Submitted:
11 September 2023
Posted:
12 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sampling Strategy, Field and Laboratory Procedures
2.2. Data Analyses
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Snelgrove, P.V.R. The Importance of Marine Sediment Biodiversity in Ecosystem Processes. Ambio 1997, 26, 578–583. [Google Scholar]
- Bilyard, G.R. The Value of Benthic Infauna in Marine Pollution Monitoring Studies. Marine Pollution Bulletin 1987, 18, 581–585. [Google Scholar] [CrossRef]
- Simonini, R.; Ansaloni, I.; Bonini, P.; Grandi, V.; Graziosi, F.; Iotti, M.; Massamba-N’Siala, G.; Mauri, M.; Montanari, G.; Preti, M.; et al. Recolonization and Recovery Dynamics of the Macrozoobenthos after Sand Extraction in Relict Sand Bottoms of the Northern Adriatic Sea. Marine Environmental Research 2007, 64, 574–589. [Google Scholar] [CrossRef]
- Manoukian, S.; Spagnolo, A.; Scarcella, G.; Punzo, E.; Angelini, R.; Fabi, G. Effects of Two Offshore Gas Platforms on Soft-Bottom Benthic Communities (Northwestern Adriatic Sea, Italy). Marine Environmental Research 2010, 70, 402–410. [Google Scholar] [CrossRef]
- Trabucco, B.; Grossi, L.; Marusso, V.; Bacci, T.; Bertasi, F.; Ceracchi, S.; Lomiri, S.; Vani, D.; Virno Lamberti, C. Macrozoobenthic Assemblages around a Marine Terminal for Re-Gasifying Liquefied Natural Gas (LNG) in the North Adriatic Sea (Italy). Journal of the Marine Biological Association of the United Kingdom 2015, 95. [Google Scholar] [CrossRef]
- Tomassetti, P.; Gennaro, P.; Lattanzi, L.; Mercatali, I.; Persia, E.; Vani, D.; Porrello, S. Benthic Community Response to Sediment Organic Enrichment by Mediterranean Fish Farms: Case Studies. Aquaculture 2016, 450, 262–272. [Google Scholar] [CrossRef]
- Spagnolo, A.; Auriemma, R.; Bacci, T.; Balković, I.; Bertasi, F.; Bolognini, L.; Cabrini, M.; Cilenti, L.; Cuicchi, C.; Cvitković, I.; et al. Non-Indigenous Macrozoobenthic Species on Hard Substrata of Selected Harbours in the Adriatic Sea. Marine Pollution Bulletin 2017. [Google Scholar] [CrossRef]
- Targusi, M.; La Porta, B.; Lattanzi, L.; La Valle, P.; Loia, M.; Paganelli, D.; Pazzini, A.; Proietti, R.; Nicoletti, L. Beach Nourishment Using Sediments from Relict Sand Deposit: Effects on Subtidal Macrobenthic Communities in the Central Adriatic Sea (Eastern Mediterranean Sea-Italy). Marine Environmental Research 2019, 144, 186–193. [Google Scholar] [CrossRef]
- Pearson, T.H.; Rosenberg, R. Macrobenthic Succession in Relation to Organic Enrichment and Pollution of the Marine Environment. Oceanography and Marine Biology: An Annual Review 1978, 16, 229–311. [Google Scholar]
- La Porta, B.; Tomassetti, P.; Lomiri, S.; Marzialetti, S.; Vani, D.; Penna, M.; Lanera, P.; Nicoletti, L. Ecology and Spatial Distribution of Selected Polychaete Species from the Italian Continental Shelf. Italian Journal of Zoology 2011, 78, 290–303. [Google Scholar] [CrossRef]
- Abele, L.G. Species Diversity of Decapod Crustaceans in Marine Habitats. Ecology 1974, 55, 156–161. [Google Scholar] [CrossRef]
- Cartes, J.E. Feeding Strategies and Partition of Food Resources in Deep-Water Decapod Crustaceans (400–2300 m). Journal of the Marine Biological Association of the United Kingdom 1998, 78, 509–524. [Google Scholar] [CrossRef]
- De Grave, S.; Pentcheff, N.; Ahyong, S.; Chan, T.Y.; Crandall, K.; Dworschak, P.; Felder, D.; Feldmann, R.; Fransen, C.; Goulding, L.; et al. A Classification of Living and Fossil Genera of Decapod Crustaceans. The Raffles bulletin of zoology 2009, supplement 21, 1–109. [Google Scholar]
- Noël, P.; Monod, T.; Laubier, L. Crustacea in the Biosphere. In Treatise on Zoology - Anatomy, Taxonomy, Biology. The Crustacea, Volume 4 Part B; Brill, 2014; pp. 3–115 ISBN 978-90-04-26493-9.
- D’Udekem d’Acoz, C. Inventaire et Distribution Des Crustacés Décapodes de l’Atlantique Nord-Oriental, de La Méditerranée et Des Eaux Continentales Adjacentes Au Nord de 25 N. Collection Patrimoines Naturels 1999. [Google Scholar]
- Froglia, F. Crustacea, Malacostraci, Decapoda. Biologia Marina Mediterranea. 2010, 17 (suppl. 1), 519–534. [Google Scholar]
- Cartes, J.E.; Sarda, F. Abundance and Diversity of Decapod Crustaceans in the Deep-Catalan Sea (Western Mediterranean). Journal of Natural History 1992, 26, 1305–1323. [Google Scholar] [CrossRef]
- Maynou, F.; Conan, G.Y.; Cartes, J.E.; Company, J.B.; Sardà, F. Spatial Structure and Seasonality of Decapod Crustacean Populations on the Northwestern Mediterranean Slope. Limnology and Oceanography 1996, 41, 113–125. [Google Scholar] [CrossRef]
- Abelló, P.; Carbonell, A.; Torres, P. Biogeography of Epibenthic Crustaceans on the Shelf and Upper Slope off the Iberian Peninsula Mediterranean Coasts: Implications for the Establishment of Natural Management Areas. Scientia Marina 2002, 66, 183. [Google Scholar] [CrossRef]
- Galil, B.; Froglia, C.; Noël, P. CIESM Atlas of Exotic Species in the Mediterranean. Vol. 2. Crustaceans: Decapods and Stomatopods. F. Briand (ed). Monaco: CIESM Publishers, 2002.
- Pipitone, C.; Arculeo, M. The Marine Crustacea Decapoda of Sicily (Central Mediterranean Sea): A Checklist with Remarks on Their Distribution. Italian Journal of Zoology 2003, 70, 69–78. [Google Scholar] [CrossRef]
- Cartes, J.E.; Maynou, F.; Moranta, J.; Massutı́, E.; Lloris, D.; Morales-Nin, B. Patterns of Bathymetric Distribution among Deep-Sea Fauna at Local Spatial Scale: Comparison of Mainland vs. Insular Areas. Progress in Oceanography 2004, 60, 29–45. [Google Scholar] [CrossRef]
- Company, J.B.; Maiorano, P.; Tselepides, A.; Politou, C.Y.; Plaity, W.; Rotllant, G.; Sardá, F. Deep-Sea Decapod Crustaceans in the Western and Central Mediterranean Sea: Preliminary Aspects of Species Distribution, Biomass and Population Structure. Scientia Marina 2004, 68, 73–86. [Google Scholar] [CrossRef]
- Politou, C.-Y.; Maiorano, P.; D’Onghia, G.; Mytilineou, C. Deep-Water Decapod Crustacean Fauna of the Eastern Ionian Sea. Belgian Journal of Zoology 2005, 135. [Google Scholar]
- Ungaro, N.; Marano, C.; Ceriola, L.; Martino, M. Distribution of Demersal Crustaceans in the Southern Adriatic Sea. Acta Adriatica (marusic@izor.hr); Vol.46 No.1 2005, 46. [Google Scholar]
- Fanelli, E.; Colloca, F.; Ardizzone, G. Decapod Crustacean Assemblages off the West Coast of Central Italy (Western Mediterranean ). Scientia Marina 2007, 71, 19–28. [Google Scholar] [CrossRef]
- Follesa, M.C.; Porcu, C.; Gastoni, A.; Mulas, A.; Sabatini, A.; Cau, A. Community Structure of Bathyal Decapod Crustaceans off South-Eastern Sardinian Deep-Waters (Central-Western Mediterranean). Marine Ecology 2009, 30, 188–199. [Google Scholar] [CrossRef]
- Torres, A.; Dos Santos, A.; Balbín, R.; Alemany, F.; Massutí, E.; Reglero, P. Decapod Crustacean Larval Communities in the Balearic Sea (Western Mediterranean): Seasonal Composition, Horizontal and Vertical Distribution Patterns. Journal of Marine Systems 2013. [Google Scholar] [CrossRef]
- Gonulal, O.; Sezgin, M.; Oztürk, B. Diversity and Bathymetric Distribution of Decapod Crustaceans Attracted to Baited Traps from the Middle Slope of the Northern Aegean Sea. Crustaceana 2014, 87. [Google Scholar] [CrossRef]
- WoRMS - World Register of Marine Species. Available online: https://www.marinespecies.org/ (accessed on 7 August 2023).
- Bianchi, C.N. Proposta di suddivisione dei mari italiani in settori biogeografici. Estratto dal Notiziario della Società Italiana di Biologia Marina. 2004, 46. [Google Scholar]
- Relini, G. (Ed.) Checklist of Flora and Fauna of the Italian Seas. Biologia Marina Mediterranea. 2010, 17 (suppl.1).
- Ocean Biodiversity Information System. Available online: https://obis.org/ (accessed on 7 August 2023).
- Türkay, M. Personal Decapoda Distribution Database for Europe. In; 2015.
- Lagardère, J.-P. Les Crevettes de côtes du Maroc; Travaux de l’Institut scientifique chérifien et de la Faculté des sciences. Sér. Zoologie; 1971.
- Zariquiey Alvarez, R. Crustáceos Decápodos Ibéricos. 1968.
- Falciai, M. Guida Dei Crostacei Decapodi d’Europa; Scienze Naturali; Franco Muzzio Editore, 1992.
- Bacci, T.; Marusso, V.; Trabucco, B.; Magaletti, E. First Record of Synalpheus Tumidomanus Africanus (Crosnier & Forest, 1965) (Caridea, Alpheidae) in Italian Waters. Crustaceana 2010, 83. [Google Scholar] [CrossRef]
- Alvarez, R.Z. Crustaceos Decapodos Ibericos. Investigacion Pesquera 1968, 32, 1–499. [Google Scholar]
- Streftaris, A.Z. & E.P., N. Globalisation in Marine Ecosystems: The Story of Non-Indigenous Marine Species across European Seas. Oceanogry and Marine Biology: an Annual Review 2005, 43, 419–453. [Google Scholar]
- AA.VV. Report of Data Collected for Posidonia Oceanica Management and Transplant. Report Action B.3. LIFE SEPOSSO (LIFE16 GIE/IT/000761); 2020.
- Lardicci, C.; Abbiati, M.; Crema, R.; Morri, C.; Bianchi, C.N.; Castelli, A. The Distribution of Polychaetes along Environmental Gradients: An Exemple from the Orbitello Lagoon, Italy. Marine Ecology 1993, 14, 35–52. [Google Scholar] [CrossRef]
- Todorova, V. , Konsulova, T. Manual for Quantitative Sampling and Sample Treatment of Marine Soft-Bottom Macrozoobenthos 2005.
- Bacci, T.; Trabucco, B.; Marzialetti, S.; Marusso, V.; Lomiri, S.; Vani, D.; Lamberti, C.V. Taxonomic Sufficiency in Two Case Studies: Where Does It Work Better? Marine Ecology 2009, 30. [Google Scholar] [CrossRef]
- Eleftheriou, A. Methods for the Study of Marine Benthos - Fourth Edition; 2013; ISBN 978-0-470-67086-6.
- Carriker, M.R. The Crucial Role of Systematics in Assessing Pollution Effects on the Biological Utilization of Estuaries. Estuarine Pollution Control and Assessment, proceedings of conference 1976, 1, 487–506. [Google Scholar]
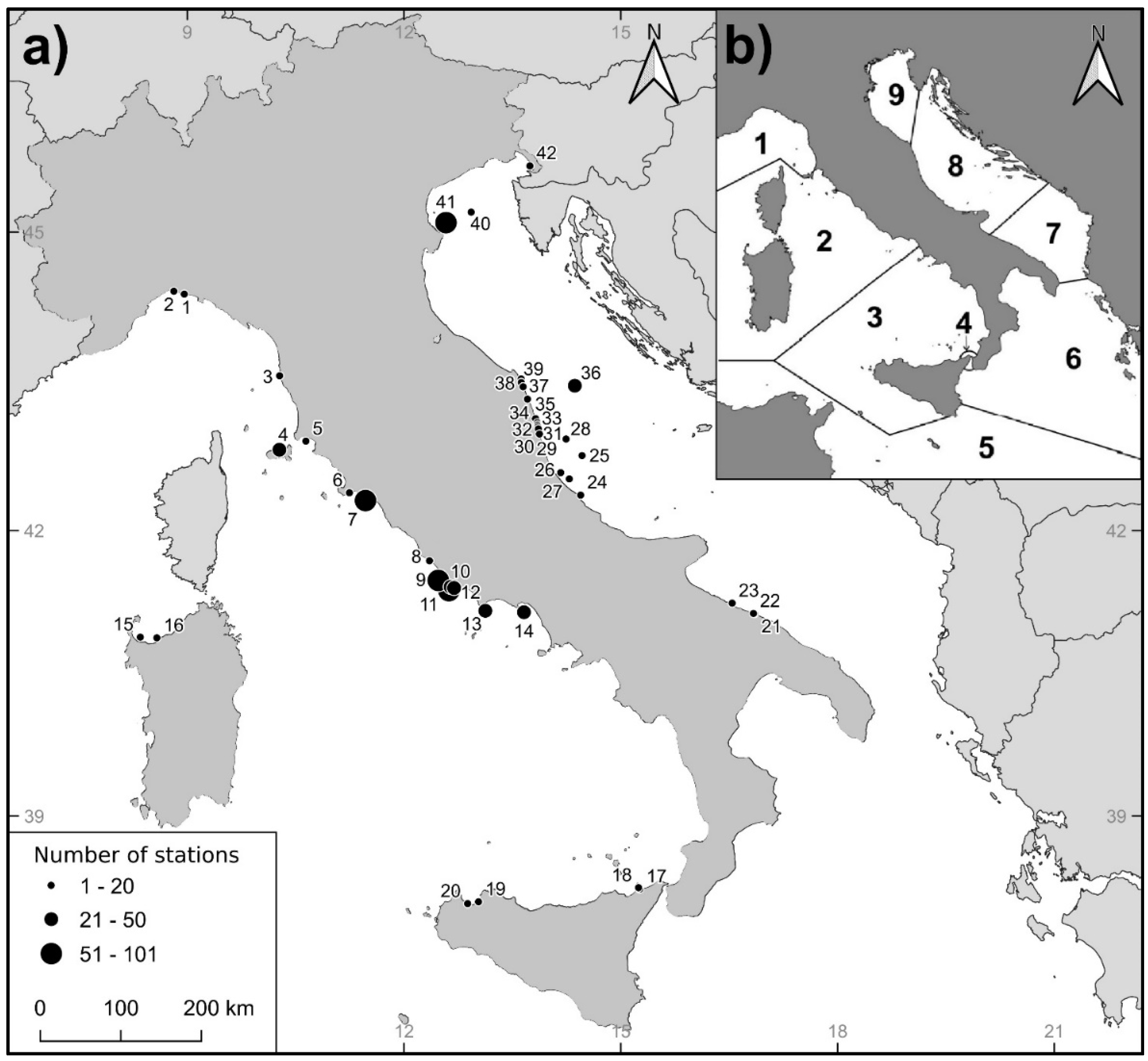
| Study areas | Sea | Biogeographic zone | Geographical coordinates | Number of stations | Depth ranges (m) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Latitude (N) | Longitude (E) | 0-10 | 10.1-20 | 20.1-30 | 30.1-50 | >50 | ||||
| 1. Genova #1 | Ligurian Sea | 1 | 44°23'16" | 08°57'34" | 3 | 0 | 3 | 0 | 0 | 0 |
| 2. Genova #2 | Ligurian Sea | 1 | 44°24'59" | 08°48'52" | 3 | 1 | 2 | 0 | 0 | 0 |
| 3. Livorno | Ligurian Sea | 1 | 43°34'25" | 10°16'44" | 3 | 2 | 1 | 0 | 0 | 0 |
| 4. Elba | N Tyrrhenian Sea | 2 | 42°49'36" | 10°16'31" | 41 | 1 | 2 | 3 | 16 | 19 |
| 5. Follonica | N Tyrrhenian Sea | 2 | 42°54'51" | 10°38'32" | 5 | 0 | 0 | 3 | 2 | 0 |
| 6. Porto Ercole | N Tyrrhenian Sea | 2 | 42°23'17" | 11°14'39" | 11 | 0 | 0 | 11 | 0 | 0 |
| 7. Montalto | C Tyrrhenian Sea | 2 | 42°18'29" | 11°28'01" | 101 | 0 | 2 | 8 | 67 | 24 |
| 8. Castel Porziano | C Tyrrhenian Sea | 2 | 41°41'21" | 12°21'10" | 12 | 3 | 3 | 3 | 3 | 0 |
| 9. Torvaianica | C Tyrrhenian Sea | 2 | 41°29'07" | 12°28'27" | 82 | 8 | 4 | 4 | 8 | 58 |
| 10. Nettuno | C Tyrrhenian Sea | 2 | 41°25'06" | 12°38'42" | 21 | 5 | 6 | 7 | 3 | 0 |
| 11. Anzio | C Tyrrhenian Sea | 2 | 41°22'45" | 12°37'17" | 87 | 0 | 0 | 0 | 80 | 7 |
| 12. Sabaudia | C Tyrrhenian Sea | 2 | 41°24'24" | 12°41'35" | 40 | 6 | 3 | 5 | 8 | 18 |
| 13. Terracina | C Tyrrhenian Sea | 2 | 41°10'12" | 13°07'32" | 50 | 9 | 3 | 2 | 13 | 23 |
| 14.Gaeta | C Tyrrhenian Sea | 2 | 41°09'18" | 13°39'37" | 33 | 0 | 4 | 2 | 10 | 17 |
| 15. Porto Torres | Sardinian Sea | 2 | 40°53'42" | 08°21'01" | 17 | 0 | 0 | 5 | 12 | 0 |
| 16. Castelsardo | Sardinian Sea | 2 | 40°53'16" | 08°34'45" | 9 | 0 | 0 | 2 | 7 | 0 |
| 17. Milazzo #1 | S Tyrrhenian Sea | 3 | 38°13'29" | 15°14'46" | 5 | 0 | 0 | 0 | 5 | 0 |
| 18. Milazzo #2 | S Tyrrhenian Sea | 3 | 38°12'38" | 15°15'49" | 8 | 4 | 2 | 1 | 1 | 0 |
| 19. Trappeto | S Tyrrhenian Sea | 3 | 38°04'16" | 13°01'54" | 14 | 0 | 0 | 14 | 0 | 0 |
| 20. Castellammare del Golfo | S Tyrrhenian Sea | 3 | 38°03'04" | 12°52'51" | 14 | 0 | 0 | 0 | 14 | 0 |
| 21. Bari #1 | S Adriatic Sea | 7 | 41°08'28" | 16°51'08" | 3 | 1 | 2 | 0 | 0 | 0 |
| 22. Bari #2 | S Adriatic Sea | 7 | 41°08'33" | 16°50'11" | 7 | 2 | 4 | 1 | 0 | 0 |
| 23. Bisceglie | S Adriatic Sea | 7 | 41°14'59" | 16°32'32" | 11 | 0 | 0 | 11 | 0 | 0 |
| 24. Ortona | C Adriatic Sea | 8 | 42°21'49" | 14°26'41" | 5 | 0 | 0 | 0 | 5 | 0 |
| 25. Pescara #1 | C Adriatic Sea | 8 | 42°46'02" | 14°27'50" | 12 | 0 | 0 | 0 | 0 | 12 |
| 26. Pescara #2 | C Adriatic Sea | 8 | 42°35'36" | 14°10'07" | 12 | 0 | 12 | 0 | 0 | 0 |
| 27. Pescara #3 | C Adriatic Sea | 8 | 42°31’46" | 14°17'17" | 15 | 0 | 0 | 6 | 5 | 4 |
| 28. S. Benedetto del Tronto | C Adriatic Sea | 8 | 42°56'05" | 14°14'36" | 10 | 0 | 0 | 0 | 0 | 10 |
| 29. Grottammare | C Adriatic Sea | 8 | 42°59'11" | 13°52'30" | 8 | 8 | 0 | 0 | 0 | 0 |
| 30. Cupramarittima | C Adriatic Sea | 8 | 43°02'16" | 13°51'36" | 8 | 8 | 0 | 0 | 0 | 0 |
| 31. Massignano | C Adriatic Sea | 8 | 43°03'29" | 13°51'13" | 4 | 4 | 0 | 0 | 0 | 0 |
| 32. Campofilone | C Adriatic Sea | 8 | 43°04'40" | 13°50'57" | 4 | 4 | 0 | 0 | 0 | 0 |
| 33. Pedaso | C Adriatic Sea | 8 | 43°06'04" | 13°50'40" | 6 | 6 | 0 | 0 | 0 | 0 |
| 34. Fermo | C Adriatic Sea | 8 | 43°08'30" | 13°49'15" | 8 | 8 | 0 | 0 | 0 | 0 |
| 35. Civitanova Marche #1 | C Adriatic Sea | 8 | 43°20'20" | 13°42'33" | 8 | 8 | 0 | 0 | 0 | 0 |
| 36. Civitanova Marche #2 | C Adriatic Sea | 8 | 43°28'27" | 14°21'51" | 36 | 0 | 0 | 0 | 0 | 36 |
| 37. Porto Recanati | C Adriatic Sea | 8 | 43°27'47" | 13°38'54" | 14 | 14 | 0 | 0 | 0 | 0 |
| 38. Numana | C Adriatic Sea | 8 | 43°30'23" | 13°37'22" | 10 | 10 | 0 | 0 | 0 | 0 |
| 39. Sirolo | C Adriatic Sea | 8 | 43°32'33" | 13°37'17" | 6 | 6 | 0 | 0 | 0 | 0 |
| 40. Chioggia #1 | N Adriatic Sea | 9 | 45°11'37" | 12°55'48" | 18 | 0 | 0 | 4 | 14 | 0 |
| 41. Chioggia #2 | N Adriatic Sea | 9 | 45°05'28" | 12°34'53" | 71 | 15 | 5 | 51 | 0 | 0 |
| 42. Trieste | N Adriatic Sea | 9 | 45°38'35" | 13°44'33" | 10 | 2 | 8 | 0 | 0 | 0 |
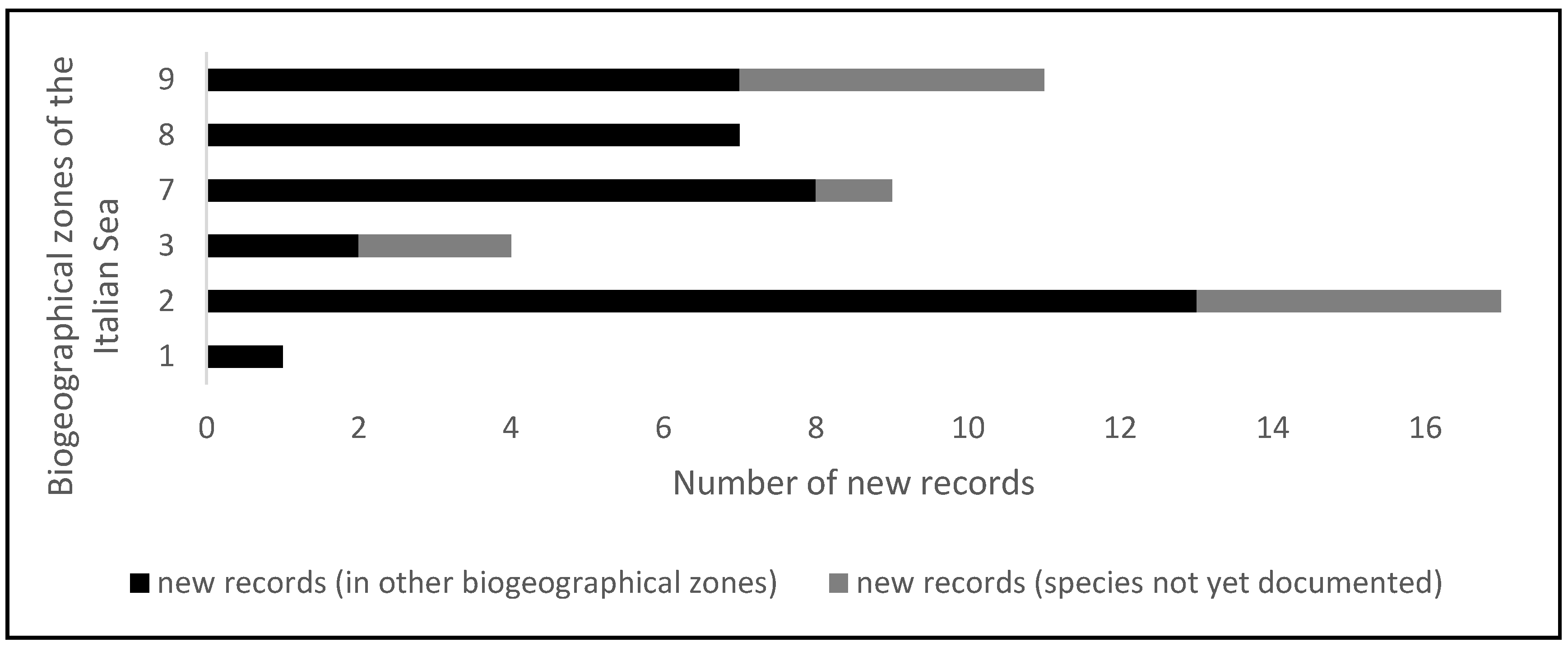
| Species | Biogeographical Zones of Italian Seas | Remarques | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| Parapenaeus longirostris (Lucas, 1846) | + | + | + | + | + | + | + | + | ||
| Sicyonia carinata (Brünnich, 1768) | + | + | + | + | + | + | + | + | + | |
| Solenocera membranacea (Risso, 1816) | + | + | + | + | + | + | + | + | + | |
| Richardina fredericii Lo Bianco, 1903 | + | + | + | + | ||||||
| Balssia gasti (Balss, 1921) | + | + | + | + | + | + | ||||
| Palaemon adspersus Rathke, 1836 | + | + | + | + | + | + | + | + | ||
| Alpheus glaber (Olivi, 1792) | + | + | + | + | + | + | + | + | + | |
| Alpheus dentipes Guérin, 1832 | + | + | + | + | + | + | + | + | + | |
| Athanas nitescens (Leach, 1814) | + | + | + | + | + | + | + | + | + | |
| Athanas nitescens var. laevirhincus (Risso, 1816) | + | |||||||||
| Synalpheus gambarelloides (Nardo, 1847) | + | + | + | + | + | + | + | + | ||
| Synalpheus africanus Crosnier & Forest, 1965 | + | |||||||||
| Eualus occultus (Lebour, 1936) | + | + | + | + | + | + | ||||
| Eualus pusiolus (Krøyer, 1841) | + | + | + | |||||||
| Eualus cranchii (Leach, 1817) | + | + | + | + | + | + | + | + | + | A1 |
| Hippolyte leptocerus (Heller, 1863) | + | + | + | + | + | + | + | |||
| Hippolyte inermis Leach, 1816 | + | + | + | + | + | + | + | + | + | |
| Chlorotocus crassicornis (A. Costa, 1871) | + | + | + | + | + | + | + | + | ||
| Processa acutirostris Nouvel & Holthuis, 1957 | + | + | + | + | + | + | + | + | + | |
| Processa canaliculata Leach, 1815 | + | + | + | + | + | + | + | |||
| Processa edulis (Risso, 1816) | + | + | + | + | + | + | + | + | + | A2 |
| Processa elegantula Nouvel & Holthuis, 1957 | + | + | + | + | + | + | ||||
| Processa intermedia Holthuis, 1951 | + | |||||||||
| Processa macrophthalma Nouvel & Holthuis, 1957 | + | + | + | + | + | + | + | + | + | |
| Processa modica carolii Williamson in Williamson & Rochanaburanon, 1979 | + | + | + | + | + | + | + | |||
| Processa nouveli nouveli Al-Adhub & Williamson, 1975 | + | + | + | + | + | + | + | |||
| Aegaeon cataphractus (Olivi, 1792) | + | + | + | + | + | + | ||||
| Crangon allmanni Kinahan, 1860 | + | + | ||||||||
| Crangon crangon (Linnaeus, 1758) | + | + | + | + | ||||||
| Philocheras monacanthus (Holthuis, 1961) | + | + | + | + | + | + | ||||
| Philocheras bispinosus (Hailstone, 1835) | + | + | + | + | + | |||||
| Philocheras sculptus (Bell, 1847) | + | + | + | + | + | |||||
| Philocheras trispinosus (Hailstone in Hailstone & Westwood, 1835) | + | + | + | + | + | |||||
| Calocaris macandreae Bell, 1846 | + | + | + | + | + | + | + | + | ||
| Callianassa subterranea (Montagu, 1808) | + | + | + | + | + | + | ||||
| Necallianassa acanthura (Caroli, 1946) | + | + | + | + | ||||||
| Necallianassa truncata (Giard & Bonnier, 1890) | + | + | + | + | + | |||||
| Gilvossius tyrrhenus (Petagna, 1792) | + | + | + | + | A3 | |||||
| Gourretia denticulata (Lutze, 1937) | + | + | + | + | + | + | + | |||
| Jaxea nocturna Nardo, 1847 | + | + | + | + | + | + | ||||
| Upogebia deltaura (Leach, 1816) | + | + | + | + | + | + | ||||
| Upogebia mediterranea Noël, 1992 | + | + | + | + | + | + | + | |||
| Upogebia pusilla (Petagna, 1792) | + | + | + | + | + | + | + | + | + | |
| Upogebia stellata (Montagu, 1808) | + | + | + | |||||||
| Upogebia tipica (Nardo, 1869) | + | + | + | + | + | + | + | |||
| Scyllarides latus (Latreille, 1803) | + | + | + | + | + | + | + | + | ||
| Calcinus tubularis (Linnaeus, 1767) | + | + | + | + | + | + | + | |||
| Dardanus arrosor (Herbst, 1796) | + | + | + | + | + | + | + | + | + | |
| Dardanus calidus (Risso, 1827) | + | + | + | + | + | + | + | |||
| Diogenes pugilator (Roux, 1829) | + | + | + | + | + | + | + | + | + | |
| Paguristes eremita (Linnaeus, 1767) | + | + | + | + | + | + | + | + | + | |
| Paguristes syrtensis de Saint Laurent, 1971 | + | + | + | + | ||||||
| Anapagurus bicorniger A. Milne-Edwards & Bouvier, 1892 | + | + | + | + | + | + | + | |||
| Anapagurus breviaculeatus Fenizia, 1937 | + | + | + | + | + | + | + | + | ||
| Anapagurus chiroacanthus (Liljeborg, 1856) | + | + | + | + | + | + | + | |||
| Anapagurus laevis (Bell, 1845) | + | + | + | + | + | + | + | + | + | |
| Anapagurus petiti Dechancé & Forest, 1962 | + | + | + | + | + | + | + | + | ||
| Anapagurus smythi Ingle, 1993 | + | + | ||||||||
| Cestopagurus timidus (Roux, 1830) | + | + | + | + | + | + | + | + | + | |
| Pagurus alatus Fabricius, 1775 | + | + | + | + | + | + | + | + | ||
| Pagurus anachoretus Risso, 1827 | + | + | + | + | + | + | + | + | + | |
| Pagurus chevreuxi (Bouvier, 1896) | + | + | + | + | ||||||
| Pagurus cuanensis Bell, 1845 | + | + | + | + | + | + | + | + | + | |
| Pagurus excavatus (Herbst, 1791) | + | + | + | + | + | + | + | + | + | |
| Pagurus forbesii Bell, 1845 | + | + | + | + | + | + | ||||
| Pagurus prideaux Leach, 1815 | + | + | + | + | + | + | + | + | + | |
| Galathea bolivari Zariquiey Álvarez, 1950 | + | + | + | + | + | + | ||||
| Galathea cenarroi Zariquiey Álvarez, 1968 | + | + | + | + | + | + | ||||
| Galathea dispersa Spence Bate, 1859 | + | + | + | + | + | + | + | + | ||
| Galathea intermedia Lilljeborg, 1851 | + | + | + | + | + | + | + | + | ||
| Galathea strigosa (Linnaeus, 1761) | + | + | + | + | + | + | + | |||
| Dactylonida curvimana (A. Milne Edwards & Bouvier, 1894) | + | + | + | A4 | ||||||
| Munida intermedia A. Milne-Edwards & Bouvier, 1899 | + | + | + | + | + | + | ||||
| Iridonita speciosa von Martens, 1878 | + | + | + | + | A5 | |||||
| Pisidia longicornis (Linnaeus, 1767) | + | + | + | + | + | + | ||||
| Pisidia bluteli (Risso, 1816) | + | + | + | + | + | + | + | + | + | |
| Ethusa mascarone (Herbst, 1785) | + | + | + | + | + | + | + | + | + | |
| Medorippe lanata (Linnaeus, 1767) | + | + | + | + | + | + | + | + | + | |
| Ilia nucleus (Linnaeus, 1758) | + | + | + | + | + | + | + | + | + | |
| Ebalia cranchii Leach, 1817 | + | + | + | + | + | |||||
| Ebalia deshayesi Lucas, 1846 | + | + | + | + | + | + | ||||
| Ebalia tuberosa (Pennant, 1777) | + | + | + | + | + | + | + | + | ||
| Ebalia edwardsii O.G. Costa, 1838 | + | + | + | + | + | + | + | + | ||
| Ebalia granulosa H. Milne Edwards, 1837 | + | + | + | + | + | + | ||||
| Ebalia tumefacta (Montagu, 1808) | + | + | ||||||||
| Achaeus cranchii Leach, 1817 | + | + | + | + | + | + | + | + | ||
| Achaeus gracilis (O.G. Costa, 1839) | + | + | + | + | + | + | + | + | ||
| Macropodia linaresi Forest & Zariquiey Álvarez, 1964 | + | + | + | + | + | + | ||||
| Macropodia rostrata (Linnaeus, 1761) | + | + | + | + | + | + | + | + | + | |
| Inachus dorsettensis (Pennant, 1777) | + | + | + | + | + | + | + | + | + | |
| Inachus parvirostris (Risso, 1816) | + | + | + | + | + | + | + | |||
| Eurynome aspera (Pennant, 1777) | + | + | + | + | + | + | + | + | + | |
| Pisa muscosa (Linnaeus, 1758) | + | + | + | + | + | + | + | + | ||
| Pisa hirticornis (Herbst, 1804) | + | + | + | + | + | + | + | |||
| Derilambrus angulifrons (Latreille, 1825) | + | + | + | + | + | + | + | + | + | |
| Parthenopoides massena (Roux, 1830) | + | + | + | + | + | + | + | + | + | |
| Spinolambrus macrochelos (Herbst, 1790) | + | + | + | + | + | + | + | |||
| Atelecyclus rotundatus (Olivi, 1792) | + | + | + | + | + | + | + | + | ||
| Atelecyclus undecimdentatus (Herbst, 1783) | + | + | ||||||||
| Corystes cassivelaunus (Pennant, 1777) | + | + | + | + | ||||||
| Primela denticulata (Montagu, 1808) | + | + | + | + | + | + | + | + | ||
| Sirpus zariquieyi Gordon, 1953 | + | + | + | + | + | + | + | + | ||
| Thia scutellata (Fabricius, 1793) | + | + | + | + | ||||||
| Bathynectes longipes (Risso, 1816) | + | + | + | + | + | + | + | + | ||
| Liocarcinus bolivari (Zariquiey Álvarez, 1948) | + | + | + | + | + | |||||
| Liocarcinus corrugatus (Pennant, 1777) | + | + | + | + | + | + | + | + | + | |
| Liocarcinus depurator (Linnaeus, 1758) | + | + | + | + | + | + | + | + | + | |
| Liocarcinus maculatus (Risso, 1827) | + | + | + | + | + | + | + | + | + | |
| Liocarcinus navigator (Herbst, 1794) | + | + | + | + | + | + | + | + | + | |
| Liocarcinus vernalis (Risso, 1827) | + | + | + | + | + | + | + | + | ||
| Liocarcinus zariquieyi (Gordon, 1968) | + | + | + | + | + | + | + | |||
| Goneplax rhomboides (Linnaeus, 1758) | + | + | + | + | + | + | + | + | + | |
| Eriphia verrucosa (Forskål, 1775) | + | + | + | + | + | + | + | + | + | |
| Monodaeus couchii (Couch, 1851) | + | + | + | + | + | + | + | |||
| Xantho pilipes A. Milne-Edwards, 1867 | + | + | + | + | + | + | + | + | + | |
| Pilumnus hirtellus (Linnaeus, 1761) | + | + | + | + | + | + | + | + | + | |
| Pilumnus minutus De Haan, 1835 | + | |||||||||
| Pilumnus spinifer H. Milne Edwards, 1834 | + | + | + | + | + | + | + | + | ||
| Brachynotus gemmellari (Rizza, 1839) | + | + | + | + | + | + | + | |||
| Brachynotus sexdentatus (Risso, 1827) | + | + | + | + | + | + | + | |||
| Nepinnotheres pinnotheres (Linnaeus, 1758) | + | + | + | + | + | + | + | + | ||
| Pinnotheres pisum (Linnaeus, 1767) | + | + | + | + | + | + | + | |||
| Palicus caronii (Roux, 1830) | + | + | + | + | + | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




