1. Introduction
Acute myocardial infarction (MI) produces massive injury to the coronary microcirculation, which leads to ischemic cardiomyopathy and generally results in myocardial necrosis. Coronary myocardial perfusion is typically required to avoid heart failure and tissue repair after MI involves a mechanical angiogenic response (1). Although the incidence of mechanical complications remains low, the associated mortality rate is high, especially among elderly patients (2). Therefore, more effective angiogenic therapies are vital for this group of patients.
Currently, adipose tissue is the most suitable source of angiogenic factors available (3) and it is an attractive candidate for tissue engineering and neovascularization (4). Although the available data has shown that aging seriously impairs the angiogenic properties of adipose tissue (5, 6), it is recognized as the most suitable source of angiogenic factors and progenitor cells to promote neovascularization in ischemic injuries because of its ease of access (6).
On the other hand, aging of the thymus is known to be a fast process in all mammals. In humans, thymic senescence begins at puberty and by 50 years of age, 80% of the thymic epithelium is replaced by adipose tissue (7). The thymus adipose tissue (TAT) is partially discarded during the aortic cannulation procedures performed as part of a cardiopulmonary bypass, which makes TAT a readily available source of fat and adipokines (8). Previously, we have shown that adult TAT from patients with ischemic cardiomyopathy produces a variety of angiogenic factors and induces proliferation and migration of human umbilical cord endothelial cells, which suggests its angiogenic potential (9). In contrast to other adipose tissues (e.g., the subcutaneous adipose tissue [SAT]), the angiogenic characteristics of TAT are significantly increased with aging (8). These data suggest that the TAT could be an ideal source of angiogenic and endothelial factors for elderly patients with ischemic cardiomyopathy. These properties are likely to be controlled by vascular endothelial growth factor A (VEGFA) because it is the angiogenic factor most highly upregulated in adult TAT as compared with SAT (8). VEGFA is the single most important regulator of blood vessel formation it is essential for embryonic angiogenesis and vasculogenesis and it is a key mediator of neovascularization (10).
MicroRNAs (miRNAs) are non-coding RNAs that regulate gene expression and have emerged as important factors in health and disease (11). The expression of miRNAs varies during all stages of myocardial ischemia-reperfusion and subsequent ischemia-reperfusion injury (12). These changes suggest that miRNAs may have a functional role in the ischemic processes and thus make for attractive therapeutic targets. Given the upregulation of angiogenic VEGFA in the TAT, and the potential involvement of miRNA regulation in the ischemic cardiomyopathy,
The primary objective of this study is to identify the specific miRNAs that exhibit differential expression in the TAT. Additionally, we aim to investigate the potential regulatory networks of these miRNAs, particularly their involvement in modulating the VEGFA pathway and contributing to age-related angiogenic processes within the TAT.
2. Results
2.1. Clinical and biological variables of both patient groups
The study was performed in two groups of patients who had a CABG surgery: 1) the elderly group (i.e., ≥70 years) with ten patients; and 2) the middle-aged group (i.e., 45 to 65 years) with 8 patients. These patients had a mean number of 3.1 grafts per patient.
Table 1 shows that there were no significant differences in all anthropometric and biochemical variables between the two groups, except for age as expected (mean age of 56.6 and 74.0 years, respectively; p<0.001).
2.2. In silico identification of miRNAs predicted to regulate VEGFA and TargetScore calculation of VEGFA miRNA binding sites
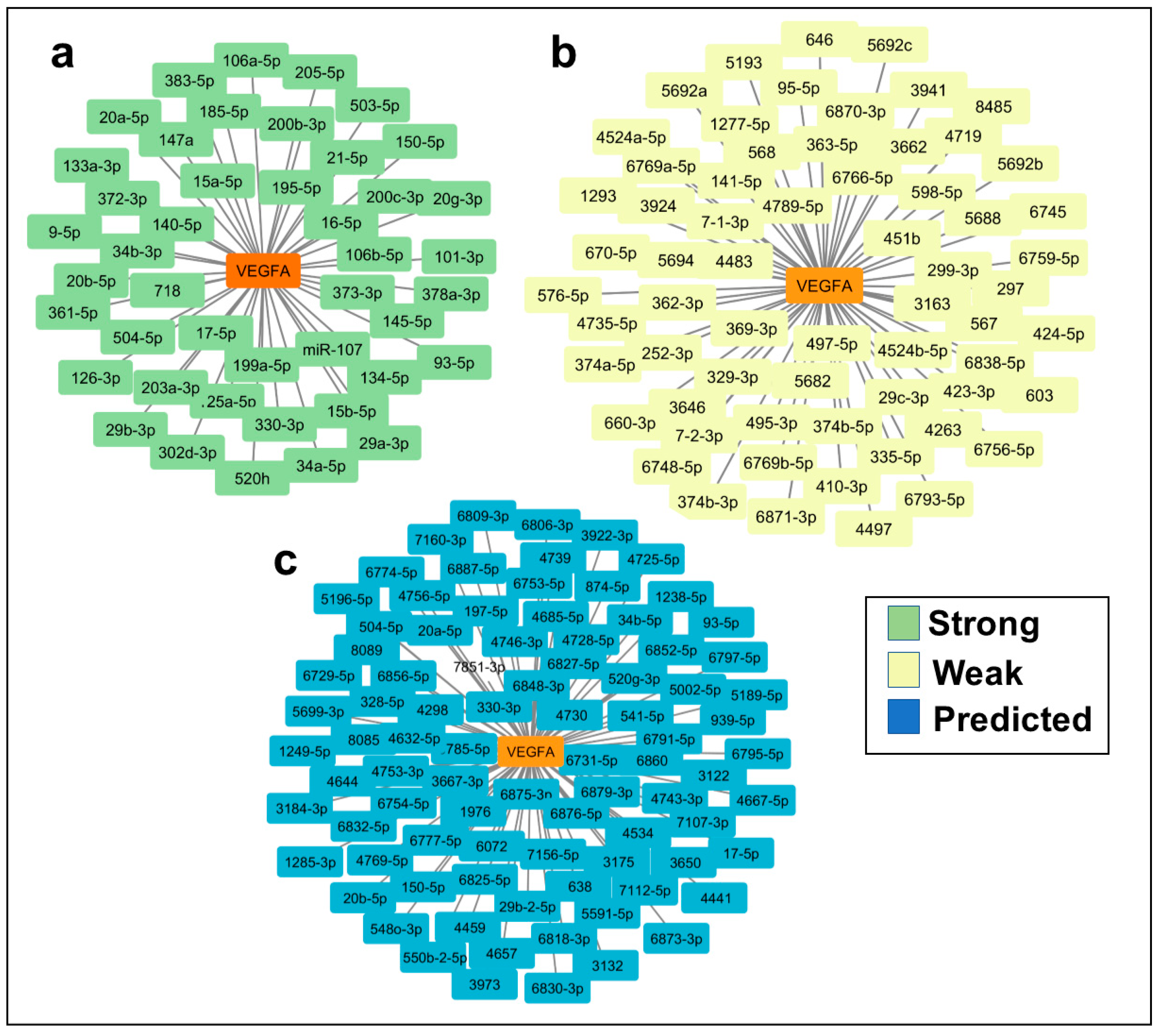
To examine the potential involvement of miRNAs in the regulation of VEGFA we performed an in silico analysis of the VEGFA gene sequence using miRTargetLink Human (13). This search identified 195 miRNAs as potential regulators of VEGFA (
Figure 1a–c, and
Table S1). Of these miRNAs, 44 were evaluated as having a high probability of involvement in VEGFA regulation (
Figure 1a), 64 were evaluated as having a low probability (
Figure 1b) and 87 as predicted target genes (
Figure 1c). Since miRNA-mRNA binding sites are not equally effective, we used the TargetScan tool (14) to calculate TargetScore values for each miRNA. This allowed us to pinpoint the five miRNAs; miR-15b-5p, miR-16-5p, miR-29a-3p, miR-29b-3p, and miR-195-5p, predicted to have the highest binding effectiveness. Consequently, these miRNAs are the most likely candidates for regulating VEGFA (
Table S2).
Previously, we have shown that miR-21-5p regulates VEGFA in adipose tissue (15). Although miR-21-5p does not have a high-probability binding site in VEGFA, we believe that it may act indirectly and therefore it was also included in subsequent analyses.
In our previous study, we established miR-21-5p's regulatory role in VEGFA within adipose tissue (15). Despite the absence of a high-probability binding site for miR-21-5p in VEGFA, we believe it may exert an indirect influence. For this reason, we believe it would be highly valuable to include it in the analysis.
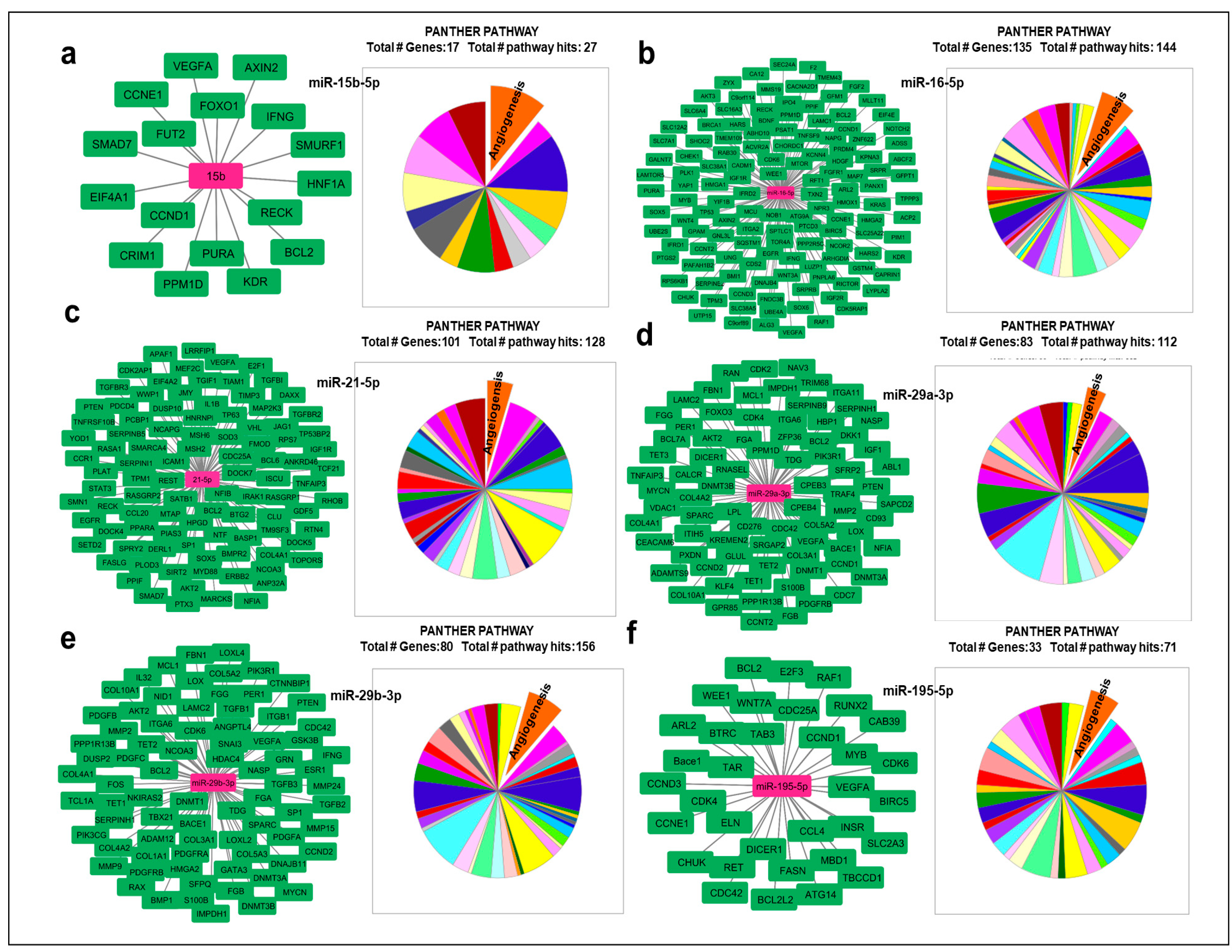
In order to find out whether these miRNAs might also regulate other angiogenic genes, we used miRTargetLinks to identify putative target genes of the six selected miRNAs (miR-15b-5p; miR-16-5p; miR-29a-3p; miR-29b-3p; miR-195-5p and miR-21-5p). We identified several target genes as having a high probability of being regulated (
Figure 2a–f), of which 25 were related to angiogenesis regulation (
Figure 2a–f and
Table S3).
2.3. Expression profile of miR-15b-5p, miR-16-5p, miR-29a-3p, miR-29b-3p, miR-195-5p and miR-21-5p in human TAT and SAT
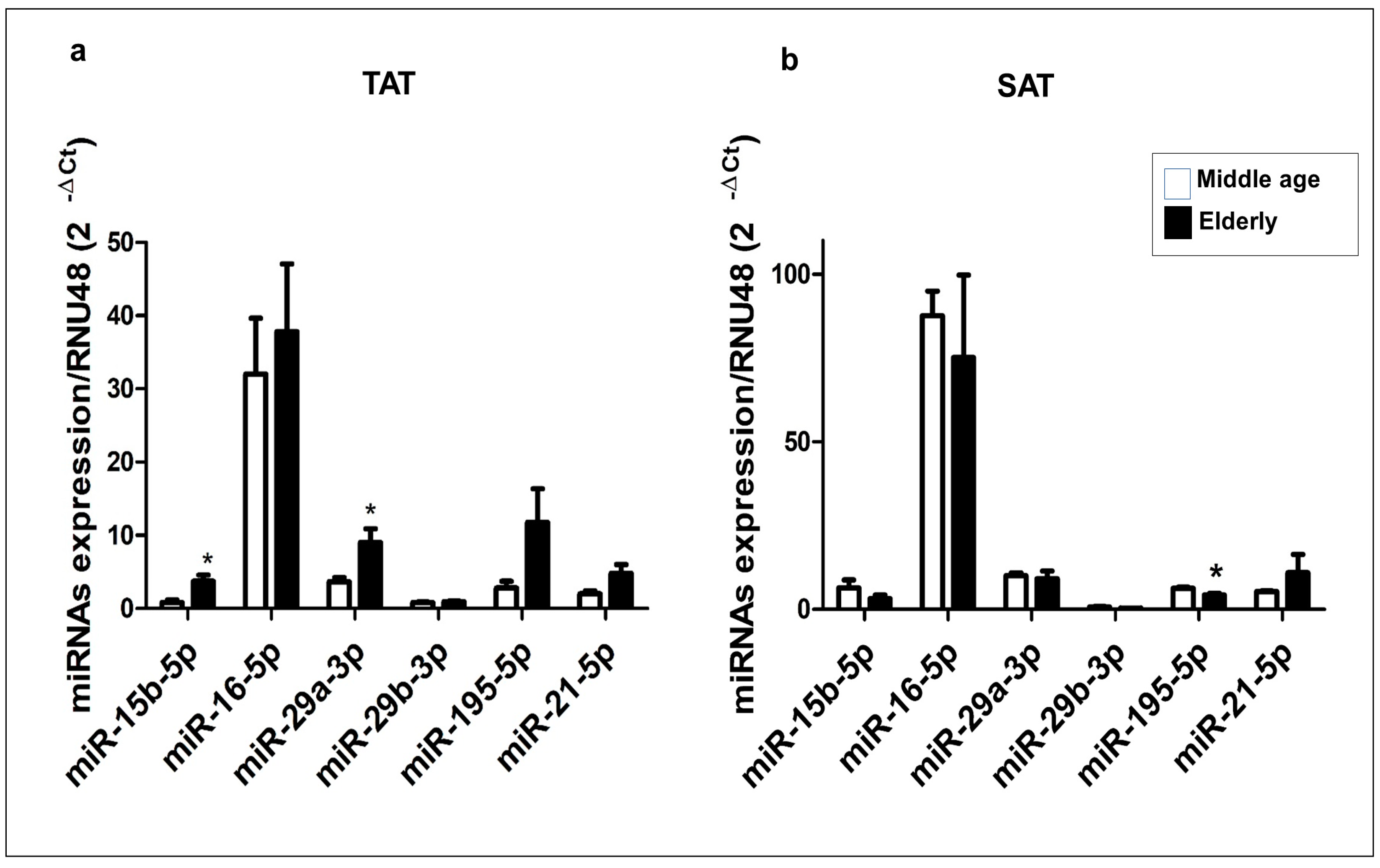
Since TAT displays an age-associated rise in VEGFA expression (8), we analyzed the expression levels of miRNAs that may be implicated in regulating this angiogenic factor in both TAT and SAT samples collected from elderly and middle-aged patient groups using RT-qPCR.
Within the TAT, we observed a notable increase in the expression levels of miR-15b-5p and miR-29a-3p in the elderly group compared to the middle-aged group (p<0.05). However, no significant differences were found in the expression of miR-16-5p, miR-21-5p, miR-195-5p, and miR-29b-3p between the two patient groups (
Figure 3a).
The SAT displayed a notably distinct miRNA expression profile compared to the TAT. Specifically, the expression level of miR-195-5p was significantly lower in the elderly group than in the middle-aged group (p<0.05), while no significant differences were observed in the expression of the remaining miRNAs (
Figure 3b). This highlights a potential association between TAT and age-specific expression of four miRNAs in relation to VEGFA, including three miRNAs that we anticipate have robust interactions with VEGFA mRNA.
2.4. mRNA expression profile of putative target genes in human TAT
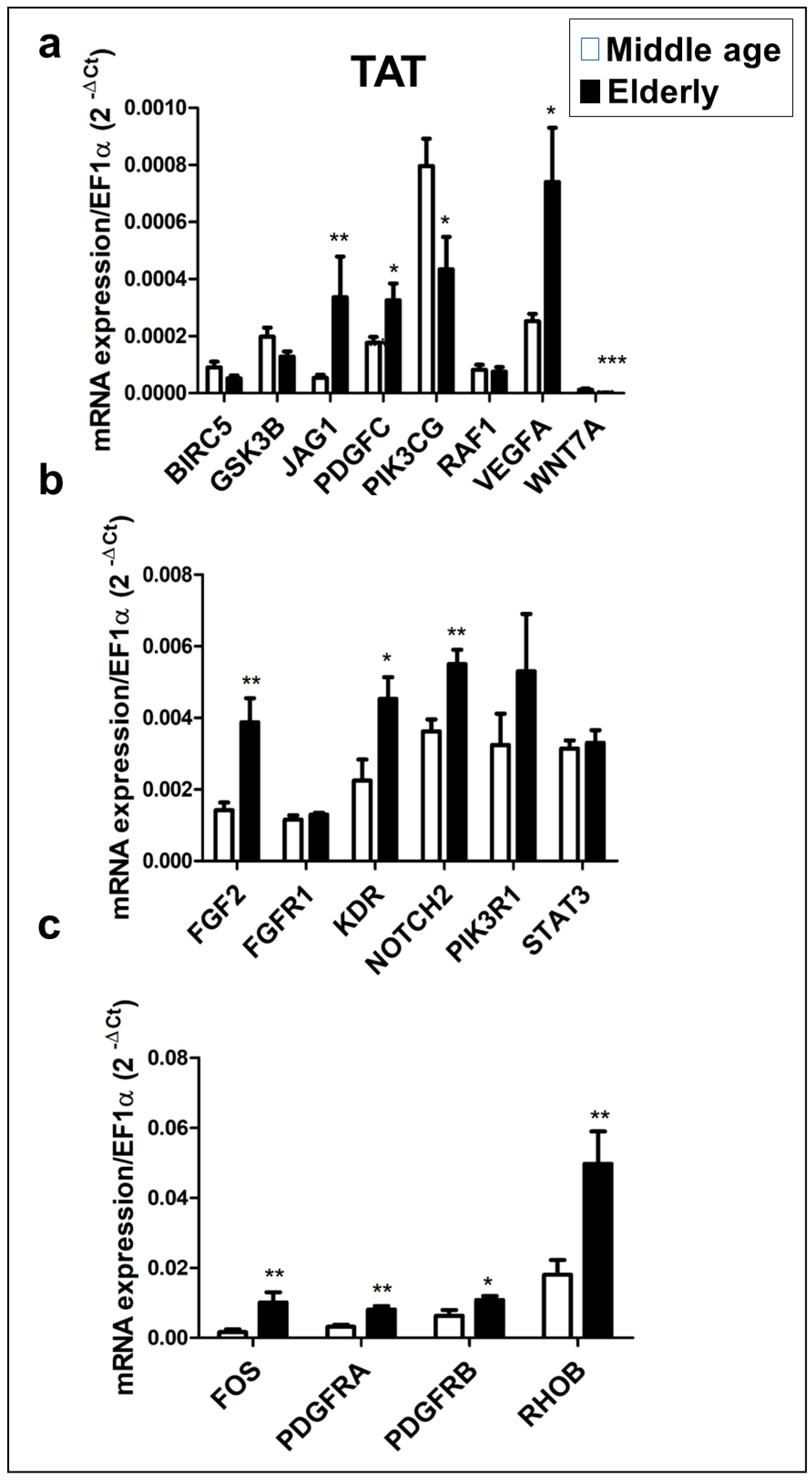
Because we previously found that the six candidate miRNAs are predicted to interact with several angiogenic genes in addition to VEGFA, we performed a RT-qPCR to compare the expression level of the predicted target genes in the TAT obtained from the elderly and middle-aged patient groups. Our analysis found that 18 of the 25 predicted target genes were expressed in the TAT, with 11 target genes showing statistically significant differences between the two patient groups (
Figure 4). The expression levels of JAG1, PDGFC, VEGFA (
Figure 4a), FGF2, KDR, NOTCH2 (
Figure 4b), FOS, PDGFRA, PDGFRB and RHOB (
Figure 4c) were significantly higher the elderly group than in the middle-aged group (p<0.05 and p<0.01). Conversely, the expression levels of PIK3CG and WNT7A (
Figure 4a) were significantly lower in the elderly group than in the middle-aged group. We found no significant differences in the expression levels of BIRC5, GSK3B, RAF1 (
Figure 4a), FGFR1, PIK3R1 and STAT3 (
Figure 4b) between the two patient groups.
2.5. Interaction network model between VEGFA, the miRNAs and target genes in human TAT of elderly patients
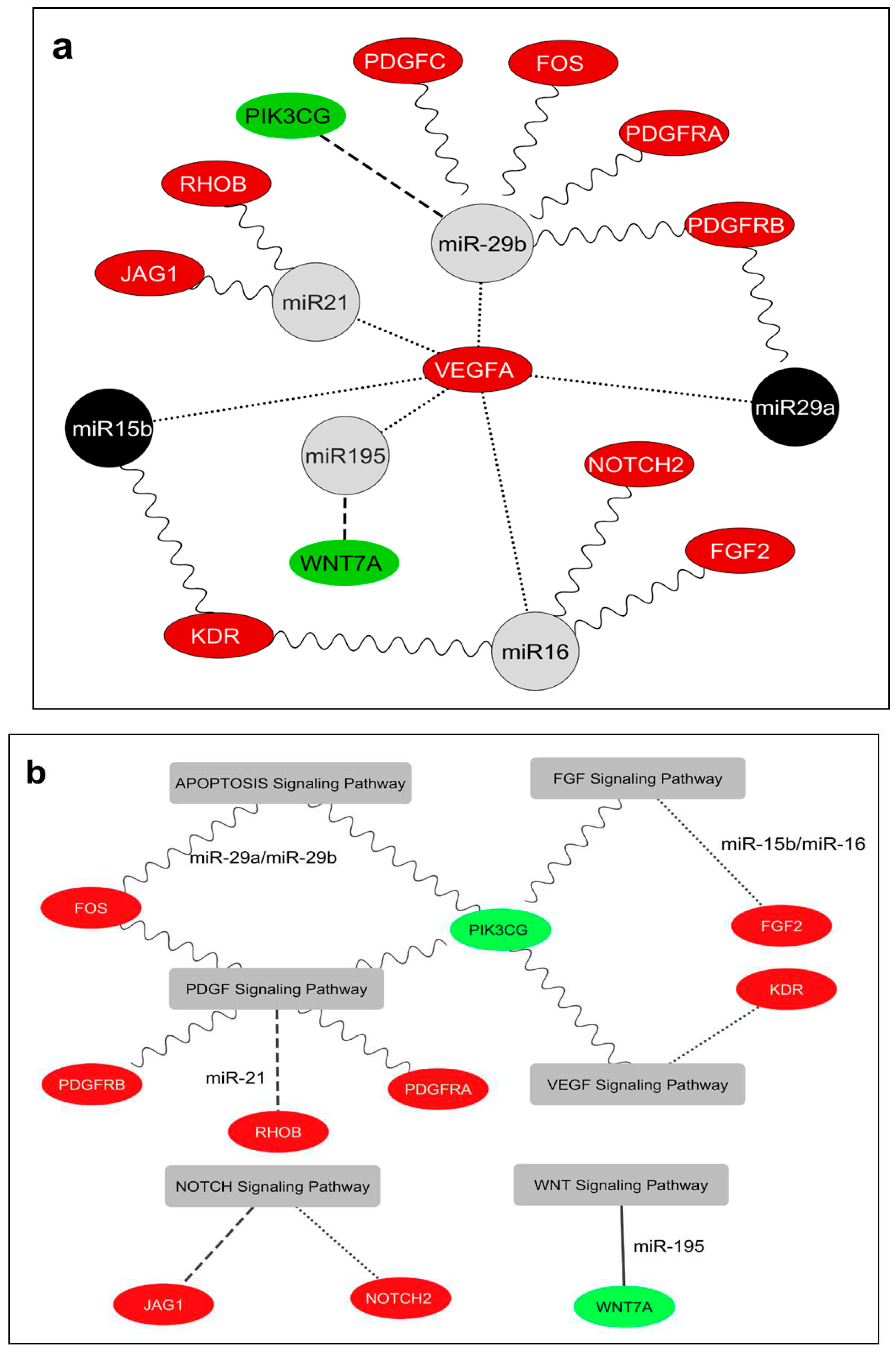
Collectively, our bioinformatics-derived data strongly indicates the presence of a complex regulatory network in the TAT. This network involves age-related differential expression of miRNAs, namely miR-15b-5p, miR-16-5p, miR-29a-3p, miR-29b-3p, miR-195-5p, and miR-21-5p, as well as a group of 12 angiogenesis-promoting genes including
PDGFC, PIK3CG, VEGFA, WNT7A, FGF2, JAG1, KDR, NOTCH2, FOS, PDGFRA, PDGFRB, and
RHOB (
Figure 5a). Additionally, through predictive bioinformatic analysis, we were able to elucidate the specific angiogenic pathways they are involved in (
Figure S1 and
Table S4). Indeed, a bivariate correlation analysis among miRNAs revealed significant positive associations: miR-21-5p and miR-195-5p (R=0.938;
p=0.0001), miR-15b-5p and miR-16-5p (R=0.890;
p=0.001), and miR-16-5p and miR-29b-3p (R=0.735;
p=0.16). On the other hand, miRNA-mRNA bivariate correlations demonstrated positive associations: miR-29b-3p and
KDR (R=0.694;
p=0.026), miR-15b-5p and
NOTCH2 (R=0.613;
p=0.05), and a negative association between miR-29a-3p and
RHOB (R=-0.641;
p=0.046).
Additionally, bivariate mRNA-mRNA correlations unveiled a negative correlation between PIK3CG and KDR (R=-.683; p=0.029), PIK3CG and FGF2 (R=-0.618; p=0.05), PIK3CG and NOTCH2 (R=-0.700; p=0.024), PIK3CG and PDGFRA (R=-0.877; p=0.001), and a positive correlation between VEGFA and KDR (R=0.741; p=.014), KDR and PDGFRB (R=0.715; p=0.02), FGF2 and PDGFRA (R=0.685; p=0.029), FOS and PDGFC (R=0.782; p=0.007), NOTCH2 and PDGFRA (R=0.766; p=0.01), NOTCH2 and PDGFRB (R=0.625; p=0.05), and PDGFC and PDGFRA (R=0.735; p=0.015).
Cytoscape software was used to draw the interaction network between miRNAs and their 12 angiogenic target genes differently expressed and considering the comparative results of expression levels of these miRNA/mRNAs between elderly and middle-aged patients. Grey nodes show the miRNAs that have similar expression levels in both elderly and middle-aged patients: Black nodes show the miRNAs that are upregulated in elderly patients compared with middle-aged patients; green nodes show the mRNAs that are downregulated in elderly patients compared with middle-aged patients; and red nodes show the mRNAs that are upregulated in elderly versus middle aged patients. Lines show potential interaction, direct or indirect, between miRNAs and their target genes; dots indicate potential upregulation of VEGFA; dashes indicate downreglated target genes; sine wave indicate potentially upregulated target genes. b) Cytoscape was used to draw the putative interaction network among miRNAs, 12 target genes and predicted pathways. Green nodes show the mRNAs that are downregulated in elderly patients compared with middle-aged patients in TAT and red nodes show the mRNAs that are upregulated in elderly versus middle aged patients in TAT. Lines show potential participation of target genes in different signaling pathways. Sine waves indicate potential regulation by miR-29a/miR-29b cluster; dots indicate potential regulation by miR-15b/miR-16 cluster; dashes indicate potential regulation by miR-21 and solid indicate potential regulation by miR-195. Hear, only predicted interactions are shown.
3. Discussion
Previously, we presented evidence that the TAT is an attractive new source of adipose tissue and angiogenic factors highlighting its angiogenic properties improve in old age, while these characteristics are impaired in the SAT (8, 16). Moreover, we also showed that VEGFA is the most upregulated angiogenic factor in the TAT in comparison with the SAT (8). The miRNAs are recognized to play a pivotal role in inducing angiogenesis for the treatment of ischemic diseases (17), and alterations in their expression during aging contributes to an age-dependent decline in vascular function and angiogenesis (18). Thus, we considered necessary to extend our previous studies by analyzing the potential role of miRNAs in the regulation of angiogenic properties in adipose tissue, especially the TAT.
Our findings suggest that the upregulation of the VEGFA pathway may be linked to changes in the expression of specific miRNAs, specifically miR-15b and miR-29a.
The miR-15 family comprises six highly conserved members: miR-15a, miR-15b, miR-16-1, miR-16-2, miR-195, and miR-497 (19). Among these, three are expressed in the TAT of elderly patients, namely miR-15b, miR-16, and miR-195. It is important to note that members of a miRNA cluster or family exhibit different expression levels and co-expression patterns, suggesting potential miRNA-miRNA interactions both within and between clusters or families. Additionally, the expression of each miRNA within the same cluster or family is dependent on the expression of other members of the cluster (20).
In this context, it has been reported that the miR-15b/miR-16 cluster contributes to the upregulation of VEGF (20-22). Therefore, based on our results, the overexpression of miR-15b and co-expression of miR-16 may be associated with an upregulation of VEGFA. Our findings also demonstrate a positive correlation in expression levels between miR-16 and miR-29b (R=0.735; p=0.016), and between miR-195 and miR-21 (R=0.938; p=0.0001). This suggests that their co-expression with members of the miR-15 or miR-29 family may promote the expression of various key genes involved in angiogenesis. Although miRNAs from the same cluster may not follow the same expression pattern, as seen with miR-15b and miR-16 or miR-29a and miR-29b, where one shows an upregulation and the other does not alter its gene expression, it cannot be ruled out that they are not involved in the regulation of the VEGFA pathway. The mere fact that they are expressed could contribute as significant members of their cluster in the regulation of VEGF, particularly in the case of the TAT.
Furthermore, it is known that the Wnt/β-catenin signaling pathway plays a significant role in the angiogenic activity of endothelial cells (ECs) (23). In relation to this, a study highlights three highly conserved members of the miR-15 family: miR-15a, miR-15b, and miR-195, which could serve as critical regulators of WNT7A. Among them, miR-15b demonstrated an inverse correlation with WNT7A (19). This phenomenon may also be occurring in the TAT of elderly patients, as our results indicate an inverse expression relationship between miR-15b and WNT7A.
Moreover, it is widely recognized that neovascularization involves the activation, proliferation, and migration of mature endothelial cells that extend the pre-existing vascular network (angiogenesis). Additionally, it is known that endothelial progenitor cells (EPCs) can promote neovascularization through the paracrine secretion of angiogenic factors and cytokines (24). Indeed, a study demonstrates the dysregulation of miRNAs in these cells, including members of the miR-29 family, which may play a pivotal role in modulating angiogenesis and the function of EPCs. This includes VEGF signaling, extracellular matrix remodeling, PI3K/AKT/MAPK signaling, the transforming growth factor-beta (TGFβ) pathway, p53, and cell cycle progression (24). In this context, we have shown a differential expression of miR-29a and VEGFA, which may align with this observation.
In our previous study, we highlighted that KDR (VEGF-R2) exhibits higher expression levels in TAT compared to SAT, with thymic expression being particularly elevated in elderly patients compared to middle-aged (8). In the current investigation, we once again demonstrate an upregulation of KDR. Our findings are in line with research indicating that the primary pro-angiogenic signal arises from VEGF-R2 activated by VEGF (25). Furthermore, another study underscores a direct regulation of KDR by miR-15b, which aligns with our results (26).
Microvascular network growth and the angiogenic properties of the TAT in elderly patients with cardiomyopathy also seem to involve the upregulation of PDGFC, FGF2, NOTCH2, FOS, JAG1 and PDGFRA target genes. In fact, all these factors are mainly involved in angiogenesis, adipogenesis, and VEGF and WNT signaling pathways (27-35). This finding is outstanding, given that in other tissues there is an impaired angiogenesis that is associated with aging and is typically accompanied by decreased expression of these angiogenic factors (36).
PI 3-Kinase plays a pivotal role in neovascularization and angiogenesis, recognized as a key signaling molecule orchestrating various cellular functions such as growth, survival, and migration. This enzyme also influences the permeability of endothelial cells and the production of nitric oxide, as evidenced by studies (37,38). The implication of this kinase in regulating the angiogenic response is complex and highly context-dependent. It can be either overexpressed or inhibited depending on a range of factors, including the cell type involved, the specific microenvironment, and the presence of other regulators. The results from this study imply that the downregulation of PI3 kinase in the TAT of elderly subjects may serve as a crucial factor within the identified miRNA/mRNA network and potentially could play a a crucial role in enhancing the angiogenic function that is specific to the TAT in these elderly individuals, as described in our previous study (8,16,39). In support of our statement, the correlation analysis has revealed a negative connection between the expression levels of the angiogenic genes; KDR, FGF2, and PDGFRA and PIK3CG.
Our results highlight an intriguing miRNA/mRNA network that may play a significant role in regulating the angiogenic functionality of the elderly TAT, as described in our previous studies (8, 16, 39). In these previous studies, we found that TAT exhibits tube formation capacity, enhanced cellular production, vascular endothelial growth factor secretion levels, and the ability to promote endothelial cell survival (40). Additionally, we have demonstrated elevated angiogenic effects of TAT compared to SAT in elderly individuals (8). Therefore, our findings provide new insights into the potential upregulation mechanism of this angiogenic function in TAT, implicating a miRNA/mRNA network well-known for its role in angiogenesis and the generation of new blood vessels. This network primarily involves a crucial factor, VEGF, known to be instrumental in inducing neovascularization. Both experimental and clinical evidence has underscored VEGF's pivotal role in promoting neovascularization (41). Furthermore, we highlight the involvement of FGF2 in this network, which has been utilized in preclinical and clinical studies to stimulate the formation of new blood vessels in damaged or poorly vascularized tissues. It can be administered directly or through tissue engineering techniques (42, 43). Additionally, PDGF has been considered in angiogenic therapy for its potential to promote the proliferation and migration of endothelial cells, thus stimulating angiogenesis in tissues requiring it. Furthermore, PDGFD has been shown to enhance the angiogenic capacity of endothelial progenitor cells, including proliferation, migration, adhesion, and tube formation, thereby contributing to angiogenesis (44). Moreover, given that miR29a and miR15b are integral components of this network, we believe that these miRNAs could play a significant role in promoting neovascularization and angiogenesis within elderly TAT.
Therefore, conducting in vitro and in vivo studies with these miRNAs to elucidate their potential role in regulating the angiogenic functionality of TAT, as well as their possible contribution to the utilization of TAT in regenerative medicine and neovascularization, holds significant promise. We believe that a comprehensive examination of its involvement in regulating the enhancement of the angiogenic function of the elderly TAT could offer fresh and compelling insights in the field of investigating the neovascularization and microvascular network development potential of adipose tissue, presenting a notable challenge and opportunity for future therapeutic advancements in regenerative medicine (45).
4. Materials and Methods
The study was approved by the local ethics committee of Málaga Regional University Hospital (code number: PI07/00288) and performed in accordance with the Ethical Principles for Medical Research Involving Human Subjects adopted in the Declaration of Helsinki by the World Medical Association and the Regulation (EU) 2016/679 of the European Parliament and of the Council 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data (General Data Protection Regulation). The written informed consents were obtained from all participants by the Spanish Thoracic and Cardiovascular Surgery Society (SECTCV).
Eighteen patients who had a coronary artery bypass graft (CABG) surgery with cardiopulmonary bypass as consequence of an ischemic cardiomyopathy were recruited from the department of Cardiology at Hospital Regional Universitario de Málaga. CABG surgery is a procedure that use the veins or arteries of the patients to bypass narrowed areas in order to restore blood flow to myocardium. Patients were divided into two groups according to aging: elderly and middle-aged groups.
All patients who were recruited were medically stable without severe ischemic injury. Patients had no infarction or prior infarction at least 6 months before surgery. None of them suffered from leg ischemic peripheral arteriopathy, which could potentially alter the expression of genes encoding angiogenic factors.
The tissue samples of SAT and TAT were removed at the beginning of the CABG practice and before the cardiac arrest. The SAT was obtained from the chest incision during the CABG. All tissue samples were immediately stored at -80ºC prior to subsequent procedures.
miRNAs were isolated from adipose tissue according to the procedure described previously (13). The mirVana™ miRNA Isolation Kit (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) was used according to the manufacturer’s guidelines. miRNA concentration and purity were determined by NanoDrop1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) software ND-1000 v3.7.1. Complementary DNA (cDNA) was obtained using the TaqMan® MicroRNA Reverse Transcription Kit (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA) (8 ng RNA/5 μL) and specific primers and probes for each miRNA were used (TaqMan® MicroRNA Assay from Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA): hsa-miR-15b-5p (assay ID 000390); hsa-miR-16-5p (assay ID 000391); hsa-miR-21-5p (assay ID 000397); hsa-miR-29a-3p (assay ID 002112); hsa-miR-29b-3p (assay ID 000413); hsa-miR-195-5p (assay ID 000494). Reverse transcription was performed as described previously (34) and miRNA expression levels were measured by Stratagene Mx3005p real-time polymerase chain-reaction system (RT-qPCR) (Agilent Technologies, Santa Clara, CA, USA). All samples were analyzed in duplicate, and the relative quantification of miRNA levels was performed using the comparative threshold cycle (Ct) method according to the manufacturer’s guidelines. hsa-RNU48 (TaqMan® MicroRNA Assay ID 001006 from Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA) was assessed using Bestkeeper© software to verify its usability as a reference gene (
http://www.gene-quantification.de/bestkeeper.html) and hsa-RNU48 was used as the endogenous control.
The RNeasy® Lipid Tissue Mini Kit (Qiagen, Washington, MD, USA) was used to isolate total mRNA from adipose tissue and RNA concentration and purity were determined using a NanoDrop1000 spectrophotometer (Thermo Fischer Scientific, Waltham, MA, USA) software ND-1000 v3.7.1. cDNA synthesis was achieved using Transcriptor Reverse Transcriptase Kit (Roche Diagnostics, Barcelona, Spain) according to the manufacturer’s instructions and the RT-qPCR performed using Ultra-Fast SYBRGreen qPCR Master mix and assayed in a Mx3005p qPCR system (Agilent Technologies, Santa Clara, CA, USA).
ΔCt values for each PCR product were established using a threshold value. The reference gene was selected using Bestkeeper© software (
http://www.gene-quantification.de/bestkeeper.html). Primers used for RT-qPCR reactions are detailed in
Bioinformatic analysis
miRTargetLink Human software (13) (website:
https://ccb-web.cs.uni-saarland.de/mirtargetlink/) was used to search for miRNAs that could be regulating
VEGFA and to predict putative miRNA target genes. The TargetScan Human 7.2 tool (14) was used to calculate TargetScore which measures conservation strength (site type: mer8 > 7mer-m8 > 7mer-A1 > 6mer); evolutionary conservation and targeting efficiency (context++ score percentile) and biological relevance (site conservation feature; P
CT) as described in (34). The PANTHER Database (Protein ANalysis Through Evolutionary Relationship) Classification System (
http://www.pantherdb.org/) was applied to annotate the biological processes of target genes. Potential interactions between miRNAs, angiogenesis and the expressed target genes and their pathways were visualized with Cytoscape v.3.2.1 software (
http://www.cytoscape.org/).
The results are expressed as the mean ± standard error of the mean (SEM). The Shapiro-Wilk test was used to assess the normal distribution of continuous variables, while variance heterogeneity was analyzed using the Levene’s test. Subsequently, data was analyzed using the Student’s t-test or U the Mann-Whitney. A bivariate correlation analysis was used to determine the association between miRNAs and their TGs. All statistical analyses were carried out with the statistical software package SPSS (version 22.0 SPSS; IBM, Chicago, IL, USA). We considered statistically significant p values equal to or less than 0.05 (p≤0.05).
5. Conclusions
In summary, our findings strongly indicate the involvement of novel epigenetic factors, particularly miR-195, miR-15b, miR-21, and miR-29a, in driving a heightened angiogenic profile in TAT among elderly patients with cardiomyopathy compared to middle-aged patients. These miRNAs likely play a pivotal role in enhancing TAT's neovascular potential with age. While further research is needed to elucidate the precise interactions between these miRNAs and co-expressed angiogenic factors in TAT, our results represent a significant stride in comprehending the intricate mechanisms underlying TAT's remarkable capacity to produce angiogenic factors. This breakthrough holds great promise for advancing regenerative angiogenic therapies, particularly for individuals with ischemic cardiomyopathy.
6. Study Limitation
One of the limitations of this study is the small sample size, which could potentially restrict the generalizability of the findings. However, we believe that despite the challenge of recruiting such specific patients, we have managed to assemble a relatively homogeneous patient group, which may offset this limitation. Additionally, the fact that the only source of thymic fat is from ischemic cardiomyopathy subjects undergoing coronary artery bypass graft (CABG) surgery with cardiopulmonary bypass precludes the possibility of conducting this type of study using a control group of healthy subjects.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, Table S1: miRNA predicted to regulate
VEGF-A, Table S2: TargetScan score calculation to miRNA-mRNA binding sites within
VEGF-A Table S3:. Gene aliases and names, Table S4:. Angiogenic pathway of differentially expressed target genes, Table S5: Gene expression primers .
Author Contributions
A.M.G. and R.E.B.-R. designed the research and analyzed the data. A.M.G., F.J.P.-M. and R.E.B.-R. wrote and revised the paper. A.M.G., S.L, and M.M.-M. performed molecular biology experiments. A.M.G. and S.L. performed bioinformatics and statistical analysis and the interpretation. J.S. is the surgeon who recruited subjects and extracted biopsies during CABG surgery. G.O.-F., P.C. -L. and R.E.B.-R. contributed to clinical analysis. J.O. and J.R.P. contributed to manuscript writing and editing. G.O.-F. and J.S. contributed to recruitment of patients and clinical analysis.
Funding
This research was supported in by the following grants: Project funded by the ISCIII and the European Regional Development Fund (ERDF-EU) (PI18/00785, PI21/01924), project funded by the Consejería de Transformación Económica, Industria, Conocimiento y Universidades-Junta de Andalucía and ERDF-EU (PI20-01274) and project funded by University of Málaga and ERDF-EU (UMA20-FEDERJA-074). S.L. is a recipient of a Plan Andaluz de Investigación, Desarrollo e Innovación post-doctoral grant from the Consejería de Economía, Conocimiento, Empresas y Universidades (DOC-01138). A.M.G. was a recipient of a Plan Propio de Investigación, Transferencia y Divulgación Científica postdoctoral grant from University of Málaga. F.J.P.-M. and R.E.B.-R. are under a contract from the ‘Nicolas Monardes’ program from the Servicio Andaluz de Salud, Consejería de Salud y Consumo-Junta de Andalucía (C1-0049-2019 and C-0030-2016, respectively). M.C.-P. was a recipient of a Juan de la Cierva Formación post-doctoral grant from the Ministerio de Ciencia, Innovación y Universidades-Gobierno de España (FJCI-2017-32194).
Institutional Review Board Statement
The study was approved by the local ethics committee of Málaga Regional University Hospital (code number: PI07/00288) and performed in accordance with the Ethical Principles for Medical Research Involving Human Subjects adopted in the Declaration of Helsinki by the World Medical Association and the Regulation (EU) 2016/679 of the European Parliament and of the Council 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data (General Data Protection Regulation). The written informed consents were obtained from all participants by the Spanish Thoracic and Cardiovascular Surgery Society (SECTCV).
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Acknowledgments
The authors wish to thank all the patients for their participation in this study. The Centro de Investigación Biomédica en Red de Fisiopatología de la Obesidad y Nutrición (CIBERobn), Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas. (CIBERdem) and Centro de Investigación Biomédica en Red de las Enfermedades Cardiovasculares (CIBERCV) are part of the Instituto de Salud del Carlos III (ISCIII).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Myocardial ischemia-reperfusion injury and the influence of inflammation. Trends Cardiovasc Med. 2022. [CrossRef]
- Damluji AA, van Diepen S, Katz JN, Menon V, Tamis-Holland JE, Bakitas M, Cohen MG, Balsam LB, Chikwe J. American Heart Association Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Surgery and Anesthesia; and Council on Cardiovascular and Stroke Nursing. Mechanical Complications of Acute Myocardial Infarction: A Scientific Statement From the American Heart Association. Circulation. 2021. [CrossRef]
- Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004. [CrossRef]
- Kondo K, Shintani S, Shibata R, Murakami H, Murakami R, Imaizumi T, Kitagawa S, Murohara T. Implantation of adipose-derived regenerative cells enhances ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2009.
- El-Ftesi S, Chang EI, Longaker MT, Gurtner GC. Aging and diabetes impair the neovascular potential of adipose-derived stromal cells. Plast Reconstr Surg. 2009. [CrossRef]
- Efimenko A, Starostina E, Kalinina N, Stolzing A. Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J Transl Med. 2011. [CrossRef]
- Kvell K, Pongracz JE. Central Immune Senescence, Reversal Potentials. In: Nagata T, editor. Senescence [Internet]. Rijeka (HR): InTech; 2012 Feb 29. Chapter 31.
- Coín Aragüez L, Murri M, Oliva Olivera W, Salas J, Mayas MD, Delgado-Lista J, Tinahones F, El Bekay R. Thymus fat as an attractive source of angiogenic factors in elderly subjects with myocardial ischemia. Age (Dordr). 2013. [CrossRef]
- Salas J, Montiel M, Jiménez E, Valenzuela M, Valderrama JF, Castillo R, González S, El Bekay R. Angiogenic properties of adult human thymus fat. Cell Tissue Res. 2009. [CrossRef]
- Holmes DIR, Zachary I. The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biology. 2005. [CrossRef]
- Bhatti GK, Khullar N, Sidhu IS, Navik US, Reddy AP, Reddy PH, Bhatti JS. Emerging role of non-coding RNA in health and disease. Metab Brain Dis. 2021. [CrossRef]
- Siebert V, Allencherril J, Ye Y, Wehrens XHT, Birnbaum Y. The Role of Non-coding RNAs in Ischemic Myocardial Reperfusion Injury. Cardiovasc Drugs Ther. 2019. [CrossRef]
- Enright AJ, John B, Gaul U, Tuschl T, Sander C, Marks DS. MicroRNA targets in Drosophila. Genome Biol. 2003. [CrossRef]
- Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015. [CrossRef]
- .Lhamyani S, Gentile AM, Giráldez-Pérez RM, Feijóo-Cuaresma M, Romero-Zerbo SY, Clemente-Postigo M, Zayed H, Olivera WO, Bermúdez-Silva FJ, Salas J, Gómez CL, Hmadcha A, Hajji N, Olveira G, Tinahones FJ, El Bekay R. miR-21 mimic blocks obesity in mice: A novel therapeutic option. Mol Ther Nucleic Acids. 2021. [CrossRef]
- Oliva-Olivera W, Coín-Aragüez L, Salas J, Lhamyani S, Gentile AM, Sarria García E, Hmadcha A, Zayed H, Vega-Rioja A, Tinahones FJ, El Bekay R. Myocardial Ischemic Subject's Thymus Fat: A Novel Source of Multipotent Stromal Cells. PLoS One. 2015. [CrossRef]
- Ding MH, Lozoya EG, Rico RN, Chew SA. The Role of Angiogenesis-Inducing microRNAs in Vascular Tissue Engineering. Tissue Eng Part A. 2020. [CrossRef]
- Che P, Liu J, Shan Z, Wu R, Yao C, Cui J, Zhu X, Wang J, Burnett MS, Wang S, Wang J. miR-125a-5p impairs endothelial cell angiogenesis in aging mice via RTEF-1 downregulation. Aging Cell. 2014. [CrossRef]
- MacLean JA 2nd, King ML, Okuda H, Hayashi K. WNT7A Regulation by miR-15b in Ovarian Cancer. PLoS One. 2016 May 19;11(5):e0156109. [CrossRef]
- Kabekkodu SP, Shukla V, Varghese VK, D' Souza J, Chakrabarty S, Satyamoorthy K. Clustered miRNAs and their role in biological functions and diseases. Biol Rev Camb Philos Soc. 2018 Nov;93(4):1955-1986. [CrossRef]
- Zhao WJ, Zhang HF, Su JY. Downregulation of microRNA-195 promotes angiogenesis induced by cerebral infarction via targeting VEGFA. Mol Med Rep. 2017. [CrossRef]
- Yang IP, Tsai HL, Huang CW, Lu CY, Miao ZF, Chang SF, Juo SH, Wang JY. High blood sugar levels significantly impact the prognosis of colorectal cancer patients through down-regulation of microRNA-16 by targeting Myb and VEGFR2. Oncotarget. 2016 Apr 5;7(14):18837-50. [CrossRef]
- Jiang L, Yin M, Wei X, Liu J, Wang X, Niu C, Kang X, Xu J, Zhou Z, Sun S, Wang X, Zheng X, Duan S, Yao K, Qian R, Sun N, Chen A, Wang R, Zhang J, Chen S, Meng D. Bach1 Represses Wnt/β-Catenin Signaling and Angiogenesis. Circ Res. 2015 Jul 31;117(4):364-375. [CrossRef]
- Desjarlais M, Dussault S, Rivera JC, Chemtob S, Rivard A. MicroRNA Expression Profiling of Bone Marrow-Derived Proangiogenic Cells (PACs) in a Mouse Model of Hindlimb Ischemia: Modulation by Classical Cardiovascular Risk Factors. Front Genet. 2020 Aug 21;11:947. [CrossRef]
- Lim HN, Jang JP, Han JM, Jang JH, Ahn JS, Jung HJ. Antiangiogenic Potential of Microbial Metabolite Elaiophylin for Targeting Tumor Angiogenesis. Molecules. 2018 Mar 2;23(3):563. [CrossRef]
- Cakmak H, Gokmen E, Bozkurt G, Kocaturk T, Ergin K. Effects of sunitinib and bevacizumab on VEGF and miRNA levels on corneal neovascularization. Cutan Ocul Toxicol. 2018 Jun;37(2):191-195. [CrossRef]
- Cignarelli A, Perrini S, Nigro P, Ficarella R, Barbaro M, Peschechera A, Porro S, Natalicchio A, Laviola L, Puglisi F, Giorgino F. Long-acting insulin analog detemir displays reduced effects on adipocyte differentiation of human subcutaneous and visceral adipose stem cells. Nutr Metab Cardiovasc Dis. 2016. [CrossRef]
- Gaebler N, Haggenmüller B, Kapapa M, Serra A, Tews D, Funcke JB, Brandt S, Ioannidis V, Schön M, Möller P, Debatin KM, Wabitsch M, Fischer-Posovszky P. Age- and BMI-Associated Expression of Angiogenic Factors in White Adipose Tissue of Children. Int J Mol Sci. 2019. [CrossRef]
- Gentile AM, Lhamyani S, Coín-Aragüez L, Clemente-Postigo M, Oliva Olivera W, Romero-Zerbo SY, García-Serrano S, García-Escobar E, Zayed H, Doblado E, Bermúdez-Silva FJ, Murri M, Tinahones FJ, El Bekay R. miR-20b, miR-296, and Let-7f Expression in Human Adipose Tissue is Related to Obesity and Type 2 Diabetes. Obesity (Silver Spring). 2019. [CrossRef]
- Hindy G, Mollet IG, Rukh G, Ericson U, Orho-Melander M. Several type 2 diabetes-associated variants in genes annotated to WNT signaling interact with dietary fiber in relation to incidence of type 2 diabetes. Genes Nutr. 2016. [CrossRef]
- Boucher JM, Ryzhova L, Harrington A, Davis-Knowlton J, Turner JE, Cooper E, Maridas D, Ryzhov S, Rosen CJ, Vary CPH, Liaw L. Pathological Conversion of Mouse Perivascular Adipose Tissue by Notch Activation. Arterioscler Thromb Vasc Biol. 2020. [CrossRef]
- Chen Z, Yu H, Shi X, Warren CR, Lotta LA, Friesen M, Meissner TB, Langenberg C, Wabitsch M, Wareham N, Benson MD, Gerszten RE, Cowan CA. Functional Screening of Candidate Causal Genes for Insulin Resistance in Human Preadipocytes and Adipocytes. Circ Res. 2020. [CrossRef]
- Yang X, Pande S, Koza RA, Friesel R. Sprouty1 regulates gonadal white adipose tissue growth through a PDGFRα/β-Akt pathway. Adipocyte. 2021. [CrossRef]
- Kakudo N, Kushida S, Suzuki K, Matsumoto N, Kusumoto K. Effect of C3 transferase on human adipose-derived stem cells. Hum Cell. 2011. [CrossRef]
- Liu J, Shi Y, Wu M, Zhang F, Xu M, He Z, Tang M. JAG1 enhances angiogenesis in triple-negative breast cancer through promoting the secretion of exosomal lncRNA MALAT1. Genes Dis. 2022 Aug 5;10(5):2167-2178. [CrossRef]
- Hodges NA, Suarez Martinez AD, Murfee WL. Understanding angiogenesis during aging: opportunities for discoveries and new models. J Appl Physiol. 2018. [CrossRef]
- Roshandel E, Noorazar L, Farhadihosseinabadi B, Mehdizadeh M, Kazemi MH, Parkhideh S. PI3 kinase signaling pathway in hematopoietic cancers: A glance in miRNA's role. J Clin Lab Anal. 2021 Apr;35(4):e23725. [CrossRef]
- Semba S, Itoh N, Ito M, Youssef EM, Harada M, Moriya T, Kimura W, Yamakawa M. Down-regulation of PIK3CG, a catalytic subunit of phosphatidylinositol 3-OH kinase, by CpG hypermethylation in human colorectal carcinoma. Clin Cancer Res. 2002 Dec;8(12):3824-31.
- Salas J, Montiel M, Jiménez E, Valenzuela M, Valderrama JF, Castillo R, González S, El Bekay R. Angiogenic properties of adult human thymus fat. Cell Tissue Res. 2009 Nov;338(2):313-8. [CrossRef]
- Oliva-Olivera W, Coín-Aragüez L, Lhamyani S, Salas J, Gentile AM, Romero-Zerbo SY, Zayed H, Valderrama J, Tinahones FJ, El Bekay R. Differences in the neovascular potential of thymus versus subcutaneous adipose-derived stem cells from patients with myocardial ischaemia. J Tissue Eng Regen Med. 2018 Mar;12(3):e1772-e1784. [CrossRef]
- Staels W, Heremans Y, Heimberg H, De Leu N. VEGF-A and blood vessels: a beta cell perspective. Diabetologia. 2019 Nov;62(11):1961-1968. [CrossRef]
- Akl MR, Nagpal P, Ayoub NM, Tai B, Prabhu SA, Capac CM, Gliksman M, Goy A, Suh KS. Molecular and clinical significance of fibroblast growth factor 2 (FGF2 /bFGF) in malignancies of solid and hematological cancers for personalized therapies. Oncotarget. 2016 Jul 12;7(28):44735-44762. [CrossRef]
- Nakamura, Y. Multiple Therapeutic Applications of RBM-007, an Anti-FGF2 Aptamer. Cells. 2021 Jun 28;10(7):1617. [CrossRef]
- Zhang J, Zhang H, Chen Y, Fu J, Lei Y, Sun J, Tang B. Platelet-derived growth factor D promotes the angiogenic capacity of endothelial progenitor cells. Mol Med Rep. 2019 Jan;19(1):125-132. [CrossRef]
- Laschke MW, Menger MD. Microvascular Fragments in Microcirculation Research and Regenerative Medicine. Tissue Eng Part B Rev. 2022 Oct;28(5):1109-1120. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).