Submitted:
21 March 2024
Posted:
22 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
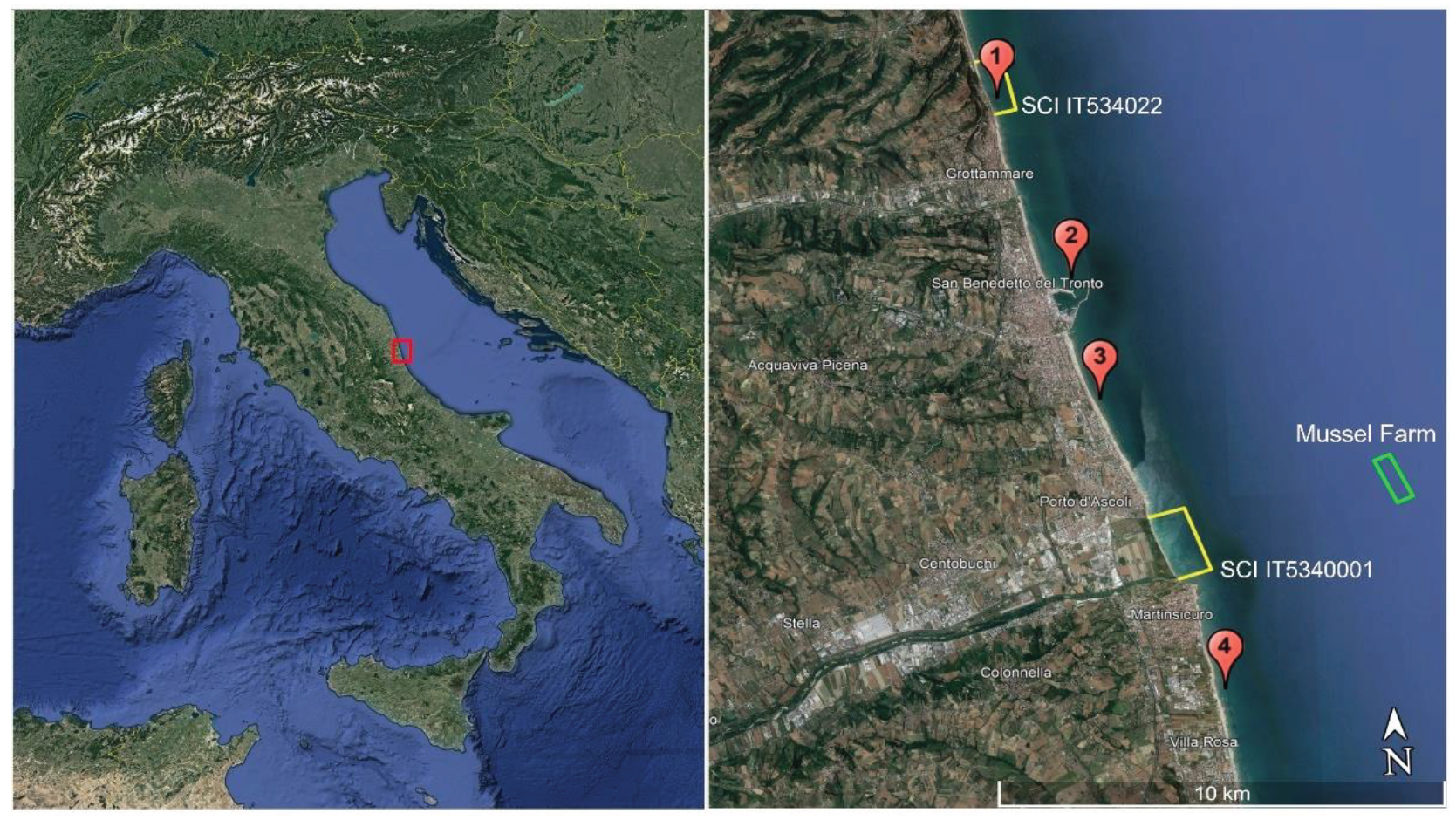
2.1. Study Area
2.2. Observations
2.3. Climatic Data
3. Results
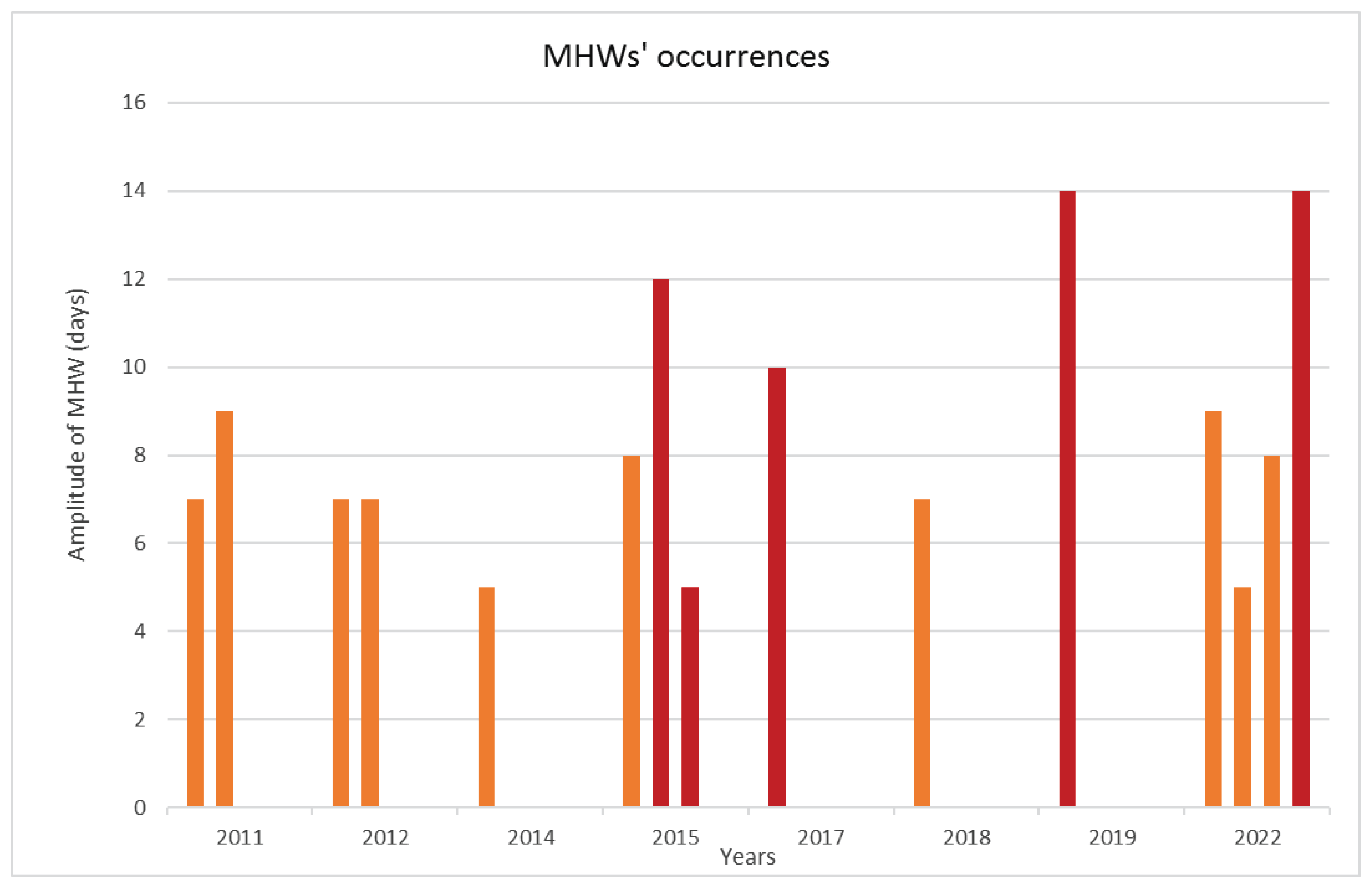
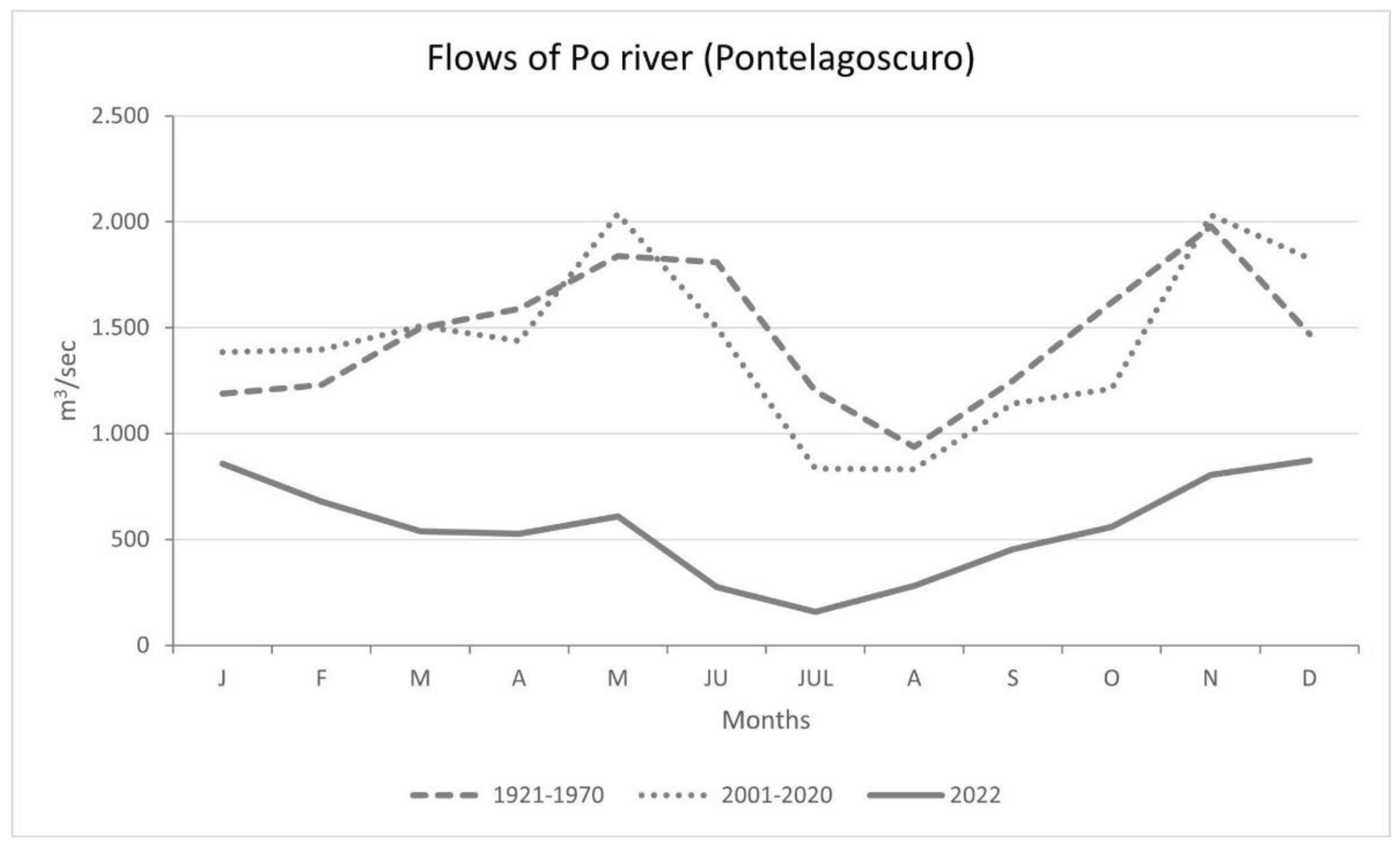
3.1. Climatic Data Analysis
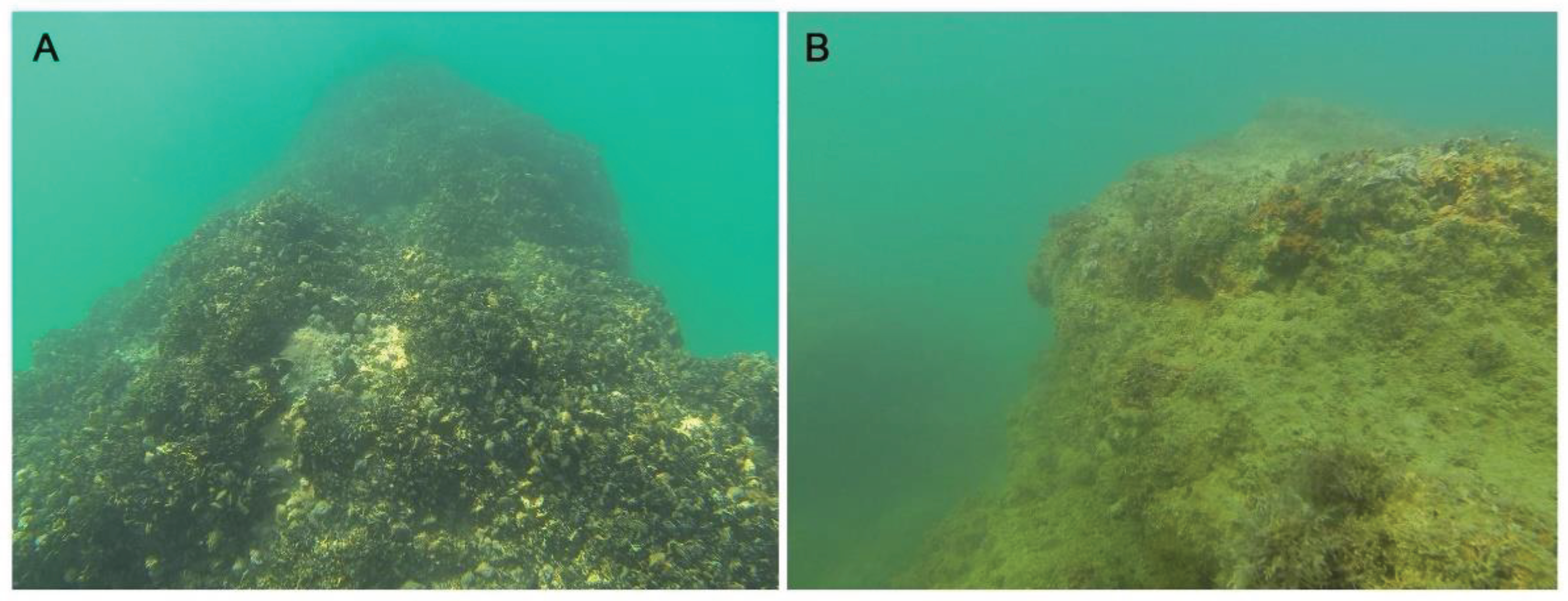
3.2. Mussel MME
4. Discussion
4.1. Climatic Data
4.2. Mussels MME
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Díaz, S.; Settele, J.; Brondizio, E.; Ngo, H.T.; Guèze, M.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.A.; Butchart, S.H.M. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-policy Platform on Biodiversity and Ecosystem Services. Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services 2019, Bonn, Germany, 39.
- Shukla, P.R.; Skea, J.; Calvo Buendia, E.; Masson-Delmotte, V.; Pörtner, H.O. Special report - Climate change and land. Intergovernmental Panel on Climate Change 2019. Available online: https://www.ipcc.ch/report/srccl/ (accessed on 20 October 2022).
- Beever, E.A.; O’Leary, J.; Mengelt, C.; West, J.M.; Julius, S.; Green, N.; Managness, D.; Petes, L.E.; Stein, B.A.; Nicotra, A.B.; et al. Improving conservation outcomes with a new paradigm for understanding species’ fundamental and realized adaptive capacity. Conserv. Lett. 2016, 9, 131–137. [Google Scholar] [CrossRef]
- Foden, W.B.; Young, B.E. IUCN SSC Guidelines for Assessing Species’ Vulnerability to Climate Change. Version 1.0. Occasional Paper of the IUCN Species Survival Commission No. 59. Cambridge, UK and Gland, Switzerland, 2016; 114. [CrossRef]
- Glick, P.; Stein, B.A.; Edelson, N.A. Scanning the Conservation Horizon: A Guide to Climate Change Vulnerability Assessment. National Wildlife Federation, Washington, DC, 2011; 168.
- Kovach, R.P.; Dunham, J.B.; Al-Chokhachy, R.; Snyder, C.D.; Letcher, B.H.; Young, J.A.; Beever, E.A.; Pederson, G.T.; Lynch, A.J.; Hitt, N.P.; et al. An integrated framework for ecological drought across riverscapes of North America. Bioscience 2019, 69, 418–431. [Google Scholar] [CrossRef]
- Jay, A.; Reidmiller, D.R.; Avery, C.W.; Barrie, D.; DeAngelo, B.J. 2018. Impacts, Risks, and Adaptation in the United States: Fourth National Climate Assessment, Volume II. U.S. Global Change Research Program, Washington, D.C., USA, pp. 33–71.
- Malhi, Y.; Franklin, J.; Seddon, N.; Solan, M.; Turner, M. G.; Field, C. B.; Knowlton, N. Climate change and ecosystems: Threats, opportunities and solutions. Philos. Trans. Royal Soc. A 2020, 375(1794), 20190104. [Google Scholar] [CrossRef] [PubMed]
- Hobday, A.J.; Oliver, E.C.; Gupta, A.S.; Benthuysen, J.A.; Burrows, M.T.; Donat, M.G.; Holbrook, N.J; Moore, P.J.; Thomsen, M.S.; Wernberg, T.; et al. Categorizing and naming marine heatwaves. Oceanogr. 2018, 31(2), 162–173. [Google Scholar] [CrossRef]
- Sparnocchia, S.; Schiano, M. E.; Picco, P.; Bozzano, R.; Cappelletti, A. The anomalous warming of summer 2003 in the surface layer of the Central Ligurian Sea (Western Mediterranean). Ann. Geophys. 2006, 24, 443–452. [Google Scholar] [CrossRef]
- Olita, A.; Sorgente, R.; Ribotti, A.; Natale, S.; Gaberšek, S. Effects of the 2003 European heatwave on the Central Mediterranean Sea surface layer: a numerical simulation. Ocean Sci. 2006, 3, 85–125. [Google Scholar]
- Pearce, A.F.; Feng, M. The rise and fall of the ‘marine heat wave’ off Western Australia during the summer of 2010/11. J. Mar. Syst. 2013, 112, 139–156. [Google Scholar] [CrossRef]
- Chen, K.; Gawarkiewicz, G.G.; Lentz, S.J.; Bane, J.M. Diagnosing the warming of the Northeastern US Coastal Ocean in 2012: a linkage between the atmospheric jet stream variability and ocean response. J. Geophys. Res. Oceans 2014, 119, 218–227. [Google Scholar] [CrossRef]
- Bond, N.A.; Cronin, M.F.; Freeland, H.; Mantua, N. Causes and impacts of the 2014 warm anomaly in the NE Pacific. Geophys. Res. Lett. 2015, 42, 3414–3420. [Google Scholar] [CrossRef]
- Di Lorenzo, E.; Mantua, N. Multi-year persistence of the 2014/15 North Pacific marine heatwave. Nat. Clim. Change 2016, 6, 1042–1047. [Google Scholar] [CrossRef]
- Oliver, E.C.J.; Donat, M.G.; Burrows, M.T.; Moore, P.J.; Smale, D.A.; Alexander, L.V.; Benthuysen, J.A.; Feng, M.; Gupta, A.S.; Hobday, A.J.; et al. Longer and more frequent marine heatwaves over the past century. Nat. Commun. 2018, 9, 1324. [Google Scholar] [CrossRef]
- Benthuysen, J.A.; Oliver, E.C.J.; Feng, M.; Marshall, A.G. Extreme marine warming across tropical Australia during austral summer 2015-16. J. Geophys. Res. Oceans 2018, 123, 1301–1326. [Google Scholar] [CrossRef]
- Wernberg, T.; Bennett, S.; Babcock, R.C.; De Bettignies, T.; Cure, K.; Depczynski, M.; Dufois, F.; Fromont, J.; Fulton, C.J.; Hovey, R.K.; et al. Climate-driven regime shift of a temperate marine ecosystem. Science 2016, 353, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Kerry, J.; Álvarez-Noriega, M.; Álvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Badcock, R.C.; Beger, M.; Bellwood, D.R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Oliver, E.; Benthuysen, J.; Bindoff, N.; Hobday, A.J.; Holbrook, N.J.; Mundy, C.N.; Perkins-Kirkpatrick, S.E. The unprecedented 2015/16 Tasman Sea marine heatwave. Nat. Commun. 2017, 8, 16101. [Google Scholar] [CrossRef] [PubMed]
- Garrabou, J.; Coma, R.; Bensossan, N.; Bally, M.; Chevaldonné, P.; Cigliano, M.; Diaz, D.; Harmelin, J.G.; Gambi, M.C.; Kersting, D.K.; et al. Mass mortality in Northwestern Mediterranean rocky benthic communities: effects of the 2003 heat wave. Glob. Chang. Biol. 2009, 15, 1090–1103. [Google Scholar] [CrossRef]
- Cavole, L.M.; Demko, A.M.; Diner, R.E.; Giddings, A.; Koester, I.; Pagniello, C.M.L.S.; Paulsen, M.L.; Ramirez-Valdez, A.; Schwenck, S.M.; Yen, N.K.; et al. Biological impacts of the 2013--2015 warm-water anomaly in the Northeast Pacific: winners, losers, and the future. Oceanography 2016, 29, 273–285. [Google Scholar] [CrossRef]
- Bonacci, O.; Bonacci, D.; Patekar, M.; Pola, M. Increasing Trends in Air and Sea Surface Temperature in the Central Adriatic Sea (Croatia). J. Mar. Sci. Eng. 2021, 9(4), 358. [Google Scholar] [CrossRef]
- Grbec, B.; Morović, M.; Matić, F.; Ninčević Gladan, Ž.; Marasović, I.; Vidjak, O.; Bojanić, N.; Čikes Keć, V.; Zorica, B.; Kusplić, G; et al. Climate regime shifts and multi-decadal variability of the Adriatic Sea pelagic ecosystem. Acta Adriat. 2015, 56, 47––66. [Google Scholar]
- Grilli, F.; Accoroni, S.; Acri, F.; Bernardi Aubry, F.; Bergami, C.; Cabrini, M.; Campanelli, A.; Giani, M.; Guicciardi, S.; Marini, M.; et al. Seasonal and Interannual Trends of Oceanographic Parameters over 40 Years in the Northern Adriatic Sea in Relation to Nutrient Loadings Using the EMODnet Chemistry Data Portal. Water 2020, 12, 2280. [Google Scholar] [CrossRef]
- Penna, N.; Cappellacci, S.; Ricci, F. The influence of the Po River discharge on phytoplankton bloom dynamics along the coastline of Pesaro (Italy) in the Adriatic Sea. Mar. Poll. Bull. 2004, 48, 321–326. [Google Scholar] [CrossRef]
- Schiano, M.E.; Sparnocchia, S.; Cappa, C.; Bozzano, R. An analysis of the climate variability over the Mediterranean Sea by means of the surface water vapour density. Int. J. Climatol. 2005, 25, 1731–1748. [Google Scholar] [CrossRef]
- Grbec, B.; Morović, M.; Beg Paklar, G.; Kušpilić, G.; Matijević, S.; Matić, F.; Gladan, Ž. The relationship between the atmospheric variability and productivity in the Adriatic Sea area. J. Mar. Biol. Assoc. U.K. 2009, 89, 1549–1558. [Google Scholar] [CrossRef]
- Giani, M.; Djakovac, T.; Degobbis, D.; Cozzi, S.; Solidoro, C.; Umani, S.F. Recent changes in the marine ecosystems of the northern Adriatic Sea. Estuar. Coast. Shelf Sci. 2012, 115, 1–13. [Google Scholar] [CrossRef]
- Djakovac, T.; Supić, N.; Bernardi Aubry, F.; Degobbis, D.; Giani, M. Mechanisms of hypoxia frequency changes in the northern Adriatic Sea during the period 1972–2012. J. Mar. Syst. 2015, 141, 179–189. [Google Scholar] [CrossRef]
- Juza, M.; Fernández-Mora, À.; Tintoré, J. Sub-Regional Marine Heat Waves in the Mediterranean Sea from Observations: Long-Term Surface Changes, Sub-Surface and Coastal Responses. Front. Mar. Sci. 2022, 9, 785771. [Google Scholar] [CrossRef]
- Kružić, P.; Popijač, A. Mass mortality events of the coral Balanophyllia europaea (Scleractinia, Dendrophylliidae) in the Mljet National Park (eastern Adriatic Sea) caused by sea temperature anomalies. Coral reefs 2015, 34, 109–118. [Google Scholar] [CrossRef]
- Di Camillo, C.G.; Cerrano, C. Mass mortality events in the NW Adriatic Sea: phase shift from slow-to fast-growing organisms. PloS one 2015, 10(5), e0126689. [Google Scholar] [CrossRef]
- Cerrano, C.; Pica, D.; Di Camillo, C.; Bastari, A.; Torsani, F. Caratterizzazione biocenotica e restituzione cartografica per l’individuazione di habitat e specie di interesse comunitario lungo la costa marchigiana. Regione Marche, Ancona, Italy, 2014; pp. 55.
- Gazeau, F.; Alliouane, S.; Bock, C.; Bramanti, L.; López Correa, M.; Gentile, M.; Hirse, T.L.; Portner, H.O.; Ziveri, P. Impact of ocean acidification and warming on the Mediterranean mussel (Mytilus galloprovincialis). Front. Mar. Sci. 2014, 1, 62. [Google Scholar] [CrossRef]
- Bracchetti, L.; Capriotti, M. Le Formazioni a Reff della costa Picena (The reef formations of the Piceno coast, central Italy). Studi Costieri 2021, 30, 83–92. [Google Scholar]
- EUMOFA, E.U. The EU fish market. Eur. Mark. Obs. Fish. Aquac. Prod, 2023. https://eumofa.eu.
- Pesaresi, S.; Biondi, E.; Casavecchia, S. Bioclimates of Italy. J. Maps 2017, 13(2), 955–960. [Google Scholar] [CrossRef]
- Fazzini, M.; Beltrando, G.; Billi, P. Intense rainfalls and flooding problems in the beach resort of San Benedetto del Tronto, Adriatic Sea, Central Italy. Proc. 3°GEOMED 2013, Antalya Turkey, 10-14 june; Ibrahim Athalay and Recept EYE, 128-131.
- Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31992L0043 (accessed on 15 September 2022).
- Dame, R.F., Kenneth, M.J. Ecology of marine bivalves: an ecosystem approach. CRC Press, Taylor & Francis, Boca Raton, FL, US, 2011; pp. 284.
- Ward, J.E.; Shumway, S.E. Separating the grain from the chaff: particle selection in suspension-and deposit-feeding bivalves. J. Exp. Mar. Biol. Ecol. 2004, 300(1-2), 83–130. [Google Scholar] [CrossRef]
- Broszeit, S.; Hattam, C.; Beaumont, N. Bioremediation of waste under ocean acidification: Reviewing the role of Mytilus edulis. Mar. Poll. Bull. 2016, 103(1-2), 5–14. [Google Scholar] [CrossRef]
- Murray, S.N.; Ambrose, R.F.; Dethier, M.N. Methods for Performing Monitoring, Impact, and Ecological Studies on Rocky Shores; MMS U.S. Department of the Interior Minerals Management Service: Pacific OCS Region, 2002; pp. 116-147.
- Hobday, A. J.; Alexander, L. V.; Perkins, S. E.; Smale, D. A.; Straub, S. C.; Oliver, E. C. J.; Benthuysen, J. A.; Burrows, M. T.; Donat, M. G.; Feng, M.; Holbrook, N. J.; Moore, P. J.; Scannell, H. A.; Sen Gupta, A; Wernberg, T. A hierarchical approach to defining marine heatwaves. Progress in Oceanography 2016, 141, 227–238. [Google Scholar] [CrossRef]
- WMO (World Meteorological Organization), 2008. Guide to Meteorological Instruments and Methods of Observation - No.8 (Seventh edition). https://www.weather.gov/media/epz/mesonet/CWOP-WMO8.pdf.
- National tide gauge network of ISPRA (Istituto Superiore per la Protezione e la Ricerca Ambientale). Available online: https://mareografico.it/?session=0S71809491267K766873MJG&syslng=ita&sysmen=-1&sysind=-1&syssub=-1&sysfnt=0&code=STAZ&idst=1W (accessed on 20 June 2023).
- Pastor, F.; Valiente, J.A.; Khodayar, S. A Warming Mediterranean: 38 Years of Increasing Sea Surface Temperature. Remote Sens. 2020, 12, 2687. [Google Scholar] [CrossRef]
- Anestis, A.; Lazou, A.; Portner, H O.; Michaelidis, B. Behavioral, metabolic, and molecular stress responses of marine bivalve Mytilus galloprovincialis during long-term acclimation at increasing ambient temperature. Am. J. Physiol. Regul. Integr. Comp. Physiol 2007, 293(2), R911-21. [Google Scholar] [CrossRef]
- Fly, E.K.; Hilbish, T.J.; Wethey, D.S.; Rognstad, R.L. Physiology and Biogeography: The Response of European Mussels (Mytilus spp.) to Climate Change. Am. Malacol. Bull. 2015, 33(1), 136–149. [Google Scholar] [CrossRef]
- Garrabou, J.; Gómez-Gras, D.; Medrano, A.; Cerrano, C.; Ponti, M.; Schlegel, R.; Bensoussan, N.; Turicchia, E.; Sini, M.; Gerovasileiou, M; et al. Marine heatwaves drive recurrent mass mortalities in the Mediterranean Sea. Global Change Biology 2022, 28(19), 5708–5725. [Google Scholar] [CrossRef]
- Parisi, M.G.; Mauro, M.; Sarà, G.; Cammarata, M. Temperature increases, hypoxia, and changes in food availability affect immunological biomarkers in the marine mussel Mytilus galloprovincialis. J. Comp. Physiol. B 2017, 187, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K. R.; Van Thiel, L. E.; Helmuth, B. Interactive effects of food availability and aerial body temperature on the survival of two intertidal Mytilus species. J. Therm. Biol. 2010, 35(4), 161–166. [Google Scholar] [CrossRef]
- Rampazzo, F.; Berto, D.; Giani, M.; Brigolin, D.; Covelli, S.; Cacciatore, F.; Boscolo Brusà, R.; Bellucci, L.C.; Pastres, R. Impact of mussel farming on sedimentary geochemical properties of a Northern Adriatic area influenced by freshwater inflows. Estuarine, Coastal and Shelf Science, 2013, 129, 49–58. [Google Scholar] [CrossRef]
- ISPRA (Istituto Superiore per la Protezione e la Ricerca Ambientale), 2021. Gli indicatori del Clima in Italia nel 2021 Anno XVII - Stato dell’ambiente 98/2022. In italian, available only in electronic format at: https://www.isprambiente.gov.it/files2022/pubblicazioni/stato-ambiente/rapporto_clima_2021.pdf.
- Pisano, A.; Marullo, S.; Artale, V.; Falcini, F.; Yang, C.; Leonelli, F.E.; Santoleri, R.; Buongiorno Nardelli, B. New Evidence of Mediterranean Climate Change and Variability from Sea Surface Temperature Observations. Remote Sens. 2020, 12, 132. [Google Scholar] [CrossRef]
- Šolić, M.; Grbec, B.; Matić, F.; Šantić, D.; Šestanović, S.; Gladan, Ž.N.; Bojanić, N.; Ordulj, M.; Jozić, S.; Vrdoljak, A. Spatio-temporal reproducibility of the microbial food web structure associated with the change in temperature: Long-term observations in the Adriatic Sea. Oceanography 2018, 161, 87–101. [Google Scholar] [CrossRef]
- Grbec, B.; Matić, F.; Beg Paklar, G.; Morović, M.; Popović, R.; Vilibić, I. Long-Term Trends, Variability and Extremes of In Situ Sea Surface Temperature Measured Along the Eastern Adriatic Coast and its Relationship to Hemispheric Processes. Pure Aappl. Geophys. 2018, 175, 4031–4046. [Google Scholar] [CrossRef]
- Fazzini, M.; Fiore, A.; Sammartino, G. Preliminary analysis of climate data and geo-hydrological instability that affected the island of Ischia on November 26, 2022. Geologia dell’Ambiente 2023, 1/2023 ISSN 1591-5352; 12-25. 26 November.
- ARPAE, 2023. Agenzia Regionale Protezione Ambientale Emilia-Romagna. Data reserved.
- www.uniurb.it. Available online (bulletins only in Italian): https://www.uniurb.it/ricerca/organizzazione-della-ricerca/strutture-della-ricerca/qualita-delle-acque-della-costa.
- Savini, D.; Occhipinti-Ambrogi, A. Consumption rates and prey preference of the invasive gastropod Rapana venosa in the Northern Adriatic Sea. Helgol Mar Res 2006, 60, 153–159. [Google Scholar] [CrossRef]
- Regional Agency for Environmental Protection of Marche Region. Relazione annuale sulla qualità delle acque di balneazione stagione 2022 (Annual report on the quality of bathing water season 2022). ARPAM, Ancona, Italy, 2022, 62, 75.
- Cerrano, C.; Bavestrello, G.; Bianchi, C.N.; Cattaneo-Vietti, R.; Bava, S.; Morganti, C.; Morri, C.; Picco, P.; Sara, G.; Schiaparelli, S.; et al. Catastrophic mass-mortality episode of gorgonians and other organisms in the Ligurian Sea (North-Western Mediterranean), summer 1999. Ecol. Lett. 2000, 3(4), 284–293. [Google Scholar] [CrossRef]
- Garrabou, J.; Gómez-Gras, D.; Medrano, A.; Cerrano, C.; Ponti, M.; Schlegel, R.; Bensoussan, N.; Turicchia, E; Sini, M.; Gerovasileiou, V.; et al. Marine heatwaves drive recurrent mass mortalities in the Mediterranean Sea. Glob. Chang. Biol. 2022, 28(19), 5708–5725. [Google Scholar] [CrossRef]
- Turner, M. G.; Calder, W. J.; Cumming, G. S.; Hughes, T. P.; Jentsch, A.; LaDeau, S.L.; Lenton, T.M.; Shuman, B.N.; Turetsky, M.R.; Ratajczak, M.; et al. Climate change, ecosystems and abrupt change: Science priorities. Philos. Trans. Royal Soc. B 2020, 375, 20190105. [Google Scholar] [CrossRef] [PubMed]
- Pagès-Escolà, M.; Hereu, B.; Garrabou, J.; Montero-Serra, I.; Gori, A.; Gómez-Gras, D.; Figuerola, B.; Linares, C. Divergent responses to warming of two common co-occurring Mediterranean bryozoan. Sci. Rep. 2018, 8(1), 17455. [Google Scholar] [CrossRef]
- Gómez-Gras, D.; Linares, C.; de Caralt, S.; Cebrian, E.; Frleta-Valić, M.; Montero-Serra, I.; Pagès-Escolà, M.; López-Sendino, P.; Garrabou, J. Response diversity in Mediterranean coralligenous assemblages facing climate change: Insights from a multi-specific thermotolerance experiment. Ecol. Evol. 2019, 9(7), 4168–4180. [Google Scholar] [CrossRef] [PubMed]
- Somero, G.N. Thermal Physiology and Vertical Zonation of Intertidal Animals: Optima, Limits, and Costs of Living. Integrative and Comp. Biol. 2002, 42(4), 780–789. [Google Scholar] [CrossRef]
- Harley, C.D. Tidal dynamics, topographic orientation, and temperature-mediated mass mortalities on rocky shores. Mar. Ecol. Prog. Ser. 2008, 371, 37–46. [Google Scholar] [CrossRef]
- Anestis, A.; Pörtner, H.O.; Karagiannis, D.; Angelidis, P.; Staikou, A.; Michelidis, B. Response of Mytilus galloprovincialis (L.) to increasing seawater temperature and to marteliosis: metabolic and physiological parameters. Comp. Biochem.Physiol. Part A Mol. Integr. Physiol. 2010, 156, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Brady, C.E.; Somero, G.N. Following the heart: temperature and salinity effects on heart rate in native and invasive species of blue mussels (genus Mytilus). J. Exp. Biol. 2006, 209, 2554–2566. [Google Scholar] [CrossRef]
- Priemel, T.; Degtyar, E.; Dean, M.; Harrington, M.J. Rapid self-assembly of complex biomolecular architectures during mussel byssus biofabrication. Nat. Commun. 2017, 8, 14539. [Google Scholar] [CrossRef] [PubMed]
- Carrington, E.; Waite, J.H.; Sarà, G.; Sebens, K.P. Mussels as a Model System for Integrative Ecomechanics. Annu. Rev. Mar. Sci. 2015, 7, 443–469. [Google Scholar] [CrossRef]
- Lachance, A.A.; Myrand, B.; Tremblay, R.; Koutitonsky, V.; Carrington, E. Biotic and abiotic factors influencing attachment strength of blue mussels Mytilus edulis in suspended culture. Aquat. Biol. 2008, 2, 119–129. [Google Scholar] [CrossRef]
- O’Donnell, M.J.; George, M.N.; Carrington, E. Mussel byssus attachment weakened by ocean acidification. Nat. Clim. Change 2013, 3(6), 587–590. [Google Scholar] [CrossRef]
- Li, Y.F.; Yang, X.Y.; Cheng, Z.Y.; Wang, L.Y.; Wang, W.X.; Liang, X.; Yang, J.L. Near-future levels of ocean temperature weaken the byssus production and performance of the mussel Mytilus coruscus. Sci. Total Environ. 2020, 1(733), 139347. [Google Scholar] [CrossRef] [PubMed]
- Zardi, G.I.; McQuaid, C.D.; Nicastro, K.R. Balancing survival and reproduction: seasonality of wave action, attachment strength and reproductive output in indigenous Perna perna and invasive Mytilus galloprovincialis mussels. Mar. Ecol. Prog. Ser. 2007, 334, 155–163. [Google Scholar] [CrossRef]
- Watson, W.G.; Margarita, E.C.; Ginoux, P.; O'Reilly, J.E.; Casey, N.W. Ocean primary production and climate: Global decadal changes. Geophys. Res. Lett. 2003, 30(15), 1809. [Google Scholar] [CrossRef]
- Martinez, M.; Mangano, M.C.; Maricchiolo, G.; Genovese, L.; Mazzola, A.; Sarà, G. Measuring the effects of temperature rise on Mediterranean shellfish aquaculture. Ecol. Indic. 2018, 88, 71–78. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D.; Plattner, G.K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. Climate Change 2013: The Physical Science Basis. Contribution of Working Group 1 to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK and New York, USA, 2013, pp. 1523.


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




