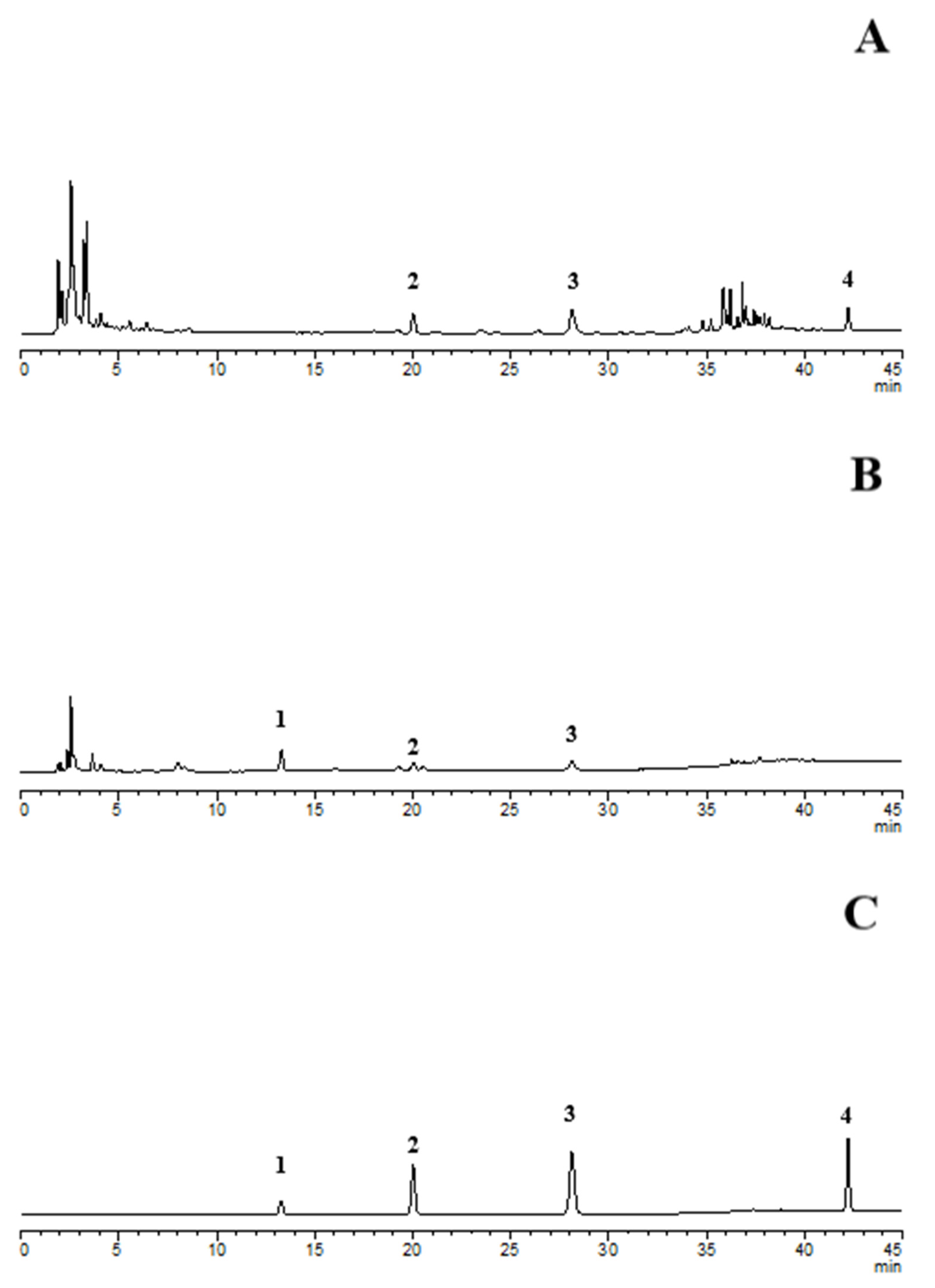
2.3. Establishment of a method for the determination of multi-component content of Gastrodia Tuder Halimasch Powder before and after fermentation
2.3.1. Chromatographic conditions
2.3.2. Preparation of reference solution
2.3.3. Preparation of test product solution
2.3.4. Investigation of linear relationship
2.3.5. Precision test
2.3.6. Stability test
2.3.7. Repeatability test
3.3.8. Sample addition recovery test
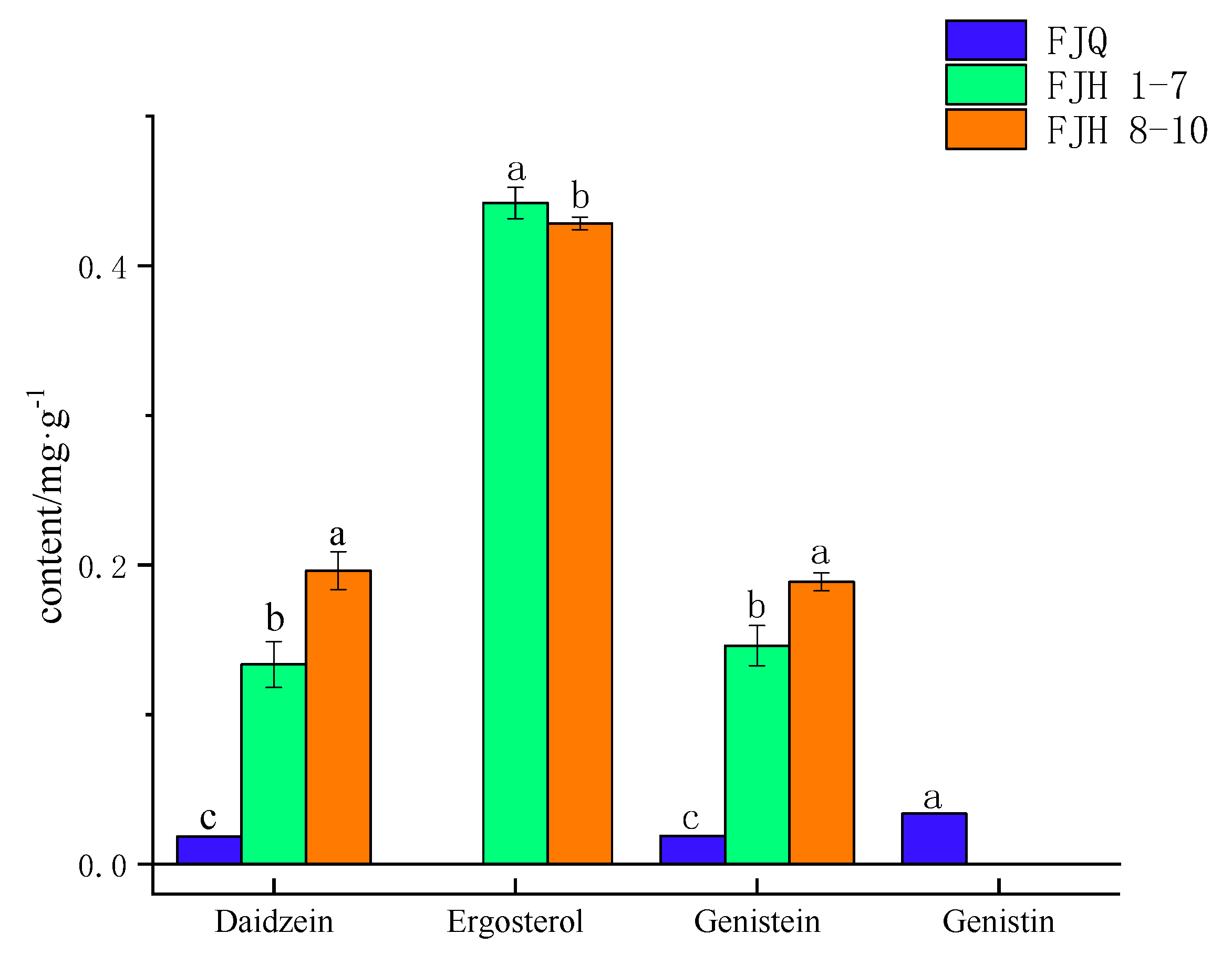
2.4. Sample Determination
3. Concluding Remarks
4. Materials and Methods
4.1. Drugs and reagents
4.2. Preparation of Gandouling tablets and standard solutions
4.3. UHPLC conditions
4.4. Mass spectrometry conditions
4.5. Data processing
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- National Pharmacopoeia Commission (Ed.). National Drug Standard Chemical landmark National Standard thirteenth. Beijing: National Pharmacopoeia Commission. 2002, 99.
- Wang R, ZHANG SY, Mu Q. Research progress on chemical constituents and biological activities of Armillaria. Chinese herbal medicine. 2016, 47, 1992–1999.
- Li IC, Lin TW, Lee TY, et al. Oral administration of Armillaria mellea mycelia promotes non-rapid eye movement and rapid eye movement sleep in rats. Journal of Fungi. 2021, 7, 371. [CrossRef]
- Geng Y, Zhu S, Cheng P, et al. Bioassay-guided fractionation of ethyl acetate extract from Armillaria mellea attenuates inflammatory response in lipopolysaccharide (LPS) stimulated BV-2 microglia. Phytomedicine. 2017, 26, 55–61. [CrossRef]
- An SS. Study on the pharmacological effects of Armillaria polysaccharides on Alzheimer’s disease through anti-apoptosis and anti-oxidation. D Jilin University, 2018.
- Baokui X, Zhang Y. The Prevention and Treatment of Polysaccharide from the Rhizomorph of Armillaria mellea on Diabetic Cataract in Rat. Agricultural Science & Technology. 2014, 15.
- Zhang T, Du Y, Liu X, et al. Study on antidepressant-like effect of protoilludane sesquiterpenoid aromatic esters from Armillaria Mellea. Natural Product Research. 2021, 35, 1042–1045. [CrossRef]
- Li Z, Wang Y, Jiang B, et al. Structure, cytotoxic activity and mechanism of protoilludane sesquiterpene aryl esters from the mycelium of Armillaria mellea[J]. Journal of ethnopharmacology. 2016, 184, 119–127. [CrossRef]
- Dorfer M, Heine D, König S, et al. Melleolides impact fungal translation via elongation factor 2. Organic & biomolecular chemistry. 2019, 17, 4906–4916.
- Aras A B, Guven M, Akman T, et al. Neuroprotective effects of daidzein on focal cerebral ischemia injury in rats. Neural regeneration research. 2015, 10, 146. [CrossRef]
- Li X, Liu RZ, Lin YF, et al. Protective effect of daidzein on cerebral ischemia-reperfusion injury in rats by inhibiting inflammatory response. When Zhen Chinese medicine. 2014, 25, 6–8.
- Cheong S H, Furuhashi K, Ito K, et al. Daidzein promotes glucose uptake through glucose transporter 4 translocation to plasma membrane in L6 myocytes and improves glucose homeostasis in Type 2 diabetic model mice. The Journal of nutritional biochemistry. 2014, 25, 136–143. [CrossRef]
- Park M H, Ju J-W, Kim M, et al. The protective effect of daidzein on high glucose-induced oxidative stress in human umbilical vein endothelial cells. Zeitschrift für Naturforschung C. 2016, 71, 21–28. [CrossRef]
- Westmark C, J. A hypothesis regarding the molecular mechanism underlying dietary soy-induced effects on seizure propensity. Frontiers in Neurology. 2014, 5, 169.
- Li Q, Zheng YJ, Zhao MS, et al. The signaling mechanism of genistein against Aβ neurotoxicity and its research progress. Chinese Journal of Gerontology. 2017, 37, 3086–3089.
- Babu P V A, Si H, Fu Z, et al. Genistein prevents hyperglycemia-induced monocyte adhesion to human aortic endothelial cells through preservation of the cAMP signaling pathway and ameliorates vascular inflammation in obese diabetic mice. The Journal of nutrition. 2012, 142, 724–730. [CrossRef]
- El-Kordy E A, Alshahrani A M. Effect of genistein, a natural soy isoflavone, on pancreatic β-cells of streptozotocin-induced diabetic rats: Histological and immunohistochemical study. Journal of microscopy and ultrastructure. 2015, 3, 108–119. [CrossRef] [PubMed]
- Hu WM, Zhang L, Li LZ, et al. Mechanism of genistein inhibition of 3T3-L1 cell adipogenic differentiation. Food science. 2016, 37, 219–224.
- Kushairi N, Tarmizi N a K A, Phan C W, et al. Modulation of neuroinflammatory pathways by medicinal mushrooms, with particular relevance to Alzheimer’s disease. Trends in Food Science & Technology. 2020, 104, 153–162.
- Xiong M, Huang Y, Liu Y, et al. Antidiabetic activity of ergosterol from Pleurotus ostreatus in KK-Ay mice with spontaneous type 2 diabetes mellitus. Molecular Nutrition & Food Research. 2018, 62, 1700444.
- Rangsinth P, Sharika R, Pattarachotanant N, et al. Potential Beneficial Effects and Pharmacological Properties of Ergosterol, a Common Bioactive Compound in Edible Mushrooms. Foods, 2023, 12, 2529.
- Sillapachaiyaporn C, Mongkolpobsin K, Chuchawankul S, et al. Neuroprotective effects of ergosterol against TNF-α-induced HT-22 hippocampal cell injury. Biomedicine & Pharmacotherapy. 2022, 154, 113596.
- Zhou, LS. Observation of therapeutic effect of Gastrodia Armillaria tablet on 100 cases of neurasthenia and hypertension. New Journal of Medicine and Pharmacy. 1978, 13. [Google Scholar]
- Dai HJ, Meng WW, Wang XQ, et al. Observation of curative effect of compound Gastrodia Armillaria tablet in treatment of tension-type headache. Chinese general clinic. 2008, 24, 365–366.
- Gong, HQ. Study on the hypoglycemic effect of Armillaria polysaccharide by oral administration and its mechanism.D Northeast Normal University, 2018.
- Hu WD, Wang SY, Xu AC, et al. Characterization and identification of chemical components of psoralea based on UHPLC-Q-TOF-MS technique. Chinese journal of traditional Chinese Medicine. 2023, 48, 2989–2999.
- Dai SY, Cui YF, Xu J, et al. UHPLC-Q-Exactive Orbitrap MS/MS Comparative analysis of alkaloids in Aconite, Aconite and aconite. Chinese journal of traditional Chinese Medicine. 2023, 48, 126–139.
- Yang J, Yuwu C, Xiaozhang F, et al. Chemical constituents of Armillaria mellea mycelium I. Isolation and characterization of armillarin and armillaridin. Planta medica. 1984, 50, 288–290. [CrossRef]
- Yang JS, Cong PZ. Study of sesquiterpene alcohol-aromatic acid esters in mycelia of Armillaria by mass spectrometry. Chemical journal. 1988, 1093–1100.
- Yang J, Chen Y, Feng X, et al. Isolation and structure elucidation of armillaricin1. Planta medica, 1989, 55, 564–565. [CrossRef] [PubMed]
- Liang N, Cai P, Wu D, et al. High-speed counter-current chromatography (HSCCC) purification of antifungal hydroxy unsaturated fatty acids from plant-seed oil and Lactobacillus cultures. Journal of agricultural and food chemistry. 2017, 65, 11229–11236. [CrossRef]
- Serrano-García I, Hurtado-Fernández E, Gonzalez-Fernandez J J, et al. Prolonged on-tree maturation vs. cold storage of Hass avocado fruit: Changes in metabolites of bioactive interest at edible ripeness. Food Chemistry. 2022, 394, 133447.
- Li J, Wang YW, Zhang Q, et al. Qualitative and quantitative analysis of Xiaoer Jiegan granules based on HPLC-Q-Exactive-MS and HPLC-MS/MS techniques. Chinese Journal of Hospital Pharmacy. 2023, 43, 868–876.
- Dong F, Li ZX, Jia CM, et al. Composition analysis of sesame oil based on UPLC/Q-TOF MS/MS. Chinese grease. 2022, 47, 130–136.
- Fang G, Zhang P, Ye XL, et al. Analysis of isoflavone glycosides and their aglycans by electrospray ion trap mass spectrometry. Journal of Second Military Medical University. 2013, 34, 1108–1115.
- Chen X, Huang ZF, Liu YH, et al. Identification of metabolites of dangerolone capsules in vivo based on UHPLC-Q/Orbitrap-MS/MS. Chinese journal of traditional Chinese Medicine. 2022, 47, 5052–5063.
- Zhao WJ, Liang YY, Wang ZJ, et al. The metabolites of genistein in rats were identified by UHPLC-LTQ-Orbitrap mass spectrometry. Journal of mass spectrometry. 2019, 40, 109–122.
- Qin WH, Yang Y, Li Q, et al. Chemical constituents of Cordyceps sinensis from Nepal were analyzed based on UPLC-Q-TOF-MS. Chinese Journal of New Drugs. 2019, 28, 1574–1581.
- Stentoft C, Vestergaard M, Løvendahl P, et al. Simultaneous quantification of purine and pyrimidine bases, nucleosides and their degradation products in bovine blood plasma by high performance liquid chromatography tandem mass spectrometry. Journal of Chromatography A. 2014, 1356, 197–210. [CrossRef]
- Dabur R, Mittal A. Detection and qualitative analysis of fatty acid amides in the urine of alcoholics using HPLC-QTOF-MS. Alcohol, 2016, 52, 71–78. [CrossRef]
- Zheng W, Shi HY, Wang P, et al. The chemical components and blood entry components of Banxia Baizhu Tianma Decoction were analyzed by UPLC-Q-Orbitrap-HRMS technique. Chinese Journal of Hospital Pharmacy. 2022, 42, 2331–2339.
- Zhang, ZT. Study on pharmacokinetics and mass spectrum lysis of piracetam. D Shenyang Pharmaceutical University. 2007.
- Li SM, Feng Y, Zeng X. Determination of ergosterone and ergosterol in Zhuling granules by HPLC-APCI-MS/MS method. Journal of Pharmaceutical Analysis. 2014, 34, 649–653.
- Wood K V, Bonham C C, Miles D, et al. Characterization of betaines using electrospray MS/MS. Phytochemistry. 2002, 59, 759–765. [CrossRef] [PubMed]
- Zhang L, Song S, Chen B, et al. Integration of UHPLC/Q-OrbitrapMS-based metabolomics and activities evaluation to rapidly explore the anti-inflammatory components from lasianthus. Heliyon. 2023, 9.


| Numbering | Class | Compound | Retention Time | Molecular Formula | Mode | Measured Value | Calculate The Value | Error/×10−6 | Fragment Ions | After Fermentation | Before Fermentation | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Other | Benzaldehyde | 0.54 | C7H6O | [M+H] | 107.04893 | 107.04914 | -1.96 | 79.05432 | + | - | |
| 2 | Amides | Phenacetin | 1.03 | C10H13NO2 | [M+H] | 180.10207 | 180.10191 | 0.89 | 138.09169 | + | - | |
| 3 | Alkaloids | Choline | 1.29 | C5H13NO | [M+H] | 104.10693 | 104.10699 | -0.58 | 60.08082, 58.06518 | + | + | |
| 4 | Nucleosides | Cytidine* | 1.38 | C9H13N3O5 | [M+H] | 244.09282 | 244.09279 | 0.12 | 112.05049 | + | + | |
| 5 | Nucleosides | Uridine* | 1.81 | C9H12N2O6 | [M+H] | 245.0768 | 245.07681 | -0.04 | 113.03452 | + | + | |
| 6 | Organic acids | Nicotinic acid | 1.86 | C6H5NO2 | [M+H] | 124.03936 | 124.0393 | 0.48 | 80.04947, 96.04435, 78.03384 | + | + | |
| 7 | Amino acids | L-Tyrosine | 2.22 | C9H11NO3 | [M+H] | 182.08092 | 182.08117 | -1.37 | 165.05461, 136.07568 | + | + | |
| 8 | Nucleosides | Adenosine* | 2.34 | C10H13N5O4 | [M+H] | 268.10406 | 268.10403 | 0.11 | 136.06178, 119.03522 | + | + | |
| 9 | Pyrrolidone derivatives | Piracetam | 2.39 | C6H10N2O2 | [M+H] | 143.08144 | 143.0815 | -0.42 | 98.05994, 126.05515, 70.06517 | + | + | |
| 10 | Nucleosides | Guanosine* | 2.45 | C10H13N5O5 | [M+H] | 284.09894 | 284.09894 | 0 | 152.0567 | + | + | |
| 11 | Nucleosides | Guanine* | 2.57 | C5H5N5O | [M+H] | 152.05667 | 152.05669 | -0.13 | 135.03012, 110.03482, 109.05079, 128.04536 | + | + | |
| 12 | Nucleosides | Uracil* | 2.8 | C4H4N2O2 | [M+H] | 113.03458 | 113.03455 | 0.27 | 96.00798 | + | + | |
| 13 | Organic acids | Methylsuccinic acid | 4.1 | C5H8O4 | [M-H] | 131.035 | 131.03498 | 0.15 | 87.0451 | + | + | |
| 14 | Nucleosides | Thymine | 4.84 | C5H6N2O2 | [M+H] | 127.05011 | 127.0502 | -0.71 | 110.03175 | + | + | |
| 15 | Amino acids | D-Phenylalanine | 4.98 | C9H11NO2 | [M-H] | 164.07172 | 164.0717 | 0.12 | 164.07172 | + | + | |
| 16 | Organic acids | 3,4-Dihydroxyphenylacetic acid | 5.23 | C8H8O4 | [M-H] | 167.03506 | 167.03498 | 0.48 | 123.04531, 122.03760 | + | + | |
| 17 | Amino acids | 3-Hydroxy-3-methylglutaricacid | 5.41 | C6H10O5 | [M-H] | 161.0455 | 161.04555 | -0.31 | 57.03456, 59.01381, 99.04536, | + | + | |
| 18 | Organic acids | Pantothenic acid | 5.79 | C9H17NO5 | [M+H] | 220.11797 | 220.11795 | 0.09 | 202.10672, 184.09637 | + | + | |
| 19 | Organic acids | 3-Hydroxy-3-methylbutanoic acid | 6.43 | C5H10O3 | [M-H] | 117.05579 | 117.05572 | 0.60 | 71.05026, 115.03962, 99.04516 | + | + | |
| 20 | Pyrrolidone derivatives | Levetiracetam | 7.05 | C8H14N2O2 | [M+H] | 171.11284 | 171.1128 | 0.23 | 126.09127, 89.07092, 72.08067 | + | + | |
| 21 | Other | Salicylic acid | 7.62 | C7H6O3 | [M-H] | 137.02437 | 137.02442 | -0.36 | 93.03452 | + | + | |
| 22 | Other | 2-Isopropylmalic acid | 7.9 | C7H12O5 | [M-H] | 175.06126 | 175.0612 | 0.34 | 115.04008, 85.06586, 113.06087 | + | + | |
| 23 | Organic acids | Terephthalic acid | 8.58 | C8H6O4 | [M-H] | 165.01938 | 165.0193 | 0.48 | 121.0296 | + | + | |
| 24 | Organic acids | benzoic acid | 9.12 | C7H6O2 | [M-H] | 121.02943 | 121.0295 | -0.58 | 93.03461 | + | + | |
| 25 | Organic acids | 5-Hydroxyindole-3-acetic acid | 9.63 | C10H9NO3 | [M+H] | 192.06543 | 192.06552 | -0.47 | 146.06000, 147.06816 | + | - | |
| 26 | Flavonoids | Genistin* | 11.53 | C21H20O10 | [M-H] | 431.09836 | 431.09837 | -0.02 | 269.04517 | + | + | |
| 27 | Flavonoids | Hispidulin | 11.89 | C16H12O6 | [M-H] | 299.0563 | 299.05611 | 0.64 | 300.05914, 284.03268, 285.03671 | + | - | |
| 28 | Flavonoids | Kaempferide | 11.93 | C16H12O6 | [M-H] | 299.05658 | 299.05611 | 0.64 | 284.03271, 285.03638 | + | + | |
| 29 | Flavonoids | Naringenin | 12.05 | C15H12O5 | [M-H] | 271.06149 | 271.0612 | 1.07 | 151.00380, 119.05028, 107.01356 | + | + | |
| 30 | Flavonoids | Daidzein* | 12.4 | C15H10O4 | [M+H] | 255.06519 | 255.06519 | 0 | 227.07022, 199.07542, 137.02338 | + | + | |
| 31 | Flavonoids | Fisetin | 12.49 | C15H10O6 | [M-H] | 285.04059 | 285.04046 | 0.46 | 135.00888, 256.03601 | + | - | |
| 32 | Flavonoids | 4′,7-Dihydroxyflavanone | 12.6 | C15H12O4 | [M+H] | 257.08096 | 257.08084 | 0.47 | 137.02336, 91.05408, 81.03345 | + | + | |
| 33 | Steroids | Estriol | 12.98 | C18H24O3 | [M+H] | 289.17941 | 289.17982 | -1.42 | 159.08067 | + | - | |
| 34 | Amino acids | Gabapentin | 13.24 | C9H17NO2 | [M+H] | 172.13322 | 172.13321 | 0.06 | 154.12212 | + | - | |
| 35 | Flavonoids | Genistein* | 13.35 | C15H10O5 | [M-H] | 269.0455 | 269.04555 | -0.19 | 241.05162, 240.04305, 225.05518, 213.05608, 197.06015 | + | + | |
| 36 | Organic acids | (15Z)-9,12,13-Trihydroxy-15-octadecenoic acid | 13.66 | C18H34O5 | [M-H] | 329.23343 | 329.23335 | 0.24 | 171.10269, 139.11282, 127.11284, 125.09731 | + | + | |
| 37 | Terpenes | Armillarinin | 14.15 | C24H29O7Cl | [M+H] | 465.16751 | 465.16746 | 0.11 | 199.01511 | + | - | |
| 38 | Terpenes | Soyasaponin I | 14.32 | C48H78O18 | [M+H] | 943.52606 | 943.52609 | -0.03 | 441.37283, 423.36234, 599.39441, 797.46826 | + | + | |
| 39 | Other | 2-Amino-1,3,4-octadecanetriol | 14.47 | C18H39NO3 | [M+H] | 318.30029 | 318.30027 | 0.06 | 300.28946 | + | + | |
| 40 | Terpenes | Armillarilin | 14.81 | C24H30O7 | [M+H] | 431.20618 | 431.20643 | -0.58 | 165.05458 | + | - | |
| 41 | Organic acids | Ethyl myristate | 14.98 | C16H32O2 | [M-H] | 255.23286 | 255.23295 | -0.35 | 255.06647, 69.03460 | + | + | |
| 42 | Terpenes | 10β,13α-dihydroxymelleolide | 15.17 | C23H28O8 | [M-H] | 431.17108 | 431.17114 | -0.14 | 167.0356 | + | - | |
| 43 | Terpenes | 4′-methoxy-8-hydroxymelledonal | 15.49 | C24H30O9 | [M-H] | 461.18134 | 461.18171 | -0.8 | 181.05078 | + | - | |
| 44 | Organic acids | 7-(2-aminophenyl)heptanoic acid | 15.64 | C13H19NO2 | [M+H] | 222.14914 | 222.14886 | 1.26 | 106.06501 | + | - | |
| 45 | Flavonoids | ar-Turmerone | 15.75 | C15H20O | [M+H] | 217.15852 | 217.15869 | -0.78 | 119.14818, 91.05412 | + | - | |
| 46 | Terpenes | Armillarin | 15.78 | C24H30O6 | [M+H] | 415.21155 | 415.21152 | 0.07 | 165.05457 | + | + | |
| 47 | Terpenes | Dehydroeburicoic acid | 16.13 | C31H48O3 | [M+H] | 469.36761 | 469.36762 | -0.02 | 451.35693 | + | - | |
| 48 | Terpenes | 10α,13α-dihydroxyarmillaridin | 16.22 | C24H29O8Cl | [M+H] | 481.1629 | 481.16237 | 1.10 | 199.01569 | + | - | |
| 49 | Other | Piptamine | 16.5 | C23H41N | [M+H] | 332.33102 | 332.33118 | -0.48 | 240.26851, 91.05411 | + | + | |
| 50 | Terpenes | Armillaribin | 16.66 | C24H28O5 | [M+H] | 397.20105 | 397.20095 | 0.25 | 232.14107, 215.14299, 187.14807, 185.13257, 171.11671, 165.05460, 131.08546 | + | + | |
| 51 | Terpenes | Armillaricin | 16.85 | C24H27O5Cl | [M+H] | 431.16205 | 431.16198 | 0.16 | 215.14302, 199.01566, 187.14810, 171.11707 | + | - | |
| 52 | Terpenes | Melleolide | 17.18 | C23H28O6 | [M+H] | 401.19601 | 401.19587 | 0.35 | 233.15363 | + | - | |
| 53 | Terpenes | coriolic acid | 17.22 | C18H32O3 | [M-H] | 295.22781 | 295.22787 | -0.2 | 277.21704 | + | + | |
| 54 | Terpenes | Armillaridin | 18.19 | C24H29O6Cl | [M+H] | 449.17239 | 449.17254 | -0.33 | 233.15359, 199.01558 | + | - | |
| 55 | Terpenes | 4′-methoxyarmillasin | 18.33 | C23H30O5 | [M-H] | 385.20212 | 385.20205 | 0.18 | 181.05067 | + | - | |
| 56 | Terpenes | 4′-demethoxyarmillaribin | 18.48 | C23H26O5 | [M-H] | 381.17075 | 381.17075 | 0 | 167.03499 | + | + | |
| 57 | Terpenes | Armillaridine | 19.05 | C24H28O6 | [M-H] | 411.18137 | 411.18131 | 0.15 | 165.05545 | + | - | |
| 58 | Amides | Linoleamide | 19.86 | C18H33NO | [M+H] | 280.26379 | 280.26349 | 1.07 | 263.23645, 245.22620 | + | + | |
| 59 | Terpenes | Oleanolic acid | 19.96 | C30H48O3 | [M-H] | 455.35297 | 455.35307 | -0.22 | 456.35941 | + | + | |
| 60 | Other | D-Sphingosine | 20.04 | C18H37NO2 | [M+H] | 300.28998 | 300.28971 | 0.90 | 282.27841, 283.26282 | + | + | |
| 61 | Terpenes | Ursolic Acid* | 20.29 | C30H48O3 | [M-H] | 455.35263 | 455.35307 | -0.97 | 456.35699 | + | + | |
| 62 | Terpenes | 1-dehydroxyarmily everninate | 20.59 | C24H32O5 | [M-H] | 399.21762 | 399.21769 | -0.18 | 181.05075 | + | - | |
| 63 | Amides | Hexadecanamide | 20.94 | C16H33NO | [M+H] | 256.26349 | 256.26349 | 0 | 74.06010, 69.06995, 57.06992, 55.05431 | + | + | |
| 64 | Organic acids | Linoleic acid | 21.37 | C18H32O3 | [M-H] | 279.23291 | 279.23295 | -0.14 | 261.22281 | + | + | |
| 65 | Amides | Oleamide | 21.43 | C18H35NO | [M+H] | 282.27917 | 282.27914 | 0.11 | 265.25272 | + | + | |
| 66 | Steroids | Ergosterol* | 21.77 | C28H44O | [M+H] | 397.34583 | 397.34649 | -1.66 | 379.33572 | + | - | |
| 67 | Terpenes | 4′-methoxy-4-dehydroxyarmillasin | 22.15 | C23H30O4 | [M-H] | 369.20715 | 369.20713 | 0.05 | 181.0507 | + | - | |
| 68 | Organic acids | Palmitic acid | 22.45 | C16H32O2 | [M-H] | 255.23296 | 255.23295 | 0.04 | 237.22198 | + | - | |
| 69 | Amides | N-octodecanoylsphinganine | 22.51 | C36H73NO3 | [M+H] | 568.56604 | 568.56632 | -0.49 | 302.30414, 284.29504 | + | + | |
| 70 | Organic acids | Palmitoleic Acid | 22.73 | C16H30O2 | [M+H] | 255.23184 | 255.23186 | -0.08 | 237.22128 | + | + | |
| 71 | Organic acids | Oleic acid | 22.87 | C18H34O2 | [M-H] | 281.24857 | 281.2486 | -0.11 | 264.34039 | + | + | |
| 72 | Organic acids | Dioctyl phthalate | 24.44 | C24H38O4 | [M+H] | 391.28439 | 391.28429 | 0.26 | 149.02319, 71.08543, 167.03371 | + | + | |
| 73 | Amides | Erucamide | 25.11 | C22H43NO | [M+H] | 338.34198 | 338.34174 | 0.71 | 321.31503 | + | + | |
| 74 | Organic acids | Linolenic acid ethyl ester | 25.31 | C20H34O2 | [M+H] | 307.26337 | 307.26316 | 0.68 | 123.11671 | + | - | |
| 75 | Organic acids | Ethyl oleate | 25.93 | C20H38O2 | [M+H] | 311.29498 | 311.29446 | 1.67 | 265.25317, 247.24187, 163.14861, | + | + | |
| 76 | Terpenes | 2′,5-epoxy-4-dehydroxyarmillaridiene | 26.28 | C24H26O5 | [M-H] | 393.17172 | 393.17075 | 2.47 | 181.05064 | + | - | |
| 77 | Alkaloids | Betaine | 26.48 | C5H11NO2 | [M+H] | 118.08617 | 118.08626 | -0.76 | 58.06517, 59.07298 | + | + | |
| Compound | Linear Equation | R² | Linear Range/μg·mL−1 |
|---|---|---|---|
| Genistin | Y=3682.9592X-745.8583 | 0.9999 | 2.1581~37.7667 |
| Daidzein | Y=3519.0934X-1460.4269 | 0.9999 | 7.1962~125.9328 |
| Genistein | Y=5724.1012X-3375.2034 | 0.9999 | 7.7379~135.4125 |
| Ergosterol | Y=1504.3237X-1830.0128 | 0.9999 | 18.8147~329.2574 |
| Compound | Weighing sample(g) | Sample content (mg/g) | Added content (mg/g) | Real measurement (mg/g) | Recovery(%) | Average recovery(%) | RSD value(%) |
|---|---|---|---|---|---|---|---|
| Genistin | 0.2497 | 0.0084 | 0.0083 | 0.0165 | 0.9741 | 97.50% | 0.39% |
| 0.2503 | 0.0084 | 0.0083 | 0.0165 | 0.9700 | |||
| 0.2501 | 0.0084 | 0.0083 | 0.0165 | 0.9753 | |||
| 0.2498 | 0.0084 | 0.0083 | 0.0165 | 0.9766 | |||
| 0.2498 | 0.0084 | 0.0083 | 0.0166 | 0.9892 | |||
| 0.2502 | 0.0084 | 0.0083 | 0.0164 | 0.9649 | |||
| Daidzein | 0.2501 | 0.0341 | 0.0343 | 0.0692 | 1.0239 | 102.97% | 0.97% |
| 0.2503 | 0.0342 | 0.0343 | 0.0685 | 1.0008 | |||
| 0.2502 | 0.0341 | 0.0343 | 0.0696 | 1.0352 | |||
| 0.2501 | 0.0341 | 0.0343 | 0.0690 | 1.0164 | |||
| 0.2498 | 0.0341 | 0.0343 | 0.0701 | 1.0491 | |||
| 0.2501 | 0.0341 | 0.0343 | 0.0702 | 1.0527 | |||
| Genistein | 0.2502 | 0.0387 | 0.0389 | 0.0766 | 0.9738 | 98.65% | 0.51% |
| 0.2501 | 0.0387 | 0.0389 | 0.0773 | 0.9915 | |||
| 0.2502 | 0.0387 | 0.0389 | 0.0767 | 0.9747 | |||
| 0.2503 | 0.0388 | 0.0389 | 0.0772 | 0.9874 | |||
| 0.2502 | 0.0387 | 0.0389 | 0.0773 | 0.9919 | |||
| 0.2499 | 0.0387 | 0.0389 | 0.0776 | 0.9997 | |||
| Ergosterol | 0.2503 | 0.1081 | 0.1081 | 0.2203 | 1.0384 | 102.87% | 0.85% |
| 0.2501 | 0.1080 | 0.1081 | 0.2192 | 1.0286 | |||
| 0.2502 | 0.1080 | 0.1081 | 0.2157 | 0.9962 | |||
| 0.2501 | 0.1080 | 0.1081 | 0.2199 | 1.0348 | |||
| 0.2502 | 0.1080 | 0.1081 | 0.2210 | 1.0451 | |||
| 0.2503 | 0.1081 | 0.1081 | 0.2193 | 1.0290 |
| Sample | Genistin(mg/g) | Daidzein(mg/g) | Genistein(mg/g) | Ergosterol(mg/g) |
|---|---|---|---|---|
| Prefermentation sample | 0.0336 | 0.0184 | 0.0188 | / |
| After fermentation sample 1 | / | 0.1391 | 0.1617 | 0.4653 |
| After fermentation sample 2 | / | 0.1364 | 0.1549 | 0.4318 |
| After fermentation sample 3 | / | 0.1390 | 0.1576 | 0.4371 |
| After fermentation sample 4 | / | 0.1116 | 0.1321 | 0.4392 |
| After fermentation sample 5 | / | 0.1092 | 0.1348 | 0.4359 |
| After fermentation sample 6 | / | 0.1487 | 0.1547 | 0.4368 |
| After fermentation sample 7 | / | 0.1498 | 0.1259 | 0.4472 |
| After fermentation sample 8 | / | 0.1938 | 0.1915 | 0.4248 |
| After fermentation sample 9 | / | 0.1820 | 0.1803 | 0.4342 |
| After fermentation sample 10 | / | 0.2127 | 0.1942 | 0.4258 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




