Submitted:
07 September 2023
Posted:
12 September 2023
You are already at the latest version
Abstract
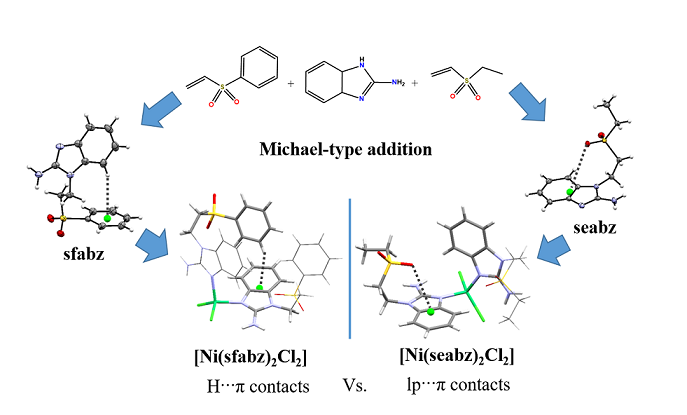
Keywords:
1. Introduction
2. Experimental
2.1. Materials and methods
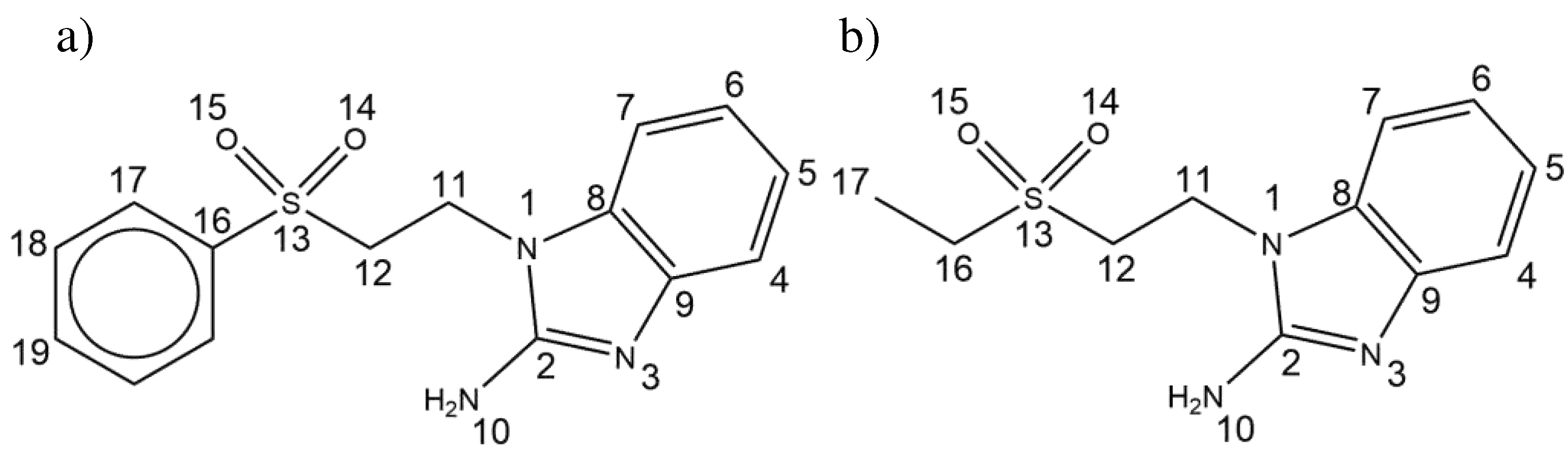
2.2. Synthesis of the ligands.
2.2.1. Synthesis of 2-amino-1-(2-phenylsulfonyl)ethylbenzimidazole (sfabz)
2.2.2. Synthesis of 2-amino-1-(2-ethylsulfonyl)ethylbenzimidazole (seabz)
2.3. Synthesis of the coordination compounds
2.3.1. [Ni(sfabz)2Cl2] (1)
2.3.2. [Ni(sfabz)2Br2] (2)
2.3.3. [Ni(seabz)2Cl2] (3)
2.3.4. [Ni(seabz)2Br2] (4)
2.3.5. [Cu(sfabz)2Cl2] (5)
2.3.6. [Cu(sfabz)2Br2] (6)
2.3.7. [Cu(seabz)2Cl2] (7)
2.3.8. [Cu(seabz)2Br2] (8)
2.3.9. [Zn(sfabz)2Cl2] (9)
2.3.10. [Zn(sfabz)2Br2] (10)
2.3.11. [Zn(seabz)2Cl2] (11)
2.3.12. [Zn(seabz)2Br2] (12)
2.3.13. [Cd(sfabz)2Cl2] (13)
2.3.14. [Cd(seabz)2Cl2] (14)
2.3.15. [Hg(sfabz)2Cl2] (15)
2.3.16. [Hg(seabz)2Cl2] (16)
2.4. Physical Measurements
2.5. Solution studies
2.6. X-ray Crystallography
2.7. Cell growth inhibition
2.7.1. Cell culture
2.7.2. In vitro growth inhibition assay
3. Results and Discussion
3.1. Spectroscopic Characterization and magnetic susceptibility
3.1.1. IR spectra.
3.1.2. Electronic spectroscopy and magnetic susceptibility.
3.1.3. NMR studies.
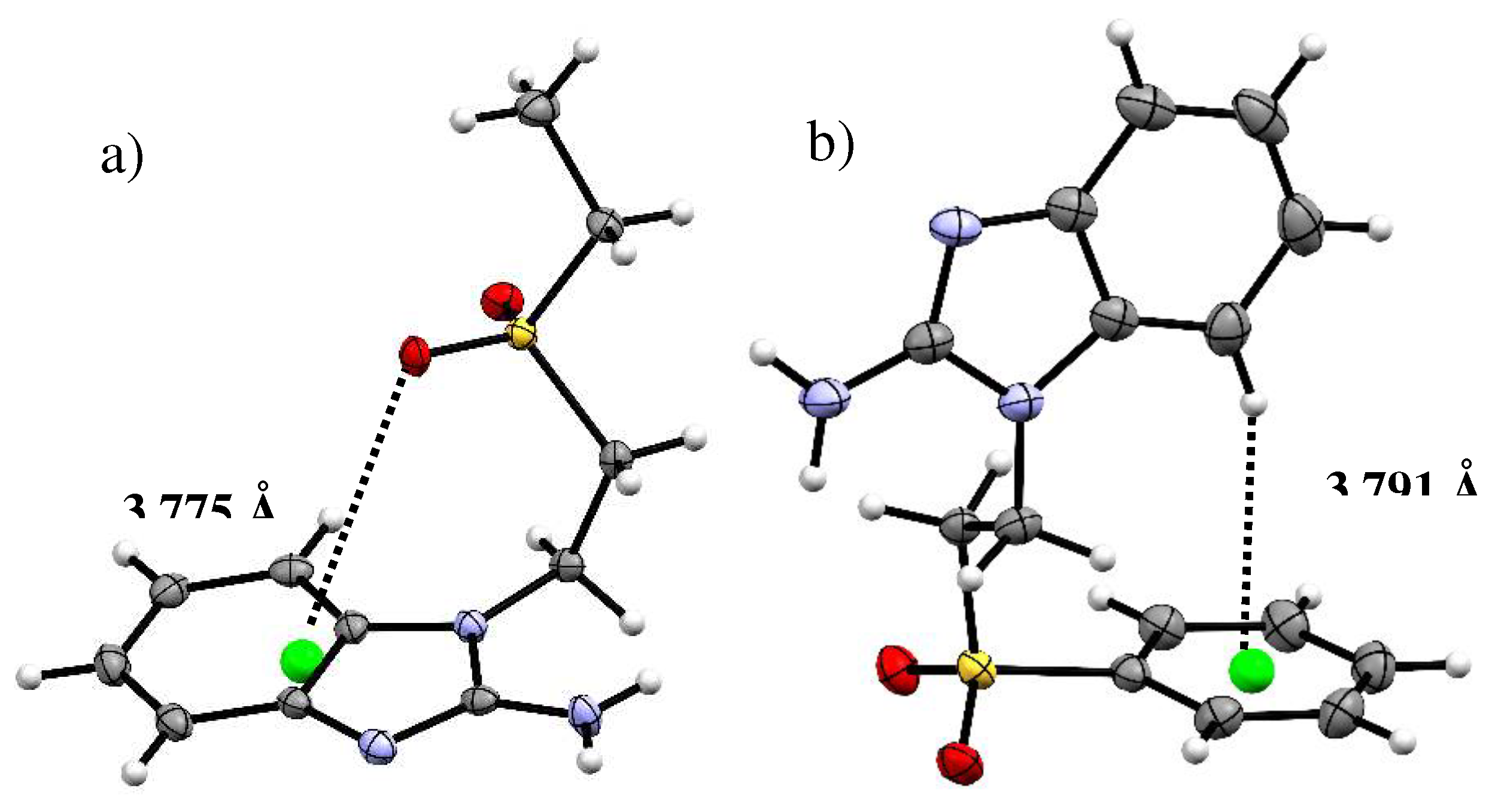
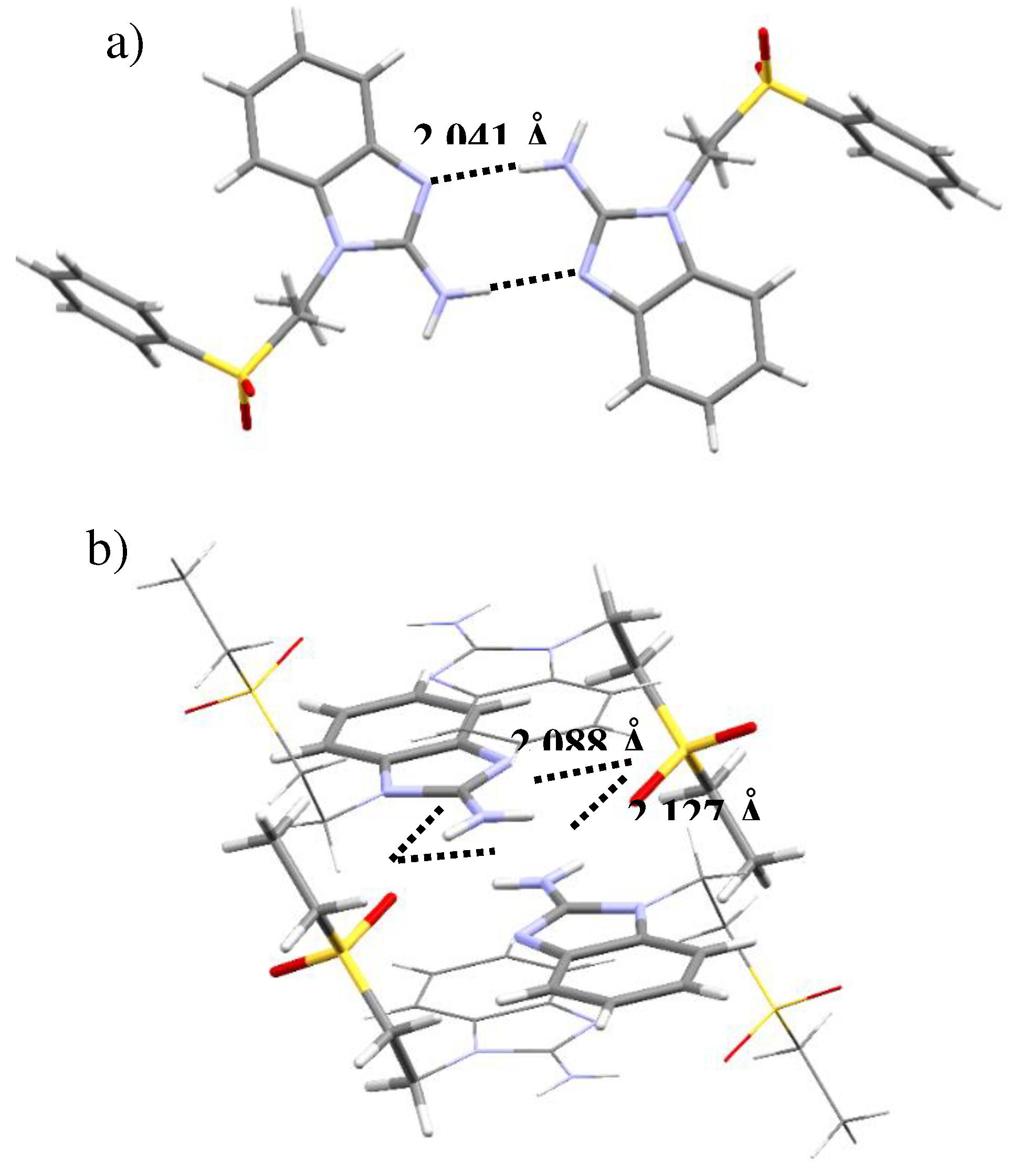
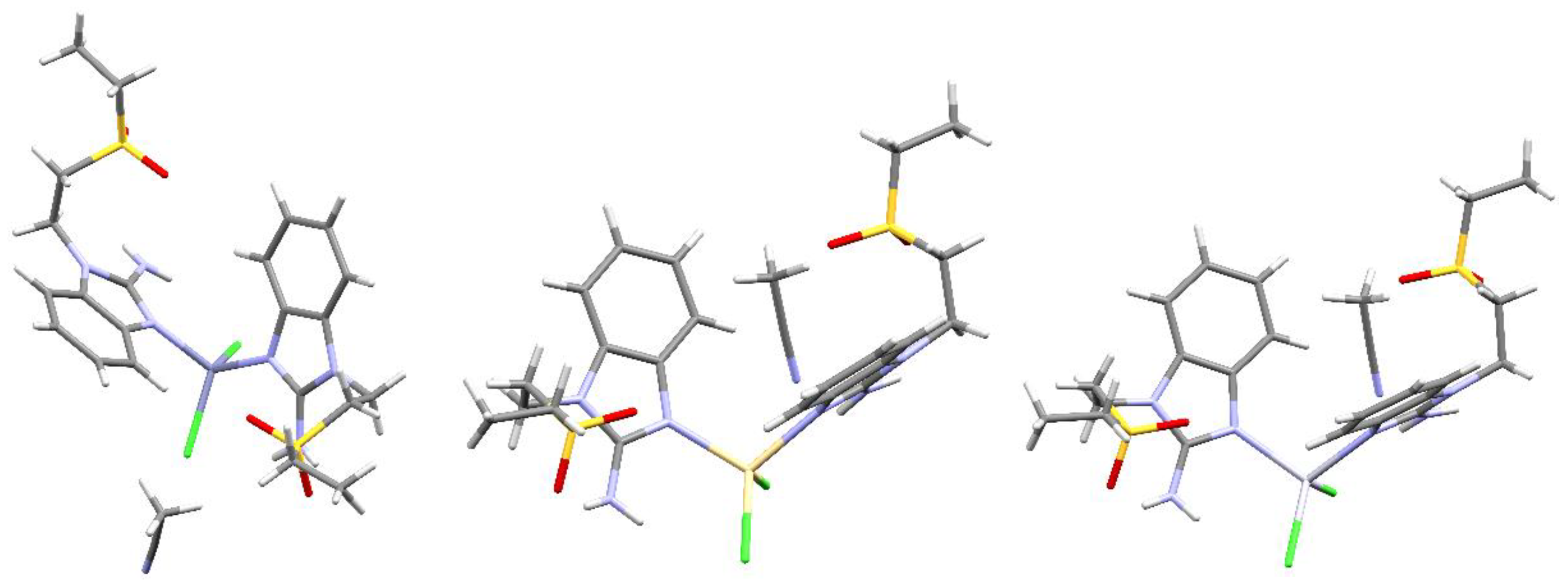
3.2. X-ray structures of the ligands and their coordination compounds
3.2.1. Crystal structure of the 2-aminobenzimidazolic ligands.
3.2.2. Crystal structure of the coordination compounds of seabz and sfabz.
3.3. Stability in solution and antiproliferative activity.
Conclusions
Supplementary Materials
Author Contributions
Data Availability Statement
Acknowledgements
Conflict of interest
Supplementary data
References
- R. A. Alderden, M. D. Hall, T. W. Hambley, J. Chem. Educ., 83 (2006), 728 –734.
- Y. Wang, X. Wang, J. Wang, Y. Zhao, W. He, Z. Guo, Inorg. Chem., 50 (2011), 12661-12668.
- Y. Qu, R. G. Kipping, N. P. Farrell, Dalton Trans., 44 (2015), 3563-3572.
- R. P. Miller, R. K. Tadagavadi, G. Ramesh, W. B. Reeves, Toxins, 2 (2010), 2490–2518.
- G. Laurell, U. Jungnelius, Laryngoscope, 100 (1990), 724–734.
- P.C.A. Bruijnincx, P.J. Sadler, Curr. Opin. Chem. Biol., 12 (2008), 197–206.
- D. Hernández-Romero, S. Rosete-Luna, A. López-Monteon, A. Chávez-Piña, N. Pérez-Hernández, J. Marroquín-Flores, A. Cruz-Navarro, G. Pesado-Gómez, D. Morales-Morales, R. Colorado-Peralta, Coord. Chem. Rev., 439 (2021), 213930-213981.
- A. Erxleben, Coord. Chem. Rev. 360 (2018), 92-121.
- Erxleben, Chimia, 71 (2017) 102-111.
- M.A. Zoroddu, J. Aaseth, G. Crisponi, S. Medici, M. Peana, V.M. Nurchi, J. Inorg. Biochem., 195 (2019), 120-129.
- B.J. Pages, D.L. Ang, E.P. Wright, J.R. Aldrich-Wright, Dalton Trans. 44 (2015), 3505-3526.
- P. Zhou, J. Huang, F. Tian, Curr. Med. Chem., 19 (2012), 226-238.
- J.P. Lu, X.H. Yuan, H. Yuan, W.L. Wang, B. Wan, S.G. Franzblau, Q.Z. Ye, Chem. Med. Chem., 6 (2011), 1041-1048.
- J.P. Lu, X.H. Yuan, Q.Z. Ye, Eur. Jour. Med. Chem., 47 (2012), 479-484.
- S.U. Rehman, T. Sarwar, M.A. Husain, H.M. Ishqi, M. Tabish, Arch. Biochem. Biophys., 576 (2015), 49-60.
- S. Burge, G.N. Parkinson, P. Hazel, A.K. Todd, S. Neidle, Nucleic Acids Res., 34(19) (2006), 5402-5415.
- J.E. Reed, A.A. Arnal, S. Neidle, R. Vilar, J. Am. Chem. Soc., 128(18) (2016), 5992-5993.
- L. Sabater, P.J. Fang, C.F. Chang, A. De Rache, E. Prado, J. Dejeu, A. Garofalo, J.H. Lin, J.L. Mergny, E. Defrancq, G. Pratviel, Dalton Trans., 44 (2015), 3701-3707.
- V.S. Stafford, K. Suntharalingam, A. Shivalingam, A.J.P. White, D.J. Mann, R. Vilar, Dalton Trans., 44 (2015), 3686-3700.
- P. Gratteri, L. Massari, E. Michelucci, R. Rigo, L. Messori, M.A. Cinellu, C. Musetti, C. Sissi, C. Bazzicalupi, Dalton Trans., 44 (2015), 3633-3639.
- J. Malina, N.P. Farrell, V. Brabec, Inorg. Chem., 53(3) (2014), 1662-1671.
- R. Navarro-Peñaloza, B. Landeros-Rivera, H. López-Sandoval, R. Castro-Ramírez, N. Barba-Behrens, Coord. Chem. Rev., 494 (2023), 215360.
- I. Alfaro-Fuentes, H. López-Sandoval, E. Mijangos, A.M. Duarte-Hernández, G. Rodríguez-López, M.I. Bernal-Uruchurtu, R. Contreras, A. Flores-Parra, N. Barba-Behrens, Polyhedron, 67 (2014), 373-380.
- I. Alfaro-Fuentes, R. Castro-Ramírez, N. Ortiz-Pastrana, R.M. Medina-Guerrero, L.C. Soler-Jiménez, I. Martínez-Rodríguez, M. Bethancourt-Lozano, L. Ibarra-Castro, N. Barba-Behrens, E.J. Fajer-Ávila, J. Inorg. Biochem., 176 (2017), 159-167.
- R. Castro-Ramírez, N. Ortiz-Pastrana, A.B. Caballero, M.T. Zimmermann, B.S. Stadelman, A.A.E. Gaertner, J.L. Brumaghim, L. Korrodi-Gregório, R. Pérez-Tomás, P. Gamez, N. Barba-Behrens, Dalton Trans., 47 (2018), 7551-7560.
- L.G. Ramírez-Palma, R. Castro-Ramírez, L. Lozano-Ramos, R. Galindo-Murillo, N. Barba-Behrens, F. Cortés-Guzmán, Dalton Trans., 52 (2023), 2087–2095.
- S. Betanzos-Lara, C. Gómez-Ruiz, L.R. Barrón-Sosa, I. Gracia-Mora, M. Flores-Álamo, N. Barba-Behrens, J. Inorg. Biochem., 114 (2012), 82-93.
- S. Betanzos-Lara, N. P. Chmel, M. T. Zimmerman, L. R. Barron-Sosa, L. Salassa, A. Rodger, J. Brumaghim, I. Gracia-Mora and N. Barba-Behrens, Dalton Trans., 44(2015), 3673-3685.
- X.J. Zhao, J. Li, B. Ding, X.G. Wang, E.C. Yang, Inorg. Chem. Comm., 30 (2007), 605-609.
- A. Esparza-Ruiz, A. Peña-Hueso, E. Mijangos, G. Osorio-Monreal, H. Nöth, A. Flores-Parra, R. Contreras, N. Barba-Behrens, Polyhedron, 30 (2011), 2090-2098.
- G. Durán-Solares, W. Fugarolas-Gómez, N. Ortíz-Pastrana, H. López-Sandoval, T. O. Villaseñor-Granados, A. Flores-Parra, P. J. Altmann, N. Barba-Behrens, J. Coord. Chem., 71 (2018), 1953-1958.
- G.M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 64 (2008), 112.
- R.C. Clark and J. S. Reid, Acta Crystallogr., Sect. A: Found. Crystallogr., 51 (1995), 887.
- C.B. Hübschle, G.M. Sheldrick, B. Dittrich, J. Appl. Crystallogr., 44 (2011), 1281–1284.
- E.A. Orellana, A. L. Kasinski, Bio-protocol, 6.21 (2016), e1984-e1984.
- S. Sudha, M. Karabacak, M. Kurt, M. Cinar, N. Sundaraganesan, Spectrochim. Acta - Part A Mol. Biomol. Spectrosc., 84 (2011), 184–195.
- A.B.P., Lever, J. Chem. Ed., 45(11) (1968), 711-712.
- G. Gliemann, Y. Wang, The Ligand Field Concept, 3rd. ed., Encyclopedia of Physical Science and Technology, 2003, pp. 523–538.
- A.B.P. Lever, Inorganic Electronic Spectroscopy, Elsevier, Amsterdam, 1984.
- R. Navarro-Peñaloza, A.B. Vázquez-Palma, H. López-Sandoval, F. Sánchez-Bartéz, I. Gracia-Mora, N. Barba-Behrens, J. Inorg. Biochem., 219 (2021), 111432.
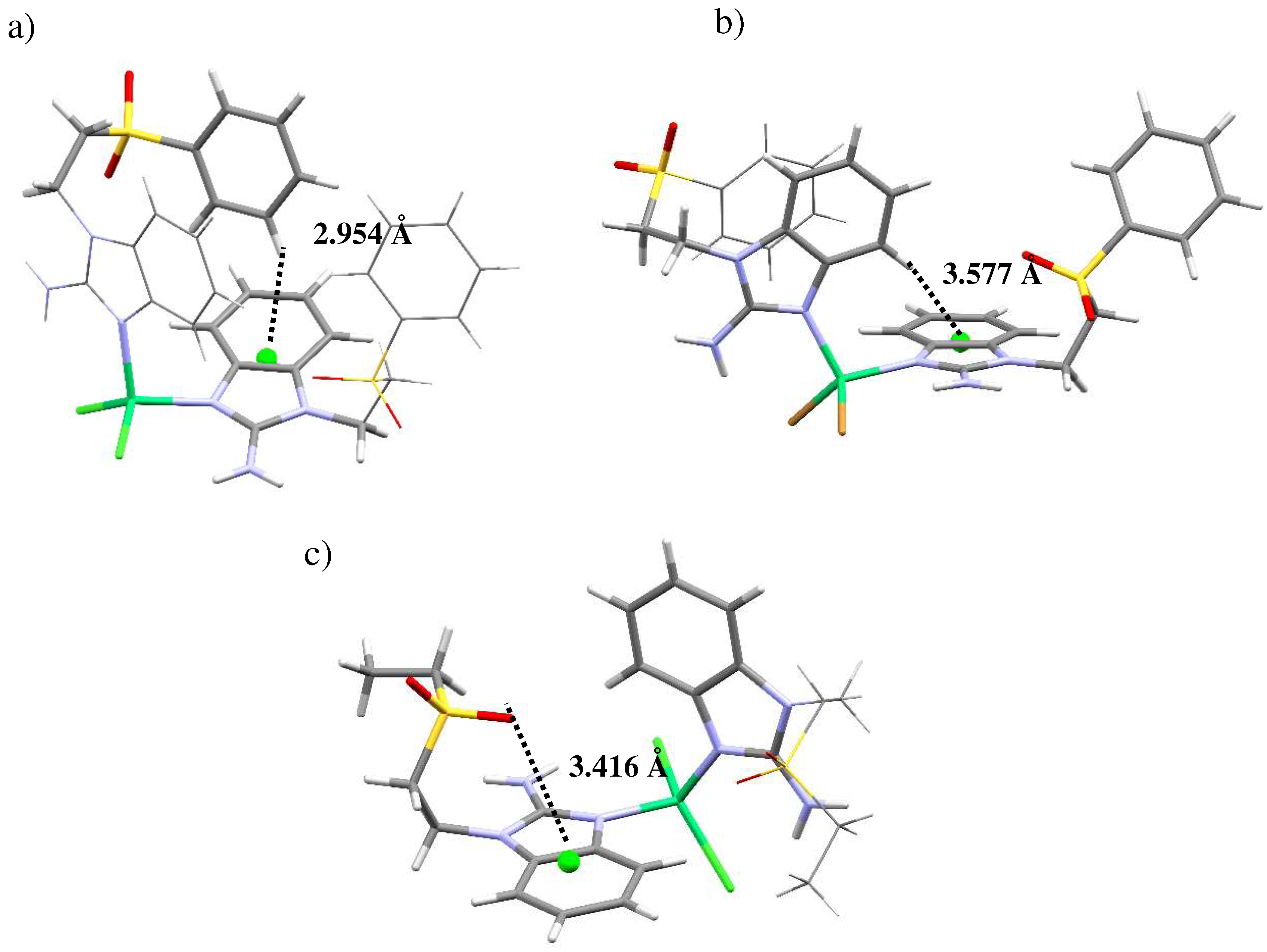
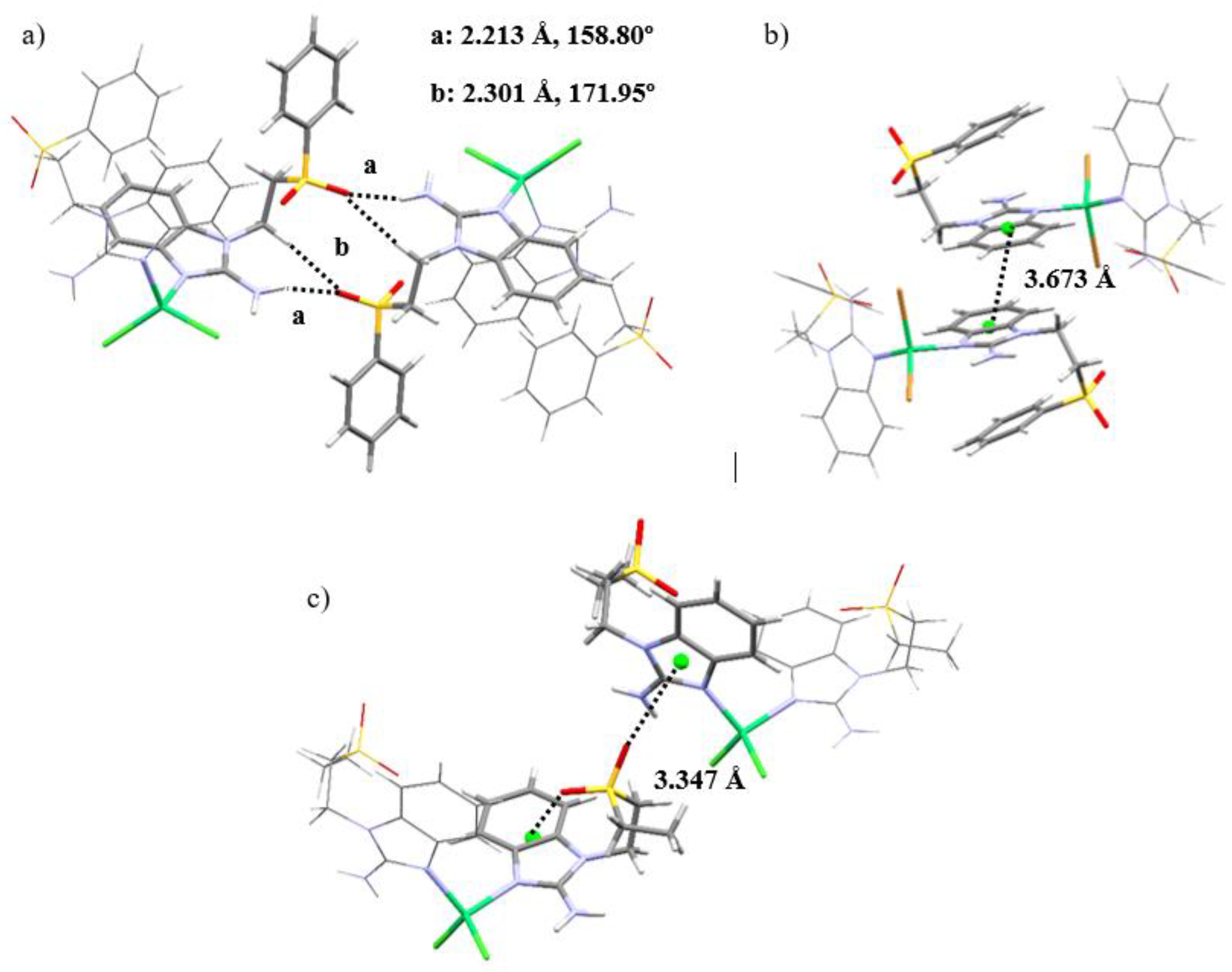
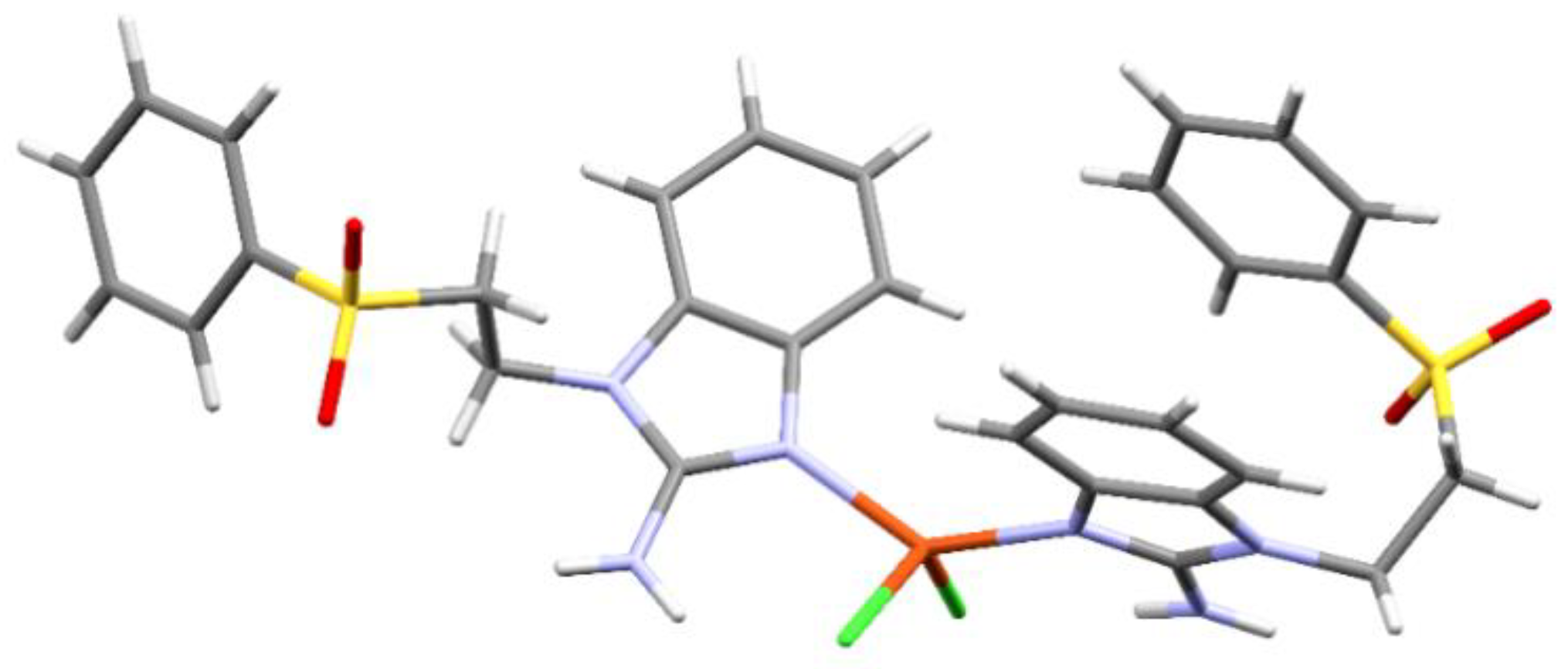
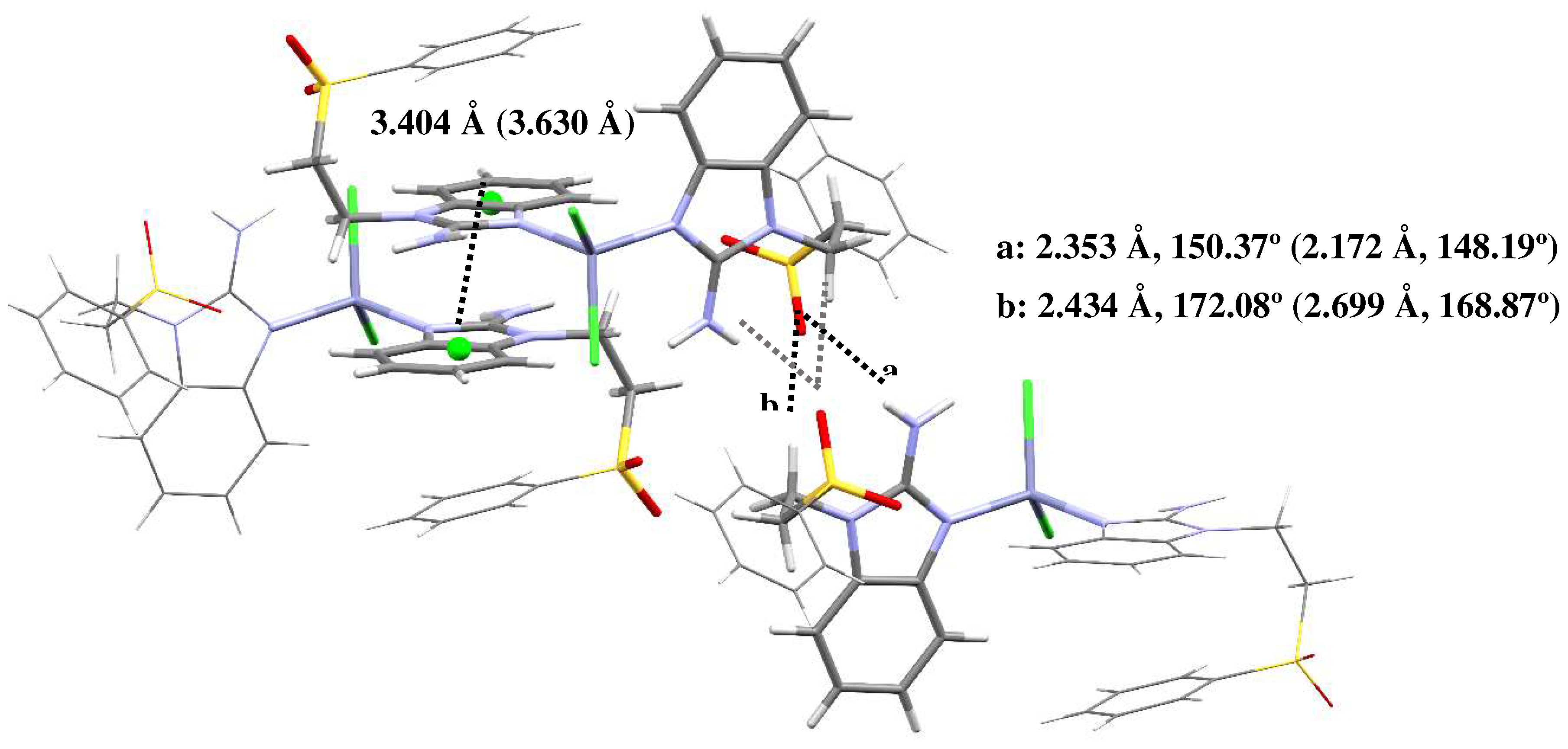
| Compound | ν1: 4T2(F)←4A2 | ν2: 4T1(F)←4A2 | ν3: 4T1(P)←4A2 | μeff (B.M.) |
| [Ni(sfabz)2Cl2] (1) | 5241 cm-1 | 9257 cm-1 | 16993 cm-1 | 3.85 |
| [Ni(sfabz)2Br2] (2) | 5143 cm-1 | 9778 cm-1 | 16135 cm-1 | 3.91 |
| [Ni(seabz)2Cl2] (3) | 5423 cm-1 | 10250 cm-1 | 16690 cm-1 | 3.60 |
| [Ni(seabz)2Br2] (4) | 5312 cm-1 | 10096 cm-1 | 16454 cm-1 | 3.64 |
| Compound | ν1: T2←Esolid state | ν1: T2←EDMSO solution | --- | μeff (B.M.) |
| [Cu(sfabz)2Cl2] (5) | 11000 cm-1 | 11049 cm-1 (905 nm) | --- | 1.88 |
| [Cu(sfabz)2Br2] (6) | 8670 cm-1 | 11481 cm-1 (871 nm) | --- | 1.91 |
| [Cu(seabz)2Cl2] (7) | 9506 cm-1 | 10989 cm-1 (910 nm) | --- | 2.15 |
| [Cu(seabz)2Br2] (8) | 8526 cm-1 | 11521 cm-1(868 nm) | --- | 2.16 |
| Position | sfabz series (Zn/Cd/Hg) | seabz series (Zn/Cd/Hg) | ||
| Δδ (ppm) | Effect | Δδ (ppm) | Effect | |
| H4 | 0.16/0.17/0.17 | deshielding | 0.13/0.21/0.17 | deshielding |
| H5 | 0.05/N.S./0.09 | deshielding | 0.11/0.10/0.14 | deshielding |
| H6 | 0.13/0.06/0.15 | deshielding | 0.08/N.S./0.09 | deshielding |
| H7 | 0.18/0.08/0.20 | deshielding | 0.13/0.08/0.14 | deshielding |
| H10 | 0.78/0.32/0.69 | deshielding | 0.84/0.38/0.65 | deshielding |
| H11 | 0.12/N.S./0.11 | deshielding | 0.11/N.S./0.08 | deshielding |
| H12 | 0.09/N.S./0.09 | deshielding | 0.09/N.S./0.06 | deshielding |
| Position | sfabz series (Zn/Cd/Hg) | seabz series (Zn/Cd/Hg) | ||
| Δδ (ppm) | Effect | Δδ (ppm) | Effect | |
| C2 | 0.1/0.2/0.2 | deshielding | 0.1/0.1/0.2 | deshielding |
| C4 | 0.7/0.2/0.9 | Zn, Hg deshieldingCd shielding | 0.8/0.2/0.8 | Zn, Hg deshieldingCd shielding |
| C5 | 1.1/0.4/1.1 | deshielding | 1.2/0.5/1.0 | deshielding |
| C6 | 2.0/1.0/1.9 | deshielding | 2.0/1.1/1.8 | deshielding |
| C7 | 1.3/0.5/1.4 | deshielding | 1.2/0.6/1.1 | deshielding |
| C8 | 1.6/0.8/1.4 | shielding | 1.6/0.9/1.3 | shielding |
| C9 | 3.9/2.0/3.7 | shielding | 4.4/2.5/3.4 | shielding |
| C11 | 0.3/0.1/0.5 | deshielding | 0.2/0.1/0.3 | deshielding |
| C12 | 0.6/0.3/0.7 | shielding | 0.6/0.3/0.5 | shielding |
| Compound | Angle | Degrees (º) | Bond | Distance (Å) |
|---|---|---|---|---|
| [Ni(sfabz)2Cl2] (1) | N-Ni-N’ | 102.0(1) | Ni-Cl | 2.233(9) |
| N-Ni-Cl | 107.91(9) | Ni-Cl’ | 2.258(1) | |
| N-Ni-Cl’ | 106.21(9) | Ni-N | 1.987(3) | |
| N’-Ni-Cl | 111.73(9) | Ni-N’ | 1.979(3) | |
| N’-Ni-Cl’ | 106.22(9) | |||
| Cl-Ni-Cl’ | 121.06(3) | |||
| [Ni(sfabz)2Br2] (2) | N-Ni-N’ | 107.94(8) | Ni-Br | 2.392(4) |
| N-Ni-Br | 109.49(6) | Ni-Br’ | 2.414(4) | |
| N-Ni-Br’ | 103.65(6) | Ni-N | 1.975(2) | |
| N’-Ni-Br | 112.06(6) | Ni-N’ | 1.969(2) | |
| N’-Ni-Br’ | 109.12(6) | |||
| Br-Ni-Br’ | 114.09(2) | |||
| [Ni(seabz)2Cl2] (3) | N-Ni-N’ | 102.00(1) | Ni-Cl | 2.256(10) |
| N-Ni-Cl | 109.09(8) | Ni-Cl’ | 2.282(9) | |
| N-Ni-Cl’ | 108.79(8) | Ni-N | 1.967(2) | |
| N’-Ni-Cl | 107.95(8) | Ni-N’ | 1.974(3) | |
| N’-Ni-Cl’ | 107.22(8) | |||
| Cl-Ni-Cl’ | 120.25(3) |
| Compound | Angle | Degrees (º) | Bond | Distance (Å) |
|---|---|---|---|---|
| [Zn(sfabz)2Cl2] (9) | N-Zn-N’ | 108.78(1) | Zn-Cl | 2.244(2) |
| N-Zn-Cl | 106.71(1) | Zn-Cl’ | 2.285(2) | |
| N-Zn-Cl’ | 111.63(1) | Zn-N | 1.996(3) | |
| N’-Zn-Cl | 115.17(1) | Zn-N’ | 1.989(4) | |
| N’-Zn-Cl’ | 106.72(1) | |||
| Cl-Zn-Cl’ | 107.91(5) | |||
| [Zn(sfabz)2Br2] (10) | N-Zn-N’ | 111.07(1) | Zn-Br | 2.399(6) |
| N-Zn-Br | 110.34(9) | Zn-Br’ | 2.430(6) | |
| N-Zn-Br’ | 106.04(9) | Zn-N | 1.999(3) | |
| N’-Zn-Br | 110.89(9) | Zn-N’ | 1.992(3) | |
| N’-Zn-Br’ | 109.85(9) | |||
| Br-Zn-Br’ | 108.52(2) |
| HCT-15 IC50 (μM) | MCF-7 IC50 (μM) | HeLaIC50 (μM) | A549IC50 (μM) | L929IC50 (μM) | |
| Sfabz | 395.4 | 406.4 | 386.3 | 360.4 | 352.5 |
| [Cu(sfabz)2Cl2] (5) | 161.3 | 136.6 | 29.8 | 153.6 | 148.3 |
| [Cu(sfabz)2Br2] (6) | 133.9 | 118.6 | 15.0 | 135.5 | 122.5 |
| [Zn(sfabz)2Cl2] (9) | 140.1 | 148.5 | 144.7 | 170.8 | 140.9 |
| [Zn(sfabz)2Br2] (10) | 144.8 | 130.2 | 189.8 | 140.6 | 137.4 |
| Seabz | 898.6 | 496.7 | 748.0 | 1311.8 | 2364.9 |
| [Cu(seabz)2Cl2] (7) | 168.8 | 147.3 | 109.7 | 166.9 | 176.7 |
| [Cu(seabz)2Br2] (8) | 159.9 | 139.1 | 142.0 | 163.0 | 147.2 |
| [Zn(seabz)2Cl2] (11) | 184.2 | 163.6 | 194.0 | 176.9 | 166.3 |
| [Zn(seabz)2Br2] (12) | 167.8 | 160.8 | 167.3 | 266.3 | 150.8 |
| cisplatin | 32.7 | 32.3 | 19.0 | 34.9 | 43.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




