1. Introduction
Aortic stenosis and coronary artery disease (CAD) share some common risk factors and some studies have suggested a similar pathophysiology process for both entities [
1]. About half of transcatheter aortic valve replacement (TAVR) candidates included in observational registries exhibit some degree of CAD [
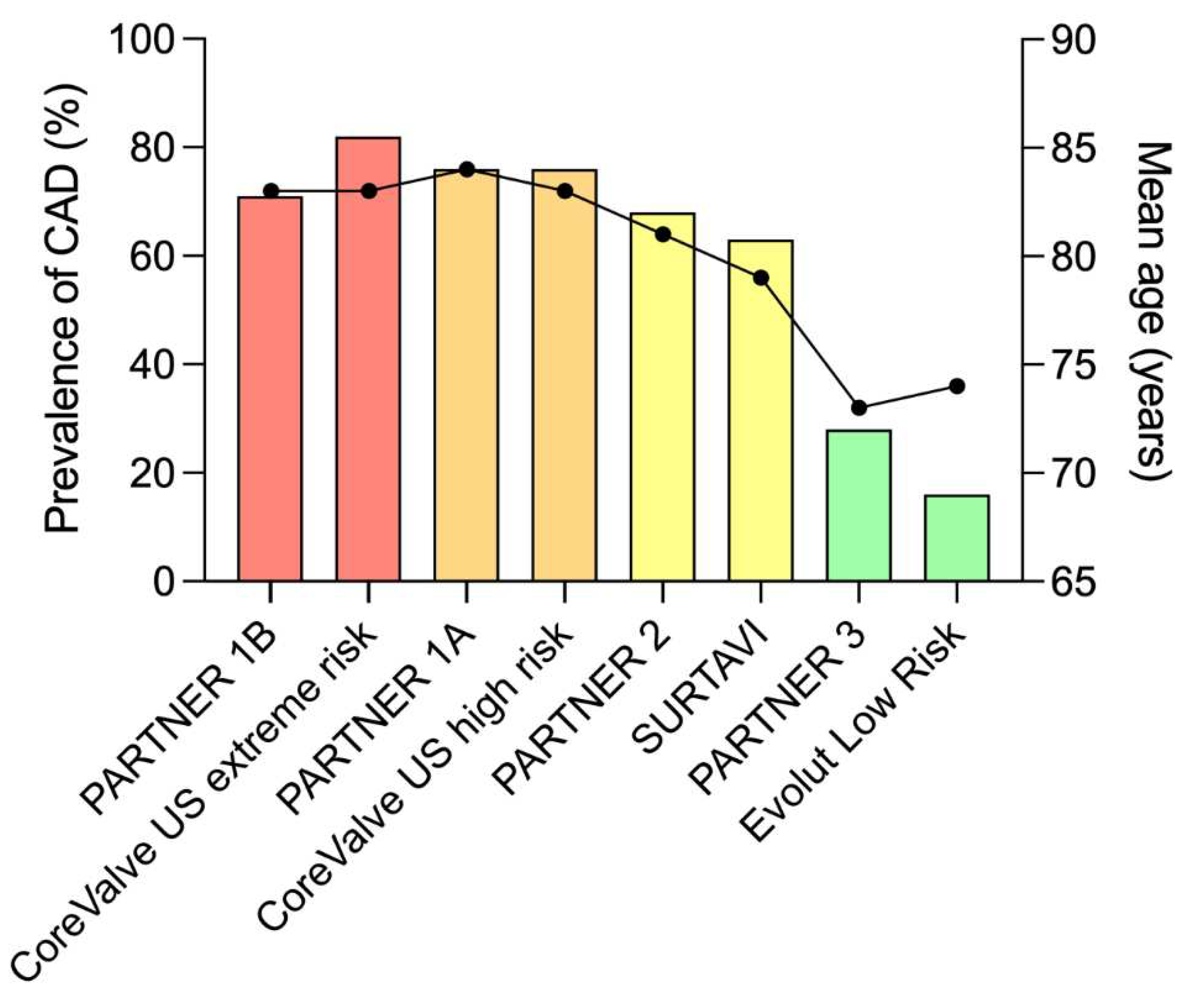
2]. In fact, the prevalence of CAD depends largely on the population studied, and within randomized trials the prevalence of CAD decreases from 81% to 15% as mean age and surgical risk of patients decreases [
3,
4,
5,
6,
7,
8,
9,
10] (
Figure 1). When present, CAD is multitruncular in half of cases and involves left main stem in 10% of the patients [
2].
2. Prognostic Impact of CAD in TAVR Candidates
Numerous observational studies aimed to assess the prognostic impact of CAD in TAVR recipients and reported conflicting results. These studies have been pooled in several meta-analyses concluding that coronary artery disease and even more its degree of severity as estimated by the SYNTAX score were markers of poor prognosis in this population. However, CAD is often associated with other comorbidities and when statistical adjustment was performed, the negative prognostic impact of CAD was no longer significant [
2]. Thus, it seems likely that CAD and its severity are more markers of comorbidity and high surgical risk than independent factors of poor prognosis.
3. Coronary Evaluation Pre-TAVR
Coronary angiography is the gold standard for the identification of coronary stenosis, either before cardiac surgery or percutaneous procedures [
11]. Nevertheless, the use of cardiac computed tomography angiography (CCTA) in the setting of pre-TAVR workup has promising preliminary data. One meta-analysis estimates that performing a cardiac computed tomography would decrease the number of pre-TAVR coronary angiograms by 37% [
12]. This percentage could increase as TAVR will be addressed to younger patients with low probability of CAD. On the other hand, the use hemodynamic assessment of coronary lesions by FFR (fractional flow reserve) or iFR (instantaneous wave-free ratio) in patients with aortic stenosis is safe and reliable and is an emerging strategy in this context [
2].
Some authors proposed that pre-TAVR coronary angiography could be performed only when CCTA suggests the presence of significant coronary stenosis. Thus, a first study concluded that coronary angiography was necessary only for a minority of TAVR candidates, with no negative clinical impact associated with the avoidance of coronary angiogram based on CCTA results [
13]. Numerous studies have since sought to investigate the performance of CCTA in this indication, showing an excellent negative predictive value at the cost of a low specificity [
12]. Assessment of previously stented segments was feasible in 70 to 90% of cases with a good diagnosis performance [
2]. Conversely, rate of false positives increased when heavily calcified segments were evaluated. Finally, a meta-analysis estimated that the performance of CCTA would reduce the number of pre-TAVR coronary angiograms by 37% [
12], and this percentage would likely increase as TAVR indication will be extended in younger patients, with a much lower probability of CAD and a lower degree of coronary calcification.
Most of TAVR candidates do not present with angina pectoris, nor have recent non-invasive ischemia testing. Therefore, in accordance with current guidelines, hemodynamic evaluation by FFR or iFR should be performed when the degree of coronary stenosis is less than 90% [
14]. However, patients with aortic stenosis were not represented in FFR studies, and scarce data are available on the functional evaluation of coronary lesions in TAVR candidates. The safety of adenosine injection (whether intravenous or intracoronary) has been well demonstrated in several studies [
2]. However, it should be noted that nitrates injection was performed only in a minority of cases. Moreover, left ventricular hypertrophy induced by aortic stenosis is likely altering coronary reserve, which could tamper results of hemodynamic assessments. Ahmad et al [
15] demonstrated that systemic flow and coronary hyperemic flow increased significantly after TAVR (compared with pre-TAVR measurement), which could lead to an underestimation of lesion severity when FFR assessment is performed prior to TAVR. In contrast, flow during the wave-free period of diastole did not change after TAVR, suggesting that iFR may not be influenced by the presence of aortic stenosis. Finally, Yamanaka et al [
16] showed, in a population of patients with severe aortic stenosis and coronary lesions previously assessed by myocardial perfusion scintigraphy, that there was a good correlation between FFR and the presence of ischemia on scintigraphy (area under the curve of 0.93). The optimal FFR threshold was 0.83, confirming that FFR likely underestimates lesion severity in the presence of aortic stenosis. These data remain preliminary, and the recent update of guidelines for the management of valvular heart disease still support the use of “classic” thresholds for FFR and iFR (0.80 and 0.89, respectively) in the presence of valvular heart disease [
11,
17].
4. Coronary Revascularization Pre-TAVR
Both European and North American guidelines recommend performing percutaneous coronary intervention (PCI) prior to TAVR for coronary stenosis of more than 70% severity in proximal coronary segments, with a recommendation grade of IIa and a level of evidence of C [
11,
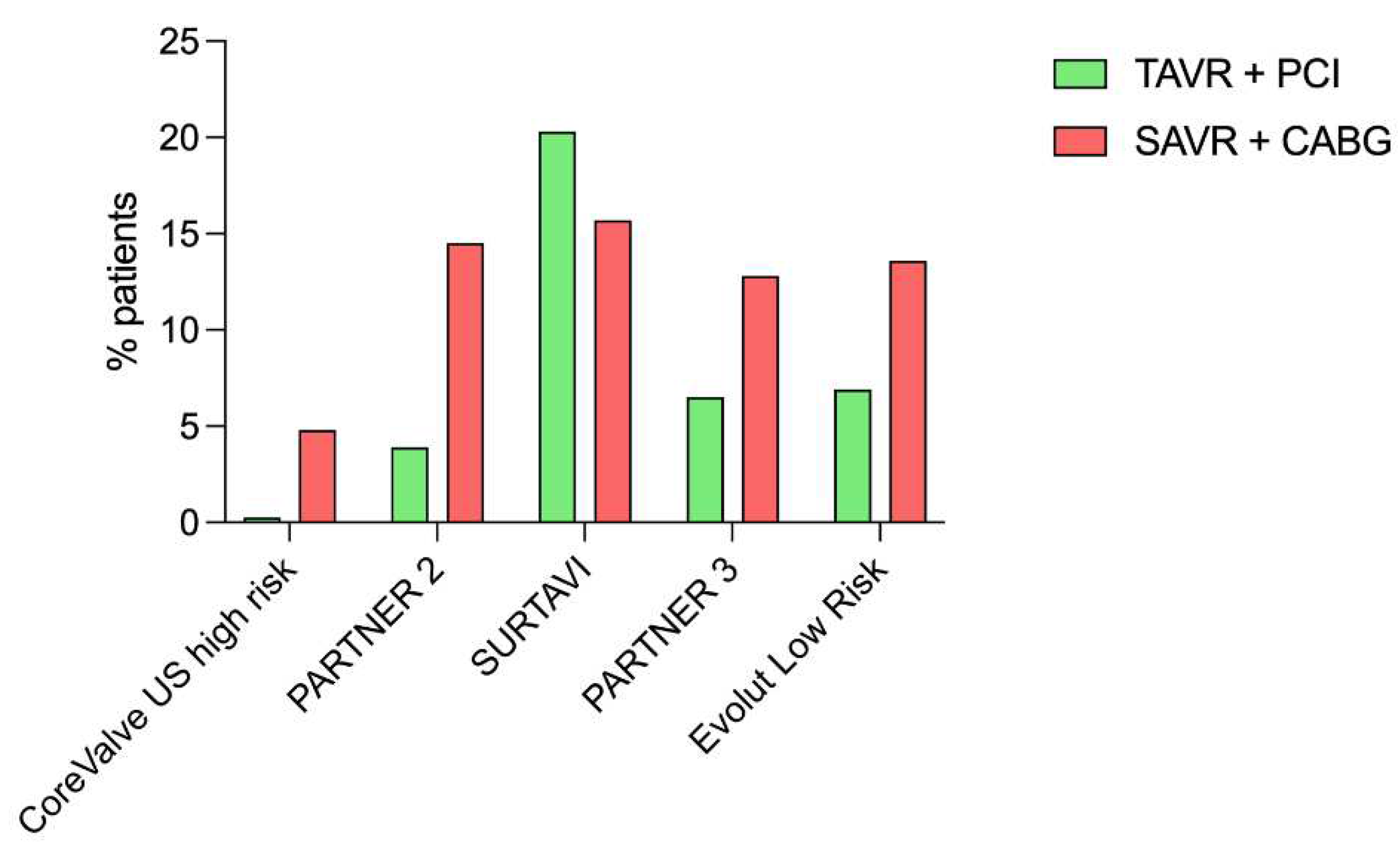
17]. Indeed, only a minority of patients included in randomized trials benefited from coronary revascularization (either by PCI or coronary artery bypass graft) associated with aortic valve replacement (
Figure 2), patients with complex coronary lesions (high SYNTAX score and/or unprotected left main) being excluded from these trials [
7,
8,
9,
10]. The largest cohort of patients who underwent pre-TAVR PCI published to date included 1197 patients with a total of 1705 coronary lesions treated, with a median follow-up of 2 years post-TAVR [
18]. Coronary lesions were frequently complex (bifurcation, ostial location, significant calcifications) with rotational atherectomy used in 7% of patients. Rates of procedural success (97.3%), intra-stent restenosis (2.3%) and stent thrombosis (0.4%) were similar to what is usually described in "all-comers" PCI. Clinical event rate (death, myocardial infarction, and stroke: MACCE) was relatively high, incomplete coronary revascularization determining an increased risk of MACCE. Witberg et al [
19] found similar results, with patients completely or reasonably incompletely revascularized (residual SYNTAX score ≤8) having a prognosis similar to patients without CAD. In contrast, patients with incomplete coronary revascularization (residual SYNTAX score >8) had a poorer prognosis. On the other hand, the REVASC-TAVI registry [
20] did not find any significant impact of the completeness of myocardial revascularization pre-TAVR. ACTIVATION trial [
21] randomized patients presenting aortic stenosis with planned TAVR and significant CAD into two arms: PCI versus medical treatment of CAD. Patients who presented an acute coronary syndrome within 1 month before inclusion, as well as those exhibiting severe angina, or an unprotected left main lesion were excluded. A single coronary lesion was revascularized in most cases (71%) and drug-eluting stents were implanted in 80% of patients. After 12 months follow-up, there was no significant difference between the two strategies regarding the primary endpoint (death or rehospitalization) without reaching the non-inferiority threshold, but there was a significantly higher rate of bleeding in the PCI arm. However, it should be noted that authors did not provide information on the use of hemodynamic tools such as FFR, nor on the completeness of myocardial revascularization, which may have mitigated a potential beneficial effect of pre-TAVR coronary revascularization. We can also assume that a positive effect of pre-TAVR PCI may require a longer follow-up to be shown.
Finally, patients with severe CAD (unprotected left main lesion and/or high SYNTAX score) were excluded from randomized trials (
Table 1), and to date only one observational study (with propensity score matching) compared outcomes of a surgical strategy (coronary artery bypass graft and surgical aortic valve replacement) with those of a transcatheter strategy (PCI followed by TAVR) in this specific population [
22]. After a median follow-up of 3 years, a similar rate of MACCE was observed between the two strategies. To note, an increased risk of repeat coronary revascularization was observed in patients benefiting from a transcatheter strategy.
In conclusion, the clinical relevance of PCIs performed prior to TAVR remains unclear, and future studies should better identify patients who benefit from pre-TAVR coronary revascularization.
5. Timing of Coronary Revascularization Pre-TA VR
When a PCI indication is established, it is usually performed upstream of TAVR, but the optimal timing of PCI prior to TAVR remains unclear. Two observational studies suggested that PCI performed during the same hospitalization as the TAVR procedure were associated with an increased risk of complications [
23,
24], while several studies have shown the feasibility and safety of TAVR and concomitant PCI [
2]. Although this later strategy has the advantage of avoiding multiple procedures and can potentially reduce the risks associated with obtaining vascular access at different time points, it increases the amount of contrast media administered at the time of the TAVR procedure and could potentially increase the risk of acute kidney injury, particularly in cases of complex CAD. Finally, A sub-analysis of the REVASC-TAVI Registry has been presented at the Cardiovascular Research Technologies (CRT) 2023 and suggested that PCI performed after TAVR showed significantly lower rates of all-cause death and a composite endpoint compared with other PCI timings relative to TAVR. In summary, no definite data exist on the timing of PCI in TAVR candidates. In the absence of further data, no specific timing strategy can be recommended in this setting. However, the presence of both complex CAD and risks factors for contrast nephropathy should probably be considered when establishing a minimum delay between procedures.
6. Acute Coronary Syndrome after TAVR
Initially neglected because of the advanced age and short life expectancy of first TAVR recipients, the issue of post-TAVR coronary events has progressively become more important and constitutes one of the last limits to the extension of TAVR to patients beyond 75 years. The first study addressing this topic was published in 2017 and reported in a single-center cohort of nearly 800 patients with a median follow-up of 2 years post-TAVR a cumulative incidence of post-TAVR ACS of 10% [
25]. Male gender, prior CAD, a non-transfemoral approach, and acute kidney injury were independent predictors of post-TAVR ACS occurrence. Several features unique to post-TAVR ACS were then promptly identified. First, ACS following TAVR are characterized by an unusually high proportion of type 2 myocardial infarction (35% of post-TAVR ACS) and a very low proportion of ST-segment elevation myocardial infarction (STEMI) (less than 10% of post-TAVR ACS) [
26]. These features are likely related to the advanced age and the high comorbidity burden of TAVR recipients in the last 10 years. Second, although relatively rare, STEMI post-TAVR are associated with a particularly poor prognosis [
26]. Finally, up to 65% of patients presenting with ACS post-TAVR do not receive coronary revascularization, and the lack of revascularization is obviously associated with an increased risk of MACCE. The low rate of coronary revascularization may be explained by the advanced age, the comorbidity burden, the high proportion of type 2 ACS, but also by potential coronary cannulation issues, related to interactions between the transcatheter valve stent and coronary ostia [
26].
Thus, one study focused on post-TAVR STEMI and compared their characteristics to those of all-comers STEMI (without TAVR history) [
27]. This study led to the following findings:
- -
All STEMI management performance indicators were significantly worse in the population with a history of TAVR, with a 33% longer door-to-balloon time, but also a significantly longer procedure time, fluoroscopy time, along with higher contrast volume and dosimetry level, reflecting an increased procedural complexity.
- -
Angioplasty failure rate was 4 times higher in the population of patients with a history of TAVR (16% versus 4%), with particularly high rates of coronary cannulation failure (6%) and lesion crossing failure (5%).
- -
Revascularization failure was an independent predictor of poor outcomes.
- -
In addition to the classical atherothrombotic mechanism, other alternative mechanisms were involved such as coronary embolism (complicating the TAVR procedure itself, or related to TAVR valve thrombosis), late migration of the TAVR valve resulting in delayed coronary occlusion (particularly with self-expanding valves), or restenosis/thrombosis of stents implanted in the work-up pre-TAVR.
All these characteristics make post-TAVR ACS a unique population, complex to manage and with poor outcomes. Coronary re-access is a central issue, whose resolution will contribute to the final expansion of TAVR indications.
7. Coronary Access after TAVR
Preliminary data on coronary canulation post-TAVR provided from case reports and small series but highlighted a highly variable success rate of selective coronary injection, ranging from 50% to 100% [
2]. In 2018, Yudi et al [
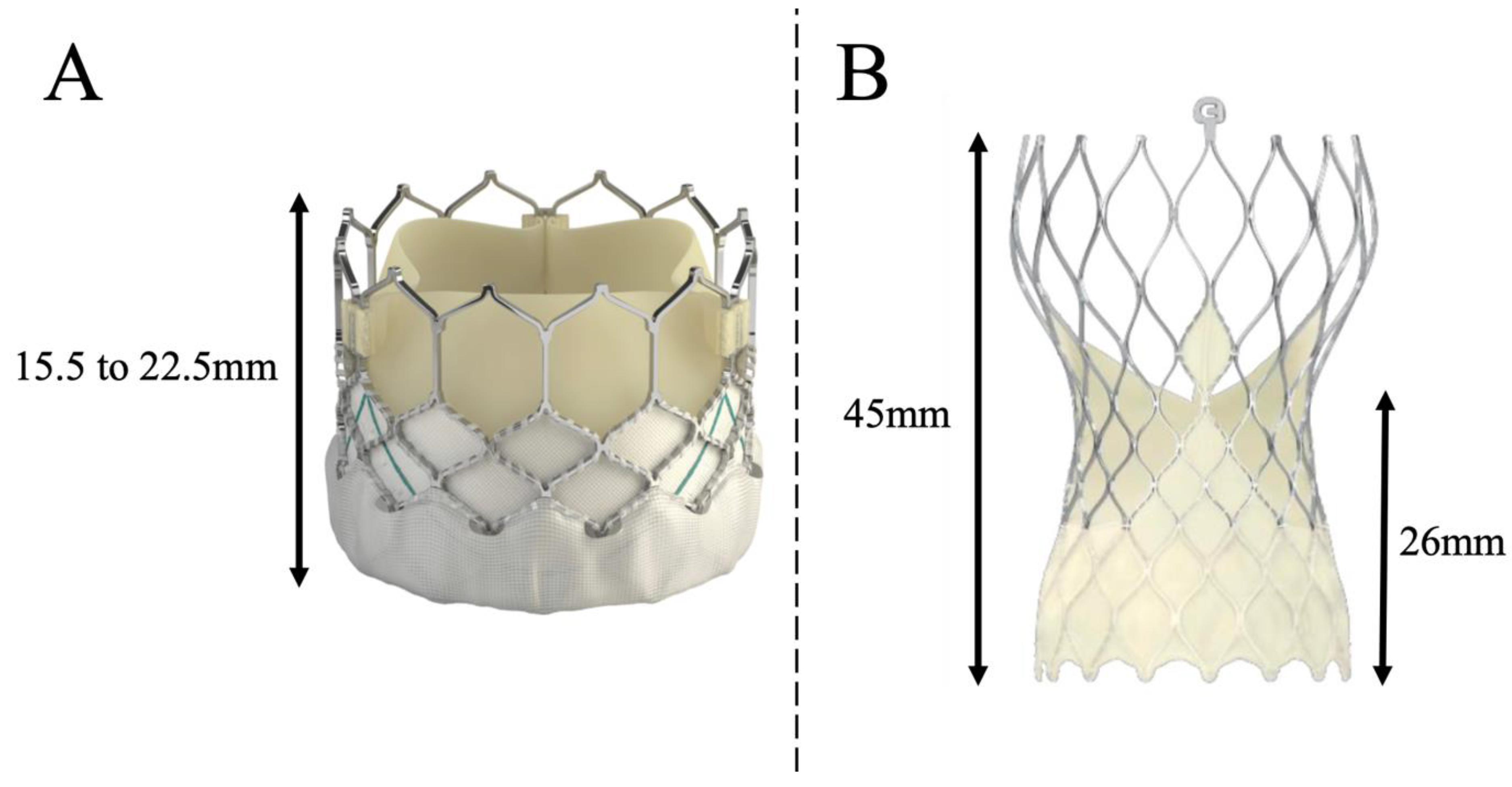
28] proposed an algorithm to successfully perform post-TAVR coronary cannulation, depending not only on the implanted transcatheter heart valve but also on the position of the commissures regarding coronary ostia. Authors recommended the systematic use of 6-Fr catheters, and the use of a left radial or femoral approach for self-expanding supra-annular EVOLUT valves. Indeed, the design of the TAVR prosthesis (
Figure 3) and its implantation technique (implantation depth, commissural alignment) will critically determine the feasibility of coronary artery cannulation after TAVR. When a SAPIEN 3 valve is implanted, the stent of the prosthesis covers the coronary ostia in 40% to 50% of cases [
29,
30], compared with 100% of cases with the EVOLUT valve [
31]. A study based on systematic post-TAVR CCTA showed that coronary ostia were in an unfavorable position (coronary ostium below the upper part of the skirt or in front of the commissures of the TAVR valve) in 8% (right coronary) to 15% (left coronary) of cases with SAPIEN 3 valves, compared with 25% (right coronary) to 35% (left coronary) of cases with EVOLUT valves [
32]. Finally, authors of the RE-ACCESS study performed systematic coronary angiography post-TAVI valve implantation, in a population composed of one-third EVOLUT valve, one-third SAPIEN 3 valve and one-third ACURATE NEO valve [
33]. They observed a cannulation failure rate of 7.7% (23 patients in total, EVOLUT valve for 22 patients). Independent factors predicting coronary cannulation failure were EVOLUT valve, implantation depth, and the ratio of TAVI valve width to sinus of Valsalva width.
In order to facilitate coronary ostia catheterization after EVOLUT valve implantation, which will always have to be performed through the stent struts of the prosthesis, a new implantation technique based on commissural alignment enabled by a cusp-overlap view implantation has been developed and rapidly popularized [
34]. This implantation technique reduces the rate of post-TAVI pacemaker implantation by minimizing implantation depth and is thought to facilitate coronary cannulation by aligning the commissures of the native valve with those of the TAVI valve. However, asymmetry of the aortic cusps is not uncommon (particularly in bicuspid valves), and an eccentric position of the coronary ostium is reported in 28% of right coronaries and 6% of left coronaries [
35]. Consequently, the commissural alignment technique may not be sufficient in some cases, and it has been proposed to perform an alignment based on a "coronary artery overlap view" rather than a "cusp overlap view", which enables one of the TAVR valve commissures to be positioned equidistant from the 2 coronary ostia, guaranteeing the best possible access to both coronaries.
8. Conclusions and Future Directions
CAD is one of the most common comorbidities among TAVR patients. Coronary revascularization of thigh coronary stenosis located on proximal trunk is currently recommended during the work-up pre-TAVR. However, uncertainties remain regarding the clinical benefit of pre-TAVR coronary revascularization, and further studies will help to better identify patients and coronary lesions that should be revascularized prior to TAVR. Finally, the management of post-TAVR coronary events is an emerging issue that will become increasingly important. The fact that a significant number of post-TAVR ACS patients will be managed in non-TAVR centers underlines the critical importance of training all interventional cardiologists in techniques that can facilitate coronary artery cannulation after TAVR.
Abbreviations
| ACS |
acute coronary syndrome |
| CAD |
coronary artery disease |
| CCTA |
cardiac computed tomography angiography |
| FFR |
fractional flow reserve |
| Ifr |
instantaneous wave-free ration |
| MACCE |
major adverse cardiovascular and cerebrovascular event |
| PCI |
percutaneous coronary intervention |
| SAVR |
surgical aortic valve replacement |
| TAVR |
transcatheter aorti valve replacement |
References
- Stewart, B.F.; Siscovick, D.; Lind, B.K.; Gardin, J.M.; Gottdiener, J.S.; Smith, V.E.; Kitzman, D.W.; Otto, C.M. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997, 29, 630–634. [Google Scholar] [CrossRef]
- Faroux, L.; Guimaraes, L.; Wintzer-Wehekind, J.; Junquera, L.; Ferreira-Neto, A.N.; Del Val, D.; Muntané-Carol, G.; Mohammadi, S.; Paradis, J.M.; Rodés-Cabau, J. Coronary Artery Disease and Transcatheter Aortic Valve Replacement: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019, 74, 362–372. [Google Scholar] [CrossRef]
- Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010, 363, 1597–1607.
- Popma JJ, Adams DH, Reardon MJ, Yakubov SJ, Kleiman NS, Heimansohn D, Hermiller J Jr, Hughes GC, Harrison JK, Coselli J, Diez J, Kafi A, Schreiber T, Gleason TG, Conte J, Buchbinder M, Deeb GM, Carabello B, Serruys PW, Chenoweth S, Oh JK; CoreValve United States Clinical Investigators. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014, 63, 1972–1981.
- Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011, 364, 2187–2198.
- Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J Jr, Kleiman NS, Chetcuti S, Heiser J, Merhi W, Zorn G, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Conte J, Maini B, Mumtaz M, Chenoweth S, Oh JK; U.S. CoreValve Clinical Investigators. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014, 370, 1790–1798.
- Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG; PARTNER 2 Investigators. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016, 374, 1609–1620.
- Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, Chetcuti S, Gleason T, Heiser J, Lange R, Merhi W, Oh JK, Olsen PS, Piazza N, Williams M, Windecker S, Yakubov SJ, Grube E, Makkar R, Lee JS, Conte J, Vang E, Nguyen H, Chang Y, Mugglin AS, Serruys PW, Kappetein AP; SURTAVI Investigators. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2017, 376, 1321–1331.
- Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, Leipsic J, Hahn RT, Blanke P, Williams MR, McCabe JM, Brown DL, Babaliaros V, Goldman S, Szeto WY, Genereux P, Pershad A, Pocock SJ, Alu MC, Webb JG, Smith CR; PARTNER 3 Investigators. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019, 380, 1695–1705.
- Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, Askew J, Sorajja P, Rovin J, Chetcuti SJ, Adams DH, Teirstein PS, Zorn GL 3rd, Forrest JK, Tchétché D, Resar J, Walton A, Piazza N, Ramlawi B, Robinson N, Petrossian G, Gleason TG, Oh JK, Boulware MJ, Qiao H, Mugglin AS, Reardon MJ; Evolut Low Risk Trial Investigators. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med. 2019, 380, 1706–1715.
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W; ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022, 43, 561–632.
- van den Boogert TPW, Vendrik J, Claessen BEPM, Baan J, Beijk MA, Limpens J, Boekholdt SAM, Hoek R, Planken RN, Henriques JP. CTCA for detection of significant coronary artery disease in routine TAVI work-up : A systematic review and meta-analysis. Neth Heart J. 2018, 26, 591–599. [CrossRef] [PubMed]
- Chieffo, A.; Giustino, G.; Spagnolo, P.; Panoulas, V.F.; Montorfano, M.; Latib, A.; Figini, F.; Agricola, E.; Gerli, C.; Franco, A.; et al. Routine Screening of Coronary Artery Disease With Computed Tomographic Coronary Angiography in Place of Invasive Coronary Angiography in Patients Undergoing Transcatheter Aortic Valve Replacement. Circ Cardiovasc Interv. 2015, 8, e002025. [Google Scholar] [CrossRef] [PubMed]
- Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Ahmad, Y.; Götberg, M.; Cook, C.; Howard, J.P.; Malik, I.; Mikhail, G.; Frame, A.; Petraco, R.; Rajkumar, C.; Demir, O.; et al. Coronary Hemodynamics in Patients With Severe Aortic Stenosis and Coronary Artery Disease Undergoing Transcatheter Aortic Valve Replacement: Implications for Clinical Indices of Coronary Stenosis Severity. JACC Cardiovasc Interv. 2018, 11, 2019–2031. [Google Scholar] [CrossRef]
- Yamanaka, F.; Shishido, K.; Ochiai, T.; Moriyama, N.; Yamazaki, K.; Sugitani, A.; Tani, T.; Tobita, K.; Mizuno, S.; Tanaka, Y.; et al. Instantaneous Wave-Free Ratio for the Assessment of Intermediate Coronary Artery Stenosis in Patients With Severe Aortic Valve Stenosis: Comparison With Myocardial Perfusion Scintigraphy. JACC Cardiovasc Interv. 2018, 11, 2032–2040. [Google Scholar] [CrossRef]
- Writing Committee Members, Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O'Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A, Toly C. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021, 77, 450–500. [Google Scholar] [CrossRef]
- Faroux, L.; Campelo-Parada, F.; Munoz-Garcia, E.; Nombela-Franco, L.; Fischer, Q.; Donaint, P.; Serra, V.; Veiga, G.; Gutiérrez, E.; Vilalta, V.; et al. Procedural Characteristics and Late Outcomes of Percutaneous Coronary Intervention in the Workup Pre-TAVR. JACC Cardiovasc Interv. 2020, 13, 2601–2613. [Google Scholar] [CrossRef]
- Witberg, G.; Regev, E.; Chen, S.; Assali, A.; Barbash, I.M.; Planer, D.; Vaknin-Assa, H.; Guetta, V.; Vukasinovic, V.; Orvin, K.; et al. The Prognostic Effects of Coronary Disease Severity and Completeness of Revascularization on Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2017, 10, 1428–1435. [Google Scholar] [CrossRef]
- Costa, G.; Pilgrim, T.; Amat Santos, I.J.; De Backer, O.; Kim, W.K.; Barbosa Ribeiro, H.; Saia, F.; Bunc, M.; Tchetche, D.; Garot, P.; et al. Management of Myocardial Revascularization in Patients With Stable Coronary Artery Disease Undergoing Transcatheter Aortic Valve Implantation. Circ Cardiovasc Interv. 2022, 15, e012417. [Google Scholar] [CrossRef]
- Patterson T, Clayton T, Dodd M, Khawaja Z, Morice MC, Wilson K, Kim WK, Meneveau N, Hambrecht R, Byrne J, Carrié D, Fraser D, Roberts DH, Doshi SN, Zaman A, Banning AP, Eltchaninoff H, Le Breton H, Smith D, Cox I, Frank D, Gershlick A, de Belder M, Thomas M, Hildick-Smith D, Prendergast B, Redwood S; ACTIVATION Trial Investigators. ACTIVATION (PercutAneous Coronary inTervention prIor to transcatheter aortic VAlve implantaTION): A Randomized Clinical Trial. JACC Cardiovasc Interv. 2021, 14, 1965–1974.
- Alperi, A.; Mohammadi, S.; Campelo-Parada, F.; Munoz-Garcia, E.; Nombela-Franco, L.; Faroux, L.; Veiga, G.; Serra, V.; Fischer, Q.; Pascual, I.; et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Complex Coronary Artery Disease. JACC Cardiovasc Interv. 2021, 14, 2490–2499. [Google Scholar] [CrossRef]
- van Rosendael PJ, van der Kley F, Kamperidis V, Katsanos S, Al Amri I, Regeer M, Schalij MJ, Ajmone Marsan, N. , Bax JJ, Delgado, V. Timing of staged percutaneous coronary intervention before transcatheter aortic valve implantation. Am J Cardiol. 2015, 115, 1726–1732. [Google Scholar] [CrossRef]
- Singh, V.; Rodriguez, A.P.; Thakkar, B.; Patel, N.J.; Ghatak, A.; Badheka, A.O.; Alfonso, C.E.; de Marchena, E.; Sakhuja, R.; Inglessis-Azuaje, I.; et al. Comparison of Outcomes of Transcatheter Aortic Valve Replacement Plus Percutaneous Coronary Intervention Versus Transcatheter Aortic Valve Replacement Alone in the United States. Am J Cardiol. 2016, 118, 1698–1704. [Google Scholar] [CrossRef]
- Vilalta, V.; Asmarats, L.; Ferreira-Neto, A.N.; Maes, F.; de Freitas Campos Guimarães, L.; Couture, T.; Paradis, J.M.; Mohammadi, S.; Dumont, E.; Kalavrouziotis, D.; et al. Incidence, Clinical Characteristics, and Impact of Acute Coronary Syndrome Following Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2018, 11, 2523–2533. [Google Scholar] [CrossRef]
- Faroux, L.; Munoz-Garcia, E.; Serra, V.; Alperi, A.; Nombela-Franco, L.; Fischer, Q.; Veiga, G.; Donaint, P.; Asmarats, L.; Vilalta, V.; et al. Acute Coronary Syndrome Following Transcatheter Aortic Valve Replacement. Circ Cardiovasc Interv. 2020, 13, e008620. [Google Scholar] [CrossRef] [PubMed]
- Faroux, L.; Lhermusier, T.; Vincent, F.; Nombela-Franco, L.; Tchétché, D.; Barbanti, M.; Abdel-Wahab, M.; Windecker, S.; Auffret, V.; Campanha-Borges, D.C.; et al. ST-Segment Elevation Myocardial Infarction Following Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2021, 77, 2187–2199. [Google Scholar] [CrossRef] [PubMed]
- Yudi MB, Sharma SK, Tang GHL, Kini, A. Coronary Angiography and Percutaneous Coronary Intervention After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2018, 71, 1360–1378. [Google Scholar] [CrossRef]
- Faroux, L.; Couture, T.; Guimaraes, C.; Junquera, L.; Del Val, D.; Muntané-Carol, G.; Wintzer-Wehekind, J.; Mohammadi, S.; Paradis, J.M.; Delarochellière, R.; et al. Interaction Between Balloon-Expandable Valves and Coronary Ostia: Angiographic Analysis and Impact on Coronary Access. J Invasive Cardiol. 2020, 32, 235–242. [Google Scholar]
- Rogers, T.; Greenspun, B.C.; Weissman, G.; Torguson, R.; Craig, P.; Shults, C.; Gordon, P.; Ehsan, A.; Wilson, S.R.; Goncalves, J.; et al. Feasibility of Coronary Access and Aortic Valve Reintervention in Low-Risk TAVR Patients. JACC Cardiovasc Interv. 2020, 13, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Couture, T.; Faroux, L.; Junquera, L.; Del Val, D.; Muntané-Carol, G.; Wintzer-Wehekind, J.; Alperi, A.; Mohammadi, S.; Paradis, J.M.; Delarochellière, R.; et al. Interaction Between Self-Expanding Transcatheter Heart Valves and Coronary Ostia: An Angiographically Based Analysis of the Evolut R/Pro Valve System. J Invasive Cardiol. 2020, 32, 123–128. [Google Scholar]
- Ochiai, T.; Chakravarty, T.; Yoon, S.H.; Kaewkes, D.; Flint, N.; Patel, V.; Mahani, S.; Tiwana, R.; Sekhon, N.; Nakamura, M.; et al. Coronary Access After TAVR. JACC Cardiovasc Interv. 2020, 13, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, M.; Costa, G.; Picci, A.; Criscione, E.; Reddavid, C.; Valvo, R.; Todaro, D.; Deste, W.; Condorelli, A.; Scalia, M.; et al. Coronary Cannulation After Transcatheter Aortic Valve Replacement: The RE-ACCESS Study. JACC Cardiovasc Interv. 2020, 13, 2542–2555. [Google Scholar] [CrossRef] [PubMed]
- Tang GHL, Zaid S, Michev I, Ahmad H, Kaple R, Undemir C, Cohen M, Lansman SL. "Cusp-Overlap" View Simplifies Fluoroscopy-Guided Implantation of Self-Expanding Valve in Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2018, 11, 1663–1665. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; De Backer, O.; Bieliauskas, G.; Wong, I.; Bajoras, V.; Xiong, T.Y.; Zhang, Y.; Kofoed, K.F.; Chen, M.; Sondergaard, L. Cusp Symmetry and Coronary Ostial Eccentricity and its Impact on Coronary Access Following TAVR. JACC Cardiovasc Interv. 2022, 15, 123–134. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).