1. Introduction
The Philadelphia (Ph) chromosome represents the most frequent cytogenetic abnormality in adults with acute lymphoblastic leukemia (ALL), with an incidence that increases with age, reaching approximately 50% in patients aged 60 years and older [
1,
2,
3]. The combination of Ph chromosome and BCR-ABL fusion gene is associated with the most unfavorable outcome, irrespective of age [
4].
Induction chemotherapy rarely determines a sustained complete remission in these patients and so, after complete hematologic remission has been achieved, allogeneic stem cell transplant, when feasible, represents the only possibility of cure.[
5]. Treatment options for Ph+ ALL have expanded over the past 15 years, mainly due to the advent of tyrosine kinase inhibitors (TKIs) that have significantly improved outcomes in various combination regimens [
6,
7,
8].
The first-generation TKI (imatinib), the second-generation TKIs (dasatinib and nilotinib), and the third-generation TKI (ponatinib) have successfully been combined with chemotherapy in prospective studies conducted in adult patients with Ph+ ALL [
9,
10,
11]. More recently, a further improvement has been achieved with a chemo-free induction/consolidation strategy based on the combination of a TKI with the CD3/CD19 bispecific antibody blinatumomab [
12,
13]. All in all, these novel regimens have determined very substantial improvements in the progression-free survival (PFS) of these patients.
In the field of survival analysis, important methodological improvements have occurred in the past two years. In particular, the IPDfromKM method [
14] (also known as “Shiny method”) has established itself as a powerful tool to reconstruct individual patient data from the graphs of Kaplan-Meier curves. The main characteristic of the Shiny method is that each Kaplan-Meier curve is analyzed through an artificial intelligence software that reconstructs patient-level data over the entire study follow-up. In this way, treatments can be compared indirectly with one another, and the results can be interpreted by application of standard survival statistics. Despite its theoretical complexity, the Shiny method is extremely easy-to-use. In fact, only three pieces of information are needed to generate a patient database from a Kaplan-Meier curve: i) the graph of the curve; ii) the total number of patients for the curve concerned; iii) the total number of events. A wide experience has rapidly accumulated on the use of this approach, particularly in the area of anti-cancer agents [
15] In this report, we applied the Shiny method to analyze the most recent survival studies focused on the treatment of Ph+ ALL with TKIs combined with low-intensity regimens, steroids, and chemo-free approaches.
2. Materials and Methods
Study Design
Our analysis had the purpose to retrieve updated information on novel therapeutic approaches for ALL and to study in comparative terms the survival outcomes observed with these treatments. After a standard PubMed search, the datasets suitable for our analysis were identified. Our analysis included the datasets in which the information on PFS was reported (with follow-up of at least 2 years) and the graph of the Kaplan-Meier curve was available. As proposed in a recent review by Hadad et al [
9], these datasets were grouped as follows: i) treatments based on TKIs combined with reduced-intensity chemotherapy (denoted as TKICHE); ii) TKIs associated to steroids with no chemotherapy (denoted as TKISTE); iii) chemotherapy-free combinations of blinatumomab plus TKIs (denoted as TKIBLI). The trials evaluating these three types of treatment were subjected to the procedure of individual-patient data reconstruction according to the Shiny method. Thereafter, the treatments identified as TKICHE, TKISTE, and TKIBLI were compared with one another using standard survival statistics. Our endpoint was PFS. The results of our analysis were summarized in a multi-trial Kaplan-Meier graph generated on the basis of all reconstructed patients.
Literature Search
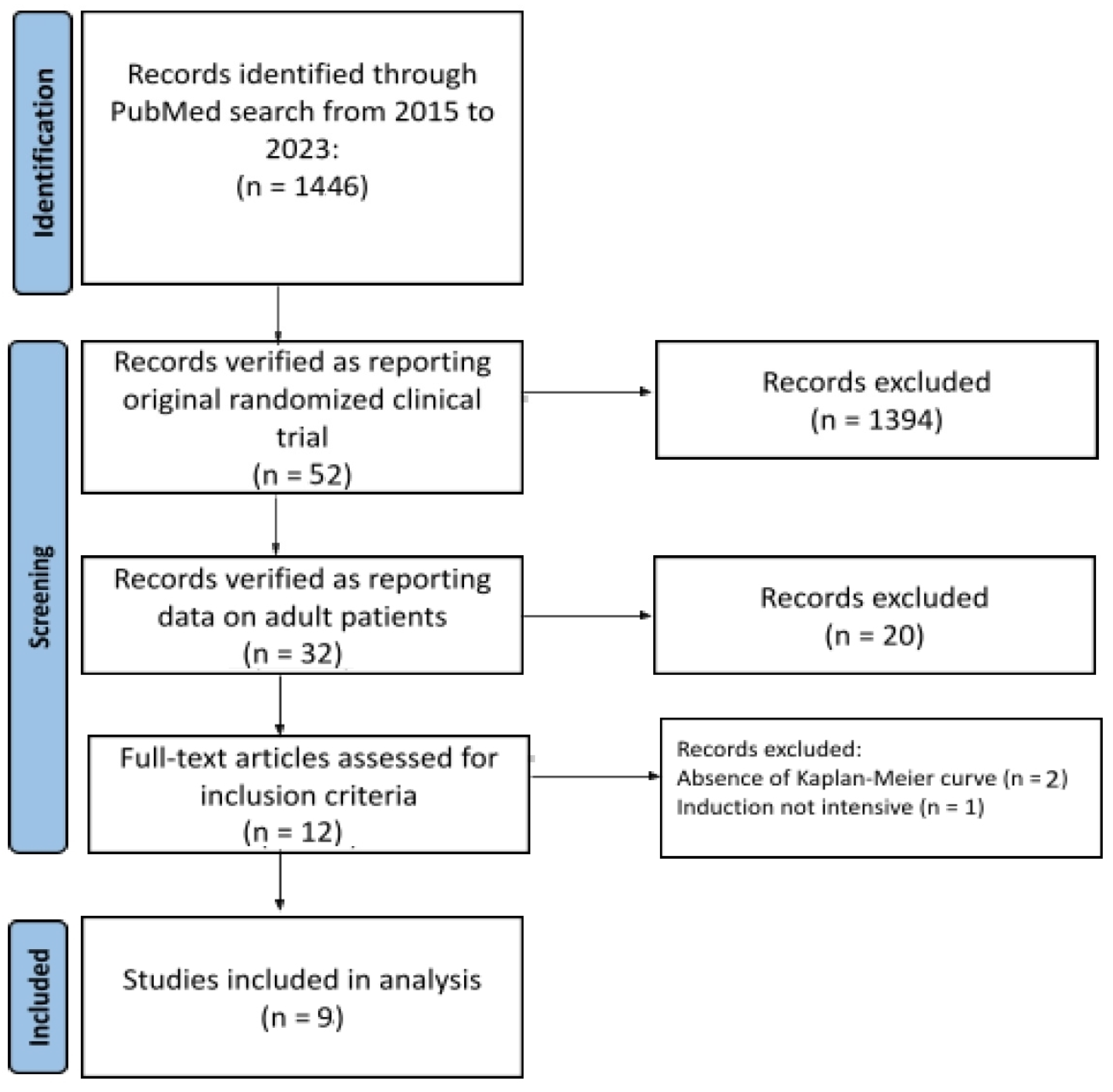
Our PubMed search covered the time interval from January 2015 to June 2023 (date of the last search: 20 June 2023; keyword, ”acute lymphoblastic leukaemia”; filters: “clinical trials” and “years from 2015 to 2023”). Original clinical trials and reviews extracted from PubMed were eligible for further scrutiny. Also abstracts were eligible if adequately indexed. Then, a further selection of this material identified some recent trials conducted in patients with ALL. When duplicate citations were found for the same trial, only the most updated dataset was included in our analysis. Trial selection was performed according to the Preferred Reporting Items for Systemic Review and Meta-Analyses (PRISMA) approach [
16]. Figure 1 shows the flow of trial selection based on the PRISMA algorithm.
Figure 1.
Literature search: flow of trial selection based on the PRISMA algorithm.
Figure 1.
Literature search: flow of trial selection based on the PRISMA algorithm.
Reconstruction of Individual Patient Data from Kaplan-Meier Survival Curves
We used the Shiny method [
14] which was implemented as in the other analyses published by our research group [
15]. Firstly, patient-level data were reconstructed from each of the treatment arms of the original trials. Thereafter, in cases where similar or identical treatments were investigated in different trials, the reconstructed patients were pooled into a single patient group. In this way, three treatment groups were formed for TKICHE, TKISTE, and TKIBLI. Finally, these three treatment groups were subjected to a standard survival statistics, in which PFS was the endpoint.
Survival Statistics
Survival statistics was carried out by standard methods using the Cox model. Head-to-head comparisons were assessed according to the hazard ratio (HR) with 95% confidence interval (CI). Statistical analyses, based on reconstructed patient-level data, were conducted under the R-platform as in our previous analyses [
15].
Assessment of Heterogeneity in Studies Pooled Together
In three separate analyses, we examined the degree of heterogeneity within the trials assigned to the TKICHE, TKISTE, and TKIBLI groups, respectively. Heterogeneity was likely to depend mainly on differences in patients’ inclusion criteria. Furthermore, in each of these three groups, heterogeneity was assessed through a post-hoc analysis aimed at estimating the degree of concordance between similar studies expected to report similar survival patterns. For this purpose, the likelihood ratio test and the concordance test were employed. Further details about this assessment of heterogeneity are presented in the Appendix.
Data Sharing Statement
For each treatment under comparison, the database of reconstructed patients is available from the author upon request.
3. Results
Our PubMed search firstly identified 12 studies [
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29]. The study by Ottman et al [
17] was excluded owing to the absence of the Kaplan-Meier curve; likewise, the study by Short et al [
29] was excluded owing to the absence of information on PFS. The study by Chalandon et al [
18] was excluded because only induction was not intensive. Hence, a total of 9 studies and 848 patients (
Table 1) were selected for our analysis [
19,
20,
21,
22,
23,
24,
25,
26,
27].
The following agents were investigated in these 9 trials:
trial [a]: dasatinib in combination with low-intensity chemotherapy (Rousselot et al [
19]);
trial [b]: nilotinib combined with low-intensity chemotherapy (Rousselot et al [
20]);
trial [c]: dasatinib plus steroids induction followed by dasatinib alone (Chiaretti et al [
21]);
trial [d]: azacytidine combined with venetoclax (DiNardo et al [
22]);
trial [e]: imatinib combined with steroids (Vignetti et al [
23]);
trial [f]: dasatinib induction therapy combined with steroids (Foà et al [
24]);
trial [g]: ponatinib plus prednisone (Martinelli et al [
25]);
trial [h]: dasatinib plus blinatumomab (Chiaretti et al [
26]);
trial [i]: ponatinib plus blinatumomab (Jabbour et al [
27]).
Table 1.
Main characteristics of the included trials.
Table 1.
Main characteristics of the included trials.
| |
First Author |
Year |
Trial |
Treatment |
Inclusion criteria |
Events |
N° of patients |
Notes |
| TKICHE |
Chaladon et al [18] |
2015 |
GRAAPH-2005 study |
Imatinib combined with low-intensity chemotherapy |
Patients aged 18 to 59 years with newly diagnosed Ph1 and/or BCRABL1–positive ALL were eligible |
65 |
135 |
Excluded from our analysis because only induction was not intensive |
| Rousselot et al [19] |
2016 |
EWALL-PH-01 international study |
Dasatinib in combination with low-intensity chemotherapy |
Patients aged 55 years or older were eligible if they had newly diagnosed Ph1 and/or BCR-ABL ALL |
40 |
71 |
Included |
| Rousselot et al [20] |
2021 |
Graaph-2014 Study |
Nilotinib combined with low-intensity chemotherapy |
Ph-Positive ALL patients aged 18-60 years old were randomized |
23 |
79 |
Included |
| Chiaretti et al [21] |
2020 |
GIMEMA LAL1509 |
Dasatinib plus steroids induction followed by dasatinib alone |
Adult Ph+ ALL patients (18-60 years). |
13 |
58 |
Included |
| |
DiNardo et al [22] |
2020 |
Volta-A |
Azacitidine combined with venetoclax |
Previously untreated patients ineligible for standard induction therapy because of coexisting conditions and/or 75 years of age or older |
161 |
286 |
Included |
| TKISTE |
Vignetti et al [23] |
2007 |
GIMEMA LAL0201-B |
Imatinib combined with steroids |
Patients with a diagnosis of ALL who were older than 60 years were eligible if they carried either the Ph chromosome or the BCR-ABL molecular translocation |
16 |
29 |
Included |
| Foà et al [24] |
2011 |
GIMEMA LAL1205
|
Dasatinib induction therapy combined with steroids |
Patients 18 years of age or older (with no upper age limit) were eligible if they had been diagnosed with Ph/BCR-ABLALL |
23 |
53 |
Included |
| Martinelli et al [25] |
2022 |
GIMEMA
LAL 1811 |
Ponatinib plus prednisone |
Patients had new-onset Ph+ ALL, and were ≥ 60 years or were ≥ 18 years but unfit for a program of intensive chemotherapy and SCT |
34 |
44 |
Included |
| TKIBLI |
Chiaretti et al [26] |
2022 |
GIMEMA LAL2217 |
Dasatinib + blinatumomab |
Ph-Positive ALL Patients, median age was 54 years (24-82; no upper age limit) |
9 |
58 |
Included |
| Jabbour et al [27] |
2023 |
NCT03263572 |
Ponatinib + blinatumomab |
Patients with newly diagnosed, relapsed/refractory Ph+ ALL or CML in lymphoid blast phase |
2 |
40 |
Included |
According to our study protocol, individual patient data from these 9 trials were reconstructed by application of the Shiny method. Then, the treatments from different trials belonging to the same pharmacological class were pooled into a single patient group. The following three groups were formed:
The regimen denoted as TKICHE (i.e. TKI plus low-intensity chemotherapy), which includes four trials, namely dasatinib plus low-intensity chemotherapy in the trial by Rousselot et al [
19], nilotinib plus low-intensity chemotherapy in the GRAAPH-2014 Study by Rousselot et al [
20], dasatinib plus steroids induction in the trial by Chiaretti et al [
21], and azacytidine plus venetoclax in the trial by DiNardo et al. [
22].
The regimen denoted as TKISTE (i.e. TKIs combined with steroids), which includes three trials, namely those by Vignetti et al [
23], Foà et al [
24] and Martinelli et al [
25].
The regimen denoted as TKIBLI (i.e. TKI plus blinatumomab), which includes two trials, namely blinatumomab combined with the second-generation dasatinib, as reported by Chiaretti et al [
26], and blinatumomab combined with the third-generation ponatinib as reported by Jabbour et al [
27].
The assignment to TKICHE of the trial by Chiaretti et al [
21] can be a matter of controversy because the regimen of this trial was chemo-free rather than based on low-intensity. Consistently with the review by Hadad et al [
9], we kept this trial by Chiaretti in the TKICHE group; likewise, the trial by DiNardo et al [
22] had a specific characteristic in that the patients were older than 75 years.
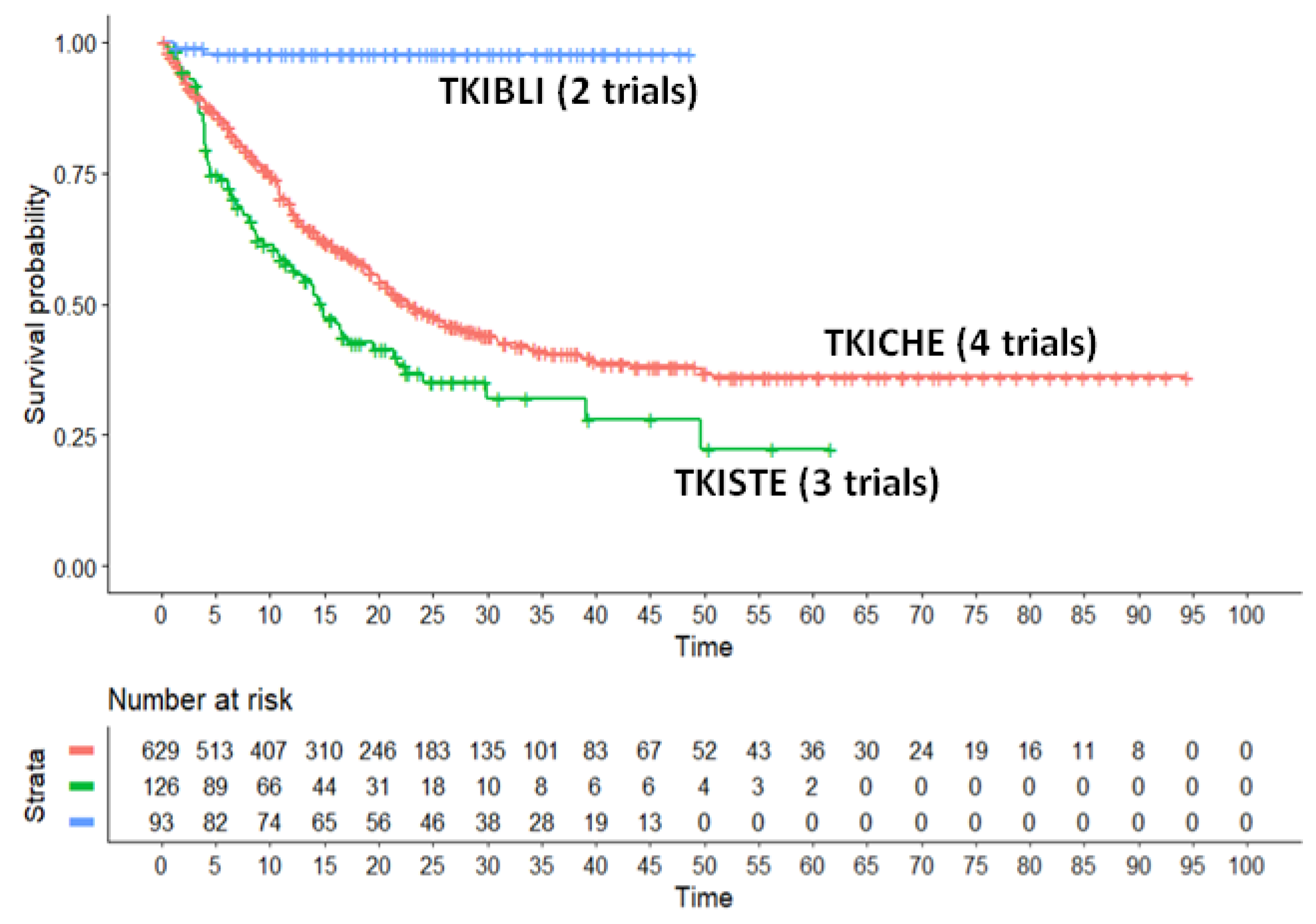
In our main analysis, these three regimens (TKICHE, TKISTE, and TKIBLI) were compared with one another based on the endpoint of PFS. The results of this analysis are shown in Figure 1, that presents the Kaplan-Meier curves generated for these three regimens from reconstructed patients. In the statistical comparisons across these three regimens, the following values of HR were estimated:
- -
HR for the comparison of TKICHE vs TKISTE = 0.6844 (95%CI, 0.5291 to 0.8852);
- -
HR for the comparison of TKIBLI vs TKICHE = 0.034 (95%CI, 0.0846 to 0.137);
- -
HR for the comparison of TKIBLI vs TKISTE = 0.023 (95%CI, 0.006 to 0.096).
All these three values of HR are statistically significant. Medians for these three regimens were 22.9 months for TKICHE (95%CI, 20.6 to 27.9), 14.7 months for TKISTE (95%CI, 11.5 to 21.7), and not computable for TKIBLI. According to these results, the advantage in PFS for TKIBLI compared with the other two regimens has a remarkable clinical relevance, along with its high level of statistical significance.
Finally, besides our main analysis, we separately assessed the degree of heterogeneity for the three regimes (TKICHE, TKISTE, and TKIBLI). For this purpose, we carried out three post-hoc analyses in which the likelihood ratio test was estimated. These post-hoc analyses are presented in detail in the Appendix.
4. Discussion
Elderly or unfit patients are not candidates for intensive chemotherapy owing to the high risk of morbidity and mortality. Hence, lower-intensity regimens have been designed especially for these patients [
19], even though they have also been explored in younger, more fit patients with newly diagnosed Ph+ ALL [
21]. Similarly, the regimens based on induction therapy with steroids plus TKIs have mainly been tested in elderly or unfit patients [
23,
24,
25].
Findings from these studies suggest that low-intensity therapies are safe and feasible in patients with Ph+, particularly those who are older and/or unfit for intensive chemotherapy or allogeneic stem cell transplantation (allo-SCT). On the other hand, blinatumomab has initially been shown to be highly effective as a single agent in patients with relapsed/refractory Ph+ ALL [
28]; thereafter, blintumomab been investigated in combination with a TKI [
26,
27] determining excellent results. The two trials by Chiaretti et al [
26] and by Jabbour et al [
27] are those included in our TKIBLI regimen.
Our analysis based on 848 patients reconstructed from 9 trials showed that the chemo-free induction/consolidation strategy, based on the sequential administration of dasatinib or ponatinib followed by blinatumomab (i.e. the TKIBLI regimen), demonstrates a clear superiority compared with TKICHE or TKISTE regimens. For example, in the updated analysis of the GIMEMA LAL2116 trial [
13], Chiaretti et al [
26] reported very favorable outcomes, with an estimated overall survival of 78% (95% CI, 66-92%) at 48 months and DFS of 75% (95% CI, 64-87%). While these findings are impressive, one should keep in mind the limited size of the patient groups enrolled in the two trials by Chiaretti et al [
26] and Jabbour et al [
27].
Also the results presented at the 2022 European Hematology Association (EHA) Congress by Short et al [
29] confirm that a chemotherapy-free regimen of simultaneous ponatinib and blinatumomab is safe and effective, for newly diagnosed Ph+ ALL; the 2-year event-free survival (EFS) and overall survival were both 93% while in the relapsed/refractory Ph+ ALL cohort, the 2-year EFS rate was 42% and the 2-year overall survival rate was 61%.
Our analysis has some limitations. The first limitation is given by the presence of different eligibility criteria among the trials that we pooled into the same group. For example, regarding the TKICHE group of trials, patients older than age 55 years were eligible in the study by Rousselot et al [
19], while patients aged more than 75 years represented 61% of those included in the Volta-A study by DiNardo et al [
22]. In the Graaph-2014 study, Rousselot et al [
20] included patients aged 18 to 60 years.
Furthermore, regarding the TKIBLI group, patients had a median age of 54 years (range, 24-82) in the GIMEMA LAL2217 trial [
26], while in the study by Jabbour et al [
27] the patients had a median age of 51 years and required to have a performance status of ≤2 without comorbidities. These characteristics of the patients included in the TKIBLI trials may have influenced the favorable outcome of this therapeutic approach compared with TKICHE and TKISTE. Finally, some caution is warranted in interpreting the results of the two TKIBLI trials because the overall number of enrolled patients was limited.
Another aspect that deserves specific comments is safety. Chemotherapy-free regimens with blinatumomab combined with second or third generation TKIs permit to avoid cytotoxic therapies, thus contributing to improve prognosis. While most imatinib adverse events tend to be mild and often resolve spontaneously, rare but serious side effects have occasionally been reported with later generation TKIs [
30]. Since most patients who do not undergo allo-SCT are recommended to receive indefinite TKI therapy, significant open questions remain concerning long-term outcomes in ALL patients besides mortality. Further prospective studies are needed to identify patients who can safely discontinue TKIs, an option that gains increasing interest with time.
5. Conclusions
In conclusion, major progress has been achieved in the management of Ph+ ALL. After the combination of TKIs with low intensive chemotherapy or steroids has considerably improved long-term survival, chemotherapy-free regimens with blinatumomab and TKIs seem to represent a further advancement that may revolutionize the landscape of Ph+ ALL. In particular, the combination of second generation dasatinib or third generation ponatinib plus blinatumomab is associated with deep and durable remissions while avoiding cytotoxic therapies and mitigating the need for allo-SCT. Our results, obtained from reconstructed patient data, suggest that this strategy might be the most effective; on the other hand, the regimens based on blinatumomab still require further investigation because this option presently is supported only by small-size studies. Finally, the present analysis confirms that the good performance of the Shiny method in improving the analysis and the interpretation of survival results in hematologic malignancies [
31,
32,
33,
34].
Author Contributions
Author AM was responsible for study conception, design and acquisition of data; author MR was responsible for data analysis and drafting; authors AM, DM and MC were responsible for data quality assurance. All authors revised the manuscript and approved the final version of the manuscript to be published.
Funding
This research received no external funding.
Data Availability Statement
For each of the 9 treatments under comparison, the database of reconstructed patients is available from the authors upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix
Appendix: Indirect Head-to-Head Efficacy Comparisons for the Three Regimens (TKICHE vs TKISTE vs TKIBLI)
This Appendix describes the methods and the results of the post-hoc analyses carried out for each of the three combination treatment regimens (TKICHE, TKISTE, and TKIBLI). PFS was the endpoint.
In these three post-hoc analyses, the regimens studied in at least two different trials were pooled into a single patient group provided that the treatments belonged to the same pharmacological class, i.e. TKICHE, TKISTE, or TKIBLI. This choice to pool similar (or even identical) combination treatments from different trials was aimed at avoiding an excessive fragmentation of our statistical results but exposed our analysis to an increased risk of underestimating between-trial variability. To manage this issue, each case of data pooling of similar or identical treatments across at least two trials was investigated by a further post-hoc PFS analysis, in which the survival data from the different trials were kept separate and heterogeneity was assessed formally. In these post-hoc analyses, the patient inclusion criteria of individual trials were also reviewed; this information has already been reported in narrative form in
Table 1. Heterogeneity in these three analyses was assessed according to the likelihood ratio and concordance test.
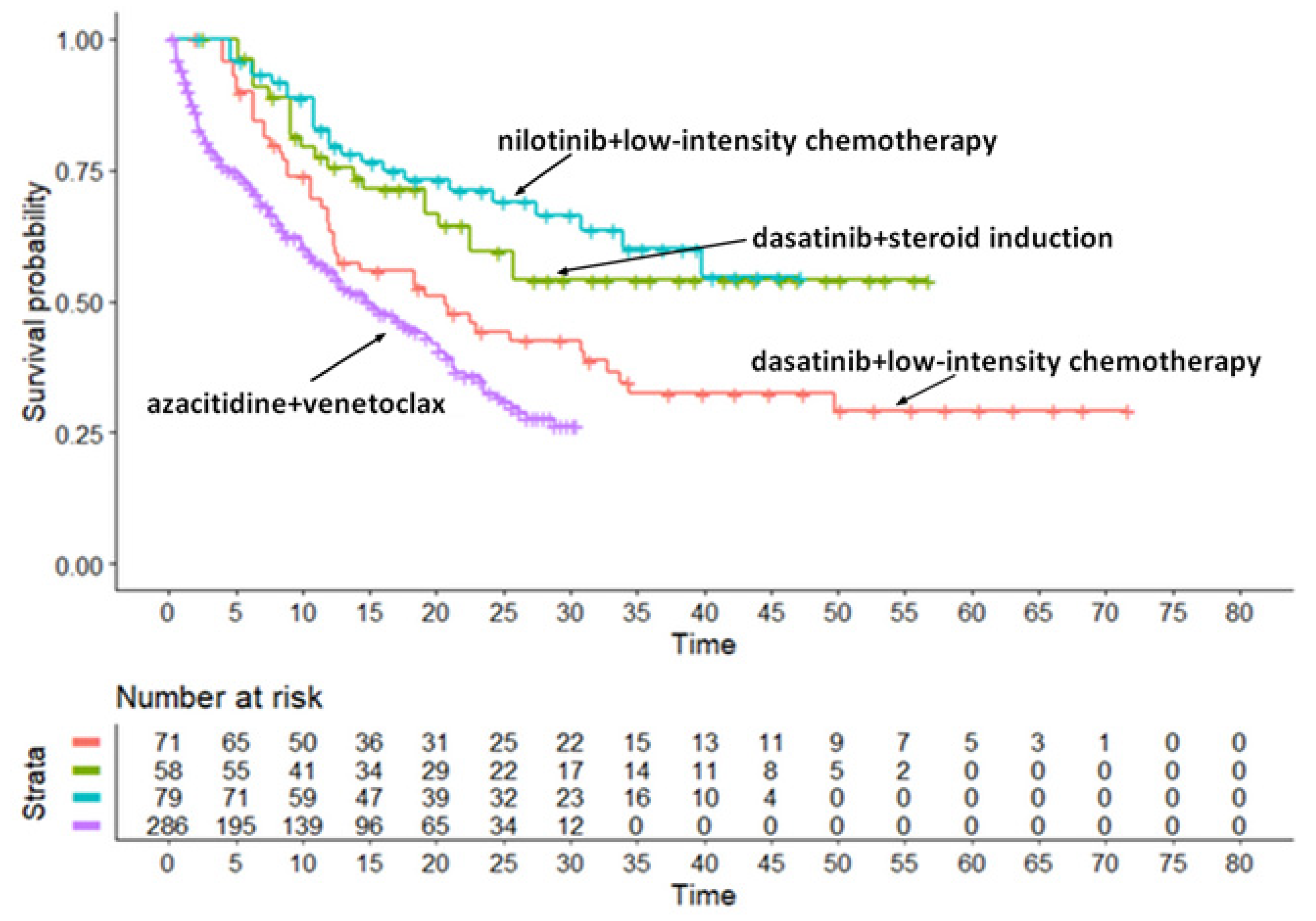
Survival Analysis for the TKICHE Regimen (4 Trials)
The first group of similar/identical treatments included the combination of TKIs plus chemotherapy according to 4 trials, namely dasatinib + chemotherapy in the EWALL-PH-01trial by Rousselot et al [
19], nilotinib + chemotherapy in the Graaph-2014 Study by Rousselot et al [
20], dasatinib+steroid induction followed by dasatinib in the LAL1509 trial by Chiaretti et al [
21], and azacytidine + venetoclax in the Volta-A trial by DiNardo et al [
22]. The post-hoc analysis for these regimens is described in Figure 1A. Medians of PFS for these four patient groups were the following:
- -
Trial [a]: 20.7 months (95%CI, 12.3 to 33.7 months) [
19];
- -
Trial [b]: not computable (95%CI, 22.5 months to not computable) [
20];
- -
Trial [c]: not computable (95%CI, 34.0 months to not computable) [
21];
- -
Trial [d]: 14.7 months (95%CI, 12.0 to 19.6) [
22].
Indirect head-to-head comparisons gave the following values of HR:
Trial [a] vs [b]: HR = 0.579 (95%CI, 0.347 to 0.966);
Trial [a] vs [c]: HR = 0.476 (95%CI, 0.289 to 0.783);
Trial [d] vs [a]: HR = 1.490 (95%CI, 1.057 to 2.102);
Trial [c] vs [b]: HR = 0.822 (95%CI, 0.398 to 1.698);
Trial [d] vs [b]: HR = 2.57 (95%CI, 1.31 to 4.831);
Trial [c] vs [d]: HR = 0.319 (95%CI, 0.174 to 0.585).
As shown above, among the 6 head-to-head indirect comparisons, 5 were significant, whereas the remaining one (comparison of [c] vs [b]) was not. The likelihood ratio test showed a quite strong heterogeneity (likelihood ratio test= 105.7 on 2 df, p<0.001), which likely depends on the presence of numerous significantly different comparisons. Concordance was 0.587 (se = 0.011). It should be kept in mind thar the patients’ age in the trial by DiNardo et al [
22] was older than that of the other three trials. This likely explains the remarkably worse survival pattern found in this trial.
Figure 1A.
Post-hoc analysis for the TKICHE regimen. The treatments shown in this Kaplan-Meier graph include dasatinib in combination with low-intensity chemotherapy (Rousselot et al [
19], trial [a]) in red, dasatinib plus steroids induction followed by dasatinib alone (Chiaretti et al [
21], trial [c]) in blue, nilotinib combined with chemotherapy (Rousselot et al [
20], trial [b]) in green, and azacitibine combined with venetoclax (DiNardo et al [
22], trial [d]) in purple. Time in months, endpoint DFS.
Figure 1A.
Post-hoc analysis for the TKICHE regimen. The treatments shown in this Kaplan-Meier graph include dasatinib in combination with low-intensity chemotherapy (Rousselot et al [
19], trial [a]) in red, dasatinib plus steroids induction followed by dasatinib alone (Chiaretti et al [
21], trial [c]) in blue, nilotinib combined with chemotherapy (Rousselot et al [
20], trial [b]) in green, and azacitibine combined with venetoclax (DiNardo et al [
22], trial [d]) in purple. Time in months, endpoint DFS.
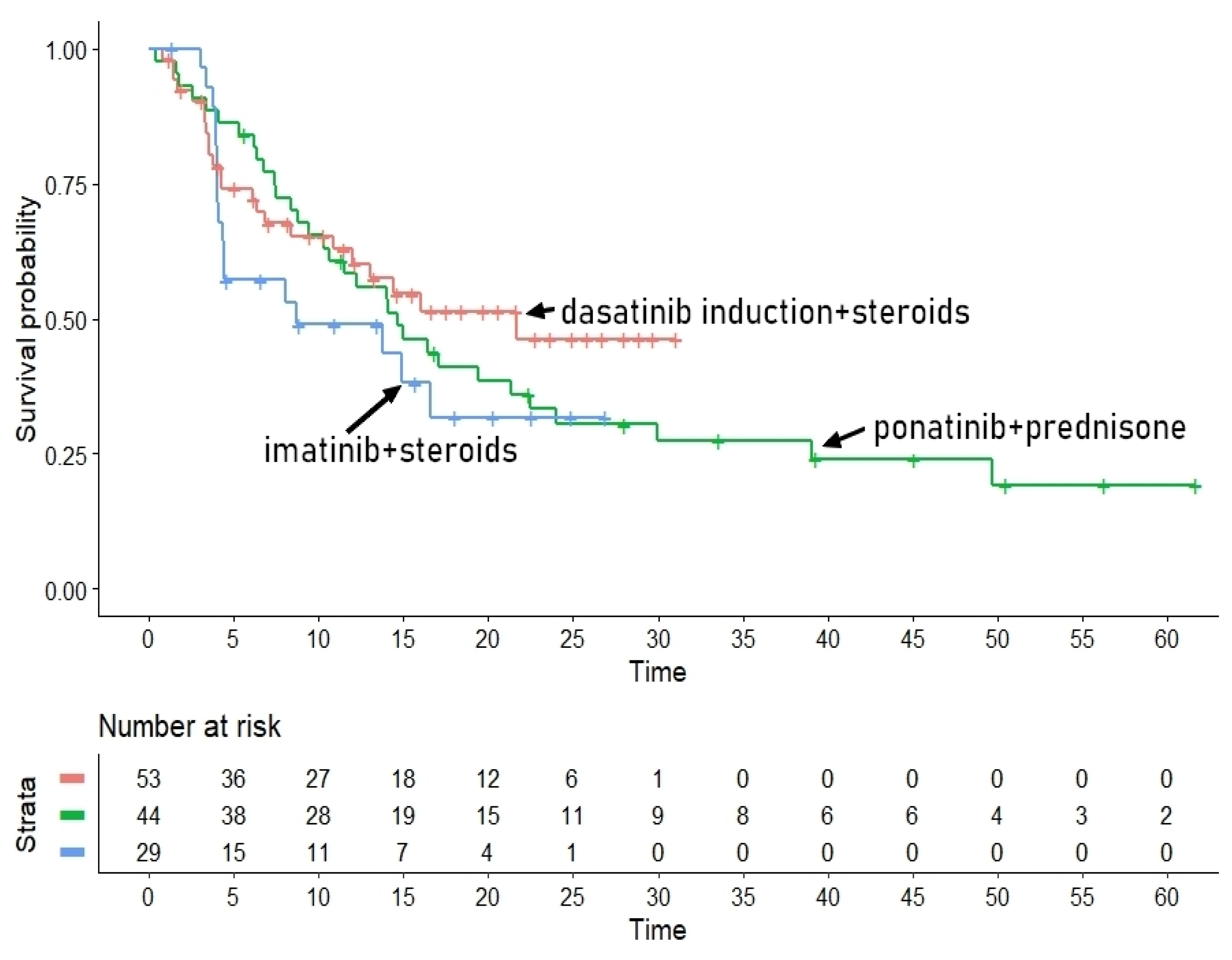
Survival Analysis for the TKISTE Regimen (3 Trials)
The second group of pharmacologically similar treatments included the combination of TKIs plus steroids, for which three trials were available, namely those by Vignetti et al21, Foà et al [
22] and Martinelli et al.[
23]. The post-hoc analysis for this regimen is described in Figure 2A. Medians of PFS for these three patient groups were the following:
- -
Trial [e]: 21.67 months (95%CI, 11.97 to not computable)
- -
Trial [f]: 14.69 months (95%CI, 10.65 to 24.0)
- -
Trial [g]: 8.66 months (95%CI, 4.38 to not computable).
The values of HR for the indirect head-to-head comparisons were the following:
- -
Trial [f] vs Trial [e]: HR= 1.22 (95%CI, 0.71 to 2.11);
- -
Trial [f] vs Trial [e]: HR= 1.53 (95%CI, 0.82 to 2.88);
- -
Trial [g] vs Trial [f]: HR=1.26 (95%CI, 0.55 to 2.89).
Figure 2A.
Post-hoc analysis for the TKISTE regimen. In blue imatinib combined with steroids (Vignetti et al [
21], trial [g]), in red dasatinib induction therapy combined with steroids (Foà et al [
22], trial [e]), and in green ponatinib plus prednisone (Martinelli et al [
23], trial [f]). Time in months, endpoint DFS.
Figure 2A.
Post-hoc analysis for the TKISTE regimen. In blue imatinib combined with steroids (Vignetti et al [
21], trial [g]), in red dasatinib induction therapy combined with steroids (Foà et al [
22], trial [e]), and in green ponatinib plus prednisone (Martinelli et al [
23], trial [f]). Time in months, endpoint DFS.
Despite the different medians of the three trials, the three head-to-head indirect comparisons did not achieve statistical significance. Finally, the likelihood ratio test (1.76 on 2 df, p=0.40) showed an acceptable homogeneity among these three patient groups. Concordance was 0.533 (se = 0.037).
Figure 2.
Kaplan-Meier survival curves generated from reconstructed patients s for three combination regimens. Treatments from different trials belonging to the same pharmacological class were pooled into a single patient group (TKICHE or TKISTE or TKIBLI). In red, TKI plus chemotherapy (TKICHE); in green, TKI plus steroids (TKISTE); in blue, TKI plus blinatumomab (TKIBLI). Time in months, endpoint DFS.
Figure 2.
Kaplan-Meier survival curves generated from reconstructed patients s for three combination regimens. Treatments from different trials belonging to the same pharmacological class were pooled into a single patient group (TKICHE or TKISTE or TKIBLI). In red, TKI plus chemotherapy (TKICHE); in green, TKI plus steroids (TKISTE); in blue, TKI plus blinatumomab (TKIBLI). Time in months, endpoint DFS.
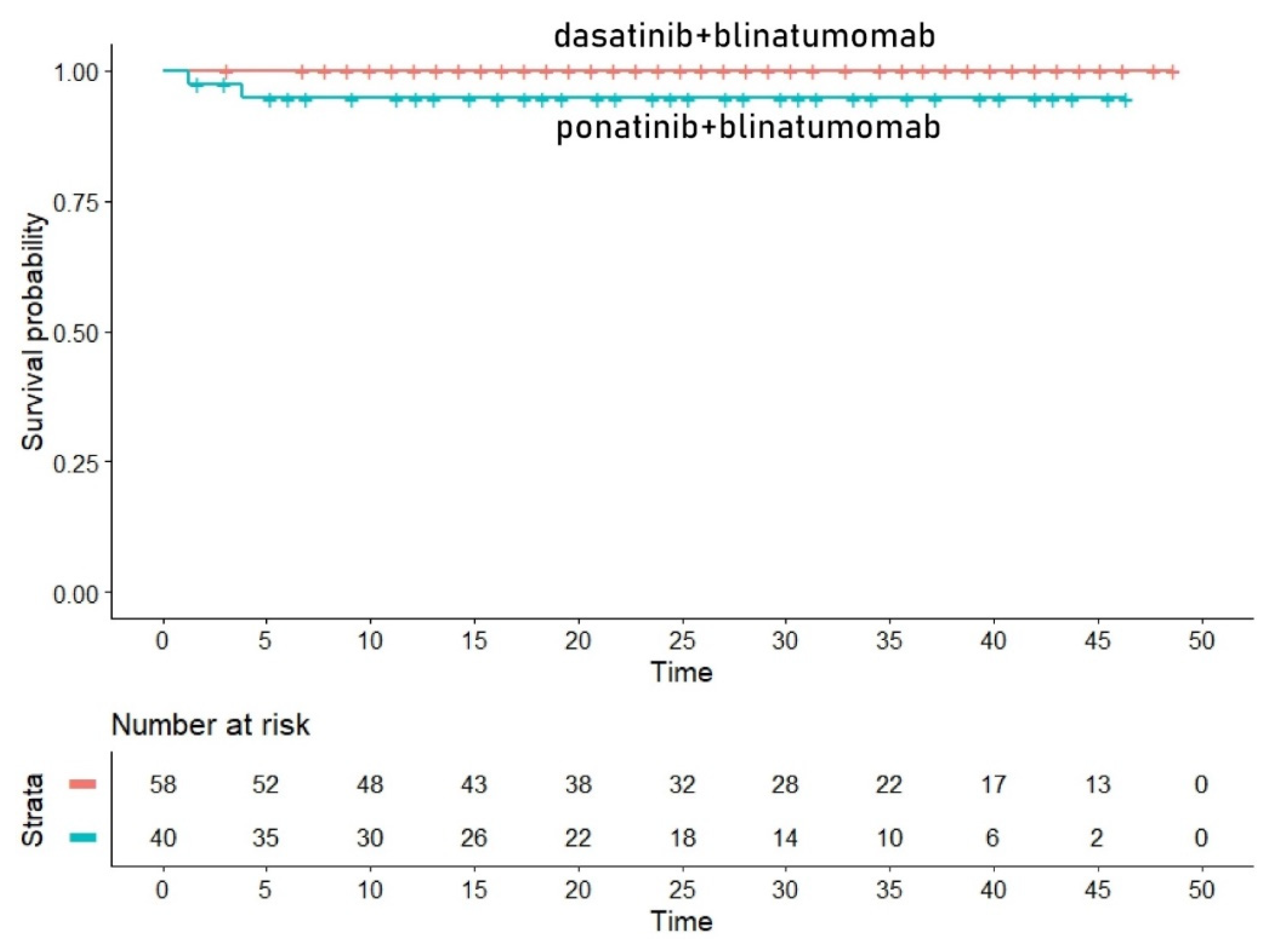
Survival Analysis for the TKIBLI Regimen (2 Trials)
Finally, the third group of similar/identical treatments regarded blinatumomab combined with a second or third generation TKI. In this group, we included two trials, namely blinatumomab combined with the second-generation dasatinib as reported by Chiaretti et al [
26] and blinatumomab combined with the third generation ponatinib reported by Jabbour et al [
27]. Quite interestingly, in this case, the two curves were nearly identical (Figure 3A). Since no events were reported in the dasatinib+blinatumomab trial by Chiaretti et al [
26], both the HR and the likelihood ratio test could not be computed. Likewise, medians could not be computed from these two curves. However, concordance was high (concordance= 0.799, se = 0.025).
Figure 3A.
Post-hoc analysis for the TKIBLI regimen. In red dasatinib plus blinatumomab (Chiaretti et al [
26], trial [h]) and in blue ponatinib plus blinatumomab (Jabbour et al [
27], trial [i]). Time in months, endpoint DFS.
Figure 3A.
Post-hoc analysis for the TKIBLI regimen. In red dasatinib plus blinatumomab (Chiaretti et al [
26], trial [h]) and in blue ponatinib plus blinatumomab (Jabbour et al [
27], trial [i]). Time in months, endpoint DFS.
References
- Chiaretti, S.; Vitale A.; Cazzaniga G.; et al. Clinico-biological features of 5202 patients with acute lymphoblastic leukemia enrolled in the Italian AIEOP and GIMEMA protocols and stratified in age cohorts. Haematologica. 2013;98(11):1702-1710. [CrossRef]
- Ravandi, F.; Kebriaei, P. Philadelphia chromosome-positive acute lymphoblastic leukemia. Hematol Oncol Clin North Am. 2009;23(5):1043-1063.
- Burmeister, T.; Schwartz, S.; Bartram, C.R.; et al. Patients’ age and BCR–ABL frequency in adult B- precursor ALL: A retrospective analysis from the GMALL study group. Blood. 2008;112(3):918-919. [CrossRef]
- Moorman, A.V.; Harrison. C.J.; Buck, G.A.; et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): Analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/ Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood. 2007;109(8):3189-3197. [CrossRef]
- Dombret, H.; Gabert, J.; Boiron, J.M.; et al. Outcome of treatment in adults Philadelphia chromosome-positive acute lymphoblastic leukemia-results of the prospective multi-center LALA-94 trial. Blood. 2002;100(7):2357-2366. [CrossRef]
- Ottmann, O.G.; Wassmann, B.; Pfeifer, H.; et al. Imatinib compared with chemotherapy as front-line treatment of elderly patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL). Cancer. 2007;109(10):2068-2076. [CrossRef]
- Talpaz, M.; Shah, N.P.; Kantarjian, H.; et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354(24):2531-2541. [CrossRef]
- Bassan, R.; Rossi, G.; Pogliani, E.M.; et al. Chemotherapy-phased imatinib pulses improve long- term outcome of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: Northern Italy Leukemia Group protocol 09/00. J Clin Oncol. 2010;28(22):3644-3652. [CrossRef]
- Haddad, F.G.; Sawyers, J.; Short, N.J. Treatment de-escalation in Philadelphia chromosome-positive B-cell acute lymphoblastic leukemia: The emerging role of chemotherapy-free regimens. Ther Adv Hematol. 2023 Feb 3;14:20406207231151294. [CrossRef]
- Chiaretti, S.; Vitale, A.; Vignetti, M.; et al. A sequential approach with imatinib.; chemotherapy and transplant for adult Ph1 acute lymphoblastic leukemia: Final results of the GIMEMA LAL 0904 study. Haematologica. 2016;101(12):1544-1552. [CrossRef]
- Jabbour, E.; Haddad, F.G.; Short, N.J.; Kantarjian, H. Treatment of Adults With Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia-From Intensive Chemotherapy Combinations to Chemotherapy-Free Regimens: A Review. JAMA Oncol. 2022;8(9):1340-1348. [CrossRef]
- Assi, R.; Kantarjian, H.; Short, N.J.; et al. Safety and Efficacy of Blinatumomab in Combination With a Tyrosine Kinase Inhibitor for the Treatment of Relapsed Philadelphia Chromosome-positive Leukemia. Clin Lymphoma Myeloma Leuk. 2017;17(12):897-901. [CrossRef]
- Foà, R.; Bassan, R.; Vitale, A.; et al. Dasatinib-Blinatumomab for Ph+ Acute Lymphoblastic Leukemia in Adults. N Engl J Med. 2020;383(17):1613-1623. [CrossRef]
- Liu, N.; Zhou, Y.; Lee, J.J. IPDfromKM: Reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2021;21(1):11. [CrossRef]
- Ossato, A.; Mengato, D.; Chiumente, M.; Messori A.; Damuzzo V. Progression-Free and Overall Survival of First-Line Treatments for Advanced Renal Cell Carcinoma: Indirect Comparison of Six Combination Regimens. Cancers (Basel). 2023 Mar 29;15(7):2029. [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann. Intern. Med. 2009; 151; W65–W94. [CrossRef]
- Ottmann, O.G.; Pfeifer, H.; Cayuela, J.M. et al. Nilotinib (Tasigna®) and Low Intensity Chemotherapy for First-Line Treatment of Elderly Patients with BCR-ABL1-Positive Acute Lymphoblastic Leukemia: Final Results of a Prospective Multicenter Trial (EWALL-PH02). Blood 2018; 132 (Supplement 1): 31. [CrossRef]
- Chalandon, Y.; Thomas, X.; Hayette S.; et al. Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph+ acute lymphoblastic leukemia. Blood. 2015;125(24):3711-3719 [published correction appears in Blood. 2015 Sep 3;126(10):1261]. [CrossRef]
- Rousselot, P.; Coudé, M.M.; Gokbuget N.; et al. Dasatinib and low-intensity chemotherapy in elderly patients with Philadelphia chromosome-positive ALL. Blood. 2016;128(6):774-782. [CrossRef]
- Rousselot P.; Chalandon Y.; Chevret S.; Cayuela, J.M.; Huguet F.; Chevallier P.; et al The Omission of High-Dose Cytarabine during Consolidation Therapy of Ph+ ALL Patients Treated with Nilotinib and Low-Intensity Chemotherapy Results in an Increased Risk of Relapses Despite Non-Inferior Levels of Late BCR-ABL1 MRD Response. First Results of the Randomized Graaph-2014 Study. Blood 2021; 138 (Supplement 1): 512. [CrossRef]
- Chiaretti, S.; Ansuinelli, M.; Vitale, A.; et al. A multicenter total therapy strategy for de novo adult Philadelphia chromosome positive acute lymphoblastic leukemia patients: Final results of the GIMEMA LAL1509 protocol. Haematologica. 2021;106(7):1828-1838. [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J Med. 2020 Aug 13;383(7):617-629. [CrossRef]
- Vignetti, M.; Fazi, P.; Cimino, G.; et al. Imatinib plus steroids induces complete remissions and prolonged survival in elderly Philadelphia chromosome-positive patients with acute lymphoblastic leukemia without additional chemotherapy: Results of the Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) LAL0201-B protocol. Blood. 2007;109(9):3676-3678. [CrossRef]
- Foà, R.; Vitale, A.; Vignetti, M.; et al. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;118(25):6521-6528. [CrossRef]
- Martinelli, G.; Papayannidis, C.; Piciocchi, A.; et al. INCB84344-201: Ponatinib and steroids in frontline therapy for unfit patients with Ph+ acute lymphoblastic leukemia. Blood Adv. 2022;6(6):1742-1753. [CrossRef]
- Chiaretti, S.; Bassan, R.; Vitale, A.; et al. Forty Months Update Of The Gimema Lal2116 (D-alba) Protocol And Ancillary Lal2217 Study For Newly Diagnosed Adult Ph+ All [abstract]. EHA Library.2022 Abstract: P353.
- Jabbour, E.; Short, N.J.; Jain, N.; et al. Ponatinib and blinatumomab for Philadelphia chromosome-positive acute lymphoblastic leukaemia: A US.; single-centre.; single-arm.; phase 2 trial. Lancet Haematol. 2023;10(1):e24-e34. [CrossRef]
- Martinelli, G.; Boissel, N.; Chevallier, P.; et al. Complete Hematologic and Molecular Response in Adult Patients With Relapsed/Refractory Philadelphia Chromosome-Positive B-Precursor Acute Lymphoblastic Leukemia Following Treatment With Blinatumomab: Results From a Phase II.; Single-Arm.; Multicenter Study. Journal of Clinical Oncology 2017 35:16.; 1795-1802. [CrossRef]
- Short NJ, Kantarjian HM, Konopleva M; et al. A phase II trial of a chemotherapy-free combination of ponatinib and blinatumomab in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL). Journal of Clinical Oncology 2022;40:7009. [CrossRef]
- Steegmann, J.L.; Baccarani, M.; Breccia, M.; et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia. 2016;30(8):1648-1671. [CrossRef]
- Messori, A.; Rivano, M.; Mengato, D.; Cancanelli, L.; Di Spazio, L.; Chiumente, M. A preliminary estimate of survival gain and cost-effectiveness of CAR-T in adult patients with acute lymphoblastic leukemia. Leuk Lymphoma. 2022;63(5):1261-1264. [CrossRef]
- Messori, A.; Chiumente, M.; Mengato, D. Chimeric Antigen Receptor T Cells in Large B-Cell Lymphoma: Analysis of Overall Survival Based on Reconstructed Patient-Level Data. Clin Ther. 2022;44(12):1626-1632. [CrossRef]
- Messori, A.; Damuzzo, V.; Leonardi, L.; Agnoletto, L.; Chiumente, M.; Mengato, D. CAR-T Treatment: Determining the Survival Gain in Patients With Relapsed or Refractory Diffuse Large B-cell Lymphoma. Clin Lymphoma Myeloma Leuk. 2020;20(7):490-491. [CrossRef]
- Messori, A. Long-term progression-free survival in patients with chronic lymphocytic leukemia treated with novel agents: An analysis based on indirect comparisons. Eur J Haematol. 2023;110(1):60-66. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).