Submitted:
07 September 2023
Posted:
12 September 2023
You are already at the latest version
Abstract
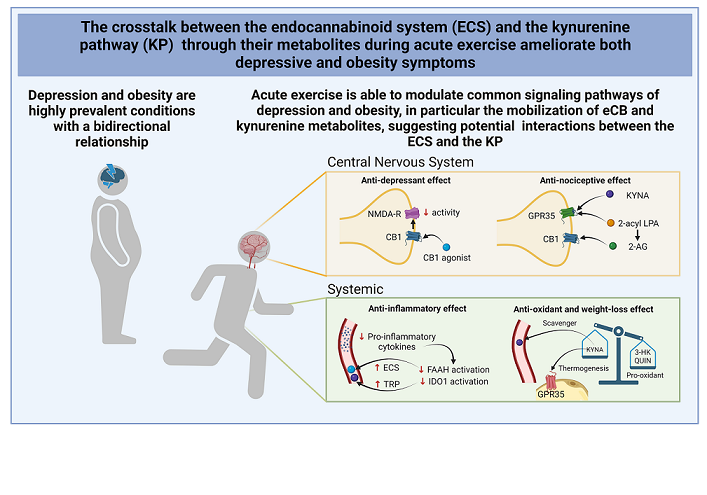
Keywords:
1. Introduction
2. Modulation of the KP and the ECS by physical exercise
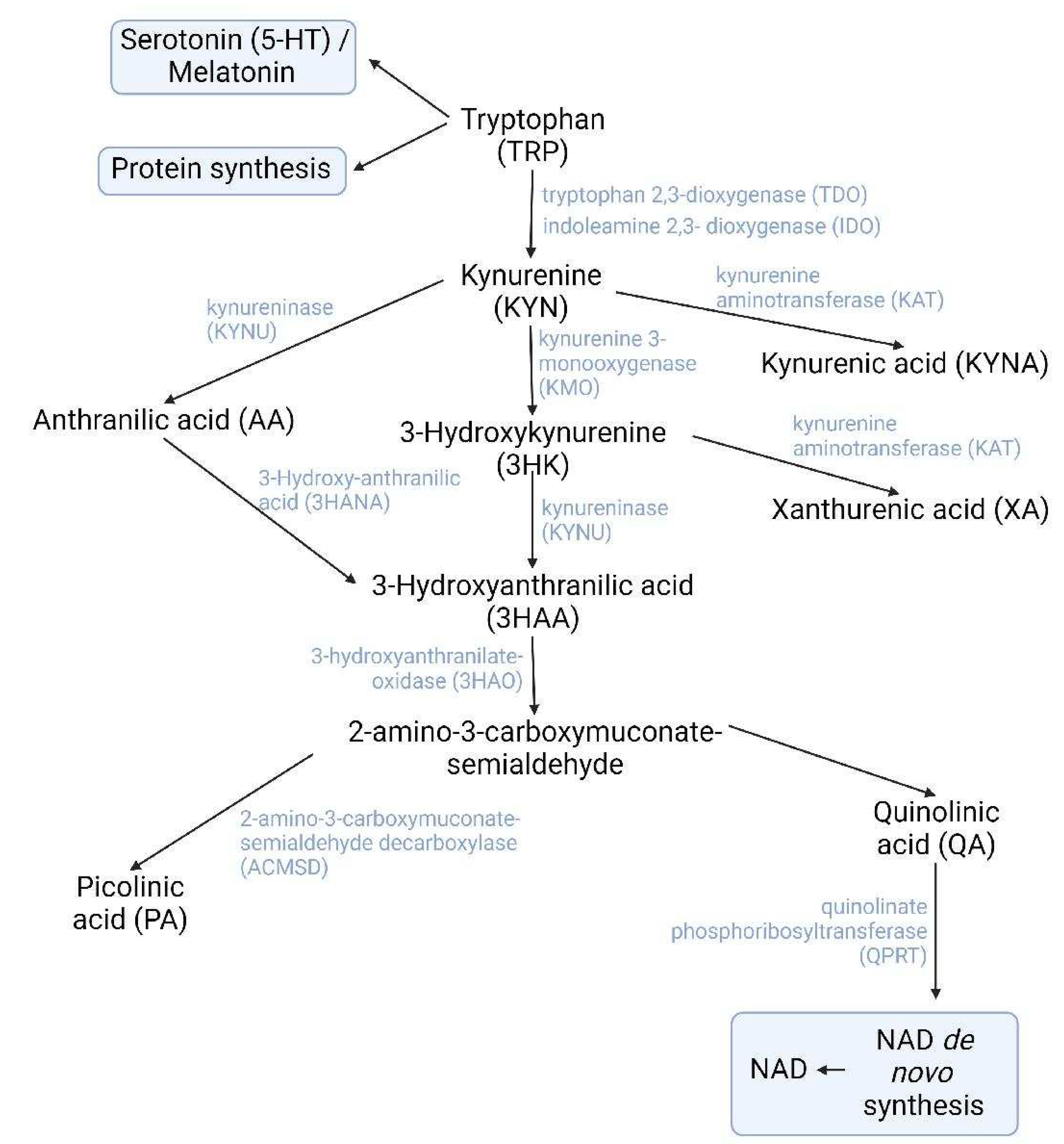
2.1. The kynurenine pathway
2.1.1. Participation of exercise-induced kynurenines in obesity
2.1.2. Participation of exercise-induced kynurenines in depression
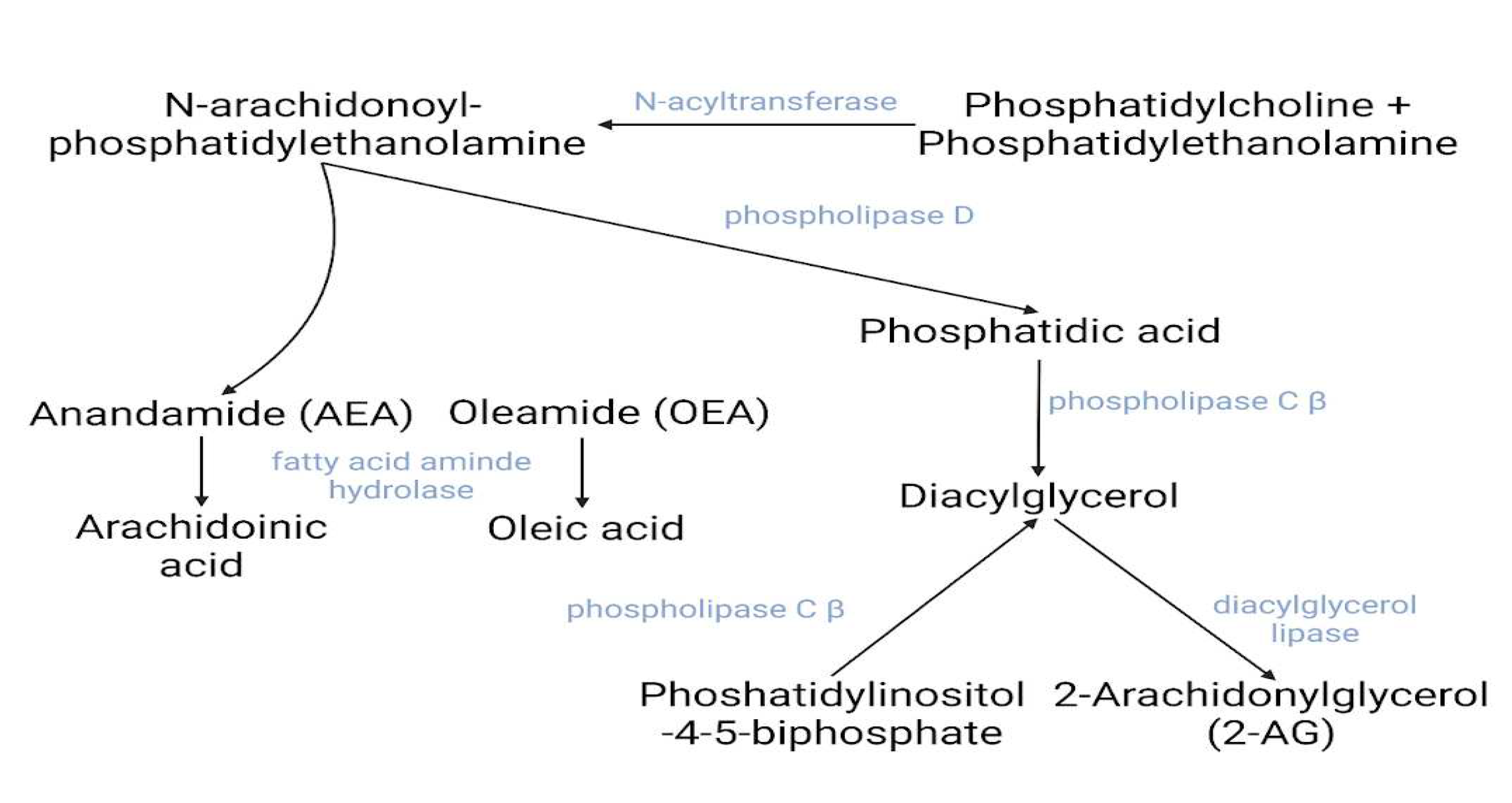
2.2. The endocannabinoid system
2.2.1. Involvement of exercise-derived endocannabinoids in obesity and depression
3. Crosstalk between the KP and the ECS in obesity and depression during exercise: potential functional interactions
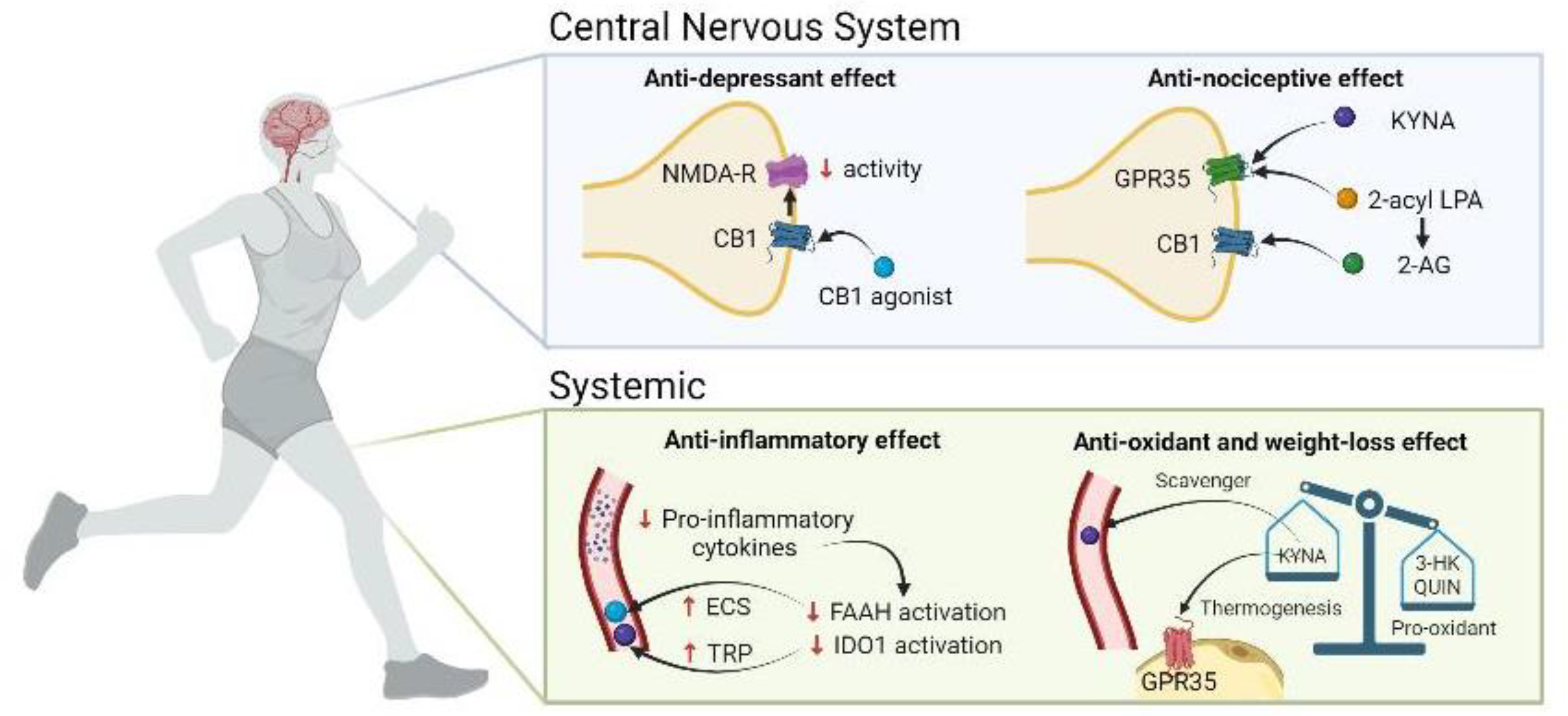
3.1. NMDAr and CB1
3.2. GPR35 and KYNA
3.3. Inflammation
3.4. Oxidative stress
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Chalder, M.; Wiles, N.J.; Campbell, J.; Hollinghurst, S.P.; Haase, A.M.; Taylor, A.H.; Fox, K.R.; Costelloe, C.; Searle, A.; Baxter, H.; Winder, R.; Wright, C.; Turner, K.M.; Calnan, M.; Lawlor, D.A.; Peters, T.J.; Sharp, D.J.; Montgomery, A.A.; Lewis, G. Facilitated physical activity as a treatment for depressed adults: randomised controlled trial. Bmj. 2012, 344, e2758. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Maes, M.; Berk, M. Inflammation-related disorders in the tryptophan catabolite pathway in depression and somatization. Adv Protein Chem Struct Biol. 2012, 88, 27–48. [Google Scholar] [PubMed]
- Huang, W.J.; Chen, W.W.; Zhang, X. Endocannabinoid system: Role in depression, reward and pain control (Review). Mol Med Rep. 2016, 14, 2899–2903. [Google Scholar] [CrossRef]
- Dadvar, S.; Ferreira, D.M.S.; Cervenka, I.; Ruas, J.L. The weight of nutrients: kynurenine metabolites in obesity and exercise. J Intern Med. 2018, 284, 519–533. [Google Scholar] [CrossRef] [PubMed]
- You, T.; Disanzo, B.L.; Wang, X.; Yang, R.; Gong, D. Adipose tissue endocannabinoid system gene expression: depot differences and effects of diet and exercise. Lipids Health Dis. 2011, 10, 194. [Google Scholar] [CrossRef]
- Heyman, E.; Gamelin, F.X.; Goekint, M.; Piscitelli, F.; Roelands, B.; Leclair, E.; Di Marzo, V.; Meeusen, R. Intense exercise increases circulating endocannabinoid and BDNF levels in humans--possible implications for reward and depression. Psychoneuroendocrinology. 2012, 37, 844–851. [Google Scholar] [CrossRef]
- Joisten, N.; Schumann, M.; Schenk, A.; Walzik, D.; Freitag, N.; Knoop, A.; Thevis, M.; Bloch, W.; Zimmer, P. Acute hypertrophic but not maximal strength loading transiently enhances the kynurenine pathway towards kynurenic acid. Eur J Appl Physiol. 2020, 120, 1429–1436. [Google Scholar] [CrossRef]
- Pagotto, U.; Marsicano, G.; Cota, D.; Lutz, B.; Pasquali, R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev. 2006, 27, 73–100. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan's metabolites in exercise, inflammation, and mental health. Science. 2017, 357. [Google Scholar] [CrossRef]
- Nagy-Grócz, G.; Zádor, F.; Dvorácskó, S.; Bohár, Z.; Benyhe, S.; Tömböly, C.; Párdutz, Á.; Vécsei, L. Interactions between the Kynurenine and the Endocannabinoid System with Special Emphasis on Migraine. Int J Mol Sci. 2017, 18. [Google Scholar] [CrossRef]
- Celik, O.; Yildiz, B.O. Obesity and physical exercise. Minerva Endocrinol (Torino). 2021, 46, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.A. Physical inactivity: associated diseases and disorders. Ann Clin Lab Sci. 2012, 42, 320–337. [Google Scholar] [PubMed]
- Metcalfe, A.J.; Koliamitra, C.; Javelle, F.; Bloch, W.; Zimmer, P. Acute and chronic effects of exercise on the kynurenine pathway in humans - A brief review and future perspectives. Physiol Behav. 2018, 194, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Valente-Silva, P.; Ruas, J.L. Tryptophan-Kynurenine Metabolites in Exercise and Mental Health. In: Spiegelman B, editor Hormones, Metabolism and the Benefits of Exercise. Cham (CH): Springer Copyright 2017, The Author(s). 2017, p. 83-91.
- Schlittler, M.; Goiny, M.; Agudelo, L.Z.; Venckunas, T.; Brazaitis, M.; Skurvydas, A.; Kamandulis, S.; Ruas, J.L.; Erhardt, S.; Westerblad, H.; Andersson, D.C. Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. Am J Physiol Cell Physiol. 2016, 310, C836–C840. [Google Scholar] [CrossRef]
- Zádor, F.; Joca, S.; Nagy-Grócz, G.; Dvorácskó, S.; Szűcs, E.; Tömböly, C.; Benyhe, S.; Vécsei, L. Pro-Inflammatory Cytokines: Potential Links between the Endocannabinoid System and the Kynurenine Pathway in Depression. Int J Mol Sci. 2021, 22. [Google Scholar] [CrossRef]
- Powers, S.K. Exercise: Teaching myocytes new tricks. J Appl Physiol (1985). 2017, 123, 460–472. [Google Scholar] [CrossRef]
- Rimer, J.; Dwan, K.; Lawlor, D.A.; Greig, C.A.; McMurdo, M.; Morley, W.; Mead, G.E. Exercise for depression. Cochrane Database Syst Rev. 2012, Cd004366. [Google Scholar]
- Hopps, E.; Caimi, G. Exercise in obesity management. J Sports Med Phys Fitness. 2011, 51, 275–282. [Google Scholar]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef]
- Ruas, J.L.; White, J.P.; Rao, R.R.; Kleiner, S.; Brannan, K.T.; Harrison, B.C.; Greene, N.P.; Wu, J.; Estall, J.L.; Irving, B.A.; Lanza, I.R.; Rasbach, K.A.; Okutsu, M.; Nair, K.S.; Yan, Z.; Leinwand, L.A.; Spiegelman, B.M. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell. 2012, 151, 1319–1331. [Google Scholar] [CrossRef]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, L.Z.; Ferreira, D.M.S.; Cervenka, I.; Bryzgalova, G.; Dadvar, S.; Jannig, P.R.; Pettersson-Klein, A.T.; Lakshmikanth, T.; Sustarsic, E.G.; Porsmyr-Palmertz, M.; Correia, J.C.; Izadi, M.; Martínez-Redondo, V.; Ueland, P.M.; Midttun, Ø.; Gerhart-Hines, Z.; Brodin, P.; Pereira, T.; Berggren, P.O.; Ruas, J.L. Kynurenic Acid and Gpr35 Regulate Adipose Tissue Energy Homeostasis and Inflammation. Cell Metab. 2018, 27, 378–392.e5. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.S.; Azzolini, M.; Lira Ruas, J. The kynurenine connection: how exercise shifts muscle tryptophan metabolism and affects energy homeostasis, the immune system, and the brain. Am J Physiol Cell Physiol. 2020, 318, C818–c30. [Google Scholar] [CrossRef] [PubMed]
- Harkin, A. Muscling in on depression. N Engl J Med. 2014, 371, 2333–2334. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.C.; Ferreira, D.M.; Ruas, J.L. Intercellular: local and systemic actions of skeletal muscle PGC-1s. Trends Endocrinol Metab. 2015, 26, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Hatazawa, Y.; Tadaishi, M.; Nagaike, Y.; Morita, A.; Ogawa, Y.; Ezaki, O.; Takai-Igarashi, T.; Kitaura, Y.; Shimomura, Y.; Kamei, Y.; Miura, S. PGC-1α-mediated branched-chain amino acid metabolism in the skeletal muscle. PLoS One. 2014, 9, e91006. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Movassat, J.; Portha, B. Emerging role for kynurenines in metabolic pathologies. Curr Opin Clin Nutr Metab Care. 2019, 22, 82–90. [Google Scholar] [CrossRef]
- Allison, D.B.; Fontaine, K.R.; Manson, J.E.; Stevens, J.; VanItallie, T.B. Annual deaths attributable to obesity in the United States. Jama. 1999, 282, 1530–1538. [Google Scholar] [CrossRef]
- Das, U.N. Is obesity an inflammatory condition? Nutrition. 2001, 17, 953–966. [Google Scholar] [CrossRef]
- Mangge, H.; Hubmann, H.; Pilz, S.; Schauenstein, K.; Renner, W.; März, W. Beyond cholesterol--inflammatory cytokines, the key mediators in atherosclerosis. Clin Chem Lab Med. 2004, 42, 467–474. [Google Scholar] [CrossRef]
- Solon-Biet, S.M.; Cogger, V.C.; Pulpitel, T.; Wahl, D.; Clark, X.; Bagley, E.; Gregoriou, G.C.; Senior, A.M.; Wang, Q.P.; Brandon, A.E.; Perks, R.; O'Sullivan, J.; Koay, Y.C.; Bell-Anderson, K.; Kebede, M.; Yau, B.; Atkinson, C.; Svineng, G.; Dodgson, T.; Wali, J.A.; Piper, M.D.W.; Juricic, P.; Partridge, L.; Rose, A.J.; Raubenheimer, D.; Cooney, G.J.; Le Couteur, D.G.; Simpson, S.J. Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat Metab. 2019, 1, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Song, J.; Gao, J.; Cheng, J.; Xie, H.; Zhang, L.; Wang, Y.H.; Gao, Z.; Wang, Y.; Wang, X.; He, J.; Liu, S.; Yu, Q.; Zhang, S.; Xiong, F.; Zhou, Q.; Wang, C.Y. Adipocyte-derived kynurenine promotes obesity and insulin resistance by activating the AhR/STAT3/IL-6 signaling. Nat Commun. 2022, 13, 3489. [Google Scholar] [CrossRef] [PubMed]
- Cussotto, S.; Delgado, I.; Anesi, A.; Dexpert, S.; Aubert, A.; Beau, C.; Forestier, D.; Ledaguenel, P.; Magne, E.; Mattivi, F.; Capuron, L. Tryptophan Metabolic Pathways Are Altered in Obesity and Are Associated With Systemic Inflammation. Front Immunol. 2020, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, K.A.; Ovchinnikova, O.; Berg, M.; Baumgartner, R.; Agardh, H.; Pirault, J.; Gisterå, A.; Assinger, A.; Laguna-Fernandez, A.; Bäck, M.; Hansson, G.K.; Ketelhuth, D.F. Inhibition of indoleamine 2, 3-dioxygenase promotes vascular inflammation and increases atherosclerosis in Apoe-/- mice. Cardiovasc Res. 2015, 106, 295–302. [Google Scholar] [CrossRef]
- Wells, G.; Kennedy, P.T.; Dahal, L.N. Investigating the Role of Indoleamine 2, 3-Dioxygenase in Acute Myeloid Leukemia: A Systematic Review. Front Immunol. 2021, 12, 651687. [Google Scholar] [CrossRef]
- Zhai, L.; Bell, A.; Ladomersky, E.; Lauing, K.L.; Bollu, L.; Sosman, J.A.; Zhang, B.; Wu, J.D.; Miller, S.D.; Meeks, J.J.; Lukas, R.V.; Wyatt, E.; Doglio, L.; Schiltz, G.E.; McCusker, R.H.; Wainwright, D.A. Immunosuppressive IDO in Cancer: Mechanisms of Action, Animal Models, and Targeting Strategies. Front Immunol. 2020, 11, 1185. [Google Scholar] [CrossRef]
- Favennec, M.; Hennart, B.; Caiazzo, R.; Leloire, A.; Yengo, L.; Verbanck, M.; Arredouani, A.; Marre, M.; Pigeyre, M.; Bessede, A.; Guillemin, G.J.; Chinetti, G.; Staels, B.; Pattou, F.; Balkau, B.; Allorge, D.; Froguel, P.; Poulain-Godefroy, O. Erratum: The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity (Silver Spring). 2016, 24, 1821. [Google Scholar] [CrossRef]
- Murr, C.; Widner, B.; Wirleitner, B.; Fuchs, D. Neopterin as a marker for immune system activation. Curr Drug Metab. 2002, 3, 175–187. [Google Scholar] [CrossRef]
- Kotake, Y.; Murakami, E. A possible diabetogenic role for tryptophan metabolites and effects of xanthurenic acid on insulin. Am J Clin Nutr. 1971, 24, 826–829. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab. 2019, 29, 27–37. [Google Scholar] [CrossRef]
- Smith, K. Mental health: a world of depression. Nature. 2014, 515, 181. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Galán, M.; De Bundel, D.; Van Eeckhaut, A.; Smolders, I.; Lindskog, M. Dysfunctional astrocytic regulation of glutamate transmission in a rat model of depression. Mol Psychiatry. 2013, 18, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, C. Disorders of memory and plasticity in psychiatric disease. Dialogues Clin Neurosci. 2013, 15, 455–463. [Google Scholar] [CrossRef]
- Sanacora, G.; Treccani, G.; Popoli, M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012, 62, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Yuen, E.Y.; Wei, J.; Liu, W.; Zhong, P.; Li, X.; Yan, Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012, 73, 962–977. [Google Scholar] [CrossRef]
- Gibney, S.M.; McGuinness, B.; Prendergast, C.; Harkin, A.; Connor, T.J. Poly I:C-induced activation of the immune response is accompanied by depression and anxiety-like behaviours, kynurenine pathway activation and reduced BDNF expression. Brain Behav Immun. 2013, 28, 170–181. [Google Scholar] [CrossRef]
- Liu, W.; Sheng, H.; Xu, Y.; Liu, Y.; Lu, J.; Ni, X. Swimming exercise ameliorates depression-like behavior in chronically stressed rats: relevant to proinflammatory cytokines and IDO activation. Behav Brain Res. 2013, 242, 110–116. [Google Scholar] [CrossRef]
- Gong, X.; Chang, R.; Zou, J.; Tan, S.; Huang, Z. The role and mechanism of tryptophan - kynurenine metabolic pathway in depression. Rev Neurosci. 2023, 34, 313–324. [Google Scholar] [CrossRef]
- Rudzki, S.J.; Cunningham, M.J. The effect of a modified physical training program in reducing injury and medical discharge rates in Australian Army recruits. Mil Med. 1999, 164, 648–652. [Google Scholar] [CrossRef]
- Savitz, J.; Ford, B.N.; Kuplicki, R.; Khalsa, S.; Teague, T.K.; Paulus, M.P. Acute administration of ibuprofen increases serum concentration of the neuroprotective kynurenine pathway metabolite, kynurenic acid: a pilot randomized, placebo-controlled, crossover study. Psychopharmacology (Berl). 2022, 239, 3919–3927. [Google Scholar] [CrossRef]
- Zhou, H.; Urso, C.J.; Jadeja, V. Saturated Fatty Acids in Obesity-Associated Inflammation. J Inflamm Res. 2020, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, T.I.M.; Instanes, J.T.; Posserud, M.R.; Ulvik, A.; Kessler, U.; Haavik, J. Changes in Tryptophan-Kynurenine Metabolism in Patients with Depression Undergoing ECT-A Systematic Review. Pharmaceuticals (Basel). 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, H.; Setoyama, D.; Kang, D.; Matsushima, J.; Kojima, R.; Fujii, Y.; Mawatari, S.; Kikuchi, J.; Sakemura, Y.; Fukuchi, J.; Shiraishi, T.; Maekawa, T.; Kato, T.A.; Asami, T.; Mizoguchi, Y.; Monji, A. The changes in kynurenine metabolites induced by rTMS in treatment-resistant depression: A pilot study. J Psychiatr Res. 2021, 138, 194–199. [Google Scholar] [CrossRef]
- Bartoli, F.; Misiak, B.; Callovini, T.; Cavaleri, D.; Cioni, R.M.; Crocamo, C.; Savitz, J.B.; Carrà, G. The kynurenine pathway in bipolar disorder: a meta-analysis on the peripheral blood levels of tryptophan and related metabolites. Mol Psychiatry. 2021, 26, 3419–3429. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Mora, P.; Pérez-De la Cruz, V.; Estrada-Cortés, B.; Toussaint-González, P.; Martínez-Cortéz, J.A.; Rodríguez-Barragán, M.; Quinzaños-Fresnedo, J.; Rangel-Caballero, F.; Gamboa-Coria, G.; Sánchez-Vázquez, I.; Barajas-Martínez, K.; Franyutti-Prado, K.; Sánchez-Chapul, L.; Ramírez-Ortega, D.; Ramos-Chávez, L.A. Serum Kynurenines Correlate With Depressive Symptoms and Disability in Poststroke Patients: A Cross-sectional Study. Neurorehabil Neural Repair. 2020, 34, 936–944. [Google Scholar] [CrossRef] [PubMed]
- da Silva Dias, I.C.; Carabelli, B.; Ishii, D.K.; de Morais, H.; de Carvalho, M.C.; Rizzo de Souza, L.E.; Zanata, S.M.; Brandão, M.L.; Cunha, T.M.; Ferraz, A.C.; Cunha, J.M.; Zanoveli, J.M. Indoleamine-2, 3-Dioxygenase/Kynurenine Pathway as a Potential Pharmacological Target to Treat Depression Associated with Diabetes. Mol Neurobiol. 2016, 53, 6997–7009. [Google Scholar] [CrossRef]
- Singh, B.; Olds, T.; Curtis, R.; Dumuid, D.; Virgara, R.; Watson, A.; Szeto, K.; O'Connor, E.; Ferguson, T.; Eglitis, E.; Miatke, A.; Simpson, C.E.; Maher, C. Effectiveness of physical activity interventions for improving depression, anxiety and distress: an overview of systematic reviews. Br J Sports Med. 2023. [Google Scholar] [CrossRef]
- Isung, J.; Granqvist, M.; Trepci, A.; Huang, J.; Schwieler, L.; Kierkegaard, M.; Erhardt, S.; Jokinen, J.; Piehl, F. Differential effects on blood and cerebrospinal fluid immune protein markers and kynurenine pathway metabolites from aerobic physical exercise in healthy subjects. Sci Rep. 2021, 11, 1669. [Google Scholar] [CrossRef]
- Paul, E.R.; Schwieler, L.; Erhardt, S.; Boda, S.; Trepci, A.; Kämpe, R.; Asratian, A.; Holm, L.; Yngve, A.; Dantzer, R.; Heilig, M.; Hamilton, J.P.; Samuelsson, M. Peripheral and central kynurenine pathway abnormalities in major depression. Brain Behav Immun. 2022, 101, 136–145. [Google Scholar] [CrossRef]
- Millischer, V.; Erhardt, S.; Ekblom, Ö.; Forsell, Y.; Lavebratt, C. Twelve-week physical exercise does not have a long-lasting effect on kynurenines in plasma of depressed patients. Neuropsychiatr Dis Treat. 2017, 13, 967–972. [Google Scholar] [CrossRef]
- Saran, T.; Mazur, A.; Łukasiewicz, J. The significance of physical activity in the prevention of depressive disorders. Psychiatr Pol. 2021, 55, 1025–1046. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Chapul, L.; Pérez de la Cruz, G.; Ramos Chávez, L.A.; Valencia León, J.F.; Torres Beltrán, J.; Estrada Camarena, E.; Carillo Mora, P.; Ramírez Ortega, D.; Baños Vázquez, J.U.; Martínez Nava, G.; Luna Angulo, A.; Martínez Canseco, C.; Wences Chirino, T.Y.; Ríos Martínez, J.; Pérez de la Cruz, V. Characterization of Redox Environment and Tryptophan Catabolism through Kynurenine Pathway in Military Divers' and Swimmers' Serum Samples. Antioxidants (Basel). 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol Psychiatry. 2016, 79, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Ruiz-Canela, M.; Guasch-Ferré, M.; Zheng, Y.; Toledo, E.; Clish, C.B.; Salas-Salvadó, J.; Liang, L.; Wang, D.D.; Corella, D.; Fitó, M.; Gómez-Gracia, E.; Lapetra, J.; Estruch, R.; Ros, E.; Cofán, M.; Arós, F.; Romaguera, D.; Serra-Majem, L.; Sorlí, J.V.; Hu, F.B.; Martinez-Gonzalez, M.A. Increases in Plasma Tryptophan Are Inversely Associated with Incident Cardiovascular Disease in the Prevención con Dieta Mediterránea (PREDIMED) Study. J Nutr. 2017, 147, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Colangeli, R.; Teskey, G.C.; Di Giovanni, G. Endocannabinoid-serotonin systems interaction in health and disease. Prog Brain Res. 2021, 259, 83–134. [Google Scholar]
- Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W. The Endocannabinoid System: A Potential Target for the Treatment of Various Diseases. Int J Mol Sci. 2021, 22. [Google Scholar] [CrossRef]
- Rossi, F.; Punzo, F.; Umano, G.R.; Argenziano, M.; Miraglia Del Giudice, E. Role of Cannabinoids in Obesity. Int J Mol Sci. 2018, 19. [Google Scholar] [CrossRef]
- Schulz, P.; Hryhorowicz, S.; Rychter, A.M.; Zawada, A.; Słomski, R.; Dobrowolska, A.; Krela-Kaźmierczak, I. What Role Does the Endocannabinoid System Play in the Pathogenesis of Obesity? Nutrients. 2021, 13. [Google Scholar] [CrossRef]
- Fuss, J.; Steinle, J.; Bindila, L.; Auer, M.K.; Kirchherr, H.; Lutz, B.; Gass, P. A runner's high depends on cannabinoid receptors in mice. Proc Natl Acad Sci U S A. 2015, 112, 13105–13108. [Google Scholar] [CrossRef]
- Matei, D.; Trofin, D.; Iordan, D.A.; Onu, I.; Condurache, I.; Ionite, C.; Buculei, I. The Endocannabinoid System and Physical Exercise. Int J Mol Sci. 2023, 24. [Google Scholar] [CrossRef]
- Raichlen, D.A.; Foster, A.D.; Seillier, A.; Giuffrida, A.; Gerdeman, G.L. Exercise-induced endocannabinoid signaling is modulated by intensity. Eur J Appl Physiol. 2013, 113, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.A.; Epstein, D.H.; Preston, K.L. Psychostimulant addiction treatment. Neuropharmacology. 2014, 87, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.; Kristensen, P.K.; Bartels, E.M.; Bliddal, H.; Astrup, A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007, 370, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Raichlen, D.A.; Foster, A.D.; Gerdeman, G.L.; Seillier, A.; Giuffrida, A. Wired to run: exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the 'runner's high'. J Exp Biol. 2012, 215, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Blázquez, P.; Rodríguez-Muñoz, M.; Garzón, J. The cannabinoid receptor 1 associates with NMDA receptors to produce glutamatergic hypofunction: implications in psychosis and schizophrenia. Front Pharmacol. 2014, 4, 169. [Google Scholar] [CrossRef]
- Hampson, D.R.; Gholizadeh, S.; Pacey, L.K. Pathways to drug development for autism spectrum disorders. Clin Pharmacol Ther. 2012, 91, 189–200. [Google Scholar] [CrossRef]
- Liu, Q.; Bhat, M.; Bowen, W.D.; Cheng, J. Signaling pathways from cannabinoid receptor-1 activation to inhibition of N-methyl-D-aspartic acid mediated calcium influx and neurotoxicity in dorsal root ganglion neurons. J Pharmacol Exp Ther. 2009, 331, 1062–1070. [Google Scholar] [CrossRef]
- Newport, D.J.; Carpenter, L.L.; McDonald, W.M.; Potash, J.B.; Tohen, M.; Nemeroff, C.B. Ketamine and Other NMDA Antagonists: Early Clinical Trials and Possible Mechanisms in Depression. Am J Psychiatry. 2015, 172, 950–966. [Google Scholar] [CrossRef]
- Morales, P.; Reggio, P.H. An Update on Non-CB(1), Non-CB(2) Cannabinoid Related G-Protein-Coupled Receptors. Cannabis Cannabinoid Res. 2017, 2, 265–273. [Google Scholar] [CrossRef]
- Zádor, F.; Nagy-Grócz, G.; Kekesi, G.; Dvorácskó, S.; Szűcs, E.; Tömböly, C.; Horvath, G.; Benyhe, S.; Vécsei, L. Kynurenines and the Endocannabinoid System in Schizophrenia: Common Points and Potential Interactions. Molecules. 2019, 24. [Google Scholar] [CrossRef]
- Zhao, P.; Abood, M.E. GPR55 and GPR35 and their relationship to cannabinoid and lysophospholipid receptors. Life Sci. 2013, 92, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Zádor, F.; Nagy-Grócz, G.; Dvorácskó, S.; Bohár, Z.; Cseh, E.K.; Zádori, D.; Párdutz, Á.; Szűcs, E.; Tömböly, C.; Borsodi, A.; Benyhe, S.; Vécsei, L. Long-term systemic administration of kynurenic acid brain region specifically elevates the abundance of functional CB(1) receptors in rats. Neurochem Int. 2020, 138, 104752. [Google Scholar] [CrossRef] [PubMed]
- Eder, K.; Baffy, N.; Falus, A.; Fulop, A.K. The major inflammatory mediator interleukin-6 and obesity. Inflamm Res. 2009, 58, 727–736. [Google Scholar] [CrossRef]
- Segarra, M.; Aburto, M.R.; Acker-Palmer, A. Blood-Brain Barrier Dynamics to Maintain Brain Homeostasis. Trends Neurosci. 2021, 44, 393–405. [Google Scholar] [CrossRef]
- Heilman, P.; Hill, M.N.; Coussons-Read, M.; Brundin, L.; Coccaro, E.F. Role of the kynurenine pathway and the endocannabinoid system as modulators of inflammation and personality traits. Psychoneuroendocrinology. 2019, 110, 104434. [Google Scholar] [CrossRef] [PubMed]
- Quin, C.; Estaki, M.; Vollman, D.M.; Barnett, J.A.; Gill, S.K.; Gibson, D.L. Probiotic supplementation and associated infant gut microbiome and health: a cautionary retrospective clinical comparison. Sci Rep. 2018, 8, 8283. [Google Scholar] [CrossRef]
- Tejeda-Martínez, A.R.; Viveros-Paredes, J.M.; Hidalgo-Franco, G.V.; Pardo-González, E.; Chaparro-Huerta, V.; González-Castañeda, R.E.; Flores-Soto, M.E. Chronic Inhibition of FAAH Reduces Depressive-Like Behavior and Improves Dentate Gyrus Proliferation after Chronic Unpredictable Stress Exposure. Behav Neurol. 2021, 2021, 6651492. [Google Scholar] [CrossRef]
- Banks, W.A.; Kastin, A.J.; Broadwell, R.D. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995, 2, 241–248. [Google Scholar] [CrossRef]
- Skorobogatov, K.; De Picker, L.; Verkerk, R.; Coppens, V.; Leboyer, M.; Müller, N.; Morrens, M. Brain Versus Blood: A Systematic Review on the Concordance Between Peripheral and Central Kynurenine Pathway Measures in Psychiatric Disorders. Front Immunol. 2021, 12, 716980. [Google Scholar] [CrossRef]
- Małkiewicz, M.A.; Szarmach, A.; Sabisz, A.; Cubała, W.J.; Szurowska, E.; Winklewski, P.J. Blood-brain barrier permeability and physical exercise. J Neuroinflammation. 2019, 16, 15. [Google Scholar] [CrossRef]
- Parastouei, K.; Aarabi, M.H.; Hamidi, G.A.; Nasehi, Z.; Kabiri-Arani, S.; Jozi, F.; Shahaboddin, M.E. A CB2 Receptor Agonist Reduces the Production of Inflammatory Mediators and Improves Locomotor Activity in Experimental Autoimmune Encephalomyelitis. Rep Biochem Mol Biol. 2022, 11, 1–9. [Google Scholar] [CrossRef]
- Quintana, F.J.; Murugaiyan, G.; Farez, M.F.; Mitsdoerffer, M.; Tukpah, A.M.; Burns, E.J.; Weiner, H.L. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2010, 107, 20768–20773. [Google Scholar] [CrossRef]
- O'Farrell, K.; Harkin, A. Stress-related regulation of the kynurenine pathway: Relevance to neuropsychiatric and degenerative disorders. Neuropharmacology. 2017, 112, 307–323. [Google Scholar] [CrossRef]
- Lee, K.J.; Jung, K.H.; Cho, J.Y.; Lee, S.T.; Kim, H.S.; Shim, J.H.; Lee, S.K.; Kim, M.; Chu, K. High-Fat Diet and Voluntary Chronic Aerobic Exercise Recover Altered Levels of Aging-Related Tryptophan Metabolites along the Kynurenine Pathway. Exp Neurobiol. 2017, 26, 132–140. [Google Scholar] [CrossRef]
- Sathyasaikumar, K.V.; Pérez de la Cruz, V.; Pineda, B.; Vázquez Cervantes, G.I.; Ramírez Ortega, D.; Donley, D.W.; Severson, P.L.; West, B.L.; Giorgini, F.; Fox, J.H.; Schwarcz, R. Cellular Localization of Kynurenine 3-Monooxygenase in the Brain: Challenging the Dogma. Antioxidants (Basel). 2022, 11. [Google Scholar] [CrossRef]
- Lovelace, M.D.; Varney, B.; Sundaram, G.; Lennon, M.J.; Lim, C.K.; Jacobs, K.; Guillemin, G.J.; Brew, B.J. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology. 2017, 112, 373–388. [Google Scholar] [CrossRef]
- Lugo-Huitrón, R.; Blanco-Ayala, T.; Ugalde-Muñiz, P.; Carrillo-Mora, P.; Pedraza-Chaverrí, J.; Silva-Adaya, D.; Maldonado, P.D.; Torres, I.; Pinzón, E.; Ortiz-Islas, E.; López, T.; García, E.; Pineda, B.; Torres-Ramos, M.; Santamaría, A.; La Cruz, V.P. On the antioxidant properties of kynurenic acid: free radical scavenging activity and inhibition of oxidative stress. Neurotoxicol Teratol. 2011, 33, 538–547. [Google Scholar] [CrossRef]
- Ostapiuk, A.; Urbanska, E.M. Kynurenic acid in neurodegenerative disorders-unique neuroprotection or double-edged sword? CNS Neurosci Ther. 2022, 28, 19–35. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Rajesh, M.; Bátkai, S.; Patel, V.; Kashiwaya, Y.; Liaudet, L.; Evgenov, O.V.; Mackie, K.; Haskó, G.; Pacher, P. CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes. Cardiovasc Res. 2010, 85, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Charytoniuk, T.; Zywno, H.; Konstantynowicz-Nowicka, K.; Berk, K.; Bzdega, W.; Chabowski, A. Can Physical Activity Support the Endocannabinoid System in the Preventive and Therapeutic Approach to Neurological Disorders? Int J Mol Sci. 2020, 21. [Google Scholar] [CrossRef]
- Danielsson, L.; Papoulias, I.; Petersson, E.L.; Carlsson, J.; Waern, M. Exercise or basic body awareness therapy as add-on treatment for major depression: a controlled study. J Affect Disord. 2014, 168, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Moraes, H.S.; Silveira, H.S.; Oliveira, N.A.; Matta Mello Portugal, E.; Araújo, N.B.; Vasques, P.E.; Bergland, A.; Santos, T.M.; Engedal, K.; Coutinho, E.S.; Schuch, F.B.; Laks, J.; Deslandes, A.C. Is Strength Training as Effective as Aerobic Training for Depression in Older Adults? A Randomized Controlled Trial. Neuropsychobiology. 2020, 79, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Fuller, O.K.; Whitham, M.; Mathivanan, S.; Febbraio, M.A. The Protective Effect of Exercise in Neurodegenerative Diseases: The Potential Role of Extracellular Vesicles. Cells. 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Sanhueza Pastén, C.; Caneo, C. Addition of aerobic exercise to antidepressant drug monotherapy for major depressive disorder in adults. Medwave. 2022, 22, e8670. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




