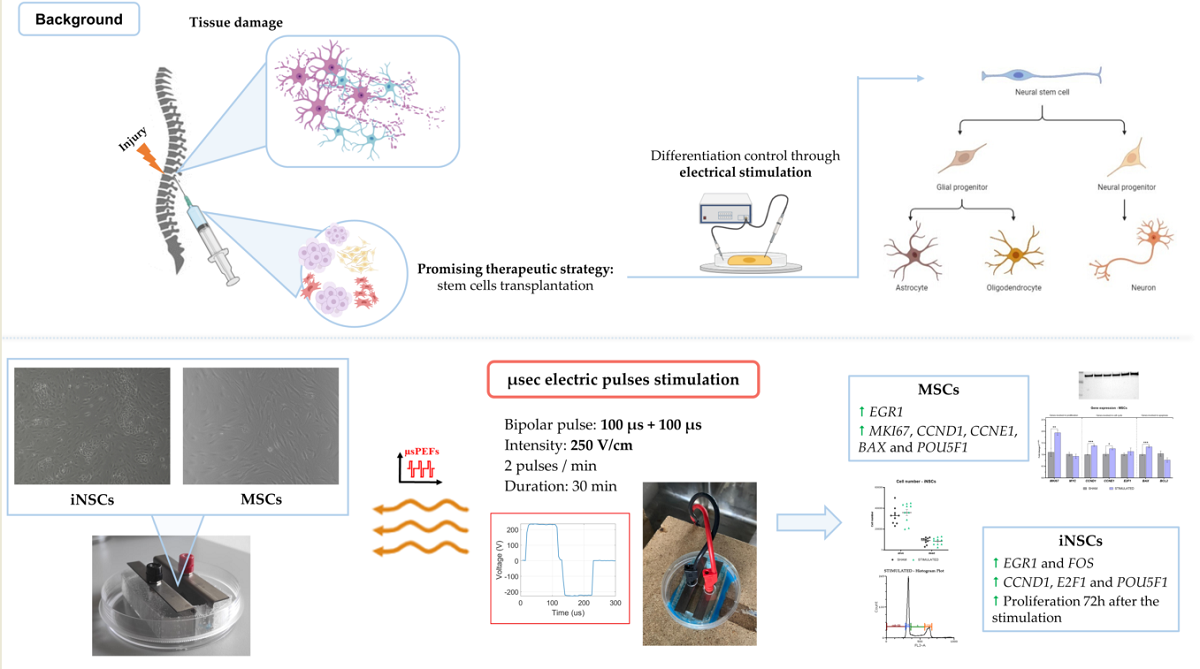
1. Introduction
Stem cells are undifferentiated or partially differentiated cells of different origins (embryonic and adult) that can differentiate in multiple cell types. Thanks to this feature, they represent a very promising therapeutic strategy for all those diseases characterized by permanently damaged and/or degenerated tissue. In the nervous system, for instance, neuronal stem cells have the ability to differentiate into neural lineage. Indeed, several evidencedemonstrate that, when transplanted, these cells can give rise to neurons and/or glial cells, which, secreting cytokines and growth factors, can sustain the tissue repair by inhibiting cell apoptosis and fibrosis [
1,
2,
3].
To effectively use stem cells in tissue regeneration, it is very important to be able to control their fate, determining their differentiation into the desired cell type. One of the methods used to differentiate stem cells is electrical stimulation [
4]. Several evidence have demonstrated how electrical stimulation applied in different current modes, such as alternating, monophasic, biphasic, continuous, as well as with different frequency, voltage, and duration ranges, allows to determine different biological responses in different cell types, stem cells included [
5,
6]. Current studies show that externally applied electrical stimulation can impact neural stem cell morphology, migration, proliferation, apoptosis, and differentiation [
7].
All living cells present natural electric fields. Bioelectricity arises from a different accumulation of electric charges across cell membrane which is kept at its homeostasis by the passive flux of ions, or their active movement though ions channels [
7]. This phenomenon highlights that the cells have a stable transmembrane potential (TMP), which makes them sensitive to the application of an external electric field that can alter this balance of charges. The response of the cells to this type of stimulation depends on the intensity, the method of application and the duration, as well as the type of cells analyzed [
8].
Ultra short electric pulses (PEF), lasting from nano to milliseconds, are delivered at high-intensity and have the ability to charge the cell membrane, creating large localized electric fields across it. When the charging goes beyond a threshold, small pores (electroporation) on the cell membrane are formed and, depending on the intensity, this process can be, or not, reversible [
9,
10,
11]. This means that by adjusting the applied intensities, it is possible to induce a reversible or irreversible electroporation [
12].
The pores formation allows direct access into the cell of molecules that usually cannot cross the plasma membrane. For example, Ca2+, can only enter the cytoplasm, or be released from the endoplasmic reticulum, through channel proteins. Applying PEF, this ion can diffuse from the extra cellular medium into the cytosol; furthermore, if the electric amplitude and the pulse duration are high enough, Ca2+ can also be released from the inner stores [
10,
13].
Calcium is one of the most important second messengers, regulating the main processes of cellular homeostasis. In stem cells it plays a pivotal role in determining cellular fate [
14]. In the nervous system its function is essential especially in the process of neurogenesis, where sequential bursts of Ca induce neuronal stem/progenitor cells to migrate, proliferate and differentiate into neurons and glia [
15,
16].
The possibility of controlling stem cells fate through bipolar microsecond electric pulses stimulation is the starting point of RISEUP FET-OPEN project (n. 964562). The idea is to transplant mesenchymal stem cells (MSCs) derived from adipose tissue and neuronal stem cells (iNSCs), obtained from induced pluripotent stem cells, and stimulate them to firstly induce the cells proliferation and then their neuronal and/or neuronal like differentiation by modulating their intracellular calcium oscillation.
In this manuscript, we present the biological response induced in adipose tissue derived MSCs and iNSCs, derived from iPSC differentiation, in response to 100 µs+ 100 µs bipolar pulses, at an intensity of 250 V/cm, delivered for 30 minutes every 30 seconds, one of the protocols that increase the number of calcium oscillation and should increase the cell proliferation.
2. Results
2.1. Lack of effect of µsPEFs on cell growth, cell cycle and apoptosis
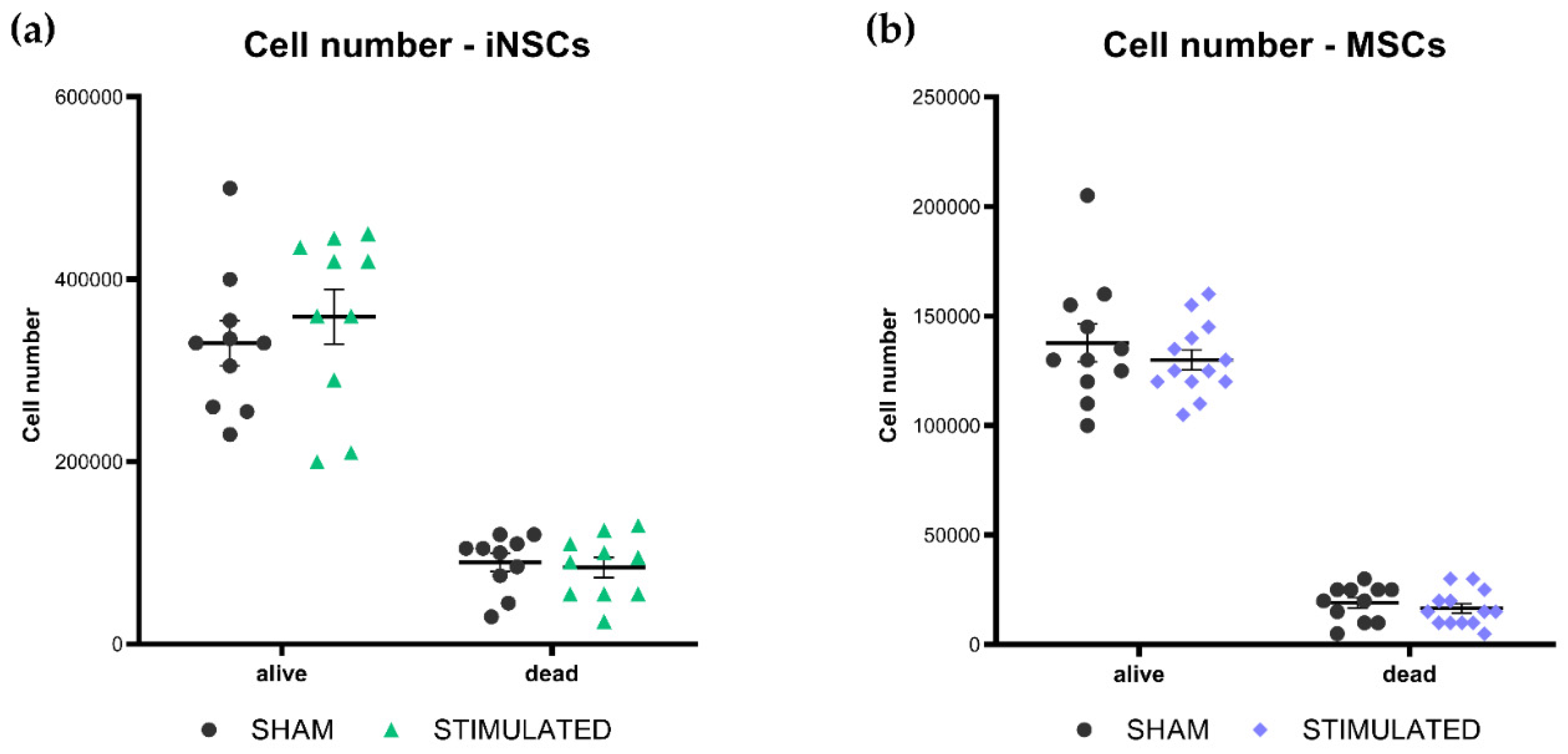
Cell growth, cell cycle, and apoptosis of iNSCs and MSCs were evaluated 24 hours after the stimulation, to characterize the general effect of µsPEFs on these cells’ viability. At the end of each experiment, cells were counted considering both alive and dead cells. Comparing the results obtained from the stimulated cells and the sham, no statistically significant variations on the cell number were observed neither in iNSCs (
Figure 1a) nor in MSCs (
Figure 1b).
A deep investigating of this result was performed evaluating cell cycle through propidium iodide staining and apoptosis considering the activation of the caspase-3 through western blot analysis.
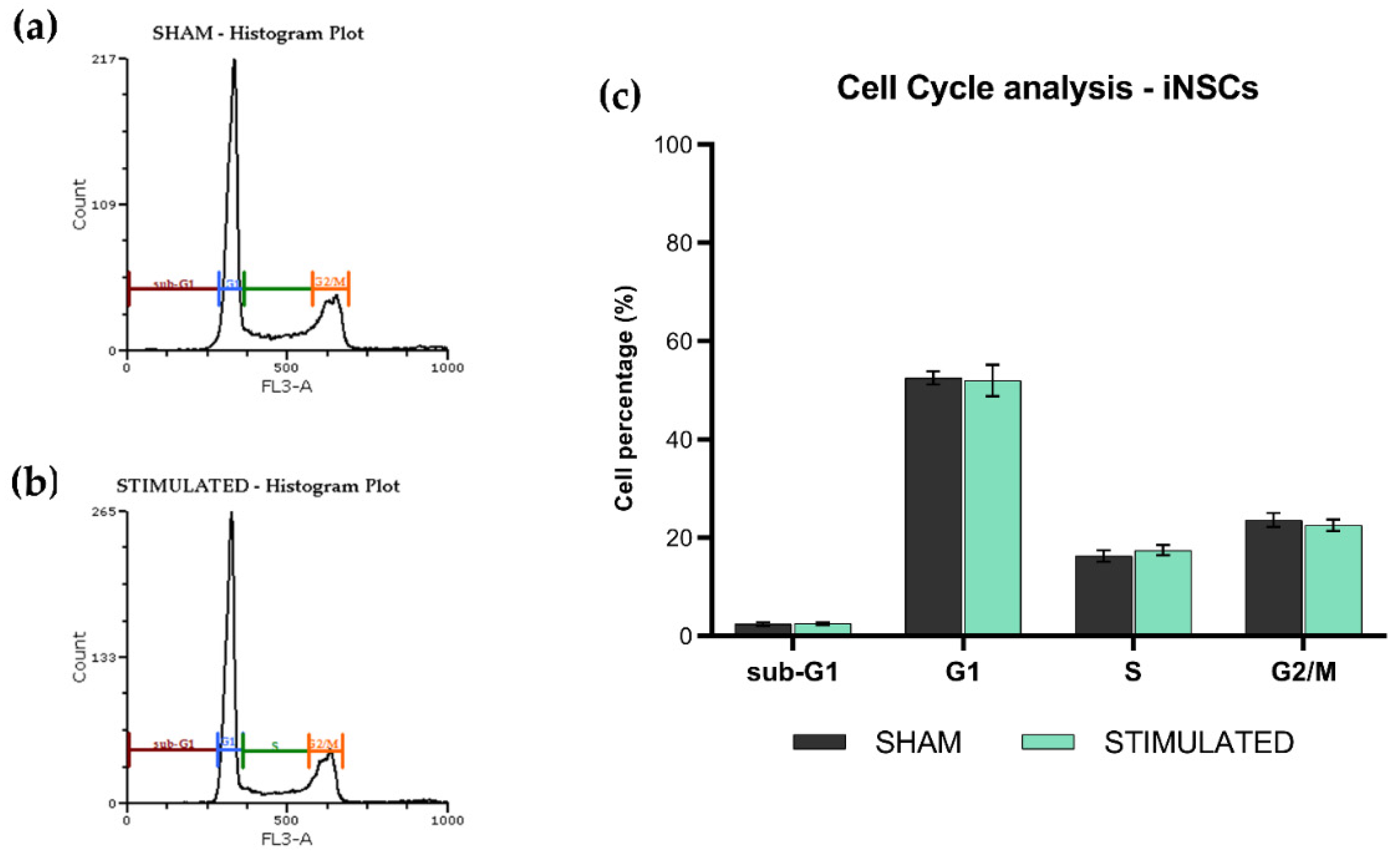
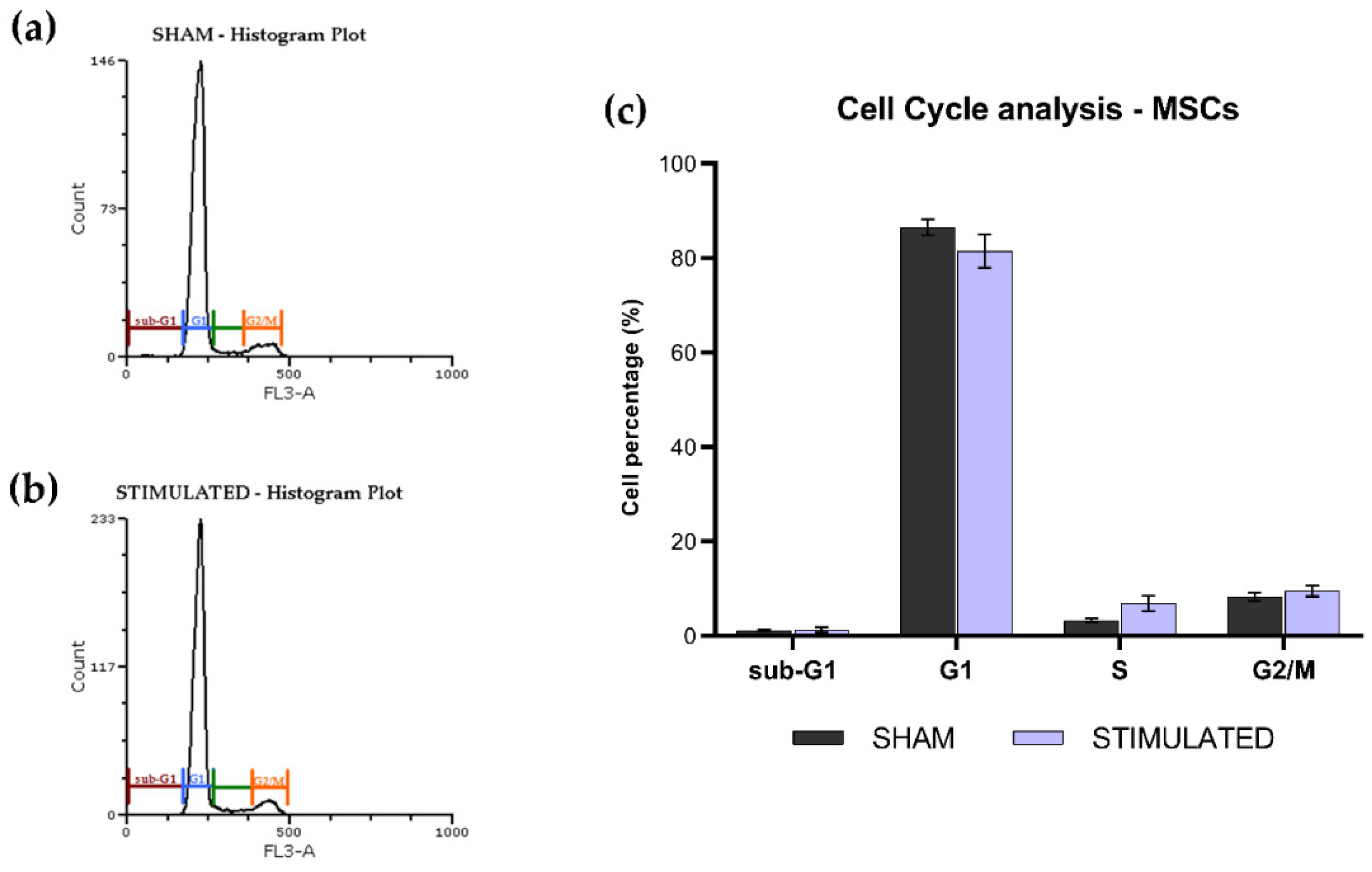
The phases of the iNSCs (
Figure 2) and MSCs (
Figure 3) cell cycle were not affected by the stimulation; the chosen protocol does not induce variations in cell cycle, proliferation (no changes on G1 phase) and mortality (no changes on sub-G1 phase) compared to the sham. Details about the performed analysis are shown in
Figure S1 (iNSCs) and S2 (MSCs).
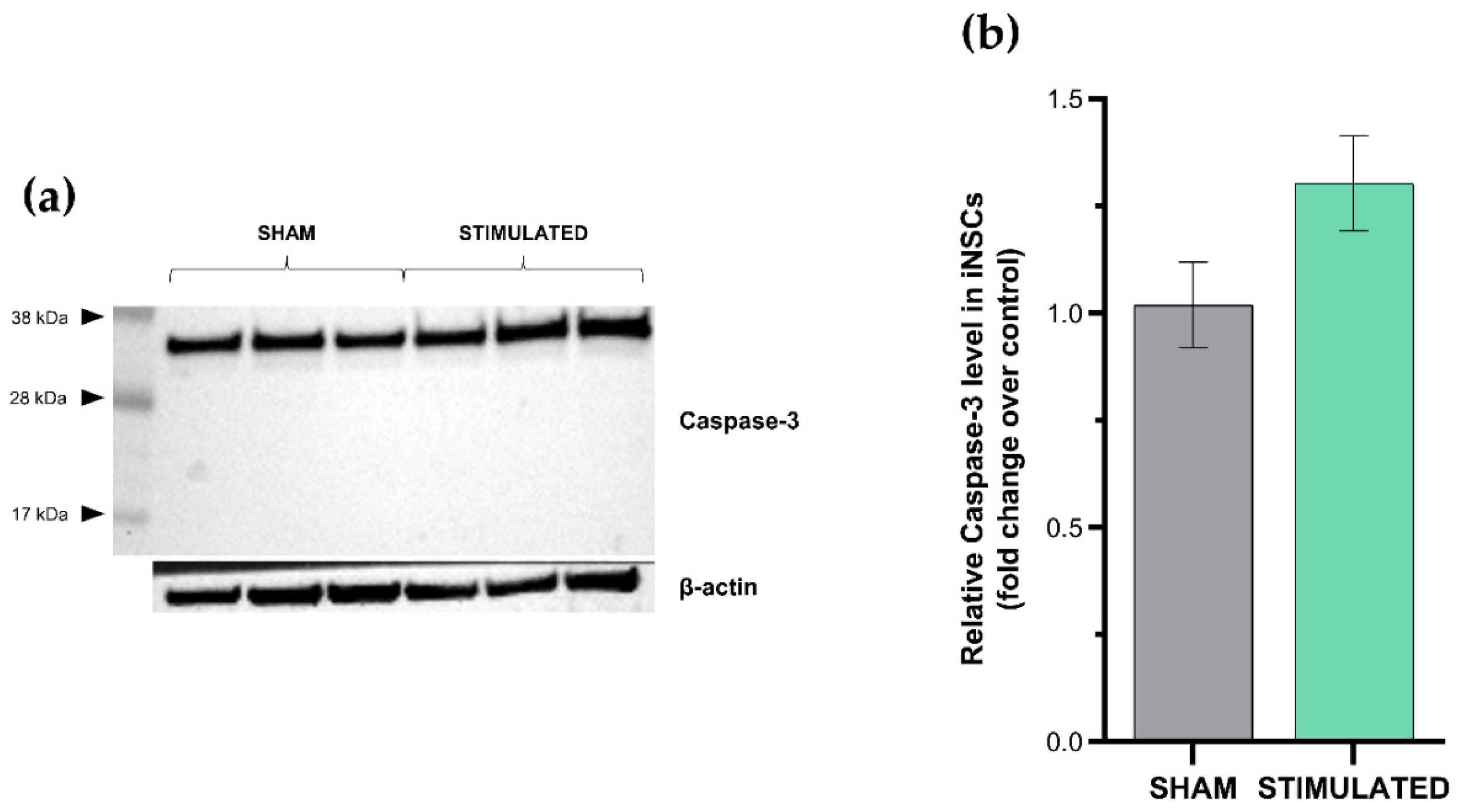
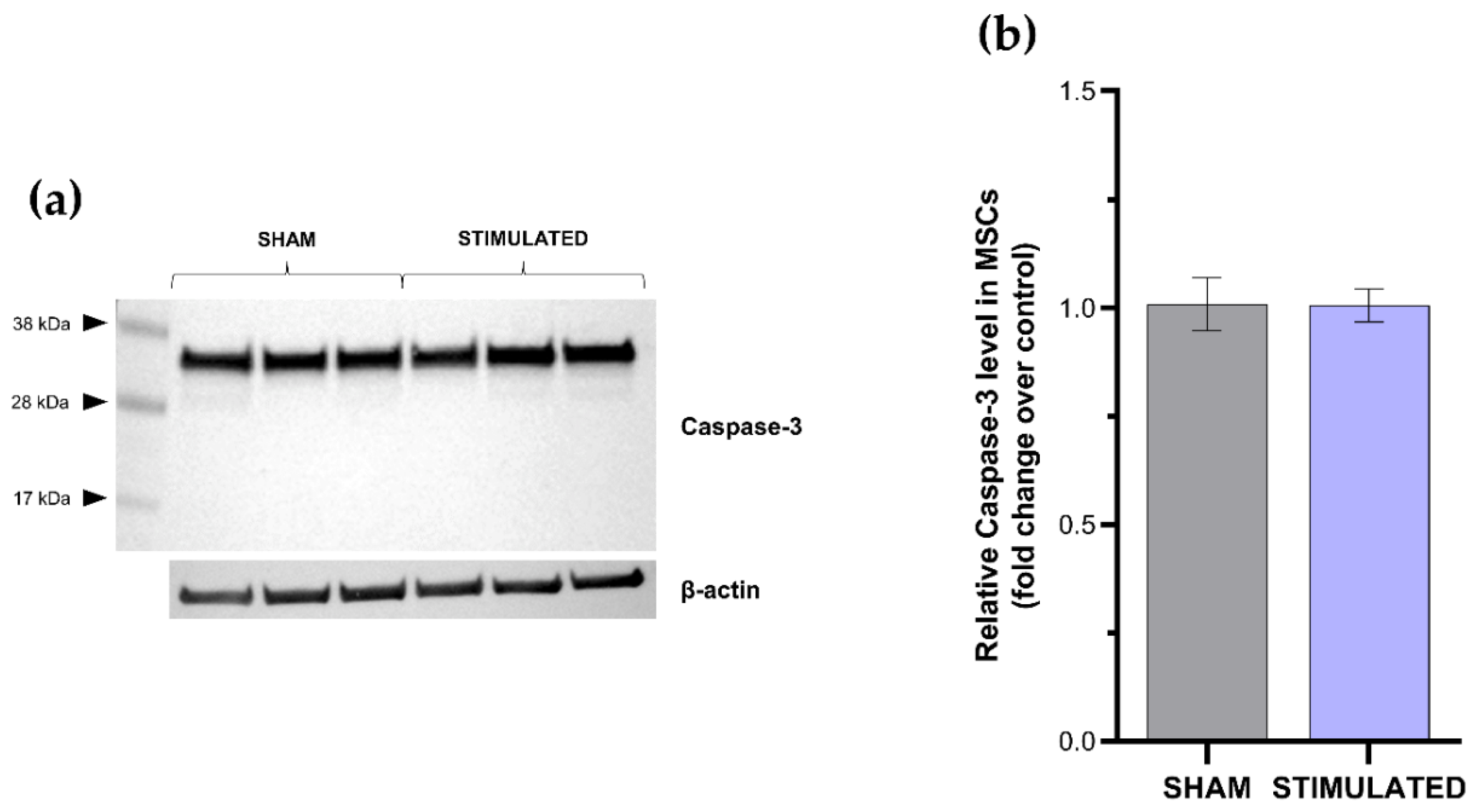
Results deriving from the apoptosis evaluation confirmed the previous highlights: no activation of apoptosis was observed in the stimulated cells compared with the sham neither in iNSCs (
Figure 4) nor in MSCs (
Figure 5). Indeed, only inactive form of caspase-3 (≈32 kDa) was detected by western blot analysis. The absence of cleaved forms (≈19-17 kDa) demonstrates that the extrinsic and intrinsic apoptosis pathways were not induced by the µsPEFs stimulation.
These results point out that a proliferative or toxic effect of the chosen stimulation protocol on iNSCs and MSCs is excluded for the time-point considered.
2.2. Gene expression
2.2.2. Different effects of µsPEFs on genes involved in proliferation, cell cycle and apoptosis
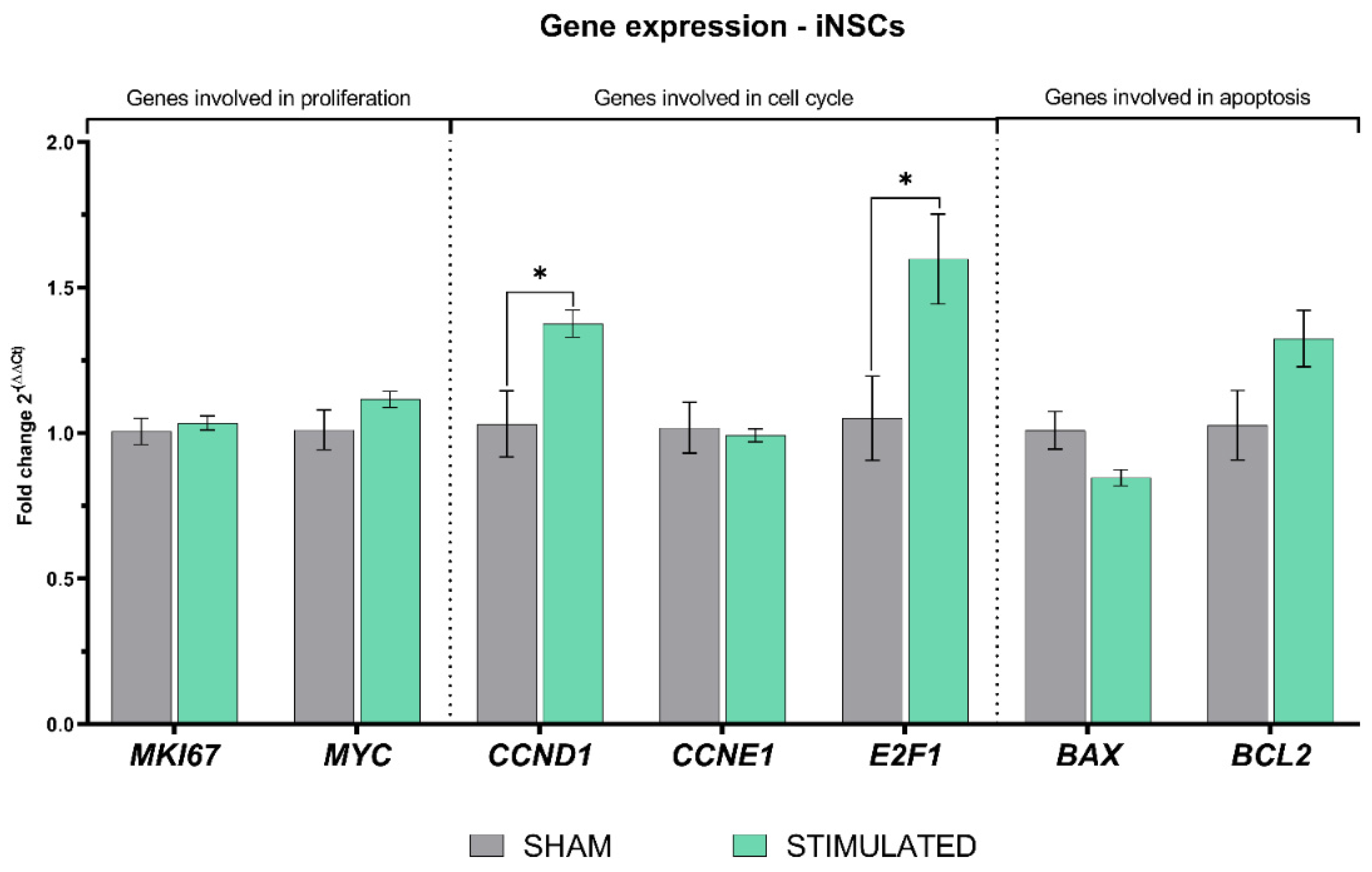
As shown in paragraph 2.1, no significant effects on cell growth, cell cycle and apoptosis were observed 24h after the µsPEFs stimulation. Despite this, a deep investigation on the expression of genes involved in these biological processes was carried out both in iNSCs and MSCs. In details, the expression of MKI67 (encoding for Ki-67) and MYC (encoding for c-Myc) was assessed as markers of cells proliferation, CCND1 (encoding for cyclinD1), CCNDE1 (encoding for cyclinE1) and E2F1 (encoding for the transcriptional factor E2f1) as genes involved in cell cycle, and BAX (encoding for the pro-apoptotic protein Bax) and BCL2 (encoding for the anti-apoptosis protein Bcl2) to highlight effects on apoptosis.
After 24h, the stimulation induced on iNSCs a statistically significant increase in
CCND1 and
E2F1 expression, encoding for proteins involved in the cell cycle G1/S transition. Instead, the other genes analyzed were not affected by the stimulation protocol chosen (details shown in
Figure 7).
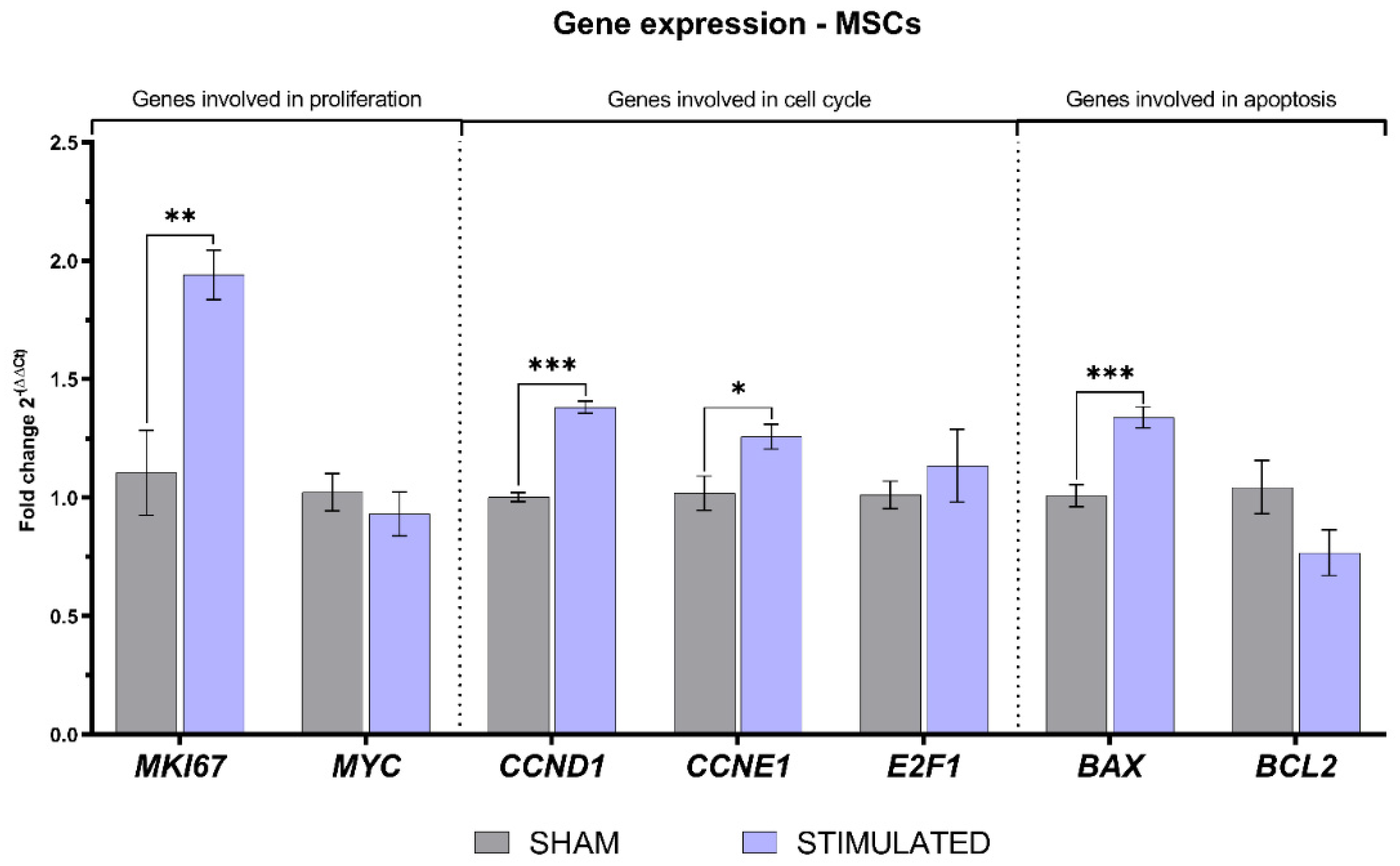
About MSCs, the expression of several considered genes was affected by the stimulation. Indeed, a statistically significant increase in the expression of the proliferative marker
MKI67 was observed, also associated with a raise in the expression of
CCND1 and
CCNE1 involved in the cell cycle G1/S transition. Regarding genes involved in apoptosis, µsPEFs induced an increased expression of
BAX, involved in the apoptotic pathway. All details about the gene expression in MSCs are shown in
Figure 8.
2.2.3. Genes involved in stemness are affected by µsPEFs
In the context of stem cells, another interesting aspect that could be affected by the µsPEFs is stemness. SOX2 (SRY-Box Transcription Factor 2) and POU5F1 (POU Class 5 Homebox 1) expression was analyzed to assess variations in pluripotency, proliferative potential, and self-renewal capacity 24h after the stimulation.
The results, shown in
Figure 9, highlight a statistically significant increase in the expression of
POU5F1 compared to the sham in both iNSCs (a) and MSCs (b).
2.3. µsPEFs have different effects on cells growth
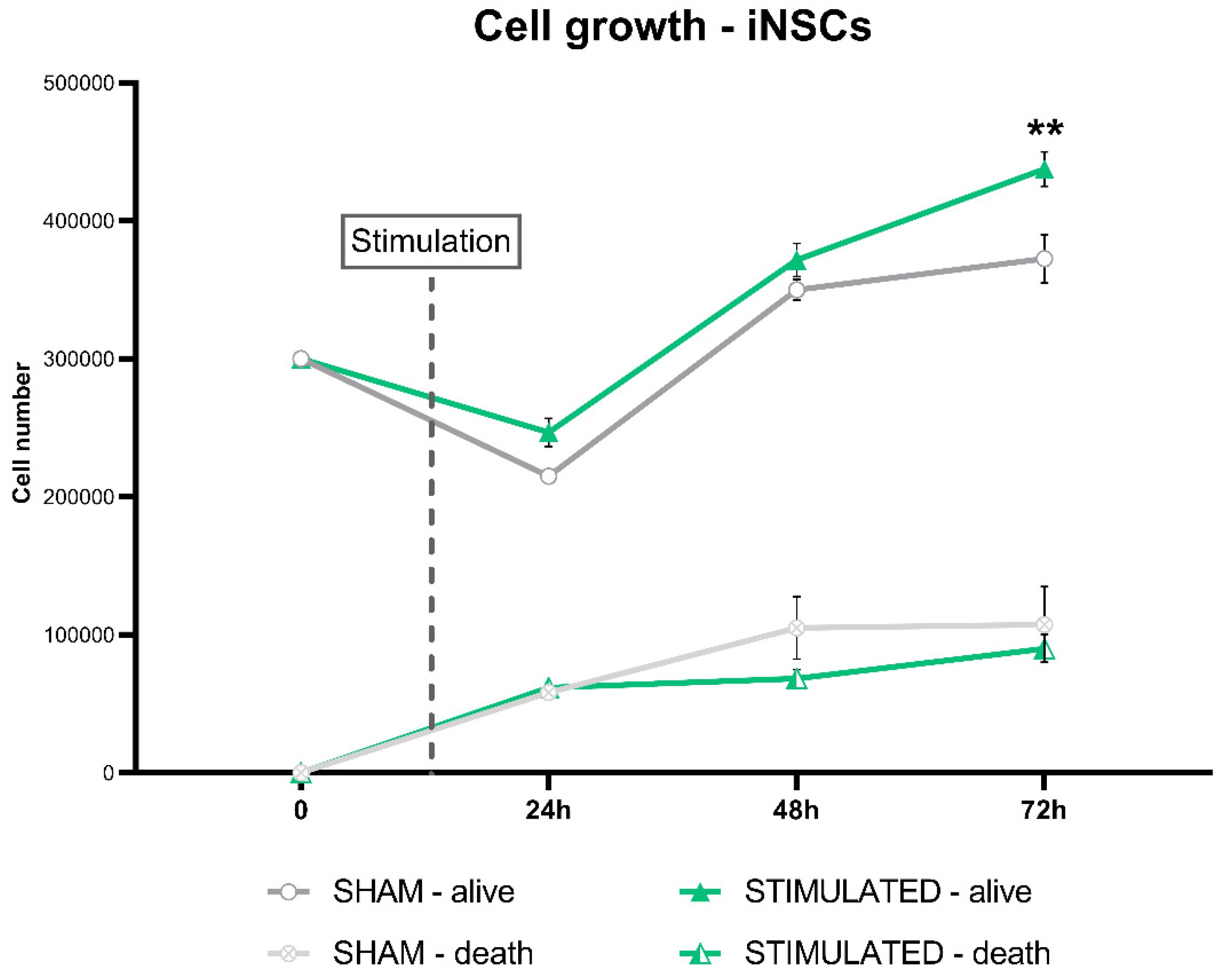
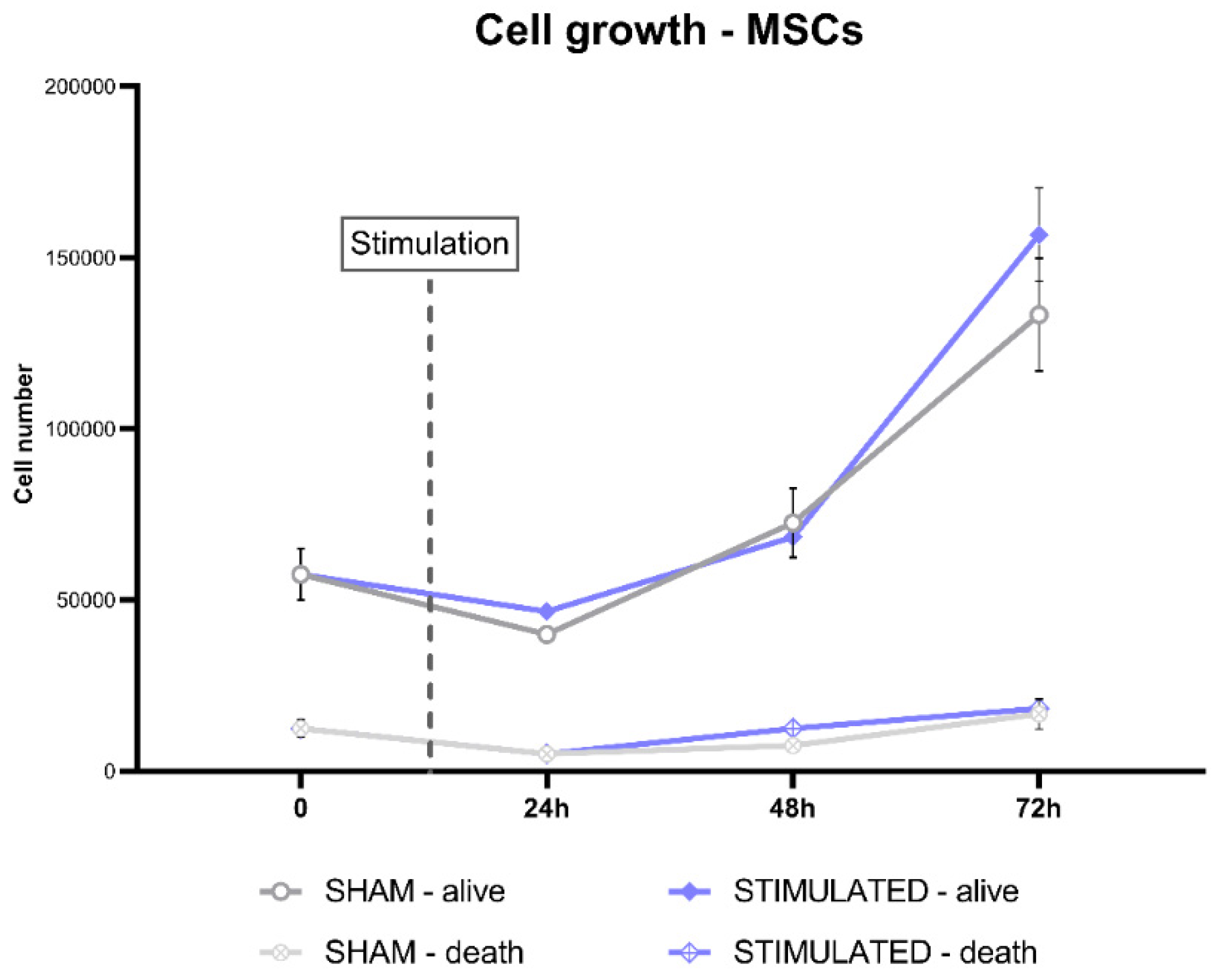
All the results obtained analyzing cell number, cell cycle, apoptosis, and gene expression after 24h from the stimulation suggest an effect of µsPEFs on the cell growth not visible after 24h. For this reason, a cell growth analysis after 24h, 48h and 72h from the stimulation was carried out for iNSCs and MSCs.
The results shown an increase in iNSCs proliferation after 72h from the stimulation, with an increase in the cell number compared to the sham (
Figure 10).
Instead, on MSCs this effect was not observed, indeed no statistically significant changes in the cell number was observed at the considered time-point (
Figure 11).
3. Discussion
Stem cells represent a very powerful tool for the regeneration of damaged tissues. Since their discovery, several studies have focused on their use in various degenerative pathologies and for tissue regeneration, for instance in the context of spinal cord injuries.
The ability of stem cells to differentiate into different cellular phenotypes is defined as potency: the higher the potency, the greater the types of cells to which the stem cell can give rise. Embryonic stem cells, for example, are totipotent, being able to give rise to all body tissues. Adult stem cells, on the other hand, have a degree of stem status that varies from multipotency (such as blood cells, adipose tissue, etc) to unipotency, i.e. cells that can only give rise to the tissue from which they were isolated.
Induced pluripotent stem cells (iPSCs) are similar to embryonic stem cells considering their potency, but are obtained from the reprogramming of differentiated adult cells, thanks to the transfection of genes codifying for four factors (named Myc, Oct3/4, Sox2 and Klf4) that induce the stemness phenotype [
17,
18].
In this work, we have used neuronal stem cells, therefore able of giving rise only to nervous tissue cells, obtained from the commitment of iPSCs. Furthermore, we used multipotent MSCs from adipose tissue. We investigated the possibility of controlling the phenotype of these stem cells using ultrashort electrical pulses in the microsecond range. These pulses are widely used in biology and in clinical practice due to their ability to interact with the cell membrane, inducing the formation of pores, without causing temperature alterations. This allows their use to transfer genetic material and/or drugs inside the cells (electrochemotherapy), or to induce irreversible electroporation, used in anticancer therapy [
19,
20].
We applied microsecond pulses with an energy density far below from electroporation threshold, but able to modulate intracellular calcium fluxes, which is another effect induced by this type of stimulation. In particular, the protocol applied, bipolar 100 µs+100 µs, 250 V/cm, can induce an increase in intracellular calcium oscillation (CNRS personal communication), and the hypotheses is that this effect can enhance cells proliferation.
Being the first time that such kind of stimulation was applied to iNSCs and MSCs, even the first time ever that iNSCs were stimulated with microsecond electric pulses, we decided to initially evaluate the response of these cells 24 hours after the stimulation, also to determine whether these pulses were safe for cells.
By assessing cell homeostasis through live cell count, cell cycle and apoptosis analysis, we found that there was no increase in proliferation, no change in cell cycle phases, and no cell death was induced. Therefore, this stimulation protocol was absolutely safe for the two types of stem cells considered and didn’t affect cells homeostasis 24 hours after the stimulation.
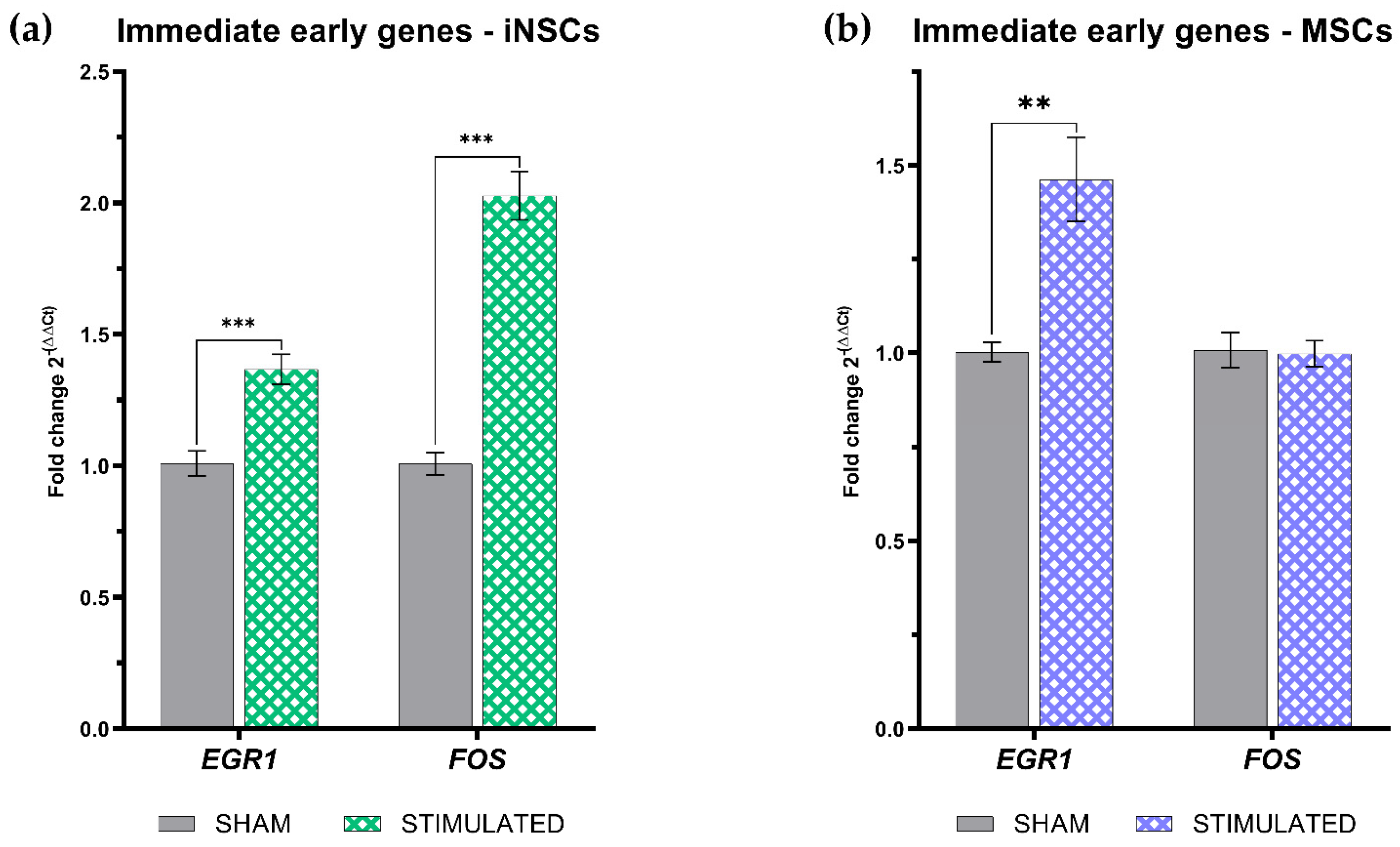
Therefore, we focused on trying to understand the type of molecular response induced. We initially analyzed the expression of two immediate early genes,
EGR-1 and
FOS. These genes encode two transcription factors that have a very short activation time and are immediately inactivated after their function. There are many conditions that induce their transcription and transduction because their function is to activate the molecular response of the cell to external stimuli [
21]. Furthermore, both these genes are involved in processes of neurogenesis and signal transmission in the central nervous system [
22,
23].
We have already demonstrated, with different stimulation protocols, that ultra short electric pulses, both microsecond and nanosecond, can induce the transcription of these IEGs [
11]; also in this case, the 100 µs + 100 µs bipolar pulses, at an intensity of 250 V/cm protocol is able to enhance the expression of both genes in iNSCs, while of only
EGR-1 in MSCs.
EGR-1 is downstream of calcium signaling, so its induction in both cell types suggests that the observed calcium fluxes could be responsible for the increase in its expression.
Subsequently, analyzing the expression of genes involved in the process of proliferation, cell cycle, apoptosis, we observed a modulation of some of them in both cell types. Furthermore, both iNSCs and MSCs showed a statistically significant increase in the
POU5F1 gene. This gene encodes the transcription factor Oct4 which possesses a POU domain and plays a fundamental role in embryonic development and stem cell pluripotency [
24]. Therefore, considering that at a "gross" level 24 hours after stimulation we did not see any effect on proliferation and cycle, but at a molecular level the expression of the genes involved in these processes were modulated, we thought that, above all for proliferation, 24 hours could not be sufficient time to observe a possible variation. For this reason, we decided to repeat the experiment by stimulating the cells and then counting them up to the next 72 hours. We thus observed that iNSCs showed a statistically significant increase in proliferation, while MSCs did not, even if they still showed an increasing trend. These results prove how the choice of time window in experiments is essential, even if not easy to establish when there are no references in literature. Another important aspect is the confirmation that the effect induced on cells, even in the case of electrical stimulation, is cell specific.
The cellular response to electrical stimulation depends on the transmembrane potential which is dynamic and changes with metabolism, cell cycles and states of differentiation. This is applicable both to differentiated cells such as neurons, which, for example, at rest stage are hyperpolarized, and to stem cells, which usually result depolarized. Also among similar cells, the effect of electrical stimulation can induce different responses. iPSC and ES, while being electrophysiologically stable, show different levels of expression of genes coding for ion channels [
25,
26]. On the contrary, MSCs differently from these types of cells, are extremely heterogeneous from the electrophysiological point of view, which is also reflected in their metabolism and differentiation capacity [
27].
Several studies have demonstrated that neuronal differentiation, both of MSCs and NSCs, can be achieved by electrical stimulation [
5,
28,
29,
30]; as well as it is possible to influence the neural lineage of differentiated NSCs through the electrical stimulation [
7]. Responsible for the response to electrical stimulation are the voltage-gated calcium channels [
29,
31]. These allow the entry of extracellular calcium following stimulation, which then acts as a second messenger internally, activating signal transduction and regulating a series of essential functions [
32,
33,
34].
Also in our work, we believe that calcium is involved in the effect induced by the microsecond electric pulses stimulation. The involvement of voltage-dependent channels would also explain the different response of the two cell lines. In fact, iNSCs have a greater number of channels, which makes them much more sensitive to the stimulation than MSCs [
29,
35,
36]. Experiments to define the transduction pathway activated are currently underway.
4. Materials and Methods
4.1. Cell culture
MSCs, adipose tissue derived, were obtained from Dr. F. André, CNRS, through the signing of a Material Transfer Agreement (MTA). iNSCs were from Dr. Moreno Manzano, CIPF, after the signing of a MTA.
Human MSCs were maintained in complete Dulbecco’s modified Eagles medium (DMEM) with high glucose concentration (4500 mg/l), completed with 10% heat-inactivated fetal bovine serum, 100 U/ml of L-glutamine, 100 U/ml penicillin and 100 U/ml streptomycin.
Human iNSCs were maintained in STEMdiff Neural Progenitor Basal Medium completed with Supplement A (50X), Supplement B (1000X) (STEMCELL Technologies, Canada), 100 U/ml of penicillin and 100 U/ml of streptomycin. To maintain their growth in adhesion, a coating of GeltrexTM LDEV-Free (Gibco, Thermo Fisher Scientific, Inc) diluted 1:50 in DMEM/F-12 was used.
Both cell lines were cultured at 37°C in a humidified incubator with 5% CO2 and used until passage 10.
4.2. Exposure system and stimulation
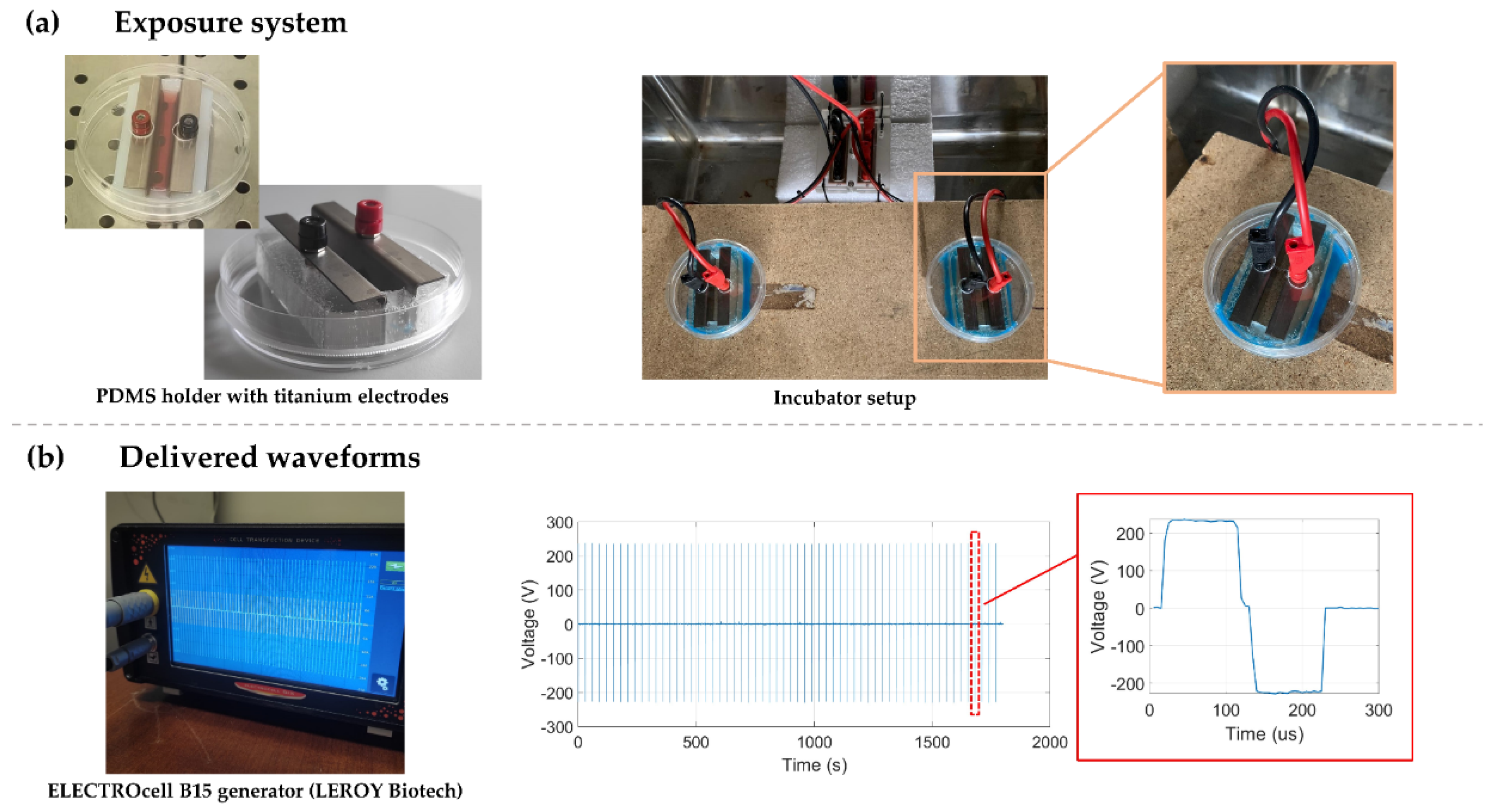
Cells were exposed to microsecond electric pulses (µsPEFs) in 10-cm Petri dishes, where a pair of titanium electrodes were placed parallel at 1 cm from each other. To maintain the electrodes in this specific configuration, polydimethylsiloxane (PDMS) (The Dow Chemical company, MI, USA) holders were fabricated. In addition, some modifications on the dish covers, allowed the direct connection of the electrodes to the generator, keeping the Petri dishes closed for the entire stimulation time (
Figure 12).
The µsPEFs were deliver by the ELECTROcell B15 generator (LEROY Biotech, France) previously connected to the titanium electrodes.
The µsPEFs were deliver by the ELECTROcell B15 generator (LEROY Biotech, France) previously connected to the titanium electrodes.
For all the biological experiments, except growth curve analysis, 3.5x10^5 iNSCs and 1.5x10^5 MSCs were seeded in the exposure systems in a final volume of 3 ml of fresh medium. After 24h for iNSCs and 48h for MSCs from the seeding, the media was changed, and 2 ml of fresh medium was added; this volume is necessary to have the right current passing through the electrodes.
Cells were stimulated with microsecond electric bipolar pulses of 100 µs + 100 µs, 2 pulses / minute were delivered for 30 minutes at an intensity of 250 V/cm. At the end, 1 ml of fresh medium was added in each exposure system and the cells were maintained in culture until the biological experiments. Stimulations were performed in a properly modified incubator to guarantee the maintenance of the specific culture conditions during the entire time of the experiment. Based on the specific biological experiment to be carried out, cells were harvested at different time points after the stimulation (the experimental protocol is described in detail in
Figure S3.
For each experiment, sham samples were prepared plating the cells in the same conditions and electrodes were placed in the PDMS insert to mimic the exposure, but no electric stimulation was applied.
4.3. Cell growth analysis
To assess the effect of the stimulation on the cell growth, the cells were counted after each experiment, comparing the stimulated one with the sham. Moreover, a growth curve was performed by counting the cells 24h, 48h and 72h from the stimulation, to assess a possible long-term effect. In this case, 3.0x10^5 iNSCs and 7,5x10^4 MSCs were seeded 24h for iNSCs and 48h for MSCs before the stimulation.
For the counting, cells were harvested, and the viability was assessed by Erythrosin B dye (Sigma-Adrich, St. Louis, MO, USA) exclusion.
4.4. Flow-cytometric analysis of cell cycle
After 24h from the stimulation, cells were harvested, washed with PBS and fixed with pre-cooled 70% ethanol. After overnight incubation at -20°C, cells were resuspended in propidium iodide (PI)/RNase staining buffer (λex = 490 nm, λem = 600 nm, BD Biosciences).
Samples were analyzed by flow cytometry using a FACSCalibur (BD Biosciences) and a minimum of 10,000 events were evaluated. Data were acquired using Cell Quest software (BD Biosciences) and analyzed using FCS Express version 7 (De Novo, CA, USA). Forward (FSC-H) and side scatterings (SSC-H) were used to exclude cellular debris from the analysis and to gate healthy cells.
4.5. Western blotting for apoptosis evaluation
Cells harvested after 24h from the stimulation were lysed in Cell Extraction Buffer (Invitrogen, Carlsbad, CA, USA) supplemented with 1.0 mM phenylmethylsulfonyl fluoride and HaltTM protease and phosphatase inhibitor cocktail (100X) (both from Pierce Biotechnology, Rockford, IL, USA) on ice for 30 min. The lysates were centrifugated at 13,000 rpm for 10 min at 4°C and the protein concentration in the supernatant was determined using the Coomassie Plus (Bradford) Assay Kit (Pierce Biotechnology, Rockford, IL, USA). An equal amount of protein for each sample was prepared adding 4X BoltTM LDS Sampe Buffer and 10X BoltTM Reducing Agent (both from Invitrogen, Carlsbad, CA, USA) before the loading onto wells of a pre-cast BoltTM Bis-Tris Plus Mini Protein Gels, 4-12%, 1.0 mm (Invitrogen, Carlsbad, CA, USA) and then transferred to nitrocellulose membranes (iBlot™ 2 Transfer Stacks, nitrocellulose, mini – Invitrogen, Carlsbad, CA, USA).
Membranes were incubated with blocking solution (TBST containing 0.05 g/ml nonfat dried milk powder and 0.05% Tween-20) for 1h at RT and then probed with anti-Caspase-3 (Cell Signaling, Danvers, MA, USA, #9662; 1:1000) and anti-beta actin (Sigma-Aldrich, St. Louis, MO, USA, #A5441, 1:5000). Bands were detected after incubation with HRP-linked goat anti-rabbit (Cell Signaling, Danvers, MA, USA, #7074, 1:3000) or anti-mouse (Cell Signaling, Danvers, MA, USA, #7076, 1:3000) secondary antibodies through the ECL detection system (Life Technologies, Carlsbad, CA, USA) in combination with the iBright
TM FL1500 Imaging System (Invitrogen, Carlsbad, CA, USA). The quantification was performed using ImageJ2 software [
37].
4.6. Gene expression analysis
To evaluate the expression of several genes following the stimulation, total RNA was extracted at different time points: for the evaluation of the immediate early genes (IEGS) FOS and EGR1, the extraction was performed after 1h from the stimulation instead, for all the other genes, after 24h from the stimulation.
For the RNA extraction, the Direct-zol RNA Miniprep Kit (Zymo Research, USA) was used following the manufacturer’s instruction. Quantitative and qualitative evaluation of the extracted RNA was performed by Nanoready Touch F3100 (Hangzhou LifeReal Biotechnology Co., Zhejiang, China) calculating the 260/230 nm and 260/280 nm absorbance ratio. Five hundred nanograms of total RNA were retrotranscribed into cDNA using the QuantiTect Reverse Transcription Kit (Qiagen, Venlo, The Netherlands?). cDNA was then diluted 1:10 for Realtime PCR analysis and amplified with specific primers using Luna® Universal qPCR Master Mix (New England Biolabs, MA, USA).
The relative abundance of the specific mRNA levels was calculated by normalizing to GAPDH expression using the 2-ΔΔCt methods and it was expressed as fold change. All reactions were run in triplicate.
The complete list of the used primer sequences is shown in
Table 1.
4.7. Statistical analysis
Data were presented as mean ± standard error of mean (SEM) calculated with n ≥ 3 replicates. All graphical and statistical data analyses were performed using GraphPad Prism version 9 (GraphPad Software, San Diego, CA, United States). P values were determined using the Two-tailed t-test, except for the cell cycle where the significant difference in the values considered was measured by variance analysis (two-way ANOVA) followed by post-hoc analysis with the Sidak test.
Significant differences were recognized as a p value < .05 and indicated with asterisks as follows: *p < .05; **p < .01; ***p < .001.
5. Conclusions
Bioelectricity and regeneration in multicellular organisms are deeply connected [
38]. The electricity generated by cells can regulate the morphogenesis in all living tissues. Organ regeneration involves careful regulation of cell division, movement, and positioning. During the embryogenesis, electrical signals serve to direct the formation of organs and limbs [
7].
Within the RISEUP project framework, we are developing microsecond stimulation protocols to control the proliferation and differentiation of iNSCs and MSCs to transplant them and achieve regeneration in spinal cord injury.
These first results demonstrate that the protocol of stimulation with microsecond pulses described here is absolutely safe and is able to induce a biological response in stem cells, even at intensities lower than those induced by electroporation. The knowledge of the biological effects induced, and of the activated molecular mechanisms, is fundamental for improving their therapeutic application, as well as developing new biomedical strategies.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Details of the cell cycle analysis performed on iNSCs.; Figure S2: Details of the cell cycle analysis performed on MSCs.; Figure S3: Schematic summary of the experimental protocol followed.
Author Contributions
Conceptualization, C.C, and G.I.; methodology, G.I., M.S.P., G.B., C.C., V.P., F.C., M.P., and C.M.; validation, G.I., and C.C.; formal analysis, G.I., G.B., and C.C.; investigation, G.I, L.C., and M.C..; resources, C.C.; data curation, G.I.; writing—original draft preparation, C.C., and G.I.; writing—review and editing, C.C, G.I., M.S.P, F.M.A, V.M.M., and P.M. ; visualization, C.C., and G.I.; supervision, C.C.; funding acquisition, C.C., F.M.A., V.M.M., P.M., and C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FET-OPEN H2020, grant number 964562
Institutional Review Board Statement
Adult human MSCs were obtained from Dr. F.M. André, METSY Laboratory CNRS. They were prepared from different donors, from surplus or wasted lipoaspirated, with informed consent from donors to be used on further experimental procedures. These lipoaspirates are surgical waste and, as such, the law (Art.L. 1245-2 du Code de la Santé Publique) establishes that there is no need to receive the authorization from an ethics committee. iNSCs, coming from adult iPSC, were provided by Dr. Moreno Manzano, CIPF, who received the authorization to their use the Ethics Committee of the Research with Medicines from the CEIM - UNIVERSITY AND POLYTECHNIC HOSPITAL in Valencia.
Data Availability Statement
Conflicts of Interest
The authors declare no conflict of interest.
References
- Assinck, P.; Duncan, G.J.; Hilton, B.J.; Plemel, J.R.; Tetzlaff, W. Cell Transplantation Therapy for Spinal Cord Injury. Nat Neurosci 2017, 20, 637–647. [Google Scholar] [CrossRef]
- Ceto, S.; Sekiguchi, K.J.; Takashima, Y.; Nimmerjahn, A.; Tuszynski, M.H. Neural Stem Cell Grafts Form Extensive Synaptic Networks That Integrate with Host Circuits after Spinal Cord Injury. Cell Stem Cell 2020, 27, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Curtis, E.; Martin, J.R.; Gabel, B.; Sidhu, N.; Rzesiewicz, T.K.; Mandeville, R.; Van Gorp, S.; Leerink, M.; Tadokoro, T.; Marsala, S.; et al. A First-in-Human, Phase I Study of Neural Stem Cell Transplantation for Chronic Spinal Cord Injury. Cell Stem Cell 2018, 22, 941–950.e6. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.K.; Basu, B. Regenerative Bioelectronics: A Strategic Roadmap for Precision Medicine. Biomaterials 2023, 301, 122271. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, J.; Dhayal, M. Rapid Neurogenic Differentiation of Human Mesenchymal Stem Cells through Electrochemical Stimulation. Bioelectrochemistry 2023, 153, 108468. [Google Scholar] [CrossRef]
- Love, M.R.; Palee, S.; Chattipakorn, S.C.; Chattipakorn, N. Effects of Electrical Stimulation on Cell Proliferation and Apoptosis. J Cell Physiol 2018, 233, 1860–1876. [Google Scholar] [CrossRef]
- Garrudo, F.F.F.; Linhardt, R.J.; Ferreira, F.C.; Morgado, J. Designing Electrical Stimulation Platforms for Neural Cell Cultivation Using Poly(Aniline): Camphorsulfonic Acid. Polymers (Basel) 2023, 15, 2674. [Google Scholar] [CrossRef]
- Katoh, K. Effects of Electrical Stimulation of the Cell: Wound Healing, Cell Proliferation, Apoptosis, and Signal Transduction. Med Sci (Basel) 2023, 11. [Google Scholar] [CrossRef]
- Joshi, R.P.; Schoenbach, K.H. Bioelectric Effects of Intense Ultrashort Pulses. Crit Rev Biomed Eng 2010, 38, 255–304. [Google Scholar] [CrossRef]
- Hanna, H.; Denzi, A.; Liberti, M.; André, F.M.; Mir, L.M. Electropermeabilization of Inner and Outer Cell Membranes with Microsecond Pulsed Electric Fields: Quantitative Study with Calcium Ions. Sci Rep 2017, 7. [Google Scholar] [CrossRef]
- Consales, C.; Merla, C.; Benassi, B.; Garcia-Sanchez, T.; Muscat, A.; André, F.M.; Marino, C.; Mir, L.M. Biological Effects of Ultrashort Electric Pulses in a Neuroblastoma Cell Line: The Energy Density Role. Int J Radiat Biol 2022, 98, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Chen, S.; Alfadhl, Y.; Chen, X.; Sun, L.; Yu, L.; Zhou, J. Pulse Width and Intensity Effects of Pulsed Electric Fields on Cancerous and Normal Skin Cells. Sci Rep 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, N.; Fujiwara, Y.; Kamezaki, T.; Katsuki, S. Variations of Intracellular Ca2+ Mobilization Initiated by Nanosecond and Microsecond Electrical Pulses in HeLa Cells. IEEE Trans Biomed Eng 2019, 66, 2259–2268. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium Signalling: Dynamics, Homeostasis and Remodelling. Nat Rev Mol Cell Biol 2003, 4, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Toth, A.B.; Shum, A.K.; Prakriya, M. Regulation of Neurogenesis by Calcium Signaling. Cell Calcium 2016, 59, 124–134. [Google Scholar] [CrossRef]
- Dayanithi, G.; Verkhratsky, A. Calcium Signalling in Stem Cells: Molecular Physiology and Multiple Roles. Cell Calcium 2016, 59, 55–56. [Google Scholar] [CrossRef]
- Bayart, E.; Cohen-Haguenauer, O. Technological Overview of IPS Induction from Human Adult Somatic Cells. Curr Gene Ther 2013, 13, 73–92. [Google Scholar] [CrossRef]
- Sharma, R. IPS Cells—The Triumphs and Tribulations. Dent J (Basel) 2016, 4, 19. [Google Scholar] [CrossRef]
- Ruzgys, P.; Novickij, V.; Novickij, J.; Šatkauskas, S. Nanosecond Range Electric Pulse Application as a Non-Viral Gene Delivery Method: Proof of Concept. Sci Rep 2018, 8. [Google Scholar] [CrossRef]
- Qian, K.; Zhong, Z. Research Frontiers of Electroporation-Based Applications in Cancer Treatment: A Bibliometric Analysis. Biomedical Engineering / Biomedizinische Technik 2023, 0. [Google Scholar] [CrossRef]
- Bahrami, S.; Drabløs, F. Gene Regulation in the Immediate-Early Response Process. Adv Biol Regul 2016, 62, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Okuno, H. Regulation and Function of Immediate-Early Genes in the Brain: Beyond Neuronal Activity Markers. Neurosci Res 2011, 69, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Minatohara, K.; Akiyoshi, M.; Okuno, H. Role of Immediate-Early Genes in Synaptic Plasticity and Neuronal Ensembles Underlying the Memory Trace. Front Mol Neurosci 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Bakhmet, E.I.; Tomilin, A.N. Key Features of the POU Transcription Factor Oct4 from an Evolutionary Perspective. Cellular and Molecular Life Sciences 2021, 78, 7339–7353. [Google Scholar] [CrossRef]
- Jiang, P.; Rushing, S.N.; Kong, C.; Fu, J.; Kuo-Ti Lieu, D.; Chan, C.W.; Deng, W.; Li, R.A. Electrophysiological Properties of Human Induced Pluripotent Stem Cells. Am J Physiol Cell Physiol 2010, 298, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xue, T.; Tsang, S.; Van Huizen, R.; Wong, C.W.; Lai, K.W.; Ye, Z.; Cheng, L.; Au, K.W.; Zhang, J.; et al. Electrophysiological Properties of Pluripotent Human and Mouse Embryonic Stem Cells. Stem Cells 2005, 23, 1526–1534. [Google Scholar] [CrossRef]
- Heubach, J.F.; Graf, E.M.; Leutheuser, J.; Bock, M.; Balana, B.; Zahanich, I.; Christ, T.; Boxberger, S.; Wettwer, E.; Ravens, U. Electrophysiological Properties of Human Mesenchymal Stem Cells. Journal of Physiology 2004, 554, 659–672. [Google Scholar] [CrossRef]
- Eftekhari, B.S.; Song, D.; Janmey, P.A. Electrical Stimulation of Human Mesenchymal Stem Cells on Conductive Substrates Promotes Neural Priming. Macromol Biosci 2023. [Google Scholar] [CrossRef]
- Wang, S.; Guan, S.; Sun, C.; Liu, H.; Liu, T.; Ma, X. Electrical Stimulation Enhances the Neuronal Differentiation of Neural Stem Cells in Three-Dimensional Conductive Scaffolds through the Voltage-Gated Calcium Ion Channel. Brain Res 2023, 1798, 148163. [Google Scholar] [CrossRef]
- Liu, Q.; Telezhkin, V.; Jiang, W.; Gu, Y.; Wang, Y.; Hong, W.; Tian, W.; Yarova, P.; Zhang, G.; Lee, S.M. yuen; et al. Electric Field Stimulation Boosts Neuronal Differentiation of Neural Stem Cells for Spinal Cord Injury Treatment via PI3K/Akt/GSK-3β/β-Catenin Activation. Cell Biosci 2023, 13. [Google Scholar] [CrossRef]
- Karatum, O.; Han, M.; Erdogan, E.T.; Karamursel, S.; Nizamoglu, S. Physical Mechanisms of Emerging Neuromodulation Modalities. J Neural Eng 2023, 20, 031001. [Google Scholar] [CrossRef] [PubMed]
- Brosenitsch, T.A.; Katz, D.M. Physiological Patterns of Electrical Stimulation Can Induce Neuronal Gene Expression by Activating N-Type Calcium Channels. The Journal of Neuroscience 2001, 21, 2571–2579. [Google Scholar] [CrossRef]
- Pall, M.L. Electromagnetic Fields Act via Activation of Voltage-Gated Calcium Channels to Produce Beneficial or Adverse Effects. J Cell Mol Med 2013, 17, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Alshawaf, A.J.; Alnassar, S.A.; Al-Mohanna, F.A. The Interplay of Intracellular Calcium and Zinc Ions in Response to Electric Field Stimulation in Primary Rat Cortical Neurons in Vitro. Front Cell Neurosci 2023, 17. [Google Scholar] [CrossRef] [PubMed]
- Pathak, M.M.; Nourse, J.L.; Tran, T.; Hwe, J.; Arulmoli, J.; Le, D.T.T.; Bernardis, E.; Flanagan, L.A.; Tombola, F. Stretch-Activated Ion Channel Piezo1 Directs Lineage Choice in Human Neural Stem Cells. Proc Natl Acad Sci U S A 2014, 111, 16148–16153. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Jung, K.H.; Kim, S.H.; Kim, K.S.; Choi, M.R.; Kim, Y.; Chai, Y.G. Functional Expression of Ion Channels in Mesenchymal Stem Cells Derived from Umbilical Cord Vein. Stem Cells 2007, 25, 2044–2052. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next Generation of Scientific Image Data. BMC Bioinformatics 2017, 18. [Google Scholar] [CrossRef]
- Levin, M.; Pezzulo, G.; Finkelstein, J.M. Endogenous Bioelectric Signaling Networks: Exploiting Voltage Gradients for Control of Growth and Form. 2017. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).