Submitted:
08 September 2023
Posted:
12 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
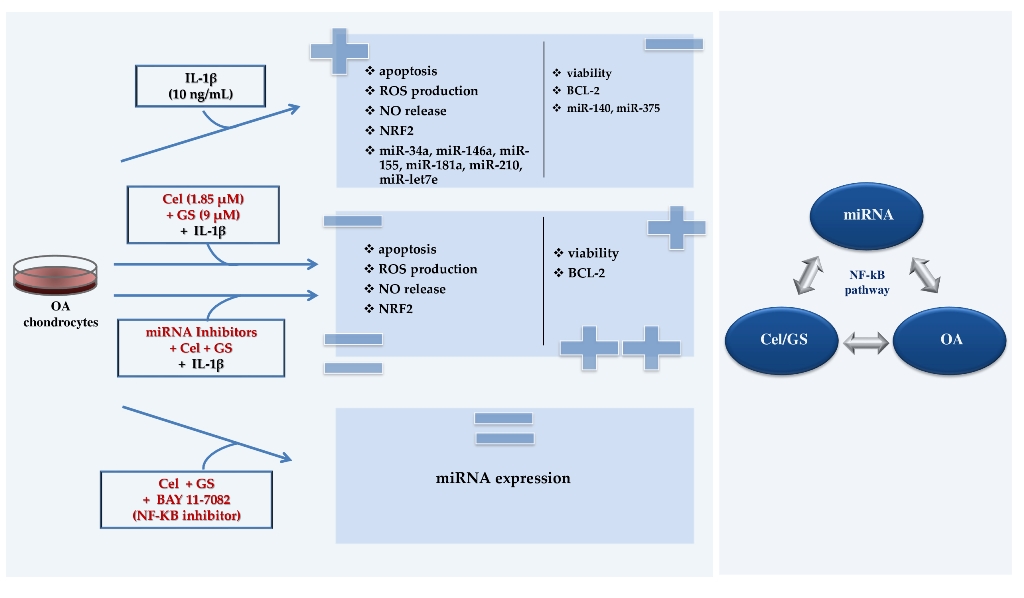
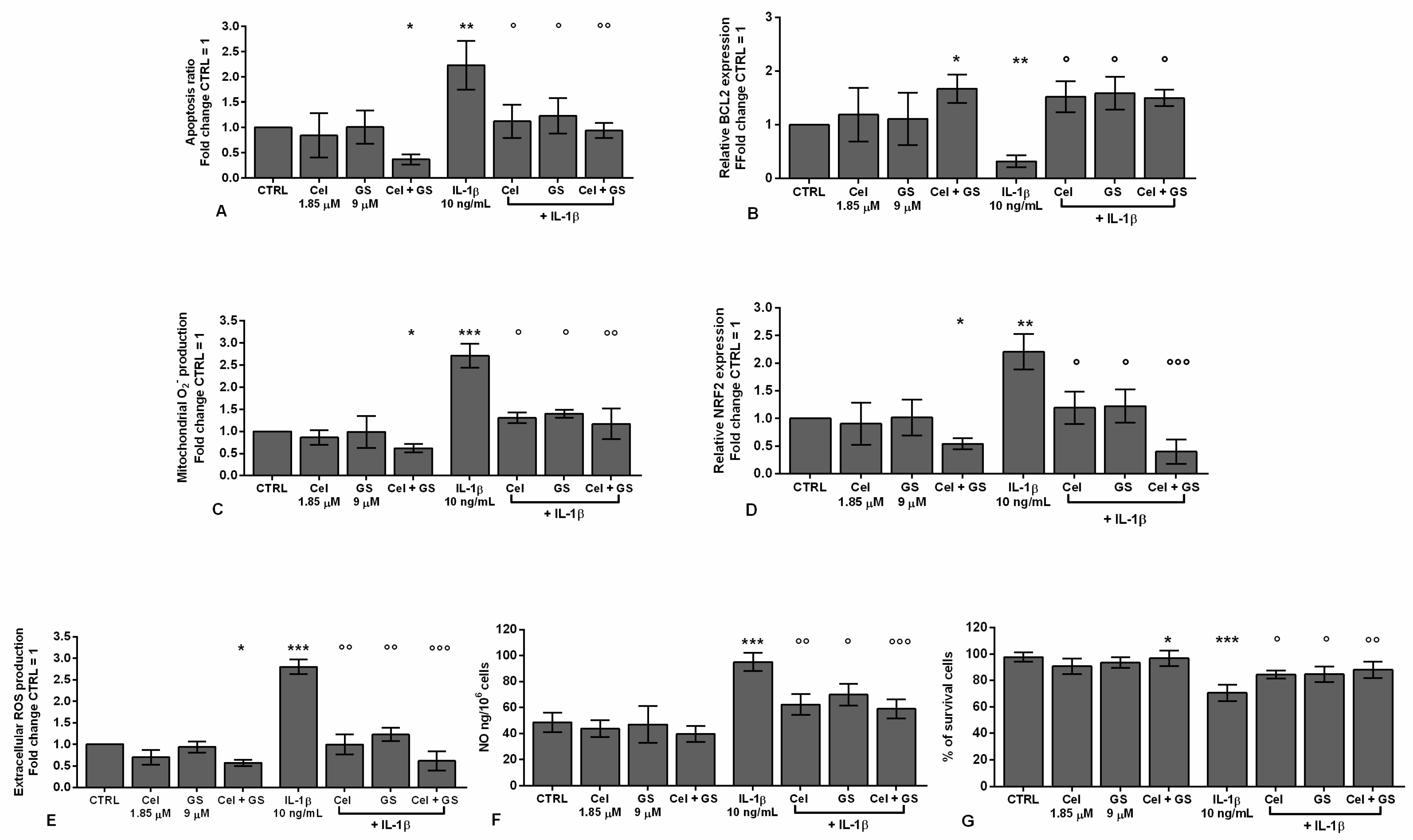
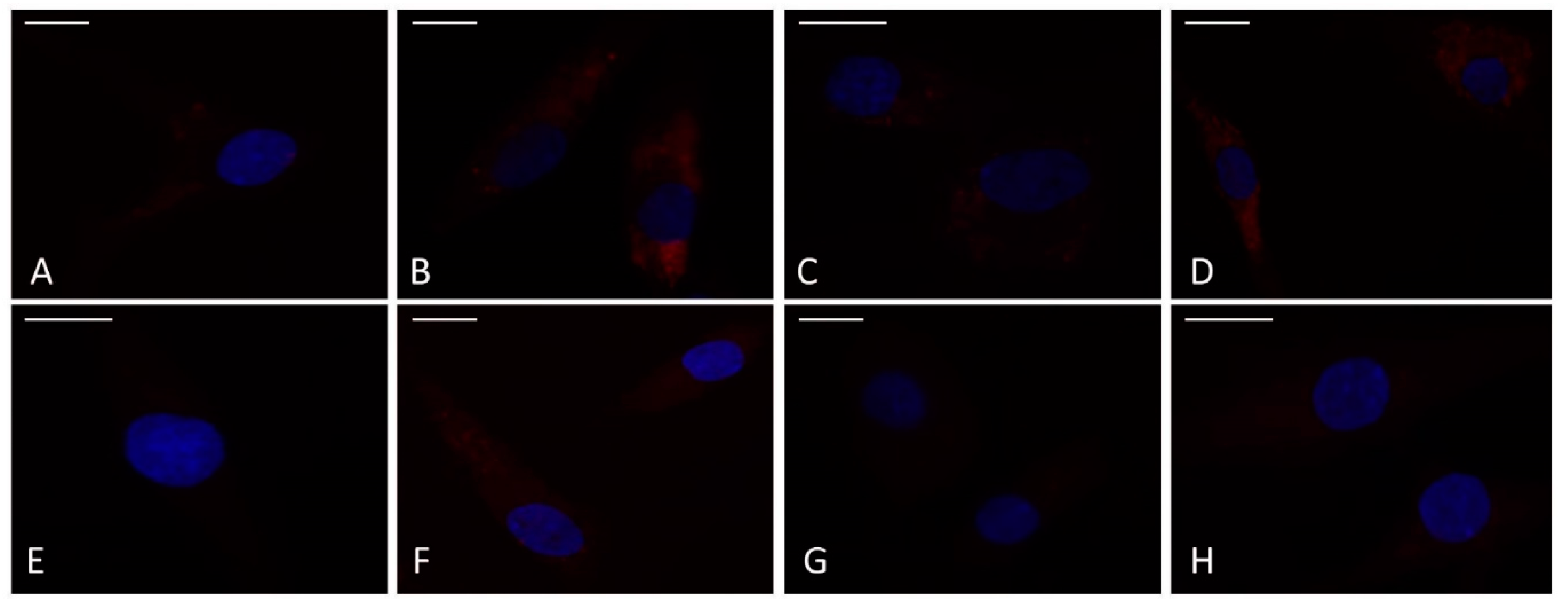
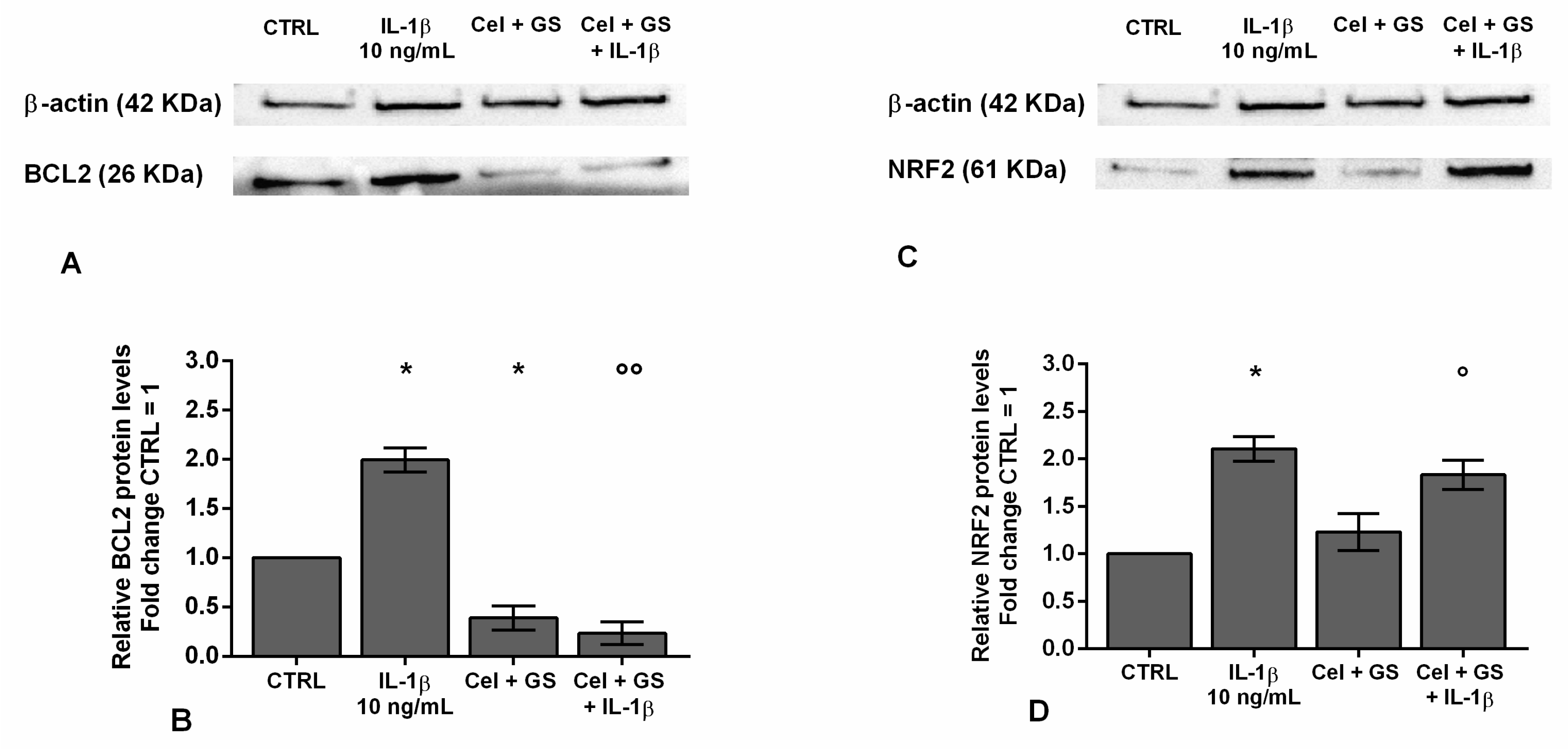
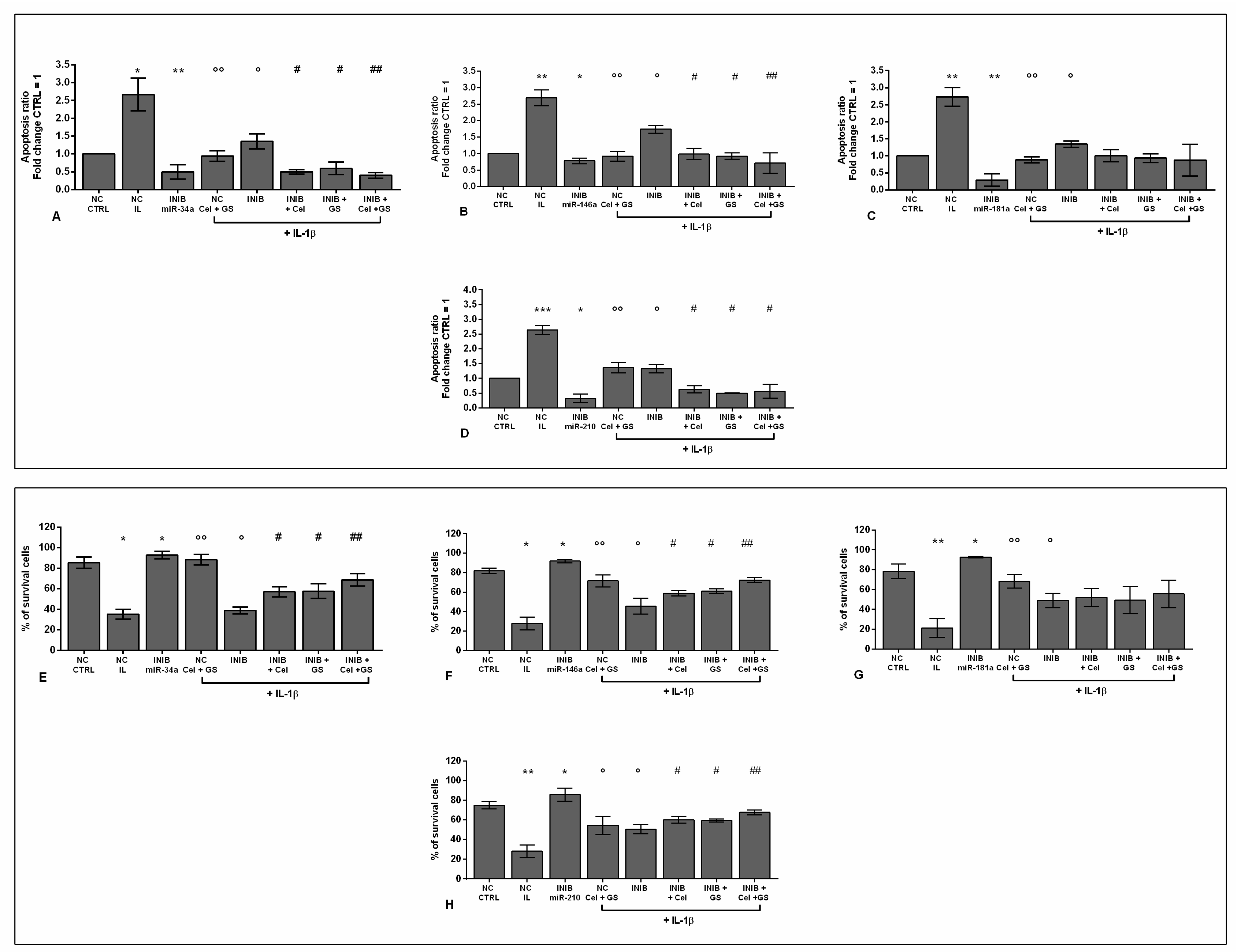
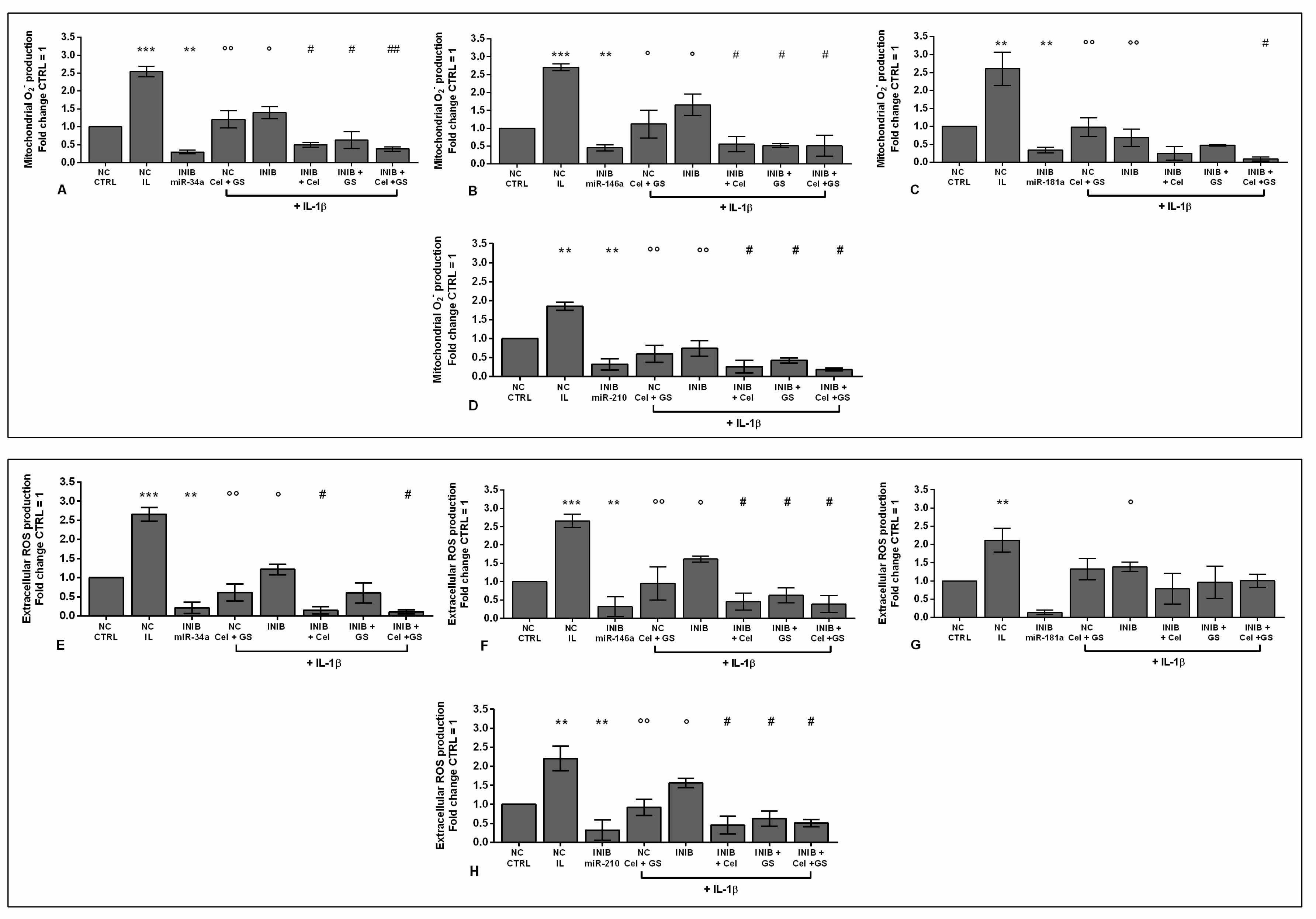
2.1. Celecoxib and GS modulate apoptosis and oxidant/antioxidant system
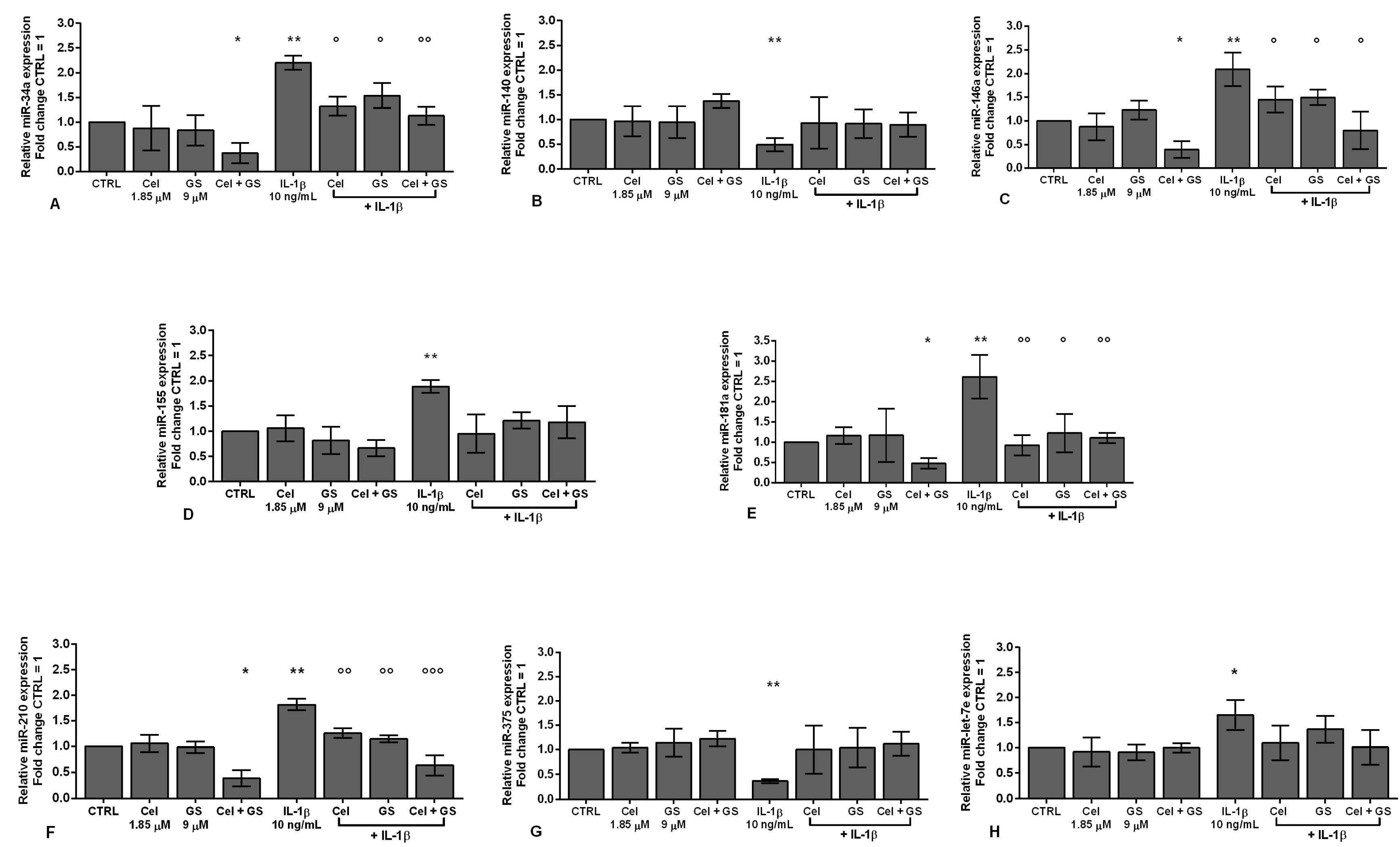
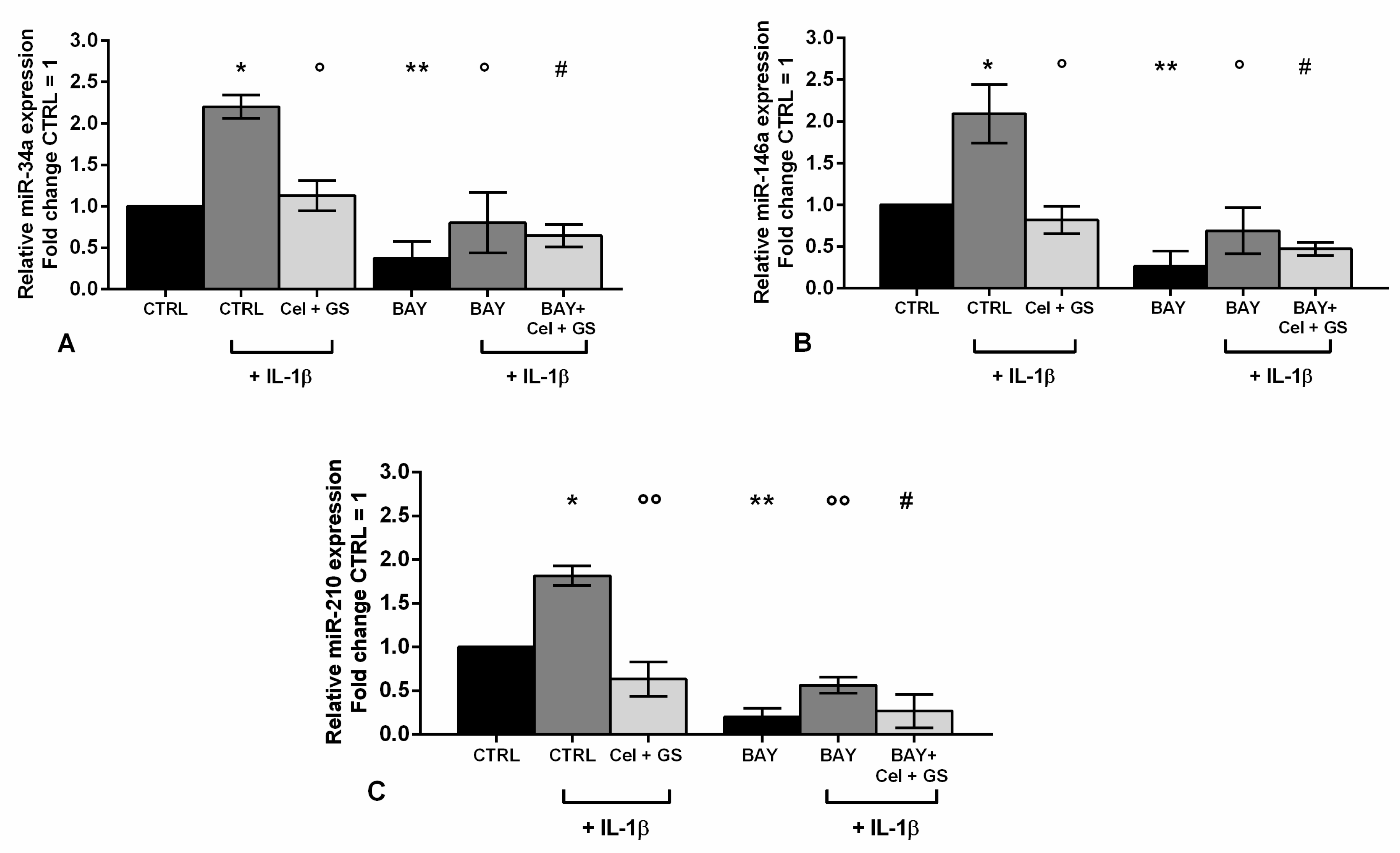
2.2. MiRNA profile modulation following celecoxib and GS treatment
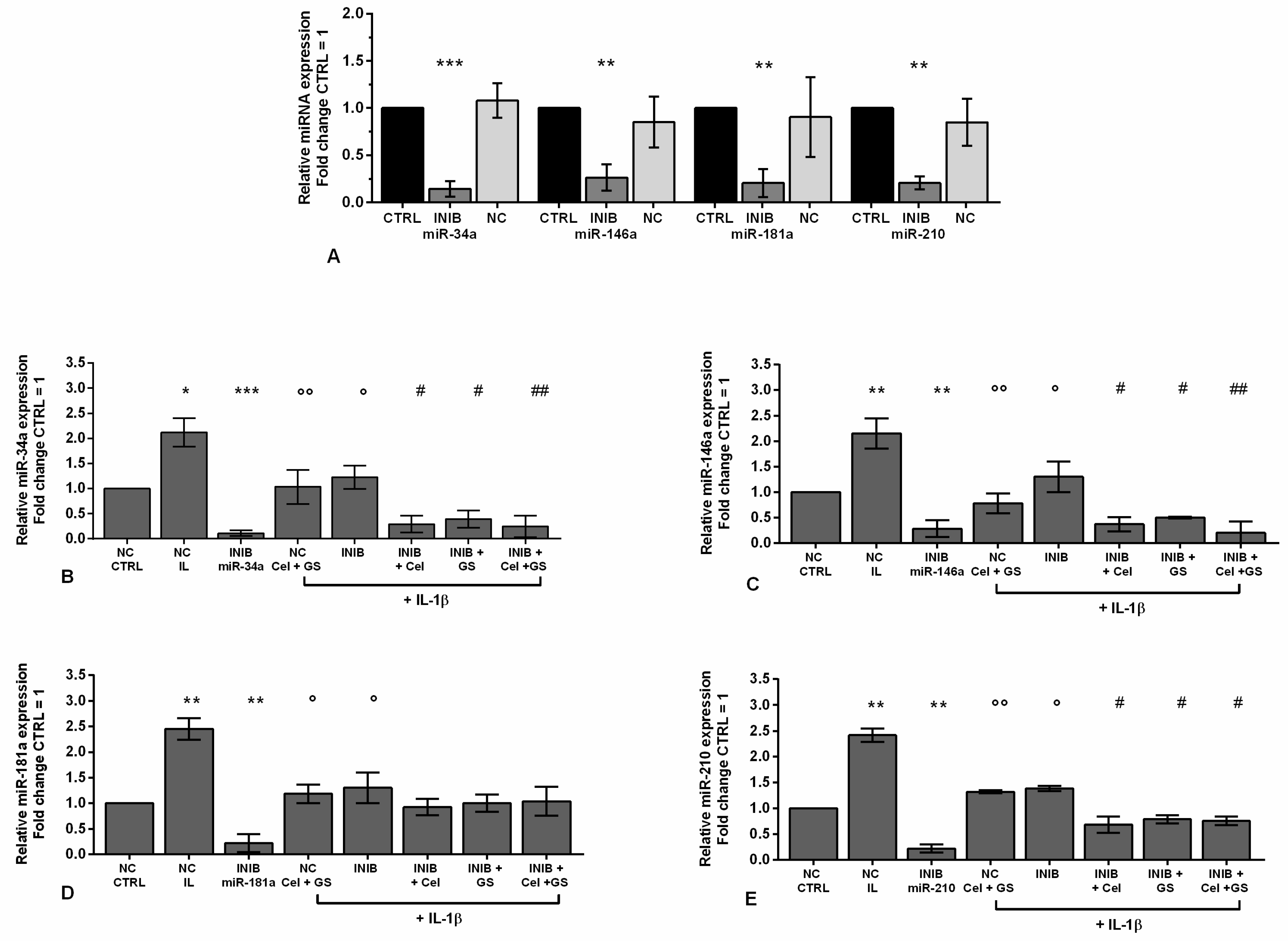
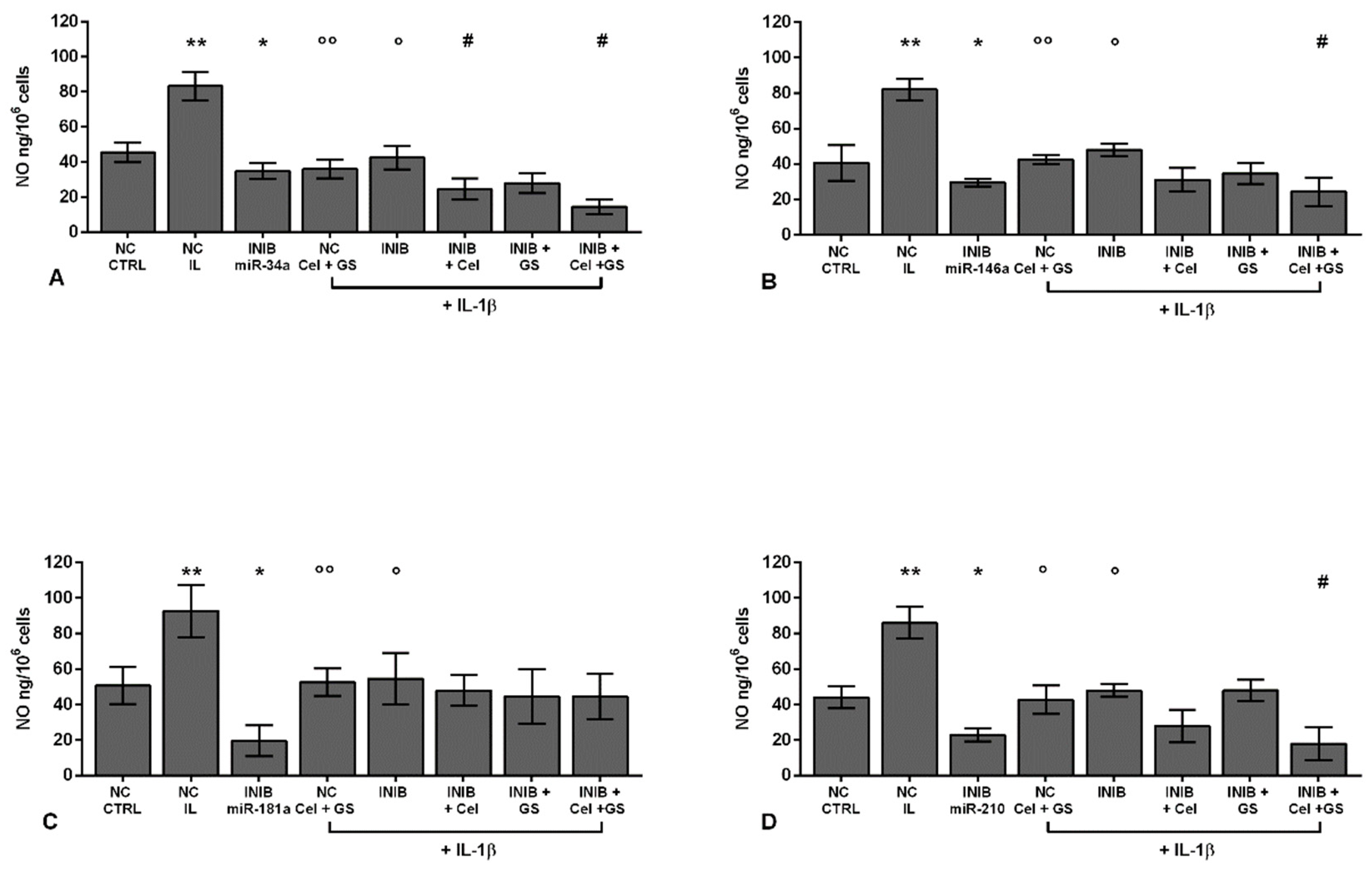
2.3. MiRNA mediate the effect induced by celecoxib and GS
2.4. NF-κB involvement in drugs-induced effects on miRNA
3. Discussion
4. Materials and Methods
4.1. Primary cultures of human OA chondrocytes
4.2. Treatment procedure
4.3. MTT assay
4.4. Apoptosis labeling and reactive oxygen species assessment
4.5. Nitric oxide detection
4.6. Quantitative real-time PCR
4.7. Immunofluorescence determination
4.8. Western blot analysis
4.9. Statistical analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matsuyama, H.; Suzuki, H.I. Systems and Synthetic microRNA Biology: From Biogenesis to Disease Pathogenesis. Int. J. Mol. Sci. 2020, 21, 132–155. [Google Scholar] [CrossRef] [PubMed]
- De Palma, A.; Cheleschi, S.; Pascarelli, N.A.; Tenti, S.; Galeazzi, M.; Fioravanti, A. Do microRNAs have a key epigenetic role in osteoarthritis and in mechanotransduction? Clin. Exp. Rheumatol. 2017, 35, 518–526. [Google Scholar] [PubMed]
- Ratneswaran, A.; Kapoor, M. Osteoarthritis year in review: genetics, genomics, epigenetics. Osteoarthritis Cartilage. 2021, 29, 151–160. [Google Scholar] [CrossRef]
- Li, J.; Gao, X.; Zhu, W.; Li, X. Integrative Analysis of the Expression of microRNA, Long Noncoding RNA, and mRNA in Osteoarthritis and Construction of a Competing Endogenous Network. Biochem. Genet. 2022, 1141–1158. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Rao, W.; Huo, S.; Fan, T.; Qiu, M.; Zhu, H.; Chen, D.; Sheng, X. MicroRNAs and long non-coding RNAs in cartilage homeostasis and osteoarthritis. Front. Cell Dev. Biol. 2022, 10, 1092776. [Google Scholar] [CrossRef] [PubMed]
- Iulian Stanciugelu, S.; Homorogan, C.; Selaru, C.; Patrascu, J.M.; Patrascu, J.M. JR; Stoica, R.; Nitusca, D.; Marian, C. Osteoarthritis and microRNAs: Do They Provide Novel Insights into the Pathophysiology of This Degenerative Disorder? Life (Basel). 2022, 12, 1914. [Google Scholar] [CrossRef]
- Díaz-Prado, S.; Cicione, C.; Muiños-López, E.; Hermida-Gómez, T.; Oreiro, N.; Fernández-López, C.; Blanco, F.J. Characterization of microRNA expression profiles in normal and osteoarthritic human chondrocytes. BMC Musculoskelet. Disord. 2012, 13, 144. [Google Scholar] [CrossRef]
- Balaskas, P.; Goljanek-Whysall, K.; Clegg, P.D.; Fang, Y.; Cremers, A.; Smagul, A.; Welting, T.J.M.; Peffers, M.J. MicroRNA Signatures in Cartilage Ageing and Osteoarthritis. Biomedicines. 2023, 11, 1189. [Google Scholar] [CrossRef]
- Ali, S.A.; Gandhi, R.; Potla, P.; Keshavarzi, S.; Espin-Garcia, O.; Shestopaloff, K.; Pastrello, C.; Bethune-Waddell, D.; Lively, S.; Perruccio, A.V. , et al. Sequencing identifies a distinct signature of circulating microRNAs in early radiographic knee osteoarthritis. Osteoarthritis Cartilage. 2020, 28, 1471–1481. [Google Scholar] [CrossRef]
- Baloun, J.; Pekáčová, A.; Švec, X.; Kropáčková, T.; Horvathová, V.; Hulejová, H.; Prajzlerová, K.; Růžičková, O.; Šléglová, O.; Gatterová, J.; et al. Circulating miRNAs in hand osteoarthritis. Osteoarthritis Cartilage. 2023, 31, 228–237. [Google Scholar] [CrossRef]
- Prasadam, I.; Batra, J.; Perry, S.; Gu, W.; Crawford, R.; Xiao, Y. Systematic Identification, Characterization and Target Gene Analysis of microRNAs Involved in Osteoarthritis Subchondral Bone Pathogenesis. Calcif. Tissue Int. 2016, 99, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Cheleschi, S.; De Palma, A.; Pecorelli, A.; Pascarelli, N.A.; Valacchi, G.; Belmonte, G.; Carta, S.; Galeazzi, M.; Fioravanti, A. Hydrostatic Pressure Regulates MicroRNA Expression Levels in Osteoarthritic Chondrocyte Cultures via the Wnt/β-Catenin Pathway. Int. J. Mol. Sci. 2017, 18, 133. [Google Scholar] [CrossRef] [PubMed]
- Cheleschi, S.; Gallo, I.; Barbarino, M.; Giannotti, S.; Mondanelli, N.; Giordano, A.; Tenti, S.; Fioravanti, A. MicroRNA Mediate Visfatin and Resistin Induction of Oxidative Stress in Human Osteoarthritic Synovial Fibroblasts Via NF-κB Pathway. Int. J. Mol. Sci. 2019, 20, 5200. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Poulet, C.; Malaise, M.; Abak, A.; Mahmud Hussen, B.; Taheriazam, A.; Taheri, M.; Hallajnejad, M. The Emerging Role of Non-Coding RNAs in Osteoarthritis. Front. Immunol. 2021, 12, 773171. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Peffers, M.J.; Ormseth, M.J.; Jurisica, I.; Kapoor, M. The non-coding RNA interactome in joint health and disease. Nat. Rev. Rheumatol. 2021, 17, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Chow, Y.Y.; Chin, K.Y. The Role of Inflammation in the Pathogenesis of Osteoarthritis. Mediators Inflamm. 2020, 8293921. [Google Scholar] [CrossRef]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: pathogenic signaling pathways and therapeutic targets. Signal Transduct. Target Ther. 2023, 8, 56. [Google Scholar] [CrossRef]
- Veronese, N.; Cooper, C.; Bruyère, O; Al-Daghri, N.M.; · Branco, J.; Cavalier, E.; Cheleschi, S.; da Silva Rosa, M.C.; Conaghan, P.G.; Dennison, E.M.; et al. Multimodal Multidisciplinary Management of Patients with Moderate to Severe Pain in Knee Osteoarthritis: A Need to Meet Patient Expectations. Drugs 2022, 82, 1347–1355. [Google Scholar] [CrossRef]
- Bruyère, O.; Honvo, G.; Veronese, N.; Arden, N.K.; Branco, J.; Curtis, E.M.; Al-Daghri, N.M.; Herrero-Beaumont, G.; Martel-Pelletier, J.; Pelletier, J.P.; et al. An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin. Arthritis Rheum. 2019, 49, 337–350. [Google Scholar] [CrossRef]
- Bassleer, C.; Rovati, L.; Franchimont, P. Stimulation of proteoglycan production by glucosamine sulfate in chondrocytes isolated from human osteoarthritic articular cartilage in vitro. Osteoarthritis Cartilage. 1998, 6, 427–34. [Google Scholar] [CrossRef]
- Largo, R.; Alvarez-Soria, M.A.; Díez-Ortego, I.; Calvo, E.; Sánchez-Pernaute, O.; Egido, J.; Herrero-Beaumont, G. Glucosamine inhibits IL-1beta-induced NFkappaB activation in human osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2003, 11, 290–298. [Google Scholar] [CrossRef]
- Chan, P.S.; Caron, J.P.; Rosa, G.J.; Orth, M.W. Glucosamine and chondroitin sulfate regulate gene expression and synthesis of nitric oxide and prostaglandin E(2) in articular cartilage explants. Osteoarthritis Cartilage. 2005, 13, 387–394. [Google Scholar] [CrossRef]
- Chiusaroli, R.; Piepoli, T.; Zanelli, T.; Ballanti, P.; Lanza, M.; Rovati, L.C.; Caselli, G. Experimental pharmacology of glucosamine sulfate. Int. J. Rheumatol. 2011, 2011, 939265. [Google Scholar] [CrossRef]
- Luo, M.; Xu, F.; Wang Q, Luo W. The inhibiting effect of glucosamine sulfate combined with loxoprofen sodium on chondrocyte apoptosis in rats with knee osteoarthritis. J. Musculoskelet. Neuronal. Interact. 2021, 21, 113–120. [Google Scholar]
- Zweers, M.C.; de Boer, T.N.; van Roon, J.; Bijlsma, J.W.; Lafeber, F.P.; Mastbergen, S.C. Celecoxib: Considerations regarding its potential disease-modifying properties in osteoarthritis. Arthritis Res. Ther 1186, 13, 239. [Google Scholar] [CrossRef]
- Nakata, K.; Hanai, T.; Take, Y.; Osada, T.; Tsuchiya, T.; Shima, D.; Fujimoto, Y. Disease-modifying effects of COX-2 selective inhibitors and non-selective NSAIDs in osteoarthritis: A systematic review. Osteoarthritis Cartilage 2018, 26, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.S.; Ahn, K.S.; Jeon, C.H.; Kim, J.; Koh, E.M. Inhibitory effect of cyclo-oxygenase-2 inhibitor on the production of matrix metalloproteinases in rheumatoid fibroblast-like synoviocytes. Rheumatol. Int. 2004, 24, 207–11. [Google Scholar] [CrossRef] [PubMed]
- Mastbergen, S.C.; Jansen, N.W.; Bijlsma, J.W.; Lafeber, F.P. Differential direct effects of cyclo-oxygenase-1/2 inhibition on proteoglycan turnover of human osteoarthritic cartilage: An in vitro study. Arthritis Res. Ther. 2006, 8, 1–9. [Google Scholar] [CrossRef]
- Tat, S.K.; Pelletier, J.P.; Lajeunesse, D.; Fahmi, H.; Duval, N.; Martel-Pelletier, J. Differential modulation of RANKL isoforms by human osteoarthritic subchondral bone osteoblasts: Influence of osteotropic factors. Bone. 2008, 43, 284–291. [Google Scholar] [CrossRef] [PubMed]
- de Boer, T.N.; Huisman, A.M.; Polak, A.A.; Niehoff, A.G.; van Rinsum, A.C.; Saris, D.; Bijlsma, J.W.; Lafeber, F.J.; Mastbergen, S.C. The chondroprotective effect of selective COX-2 inhibition in osteoarthritis: Ex vivo evaluation of human cartilage tissue afterin vivo treatment. Osteoarthritis Cartilage. 2009, 17, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, A.; Tinti, L.; Pascarelli, N.A.; Di Capua, A.; Lamboglia, A.; Cappelli, A.; Biava, M.; Giordani, A.; Niccolini, S.; Galeazzi, M.; Anzini, M. In Vitro effects of VA441, a new selective cyclooxygenase-2 inhibitor, on human osteoarthritic chondrocytes exposed to IL-1β. J. Pharmacol. Sci. 2012, 120, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Cheleschi, S.; Calamia, V.; Fernandez-Moreno, M.; Biava, M.; Giordani, A.; Fioravanti, A.; Anzini, M.; Blanco, F. In vitro comprehensive analysis of VA692 a new chemical entity for the treatment of osteoarthritis. Int. Immunopharmacol. 2018, 64, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Jiang, H.; Jian, X.; Zhang, W. Change of miRNA expression profiles in patients with knee osteoarthritis before and after celecoxib treatment. J. Clin. Lab. Anal. 2019, 33, e22648. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Suzuki, H.; Imaeda, H.; Matsuzaki, J.; Hirata, K.; Tsugawa, H.; Hibino, S.; Kanai, Y.; Saito, H.; Hibi, T. The tumor suppressor microRNA-29c is downregulated and restored by celecoxib in human gastric cancer cells. Int. J. Cancer. 2013, 132, 1751–1760. [Google Scholar] [CrossRef]
- Chen, X.; Peng, D.; Shen, Y.; Liu, B.; Zhou, H.; Tao, H.; Huang, J. The potential combinational effect of miR-34a with celecoxib in osteosarcoma. Anticancer Drugs. 2017, 28, 888–897. [Google Scholar] [CrossRef]
- Cheleschi, S.; Tenti, S.; Giannotti, S.; Veronese, N.; Reginster, J.Y.; Fioravanti, A. A Combination of Celecoxib and Glucosamine Sulfate Has Anti-Inflammatory and Chondroprotective Effects: Results from an In Vitro Study on Human Osteoarthritic Chondrocytes. Int. J. Mol. Sci. 2021, 22, 8980. [Google Scholar] [CrossRef]
- Walter, M.F.; Jacob, R.F.; Day, C.A.; Dahlborg, R.; Weng, Y.; Mason, R.P. Sulfone COX-2 inhibitors increase susceptibility of human LDL and plasma to oxidative modification: comparison to sulfonamide COX-2 inhibitors and NSAIDs. Atherosclerosis. 2004, 177, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhang, Z.; Sheng, P.; Mobasheri, A. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res. Rev. 2021, 66, 101249. [Google Scholar] [CrossRef]
- Tudorachi, N.B.; Totu, E.E.; Fifere, A.; Ardeleanu, V.; Mocanu, V.; Mircea, C.; Isildak, I.; Smilkov, K.; Cărăuşu, E.M. The implication of reactive oxygen species and antioxidants in knee osteoarthritis. Antioxidants (Basel). 2021, 10, 985. [Google Scholar] [CrossRef]
- Coaccioli, S.; Sarzi-Puttini, P.; Zis, P.; Rinonapoli, G.; Varrassi, G. Osteoarthritis: New insight on its pathophysiology. J. Clin. Med. 2022, 11, 6013. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Héraud, F.; Héraud, A.; Harmand, M.F. Apoptosis in normal and osteoarthritic human articular cartilage. Ann. Rheum. Dis. 2000, 59, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.J.; Lu, J.W.; Lee, C.H. , Lee, H.S.; Chu, Y.H.; Ho, Y.J.; Liu, F.C.; Huang, C.J.; Wu, C.C.; Wang, C.C. Cardamonin Attenuates Inflammation and Oxidative Stress in Interleukin-1β-Stimulated Osteoarthritis Chondrocyte through the Nrf2 Pathway. Antioxidants (Basel). 2021, 10, 862. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Yao, C.; Liu, Y.; Yuan, J.; Wu, L.; Hosoi, K.; Yu, S.; Huang, C.; Wei, H. , Chen, G. Arsenic trioxide induces expression of BCL-2 expression via NF-κB and p38 MAPK signaling pathways in BEAS-2B cells during apoptosis. Ecotoxicol. Environ. Saf. 2021, 222, 112531. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Matsuda, K.; Matsusaka, K.; Nakajima, M.; Takeno, Y.; Miyazaki, T.; Shintaku, T.; Yoda, N.; Saito, T.; Ikeda, E.; et al. Anti-proliferating and apoptosis-inducing activity of chemical compound FTI-6D in association with p53 in human cancer cell lines. Chem. Biol. Interact. 2023, 369, 110257. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Soria, M.A.; Herrero-Beaumont, G.; Moreno-Rubio, J.; Calvo, E.; Santillana, J.; Egido, J.; Largo, R. Long-term NSAID treatment directly decreases COX-2 and mPGES-1 production in the articular cartilage of patients with osteoarthritis. Osteoarthritis Cartilage. 2008, 16, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Valvason, C.; Musacchio, E.; Pozzuoli, A.; Ramonda, R.; Aldegheri, R.; Punzi, L. Influence of glucosamine sulphate on oxidative stress in human osteoarthritic chondrocytes: effects on HO-1, p22(Phox) and iNOS expression. Rheumatology (Oxford). 2008, 47, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Tan, C.; An, H.; Jiang, D.; Quan, Z.; Tang, K.; Luo, X. Selective COX-2 inhibitor ameliorates osteoarthritis by repressing apoptosis of chondrocyte. Med. Sci. Monit. 2012, 18, BR247–52. [Google Scholar] [CrossRef]
- Zhong, X.; Li, P.; Li, J.; He, R.; Cheng, G.; Li, Y. Downregulation of microRNA-34a inhibits oxidized low-density lipoprotein-induced apoptosis and oxidative stress in human umbilical vein endothelial cells. Int. J. Mol. Med. 2018, 42, 1134–1144. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, C.; Yang, Y.; Hou, D.; Zhu. Role of miR-181a in the process of apoptosis of multiple malignant tumors: A literature review. Adv. Clin. Exp. Med. 2018, 27, 263–270. [Google Scholar] [CrossRef]
- Cheng, D.L.; Fang, H.X.; Liang, Y.; Zhao, Y.; Shi, C.S. MicroRNA-34a promotes iNOS secretion from pulmonary macrophages in septic suckling rats through activating STAT3 pathway. Biomed. Pharmacother. 2018, 105, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Endisha, H.; Datta, P.; Sharma, A.; Nakamura, S.; Rossomacha, E.; Younan, C. MicroRNA-34a-5p Promotes Joint Destruction During Osteoarthritis. Arthritis Rheumatol. 2021, 73, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Cheleschi, S.; Barbarino, M.; Gallo, I.; Tenti, S.; Bottaro, M.; Frati, E.; Giannotti, S.; Fioravanti, A. Hydrostatic Pressure Regulates Oxidative Stress through microRNA in Human Osteoarthritic Chondrocytes. Int. J. Mol. Sci. 2020, 21, 3653. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.N.; Lu, S.; Fu, C.M. MiR-146a expression profiles in osteoarthritis in different tissue sources: a meta-analysis of observational studies. J. Orthop. Surg. Res. 2022, 17, 148. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Su, W.; Xia, H.; Wang, Z.; Su, C.; Su, B. Synovial Fluid MicroRNA-210 as a Potential Biomarker for Early Prediction of Osteoarthritis. Biomed. Res. Int. 2019, 2019, 7165406. [Google Scholar] [CrossRef]
- Liu, S.C.; Chuang, S.M.; Hsu, C.J.; Tsai, C.H.; Wang, S.W.; Tang, C.H. CTGF increases vascular endothelial growth factor-dependent angiogenesis in human synovial fibroblasts by increasing miR-210 expression. Cell Death Dis. 2014, 5, e1485. [Google Scholar] [CrossRef]
- Yang, M.; Yan, X.; Yuan, F.Z.; Ye, J.; Du, M.Z.; Mao, Z.M.; Xu, B.B.; Chen, Y.R.; Song, Y.F.; Fan, B.S.; Yu, J.K. MicroRNA-210-3p Promotes Chondrogenic Differentiation and Inhibits Adipogenic Differentiation Correlated with HIF-3α Signalling in Bone Marrow Mesenchymal Stem Cells Biomed. Res. Int. 2021, 2021, 6699910. [Google Scholar] [CrossRef]
- Kim, D.; Song, J.; Kim, S.; Chun, C.H.; Jin, E.J. MicroRNA-34a regulates migration of chondroblast and IL-1β-induced degeneration of chondrocytes by targeting EphA5. Biochem. Biophys Res. Commun. 2011, 415, 551–557. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Z.; Chen, S.; Zang, X.; Miao, J. Interleukin-1β/nuclear factor-κB signaling promotes osteosarcoma cell growth through the microRNA-181b/phosphatase and tensin homolog axis. J. Cell Biochem. 2019, 20, 1763–1772. [Google Scholar] [CrossRef]
- Zhao, G.; Gu, W. Effects of miR-146a-5p on chondrocyte interleukin-1β-induced inflammation and apoptosis involving thioredoxin interacting protein regulation. J. Int. Med. Res. 2020, 48, 300060520969550. [Google Scholar] [CrossRef]
- Chen, W.C.; Lin, M.S.; Ye, Y.L.; Gao, H.J.; Song, Z.Y.; Shen, X.Y. microRNA expression pattern and its alteration following celecoxib intervention in human colorectal cancer. Exp. Ther. Med. 2012, 3, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Wang, M.; Zhao, J.; Zhang, H.; Zhou, C.; Jin, L.; Zhang, Y.; Qiu, X.; Ma, B.; Fan, Q. MicroRNA-34a affects chondrocyte apoptosis and proliferation by targeting the SIRT1/p53 signaling pathway during the pathogenesis of osteoarthritis. Int. J. Mol. Med. 2016, 38, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Fu, Y.Y.; Shi, M.Y.; Li, H.X. Down-regulation of miR-181a can reduce heat stress damage in PBMCs of Holstein cows. In Vitro Cell. Dev. Biol. Anim. 2016, 52, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chu, A.; Feng, Y.; Chen, L.; Shao, Y.; Luo, Q.; Deng, X.; Wu, M.; Shi, X.; Chen, Y. A MicroRNA-146a: A Comprehensive Indicator of Inflammation and Oxidative Stress Status Induced in the Brain of Chronic T2DM Rats. Front. Pharmacol. 2018, 9, 478. [Google Scholar] [CrossRef]
- Cai, F,; Chen, M.; Zha, D.; Zhang, P.; Zhang, X.; Cao, N.; Wang, J.; He, Y.; Fan, X.; Zhang, W; et al. Curcumol potentiates celecoxib-induced growth inhibition and apoptosis in human non-small cell lung cancer. Oncotarget. 2017, 8, 115526-115545. [CrossRef]
- Tudor, D.V.; Bâldea, I.; Olteanu, D.E.; Fischer-Fodor, E.; Piroska, V.; Lupu, M.; Călinici, T.; Decea, R.M.; Filip, G.A. Celecoxib as a valuable adjuvant in cutaneous melanoma treated with trametinib. Int. J. Mol. Sci. 2021, 22, 4387. [Google Scholar] [CrossRef]
- Imagawa, K.; de Andrés, M.C.; Hashimoto, K.; Pitt, D.; Itoi, E.; Goldring, M.B.; Roach, H.I.; Oreffo, R.O. The epigenetic effect of glucosamine and a nuclear factor-kappa B (NF-kB) inhibitor on primary human chondrocytes–implications for osteoarthritis. Biochem. Biophys. Res. Commun. 2011, 405, 362–367. [Google Scholar] [CrossRef]
- Kucharz, E.J.; Kovalenko, V.; Szántó, S.; Bruyère, O.; Cooper, C.; Reginster, J.Y. A review of glucosamine for knee osteoarthritis: why patented crystalline glucosamine sulfate should be differentiated from other glucosamines to maximize clinical outcomes. Curr. Med. Res. Opin. 2016, 32, 997–1004. [Google Scholar] [CrossRef]
- Calamia, V.; Mateos, J.; Fernández-Puente, P.; Lourido, L.; Rocha, B.; Fernández-Costa, C.; Montell, E.; Vergés, J.; Ruiz-Romero, C.; Blanco, F. J Pharmacoproteomic study of the effects of chondroitin and glucosamine sulfate on human articular chondrocytes. Arthritis Res Ther. 2010, 12, R138. [Google Scholar] [CrossRef]
- Qi, J.; Qiao, Y.; Wang, P.; Li, S.; Zhao, W.; Gao, C. microRNA-210 negatively regulates LPS-induced production of proinflammatory cytokines by targeting NF-κB1 in murine macrophages. FEBS Lett. 2012, 586, 1201–1207. [Google Scholar] [CrossRef]
- Zhou, B.; Li, H.; Shi, J. miR-27 inhibits the NF-κB signaling pathway by targeting leptin in osteoarthritic chondrocytes. Int. J. Mol. Med. 2017, 40, 523–530. [Google Scholar] [CrossRef]
- Altman, R.; Alarcón, G.; Appelrouth, D.; Bloch, D.; Borenstein, D.; Brandt, K.; Brown, C.; Cooke, T.D.; Daniel, W.; Feldman, D.; et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991, 34, 505–514. [Google Scholar] [CrossRef]
- Mankin, H.J.; Dorfman, H.; Lippiello, L.; Zarins, A. Biochemical and metabolic abnormalities in articular cartilage from osteoarthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J. Bone Joint Surg. Am. 1971, 53, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Cheleschi, S.; Tenti, S.; Lorenzini, S.; Seccafico, I.; Barbagli, S.; Frati, E.; Fioravanti, A. Synovial Fluid Regulates the Gene Expression of a Pattern of microRNA via the NF-κB Pathway: An In Vitro Study on Human Osteoarthritic Chondrocytes. Int. J. Mol. Sci. 2022, 23, 8334. [Google Scholar] [CrossRef] [PubMed]
- Pascarelli, N.A.; Moretti, E.; Terzuoli, G.; Lamboglia, A.; Renieri, T.; Fioravanti, A.; Collodel, G. Effects of gold and silver nanoparticles in cultured human osteoarthritic chondrocytes. J. Appl. Toxicol. 2013, 33, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.T.B.; Clark, I.M.; and Le, L.T.T. MicroRNA-Based Diagnosis and Therapy. Int. J. Mol. Sci. 2022, 23, 7167. [Google Scholar] [CrossRef]
- Veronese, N.; Ecarnot, F.; Cheleschi, S.; Fioravanti, A.; Maggi, S. Possible synergic action of non-steroidal anti-inflammatory drugs and glucosamine sulfate for the treatment of knee osteoarthritis: a scoping review. BMC Musculoskelet. Disord. 2022, 23, 1084. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




