1. Introduction
Biodegradable materials based on biopolymers are widely used in the creation of products for medical and biological purposes: absorbable sutures, implants for plastic surgery, matrices for cell and tissue engineering, and regenerative medicine [
1,
2,
3,
4,
5]. The creation of materials for innovative medical technologies requires the use of biopolymers and adequate technologies for their processing, which ensure their effective functioning in the human body.
Electrospinning has the greatest promise as such a technology, since it allows fabrication of a nanofibrous matrix with desired fiber diameter and porosity which should be identical to native ECM fibers and allow high adhesion, rates for cell infiltration and mass transport [
6]. Biocompatible fiber-forming polymers are used as spinning solutions - most often proteins and polysaccharides. Synthetic biodegradable polyesters are sometimes added to improve fiber formation [
7], but this is best avoided. Fibrous materials derived from polysaccharides and proteins such as chitosan and fibroin mimic the body's natural environment and thus provide optimal conditions for tissue growth and regeneration [
8,
9,
10,
11,
12]. The possibility of using chitosan substrates in cellular technologies as a matrix for tissue engineering was studied in a number of works, which is a consequence of its biological and physicochemical properties, as well as the presence of amino groups, which determine the possibility of its simple chemical modifications [
13,
14,
15,
16]. SF is interesting in that it's not immunogenic, has good biocompatibility, it's nontoxic, and it's capable of biodegradation without causing side effects [
1,
3]. Important advantages of SF include the ability to process through aqueous solutions, and transfer to a water-insoluble form without the use of chemical cross-linking reagents, as described in articles [
17,
18]. As the properties of silk fibroin are being studied, it is increasingly being used in various fields of biomedicine, mainly due to its mechanical strength, elasticity, biocompatibility and controlled biodegradability [
19]. These properties are especially important for tissue engineering. Quite a lot of works have been published on the processing of SF by electrospinning from solutions in various solvents, including from aqueous and mixed solutions [
12,
20,
21,
22]. For this purpose, regenerated SF from the cocoons of the silkworm Bombyx mori is used.
Materials from regenerated SF are soluble in water and require additional hydrophobization. A well-known method for chitosan to prevent solubility, improve water resistance and mechanical properties - chemical cross-linking with non-toxic cross-linking reagents, such as genipin - may not be effective enough for SF due to the low content of primary amino groups in this protein [
23], however, the addition of chitosan can contribute to the effectiveness modifications with bifunctional reagents. In addition, given that several possible conformational states are known for SF: water-soluble α-helices or statistical coil conformations and insoluble β-folded structures, creating conditions for the α→β conformational transition in the process of modifying fibrous materials from regenerated SF will allow one to influence on the solubility of the polymer material.
Thus, in order to obtain SF-containing biopolymer matrices from aqueous solutions, in addition to the molding process itself, it is necessary to study the conditions of SF modification leading to the loss of its solubility in water. The aim of this study was to develop an optimal method for obtaining water-insoluble fibrous matrices intended for use in regenerative medicine and tissue engineering, combining chemical cross-linking with a naturally occurring agent Gp and conformational transition in SF, as well as to evaluate in vitro the biocompatibility of fibrous matrices during cell cultivation.
2. Materials and Methods
2.1. Chemicals
Chitosan (Mw 190 kDa, degree of deacetylation 87%) was purchased from «Roeper» (Germany). Raw silk in the form of cocoons and threads was purchased in China. Genipin was purchased from Sigma (St. Louis, MO, USA).
Preparation of regenerated fibroin. To obtain fibroin solutions, raw silk was treated in 0.02M sodium carbonate for 1 hour to purify the fibers from contamination, remove sericin and residual fats. Next, the silk was washed three times in distilled water, then kept in distilled water at a temperature of 90-100°C for up to 2 hours, and left to dry at room temperature for a day. The purified silk was dissolved in 9M lithium bromide at a temperature of 70oC for 1 hour. The resulting solution was centrifuged and filtered to get rid of the insoluble conglomerates, then dialized against water for three days with a change of medium every 3-4 hours to remove salts, freeze-dried and a water-soluble fibroin powder was obtained.
2.2. Preparation of fibroin solutions and mixed solutions of fibroin and chitosan
SF solutions were prepared in water using exact weights of dry polymer (±0.0002 g). The dissolution was carried out on a magnetic stirrer in flasks of the required volume.
To prepare mixtures containing SF and chitosan with a ratio of 5:1 an accurate weight of chitosan was dispersed in distilled water, an accurate weight of fibroin was added, stirring was continued in water for 30 minutes, then the calculated amount of glacial acetic acid was added.
2.3. Dynamic viscosity of fibroin solutions
Dynamic viscosity was determined using vibroviscometer SV-10 (AND, Japan), this device after calibration shows values with an accuracy of 0.1%.
2.4. Measurement of turbidity
Turbidity is assessed by measuring the absorbance of aqueous solutions of SF. Absorbance was measured at λ = 400 nm using a Spectronic Genesys 10UV (Spectronic Instruments, Inc. Rochester, N.Y., USA).
2.5. Electrical conductivity of fibroin solutions
The determination of electrical conductivity was carried out on an “Expert-002” conductometer manufactured by Ekoniks (Russia) using a submerged electrode. 5 ml of the test solution was taken and poured into the measuring cell, the electrode was immersed, and the electrical conductivity of the solution was measured 3-4 times until converging results were determined.
2.6. Thermogravimetric analysis
The materials were analyzed by the dynamic method (TDM) (when the furnace temperature changed over time at a constant heating rate) on a TA Instruments TDM Q50 analyzer in the temperature range from 0 to 600°C at a heating rate of 10°C/min in a nitrogen atmosphere.
2.7. Study of gel formation in fibroin solutions when cross-linked with genipin
Gelation in the systems chitosan/SF solution - Gp was studied at different molar ratios of the crosslinking agent per chitosan amino group. The point of gelation in the system was taken as the time at which the mixture of chitosan with fibroin stopped flowing under its own weight. The results obtained were presented as the dependence of the gelation time on the ratio of the crosslinking agent Gp/NH2.
2.8. Obtaining fibrous matrices by electrospinning
The fibrous material was obtained by electrospinning method utilizing the free surface of a polymer solution-coated electrode in a strong electrostatic field on a Nanospider NS-Lab (Elmarco, Czech Republic). Electrospinning was carried out from aqueous solutions of fibroin from the electrode surface onto substrates in the form of non-woven material.
2.9. Studying of fibroin matrix morphology by confocal laser scanning microscopy
The structures of the fibrous samples were analyzed by confocal laser scanning microscopy (CLSM) using Nikon TE-2000 inverted microscope equipped with an EZ-C1 confocal laser (Nikon, Tokyo, Japan). The fibrous samples were treated with 96% ethanol for 10 minutes and stained with fluorescamine. A solution of fluorescamine (0.3 mg/ml in acetone) was added to the samples and incubated for 10 min at room temperature and then washed with saline. The excitation wavelength was 408 nm and fluorescence signals were collected at 515±30 nm.
2.10. Studying the structure of fibrous matrices by AFM.
The images of the fiber surface and their diameters were obtained using an atomic force microscope (AFM) based on the NtegraPrima micro-console system (NT-MDT, Russia) in the cantilever semi-contact mode. The obtained data were subjected to processing and comparative analysis in the Nova SPM control program based on the INTEGRA platform and Solver.
2.11. Cell culture
In the current study ASC52telo, mesenchymal stem cells immortalized with human telomerase (hTERT) (hTERT-MSC) were used. The cells were kindly provided by Prof. Efimenko from Moscow State University. The cells were cultured in αMEM in a 5% CO2 humidified atmosphere at 37°C (CO2 incubator Heraeus B5060 EK/CO2, Hanau, Germany). We used media supplemented with 10% FBS, 2 mM L-glutamine, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol, 100 μg/mL streptomycin and 100 U/mL penicillin.
2.12. Morphology of cells after 3 days of cultivation in fibrous matrices
The fibrous samples were sterilized by incubation in 96% ethanol for 1 hour. After that, the samples were washed 3 times with saline and incubated in 1 ml of DMEM + 10% FBS for 1 hour. Cell suspension (20 µl, 106 cells/ml) in culture medium containing 10% serum was added to the fibrous samples. In 1 hour, another 100 µl of medium was added to each well. Next, the plate was placed in a CO2 incubator and cultivated under standard conditions for 3 days. After 3 days of cell culture on the matrices, the samples were incubated in 100 μl of culture medium (without serum) containing Calcein AM (1 μg/ml) and DAPI (10 μg/ml) for 30 minutes at 37°C. Further, the samples were studied using confocal laser scanning microscope (Nikon TE-2000, Tokyo, Japan) with excitation and emission at 408/515±30 and 488/590±50 nm for DAPI and Calcein AM, respectively.
3. Results
3.1. Effect of SF Solution concentration on fibrous matrices from regenerated fibroin electrospinning
It is advisable to use non-toxic solvents to obtain SF fibrous matrices for tissue engineering by electrospinning [
24,
25]. When using aqueous solutions, the most important factor determining the possibility of molding is the concentration of the solution [
25,
26]. Regenerated SF obtained using concentrated LiBr solutions according to the procedure described in 2.1 takes the form of random tangles and α-helices in aqueous solutions [
23,
27,
28], which interact weakly with each other. The formation of the mesh required for fiber formation is possible only at high concentrations of SF.
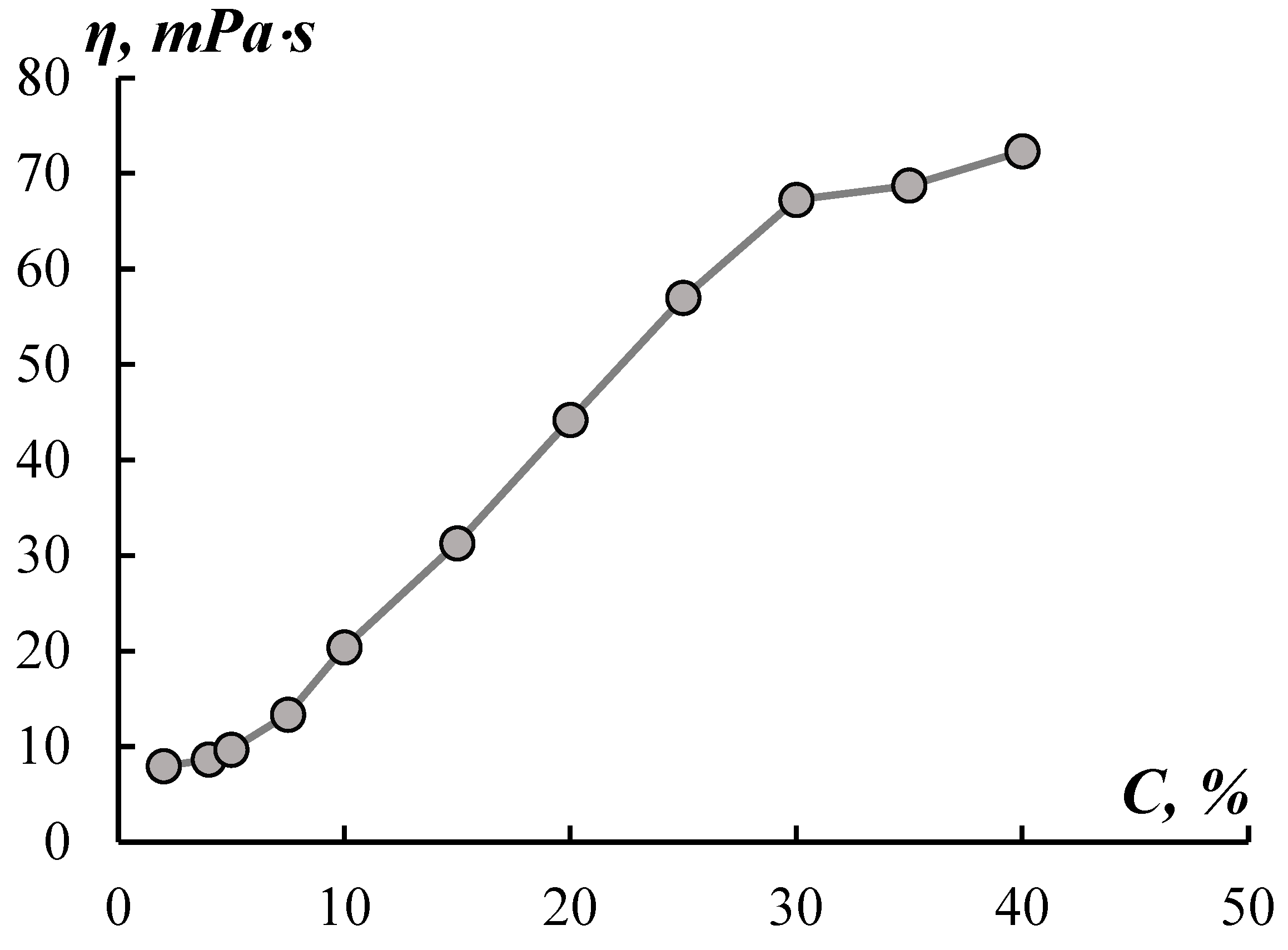
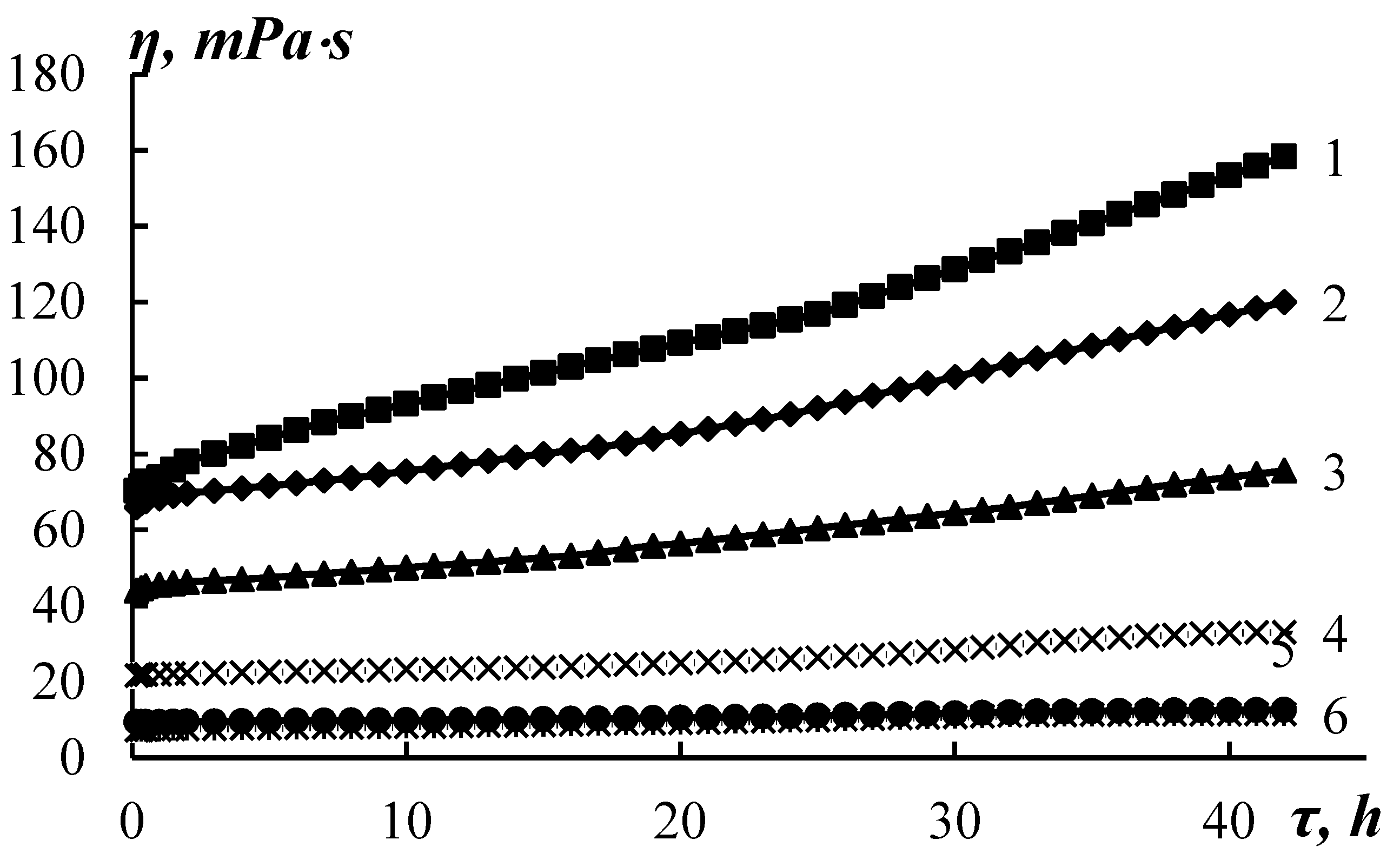
Figure 1 shows the concentration dependences of the dynamic viscosity of an aqueous solution of fibroin.
The curve η=f(C) has several sections with different concentration dependency. An intensive increase in viscosity corresponding to the formation of the fluctuation grid is observed in the concentration region above 5%, which is typical for proteins. This type of dependence cannot be simply due to an increase in the number of ionizing groups, since the electrical conductivity of the solution changes monotonously, and the pH does not exceed 0.1 units with a change in concentration by 10% (
Table 1).
A sharp slowdown in viscosity growth after 30% concentration (
Figure 1) may be associated with the beginning of conformational rearrangements: the transition of fibroin from the globular conformation characteristic of dilute solutions to the anisotropic conformation of the α-helix and the appearance of β-folded structures. This assumption is confirmed by the results of nephelometric studies (
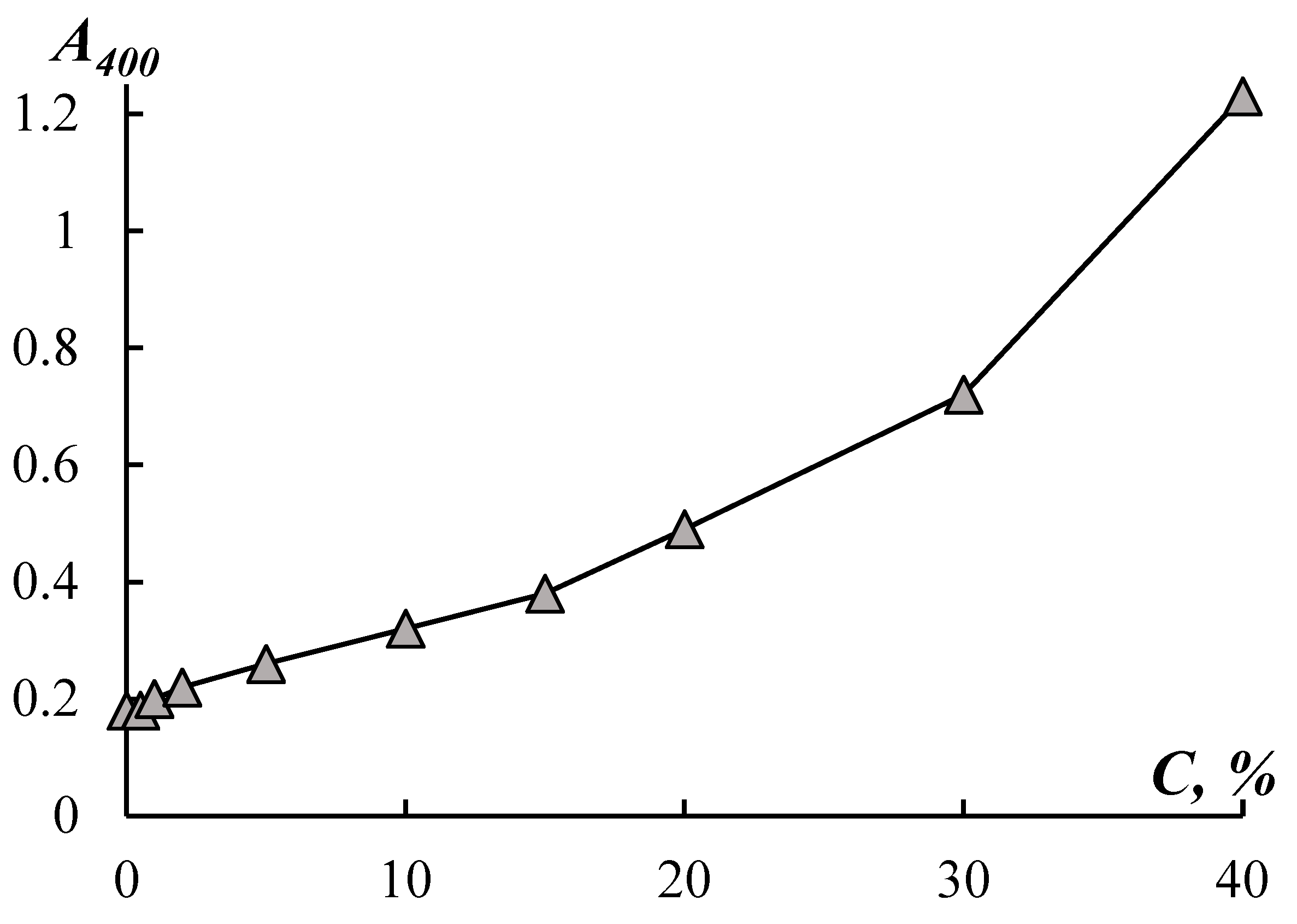
Figure 2) – a sharp increase in the optical density of the solution in this concentration region.
Electrospinning was performed by method utilizing the free surface of a polymer solution-coated electrode.
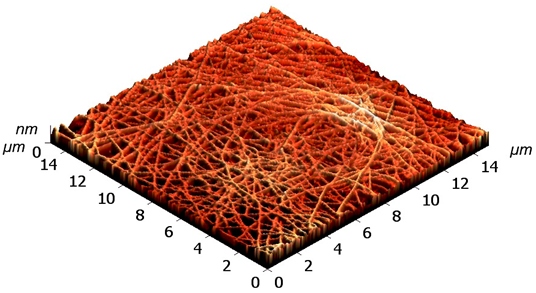
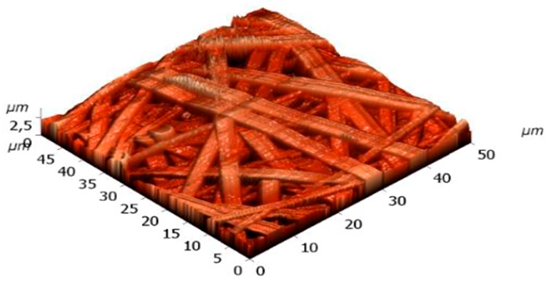
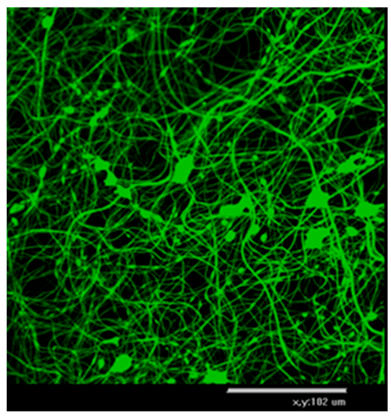
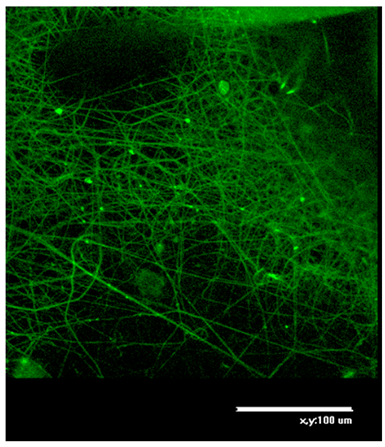
Table 2 shows AFM-images of electrospun fibers from SF solutions with different concentrations or viscosities, ranging from about 10% (w/w) to about 30% (w/w).
From solutions with a concentration below 10%, fiber formation did not occur even at an electrospinning voltage of 30 kV, only splashing of the solution was observed. AFM-images showed that SF fibers are arranged randomly, intersect with each other, forming numerous pores, the size of which increases with increasing fiber thickness. As shown in
Table 2, at concentration of 10%, an electrospun fibrous material consisting of fibers with an average diameter of 0.61 µm is formed. An increase in the concentration of the solution leads to a sharp increase in the diameter of the fibers. Non-woven mats at the voltage of 23.0-26.4 kV with a diameter of 2.80±0.2 µm were obtained from a solution with a concentration of 20%. The fibers had a belt-like morphology instead of the usual wire morphology. This is usually due to incomplete evaporation of a solvent with low vapor pressure from fibers of large thickness. At a SF solution concentration of 30%, the morphology of the resulting material loses its pronounced fibrous structure (
Table 2): the fibers probably stick together due to incomplete evaporation of water from a thicker jet of a viscous SF solution. Fibrous matrices obtained from a solution with a concentration of 10% were not strong enough and were destroyed during subsequent manipulations associated with the modification of SF in order to reduce solubility. The solution with a concentration of 20% (w/w) was chosen to fabricate non-woven mats, their subsequent modification and to evaluation in vitro biocompatibility of fibrous matrices during cell cultivation.
3.2. Hydrophobization of electrospun with SF fibers with ethanol solution
The resulting electrospun SF fibers are water soluble and require additional hydrophobization. The conversion of fibroin to insoluble form can be accomplished by a post-treatment to change the random coil conformation to β-sheet which is more stable and insoluble in water. Such post-treatment by treating the formed fibers of silk fibroin with methanol or ethanol [
29,
30]. Methanol is a toxic solvent, therefore, a method of forming water-resistant materials from fibroin with ethanol is used.
The change in the structure of the protein during the conformational transition occurs as a result of the redistribution of hydrogen bonds in favor of intermolecular bonds, which maintain a β-stacked conformation and give the material resistance to water. The conformational stability of silk fibroin in an aqueous-alcoholic solution is determined by interactions of amino acid residues with each other and with a solvent. Aggregation of hydrophobic side chains is disrupted by breaking the hydrogen bond network of water with alcohol and binding the alcohol to the hydrophobic group [
27,
29]. The formation of a β-stacked conformation is a process of rearrangement of hydrogen bonds, with their formation between peptide units of different macromolecules.
Based on preliminary experiments to study the solubility of electrospun fibers from SF and the results described in [
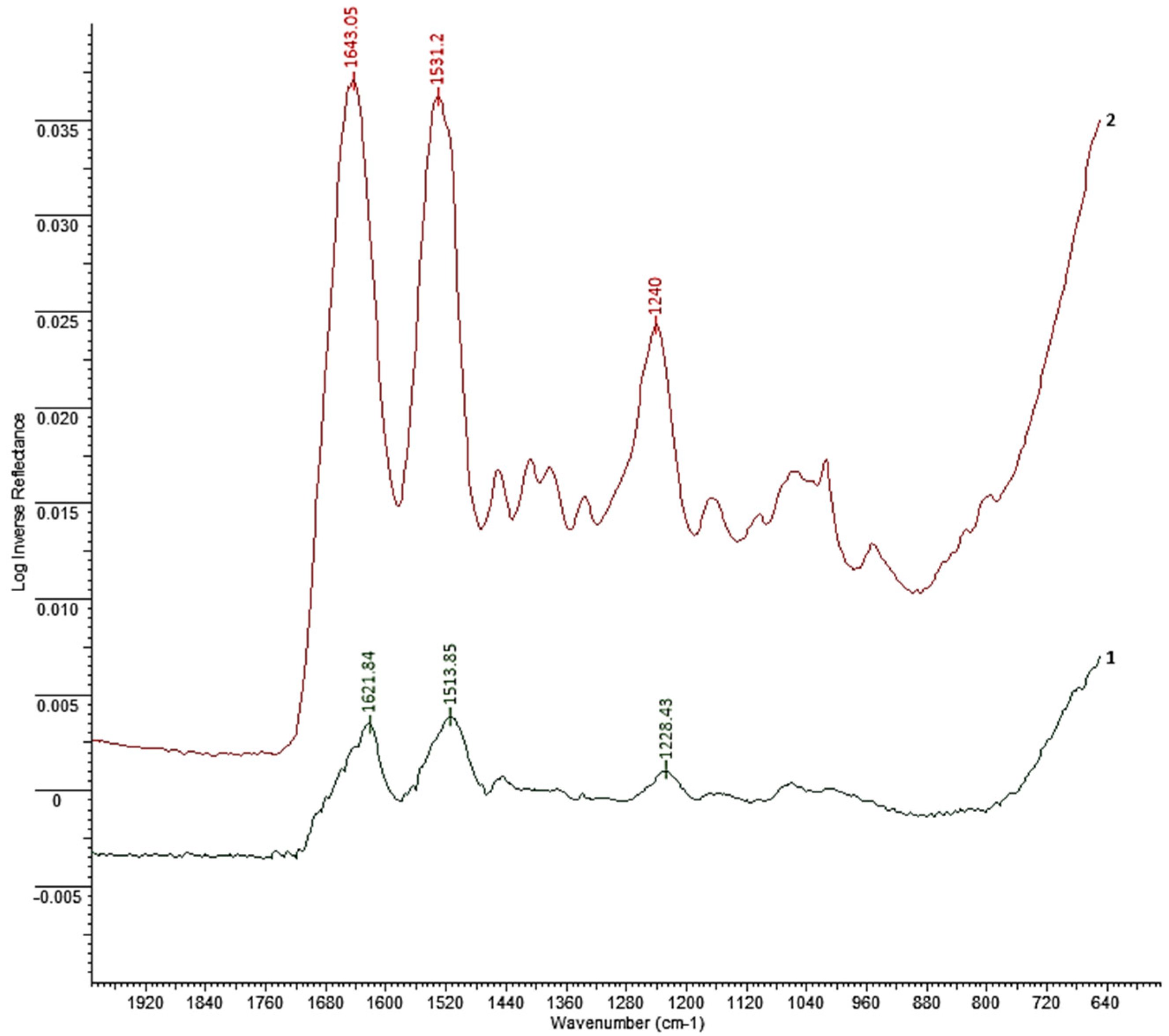
31], a 80% aqueous ethanol solution was selected for the treatment of the fibers, in which the material was held for 2 hours. Structural changes in fibroin induced by ethanol treatment confirmed by FTIR spectroscopy (
Figure 3).
As stated previously, fibroin can exist as random coil/globules (low solute concentration) with α-structure (silk I), and β-form (silk II, ordered as antiparallel β-folded sheets, formed by physical shear or exposure to solvents such as ethanol).
Figure 3 shows the FTIR spectra of electrospun fibrous fibroin mats: initial and treated with 80% ethanol solution, which confirm the effect of ethanol on the conformational transformation of regenerated silk fibroin. Fibroin fibrous materials initially have a mainly amorphous structure with the characteristic structure of silk I. The untreated sample (curve 2) shows strong bands at 1531.2 cm
-1 (amide II), and 1643.05 cm
-1 (amide I), characteristic of the α-helix and random coil conformation [
32]. The material treated with 80% ethanol (curve 1) shows bands shifting and appearance at 1513.85 and 1621.84 cm
-1 characteristic of the β-sheet conformation [
33]. These changes indicate the transformation of random helices into an ordered β-sheet upon treatment with aqueous ethanol solution. Actually, water might act as swelling agent towards the compact and dense silk fibroin [
34], promoting ethanol penetration and rearrangements of inter- and intramolecular hydrogen bonds. The data obtained are consistent with those described in the literature earlier [
30,
31,
32,
33,
34,
35].
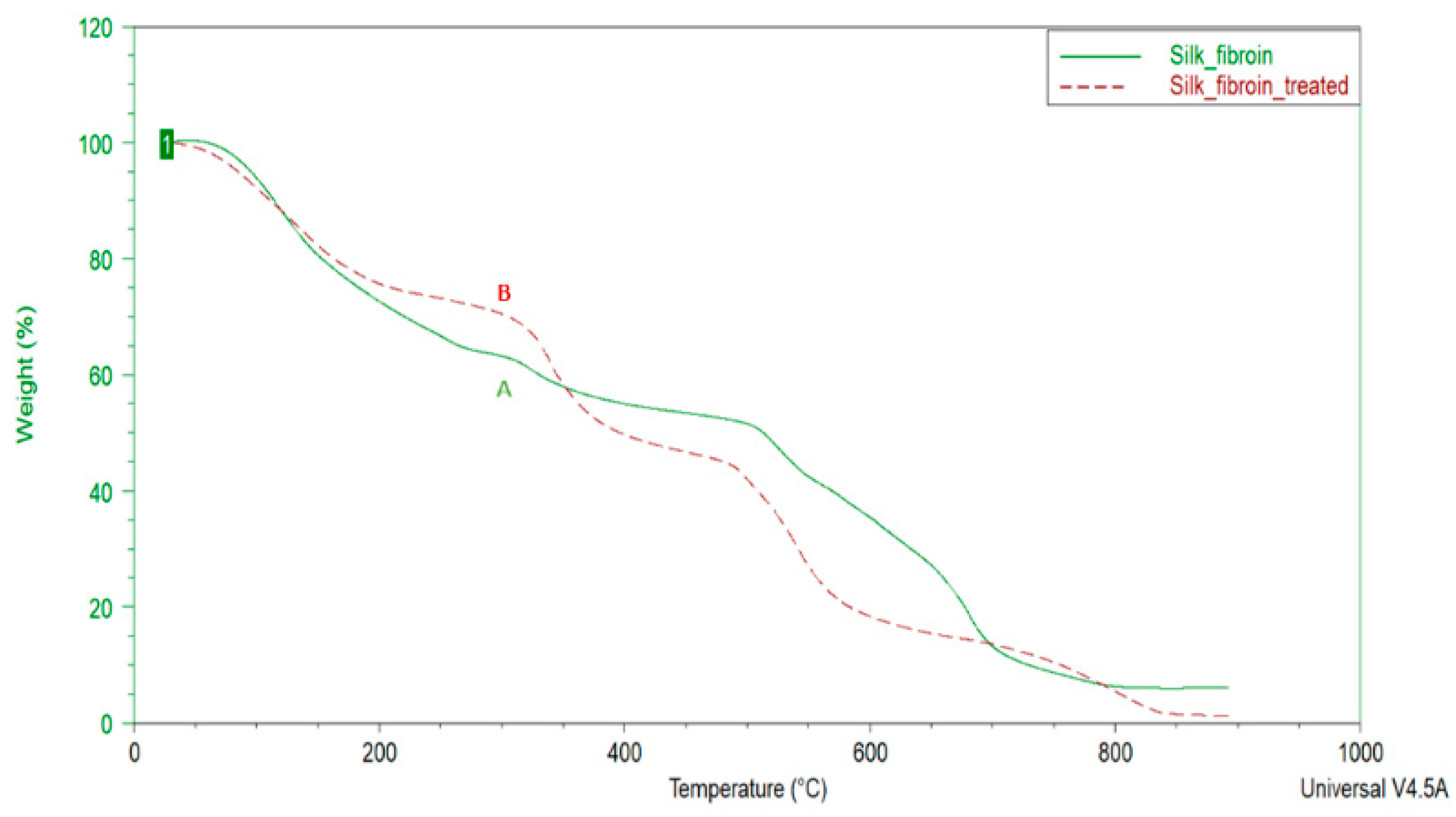
In this work, the untreated and 80% alcohol treated electrospun fibrous matrices were analyzed by the method of thermogravimetric analysis (TGA). The method of thermogravimetric analysis is based on continuous recording of the change in the mass of the sample depending on the temperature under conditions of its programmed change.
The untreated and 80% alcohol treated electrospun fibrous matrices showed a trend of weight loss with increase in temperature in the TGA plots (
Figure 4). The degradation of the untreated untreated and alcohol treated matrices when heated to 150
oC was attributed to the loss of bound water. In this region, the fibrous matrix sample lost mass more slowly than the untreated sample. It is manifested that the water molecules are much stronger bonded with the β-sheet (silk II) structure of fibroin. The main weight loss both for the untreated and treated samples was observed from 150
oC to 450
oC and was attributed to the degradation of silk structure.
The observed reduction in the rate of mass loss resulting from treatment with 80% ethanol solution along with FTIR-spectroscopy results indicate that that treatment with aqueous-ethanol solution electrospun fibrous matrices causes the conditional transition of fibroin into β-folded conformation and results in hydrophobization of the material.
3.3. Use of genipine crosslinking reagent to obtaining water-insoluble fibrous matrices from regenerated fibroin
Another way to prevent solubility, improve water resistance and mechanical properties is chemical crosslinking with non-toxic crosslinking reagents. It is known that chemical crosslinking of chitosan with a natural-origin crosslinking reagent, genipin, causes gelation in its solutions, and during post-treatment leads to the production of water-insoluble fibrous, film materials and porous cryogels based on [
15,
16,
37]. There are different ideas about the mechanism of reaction of amino-containing biopolymers with Gp [
37,
39,
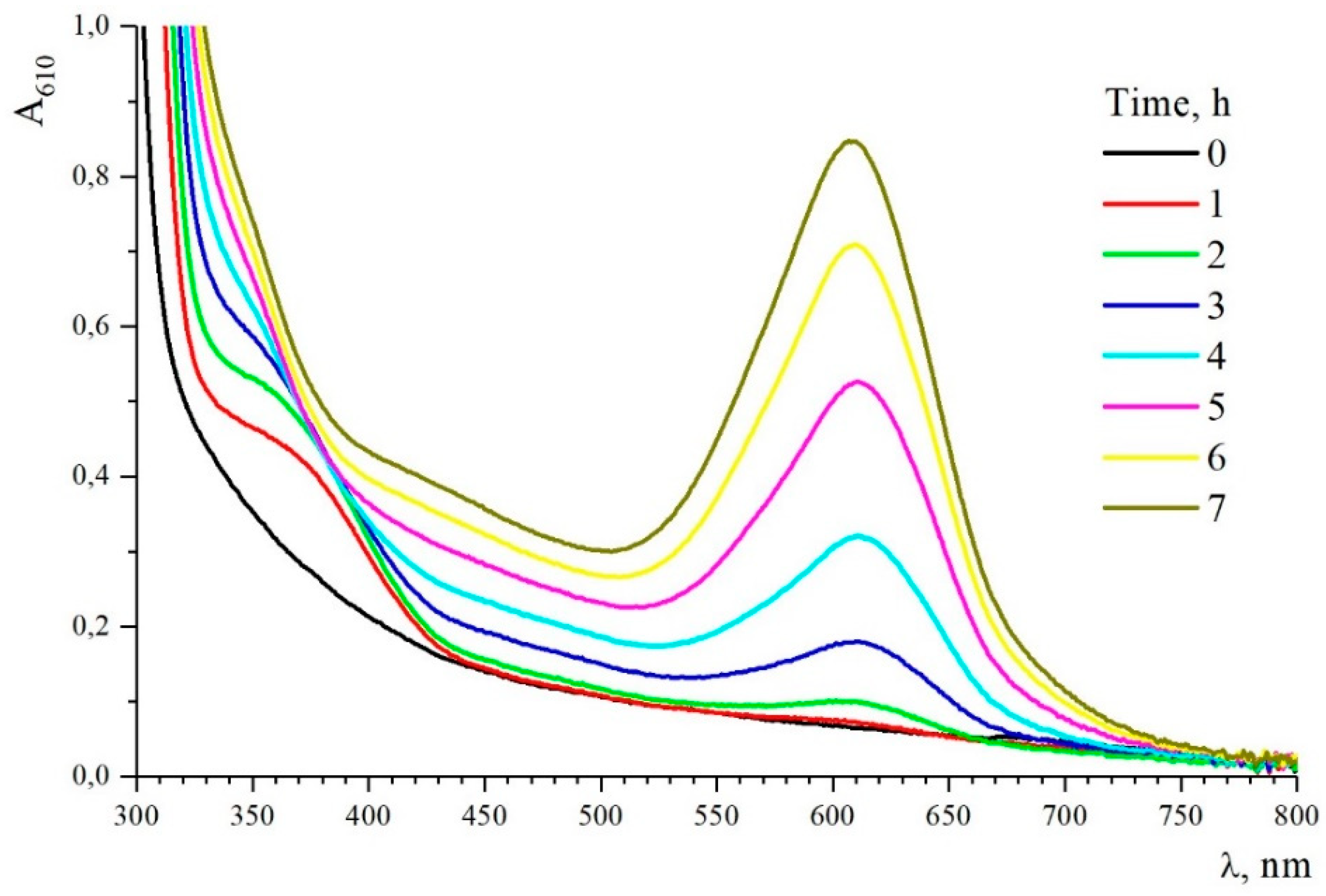
40,41]. Summarizing the literature and considering that the interaction of SF with Gp not only leads to an increase in viscosity, but also the appearance of absorption in various regions of the spectrum, including the visible region (
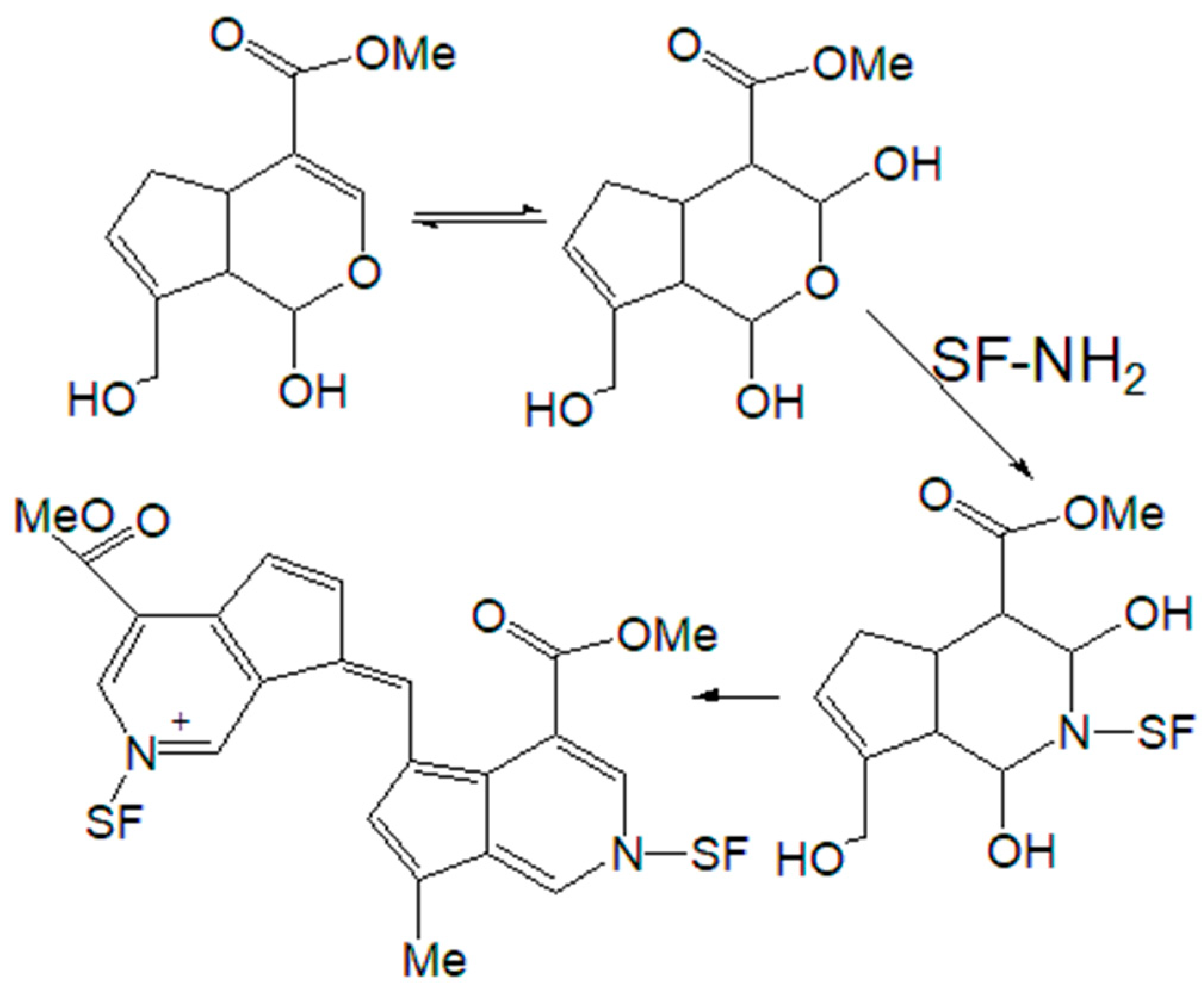
Figure 5) and the staining of the material in blue, scheme of the reaction of crosslinking fibroin with genipin can be represented by
Figure 6.
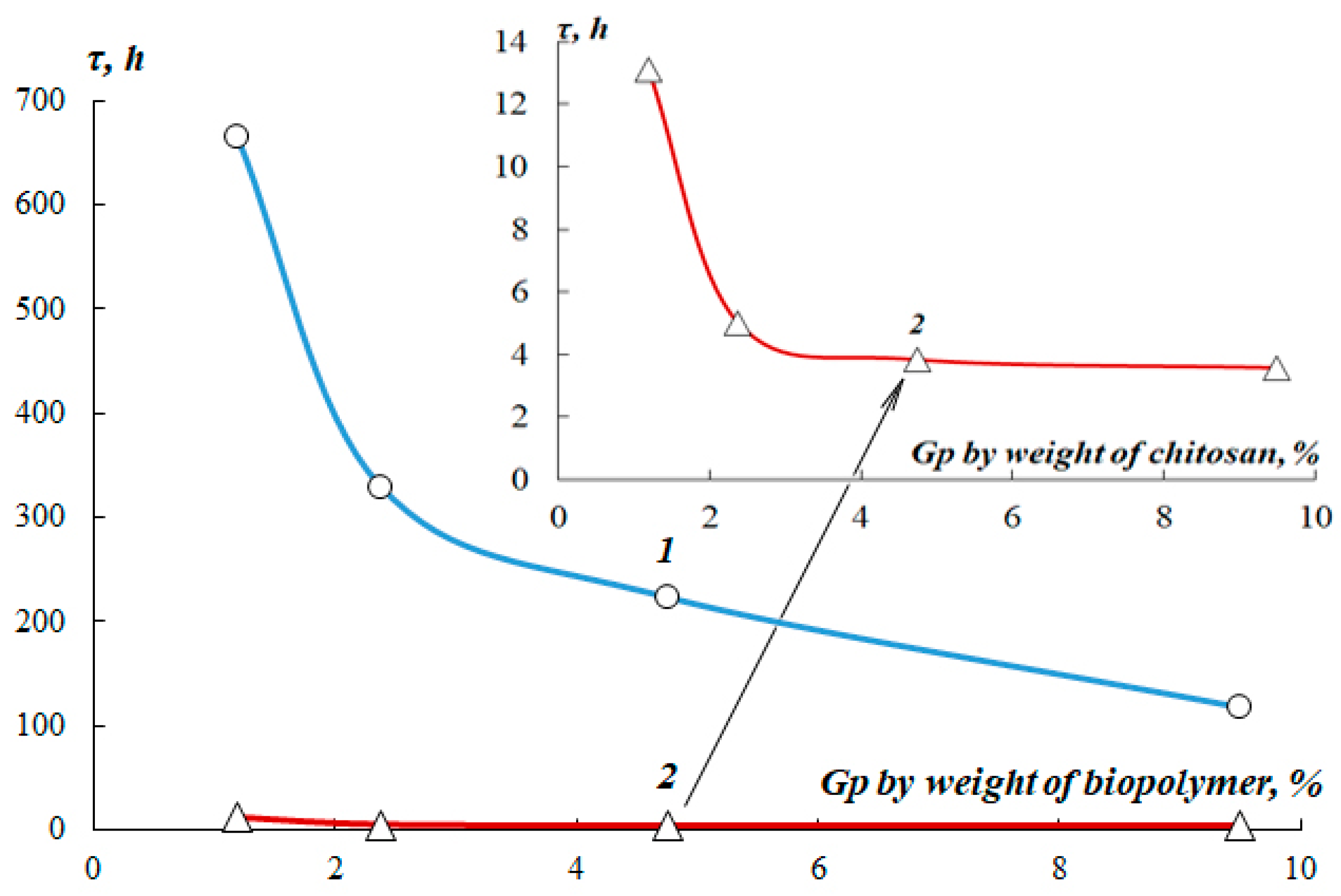
The gelation process in equiconcentrated fibroin and chitosan solutions was investigated. Gelling time dependence in 2% SF solutions at pH 7.3 and chitosan at pH 5.6. in the process of crosslinking with Gp, the content of crosslinking agent is shown in
Figure 7.
As can be seen from the data obtained, the gelation time in a 2% SF solution, even with a high content of Gp and a pH of 7.2, is 20-50 times higher than in an equiconcentrated chitosan solution and is several days. This is due to the low content of primary amino groups in the fibroin molecule. Protein crosslinking using Gp occurs primarily with the participation of amino groups of basic amino acids and, above all, lysine, whose content in regenerated fibroin is only 0.3-0.5% mol [
23,42]. The terminal amino groups of the protein are not always available for modification, therefore, it is natural that under conditions leading to the gelation of chitosan, the addition of Gp to an equi-concentrated solution of SF does not lead to gelation, although it causes the appearance of a blue color.
On
Figure 8 kinetic curves of change in viscosity of SF solutions in the process of crosslinking with Gp at the same content of crosslinking agent 10.9% of fibroin weight in a wide range of SF concentrations obtained on a vibration viscometer are given. Within 24 hours, a marked change in viscosity was observed only starting with the 10% concentration. During measurements (42 hours) in the presence of Gp, even 40% SF solutions did not lose their flow ability. However, the color change to green or blue (depending on the concentration of the solution) occurred, which indicates a secondary reaction with the formation of a product absorbing in the λ=600 nm region.
To accelerate the intensification of crosslinking leading to the formation of water-insoluble biomedical materials, the present paper proposes the use of chitosan-containing systems based on SF solutions. Dehummed and regenerated fibroin is soluble throughout the pH range. Chitosan is soluble in water only in an acidic environment when its primary amino groups protonate and the macromolecule acquires a positive charge. Mixed solutions of chitosan and fibroin in dilute acetic acid prepared according to the procedure described in 2.2 were used to obtain fibrous matrices by electrospinning.
The concentration of SF in the solution was 10%, such a concentration was chosen based on the fact that the addition of chitosan leads to a sharp increase in viscosity, which prevents droplet formation on the free surface of the solution and the formation of extended fibers on the receiving electrode. For the electrospinning of fibrous mats from a mixture of SF and chitosan, solutions with the physicochemical characteristics given in
Table 3 were used. Electrospinning took place at the voltage of 26 kV.
Gp is soluble in both water and ethanol, and this fact allows the use of solutions of Gp in 80% aqueous ethanol solution to modify the electrospun fibrous matrices from SF and chitosan in order to prevent their dissolution during cell culture. Conditions for redistribution of hydrogen bonds accompanying the formation of β sheets are realized in 80% aqueous ethanol solution, and the presence of chitosan in the material contributes to intensification of the process of crosslinking with Gp and inclusion of macromolecules of SF in the three-dimensional grid of the crosslinked biopolymer. The fibrous matrices were kept in 0.95% Gp solution at 2 μl/mg for 72 hours, then washed with phosphate buffer solution (pH 7.4).
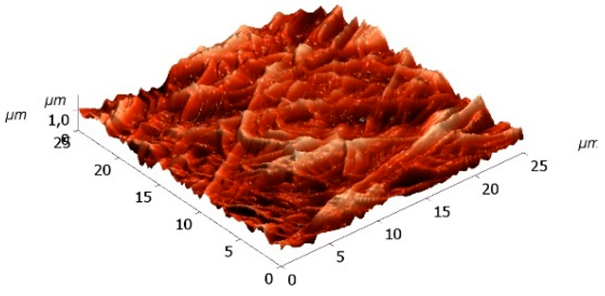
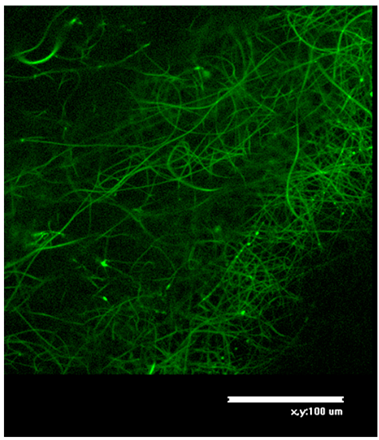
Morphology of received electrospun fibrous matrices treated with 80% ethanol and crosslinked with Gp showed in
Table 4.
According to the procedure described in 2.9, of morphology of source electrospun fibrous matrices and after washing with phosphate buffer solution (pH 7.4) was studied by confocal laser microscopy. Morphology of electrospun fibrous matrices based on 100% fibrin after washing with phosphate buffer solution showed the presence of defects, due to partial dissolution of the material, despite the fact that visually the material retained its integrity. That is, hydrophobization of SF fibers when treated with a Gp solution in 80% ethanol solution was insufficient, probably due to the low content of Gp amino groups available for crosslinking in SF. The introduction of chitosan, which in the process of crosslinking with Gp promotes the inclusion of SF in the three-dimensional network of crosslinked biopolymers, improve water resistance and reduce defective material (
Table 4).
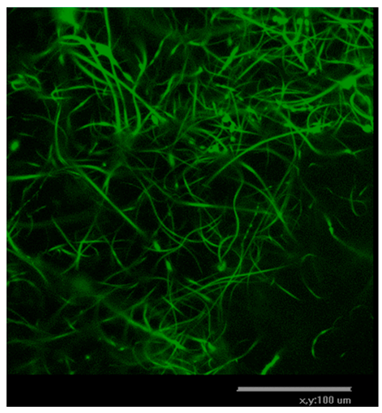
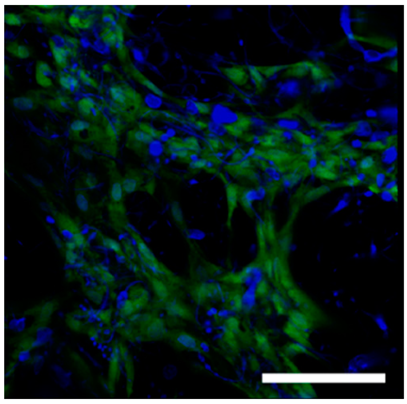
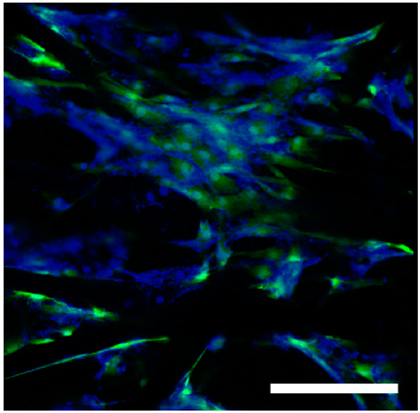
Polysaccharide and protein-derived fibrous materials mimic the body's natural environment and thus must provide optimal conditions for tissue growth and regeneration. To evaluate the biocompatibility of the fibrous matrices hTERT-MSCs were seeded on fibers and cultured for 3 days. Before use the fibrous samples were treated with 96% ethanol for 10 minutes. Cell morphology and distribution were monitored using transmission light and confocal microscopy. In order to assess the viability of hTERT-MSCs cultivated in the fibrous matrices, cells were stained with vital dye Calcein AM and DAPI after three days of cultivation (see
Table 4). The fluorescence micrographs showed that the cells penetrated through the fiber matrix and adhered and spread on the fibers. Moreover, conversion of non-fluorescent Calcein AM to green fluorescent dye Calcein, which occurs in living cells only, indicated that those labelled hTERT-MSCs were viable after 3 days of cell culture within the fibers.
4. Conclusions
Fibrous mats from SF were obtained from water solutions with a concentration of 10 and 20% using the electrospinning method. Materials from regenerated SF are soluble in water and require additional hydrophobization. Two methods were studied to prevent solubility of fibroin-based matrices: conversion of fibroin to the β-conformation by treatment with an ethanol solution and chemical cross-linking with genipin. Using the TGA method, a stronger binding of water to the material was found after its treatment with 80% ethanol solution, which, along with the results of FTIR-spectroscopy, indicate that that treatment with aqueous-ethanol solution electrospun fibrous matrices causes the conformational transition of fibroin into β-folded conformation and leads to hydrophobisation of material. The interaction of Gp with SF leads to the appearance of a characteristic blue color, but does not call for the gelation of solutions. To speed up the crosslinking reaction with Gp, it is proposed to use chitosan-containing systems and modify fibrous materials by treating with a solution of Gp in 80% ethanol. At the same time, on the one hand, conditions are created for redistribution of hydrogen bonds accompanying the formation of β sheets, and the presence of chitosan in the material contributes to intensification of the process of crosslinking with jenipin and the inclusion of macromolecules of fibroin in the three-dimensional network of the crosslinked biopolymer. Thus, the method of producing water-insoluble fibrous matrices for use in regenerative medicine and tissue engineering is optimized, combining chemical crosslinking with a naturally occurring crosslinking reagent genipine and α→β a conformational transition in fibroin. Electrospun fiber matrices based on regenerated SF, modified by cross-linking with Gp in water-alcohol solutions, promote cell growth and proliferation and may be promising for tissue engineering.
Author Contributions
Conceptualization, Nataliya Kildeeva and Elena Markvicheva; methodology, Nikita Sazhnev; software, Nikita Sazhnev.; validation, Maria Drozdova, Vasilina Zakharova. and Evgeniya Svidchenko; formal analysis, Vasilina Zakharova.; investigation, Nikolay Surin.; resources, Nikita Sazhnev; data curation, Nikolay Surin.; writing—original draft preparation, Nikita Sazhnev, Vasilina Zakharova, Maria Drozdova ; writing—review and editing, Nataliya Kildeeva; visualization, Nikita Sazhnev.; supervision, Nikolay Surin; project administration, Nataliya Kildeeva.; funding acquisition, Elena Markvicheva. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Science Foundation (project No. 22-13-00261).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data is available within the manuscript.
Acknowledgments
The center of collective use, Enikolopov Institute of Synthetic Polymeric Materials of Russian Academy of Sciences, Moscow, Russia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Altman, G.H. et al. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y. et al. flexible humidity sensor based on natural biocompatible silk fibroin films. Advanced Materials Technologies 2021, 6, 2001053. [Google Scholar] [CrossRef]
- Zheng, H., Zuo, B. Functional silk fibroin hydrogels: preparation, properties and applications. Journal of Materials Chemistry B 2021, 9, 1238–1258. [CrossRef] [PubMed]
- Long, S. , Xiao, Y., Zhang, X. Progress in preparation of silk fibroin microspheres for biomedical applications. Pharmaceutical Nanotechnology 2020, 8, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Farokhi, M. et al. Silk fibroin scaffolds for common cartilage injuries: possibilities for future clinical applications. European Polymer Journal 2019, 115, 251–267. [CrossRef]
- Li, D., Wang, Y., Xia, Y. Electrospinning nanofibers as uniaxially aligned arrays and layer-by-layer stacked films. Advanced materials 2004, 16, 361–366. [CrossRef]
- Yang, N. et al. Polyvinyl alcohol/silk fibroin/borax hydrogel ionotronics: a highly stretchable, self-healable, and biocompatible sensing platform. ACS applied materials & interfaces 2019, 11, 23632–23638.
- Shi, W. et al. Functional tissue-engineered bone-like graft made of a fibrin scaffold and TG2 gene-modified EMSCs for bone defect repair. NPG Asia Materials 2021, 13, 28. [CrossRef]
- Yang, W. et al. Preparation and characterisation of a novel silk fibroin/hyaluronic acid/sodium alginate scaffold for skin repair. International journal of biological macromolecules 2019, 130, 58–67. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S. et al. Physico-chemical characterization and biological tests of collagen/silk fibroin/chitosan scaffolds cross-linked by dialdehyde starch. Polymers 2020, 12, 372. [Google Scholar] [CrossRef]
- Roseti, L. et al. Scaffolds for bone tissue engineering: state of the art and new perspectives. Materials Science and Engineering: C 2017, 78, 1246–1262. [CrossRef] [PubMed]
- Kasoju, N. , Bora, U. Silk fibroin in tissue engineering. Advanced healthcare materials 2012, 1, 393–412. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Progress in polymer science 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Wang, J., Zhuang, S. Chitosan-based materials: Preparation, modification and application. Journal of Cleaner Production 2022, 355, 131825. [CrossRef]
- Sazhnev, N.A. et al. Preparation of chitosan cryostructurates with controlled porous morphology and their use as 3D-scaffolds for the cultivation of animal cells. Applied Biochemistry and Microbiology 2018, 54, 459–467. [CrossRef]
- Kildeeva, N.; et al. Influence of genipin crosslinking on the properties of chitosan-based films. Polymers 2020, 12, 1086. [Google Scholar] [CrossRef]
- Asakura, T. et al. Refinement of repeated β-turn structure for silk I conformation of Bombyx mori silk fibroin using 13C solid-state NMR and X-ray diffraction methods. Macromolecules 2005, 38, 7397–7403. [CrossRef]
- Asakura, T. Structure of silk I (Bombyx mori silk fibroin before spinning)-type II β-turn, not α-helix. Molecules 2021, 26, 3706. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; et al. Preparation of undegraded native molecular fibroin solution from silkworm cocoons. Materials Science and Engineering: C 2001, 14, 41–46. [Google Scholar] [CrossRef]
- Farokhi, M.; et al. Functionalized silk fibroin nanofibers as drug carriers: Advantages and challenges. Journal of Controlled Release 2020, 321, 324–347. [Google Scholar] [CrossRef]
- Chen, K. et al. Silk fibroin combined with electrospinning as a promising strategy for tissue regeneration. Macromolecular Bioscience 2023, 23, 2200380. [Google Scholar] [CrossRef] [PubMed]
- Yerra, A. , Dadala, M.M. Silk fibroin electrospun nanofiber blends with antibiotics and polyvinyl alcohol for burn wound healing. Journal of Applied Polymer Science 2022, 139, 51930. [Google Scholar] [CrossRef]
- Sashina, E.S. et al. Structure and solubility of natural silk fibroin. Russian journal of applied chemistry 2006, 79, 869–876. [Google Scholar] [CrossRef]
- Li, G. , Sun, S. Silk fibroin-based biomaterials for tissue engineering applications. Molecules 2022, 27, 2757. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J. et al. Electrospinning and rheology of regenerated Bombyx mori silk fibroin aqueous solutions: The effects of pH and concentration. Polymer 2008, 49, 2880–2885. [CrossRef]
- Singh, B.K. , Dutta, P.K. Chitin, chitosan, and silk fibroin electrospun nanofibrous scaffolds: a prospective approach for regenerative medicine. Chitin and chitosan for regenerative medicine 2016, 151–189. [Google Scholar]
- Kishimoto, Y. et al. Electrospinning of silk fibroin from all aqueous solution at low concentration. Materials Science and Engineering: C 2017, 73, 498–506. [CrossRef]
- Elango, J. et al. The Relationship of Rheological Properties and the Performance of Silk Fibroin Hydrogels in Tissue Engineering Application. Process Biochemistry 2022. [Google Scholar] [CrossRef]
- Tsukada, M. et al. Structure and molecular conformation of tussah silk fibroin films: Effect of methanol. Journal of Polymer Science Part B: Polymer Physics 1995, 33, 1995–2001. [CrossRef]
- Shu, T. et al. Moderate conformational transition promotes the formation of a self-reinforced highly oriented silk fibroin network structure. Soft Matter. 2021, 17, 9576–9586. [CrossRef]
- Chen, X. et al. Conformation transition kinetics of Bombyx mori silk protein. Proteins: Structure, Function, and Bioinformatics 2007, 68, 223–231. [CrossRef] [PubMed]
- Zuo, B., Liu, L., Wu, Z. Effect on properties of regenerated silk fibroin fiber coagulated with aqueous methanol/ethanol. Journal of applied polymer science 2007, 106, 53–59. [CrossRef]
- Nogueira, G.M. et al. Preparation and characterization of ethanol-treated silk fibroin dense membranes for biomaterials application using waste silk fibers as raw material. Bioresource technology 2010, 101, 8446–8451. [CrossRef] [PubMed]
- Mo, C. et al. The effect of water on the conformation transition of Bombyx mori silk fibroin. Vibrational Spectroscopy 2009, 51, 105–109. [Google Scholar] [CrossRef]
- Li, M. et al. Controlling molecular conformation of regenerated wild silk fibroin by aqueous ethanol treatment. Polymers for Advanced Technologies 2003, 14, 694–698. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G. et al. Genipin as an emergent tool in the design of biocatalysts: mechanism of reaction and applications. Catalysts 2019, 9, 1035. [CrossRef]
- Yu, Y. et al. Genipin-cross-linked hydrogels based on biomaterials for drug delivery: A review. Biomaterials science 2021, 9, 1583–1597. [Google Scholar] [CrossRef]
- Pizzolitto, C. et al. On the mechanism of genipin binding to primary amines in lactose-modified chitosan at neutral pH. International Journal of Molecular Sciences 2020, 21, 6831. [CrossRef]
- Bucciarelli, A. et al. A genipin crosslinked silk fibroin monolith by compression molding with recovering mechanical properties in physiological conditions. Cell Reports Physical Science 2021, 2. [Google Scholar] [CrossRef]
- Zhou, C.Z. et al. Silk fibroin: structural implications of a remarkable amino acid sequence. Proteins: Structure, Function, and Bioinformatics 2001, 44, 119–122. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).