Submitted:
11 September 2023
Posted:
12 September 2023
You are already at the latest version
Abstract
Keywords:
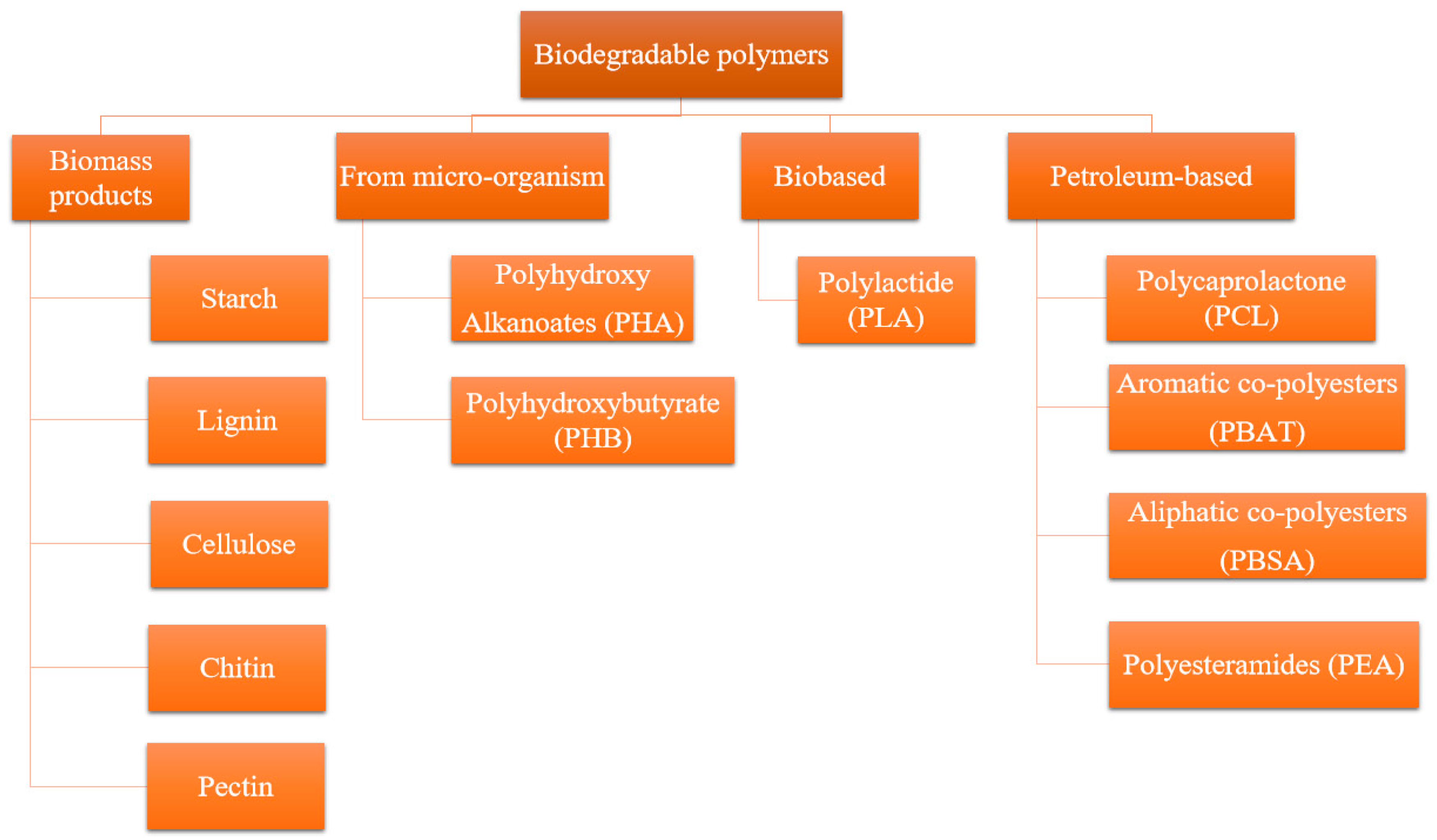
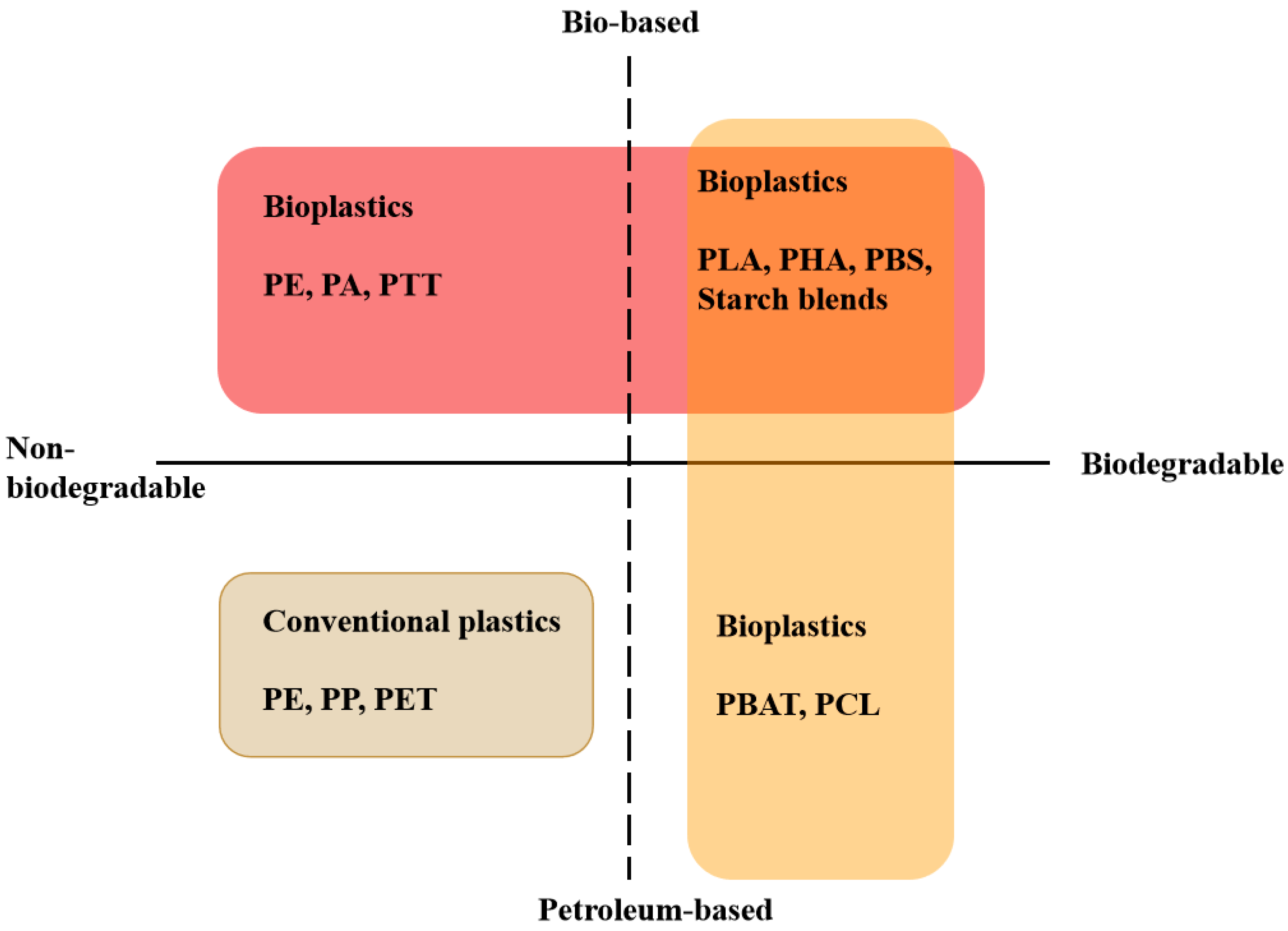
1. Introduction
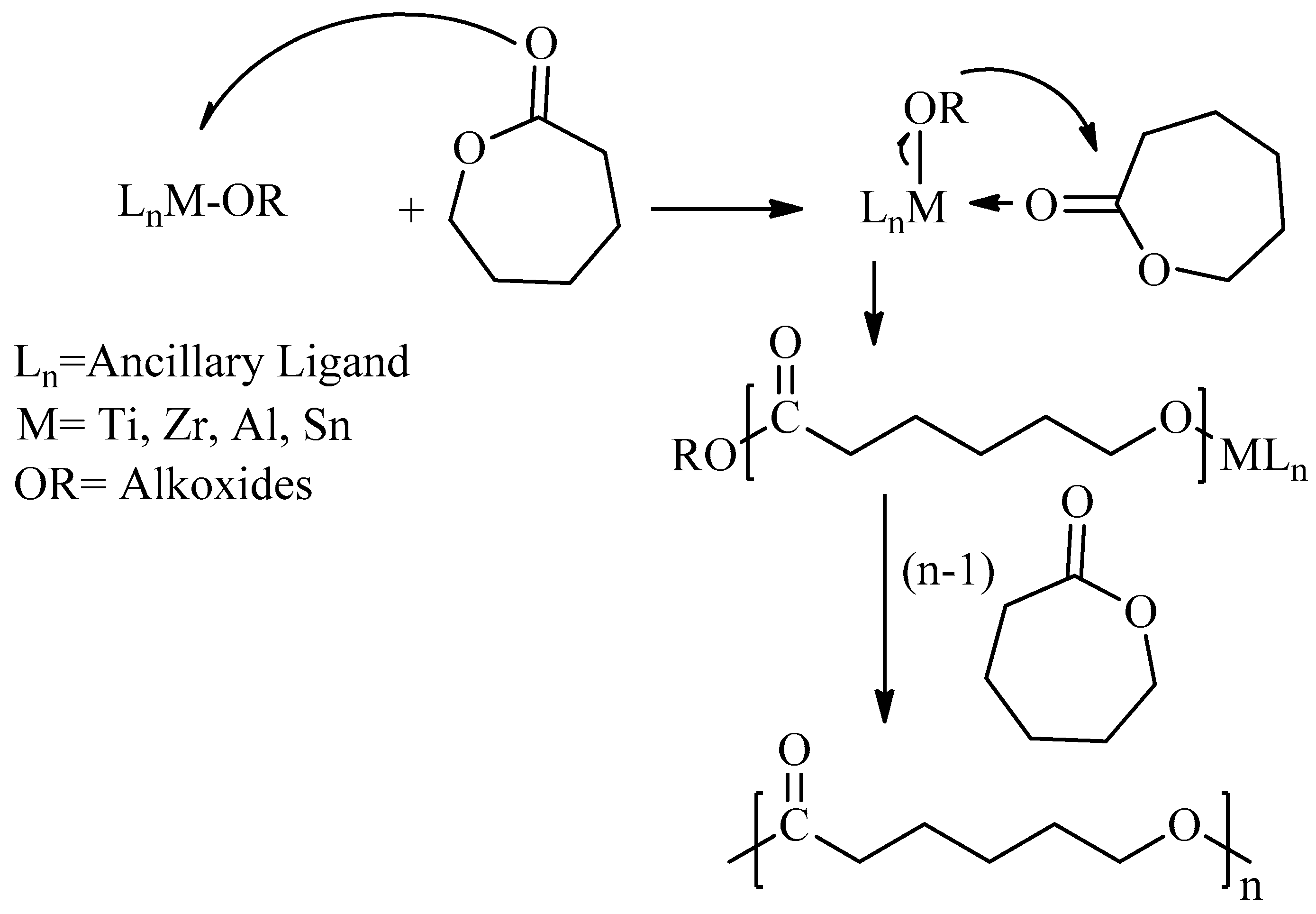
2. Chemical Synthesis of PCL
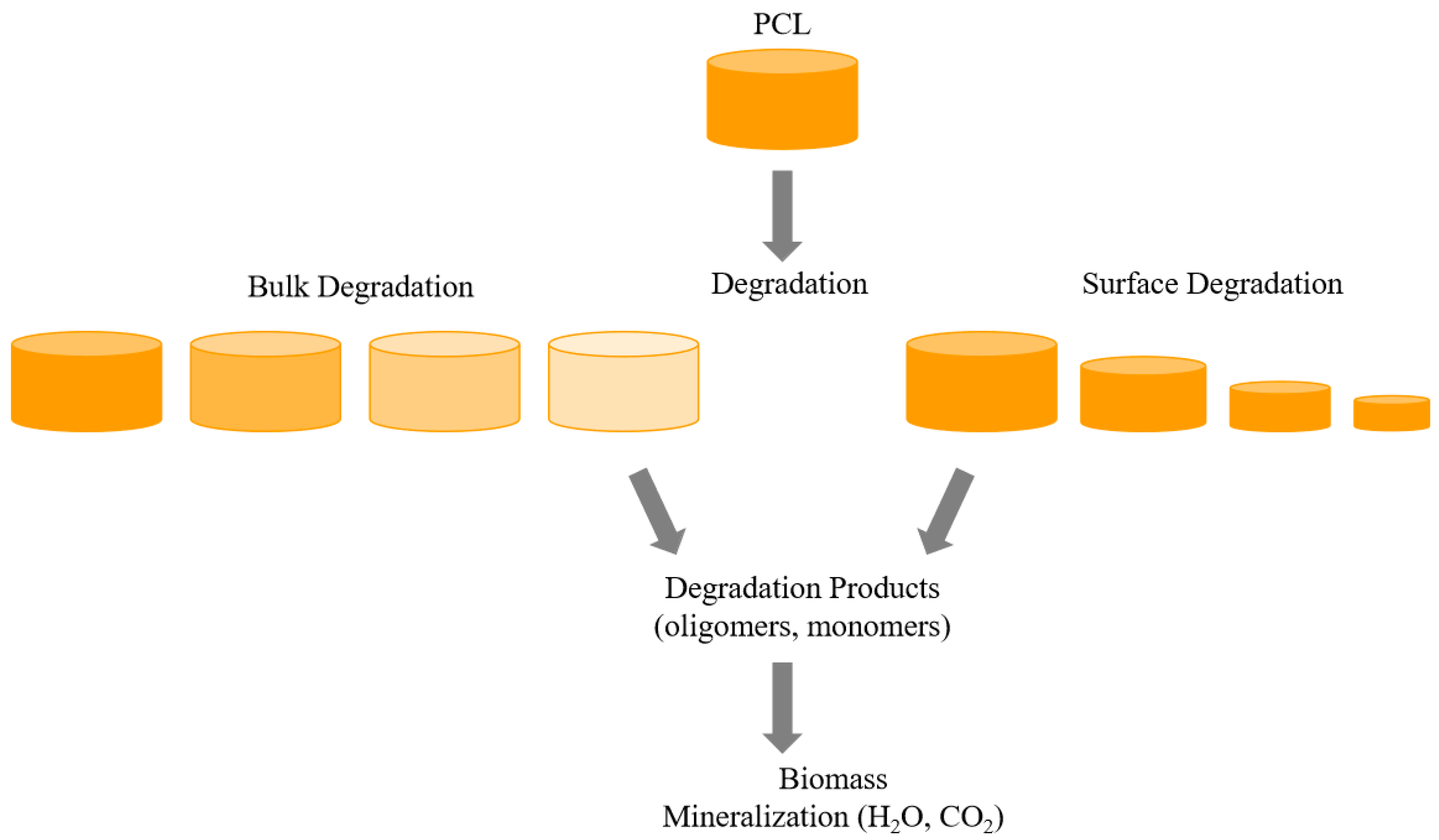
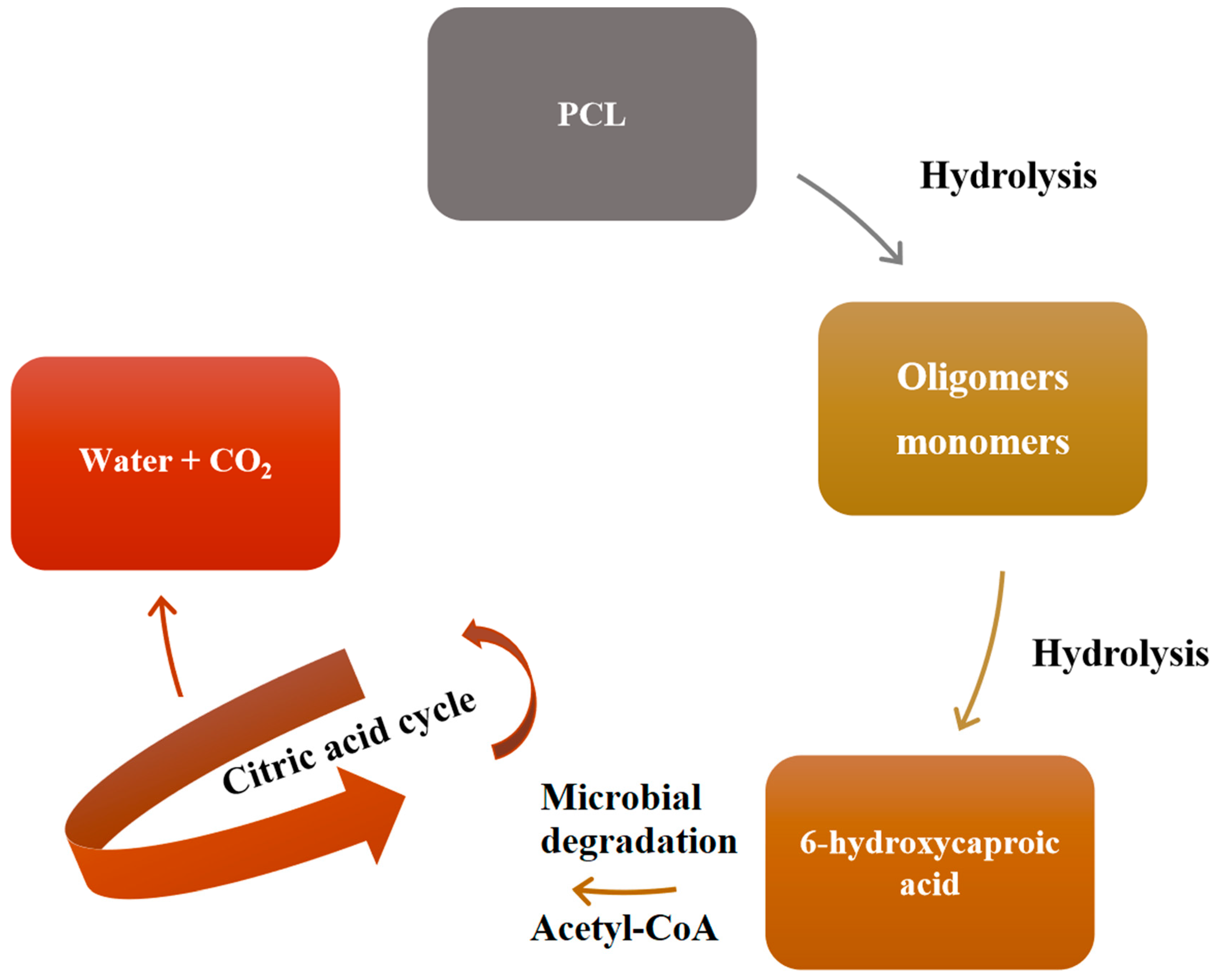
3. Degradation of PCL
4. Material Properties of PCL
5. PCL Blends/Composites
5.1. PCL Reinforced with Other Polymers
5.2. PCL Reinforced with Metal Oxides
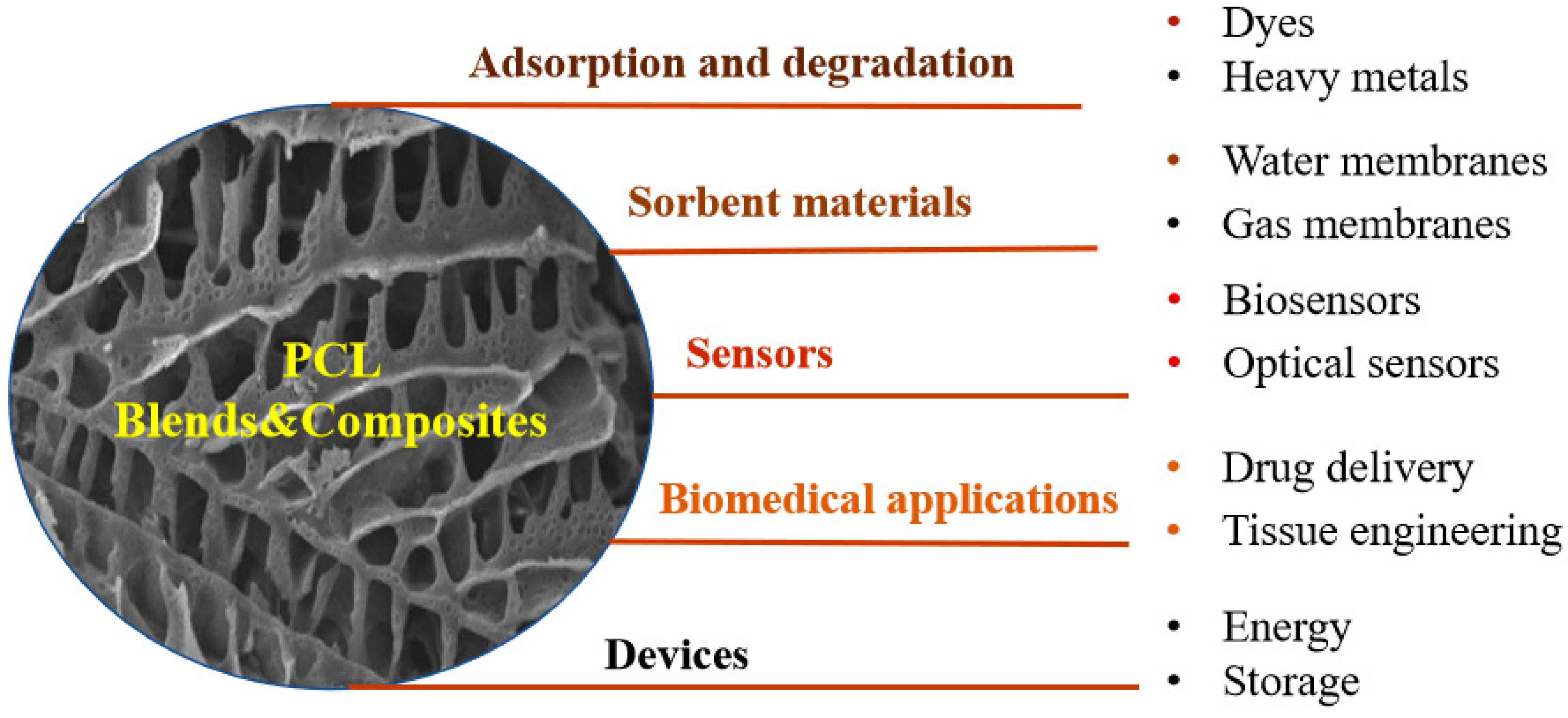
6. Applications of PCL Blends/Composites
6.1. Pollutant Removal Applications of PCL Blends/Composites
6.1.1. Dye Removal by PCL Blends
6.1.2. Dye Removal by PCL/Metal Oxide Composites
6.1.3. Heavy Metal Removal by PCL Composites/Blends
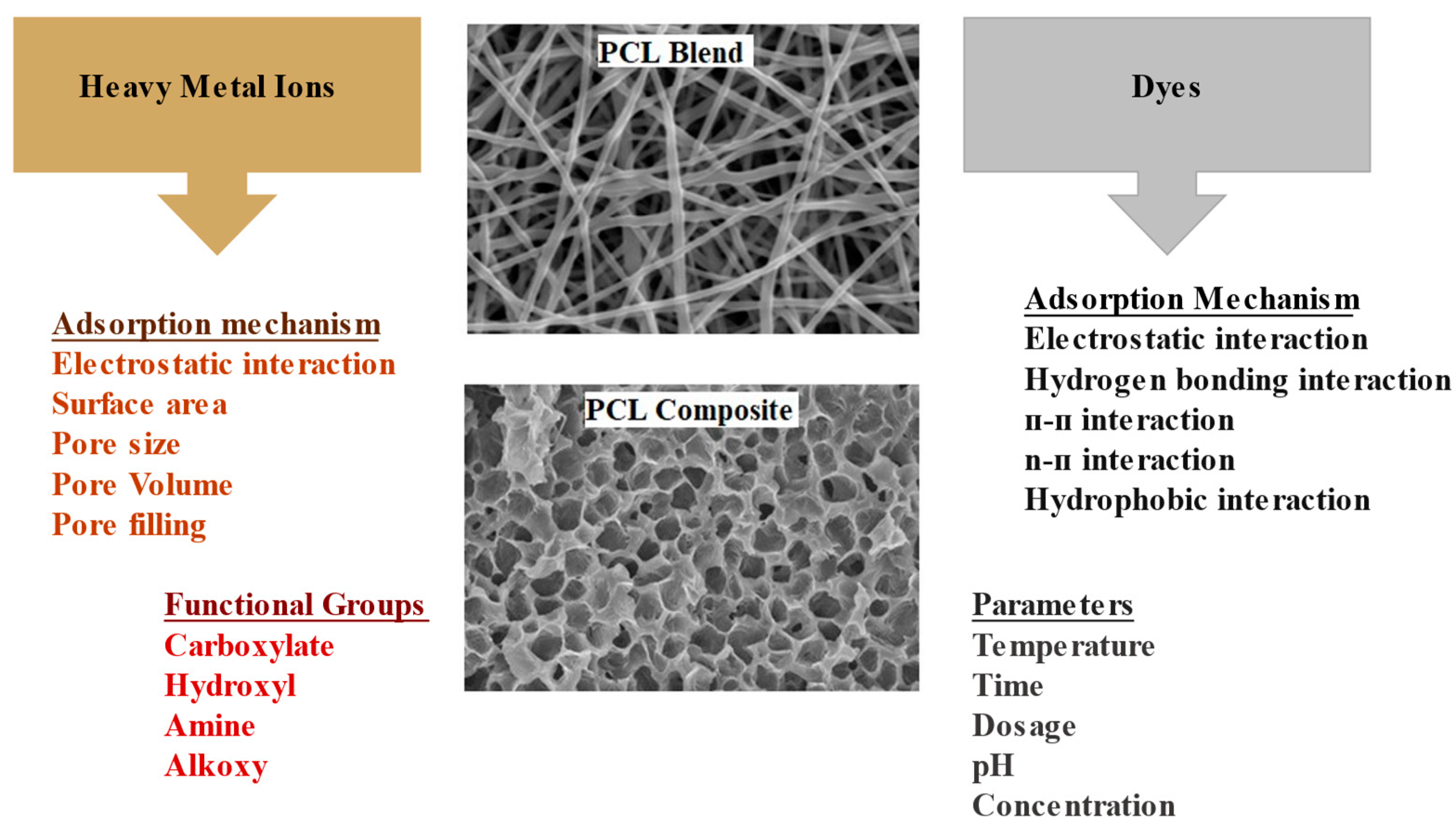
6.1.4. Adsorption Mechanism of Dyes/Metal Ions onto PCL Composites/Blends
| PCL-Blends/Composites | Time | Dyes | Adsorption Capacity, mg.g-1 | Ref. |
|---|---|---|---|---|
| PCL/(50%)PCD | 2h | MB | 11.2 | [85] |
| CS/P5K Nanofibrous Membranes | 2h | CR | 291.55 | [87] |
| PCL-PEG-PCL/Bentonite A2 | 1.5h | MB | 600 | [88] |
| Bacteria/PCL Webs | 48h | Setazol Blue BRF-X | 109.75 | [91] |
| PCL/PEO@PDA-45 | 45h | MO | 60.22 | [92] |
| GO-CNT@PCL | 6h | MO | 80 | [93] |
| Tri-PEG-PCL | 2h | MB | 193.51 | [94] |
| Multi-PEG-PCL | 2h | MB | 256.01 | [94] |
| m-RHAp-PCL Microspheres | 55h | MG | 609.76 | [103] |
| CS/PCL-b-PEG/SA@PDA-24 | 24h | CR | 598.8 | [104] |
| PCL-FeTA-APTES | - | MB | 32.04 | [128] |
| PCL-BIX80 | 2h | MB | 79 | [135] |
| PCL-BIX80 | 2h | BG | 254 | [135] |
| PCL/PEI/TTL | 8h | MG | 36.5 | [136] |
| PCL-Blends/Composites | Time | Heavy Metal Ions | Adsorption Capacity, mg.g-1 | Ref. |
|---|---|---|---|---|
| PCL/30%SA Filaments | 30 days | Cu(II) | 93.3 | [112] |
| CA/10%PCL Membrane | 6h | Pb(II) | 70.50 | [113] |
| CA–PCL/CS | ? | Cr(VI) | 126 | [114] |
| PCL/Clay/Zeolite | 2h | Pb(II) | 19.92 | [116] |
| Coffee/PCL | 4h | Cu(II) | 25.91 | [129] |
| CQD/PAN/PCL | ? | Cu(II) | 63.45 | [132] |
| 10%PCL/5%Clay Fiber | 72h | Cd(II), Cr(III), Pb(II) | 29.59, 27.23, 32.88 | [137] |
| PCL-CuHCF | 40 min | Cs(I), Co(II) | 178.7, 85.06 | [138] |
| PCL/Mg micromotor | 3 min | Ag(I) | 0.635 | [139] |
| Fe-MMt/PCL | 6h | Hg(II) | 14.25 | [140] |
| CMKC-coated PCL scaffolds | 1 min | Ca(II) | 2.186 | [141] |
| TPCL | 30 min | Pb(II), Cd(II) | 10.27, 5.81 | [142] |
6.2. Other uses like biomedical applications
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Pap, S.; Taggart, M.A.; Boyd, K.G.; James, N.A.; Gibb, S.W. A review of the potential utilisation of plastic waste as adsorbent for removal of hazardous priority contaminants from aqueous environments. Environ. Pollut. 2020, 258, 113698. [Google Scholar] [CrossRef] [PubMed]
- Moharir, R.V.; Kumar, S. Challenges associated with plastic waste disposal and allied microbial routes for its effective degradation: a comprehensive review. J. Clean. Prod. 2019, 208, 65–76. [Google Scholar] [CrossRef]
- Zhai, Z.; Du, X.; Zheng, H.; Long, Y. Biodegradable polymeric materials for flexible and degradable electronics. Front. Electron. 2022, 3, 985681. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A sustainable material for food and medical applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef]
- Nanda, S.; Patra, B.R.; Patel, R.; Bakos, J.; Dalai, A.K. Innovations in applications and prospects of bioplastics and biopolymers: a review. Environ. Chem. Lett. 2022, 20, 379–395. [Google Scholar] [CrossRef]
- Kurakula, M.; Rao, G.K.; Yadav, K.S. Fabrication and characterization of polycaprolactone-based green materials for drug delivery. In Ahmed S (ed) Applications of Advanced Green Materials. Woodhead Publishing, Cambridge, 2021, 395-423.
- Siddiqui, N.; Kishori, B.; Rao, S.; Anjum, M.; Hemanth, V.; Das, S.; Jabbari, E. Electropsun polycaprolactone fibres in bone tissue engineering: a review. Mol. Biotechnol. 2021, 63, 363–388. [Google Scholar] [CrossRef]
- Hsissou, R.; Seghiri, R.; Benzekri, Z.; Hilali, M.; Rafik, M.; Elharfi, A. Polymer composite materials: A comprehensive review. Compos. Struct. 2021, 262, 113640. [Google Scholar] [CrossRef]
- Ghosal, K.; Manakhov, A.; Zajíčková, L.; Thomas, S. Structural and surface compatibility study of modified electrospun poly (ε-caprolactone)(PCL) composites for skin tissue engineering. AAPS PharmSciTech. 2017, 18, 72–81. [Google Scholar] [CrossRef]
- Olsen, P.; Herrera, N.; Berglund, L.A. Polymer grafting inside wood cellulose fibers by improved hydroxyl accessibility from fiber swelling. Biomacromolecules 2019, 21, 597–603. [Google Scholar] [CrossRef]
- de Oliveira Aguiar, V.; de Fatima Vieira Marques, M. Composites of polycaprolactone with cellulose fibers: morphological and mechanical evaluation. Macromol. Symp. 2016, 367, 101–112. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Zuhri, M.Y.M.; Norrrahim, M.N.F.; Misenan, M.S.M.; Jenol, M.A.; Samsudin, S.A.; Omran, A.A.B. Natural fiber-reinforced polycaprolactone green and hybrid biocomposites for various advanced applications. Polymers 2022, 14, 182. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Qasim, M.; Ali, A. Engineering and polymeric composition of drug-eluting suture: A review. J. Biomed. Mater. Res A. 2021, 109, 2065–2081. [Google Scholar] [CrossRef] [PubMed]
- El Fawal, G.; Hong, H.; Mo, X.; Wang, H. Fabrication of scaffold based on gelatin and polycaprolactone (PCL) for wound dressing application. J. Drug. Deliv. Sci. Technol. 2021, 63, 102501. [Google Scholar] [CrossRef]
- Moore, M.J.; Tan, R.P.; Yang, N.; Rnjak-Kovacina, J.; Wise, S.G. Bioengineering artificial blood vessels from natural materials. Trends Biotechnol. 2022, 40, 693–707. [Google Scholar] [CrossRef]
- Salehi, A.O.M.; Keshel, S.H.; Sefat, F.; Tayebi, L. Use of polycaprolactone in corneal tissue engineering: A review. Mater. Today. Commun. 2021, 27, 102402. [Google Scholar] [CrossRef]
- Banimohamad-Shotorbani, B.; Rahmani Del Bakhshayesh, A.; Mehdipour, A.; Jarolmasjed, S.; Shafaei, H. The efficiency of PCL/HAp electrospun nanofibers in bone regeneration: A review. J. Med. Eng. Technol. 2021, 45, 511–531. [Google Scholar] [CrossRef]
- Bangar, S.P.; Whiteside, W.S.; Ashogbon, A.O.; Kumar, M. Recent advances in thermoplastic starches for food packaging: A review. Food Packag. Shelf Life 2021, 30, 100743. [Google Scholar] [CrossRef]
- Sharma, V.; Borkute, G.; Gumfekar, S.P. Biomimetic nanofiltration membranes: Critical review of materials, structures, and applications to water purification. Chem. Eng. J. 2022, 433, 133823. [Google Scholar] [CrossRef]
- Arif, U.; Haider, S.; Haider, A.; Khan, N.; Alghyamah, A.A.; Jamila, N.; Kang, I.K. Biocompatible polymers and their potential biomedical applications: A review. Curr. Pharm. Des. 2019, 25, 3608–3619. [Google Scholar] [CrossRef]
- Müller, K.; Zollfrank, C.; Schmid, M. Natural polymers from biomass resources as feedstocks for thermoplastic materials. Macromol. Mater. Eng. 2019, 304, 1800760. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: a review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef] [PubMed]
- Kayan, A. Recent studies on single site metal alkoxide complexes as catalysts for ring opening polymerization of cyclic compounds. Catal. Surv. Asia 2020, 24, 87–103. [Google Scholar] [CrossRef]
- Kaluzynski, K.; Pretula, J.; Lewinski, P.; Kaźmierski, S.; Penczek, S. Catalysis in polymerization of cyclic esters. Catalyst and initiator in one molecule. Polymerization of ε-caprolactone. J. Catal. 2020, 392, 97–107. [Google Scholar] [CrossRef]
- Thakur, M.; Majid, I.; Hussain, S.; Nanda, V. Poly (ε-caprolactone): A potential polymer for biodegradable food packaging applications. Packag. Technol. Sci. 2021, 34, 449–461. [Google Scholar] [CrossRef]
- Grobelny, Z.; Golba, S.; Jurek-Suliga, J. Mechanism of ε-caprolactone polymerization in the presence of alkali metal salts: investigation of initiation course and determination of polymers structure by MALDI-TOF mass spectrometry. Polym. Bull. 2019, 76, 3501–3515. [Google Scholar] [CrossRef]
- Cama, G.; Mogosanu, D.E.; Houben, A.; Dubruel, P. Synthetic biodegradable medical polyesters: poly-ε-caprolactone. In: Zhang X (ed) Science and Principles of Biodegradable and Bioresorbable Medical Polymers, 1st edn. Woodhead Publishing, Cambridge. 2017; 79–105. [Google Scholar]
- Penczek, S.; Pretula, J. Activated Monomer Mechanism (AMM) in Cationic Ring-Opening Polymerization. The Origin of the AMM and Further Development in Polymerization of Cyclic Esters. ACS Macro. Lett. 2021, 10, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Cho, Y.; Kim, D.; Mehrkhodavandi, P. Cationic aluminum, gallium, and indium complexes in catalysis. Catal. Sci. Technol. 2021, 11, 62–91. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.; Chen, C.; Huang, P.; Dai, H.; Jiang, W.; Zhou, Y. Living Cationic Polymerization of ε-Caprolactone Catalyzed by a Metal-free Lewis Acid of Trityl Tetrafluoroborate. Macromolecules 2023, 56, 501–509. [Google Scholar] [CrossRef]
- Grobelny, Z.; Jurek-Suliga, J.; Golba, S. The influence of hydroxylic compounds on cationic polymerization of ɛ-caprolactone mediated by iron (III) chloride in tetrahydrofuran solution. Polym. Bull. 2023, 80, 6307–6326. [Google Scholar] [CrossRef]
- Stridsberg, K.M.; Ryner, M.; Albertsson, A.C. Controlled ring-opening polymerization: polymers with designed macromolecular architecture. Adv. Polym. Sci. 2002, 157, 41–65. [Google Scholar]
- Punyodom, W.; Limwanich, W.; Meepowpan, P.; Thapsukhon, B. Ring-opening polymerization of ε-caprolactone initiated by tin (II) octoate/n-hexanol: DSC isoconversional kinetics analysis and polymer synthesis. Des. Monomers Polym. 2021, 24, 89–97. [Google Scholar] [CrossRef] [PubMed]
- McCollum, A.M.; Longo, A.M.; Stahl, A.E.; Butler, A.S.; Rheingold, A.L.; Cundari, T.R.; Fritsch, J.M. Synthesis, spectroscopy, and crystallography of mononuclear, five-coordinate aluminum complexes that act as cyclic ester polymerization initiators. Polyhedron 2021, 204, 115233. [Google Scholar] [CrossRef]
- Yildiz, B.C.; Kayan, A. Preparation of single-site tin (IV) compounds and their use in the polymerization of ε-caprolactone. Des. Monomer Polym. 2017, 20, 89–96. [Google Scholar] [CrossRef]
- Gökalp, Y.; Kayan, A. Synthesis and characterization of Ti-/Zr-diphenylpropanedione complexes and their application in the ring opening polymerization of Ɛ-caprolactone. J. Turk. Chem. Soc. A Chem. 2018, 5, 1095–1104. [Google Scholar] [CrossRef]
- Yildiz, B.C.; Kayan, A. Ti (IV)-silyliminophenolate catalysts for ϵ-caprolactone and L-Lactide polymerization. Sustain. Chem. Pharm. 2021, 21, 100416. [Google Scholar] [CrossRef]
- Lyubov, D.M.; Tolpygin, A.O.; Trifonov, A.A. Rare-earth metal complexes as catalysts for ring-opening polymerization of cyclic esters. Coord. Chem. Rev. 2019, 392, 83–145. [Google Scholar] [CrossRef]
- Appavoo, D.; Omondi, B.; Guzei, I.A. van Wyk, J.L.; Zinyemba, O.; Darkwa, J. Bis (3, 5-dimethylpyrazole) copper (II) and zinc (II) complexes as efficient initiators for the ring opening polymerization of ε-caprolactone and d, l-lactide. Polyhedron 2014, 69, 55–60. [Google Scholar] [CrossRef]
- Paradiso, V.; Capaccio, V.; Lamparelli, D.H.; Capacchione, C. [OSSO]-bisphenolate metal complexes: A powerful and versatile tool in polymerization catalysis. Coord. Chem. Rev. 2021, 429, 213644. [Google Scholar] [CrossRef]
- Wang, X.; Thevenon, A.; Brosmer, J.L.; Yu, I.; Khan, S.I.; Mehrkhodavandi, P.; Diaconescu, P.L. Redox control of group 4 metal ring-opening polymerization activity toward L-lactide and ε-caprolactone. J. Am. Chem. Soc. 2014, 136, 11264–11267. [Google Scholar] [CrossRef]
- Shao, J.; Zhou, H.; Wang, Y.; Luo, Y.; Yao, Y. Lanthanum complexes stabilized by a pentadentate Schiff-base ligand: synthesis, characterization, and reactivity in statistical copolymerization of ε-caprolactone and L-lactide. Dalton. Trans. 2020, 49, 5842–5850. [Google Scholar] [CrossRef]
- Vidal, J.L.; Yavitt, B.M.; Wheeler, M.D.; Kolwich, J.L.; Donovan, L.N.; Sit, C.S.; Kerton, F.M. (2022) Biochar as a sustainable and renewable additive for the production of Poly (ε-caprolactone) composites. Sustain. Chem. Pharm. 2022, 25, 100586. [Google Scholar] [CrossRef]
- Heimowska, A.; Morawska, M.; Bocho-Janiszewska, A. Biodegradation of poly (ε-caprolactone) in natural water environments. Pol. J. Chem. Technol. 2017, 19, 120–126. [Google Scholar] [CrossRef]
- Khan, I.; Dutta, J.R.; Ganesan, R. Lactobacillus sps. lipase mediated poly (ε-caprolactone) degradation. Int. J. Biol. Macromol. 2017, 95, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Aris, M.H.; Annuar, M.S.M.; Ling, T.C. Lipase-mediated degradation of poly-ε-caprolactone in toluene: Behavior and its action mechanism. Polym. Degrad. Stab. 2016, 133, 182–191. [Google Scholar] [CrossRef]
- Ma, Q.; Shi, K.; Su, T.; Wang, Z. Biodegradation of polycaprolactone (PCL) with different molecular weights by Candida antarctica lipase. J. Polym. Environ. 2020, 28, 2947–2955. [Google Scholar] [CrossRef]
- Woodard, L.N. Grunlan, M.A. Hydrolytic degradation and erosion of polyester biomaterials. ACS Macro. Lett. 2018, 7, 976–982. [Google Scholar] [CrossRef]
- Singh, N.K.; Purkayastha, B.D.; Roy, J.K.; Banik, R.M.; Yashpal, M.; Singh, G.; Maiti, P. Nanoparticle-induced controlled biodegradation and its mechanism in poly (ε-caprolactone). ACS Appl. Mater. Interfaces. 2010, 2, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P,; Agate, S. ; Velev, O.D.; Lucia. L.; Pal. L. A critical review of the performance and soil biodegradability profiles of biobased natural and chemically synthesized polymers in industrial applications. Environ. Sci. Technol. 2022, 56, 2071–2095. [Google Scholar] [CrossRef]
- Liu, Q.; Yuan, S.; Guo, Y.; Narayanan, A.; Peng, C.; Wang, S.; Joy, A. Modulating the crystallinity, mechanical properties, and degradability of poly (ε-caprolactone) derived polyesters by statistical and alternating copolymerization. Polym. Chem. 2019, 10, 2579–2588. [Google Scholar] [CrossRef]
- Magazzini, L.; Grilli, S.; Fenni, S.E.; Donetti, A.; Cavallo, D.; Monticelli, O. The Blending of Poly (glycolic acid) with Polycaprolactone and Poly (l-lactide): Promising Combinations. Polymers 2021, 13, 2780. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Yusoh, K. A review on the recent research of polycaprolactone (PCL). Trans Tech Publications Ltd. In Adv Mater Res. 2016, 1134, 249–255. [Google Scholar] [CrossRef]
- Sin, L.T.; Rahmat, A.R.; Rahman, W.A. In: Ebnesajjad S(ed) Handbook of Biopolymers and Biodegradable Plastics, 1st edn. Elsevier, Amsterdam, 2013, 11-54.
- Guarino, V.; Gentile, G.; Sorrentino, L.; Ambrosio, L. Polycaprolactone: synthesis, properties, and applications. Encyclopedia Polym. Sci. Technol. 2017, 1–36. [Google Scholar]
- Espinoza, S.M.; Patil, H.I.; San Martin Martinez, E.; Casañas Pimentel, R.; Ige, P.P. Poly-ε-caprolactone (PCL), a promising polymer for pharmaceutical and biomedical applications: Focus on nanomedicine in cancer. Int. J. Polymeric Mater. Polym. Biomater. 2020, 69, 85–126. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Boccaccini, A.R. Nature-Derived and Synthetic Additives to poly (ɛ-Caprolactone) Nanofibrous Systems for Biomedicine; an Updated Overview. Front. Chem. 2022, 9, 809676. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Dargaville, T.R. Ivanovski, S.; Hutmacher, D.W. Degradation mechanisms of polycaprolactone in the context of chemistry, geometry and environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Taniguchi, H.; Kurokawa, N.; Inukai, S.; Hotta, A. Structures and mechanical properties of electrospun cellulose nanofibers/poly (ε-caprolactone) composites. J. Appl. Polym. Sci. 2020, 137, 49307. [Google Scholar] [CrossRef]
- Clamor, C.; Cattoz, B.N.; Wright, P.M.; O’Reilly, R.K.; Dove, A.P. Controlling the crystallinity and solubility of functional PCL with efficient post-polymerisation modification. Polym. Chem. 2021, 12, 1983–1990. [Google Scholar] [CrossRef]
- Goel, V.; Luthra, P.; Kapur, G.S.; Ramakumar, S.S.V. Biodegradable/bio-plastics: myths and realities. J. Polym. Environ. 2021, 29, 3079–3104. [Google Scholar] [CrossRef]
- Nandakumar, A.; Chuah, J.A.; Sudesh, K. Bioplastics: a boon or bane? Renew. Sustain. Energy Rev. 2021, 147, 111237. [Google Scholar] [CrossRef]
- Moshood, T.D.; Nawanir, G.; Mahmud, F.; Mohamad, F.; Ahmad, M.H.; AbdulGhani, A. Sustainability of biodegradable plastics: New problem or solution to solve the global plastic pollution. Curr. Res. Green Sustain. Chem. 2022, 5, 100273. [Google Scholar] [CrossRef]
- Adeli-Sardou, M.; Yaghoobi, M.M.; Torkzadeh-Mahani, M.; Dodel, M. Controlled release of lawsone from polycaprolactone/gelatin electrospun nano fibers for skin tissue regeneration. Int. J. Biol. Macromol. 2019, 124, 478–491. [Google Scholar] [CrossRef]
- Leonés, A.; Mujica-Garcia, A.; Arrieta, M.P.; Salaris, V.; Lopez, D.; Kenny, J.M.; Peponi, L. Organic and inorganic PCL-based electrospun fibers. Polymers 2020, 12, 1325. [Google Scholar] [CrossRef]
- Faruk, O.; Bledzki, A.K.; Fink, H.P.; Sain, M. Biocomposites reinforced with natural fibers: 2000–2010. Prog. Polym. Sci. 2012, 37, 1552–1596. [Google Scholar] [CrossRef]
- Ludueña, L.; Vázquez, A.; Alvarez, V. Effect of lignocellulosic filler type and content on the behavior of polycaprolactone based eco-composites for packaging applications. Carbohydr. Polym. 2012, 87, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Boujemaoui, A.; Cobo Sanchez, C.; Engström, J.; Bruce, C.; Fogelström, L.; Carlmark, A.; Malmström, E. Polycaprolactone nanocomposites reinforced with cellulose nanocrystals surface-modified via covalent grafting or physisorption: a comparative study. ACS Appl. Mater. Interfaces 2017, 9, 35305–35318. [Google Scholar] [CrossRef] [PubMed]
- Morouço, P.; Biscaia, S.; Viana, T.; Franco, M.; Malça, C.; Mateus, A.; Alves, N.M. Fabrication of Poly (-caprolactone) Scaffolds Reinforced with Cellulose Nanofibers, with and without the Addition of Hydroxyapatite Nanoparticles. BioMed. Res. Int. 2016, 2016, 1596157. [Google Scholar] [CrossRef]
- Yu, Y.; Gao, X.; Jiang, Z.; Zhang, W.; Ma, J.; Liu, X.; Zhang, L. Homogeneous grafting of cellulose with polycaprolactone using quaternary ammonium salt systems and its application for ultraviolet-shielding composite films. RSC Adv. 2018, 8, 10865–10872. [Google Scholar] [CrossRef]
- Mi, H.Y.; Jing, X.; Peng, J.; Salick, M.R.; Peng, X.F. Turng, L.S. Poly (ε-caprolactone)(PCL)/cellulose nano-crystal (CNC) nanocomposites and foams. Cellulose 2014, 21, 2727–2741. [Google Scholar] [CrossRef]
- Hashiwaki, H.; Teramoto, Y.; Nishio, Y. Fabrication of thermoplastic ductile films of chitin butyrate/poly (ɛ-caprolactone) blends and their cytocompatibility. Carbohydr. Polym. 2014, 114, 330–338. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef]
- San, I.S.; Rezaei, M.; Khoshfetrat, A.B.; Razzaghi, D. Preparation and characterization of polycaprolactone/chitosan-g-polycaprolactone/hydroxyapatite electrospun nanocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2021, 182, 1638–1649. [Google Scholar]
- Manoel, A.F.; Claro, P.I.; Galvani, F.; Mattoso, L.H.; Marconcini, J.M.; Mantovani, G.L. Poly (ε-caprolactone) blended with thermoplastic waxy starch matrix reinforced with cellulose nanocrystals from Macauba (Acrocomia spp.) Rachis. Ind. Crops. Prod. 2022, 177, 114446. [Google Scholar] [CrossRef]
- Peterson, C.H.; Werber, J.R.; Lee, H.K.; Hillmyer, M.A. Tailored Mesoporous Microspheres by Polymerization-Induced Microphase Separation in Suspension. ACS Appl. Polym. Mater. 2022, 4, 4219–4233. [Google Scholar] [CrossRef]
- Li, W.; Zong, Y.; Liu, Q.; Sun, Y.; Li, Z.; Wang, H.; Li, Z. A highly stretchable and biodegradable superamphiphobic fluorinated polycaprolactone nanofibrous membrane for antifouling. Prog. Org. Coat. 2020, 147, 105776. [Google Scholar] [CrossRef]
- Rusli, M.S.I.C.; Hassan, M.I.; Sultana, N.; Ismail, A.F. Characterization of PCL/zeolite electrospun membrane for the removal of silver in drinking water. J. Teknol. 2017, 79, 89–95. [Google Scholar]
- Huang, Y.; Dan, N.; Dan, W.; Zhao, W. Reinforcement of polycaprolactone/chitosan with nanoclay and controlled release of curcumin for wound dressing. ACS Omega. 2019, 4, 22292–22301. [Google Scholar] [CrossRef]
- Karimian, R.; Mehrabani, M.G.; Mehramuz, B.; Ganbarov, K.; Ejlali, L.; Tanomand, A.; Kafil, H.S. Poly (ε-Caprolactone)/cellulose nanofiber blend nanocomposites containing ZrO2 nanoparticles: A new biocompatible wound dressing bandage with antimicrobial activity. Adv. Pharm. Bull. 2020, 10, 577. [Google Scholar]
- Ramírez-Cedillo, E.; Ortega-Lara, W.; Rocha-Pizaña, M.R.; Gutierrez-Uribe, J.A.; Elías-Zúñiga, A.; Rodríguez, C.A. Electrospun polycaprolactone fibrous membranes containing Ag, TiO2 and Na2Ti6O13 particles for potential use in bone regeneration. Membranes 2019, 9, 12. [Google Scholar] [CrossRef]
- del Ángel-Sánchez, K.; Borbolla-Torres, C.I.; Palacios-Pineda, L.M.; Ulloa-Castillo, N.A.; Elías-Zúñiga, A. Development, fabrication, and characterization of composite polycaprolactone membranes reinforced with TiO2 nanoparticles. Polymers 2019, 11, 1955. [Google Scholar] [CrossRef]
- Scaffaro, R.; Gammino, M.; Maio, A. Wet electrospinning-aided self-assembly of multifunctional GO-CNT@ PCL core-shell nanocomposites with spider leg bioinspired hierarchical architectures. Compos. Sci. Technol. 2022, 221, 109363. [Google Scholar] [CrossRef]
- Thamer, B.M.; Aldalbahi, A.; Moydeen, A.M.; Rahaman, M.; El-Newehy, M.H. Modified electrospun polymeric nanofibers and their nanocomposites as nanoadsorbents for toxic dye removal from contaminated waters: A review. Polymers 2021, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Wang, R.; Yin, J.; Jiao, T.; Huang, H.; Zhao, X.; Peng, Q. Fabrication and highly efficient dye removal characterization of beta-cyclodextrin-based composite polymer fibers by electrospinning. Nanomaterials 2019, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Staszak, M.; Jankowska, K.; Kaźmierczak, K.; Degórska, O.; Nguyen, L.N.; Jesionowski, T. The response surface methodology for optimization of tyrosinase immobilization onto electrospun polycaprolactone–chitosan fibers for use in bisphenol A removal. Int. J. Biol. Macromol. 2020, 165, 2049–2059. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xiang, C.; He, N.; Zhang, J.; Li, L.; Dong, S. Chitosan/poly (ε-caprolactone)-block-poly (ethylene glycol) copolymer electrospun membrane for the adsorption of dyes. New. J. Chem. 2020, 44, 20458–20469. [Google Scholar] [CrossRef]
- Draoua, Z.; Harrane, A.; Adjdir, M. Preparation, characterization and application of the nanocomposite PCL-PEG-PCL/Bentonite for the removal of methylene blue (MB) dye. Res. Chem. Intermed. 2021, 47, 4635–4655. [Google Scholar] [CrossRef]
- Karagoz, S.; Kiremitler, N.B.; Sakir, M.; Salem, S.; Onses, M.S.; Sahmetlioglu, E.; Yilmaz, E. Synthesis of Ag and TiO2 modified polycaprolactone electrospun nanofibers (PCL/TiOf-Ag NFs) as a multifunctional material for SERS, photocatalysis and antibacterial applications. Ecotoxicol. Environ. Saf. 2020, 188, 109856. [Google Scholar] [CrossRef]
- Nivedita, S.; Joseph, S. Optimization of process parameters using response surface methodology for PCL based biodegradable composite membrane for water purification. Arab. J. Sci. Eng. 2020, 45, 7347–7360. [Google Scholar] [CrossRef]
- Sarioglu, O.F.; San Keskin, N.O.; Celebioglu, A.; Tekinay, T.; Uyar, T. Bacteria immobilized electrospun polycaprolactone and polylactic acid fibrous webs for remediation of textile dyes in water. Chemosphere 2017, 184, 393–399. [Google Scholar] [CrossRef]
- Wang, C.; Yin, J.; Wang, R.; Jiao, T.; Huang, H.; Zhou, J.; Peng, Q. Facile preparation of self-assembled polydopamine-modified electrospun fibers for highly effective removal of organic dyes. Nanomaterials 2019, 9, 116. [Google Scholar] [CrossRef]
- Scaffaro, R.; Gammino, M.; Maio, A. Wet electrospinning-aided self-assembly of multifunctional GO-CNT@ PCL core-shell nanocomposites with spider leg bioinspired hierarchical architectures. Compos. Sci. Technol. 2022, 221, 109363. [Google Scholar] [CrossRef]
- Yousfi, R.E.; Achalhi, N.; Benahmed, A.; El Idrissi, A. Synthesis, characterization of multi-arm copolymers and linear blocks based on PEG and PCL: Effect of topology on dye adsorption. Mater. Today: Proc. 2023, 72, 3650–3661. [Google Scholar] [CrossRef]
- Xue, W.; Hu, Y.; Wang, F.; Yang, X.; Wang, L. Fe3O4/poly (caprolactone)(PCL) electrospun membranes as methylene blue catalyst with high recyclability. Colloids Surf. A: Physicochem. Eng. Asp. 2019, 564, 115–121. [Google Scholar] [CrossRef]
- Alrafai, H.A.; Al-Ahmed, Z.A.; Ahmed, M.K.; Afifi, M.; Shoueir, K.R.; Abu-Rayyan, A. The degradation of methylene blue dye using copper-doped hydroxyapatite encapsulated into polycaprolactone nanofibrous membranes. New J. Chem. 2021, 45, 16143–16154. [Google Scholar] [CrossRef]
- Elias, E.; Sarathchandran, C.; Joseph, S.; Zachariah, A.K.; Thomas, J.; Devadasan, D.; Thomas, S. Photoassisted degradation of rhodamine B using poly (ε-caprolactone) based nanocomposites: Mechanistic and kinetic features. J. Appl. Polym. Sci. 2021, 138, 50612. [Google Scholar] [CrossRef]
- Marković, D.; Milovanović, S.; Radoičić, M.B.; Radovanović, Ž.; Žižović, I.T.; Šaponjić, Z.; Radetić, M.M. Removal of textile dyes from water by TiO2 nanoparticles immobilized on poly (ε-caprolactone) beads and foams. J. Serb. Chem. Soc. 2018, 83, 1379–1389. [Google Scholar] [CrossRef]
- Geravand, M.H.A.; Saljoughi, E.; Mousavi, S.M.; Kiani, S. Biodegradable polycaprolactone/MXene nanocomposite nanofiltration membranes for the treatment of dye solutions. J. Taiwan Inst. Chem. Eng. 2021, 128, 124–139. [Google Scholar] [CrossRef]
- Tu, H.; Li, D.; Yi, Y.; Liu, R.; Wu, Y.; Dong, X.; Deng, H. Incorporation of rectorite into porous polycaprolactone/TiO2 nanofibrous mats for enhancing photocatalysis properties towards organic dye pollution. Compos. Commun. 2019, 15, 58–63. [Google Scholar] [CrossRef]
- Xie, J.; Hung, Y.C. UV-A activated TiO2 embedded biodegradable polymer film for antimicrobial food packaging application. Lwt 2018, 96, 307–314. [Google Scholar] [CrossRef]
- Saeed, K.; Khan, N.; Shah, T.; Sadiq, M. Morphology, Properties and Application of Iron Oxide/Polycaprolactone Nanocomposites. J. Chem. Soc. Pak. 2021, 43, 34–40. [Google Scholar]
- Yan, B.; Wang, X.; Zhang, X.; Liu, S.; Lu, H.; Ran, R. One-step preparation of hydroxyapatite-loaded magnetic Polycaprolactone hollow microspheres for malachite green adsorption by Pickering emulsion template method. Colloids Surf. A: Physicochem. Eng. Asp. 2022, 639, 128347. [Google Scholar] [CrossRef]
- Chen, J.; Liu, L. Huang, J.; Sheng, C.; Li, L. Porous multicomponent chitosan/poly (ε-caprolactone)-block poly (ethylene glycol)/SiO2 aerogel@ polydopamine membrane for Congo red adsorption. Mater. Today Chem. 2022, 23, 100661. [Google Scholar] [CrossRef]
- Radwan, H.A.; Ismail, R.A. Abdelaal, S.A.; Al Jahdaly, B.A.; Almahri, A.; Ahmed, M.K.; Shoueir, K. Electrospun polycaprolactone nanofibrous webs containing Cu–Magnetite/Graphene oxide for cell viability, antibacterial performance, and dye decolorization from aqueous solutions. Arab. J. Sci. Eng. 2022, 47, 303–318. [Google Scholar] [CrossRef]
- Xue, W.; Hu, Y.; Wang, F.; Yang, X.; Wang, L. Fe3O4/poly (caprolactone)(PCL) electrospun membranes as methylene blue catalyst with high recyclability. Colloids Surf. A: Physicochem. Eng. Asp. 2019, 564, 115–121. [Google Scholar] [CrossRef]
- Pekdemir, M.E.; Tanyol, M.; Torğut, G. Preparation of ε-Caprolactone/Fe3O4 Magnetic Nanocomposite and Its Application to the Remazol Brilliant Violet 5R Dye Adsorption from Wastewaters by Using RSM. J. Polym. Environ. 2022, 30, 4225–4237. [Google Scholar] [CrossRef]
- He, N.; Li, L.; Chen, J.; Zhang, J.; Liang, C. Extraordinary superhydrophobic polycaprolactone-based composite membrane with an alternated micro–nano hierarchical structure as an eco-friendly oil/water separator. ACS Appl. Mater. Interfaces 2021, 13, 24117–24129. [Google Scholar] [CrossRef] [PubMed]
- Uzunok, S.; Sonmez, H.B. Reusable polycaprolactone based sorbents with different cross-linking densities for the removal of organic pollutants. J. Environ. Chem. Eng. 2023, 11, 109287. [Google Scholar] [CrossRef]
- Eom, J.; Kwak, Y.; Nam, C. Electrospinning fabrication of magnetic nanoparticles-embedded polycaprolactone (PCL) sorbent with enhanced sorption capacity and recovery speed for spilled oil removal. Chemosphere 2022, 303, 135063. [Google Scholar] [CrossRef]
- Seema, K.M.; Mamba, B.B.; Njuguna, J.; Bakhtizin, R.Z.; Mishra, A.K. Removal of lead (II) from aqueous waste using (CD-PCL-TiO2) bio-nanocomposites. Int. J. Biol. Macromol. 2018, 109, 136–142. [Google Scholar] [CrossRef]
- Liakos, I.L.; Mondini, A.; Del Dottore, E.; Filippeschi, C.; Pignatelli, F.; Mazzolai, B. 3D printed composites from heat extruded polycaprolactone/sodium alginate filaments and their heavy metal adsorption properties. Mater. Chem. Front. 2020, 4, 2472–2483. [Google Scholar] [CrossRef]
- Aquino, R.R.; Tolentino, M.S.; Elacion, R.M.P.D.; Ladrillono, R.; Laurenciana, T.R.C.; Basilia, B.A. Adsorptive removal of lead (Pb2+) ion from water using cellulose acetate/polycaprolactone reinforced nanostructured membrane. In IOP Conf. Ser: Earth Environ. Sci. 2018, 191, 012139. [Google Scholar] [CrossRef]
- Ma, L.; Shi, X.; Zhang, X.; Dong, S.; Li, L. Electrospun cellulose acetate–polycaprolactone/chitosan core–shell nanofibers for the removal of Cr (VI). Phys. Status Solidi (a) 2019, 216, 1900379. [Google Scholar] [CrossRef]
- Benhacine, F.; Abdellaoui, N.; Arous, O.; Hadj-Hamou, A.S. Behaviours of poly (ε-caprolactone)/silver-montmorillonite nanocomposite in membrane ultrafiltration for wastewater treatment. Environ. Technol. 2020, 41, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- Irandoost, M.; Pezeshki-Modaress, M.; Javanbakht, V. Removal of lead from aqueous solution with nanofibrous nanocomposite of polycaprolactone adsorbent modified by nanoclay and nanozeolite. J. Water Process Eng. 2019, 32, 100981. [Google Scholar] [CrossRef]
- Palacios Hinestroza, H.; Urena-Saborio, H.; Zurita, F.; Guerrero de Leon, A.A.; Sundaram, G.; Sulbarán-Rangel, B. Nanocellulose and polycaprolactone nanospun composite membranes and their potential for the removal of pollutants from water. Molecules 2020, 25, 683. [Google Scholar] [CrossRef]
- Lan, X.; Wang, H.; Liu, Y.; Chen, X.; Xiong, J.; Mai, R.; Tang, Y. Biodegradable trilayered micro/nano-fibrous membranes with efficient filtration, directional moisture transport and antibacterial properties. Chem. Eng. J. 2022, 447, 137518. [Google Scholar] [CrossRef]
- Song, W.; Qian, L.; Gao, B.; Zhu, Y.; Zhu, M.; Zhao, Y.; Miao, Z. Ionic liquid-based amphiphilic conetwork with mechanical toughness: a promising candidate for dye removal. J. Mater. Sci. 2019, 54, 6212–6226. [Google Scholar] [CrossRef]
- Kayan, G.O.; Kayan, A. Inorganic–Organic Hybrid Materials of Zirconium and Aluminum and Their Usage in the Removal of Methylene Blue. J. Inorg. Organomet. Polym. Mater. 2021, 31, 3613–3623. [Google Scholar] [CrossRef]
- Kayan, G.O.; Kayan, A. Polyhedral Oligomeric Silsesquioxane and Polyorganosilicon Hybrid Materials and Their Usage in the Removal of Methylene Blue Dye. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2781–2792. [Google Scholar] [CrossRef]
- Mohammad, N.; Atassi, Y. Adsorption of methylene blue onto electrospun nanofibrous membranes of polylactic acid and polyacrylonitrile coated with chloride doped polyaniline. Sci. Rep. 2020, 10, 13412. [Google Scholar] [CrossRef]
- Bahalkeh, F.; Mehrabian, R.Z.; Ebadi, M. Removal of Brilliant Red dye (Brilliant Red E-4BA) from wastewater using novel Chitosan/SBA-15 nanofiber. Int. J. Biol. Macromol. 2020, 164, 818–825. [Google Scholar] [CrossRef]
- Qi, F.F.; Ma, T.Y.; Liu, Y.; Fan, Y.M.; Li, J.Q.; Yu, Y.; Chu, L.L. 3D superhydrophilic polypyrrole nanofiber mat for highly efficient adsorption of anionic azo dyes. Microchem. J. 2020, 159, 105389. [Google Scholar] [CrossRef]
- Lin, Y.; Tian, Y.; Sun, H.; Hagio, T. Progress in modifications of 3D graphene-based adsorbents for environmental applications. Chemosphere 2021, 270, 129420. [Google Scholar] [CrossRef]
- Kayan, G.O.; Kayan, A. Composite of natural polymers and their adsorbent properties on the dyes and heavy metal ions. J. Polym. Environ. 2021, 29, 3477–3496. [Google Scholar] [CrossRef]
- Baig, M.T.; Kayan, A. Eco-friendly novel adsorbents composed of hybrid compounds for efficient adsorption of methylene blue and Congo red dyes: Kinetic and thermodynamic studies. Sep. Sci. Technol. 2023, 58, 862–883. [Google Scholar] [CrossRef]
- Hussain, Z.; Ullah, S.; Yan, J.; Wang, Z.; Ullah, I.; Ahmad, Z.; Pei, R. Electrospun tannin-rich nanofibrous solid-state membrane for wastewater environmental monitoring and remediation. Chemosphere 2022, 307, 135810. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, H.B.; Oh, Y.; Choi, A.J.; Seo, T.H.; Kim, Y.K.; Lee, M.W. Used coffee/PCL composite filter for Cu (II) removal from wastewater. J. Water Process Eng. 2022, 50, 103253. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, L.C.; Quintanilla-Carvajal, M.X.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A. Jiménez-Junca, C. Preparation and Characterization of an Electrospun Whey Protein/Polycaprolactone Nanofiber Membrane for Chromium Removal from Water. Nanomaterials 2022, 12, 2744. [Google Scholar] [CrossRef] [PubMed]
- Dela Peña, E.M.B.; Araño, K.; Dela Cruz, M.L.; de Yro, P.A.; Diaz, L.J.L. The design of a bench-scale adsorbent column based on nanoclay-loaded electrospun fiber membrane for the removal of arsenic in wastewater. Water Environ. J. 2021, 35, 937–942. [Google Scholar] [CrossRef]
- Mabborang, C.M.P.; Padrigo, J.N.B.; Quiachon, G.M.; de Yro, P.A.N. Synthesis and Characterization of Electrospun Carbon Quantum Dots–Polyacrylonitrile/Polycaprolactone Composite Nanofiber Membranes for Copper (II) Adsorption. Key Eng. Mater. 2021, 878, 3–8. [Google Scholar] [CrossRef]
- Tonsomboon, K.; Noppakuadrittidej, P.; Sutikulsombat, S.; Petdum, A.; Panchan, W.; Wanichacheva, N.; Karoonuthaisiri, N. Turn-On fluorescence resonance energy transfer (FRET)-based electrospun fibrous membranes: Rapid and ultrasensitive test strips for on-site detection of Mercury (II) ion. Sens. Actuators B: Chem. 2021, 344, 130212. [Google Scholar] [CrossRef]
- Bernhardt, K.T.; Collins, H.G.; Balija, A.M. Biorenewable triblock copolymers consisting of l-lactide and ε-caprolactone for removing organic pollutants from water: a lifecycle neutral solution. BMC chem. 2019, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Domingues, J.T.; Orlando, R.M.; Sinisterra, R.D.; Pinzon-Garcia, A.D.; Rodrigues, G.D. Polymer-bixin nanofibers: A promising environmentally friendly material for the removal of dyes from water. Sep. Purif. Technol. 2020, 248, 117118. [Google Scholar] [CrossRef]
- Kolak, S.; Birhanlı, E.; Boran, F.; Bakar, B.; Ulu, A.; Yeşilada, Ö.; Ateş, B. Tailor-made novel electrospun polycaprolactone/polyethyleneimine fiber membranes for laccase immobilization: An all-in-one material to biodegrade textile dyes and phenolic compounds. Chemosphere 2023, 313, 137478. [Google Scholar] [CrossRef] [PubMed]
- Roque-Ruiz, J.H.; Cabrera-Ontiveros, E.A.; Torres-Pérez, J.; Reyes-López, S.Y. Preparation of PCL/clay and PVA/clay electrospun fibers for cadmium (Cd+2), chromium (Cr+3), copper (Cu+2) and lead (Pb+2) removal from water. Water Air Soil Pollut. 2016, 227, 286. [Google Scholar] [CrossRef]
- Saberi, R.; Sadjadi, S.; Ammari Allahyari, S.; Charkhi, A. Poly (ε-caprolactone) electrospun nanofibers decorated with copper hexacyanoferrate as an ion exchanger for effective cesium ion removal. Sep. Sci. Technol. 2022, 57, 897–909. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, D.; Li, J.; Xu, X.; Zhang, H.; Duan, R.; Dong, B. One-step synthesis of PCL/Mg Janus micromotor for precious metal ion sensing, removal and recycling. J. Mater. Sci. 2019, 54, 7322–7332. [Google Scholar] [CrossRef]
- Somera, L.R.; Cuazon, R.; Cruz, J.K.; Diaz, L.J. Kinetics and isotherms studies of the adsorption of Hg (II) onto iron modified montmorillonite/polycaprolactone nanofiber membrane. IOP Conf. Ser.: Mater. Sci. Eng. 2019, 540, 012005. [Google Scholar] [CrossRef]
- Ataie, M.; Nourmohammadi, J.; Seyedjafari, E. Carboxymethyl carrageenan immobilized on 3D-printed polycaprolactone scaffold for the adsorption of calcium phosphate/strontium phosphate adapted to bone regeneration. Int. J. Biol. Macromol. 2022, 206, 861–874. [Google Scholar] [CrossRef]
- Körpınar, B.; Yayayürük, A.E.; Yayayürük, O.; Akat, H. Thiol–ended polycaprolactone: synthesis, preparation and use in Pb (II) and Cd (II) removal from water samples. Mater. Today Commun. 2021, 29, 102908. [Google Scholar] [CrossRef]
- Echeverria Molina, M.I.; Malollari, K.G.; Komvopoulos, K. Design challenges in polymeric scaffolds for tissue engineering. Front. Bioeng. Biotechnol. 2021, 9, 617141. [Google Scholar] [CrossRef]
- Das, P.; Remigy, J.C.; Lahitte, J.F. van der Meer, A.D.; Garmy-Susini, B.; Coetsier, C.; Bacchin, P. Development of double porous poly (ε-caprolactone)/chitosan polymer as tissue engineering scaffold. Mater. Sci. Eng. C 2020, 107, 110257. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tian, L.; Luo, B.; Ramakrishna, S.; Kai, D.; Loh, X.J.; Mo, X. Engineering PCL/lignin nanofibers as an antioxidant scaffold for the growth of neuron and Schwann cell. Colloids Surf. B: Biointerfaces 2018, 169, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.S.; Khorshidian, A.; Beram, F.M.; Derakhshani, A.; Esmaeili, J.; Barati, A. 3D printed chitosan/polycaprolactone scaffold for lung tissue engineering: Hope to be useful for COVID-19 studies. RSC Adv. 2021, 11, 19508–19520. [Google Scholar] [CrossRef] [PubMed]
- Salami, M.A.; Kaveian, F.; Rafienia, M.; Saber-Samandari, S.; Khandan, A.; Naeimi, M. Electrospun polycaprolactone/lignin-based nanocomposite as a novel tissue scaffold for biomedical applications. J. Medical Signals Sens. 2017, 7, 228. [Google Scholar]
- Zhang, Y.; Liao, J.; Fang, X.; Bai, F.; Qiao, K.; Wang, L. Renewable high-performance polyurethane bioplastics derived from lignin–poly (ε-caprolactone). ACS Sustain. Chem. Eng. 2017, 5, 4276–4284. [Google Scholar] [CrossRef]
- Kotcharat, P.; Chuysinuan, P.; Thanyacharoen, T.; Techasakul, S.; Ummartyotin, S. Development of bacterial cellulose and polycaprolactone (PCL) based composite for medical material. Sustain. Chem. Pharm. 2021, 20, 100404. [Google Scholar] [CrossRef]
- Liao, N.; Unnithan, A.R.; Joshi, M.K.; Tiwari, A.P.; Hong, S.T.; Park, C.H.; Kim, C.S. Electrospun bioactive poly (ɛ-caprolactone)–cellulose acetate–dextran antibacterial composite mats for wound dressing applications. Colloids Surf. A: Physicochem. Eng. Asp. 2015, 469, 194–201. [Google Scholar] [CrossRef]
- Gorrasi, G.; Bugatti, V.; Viscusi, G.; Vittoria, V. Physical and barrier properties of chemically modified pectin with polycaprolactone through an environmentally friendly process. Colloid. Polym. Sci. 2021, 299, 429–437. [Google Scholar] [CrossRef]
- Hamzah, M.S.A.; Austad, A.; Abd Razak, S.I.; Nayan, N.H.M. Tensile and wettability properties of electrospun polycaprolactone coated with pectin/polyaniline composite for drug delivery application. Int. J. Struct. Integr. 2019, 10, 704–713. [Google Scholar] [CrossRef]
- Homocianu, M.; Pascariu, P. Electrospun polymer-inorganic nanostructured materials and their applications. Polym. Rev. 2020, 60, 493–541. [Google Scholar] [CrossRef]
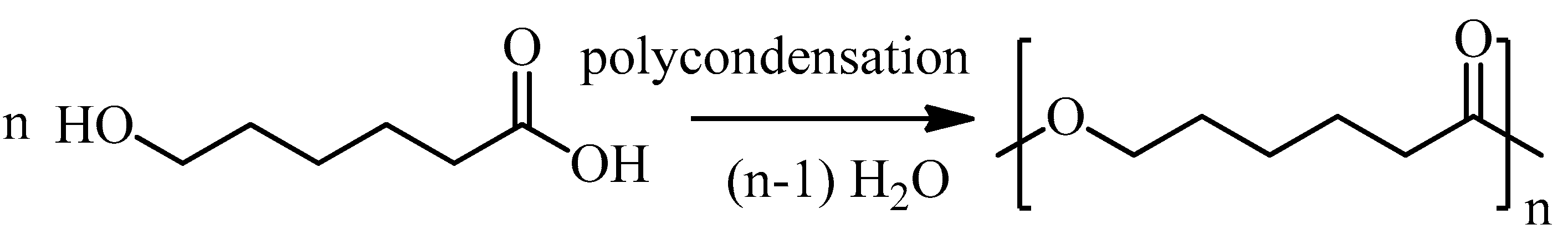
| 1H-NMR results (in CDCl3) | δ(ppm): 4.1 (t, J = 6.7 Hz, εCH2O), 2.3 (t, J = 7.5 Hz, αCH2), 1.6 (m, J = 7.3 Hz, β,δCH2), 1.4 (m, J = 7.3 Hz, γCH2) |
| 13C NMR results (in CDCl3) | δ(ppm): 174 (C=O), 64 (εCH2O), 34 (αCH2), 28 (δCH2), 26 (βCH2), 25 (γCH2). [O=C-αCH2βCH2γCH2δCH2εCH2O-]. |
| FTIR results (cm-1) | 2936 (CH, asym str), 2865 (CH, sym str), 1723 (C=O, asym str), 1472, 1418, 1394, 1366 (C=O, sym str) 1288, 1240 (C–O–C str vib), 1162 (C–O–C str vib), 1100, 1046, 1018 cm-1 etc. |
| Properties | Values |
|---|---|
| Molecular weight, Mn, g/mol | 3x103 to 8x104 |
| Density, g/cm3 | 1.07 to 1.20 |
| Melting temperature (Tm), oC | 56 to 65 |
| Glass transition temp. (Tg), oC | -65 to -60 |
| Decomposition temp. (Td), oC | 350 |
| Degradation time, years | 2-3 |
| Inherent viscosity, cm3/g | 100–130 |
| Intrinsic viscosity, cm3/g | 0.9 |
| Tensile strength, σm, MPa | 4-785 |
| Tensile elastic modulus, Et, MPa | 250-440 |
| Yield stress, σy, kN/m2 | 8.2x103-1.1x104 |
| Young modulus, MPa | 210-440 |
| Elongation at break, % | 20–1000 |
| Conformation | Extended and zigzag |
| Degree of crystallinity | Semicrystalline (50%) |
| Water vapor permeability, g/m2·day | 783 |
| Oxygen transmission rate, m3/m2·day | 486 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).