Introduction
The ubiquitin proteasome system (UPS) is a highly regulated and dynamic process that utilizes a three-step enzymatic cascade to attach small molecules of the ubiquitin family onto proteins to alter their function and/ or mark ubiquitinated proteins for proteasomal degradation. The extensive Ubiquitin and Ubiquitin-like Conjugation Database (UUCD) lists enzymes involved in ubiquitin post-translational modification of proteins1. In eukaryotes, this includes one human ubiquitin-activating (E1) enzyme, 43 E2 ubiquitin-conjugating enzymes, 468 enzymes with E3 ligase activity, 538 E3 ligase adaptors, and approx. 100 de-ubiquitin enzymes (DUBs)2, 3. Ubiquitin is one of several ubiquitin-like protein modifiers that also includes ubiquilins, SUMO, NEDD8, and ISG154. While cellular regulators in their own right, these post-translational modifiers can cross-communicate with ubiquitin through modifications or are being modified by (poly)ubiquitin5. Ubiquitin has eight ubiquitination sites, including seven lysine (K) residues (K6, K11, K27, K29, K33, K48, K63) and a primary amine at the N-terminus5. While mono-ubiquitination and K48- and K63-linked polyubiquitination are the most abundant forms, multiple other types of ubiquitination exist which have distinct functional outcomes6. Monoubiquitination refers to the attachment of a single ubiquitin molecule to a target protein and serves as a signal for protein recognition, complex formation, or allosteric regulation. The addition of a single ubiquitin moiety during mono-ubiquitination can influence the localization, activity, or interaction of the modified protein within the cell. Polyubiquitination refers to the formation of a covalently linked ubiquitin molecule chain attached to a specific lysine residue of ubiquitin. When a protein is polyubiquitinated with K48-linked ubiquitin chains, it is recognized by the proteasome and targeted for degradation. Unlike K48-linked ubiquitin chains, K63-linked ubiquitin chains do not target proteins for degradation but enable context specific functions of K63 ubiquitinated proteins in cellular signaling, intracellular trafficking, autophagy, and DNA damage responses7, 8. K63-linked ubiquitin chains serve as scaffolds for protein-protein interactions, modulate enzyme activity, and regulate the localization and function of target proteins9, 10.
Deubiquitinating enzymes (DUBs) contribute to the regulation of a variety of biological processes, including proteasomal degradation of proteins, cell cycle regulation, histone modifications, transcriptional and translational control, protein trafficking, macro- and mitophagy, DNA damage response, epigenetic processes, and immune response signaling5. DUBs reverse the process of protein ubiquitination by selectively removing ubiquitin molecules or chains from proteins. Hence, DUBs are editors of the ubiquitin code and remove single ubiquitin molecules, entire ubiquitin chains, or ubiquitin branches from a ubiquitinated protein by cleaving ubiquitin substrate bonds and ubiquitin-ubiquitin peptide bonds11, 12 The approx. 100 putative DUBs identified so far in the human proteome are classified into five major families based on their structural and functional characteristics13-15. The ubiquitin-specific proteases (USP) are the largest subclass of DUBs, with currently 54 members in humans16. USPs contain a conserved catalytic domain known as the ubiquitin-specific protease domain and exhibit specificity towards different types of ubiquitin linkages. Ubiquitin carboxy-terminal hydrolases (UCH) family members (4 members) possess a distinct catalytic domain called the UCH domain. UCHL members are involved in the processing of ubiquitin precursors and the removal of ubiquitin from proteins17-19. DUBs of the ovarian tumor proteases (OTU) family (16 members) contain an ovarian tumor (OTU) domain and are involved in various cellular processes, including immune signaling, pathogen infection, and DNA damage response20-22. The Machado-Joseph disease proteases (MJD) family of DUB proteins (4 members) possess a Josephin domain, prefer K48/ K63 linkages, and are associated with neurodegenerative disorders, particularly Machado-Joseph disease23. The JAB1/ MPN/ Mov34 (JAMM) metalloenzyme family (16 members) of DUBs contains a metalloprotease domain and prefers targeting K63 ubiquitination sites. JAMM member CSN5 is a deNEDDylase24-26. The MINDY family is a recent DUB addition, with two of the 4 family members containing a “motif interacting with ubiquitin” (MIU) which assists in the enzymatic cleave of long K48 polyubiquitin chains27. Finally, a diverse group of ubiquitin-like proteases (ULPs) targets ubiquitin-like modifiers other than ubiquitin and comprises SENP (sentrin/ SUMO-specific protease), DeSI (deSUMOylating isopeptidase) families], and NEDD828-30.
DUBs are essential for the dynamic and coordinated actions of the UPS and ensure proper functions of virtually all cellular processes, including the control of cellular levels of key regulatory transcription factors, growth factors, morphogens, cell cycle regulators, and the balance of factors regulating cell survival. The enzymatic removal of ubiquitin groups by DUBs is critical for reversible ubiquitination and the recycling of unbound ubiquitin to the UPS and ERAD (endoplasmic reticulum-(ER) associated degradation) pathways. Cellular DUB activity determines the coordinated regulation of both the UPS and ERAD pathways in a tissue region- and context-specific manner31.
Extending throughout eukaryotic cells, the ER is the largest cellular organelle and composed of a series of sheet like and tubular structures that form close contacts with other organelles, in particular the nucleus and mitochondria32. The ER can be subdivided into two types, the smooth and the rough ER. While the smooth ER facilitates lipid synthesis and hormone synthesis, the rough ER is the site of protein folding, modification, and quality control32. Signal peptides direct newly synthesized proteins to the ER lumen where ER localized chaperones and enzymes facilitate protein folding and modifications. Correctly folded and processed proteins are then shuttled, via transport vesicles, to the Golgi apparatus and from there to their final destination33. The folding and modification of proteins is highly dependent on the maintenance of a stable ER environment. Exposure to stresses, such as oxygen and glucose deprivation or loss of ER calcium lowers protein folding efficiency resulting in the accumulation of unfolded/ misfolded proteins34. ER function can also be compromised by protein folding demands exceeding capacity. An example being viral infections where the capacity of the ER to facilitate protein folding is overwhelmed, giving rise to misfolded proteins35. Irrespective of the initiating stimulus, the buildup of misfolded/ unfolded proteins is commonly referred to as ER stress. Cells combat ER stress by initiating an adaptive, highly conserved stress response referred to as the Unfolded Protein Response or UPR. The UPR is controlled by three ER anchored transmembrane receptors, Inositol-requiring enzyme 1 (IRE1), protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) and activating transcription factor 6 (ATF6). These three ER-based receptors monitor ER health. In an unstressed setting, each of these stress sensors is held in an “off” position by binding of their luminal domain to the ER chaperone Grp78 (BIP, HSPA5)36, 37. Upon ER stress, Grp78 dissociates from IRE1, PERK, and ATF6 which stimulates their transition from inactive to active states36, 37. Downstream signaling pathways orchestrated by IRE1, PERK, and ATF6 function in a co-operative, complementary manner to support the refolding of those proteins that can be refolded. Those proteins beyond repair are removed via the ER associated degradation or ERAD pathway34. Ultimately, the objective of the UPR is to reduce ER stress, thereby restoring ER homeostasis.
Cancer cells frequently endure both external stressors (e.g., hypoxia and glucose deprivation) and internal stresses triggered by their high proliferation and metabolic rate. To thrive under such conditions and escape immune responses, cancer cells engage and coordinate adaptive responses, including UPS, ERAD, UPR, DNA damage repair38-41. Although these responses may initially be engaged to aid cellular stress adaptation, cancer cells usurp and/ or co-opt these pathways for their benefit in numerous ways. We recently identified distinct gene expression changes in ubiquitin ligases and ligase adaptors in different human brain tumors and subtypes42, 43. Sustained UPR signaling has been reported in diverse cancers including breast, prostate, and brain cancers and emerging evidence links ERAD and UPR to an array of pro-tumorigenic processes, including angiogenesis, metastasis, and cancer stem cell expansion38.
DUBs are an integral part of the UPS but their role in human brain tumors is incompletely understood. Understanding the role of DUBs in brain tumors could yield new therapeutic avenues. In the present study, we have analyzed the gene expression profiles of 99 human DUBs belonging to 7 subgroups listed in the HUGO (Human genome organization) classification. The objective of the current study was to examine the differential gene expression of these DUBs in publicly available datasets of selected human neuronal system tumors, including pediatric (craniopharyngioma (CPh), ependymoma (EPN), medulloblastoma (MB), adult brain tumors (astrocytoma (AS), oligodendroglioma (ODG), glioblastoma (GBM), and neuroblastoma (NBT) as the most common childhood extra-cranial solid tumor arising from the developing sympathetic nervous system44, 45. Some of the datasets had available gene expression data of non-tumor tissues for comparison while other datasets had available age data for each subject. This allowed for plotting gene expression by tumor subgroup and by age for each DUB and enabled the comparison of gene expression in pediatric vs adult age groups. We sought to determine whether expression of DUB genes was selective for specific tumors, specific subgroups of tumors, or specific age groups of subjects with these brain tumors. For those datasets with survival data, we determined whether DUB gene expression was statistically associated with survival. Top DUB hits identified in these bioinformatic screens were interrogated for their association with ERAD, UPR, and DNA repair. This is the first comprehensive report with a focus on DUB family members in selected pediatric and adult brain tumors, their relationship with ERAD, UPR, DNA damage repair pathways, and their suitability as potential biomarkers and therapeutic targets.
Methods
We utilized the human genome list of 99 deubiquitinases to determine the expression of these DUB genes in publicly available datasets of brain tumors. Differential expression of DUB genes was examined in these datasets made available in the R2 Genomics Analysis and Visualization platform (
https://r2/amc/nl). We used the AS, GBM, ODG, and a non-tumor group in the mixed glioma dataset of Sun et al (Geo ID: GSE4290). Differentially expressed DUB genes were considered significant at p < 0.001 as determined by Analysis of Variance (ANOVA) through the R2 Genomics site and plotted using the Morpheus heatmap and cluster analysis program at the Broad Institute website (
https://software.broadinstitute.org/morpheus). DUB gene expression of classic, mesenchymal, and proneural GBM subtypes were examined in the TCGA GBM dataset (R2 ID: Tumor Glioblastoma TCGA 540). Survival data associated with differentially expressed DUB genes was examined in the French glioma data (GSE16022)
46. The Pfister dataset (GSE64415) was used to examine differentially expressed DUB genes in ependymoma. A dataset of Donson (GSE94349) was used to examine differential expression of DUB genes in CPh, while the datasets of Cavalli et al.
47 (GSE85217) and Weishaupt et al.
48 (GSE124814) were used to examine DUB gene expression in MB subgroups. The Cavalli dataset had extensive data on the age of subjects in the various subgroups, which we used to determine the age distribution for the most highly significant differentially expressed DUB genes. Those DUB genes statistically associated with survival of MB patients were also determined in the Cavalli dataset. The Weishaupt data (Swartling dataset in the R2 Genomics database) allowed for a comparison of genes between MB tumor tissue and non-tumor tissue. The NBT dataset of Fischer
49 (GSE120572) was used to evaluate various treatment effects on DUB expression. The Cytoscape program was applied to identify gene ontology (GO) pathways, including ERAD, DNA repair, immune response pathways, and genes coding for differentially expressed DUBs that were associated with brain tumors.
Results
Cytoscape analysis of the 99 DUB genes (GO biological pathways) identified several functional groups of DUBs involved in the deubiquitination of lysine (K) residues K6/ K11/ K27/ K29/ K48/ K63 ubiquitinated proteins. The most significant biological pathways, other than deubiquitination itself, included DNA repair, DNA methylation, the regulation of ER stress and ERAD pathway, death receptor signaling, and the regulation of immune and cytokine responses.
Adult Glioma Show Differential Expression of DUBs
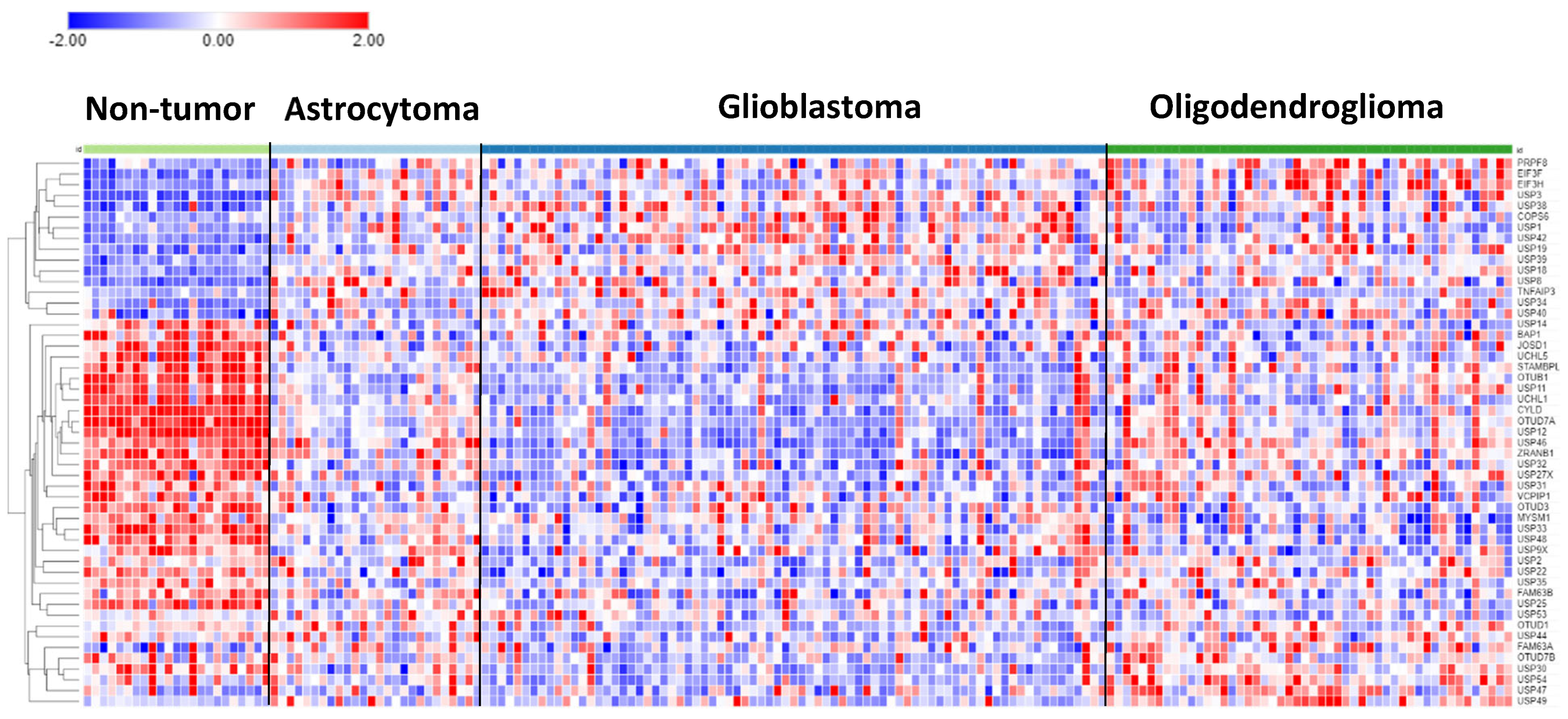
In the Sun mixed glioma dataset, expression of 51 of the 99 DUB genes (HUGO classification) were significantly different (p < 0.001) between the four groups in the dataset (non-tumor, AS, GBM, ODG). The heatmap (
Figure 1) shows the gene expression profiles of the four groups.
Table 1 shows the DUB genes that were most significantly different between the non-tumor group and each of the other three adult glioma groups. Seventeen of the 99 DUB genes were differentially expressed (p < 0.001) between AS, ODG, and GBM (
USP46, USP54, ZRANB1, USP1, OTUD7A, TNFAIP3, USP27x, USP30, EIF3H, USP49, OTUD1, USP11, OTUB1, CYLD, USP12, USP2, USP47; in order of
p value as determined by ANOVA).
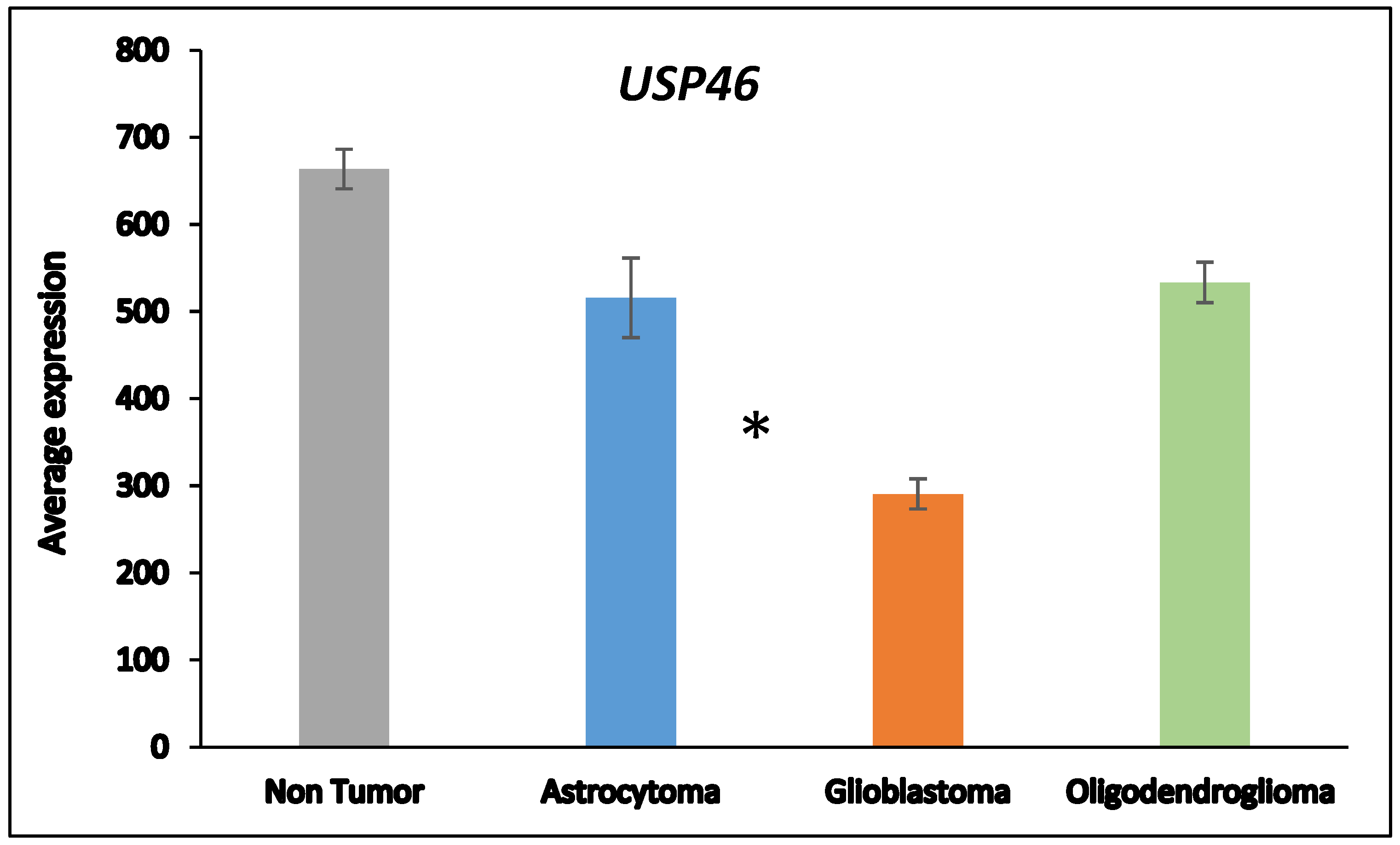
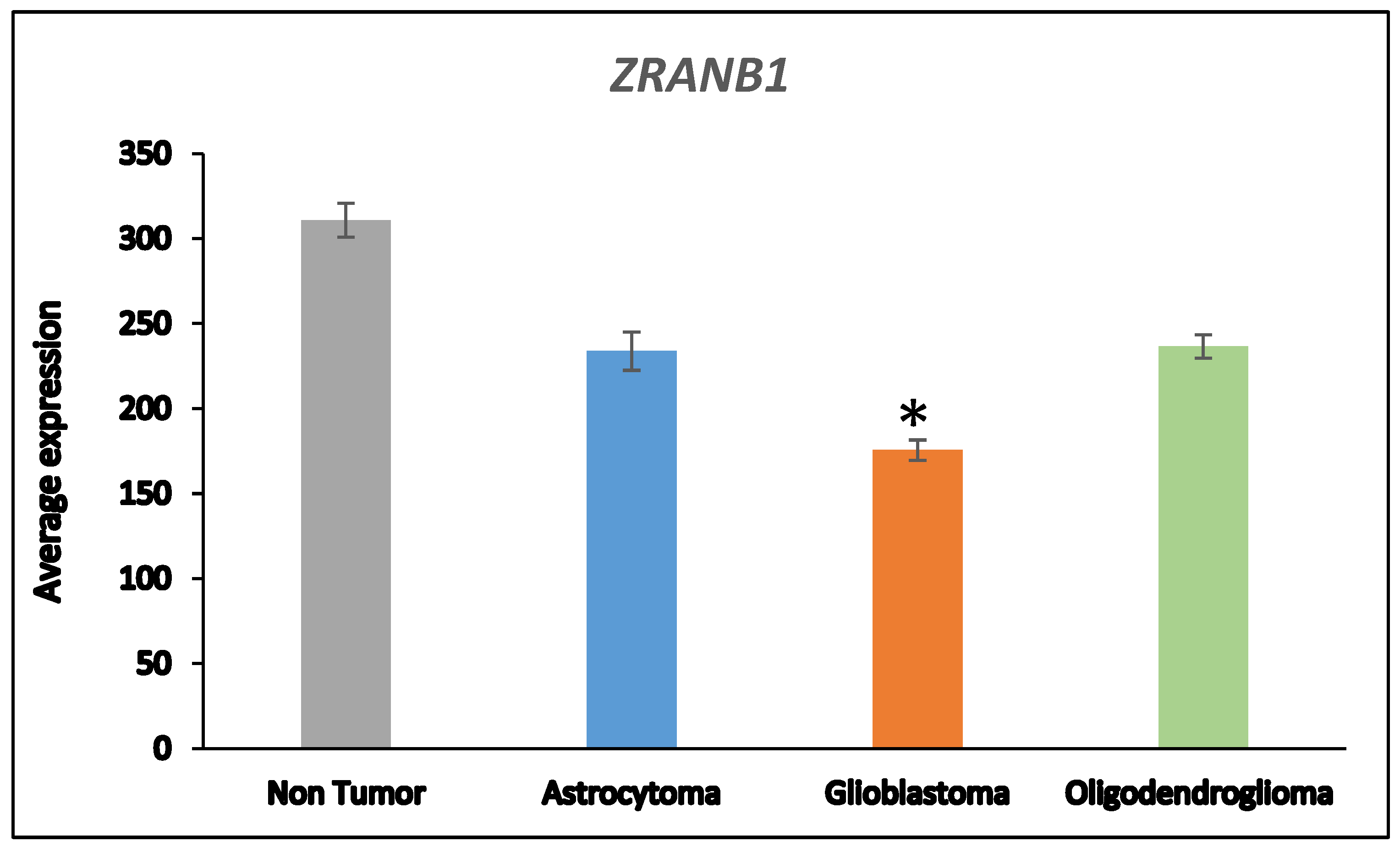
Next, we asked whether the differences in DUB gene expression between AS and GBM were related to the progression from AS to GBM which is associated with several changes in the transcriptome
50. ANOVA showed that the expression of two DUB genes,
USP46 and
ZRANB1, differed at a high level of significance (
p < 0.001) between the AS and GBM group (
Figure 2).
USP46 was among the top 100 of all differentially expressed genes between the AS and GBM groups in the Sun mixed glioma dataset. Of all
18,896 genes in the French dataset,
ZRANB1 and
USP46 expression were ranked 3
rd and 2725
th respectively when associated with survival. ZRANB1 belongs to the OTU class of DUBs and has been reported as an EZH2 (Enhance of zeste homolog 2) DUB
51. EZH2 inhibitors are currently tested for cancer therapy and brain permeable derivatives may offer new avenues in the treatment of brain tumors
52, 53.
Chromosome 10 and DUB Expression in Astrocytic Glioma
The loss of chromosome 10 in primary GBM or loss of the q arm of chromosome 10 in secondary GBM is a common finding54, 55. This has led to the hypothesis that the loss of one or more tumor suppressor genes on the q arm of chromosome 10 may contribute to GBM development. The most significant differentially expressed pathway between the AS and GBM group in the Sun dataset was the “D-glutamine and D-glutamate” pathway which was represented by differential expression of two genes, GLUD1 and GLUD2 (glutamate dehydrogenase 1 and 2). GLUD1 is located on chromosome 10 and codes for the mitochondrial matrix enzyme glutamate dehydrogenase 1. Several differentially expressed DUB genes, including ZRANB1, are also located on chromosome 10. The HUGO list of DUB genes includes two DUBs located on the p arm of chromosome 10 (OTUD1 and MINDY3/ FAM188A) and three DUBs that are located on the q arm of chromosome 10 (ZRANB1, USP54, and STAMBPL1). Expression of ZRANB1 and USP54 was depressed in the GBM group compared to the AS and non-tumor groups. Similary, GLUD1 expression was lowered to 52.7% and 52.2% compared to the NT and AS groups, respectively. While a role for specific DUBs in the regulation of GLUD1 has not been established, among all 99 DUB genes we observed highest correlations of GLUD1 expression with USP46 (r = 0.74, p = 1.74e-30) and ZRANB1 (r = 0.70, p = 1.01e-26). This may suggest a possible new role for USP46 and/ or ZRANB1 in the regulation of GLUD1 in astrocytic glioma (AS and GBM).
Differential Expression of DUBs in GBM Subtypes
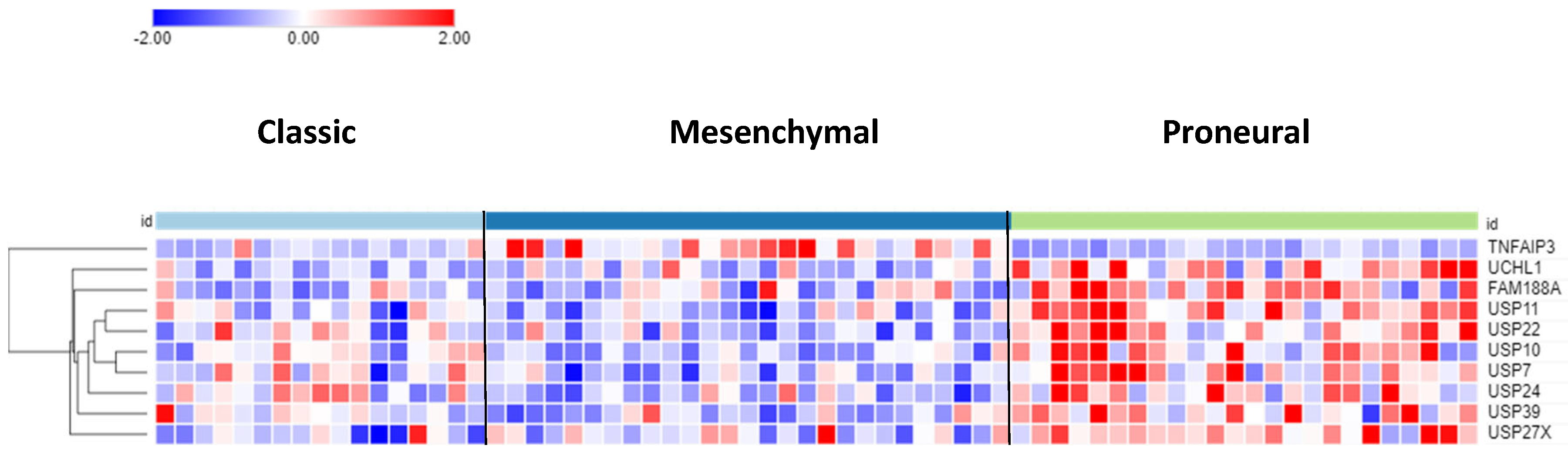
The differential expression of DUB genes was determined in three GBM subtypes from a TCGA dataset in the R2 genomics platform.
TNFAIP3 showed the most significant difference (p = 7.68e-09) in expression with elevated expression in the mesenchymal GBM subtype (
Figure 3). The DUBs with the most significantly elevated gene expression in the proneural GBM group were
USP11,
USP22, and
USP7.
Relationship Between DUB Expression and Survival (French Dataset)
Survival data were not available in the Sun mixed glioma dataset. Hence, we used the glioma dataset (GSE16011) of French et al.
46 (N = 284) to determine survival data associated with DUB gene expression. Kaplan Meier curves showed that the expression of 23 DUB genes was significantly associated (p < 0.001) with survival in glioma patients.
Table 2 lists the Chi square, p values, and hazard ratios for these DUB genes. The most significantly downregulated DUB,
ZRANB1 (as would be expected with loss of chromosome 10 or its q arm), was associated with worse survival. p
Role of DUBs in ER Stress and ERAD Signaling in Glioma
Employing the R2 genomics platform to query the 99 DUBs for their association with the GO category of
regulation of ERAD pathway, we identified three DUB genes in this category as differentially expressed in the glioma dataset:
USP14,
USP19, and
USP25 (
Table 3); it should be noted, however, that only
USP14 was among the most significant in
Table 1. In the French dataset high
USP14 expression was associated with worse survival (
Table 2). The role of USP14 and USP19 proteins in ER stress has been illustrated in a review by Qu et al.
31. USP19 is reported to inhibit the unfolded protein response
56 and to deubiquitinate the E3 ligase HRD1
57, a component of the ERAD pathway. USP14 binds to IRE1 and is reported to be an inhibitor of the ERAD pathway
58. USP25 deubiquitinates selected ERAD substrates
59. Another DUB reported by Qu et al. to regulate ER stress induced apoptosis is BAP1
31. Differential
BAP1 gene expression is also shown in
Table 3.
DUBs in the Regulation of Immune Responses in Glioma
Among the 99 DUB genes, four differentially expressed genes were identified in the Sun glioma dataset that associated with the GO category of
Regulation of the immune response. This included
OTUD7A, F = 69.909, p = 1.31e-29,
CYLD, F = 50.614, p = 1.69e-23,
TNFAIP3, F = 11.84, p = 4.34e-07, and
USP18, F = 8.14, p = 4.21e-05 (
Figure 4). Notably, expression of
OTUD7A was not only most significantly different of the DUBs in the GO category of
Regulation of immune response but was also the most significant of any of the 512 genes in this category in the Sun dataset.
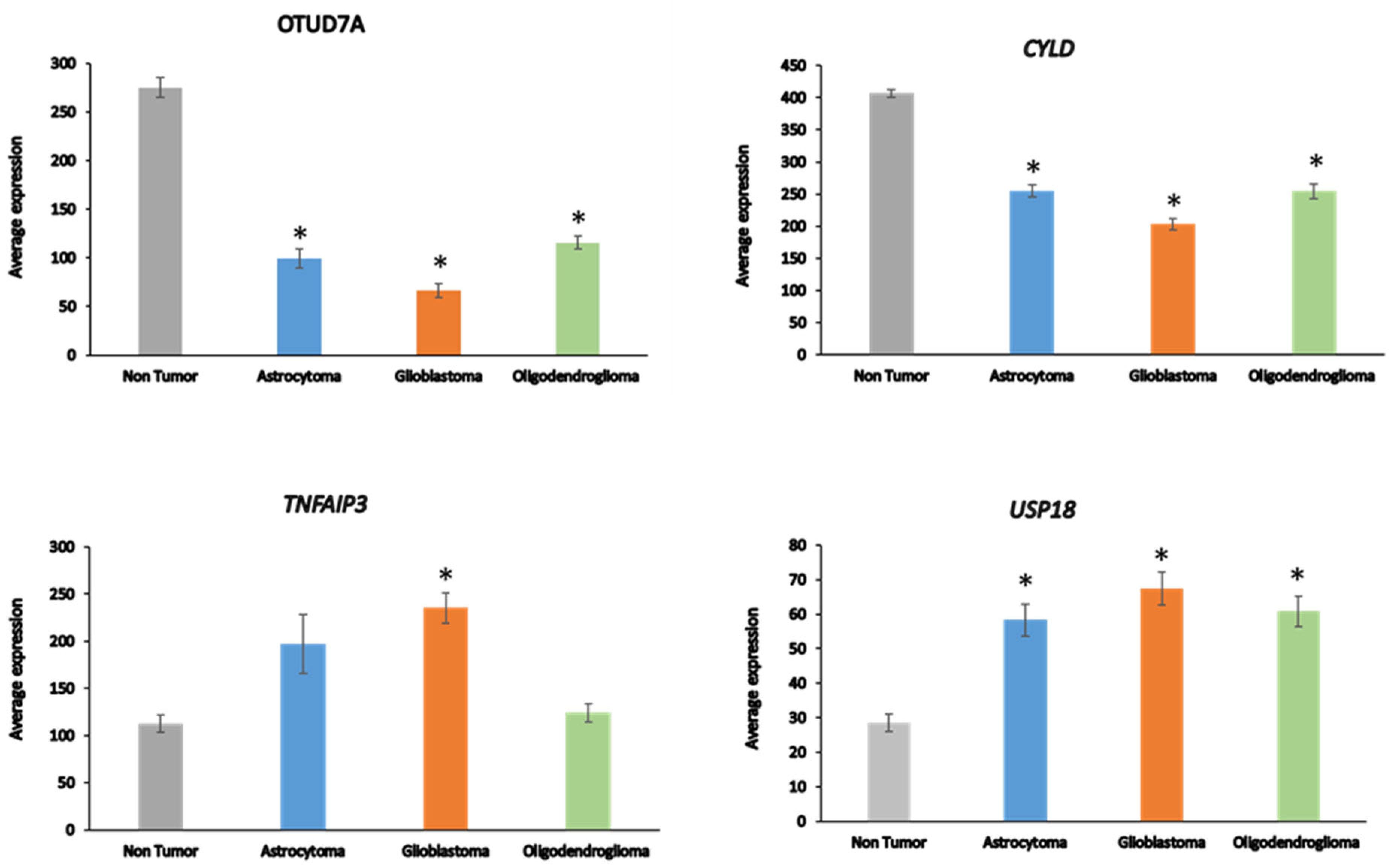
OTUD7A expression was significantly depressed in AS, GBM, and ODG (
Figure 4), as was
OTUB1 in all three types of gliomas compared to non-tumor tissues in the Sun dataset (
Table 1). OTUB1 deubiquitinase function was recently associated with the regulation of immune responses and contributes to immunosuppression in cancers via the programmed death ligand 1 (PD-L1) protein
60. Decreased
OTUB7A and OTUB1 gene expression may both affect immune responses and DNA damage repair functions (see below) in glioma
60, 61.
The OTUD7a gene is located on chromosome 15q13.3. Microdeletion of this chromosomal region results in abnormalities of neuronal development62, 63. CYLD is a tumor suppressor that contributes to regulation of NF-kB64. TNFAIP3 plays a role in several aspects of the immune response, including regulation of NF-κB and regulation of inflammation65. TNFAIP3 deletions have been associated with Epstein-Barr viral infection in lymphomas66. USP18 regulates interferon signaling by binding to one of its receptors (IFNAR2)67.
DUBs and DNA Repair in Glioma
Ten of the 99 DUB genes analyzed in the Sun dataset were differentially expressed genes associated with the GO category of
DNA repair:
OTUB1,
UCHL5,
USP3,
USP1,
USP51,
COPS5,
COPS6,
USP10,
USP47,
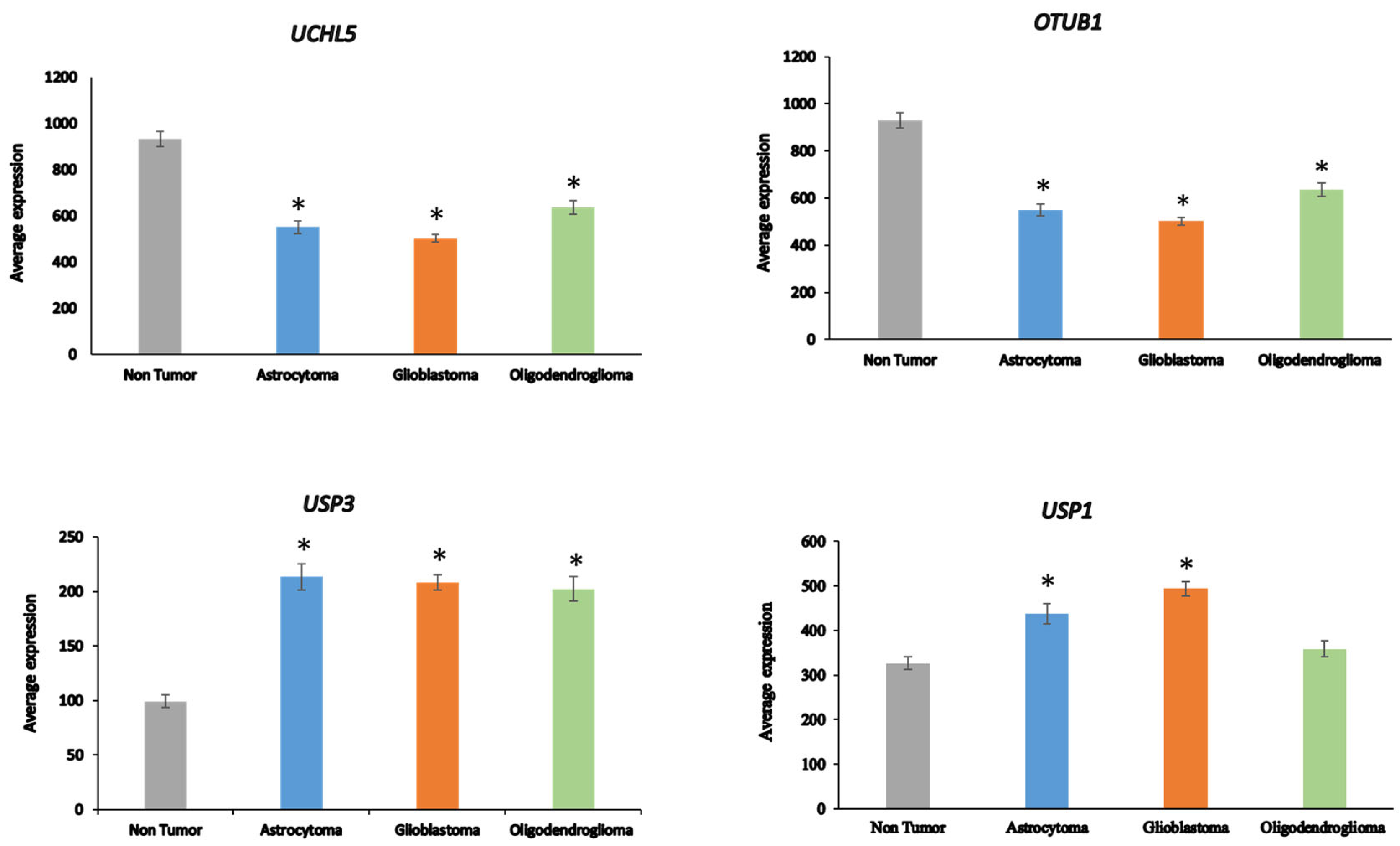
USP43 (in order of significance in ANOVA). The expression of
OTUB1 and
UCHL5 was significantly decreased (p < 0.001) in AS, GBM, and ODG compared to non-tumor tissue, while the expression of
USP3 was significantly elevated (p < 0.001) (
Figure 5). Expression of
USP1 was markedly increased in GBM and, to a lesser extent, in AS compared to the non-tumor group. Several DUBs, including
USP1,
OTUB1,
UCHL5, and
USP47, have been included in a list of 16 DUBs reported to be involved in specific DNA damage repair pathways
68. OTUD7A (Cezanne2) has also recently been reported to contribute to the DNA damage response to double strand break (DSB) repair
69 and expression of
OTUD7A was substantially reduced in gliomas compared to non-tumor brain tissue (
Figure 4). While linked to several DNA repair pathways
68,
USP7 and
USP24 expression was not significantly different among glioma groups or between glioma and the non-tumor control group in the Sun dataset, suggesting that these two DUBs may not be critical factors in DNA damage repair pathways in glioma.
DUBs in Ependymoma
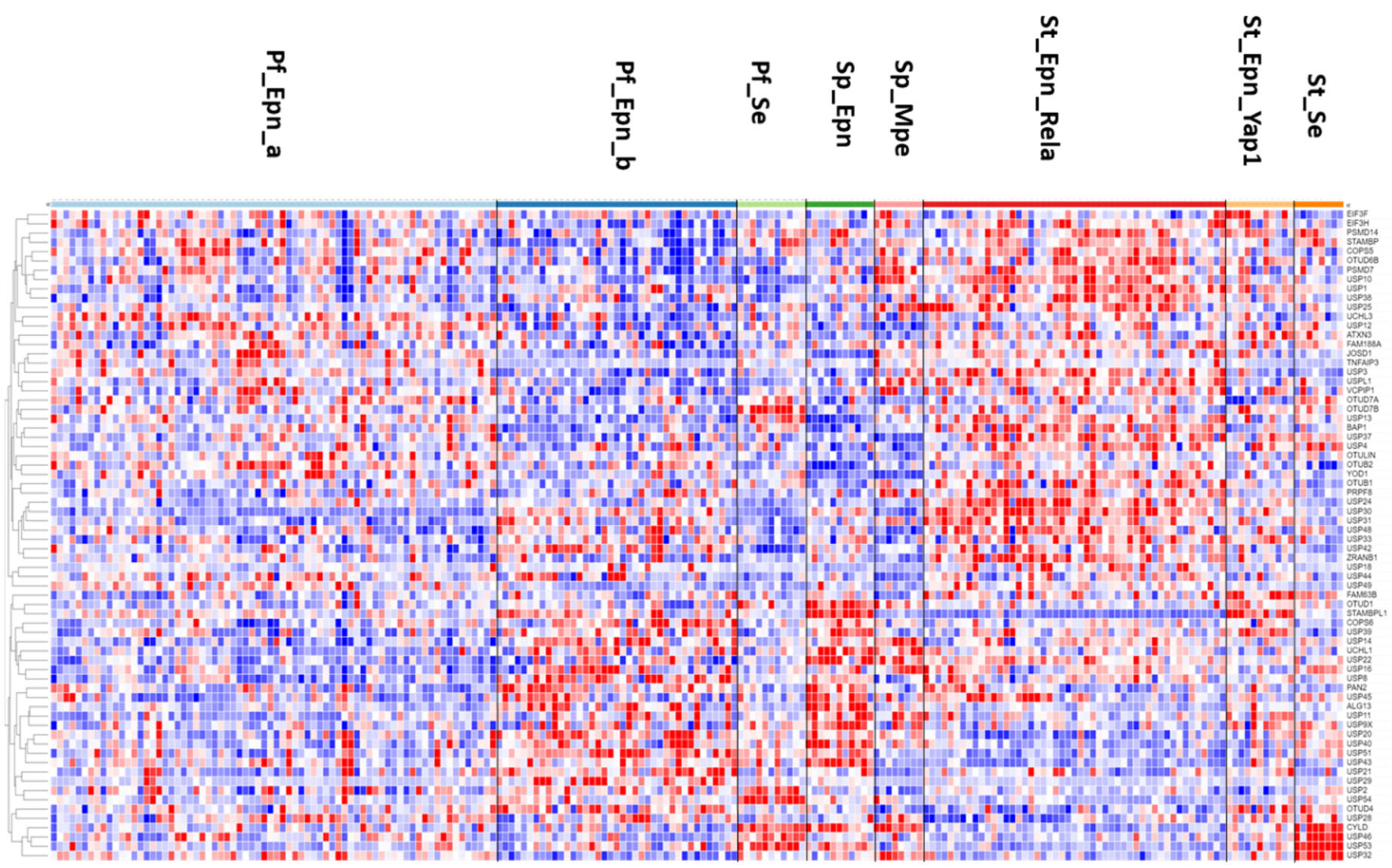
The heatmap and cluster analysis shown in
Figure 6 illustrates clusters of DUB gene expression that differ significantly between the EPN subgroups of the Pfister dataset. Of all molecular subgroups in this dataset, the largest groups were the posterior fossa groups, Pf_Epn_a (N = 72) and Pf_Epn_b (N = 39) followed by the supratentorial group (St_Epn_Rela) (N =49). Among all 99 DUBs,
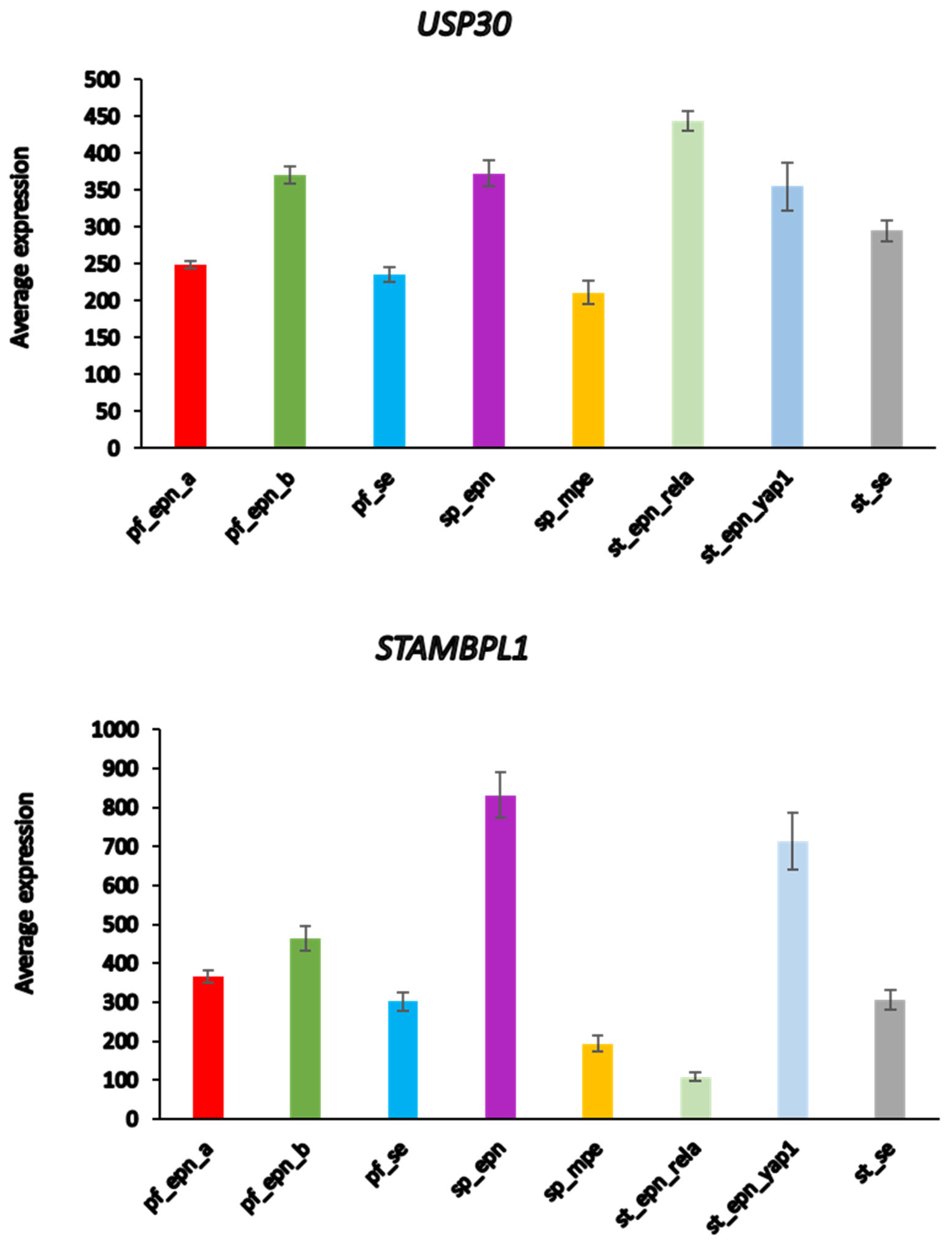
USP30 and
STAMBPL1 were most significantly different in these 3 EPN subgroups.
USP30 expression most significantly distinguished Pf_Epn_a and Pf_Epn_b, whereas
STAMBPL1 expression was depressed compared to the other subgroups (
Figure 7).
The UPS30 protein is located on the mitochondrial outer membrane70 and serves as an inhibitor of mitophagy71, 72 by blocking the action of the E3 ligase PARKIN73. STAMBPL1 is a K63 specific DUB reported to be expressed higher in cancer tissue than in adjacent control tissues74, 75.
The heatmap of DUB expression in EPN subgroups (
Figure 6) showed a cluster with relatively elevated DUB expression in the St_Rela subgroup (red squares in heatmap) and a cluster in which DUB expression was relatively depressed (blue squares in heatmap). Cytoscape analysis of GO biological pathways identified several DUBs associated with
histone deubiquitination in both clusters. This included the upregulated expression of
BAP1,
USP25,
USP3,
USP49 (red squares) and downregulated expression of
USP16,
USP21,
USP22, and
USP51 (blue squares) (
Figure 6). Cytoscape Reactome pathway analysis of differentially expressed DUB genes in
Figure 6 identified the expression of three DUB genes,
CYLD,
USP2, and
USP21, to be associated with the TRAF2:RIP1 complex in Tumor necrosis factor receptor (TNFR) signaling and apoptosis. The UPS2 protein has been labeled a “master regulator of apoptosis” since USP2 can remove ubiquitin chains from RIP1 and TRAF2, regulate TNF-TNFR1 mediated cell death, and upregulate the transcription of IkBα
76. Like USP2, USP21 was also reported to deubiquitinate RIP1
77 and a selective USP21 inhibitor, compound BAY-805, may have therapeutic potential in cancer
78. Both, CYLD and TNFAIP3 have been shown to also contribute to the regulation of NF-kB
64, 66. Notably, the supratentorial molecular subgroup St_Se of EPN was unique in that it showed increased expression of a cluster of four DUB genes,
CYLD,
USP46,
USP53,
USP32 (
Figure 6). This may be considered a new gene signature for this WHO grade I subependymoma (Se) subgroup
79. Among the significant differentially expressed DUBs in the EPN dataset were five genes associated with the
Regulation of ERAD pathway, including
ATXN3,
USP3,
USP14,
USP25, and OTUD2/
YOD1. Since the Pfister ependymoma dataset did not include non-tumor subjects or survival data, these comparisons were not possible for the DUB genes.
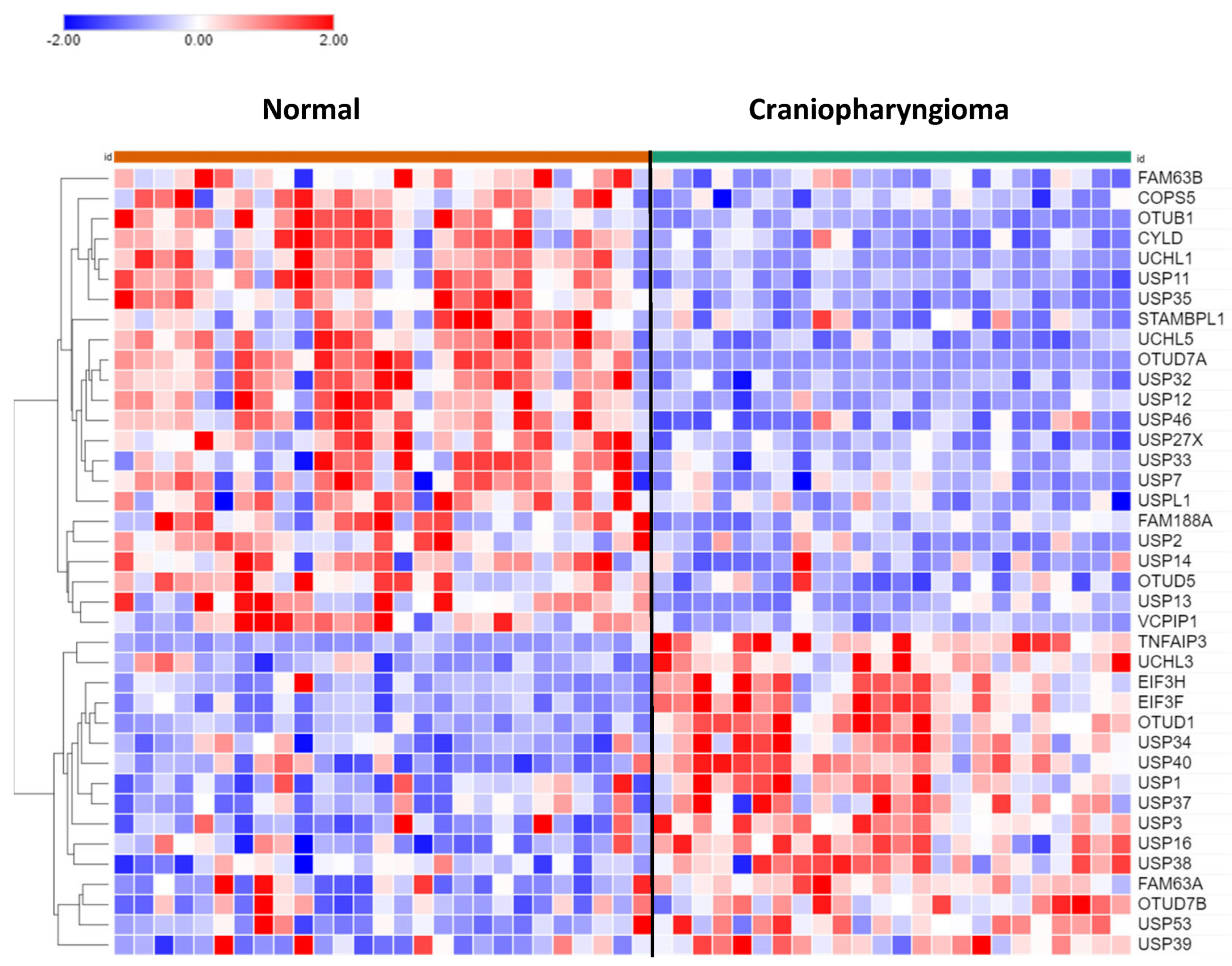
DUBs in Craniopharyngioma
Of the 99 DUB genes analyzed, 39 DUBs were differentially expressed between normal brain and CPh. Other than DUB activity itself, histone deubiquitination (USP3, USP7, USP16) was the most significant GO pathway identified by Cytoscape.
USP13 (F = 24.25, p = 1.00 e-05) and USP14 (F = 11.88, p = 1.17e-03) expression were both depressed in CPh compared to non-tumor tissue (
Figure 8). Of note, differential expression of USP14 was also observed in mixed gliomas and in EPN. USP14 protein was reported to be an inhibitor of the ERAD pathway by binding to IRE1a and inhibiting the phosphorylation of this ER stress activated kinase
80.
DUB Regulation of Immune Response in Craniopharyngioma
In the dataset of CPh, the DUB genes TNFAIP3, OTUD7A, and CYLD were identified by Cytoscape to be associated with the Regulation of immune response pathway. TNFAIP expression was upregulated about 5-fold (F = 78.84, p = 9.93e-12) compared to non-tumor tissue. TNFAIP3 has been identified as a druggable targeted for melanoma in mice81 and in inflammatory lung disease82. Expression of OTUD7A (aka Cezanne2) in CPh was significantly downregulated to 6.8% of normal non-tumor values (F = 81.52, p = 5.34e-12). Located on chromosome 15, OTUD7A is one of six genes that contribute to the 15q13.3 microdeletion syndrome, which is associated with neurodevelopmental and psychiatric disorders83, 84. Whether OTUD7A protein may qualify as tumor suppressor for the development of CPh tumors (and gliomas, see above), as these data may suggest, requires further studies. Expression of the DUB and tumor suppressor CYLD was significantly reduced (>50%) in CPh compared to normal brain tissue. CYLD is an inhibitor of the immune response and NF-kB signaling85-87.
DUBs and Medulloblastoma
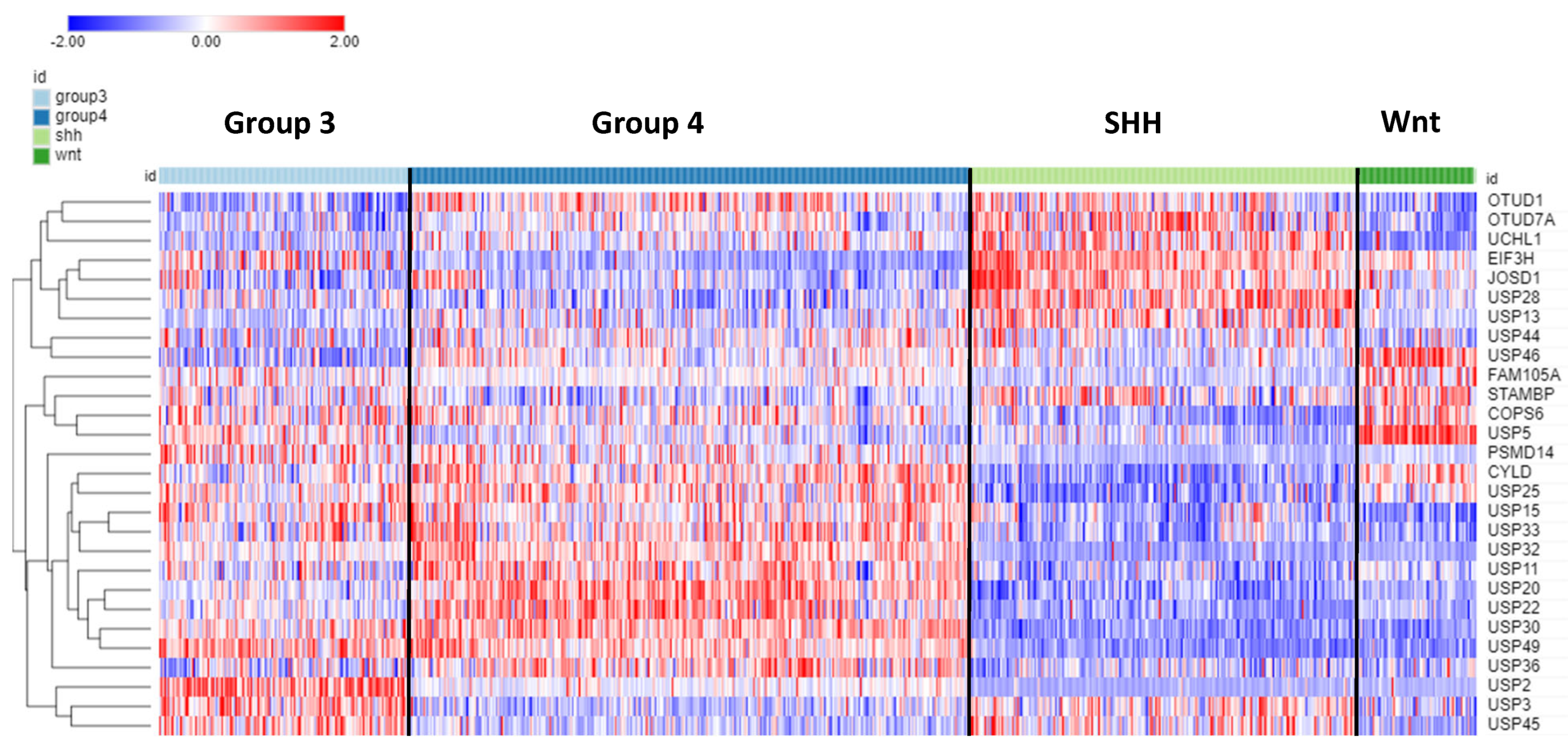
Of the 99 DUB genes, 78 were differentially expressed (p < 0.001) among the four subgroups of MB.
Table 4 shows the top ten DUB genes of each MB subgroup most significantly different from non-tumor tissues in the Swartling dataset. The heatmap in
Figure 9 illustrates the distribution of DUB expression among the four MB subgroups (Group 3, Group 4, SHH, and WNT) in the Cavalli dataset. The most significant differentially expressed DUB gene by subgroups in the Cavalli dataset was
USP2 (F = 271.00, p = 1.57e-119) (
Figure 9), which was upregulated in Group 3 MB compared to the other subgroups. USP2 protein removes ubiquitin from several proteins, including the E3 ubiquitin ligase MDM2
88, cyclin D (CCND1)
89, and the circadian clock gene PER1
90. While the Cavalli MB dataset does not include non-tumor controls, the Donson dataset showed that
USP2 expression was elevated compared to non-tumor brain. This was confirmed in the large meta-analysis of Weishaupt et al.
48 available in the R2 genomics site as the Swartling dataset, which identified
USP2 as the most significant differentially expressed gene in the five groups (non-tumor, Group 3, Group 4, SHH, Wnt). The role of USP2 in the deubiquitination of clock proteins regulating the circadian rhythm of pathways is well documented
91, 92. USP2 may function as an oncogene in breast and gastric cancer by inhibiting autophagy and this DUB has been proposed as a therapeutic target in breast cancer
93, 94.
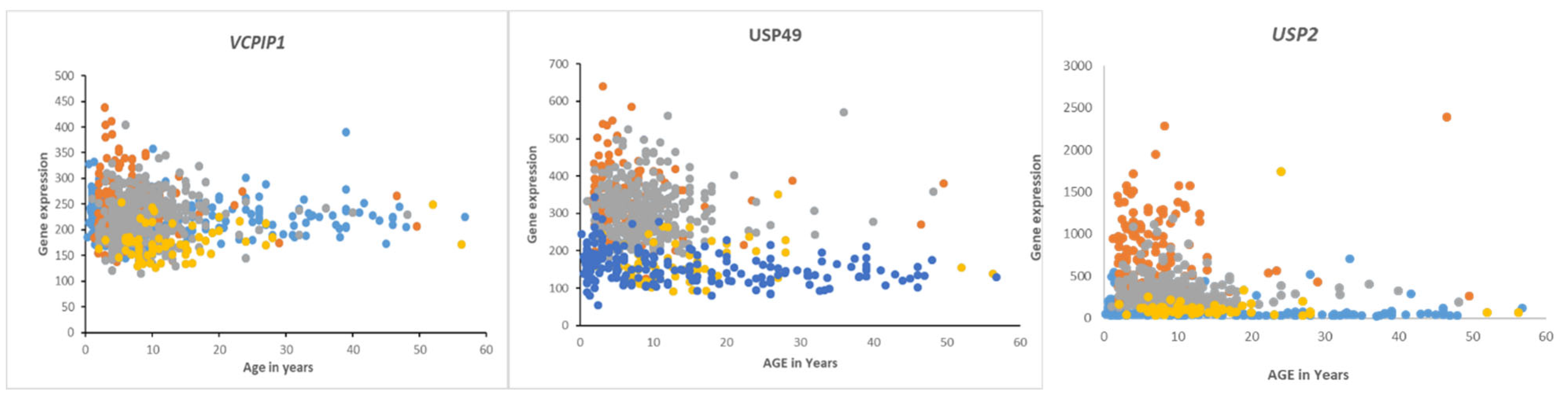
USP2 expression was also differentially expressed according to age groups (F = 13.73, p = 1.01e-08). The age distribution for the four MB subgroups showed elevated
USP2 expression primarily in infants and children (
Figure 10) and high expression of USP2 was associated with poor survival (
Table 4). USP2 may be a lucrative therapeutic target in patients with Group 3 MB.
Survival in Medulloblastoma Subgroups and DUB Expression
Overall survival in the Cavalli dataset, as determined by the R2 genomics platform, was best in the Wnt group, worst in Group 3, and intermediate in SHH and Group 4, confirming previously determined survival times
47. DUB genes that were significantly associated with survival (p < 0.01, for Kaplan-Meier curves) are listed in
Table 5 and includes DUB genes not differentially expressed between subgroups. DUB genes with the most significant Kaplan-Meier curves (high vs low) and hazard ratios included
VCPIP1,
USP49, and
USP2. High expression of these genes was associated with worse survival (
Table 4). While high expression of
VCPIP1 was associated with worse survival in the Cavalli dataset, expression of
VCPIP1 in none of the four MB groups was significantly higher than in non-tumor tissues in the Swartling dataset. High expression of USP49 was observed in infants and young children in Groups 3 and 4, whereas high expression of
USP2 was primarily observed in infants and young children in Group 3 (
Figure 10).
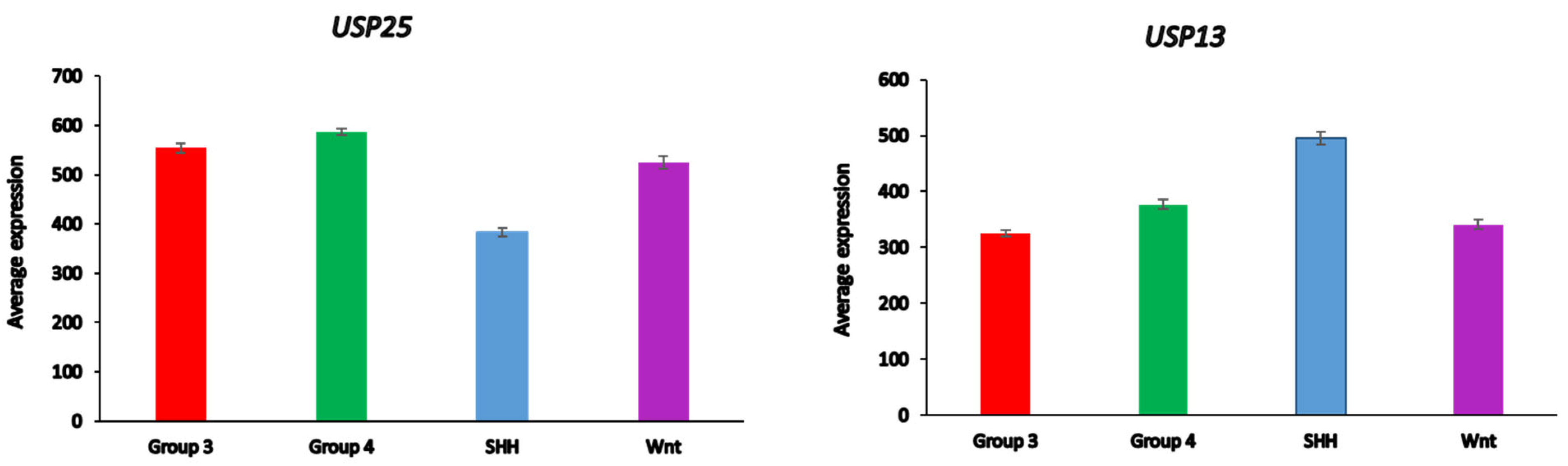
Medulloblastoma DUBs and ERAD
Differential expression of several DUB genes (
USP13,
USP14, USP25, USP19,
OTUD2 (alias
YOD1)) in the Cavalli dataset was associated with the GO category of
Regulation of ERAD pathway. This list of genes includes three DUBs (
USP14,
USP19,
USP25) shared with the list of differential expressed ERAD genes in glioma (
Table 3). USP25 was the only ERAD-associated DUB gene among the top ten DUB genes in the SHH MB group (
Table 4). Our data implicate both
USP25 and
USP13 with ERAD in the SHH group of MB (
Figure 11). In the Swartling dataset the expression of
USP25 was downregulated in the SHH group compared to non-tumor controls (t = 14.37,
p = 3.17e-41), while the expression of
USP13 was elevated compared to non-tumor tissues (t = 11.36,
p = 1.41e-27). Expression of
USP14, but not
USP19 and
OTUD2, was also reduced in the SHH group versus non-tumor tissues, but at a much lower level of significance (t = 3.35, p = 8.64e-04) (data not shown).
Medulloblastoma DUBs and the Immune Response
Five differentially expressed DUB genes were associated with the GO category of
Regulation of immune response in the Cavalli dataset. This included DUB genes
CYLD (F = 141.44,
p = 8.53e-73),
PSMD14 (F = 58.20,
p = 7.15e-34),
OTUD7A (F = 66.35,
p = 4.14e-38),
USP18 (F = 37.27,
p = 1.78e-22), and
PSMD7 (F= 16.56, p = 1.96e-10).
Table 6 shows the genes that were significantly elevated or depressed compared to non-tumors samples in the Swartling dataset.
CYLD is an inhibitor of the immune response, alters NF-kB signaling, and affects the development and Th2 conversion of Treg cells86, 87, 95. PSMD14 and PSMD7 are DUB components of the proteasome and PSMD14 is a druggable target that specifically deubiquitinates at K63 and suppresses autophagy by affecting vesicular retrograde transport from the Golgi to the ER96, 97. OTUD7A contributes to neuronal development62, 63 and USP18 regulates the immune response by binding to the interferon receptor IFNAR267.
Medulloblastoma DUBs and DNA Damage Repair
Twelve of the 99 DUB genes were identified as differentially expressed in MB compared to non-tumor tissue (p < 0.0001) for the GO category of
DNA repair in the Swartling dataset including
USP1,
OTUB1,
UCHL5,
USP7, and
PSMD14 (aka
POH1) (
Table 7). The DUB proteins USP1, OTUB1, UCHL5, USP7, and PSMD14 were reported to contribute to double strand break repair, USP1 to Fanconi anemia pathway, USP1 and USP7 to translesion repair, USP7 and USP47 to base excision repair, and USP7 and USP45 to nucleotide excision repair
68. In addition, the USP28 protein was also found to contribute to the DNA damage response
98. A highly significant reduction of
USP28 expression in Group 3, Group 4, and Wnt MB groups (
Table 7) may point to an impaired DNA damage response in these MB groups.
PSMD14 was the most significantly upregulated DUB gene in Group 3 MB (
Table 7)
99. A subtype-specific analysis revealed that
PSMD14 over-expression was limited to selected subtypes of Group 3 (Group 3 beta and gamma) and Group 4 (Group 4 alpha).
DUBs in Neuroblastoma (Fischer Dataset)
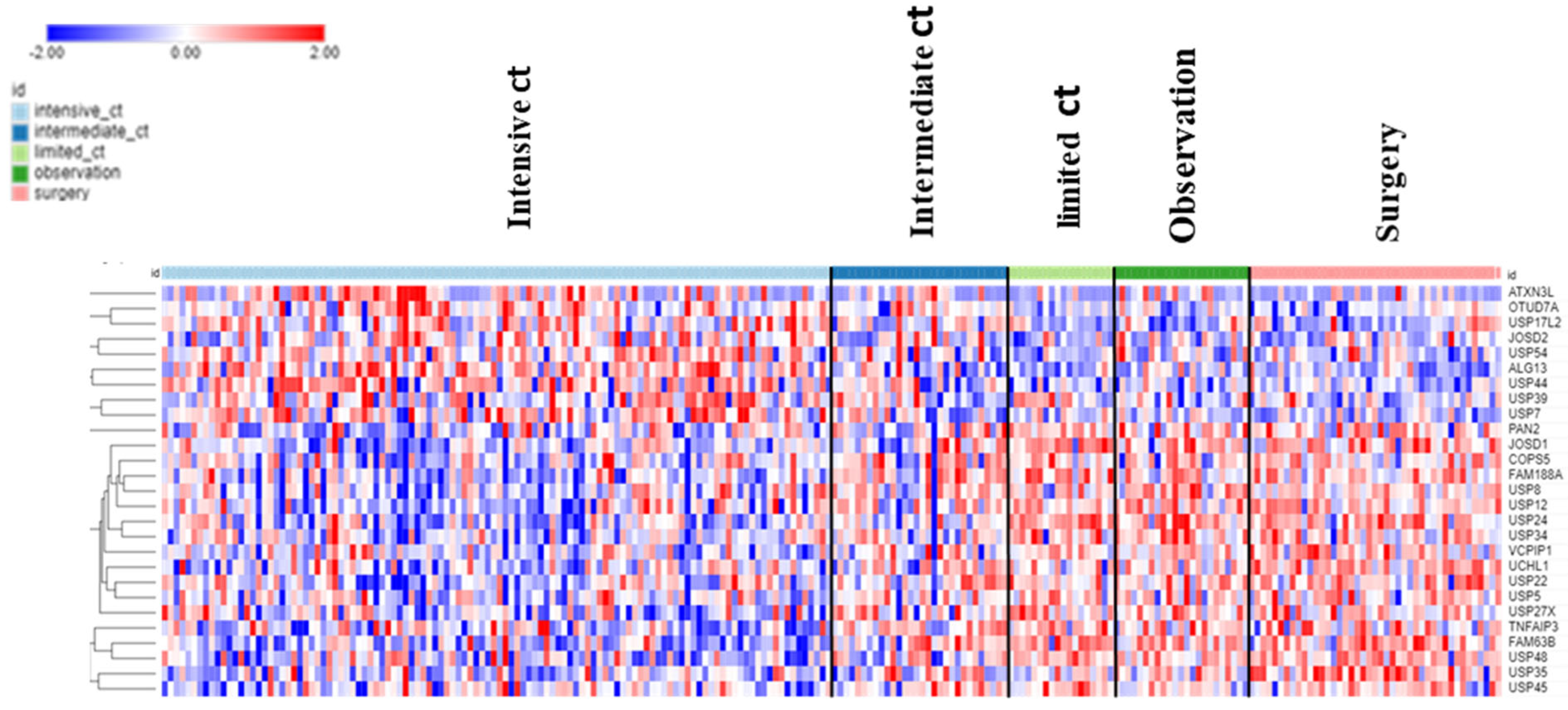
The dataset of Fischer on NBT allowed the examination of DUB genes in patients with various treatments. Intensive chemotherapy of NBT was associated with increased expression of selected DUB genes and a decrease in several other DUB members compared to the observation group. Intriguingly, limited chemotherapy or surgery had no significant effect on the expression of DUB genes compared to observation group alone (
Figure 12).
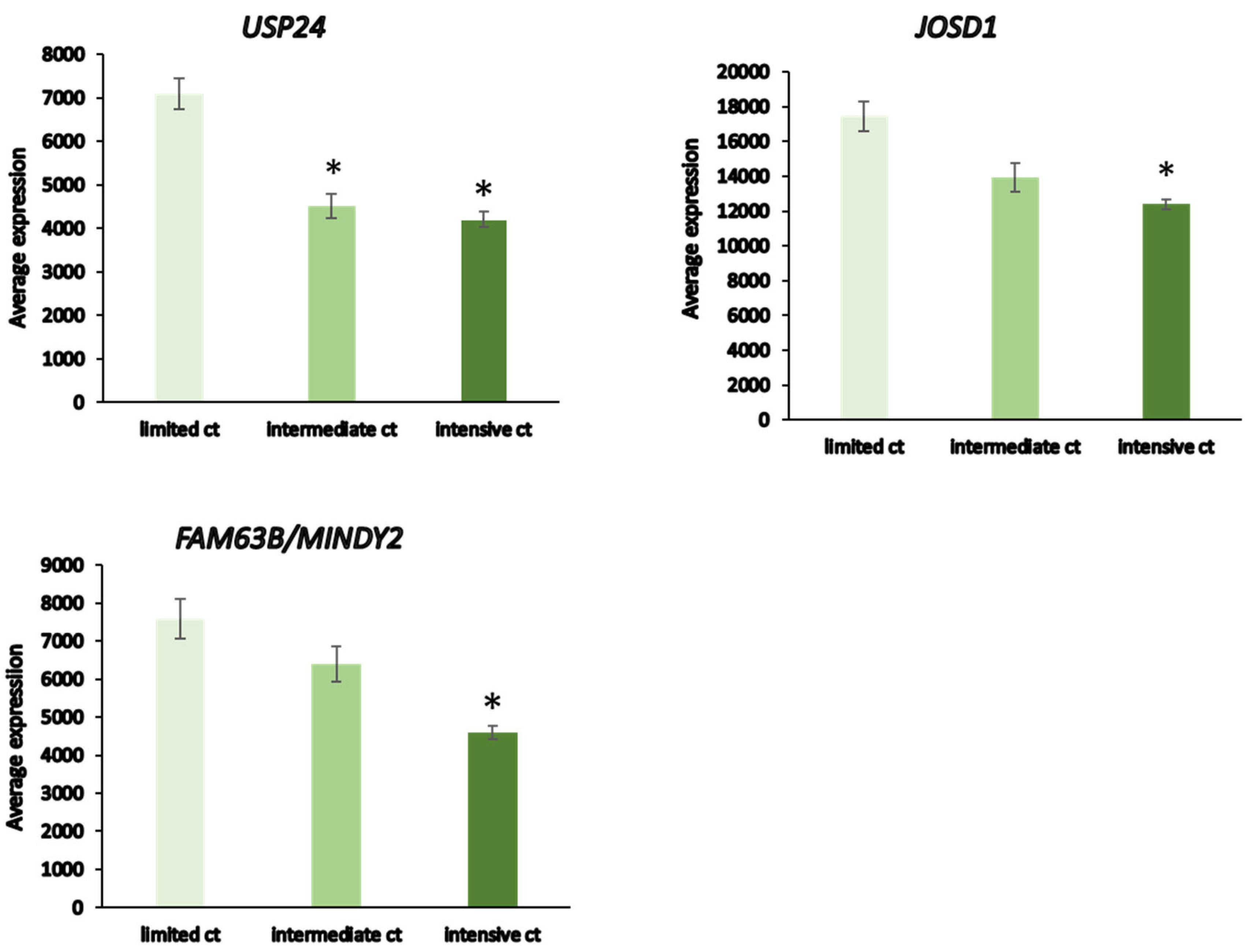
Patients receiving intensive chemotherapy of their NBT showed significantly reduced tumor tissue expression of
USP24,
USP34,
MINDY2,
USP8,
JOSD1,
USP52, and
USP12 when compared to the observation group. The most significant differences in DUB expression between limited and intensive chemotherapy included
USP24,
JOSD1, and
MINDY2 (
Figure 13). Notably, there were many other genes in the Fischer dataset that showed differential expression between the limited and intensive chemotherapy groups, the most significant of them being
MDGA1 (F = 128.15, p = 4.24e-21) with approximately 4-fold difference.
MDGA1 is expressed mainly in neurons and astrocytes of the brain.
Discussion
The complex functional roles of DUBs in tumor biology is gradually emerging100. Here we present a comprehensive gene expression profiling of 99 DUB family members in six different brain tumor entities that span different molecular subtypes and age groups. We also included gene expression data from different treatment groups of NBT, the most common extracranial sympathetic nervous system tumor in childhood, to better understand the emerging role of deubiquitinases in pediatric and adult CNS tumors. While we observed pronounced gene expression changes for several DUBs, for brevity, we will only discuss those selected DUB members with highest differential expression. Wherever possible, we have focused on known brain-related functions for these DUBs, with a particular emphasis on clinically relevant pathomechanisms, such as the ERAD pathway, immune system, and DNA damage repair.
GBM displayed a distinct downregulation of USP46 and ZRANB1. Ubiquitously expressed throughout the mammalian brain, USP46 is involved in the formation of synapses and neuronal morphogenesis by regulating both excitatory and inhibitory synaptic transmission101. By deubiquitinating K63 ubiquitinated glutaminergic AMPARs (α-amino-3-hydroxy-5methyl-4-isoxazolepropionic acid) receptors GluA1 and GluA2, which are considered to mediate most of the excitatory synaptic transmission in the brain, USP46 upregulates the intracellular trafficking, cell surface density, and signal intensity of AMPARs101, 102. These receptors are critical for perivascular brain invasion, promote plasticity and growth of GBM and coincide with poor prognosis103-105. USP46 also interferes with the neuronal activity-dependent ubiquitination and trafficking of GABAA receptors. Loss of USP46 coincides with reduced expression of glutamic acid decarboxylase (GAD67) which synthesizes GABA106. Recently, higher expression of the non-coding (nc)RNA USP46-AS1 has been linked to increased overall survival in glioma107. It is tempting to speculate that the marked reduction in USP46 gene expression in GBM, but not AS, coincides with the acquisition of altered receptor density in the plasma membrane and synaptic activity during dedifferentiation from high-grade AS to GBM. GABAA receptor activity was reported to inhibit glioma growth and lowest levels of GABAA receptors were reported in GBM compared to lower grade glioma108, 109.
While the role of ZRANB1 (zinc finger RANBP2-type containing 1, TRABID) in glioma is still unclear, it is likely multifactorial in nature. In breast cancer, this K29- and K33-specific DUB binds, deubiquitinates, and stabilizes enhancer of zeste homologue (EZH2) catalytic component of the gene silencing Polycomb repressive complex 2 (PRC2) to promote growth, resulting in poor prognosis51. USP1 in glioma110, as well as USP7 and USP34, also converge on EZH2 to promote tumorigenesis111. Tight regulation of ZRANB1 expression is critical in glioma. Reduced expression of ZRANB1 may confer a survival advantage to GBM by reducing UPR through the recruitment of p62 to K33-ubiquitated protein aggregates for autophagic removal 112. However, in solid tumors lower ZRANB1 levels coincide with epigenetic regulation that promotes interferon and inflammatory immune cell responses in the tumor microenvironment113-115. Lower ZRANB1 levels also attenuate deubiquitination of K29-linked polyubiquitinated repair factor 53BP1. Proteasomal removal of this DNA repair factor mitigates genomic instability by preventing 53BP1 from blocking homologous recombination repair at double strand DNA breaks116. Further studies are needed to establish the role of ZRANB1 in GBM.
Selected GBM, EPN, CPh, and MB subtypes showed distinct expression of specific DUB genes. Among 10 differentially expressed DUBs in the three GBM subtypes, only TNFAIP3, aka A20, was upregulated in mesenchymal GBM. Possessing both DUB and E3-ligase activities117, 118, TNFAIP3 is an important player in a diverse array of diseases119 and a key negative regulator of NFkB signaling downstream of TNF receptors, interleukin 1 receptor (IL-1R), pathogen recognition receptors (PRRs), NOD-like receptors (NLRs), T- and B-cell receptors, and CD40120, 121. TNFAIP3 regulates glioma stem cell survival, increases resistance to alkylating agents, and is considered a poor prognostic marker in GBM122, 123. While TNFAIP3 upregulation was a unique mesenchymal feature among GBM subtypes, DUB genes significantly associated with immune cell functions were identified in other GBM subtypes, the St_se subgroup of EPN, MB, and in CPh. This included TNFAIP3, CYLD (Cylindromatosis), another negative regulator of NF-kB signaling87, and the critical neurodevelopmental factor and putative tumor suppressor OTUD7A/ Cezanne-263. These data suggest a redundant role for several DUBs in targeting NF-kB signaling as a mechanism to regulate inflammatory and immune responses in intra- and extracranial nervous system brain tumors.
Among the four MB subgroups, we identified USP2 to be selectively upregulated primarily in infants and children within Group 3 MB (
Figure 9). Group 3 MB frequently have elevated MYC levels due to MYC overexpression or MYC gene amplifications and these patients have the worst prognosis of all MB groups with less than 50% survival
124, 125. In the Cavalli dataset, MYC expression was elevated most in the Group 3 gamma subtype. As may be expected, USP2 DUB functions target a wide range of interconnected pathways in a tissue-specific manner
126. Relevant USP2 functions in tumorigenesis target the metabolic (e.g., fatty acids) and p53 pathways, EMT, cell cycle control, and maintenance of genome stability
126. High USP2 levels resulted in the downregulation of several miRs, including
MYC-targeting miR-34b/c, which resulted in deubiquitination of MDM2 and elevated MYC levels with subsequent p53 inactivation in prostate cancer cells
127. Hence, it is conceivable that higher USP2 expression may contribute to higher MYC protein levels in Group 3 MB patients.
Emerging research is starting to unravel the complex and clinically relevant relationships between UPR, ER and DNA stress signaling, chronic inflammation, and immune responses in primary brain tumors and their microenvironment
128-131. We identified a selected group of DUBs (USP13, USP14, USP19, USP25, OTUD2/ YOD1) associated with the
Regulation of ERAD pathway across several adult and pediatric primary brain tumors (GBM, EPN, CPh, MB). A recent TCGA-based gene expression profiling interactive analysis (GEPIA;
http://gepia. cancer-pku.cn/) of low-grade glioma and GBM identified lower expression of all but one (USP25) of these USP Dub members in GBM
132. There was a strong correlation for higher expression of USP14 and worse prognosis in GBM patients
132. In addition to its roles at the ER
58, USP14 (and UCH37) engage in polyubiquitin chain trimming which can delay proteasomal degradation by weakening the affinity of ubiquitin chains with ubiquitin-binding receptors at the proteasome
133. Hence, USP14 has been targeted with a small molecule inhibitor
134 or selected USP14 aptamers
135 to enhance proteasomal activity and degradation of proteotoxicity. Although USP14 downregulation in several tumor types was shown to reduce tumor burden in mice, data are lacking for brain tumors
136, 137. OTUD2/ YOD1 is another DUB with regulator functions in the ERAD pathway and linked to injury-induced ER stress responses
138, 139. This includes a regulatory role of the inflammatory cytokine IL1 and p62 NFkB signaling axis through interaction with the E3 ligase TRAF6
140, which may contribute to OTUD2/ YOD1 deubiquitinating activity in attenuating neurogenic proteotoxicity
141. In glioma, OTUD2/ YOD1 has been identified as a target of miR-190a-3p. Blocking miR-190a-3p or the overexpression of its target OTUD2/ YOD1 attenuated the proliferation and migration of glioma cells
142. While the underlying mechanism is currently unknown, YAP and TAZ, the transcriptional coactivators and effectors of the Hippo signaling pathway, have been identified as downstream targets of an miR21-OTUD2-YAP/ TAZ axis in hepatocellular carcinoma
143, thus, potentially linking OTUD2/ YOD1 to glioma stem cell maintenance and proliferation
144.
Among the selected DUBs significantly linked to the DNA damage repair pathway, the glioma and MB data sets shared several DUBs, including
USP1,
USP47,
UCHL5,
OTUD1, which cover five major DNA damage repair pathways (BER, NER, FA, TLS, DSB)
68. USP1 targets FANCD2/FANCI to regulate the Fanconi anemia pathway (FA)
145 and, together with USP7 targets translesional DNA repair (TLS)
146, 147. USP7, a DDR-associated DUB exclusively altered in MB, and USP47 target the base excision repair pathway (BER)
148, 149, while USP7 and USP45 have regulate roles in nucleotide excision repair (NER)
150, 151.
UCHL5 and
OTUD1 were reported to increase or decrease double strand break repair (DSB), respectively
152, 153. Expressed among the top genes in group 3 MB and highly significantly associated with poor survival in MB groups 3 and 4 (
Table 4 and
Table 5),
PSMD14 (aka
POH1) was also significantly associated with immune responses and DNA repair, particularly in MB groups 3 and 4 (
Table 6 and
Table 7). PSMD14 was shown to fortify tumor cells against DNA damaging drugs by promoting a switch from non-homologous end-joining to homologous recombination
154, 155. This identifies proteasomal PSMD14 as a key DUB in regulating ubiquitin conjugation in response to DNA damage and demonstrates the intricate relationships between the proteasome and DNA damage responses.
Changes in DUB expression also occur during treatment of extracranial NBT sympathetic nervous tumors. Our analysis of a data set from NBT undergoing different treatment options identified significantly reduced expression of seven (
USP24,
USP34,
MINDY2,
USP8,
JOSD1,
USP52,
USP12) DUBs in treated versus non-treated NBT group. Intriguingly,
USP24,
JOSD1, and
MINDY2 showed the most significant downregulation during intensive versus limited chemotherapy (
Figure 12). USP24 has recently been identified as a novel tumor suppressor in NBT that targets collapsin response mediator protein 2 (CRMP2), which promotes axon growth, guidance, and neuronal polarity but also affects T cell polarization and migration
156, 157. Deubiquitination of CRMP2 by USP24 ensured proper spindle pole assembly and block chromosomal instability and aneuploidy observed upon USP24 knockdown in NBT
158. A glimpse into possible additional cellular strategies in response to intensive treatment regimes in NBT cells comes from findings that USP24 downregulation increases autophagy flux in cells
159. The biological roles of Machado-Joseph DUB member JOSD1 are complex
23. While data on JOSD1 in NBT are lacking, JOSD1 can deubiquitinate and stabilize Snail protein to promote EMT and tissue invasion of lung cancer cells
160. A small molecule inhibitor of JOSD1 was shown to induce cell death of JAK2-V617F-positive primary acute myeloid leukemia (AML) cells
161. Downregulation of JOSD1 in treated NBT may also affect regulatory mechanisms of interferon-1 mediated inflammatory cytokine responses
162. The role of evolutionarily conserved MINDY1/2 family of DUBs in brain tumors is currently unknown. However, MINDY1 DUB activity promotes the proliferation of bladder cancer cells by stabilizing MINDY1 interaction partner YAP protein and critical transcriptional regulator of the Hippo pathway. YAP overexpression in MINDY1 depleted cells was able to rescue this proliferation
163. In human breast cancer cells, MINDY1 stabilized estrogen receptor alpha (ERa) and promoted ERa mediated proliferation
164.
In conclusion, the role of DUBs as relevant modulators of tumor relevant cellular and immunomodulatory pathways in brain tumors is evolving. Selective DUB targeting strategies may provide important synergistic therapeutic potential in the future.
Author Contributions
Conceptualization, TK and JV; methodology JV; formal analysis JV; data curation JV; writing – original draft preparation TK and JV; writing – review and editing TK, SHK, SEL, JV; visualization JV,TK; project administration JV. All authors have read and agreed to the final version of the manuscript.
Funding
TK is grateful to the Natural Sciences and Engineering Research Council of Canada (NSERC) for funding.
Data Availability Statement
The data referred to in this manuscript are publicly available at the R2 Genomics Analysis and Visualization Platform (
http://r2.amc.nl) and at the NIH GEO database and are available on reasonable request from the first author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gao, T.; Liu, Z.; Wang, Y.; Cheng, H.; Yang, Q.; Guo, A.; Ren, J.; Xue, Y. UUCD: A family-based database of ubiquitin and ubiquitin-like conjugation. Nucleic Acids Res 2013, 41 (Database issue), D445–451. [Google Scholar] [CrossRef]
- van der Veen, A.G.; Ploegh, H.L. Ubiquitin-like proteins. Annu Rev Biochem 2012, 81, 323–357. [Google Scholar] [CrossRef]
- Varshavsky, A. The Ubiquitin System, Autophagy, and Regulated Protein Degradation. Annu Rev Biochem 2017, 86, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Dikic, I.; Schulman, B.A. An expanded lexicon for the ubiquitin code. Nat Rev Mol Cell Biol 2023, 24, 273–287. [Google Scholar] [CrossRef]
- Swatek, K.N.; Komander, D. Ubiquitin modifications. Cell Res 2016, 26, 399–422. [Google Scholar] [CrossRef]
- Tracz, M.; Bialek, W. Beyond K48 and K63: Non-canonical protein ubiquitination. Cell Mol Biol Lett 2021, 26, 1. [Google Scholar] [CrossRef]
- Jacobson, A.D.; Zhang, N.Y.; Xu, P.; Han, K.J.; Noone, S.; Peng, J.; Liu, C.W. The lysine 48 and lysine 63 ubiquitin conjugates are processed differently by the 26 s proteasome. J Biol Chem 2009, 284, 35485–35494. [Google Scholar] [CrossRef]
- Kwon, Y.T.; Ciechanover, A. The Ubiquitin Code in the Ubiquitin-Proteasome System and Autophagy. Trends Biochem Sci 2017, 42, 873–886. [Google Scholar] [CrossRef]
- Dosa, A.; Csizmadia, T. The role of K63-linked polyubiquitin in several types of autophagy. Biol Futur 2022, 73, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gong, Z.; Jiang, W.X.; Yang, J.; Zhu, W.K.; Guo, D.C.; Zhang, W.P.; Liu, M.L.; Tang, C. Lys63-linked ubiquitin chain adopts multiple conformational states for specific target recognition. Elife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Suresh, H.G.; Pascoe, N.; Andrews, B. The structure and function of deubiquitinases: Lessons from budding yeast. Open Biol 2020, 10, 200279. [Google Scholar] [CrossRef] [PubMed]
- Mevissen, T.E. T.; Komander, D. Mechanisms of Deubiquitinase Specificity and Regulation. Annu Rev Biochem 2017, 86, 159–192. [Google Scholar] [CrossRef]
- Komander, D.; Clague, M.J.; Urbe, S. Breaking the chains: Structure and function of the deubiquitinases. Nat Rev Mol Cell Biol 2009, 10, 550–563. [Google Scholar] [CrossRef]
- Lange, S.M.; Armstrong, L.A.; Kulathu, Y. Deubiquitinases: From mechanisms to their inhibition by small molecules. Mol Cell 2022, 82, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Meng, T.; Chen, L.; Wei, W.; Wang, P. The role of ubiquitination in tumorigenesis and targeted drug discovery. Signal Transduct Target Ther 2020, 5, 11. [Google Scholar] [CrossRef]
- Davis, M.I.; Simeonov, A. Ubiquitin-Specific Proteases as Druggable Targets. Drug Target Rev 2015, 2, 60–64. [Google Scholar] [PubMed]
- Rong, C.; Zhou, R.; Wan, S.; Su, D.; Wang, S.L.; Hess, J. Ubiquitin Carboxyl-Terminal Hydrolases and Human Malignancies: The Novel Prognostic and Therapeutic Implications for Head and Neck Cancer. Front Oncol 2020, 10, 592501. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.C.; Riddle, S.M.; Cohen, R.E.; Hill, C.P. Structural basis for the specificity of ubiquitin C-terminal hydrolases. EMBO J 1999, 18, 3877–3887. [Google Scholar] [CrossRef]
- Larsen, C.N.; Price, J.S.; Wilkinson, K.D. Substrate binding and catalysis by ubiquitin C-terminal hydrolases: Identification of two active site residues. Biochemistry 1996, 35, 6735–6744. [Google Scholar] [CrossRef]
- Makarova, K.S.; Aravind, L.; Koonin, E.V. A novel superfamily of predicted cysteine proteases from eukaryotes, viruses and Chlamydia pneumoniae. Trends Biochem Sci 2000, 25, 50–52. [Google Scholar] [CrossRef]
- Du, J.; Fu, L.; Sui, Y.; Zhang, L. The function and regulation of OTU deubiquitinases. Front Med 2020, 14, 542–563. [Google Scholar] [CrossRef] [PubMed]
- Schluter, D.; Schulze-Niemand, E.; Stein, M.; Naumann, M. Ovarian tumor domain proteases in pathogen infection. Trends Microbiol 2022, 30, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Zhao, C.; Ge, F.; Li, Y.; Cao, J.; Ying, M.; Lu, J.; He, Q.; Yang, B.; Dai, X.; et al. Machado-Joseph Deubiquitinases: From Cellular Functions to Potential Therapy Targets. Front Pharmacol 2020, 11, 1311. [Google Scholar] [CrossRef]
- Patterson-Fortin, J.; Shao, G.; Bretscher, H.; Messick, T.E.; Greenberg, R.A. Differential regulation of JAMM domain deubiquitinating enzyme activity within the RAP80 complex. J Biol Chem 2010, 285, 30971–30981. [Google Scholar] [CrossRef]
- Pan, X.; Wu, S.; Wei, W.; Chen, Z.; Wu, Y.; Gong, K. Structural and Functional Basis of JAMM Deubiquitinating Enzymes in Disease. Biomolecules 2022, 12. [Google Scholar] [CrossRef]
- Dubiel, W.; Chaithongyot, S.; Dubiel, D.; Naumann, M. The COP9 Signalosome: A Multi-DUB Complex. Biomolecules 2020, 10. [Google Scholar] [CrossRef]
- Abdul Rehman, S.A.; Kristariyanto, Y.A.; Choi, S.Y.; Nkosi, P.J.; Weidlich, S.; Labib, K.; Hofmann, K.; Kulathu, Y. MINDY-1 Is a Member of an Evolutionarily Conserved and Structurally Distinct New Family of Deubiquitinating Enzymes. Mol Cell 2016, 63, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Hickey, C.M.; Wilson, N.R.; Hochstrasser, M. Function and regulation of SUMO proteases. Nat Rev Mol Cell Biol 2012, 13, 755–766. [Google Scholar] [CrossRef]
- Tokarz, P.; Wozniak, K. SENP Proteases as Potential Targets for Cancer Therapy. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Mendoza, H.M.; Shen, L.N.; Botting, C.; Lewis, A.; Chen, J.; Ink, B.; Hay, R.T. NEDP1, a highly conserved cysteine protease that deNEDDylates Cullins. J Biol Chem 2003, 278, 25637–25643. [Google Scholar] [CrossRef]
- Qu, J.; Zou, T.; Lin, Z. The Roles of the Ubiquitin-Proteasome System in the Endoplasmic Reticulum Stress Pathway. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Shibata, Y.; Voeltz, G.K.; Rapoport, T.A. Rough sheets and smooth tubules. Cell 2006, 126, 435–439. [Google Scholar] [CrossRef]
- Schwarz, D.S.; Blower, M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell Mol Life Sci 2016, 73, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Almanza, A.; Carlesso, A.; Chintha, C.; Creedican, S.; Doultsinos, D.; Leuzzi, B.; Luis, A.; McCarthy, N.; Montibeller, L.; More, S.; et al. Endoplasmic reticulum stress signalling - from basic mechanisms to clinical applications. FEBS J 2019, 286, 241–278. [Google Scholar] [CrossRef]
- He, B. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ 2006, 13, 393–403. [Google Scholar] [CrossRef]
- Shen, J.; Chen, X.; Hendershot, L.; Prywes, R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell 2002, 3, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Bertolotti, A.; Zhang, Y.; Hendershot, L.M.; Harding, H.P.; Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol 2000, 2, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Madden, E.; Logue, S.E.; Healy, S.J.; Manie, S.; Samali, A. The role of the unfolded protein response in cancer progression: From oncogenesis to chemoresistance. Biol Cell 2019, 111, 1–17. [Google Scholar] [CrossRef]
- Park, J.; Cho, J.; Song, E.J. Ubiquitin-proteasome system (UPS) as a target for anticancer treatment. Arch Pharm Res 2020, 43, 1144–1161. [Google Scholar] [CrossRef]
- Jurkovicova, D.; Neophytou, C.M.; Gasparovic, A.C.; Goncalves, A.C. DNA Damage Response in Cancer Therapy and Resistance: Challenges and Opportunities. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Pilger, D.; Seymour, L.W.; Jackson, S.P. Interfaces between cellular responses to DNA damage and cancer immunotherapy. Genes Dev 2021, 35, 602–618. [Google Scholar] [CrossRef] [PubMed]
- Vriend, J.; Klonisch, T. Genes of the Ubiquitin Proteasome System Qualify as Differential Markers in Malignant Glioma of Astrocytic and Oligodendroglial Origin. Cell Mol Neurobiol 2023, 43, 1425–1452. [Google Scholar] [CrossRef] [PubMed]
- Vriend, J.; Thanasupawat, T.; Sinha, N.; Klonisch, T. Ubiquitin Proteasome Gene Signatures in Ependymoma Molecular Subtypes. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Maris, J.M.; Hogarty, M.D.; Bagatell, R.; Cohn, S.L. Neuroblastoma. Lancet 2007, 369, 2106–2120. [Google Scholar] [CrossRef] [PubMed]
- Park, J.R.; Bagatell, R.; London, W.B.; Maris, J.M.; Cohn, S.L.; Mattay, K.K.; Hogarty, M.; Committee, C.O. G. N. Children's Oncology Group's 2013 blueprint for research: Neuroblastoma. Pediatr Blood Cancer 2013, 60, 985–993. [Google Scholar] [CrossRef]
- Gravendeel, L.A.; Kouwenhoven, M.C.; Gevaert, O.; de Rooi, J.J.; Stubbs, A.P.; Duijm, J.E.; Daemen, A.; Bleeker, F.E.; Bralten, L.B.; Kloosterhof, N.K.; et al. Intrinsic gene expression profiles of gliomas are a better predictor of survival than histology. Cancer Res 2009, 69, 9065–9072. [Google Scholar] [CrossRef]
- Cavalli, F.M. G.; Remke, M.; Rampasek, L.; Peacock, J.; Shih, D.J. H.; Luu, B.; Garzia, L.; Torchia, J.; Nor, C.; Morrissy, A.S.; et al. Intertumoral Heterogeneity within Medulloblastoma Subgroups. Cancer Cell 2017, 31, 737–754. [Google Scholar] [CrossRef]
- Weishaupt, H.; Johansson, P.; Sundstrom, A.; Lubovac-Pilav, Z.; Olsson, B.; Nelander, S.; Swartling, F.J. Batch-normalization of cerebellar and medulloblastoma gene expression datasets utilizing empirically defined negative control genes. Bioinformatics 2019, 35, 3357–3364. [Google Scholar] [CrossRef]
- Ackermann, S.; Cartolano, M.; Hero, B.; Welte, A.; Kahlert, Y.; Roderwieser, A.; Bartenhagen, C.; Walter, E.; Gecht, J.; Kerschke, L.; et al. A mechanistic classification of clinical phenotypes in neuroblastoma. Science 2018, 362, 1165–1170. [Google Scholar] [CrossRef]
- Seifert, M.; Schackert, G.; Temme, A.; Schrock, E.; Deutsch, A.; Klink, B. Molecular Characterization of Astrocytoma Progression Towards Secondary Glioblastomas Utilizing Patient-Matched Tumor Pairs. Cancers (Basel) 2020, 12. [Google Scholar] [CrossRef]
- Zhang, P.; Xiao, Z.; Wang, S.; Zhang, M.; Wei, Y.; Hang, Q.; Kim, J.; Yao, F.; Rodriguez-Aguayo, C.; Ton, B.N.; et al. ZRANB1 Is an EZH2 Deubiquitinase and a Potential Therapeutic Target in Breast Cancer. Cell Rep 2018, 23, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Du, W.; Guo, W. EZH2: A novel target for cancer treatment. J Hematol Oncol 2020, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Straining, R.; Eighmy, W. Tazemetostat: EZH2 Inhibitor. J Adv Pract Oncol 2022, 13, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, H.; Reis, R.M.; Nakamura, M.; Colella, S.; Yonekawa, Y.; Kleihues, P.; Ohgaki, H. Loss of heterozygosity on chromosome 10 is more extensive in primary (de novo) than in secondary glioblastomas. Lab Invest 2000, 80, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Balesaria, S.; Brock, C.; Bower, M.; Clark, J.; Nicholson, S.K.; Lewis, P.; de Sanctis, S.; Evans, H.; Peterson, D.; Mendoza, N.; et al. Loss of chromosome 10 is an independent prognostic factor in high-grade gliomas. Br J Cancer 1999, 81, 1371–1377. [Google Scholar] [CrossRef]
- Wiles, B.; Miao, M.; Coyne, E.; Larose, L.; Cybulsky, A.V.; Wing, S.S. USP19 deubiquitinating enzyme inhibits muscle cell differentiation by suppressing unfolded-protein response signaling. Mol Biol Cell 2015, 26, 913–923. [Google Scholar] [CrossRef]
- Harada, K.; Kato, M.; Nakamura, N. USP19-Mediated Deubiquitination Facilitates the Stabilization of HRD1 Ubiquitin Ligase. Int J Mol Sci 2016, 17. [Google Scholar] [CrossRef]
- Nagai, A.; Kadowaki, H.; Maruyama, T.; Takeda, K.; Nishitoh, H.; Ichijo, H. USP14 inhibits ER-associated degradation via interaction with IRE1alpha. Biochem Biophys Res Commun 2009, 379, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Blount, J.R.; Burr, A.A.; Denuc, A.; Marfany, G.; Todi, S.V. Ubiquitin-specific protease 25 functions in Endoplasmic Reticulum-associated degradation. PLoS ONE 2012, 7, e36542. [Google Scholar] [CrossRef]
- Zhu, D.; Xu, R.; Huang, X.; Tang, Z.; Tian, Y.; Zhang, J.; Zheng, X. Deubiquitinating enzyme OTUB1 promotes cancer cell immunosuppression via preventing ER-associated degradation of immune checkpoint protein PD-L1. Cell Death Differ 2021, 28, 1773–1789. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, Y.; Gu, L.; Chang, Z.; Li, G.M. OTUB1 stabilizes mismatch repair protein MSH2 by blocking ubiquitination. J Biol Chem 2021, 296, 100466. [Google Scholar] [CrossRef]
- Unda, B.K.; Chalil, L.; Yoon, S.; Kilpatrick, S.; Irwin, C.; Xing, S.; Murtaza, N.; Cheng, A.; Brown, C.; Afonso, A.; et al. Impaired OTUD7A-dependent Ankyrin regulation mediates neuronal dysfunction in mouse and human models of the 15q13.3 microdeletion syndrome. Mol Psychiatry 2023, 28, 1747–1769. [Google Scholar] [CrossRef]
- Yin, J.; Chen, W.; Chao, E.S.; Soriano, S.; Wang, L.; Wang, W.; Cummock, S.E.; Tao, H.; Pang, K.; Liu, Z.; et al. Otud7a Knockout Mice Recapitulate Many Neurological Features of 15q13.3 Microdeletion Syndrome. Am J Hum Genet 2018, 102, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Bonacci, T.; Emanuele, M.J. Dissenting degradation: Deubiquitinases in cell cycle and cancer. Semin Cancer Biol 2020, 67 (Pt 2) Pt 2, 145–158. [Google Scholar] [CrossRef]
- Das, T.; Chen, Z.; Hendriks, R.W.; Kool, M. A20/Tumor Necrosis Factor alpha-Induced Protein 3 in Immune Cells Controls Development of Autoinflammation and Autoimmunity: Lessons from Mouse Models. Front Immunol 2018, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Sato, Y.; Takata, K.; Nomoto, J.; Nakamura, S.; Ohshima, K.; Takeuchi, T.; Orita, Y.; Kobayashi, Y.; Yoshino, T. A20 (TNFAIP3) deletion in Epstein-Barr virus-associated lymphoproliferative disorders/lymphomas. PLoS ONE 2013, 8, e56741. [Google Scholar] [CrossRef]
- Malakhova, O.A.; Kim, K.I.; Luo, J.K.; Zou, W.; Kumar, K.G.; Fuchs, S.Y.; Shuai, K.; Zhang, D.E. UBP43 is a novel regulator of interferon signaling independent of its ISG15 isopeptidase activity. EMBO J 2006, 25, 2358–2367. [Google Scholar] [CrossRef] [PubMed]
- Le, J.; Perez, E.; Nemzow, L.; Gong, F. Role of deubiquitinases in DNA damage response. DNA Repair (Amst) 2019, 76, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.P.; Chen, J.; Tse, W.K. F. Role of Deubiquitinases in Human Cancers: Potential Targeted Therapy. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Hirose, S. Regulation of mitochondrial morphology by USP30, a deubiquitinating enzyme present in the mitochondrial outer membrane. Mol Biol Cell 2008, 19, 1903–1911. [Google Scholar] [CrossRef]
- Wauer, T.; Swatek, K.N.; Wagstaff, J.L.; Gladkova, C.; Pruneda, J.N.; Michel, M.A.; Gersch, M.; Johnson, C.M.; Freund, S.M.; Komander, D. Ubiquitin Ser65 phosphorylation affects ubiquitin structure, chain assembly and hydrolysis. EMBO J 2015, 34, 307–325. [Google Scholar] [CrossRef]
- Cunningham, C.N.; Baughman, J.M.; Phu, L.; Tea, J.S.; Yu, C.; Coons, M.; Kirkpatrick, D.S.; Bingol, B.; Corn, J.E. USP30 and parkin homeostatically regulate atypical ubiquitin chains on mitochondria. Nat Cell Biol 2015, 17, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Bingol, B.; Tea, J.S.; Phu, L.; Reichelt, M.; Bakalarski, C.E.; Song, Q.; Foreman, O.; Kirkpatrick, D.S.; Sheng, M. The mitochondrial deubiquitinase USP30 opposes parkin-mediated mitophagy. Nature 2014, 510, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Yoshikawa, A.; Yamagata, A.; Mimura, H.; Yamashita, M.; Ookata, K.; Nureki, O.; Iwai, K.; Komada, M.; Fukai, S. Structural basis for specific cleavage of Lys 63-linked polyubiquitin chains. Nature 2008, 455, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xu, C.; Yang, W.; Chen, J.; Ou, Y.; Guan, Y.; Guan, J.; Liu, Y. E3 ligase RNF167 and deubiquitinase STAMBPL1 modulate mTOR and cancer progression. Mol Cell 2022, 82, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Mahul-Mellier, A.L.; Pazarentzos, E.; Datler, C.; Iwasawa, R.; AbuAli, G.; Lin, B.; Grimm, S. De-ubiquitinating protease USP2a targets RIP1 and TRAF2 to mediate cell death by TNF. Cell Death Differ 2012, 19, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Tan, X.; Wang, H.; Sun, W.; Shi, Y.; Burlingame, S.; Gu, X.; Cao, G.; Zhang, T.; Qin, J.; et al. Ubiquitin-specific peptidase 21 inhibits tumor necrosis factor alpha-induced nuclear factor kappaB activation via binding to and deubiquitinating receptor-interacting protein 1. J Biol Chem 2010, 285, 969–978. [Google Scholar] [CrossRef]
- Goricke, F.; Vu, V.; Smith, L.; Scheib, U.; Bohm, R.; Akkilic, N.; Wohlfahrt, G.; Weiske, J.; Bomer, U.; Brzezinka, K.; et al. Discovery and Characterization of BAY-805, a Potent and Selective Inhibitor of Ubiquitin-Specific Protease USP21. J Med Chem 2023, 66, 3431–3447. [Google Scholar] [CrossRef]
- Pajtler, K.W.; Mack, S.C.; Ramaswamy, V.; Smith, C.A.; Witt, H.; Smith, A.; Hansford, J.R.; von Hoff, K.; Wright, K.D.; Hwang, E.; et al. The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta Neuropathol 2017, 133, 5–12. [Google Scholar] [CrossRef]
- Hyrskyluoto, A.; Bruelle, C.; Lundh, S.H.; Do, H.T.; Kivinen, J.; Rappou, E.; Reijonen, S.; Waltimo, T.; Petersen, A.; Lindholm, D.; et al. Ubiquitin-specific protease-14 reduces cellular aggregates and protects against mutant huntingtin-induced cell degeneration: Involvement of the proteasome and ER stress-activated kinase IRE1alpha. Hum Mol Genet 2014, 23, 5928–5939. [Google Scholar] [CrossRef]
- Giordano, M.; Roncagalli, R.; Bourdely, P.; Chasson, L.; Buferne, M.; Yamasaki, S.; Beyaert, R.; van Loo, G.; Auphan-Anezin, N.; Schmitt-Verhulst, A.M.; et al. The tumor necrosis factor alpha-induced protein 3 (TNFAIP3, A20) imposes a brake on antitumor activity of CD8 T cells. Proc Natl Acad Sci U S A 2014, 111, 11115–11120. [Google Scholar] [CrossRef] [PubMed]
- Momtazi, G.; Lambrecht, B.N.; Naranjo, J.R.; Schock, B.C. Regulators of A20 (TNFAIP3): New drug-able targets in inflammation. Am J Physiol Lung Cell Mol Physiol 2019, 316, L456–L469. [Google Scholar] [CrossRef]
- van Bon, B.W. M.; Mefford, H.C.; de Vries, B.B. A.; Schaaf, C.P. 15q13.3 Recurrent Deletion. In GeneReviews((R)), Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J. H., Gripp, K.W., Amemiya, A. Eds.; 1993.
- Uddin, M.; Unda, B.K.; Kwan, V.; Holzapfel, N.T.; White, S.H.; Chalil, L.; Woodbury-Smith, M.; Ho, K.S.; Harward, E.; Murtaza, N.; et al. OTUD7A Regulates Neurodevelopmental Phenotypes in the 15q13.3 Microdeletion Syndrome. Am J Hum Genet 2018, 102, 278–295. [Google Scholar] [CrossRef]
- Lim, J.H.; Ha, U.H.; Woo, C.H.; Xu, H.; Li, J.D. CYLD is a crucial negative regulator of innate immune response in Escherichia coli pneumonia. Cell Microbiol 2008, 10, 2247–2256. [Google Scholar] [CrossRef]
- Deng, M.; Dai, W.; Yu, V.Z.; Tao, L.; Lung, M.L. Cylindromatosis Lysine 63 Deubiquitinase (CYLD) Regulates NF-kB Signaling Pathway and Modulates Fibroblast and Endothelial Cells Recruitment in Nasopharyngeal Carcinoma. Cancers (Basel) 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.C. CYLD: A tumor suppressor deubiquitinase regulating NF-kappaB activation and diverse biological processes. Cell Death Differ 2010, 17, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, L.F.; Sparks, A.; Allende-Vega, N.; Xirodimas, D.P.; Lane, D.P.; Saville, M.K. The deubiquitinating enzyme USP2a regulates the p53 pathway by targeting Mdm2. EMBO J 2007, 26, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Zhao, W.; Gu, W. Suppression of cancer cell growth by promoting cyclin D1 degradation. Mol Cell 2009, 36, 469–476. [Google Scholar] [CrossRef]
- Yang, Y.; Duguay, D.; Fahrenkrug, J.; Cermakian, N.; Wing, S.S. USP2 regulates the intracellular localization of PER1 and circadian gene expression. J Biol Rhythms 2014, 29, 243–256. [Google Scholar] [CrossRef]
- Scoma, H.D.; Humby, M.; Yadav, G.; Zhang, Q.; Fogerty, J.; Besharse, J.C. The de-ubiquitinylating enzyme, USP2, is associated with the circadian clockwork and regulates its sensitivity to light. PLoS ONE 2011, 6, e25382. [Google Scholar] [CrossRef]
- Stojkovic, K.; Wing, S.S.; Cermakian, N. A central role for ubiquitination within a circadian clock protein modification code. Front Mol Neurosci 2014, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Lee, H.J.; Saha, S.; Ruan, D.; Guo, H.; Chan, C.H. Inhibition of USP2 eliminates cancer stem cells and enhances TNBC responsiveness to chemotherapy. Cell Death Dis 2019, 10, 285. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Wang, J.; Wang, X.; Cai, S.; Guo, Y.; Ye, L.; Li, D.; Hu, A.; Jin, S.; Yuan, B.; et al. Therapeutic targeting of the USP2-E2F4 axis inhibits autophagic machinery essential for zinc homeostasis in cancer progression. Autophagy 2022, 18, 2615–2635. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Zou, L.; Yang, R.; Han, J.; Wan, Q.; Zhang, X.; El Baghdady, S.; Roman, A.; Elly, C.; Jin, H.S.; et al. The deubiquitinase CYLD controls protective immunity against helminth infection by regulation of Treg cell plasticity. J Allergy Clin Immunol 2021, 148, 209–224. [Google Scholar] [CrossRef]
- Bustamante, H.A.; Cereceda, K.; Gonzalez, A.E.; Valenzuela, G.E.; Cheuquemilla, Y.; Hernandez, S.; Arias-Munoz, E.; Cerda-Troncoso, C.; Bandau, S.; Soza, A.; et al. The Proteasomal Deubiquitinating Enzyme PSMD14 Regulates Macroautophagy by Controlling Golgi-to-ER Retrograde Transport. Cells 2020, 9. [Google Scholar] [CrossRef]
- Bustamante, H.A.; Albornoz, N.; Morselli, E.; Soza, A.; Burgos, P.V. Novel insights into the non-canonical roles of PSMD14/POH1/Rpn11 in proteostasis and in the modulation of cancer progression. Cell Signal 2023, 101, 110490. [Google Scholar] [CrossRef]
- Zhang, D.; Zaugg, K.; Mak, T.W.; Elledge, S.J. A role for the deubiquitinating enzyme USP28 in control of the DNA-damage response. Cell 2006, 126, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Jacq, X.; Kemp, M.; Martin, N.M.; Jackson, S.P. Deubiquitylating enzymes and DNA damage response pathways. Cell Biochem Biophys 2013, 67, 25–43. [Google Scholar] [CrossRef]
- Liang, X.W.; Wang, S.Z.; Liu, B.; Chen, J.C.; Cao, Z.; Chu, F.R.; Lin, X.; Liu, H.; Wu, J.C. A review of deubiquitinases and thier roles in tumorigenesis and development. Front Bioeng Biotechnol 2023, 11, 1204472. [Google Scholar] [CrossRef]
- Huo, Y.; Khatri, N.; Hou, Q.; Gilbert, J.; Wang, G.; Man, H.Y. The deubiquitinating enzyme USP46 regulates AMPA receptor ubiquitination and trafficking. J Neurochem 2015, 134, 1067–1080. [Google Scholar] [CrossRef]
- Anggono, V.; Huganir, R.L. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr Opin Neurobiol 2012, 22, 461–469. [Google Scholar] [CrossRef]
- Ishiuchi, S.; Tsuzuki, K.; Yoshida, Y.; Yamada, N.; Hagimura, N.; Okado, H.; Miwa, A.; Kurihara, H.; Nakazato, Y.; Tamura, M.; et al. Blockage of Ca(2+)-permeable AMPA receptors suppresses migration and induces apoptosis in human glioblastoma cells. Nat Med 2002, 8, 971–978. [Google Scholar] [CrossRef]
- Piao, Y.; Lu, L.; de Groot, J. AMPA receptors promote perivascular glioma invasion via beta1 integrin-dependent adhesion to the extracellular matrix. Neuro Oncol 2009, 11, 260–273. [Google Scholar] [CrossRef]
- Krishna, S.; Choudhury, A.; Keough, M.B.; Seo, K.; Ni, L.; Kakaizada, S.; Lee, A.; Aabedi, A.; Popova, G.; Lipkin, B.; et al. Glioblastoma remodelling of human neural circuits decreases survival. Nature 2023, 617, 599–607. [Google Scholar] [CrossRef]
- Tomida, S.; Mamiya, T.; Sakamaki, H.; Miura, M.; Aosaki, T.; Masuda, M.; Niwa, M.; Kameyama, T.; Kobayashi, J.; Iwaki, Y.; et al. Usp46 is a quantitative trait gene regulating mouse immobile behavior in the tail suspension and forced swimming tests. Nat Genet 2009, 41, 688–695. [Google Scholar] [CrossRef]
- Xian, J.; Zhang, Q.; Guo, X.; Liang, X.; Liu, X.; Feng, Y. A prognostic signature based on three non-coding RNAs for prediction of the overall survival of glioma patients. FEBS Open Bio 2019, 9, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Smits, A.; Jin, Z.; Elsir, T.; Pedder, H.; Nister, M.; Alafuzoff, I.; Dimberg, A.; Edqvist, P.H.; Ponten, F.; Aronica, E.; et al. GABA-A channel subunit expression in human glioma correlates with tumor histology and clinical outcome. PLoS ONE 2012, 7, e37041. [Google Scholar] [CrossRef] [PubMed]
- Blanchart, A.; Fernando, R.; Haring, M.; Assaife-Lopes, N.; Romanov, R.A.; Andang, M.; Harkany, T.; Ernfors, P. Endogenous GAB(AA) receptor activity suppresses glioma growth. Oncogene 2017, 36, 777–786. [Google Scholar] [CrossRef]
- Ma, L.; Lin, K.; Chang, G.; Chen, Y.; Yue, C.; Guo, Q.; Zhang, S.; Jia, Z.; Huang, T.T.; Zhou, A.; et al. Aberrant Activation of beta-Catenin Signaling Drives Glioma Tumorigenesis via USP1-Mediated Stabilization of EZH2. Cancer Res 2019, 79, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, M.; Wang, D.; Hou, P.; Chen, X.; Chu, S.; Chai, D.; Zheng, J.; Bai, J. Post-translational modifications of EZH2 in cancer. Cell Biosci 2020, 10, 143. [Google Scholar] [CrossRef] [PubMed]
- Nibe, Y.; Oshima, S.; Kobayashi, M.; Maeyashiki, C.; Matsuzawa, Y.; Otsubo, K.; Matsuda, H.; Aonuma, E.; Nemoto, Y.; Nagaishi, T.; et al. Novel polyubiquitin imaging system, PolyUb-FC, reveals that K33-linked polyubiquitin is recruited by SQSTM1/p62. Autophagy 2018, 14, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Chen, H.H.; Wang, W.J.; Chen, H.Y.; Huang, W.S.; Kao, C.H.; Lee, S.R.; Yeat, N.Y.; Yan, R.L.; Chan, S.J.; et al. TRABID inhibition activates cGAS/STING-mediated anti-tumor immunity through mitosis and autophagy dysregulation. Nat Commun 2023, 14, 3050. [Google Scholar] [CrossRef] [PubMed]
- Afonina, I.S.; Beyaert, R. Trabid epigenetically drives expression of IL-12 and IL-23. Nat Immunol 2016, 17, 227–228. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Xie, X.; Xiao, Y.; Hu, H.; Zou, Q.; Cheng, X.; Sun, S.C. Epigenetic regulation of the expression of Il12 and Il23 and autoimmune inflammation by the deubiquitinase Trabid. Nat Immunol 2016, 17, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhou, Y.; Pan, P.; Yu, H.; Wang, Z.; Li, L.L.; Wang, B.; Yan, Y.; Pan, Y.; Ye, Q.; et al. TRABID overexpression enables synthetic lethality to PARP inhibitor via prolonging 53BP1 retention at double-strand breaks. Nat Commun 2023, 14, 1810. [Google Scholar] [CrossRef] [PubMed]
- Wertz, I.E.; O'Rourke, K.M.; Zhou, H.; Eby, M.; Aravind, L.; Seshagiri, S.; Wu, P.; Wiesmann, C.; Baker, R.; Boone, D.L.; et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature 2004, 430, 694–699. [Google Scholar] [CrossRef]
- Wertz, I.E.; Newton, K.; Seshasayee, D.; Kusam, S.; Lam, C.; Zhang, J.; Popovych, N.; Helgason, E.; Schoeffler, A.; Jeet, S.; et al. Phosphorylation and linear ubiquitin direct A20 inhibition of inflammation. Nature 2015, 528, 370–375. [Google Scholar] [CrossRef]
- Abbasi, A.; Forsberg, K.; Bischof, F. The role of the ubiquitin-editing enzyme A20 in diseases of the central nervous system and other pathological processes. Front Mol Neurosci 2015, 8, 21. [Google Scholar] [CrossRef]
- Catrysse, L.; Vereecke, L.; Beyaert, R.; van Loo, G. A20 in inflammation and autoimmunity. Trends Immunol 2014, 35, 22–31. [Google Scholar] [CrossRef]
- Opipari, A.W., Jr.; Boguski, M.S.; Dixit, V.M. The A20 cDNA induced by tumor necrosis factor alpha encodes a novel type of zinc finger protein. J Biol Chem 1990, 265, 14705–14708. [Google Scholar] [CrossRef]
- Bredel, M.; Bredel, C.; Juric, D.; Duran, G.E.; Yu, R.X.; Harsh, G.R.; Vogel, H.; Recht, L.D.; Scheck, A.C.; Sikic, B.I. Tumor necrosis factor-alpha-induced protein 3 as a putative regulator of nuclear factor-kappaB-mediated resistance to O6-alkylating agents in human glioblastomas. J Clin Oncol 2006, 24, 274–287. [Google Scholar] [CrossRef]
- Hjelmeland, A.B.; Wu, Q.; Wickman, S.; Eyler, C.; Heddleston, J.; Shi, Q.; Lathia, J.D.; Macswords, J.; Lee, J.; McLendon, R.E.; et al. Targeting A20 decreases glioma stem cell survival and tumor growth. PLoS Biol 2010, 8, e1000319. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Schwalbe, E.C.; Williamson, D.; Sill, M.; Hovestadt, V.; Mynarek, M.; Rutkowski, S.; Robinson, G.W.; Gajjar, A.; Cavalli, F.; et al. Second-generation molecular subgrouping of medulloblastoma: An international meta-analysis of Group 3 and Group 4 subtypes. Acta Neuropathol 2019, 138, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Russell, A.J.; Liu, B.; George, J.; Liu, P.Y.; Liu, T.; DeFazio, A.; Bowtell, D.D.; Oberthuer, A.; London, W.B.; et al. A Myc Activity Signature Predicts Poor Clinical Outcomes in Myc-Associated Cancers. Cancer Res 2017, 77, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H.; Hashimoto, M. USP2-Related Cellular Signaling and Consequent Pathophysiological Outcomes. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Benassi, B.; Flavin, R.; Marchionni, L.; Zanata, S.; Pan, Y.; Chowdhury, D.; Marani, M.; Strano, S.; Muti, P.; Blandino, G.; et al. MYC is activated by USP2a-mediated modulation of microRNAs in prostate cancer. Cancer Discov 2012, 2, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Petrosyan, E.; Fares, J.; Fernandez, L.G.; Yeeravalli, R.; Dmello, C.; Duffy, J.T.; Zhang, P.; Lee-Chang, C.; Miska, J.; Ahmed, A.U.; et al. Endoplasmic Reticulum Stress in the Brain Tumor Immune Microenvironment. Mol Cancer Res 2023, 21, 389–396. [Google Scholar] [CrossRef]
- Mitchell, D.; Shireman, J.; Sierra Potchanant, E.A.; Lara-Velazquez, M.; Dey, M. Neuroinflammation in Autoimmune Disease and Primary Brain Tumors: The Quest for Striking the Right Balance. Front Cell Neurosci 2021, 15, 716947. [Google Scholar] [CrossRef]
- Pelizzari-Raymundo, D.; Doultsinos, D.; Pineau, R.; Sauzay, C.; Koutsandreas, T.; Langlais, T.; Carlesso, A.; Gkotsi, E.; Negroni, L.; Avril, T.; et al. A novel IRE1 kinase inhibitor for adjuvant glioblastoma treatment. iScience 2023, 26, 106687. [Google Scholar] [CrossRef]
- Flores-Santibanez, F.; Rennen, S.; Fernandez, D.; De Nolf, C.; Van De Velde, E.; Gaete Gonzalez, S.; Fuentes, C.; Moreno, C.; Figueroa, D.; Lladser, A.; et al. Nuanced role for dendritic cell intrinsic IRE1 RNase in the regulation of antitumor adaptive immunity. Front Immunol 2023, 14, 1209588. [Google Scholar] [CrossRef]
- Liang, W.; Fang, J.; Zhou, S.; Hu, W.; Yang, Z.; Li, Z.; Dai, L.; Tao, Y.; Fu, X.; Wang, X. The role of ubiquitin-specific peptidases in glioma progression. Biomed Pharmacother 2022, 146, 112585. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.; Hathaway, N.A.; Tone, Y.; Crosas, B.; Elsasser, S.; Kirkpatrick, D.S.; Leggett, D.S.; Gygi, S.P.; King, R.W.; Finley, D. Deubiquitinating enzyme Ubp6 functions noncatalytically to delay proteasomal degradation. Cell 2006, 127, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Lee, M.J.; Park, S.; Oh, D.C.; Elsasser, S.; Chen, P.C.; Gartner, C.; Dimova, N.; Hanna, J.; Gygi, S.P.; et al. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature 2010, 467, 179–184. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, S.K.; Jiang, Y.; Choi, W.H.; Hong, C.; Kim, D.E.; Lee, M.J. Facilitated Tau Degradation by USP14 Aptamers via Enhanced Proteasome Activity. Sci Rep 2015, 5, 10757. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, C.; Gu, C.; Li, Q.; Wu, N. Function of Deubiquitinating Enzyme USP14 as Oncogene in Different Types of Cancer. Cell Physiol Biochem 2016, 38, 993–1002. [Google Scholar] [CrossRef]
- Ma, Y.S.; Wang, X.F.; Zhang, Y.J.; Luo, P.; Long, H.D.; Li, L.; Yang, H.Q.; Xie, R.T.; Jia, C.Y.; Lu, G.X.; et al. Inhibition of USP14 Deubiquitinating Activity as a Potential Therapy for Tumors with p53 Deficiency. Mol Ther Oncolytics 2020, 16, 147–157. [Google Scholar] [CrossRef]
- Kaokhum, N.; Pinto-Fernandez, A.; Wilkinson, M.; Kessler, B.M.; Ismail, H.M. The Mechano-Ubiquitinome of Articular Cartilage: Differential Ubiquitination and Activation of a Group of ER-Associated DUBs and ER Stress Regulators. Mol Cell Proteomics 2022, 21, 100419. [Google Scholar] [CrossRef]
- Ernst, R.; Mueller, B.; Ploegh, H.L.; Schlieker, C. The otubain YOD1 is a deubiquitinating enzyme that associates with p97 to facilitate protein dislocation from the ER. Mol Cell 2009, 36, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Schimmack, G.; Schorpp, K.; Kutzner, K.; Gehring, T.; Brenke, J.K.; Hadian, K.; Krappmann, D. YOD1/TRAF6 association balances p62-dependent IL-1 signaling to NF-kappaB. Elife 2017, 6. [Google Scholar] [CrossRef]
- Tanji, K.; Mori, F.; Miki, Y.; Utsumi, J.; Sasaki, H.; Kakita, A.; Takahashi, H.; Wakabayashi, K. YOD1 attenuates neurogenic proteotoxicity through its deubiquitinating activity. Neurobiol Dis 2018, 112, 14–23. [Google Scholar] [CrossRef]
- Zhou, L.; Li, L.; Chen, Y.; Chen, C.; Zhi, Z.; Yan, L.; Wang, Y.; Liu, B.; Zhai, Q. miR-190a-3p Promotes Proliferation and Migration in Glioma Cells via YOD1. Comput Math Methods Med 2021, 2021, 3957738. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, W.; Song, Y.; Kim, J.R.; Cho, K.; Moon, H.; Ro, S.W.; Seo, E.; Ryu, Y.M.; Myung, S.J.; et al. Deubiquitinase YOD1 potentiates YAP/TAZ activities through enhancing ITCH stability. Proc Natl Acad Sci U S A 2017, 114, 4691–4696. [Google Scholar] [CrossRef] [PubMed]
- Masliantsev, K.; Karayan-Tapon, L.; Guichet, P.O. Hippo Signaling Pathway in Gliomas. Cells 2021, 10. [Google Scholar] [CrossRef]
- Nijman, S.M.; Huang, T.T.; Dirac, A.M.; Brummelkamp, T.R.; Kerkhoven, R.M.; D'Andrea, A.D.; Bernards, R. The deubiquitinating enzyme USP1 regulates the Fanconi anemia pathway. Mol Cell 2005, 17, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.T.; Nijman, S.M.; Mirchandani, K.D.; Galardy, P.J.; Cohn, M.A.; Haas, W.; Gygi, S.P.; Ploegh, H.L.; Bernards, R.; D'Andrea, A.D. Regulation of monoubiquitinated PCNA by DUB autocleavage. Nat Cell Biol 2006, 8, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Kashiwaba, S.; Kanao, R.; Masuda, Y.; Kusumoto-Matsuo, R.; Hanaoka, F.; Masutani, C. USP7 Is a Suppressor of PCNA Ubiquitination and Oxidative-Stress-Induced Mutagenesis in Human Cells. Cell Rep 2015, 13, 2072–2080. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.L.; Dianova, II; Khoronenkova, S. V.; Edelmann, M.J.; Kessler, B.M.; Dianov, G.L. USP47 is a deubiquitylating enzyme that regulates base excision repair by controlling steady-state levels of DNA polymerase beta. Mol Cell 2011, 41, 609–615. [Google Scholar] [CrossRef]
- Zhao, Y.; Majid, M.C.; Soll, J.M.; Brickner, J.R.; Dango, S.; Mosammaparast, N. Noncanonical regulation of alkylation damage resistance by the OTUD4 deubiquitinase. EMBO J 2015, 34, 1687–1703. [Google Scholar] [CrossRef]
- Chitale, S.; Richly, H. Timing of DNA lesion recognition: Ubiquitin signaling in the NER pathway. Cell Cycle 2017, 16, 163–171. [Google Scholar] [CrossRef]
- Perez-Oliva, A.B.; Lachaud, C.; Szyniarowski, P.; Munoz, I.; Macartney, T.; Hickson, I.; Rouse, J.; Alessi, D.R. USP45 deubiquitylase controls ERCC1-XPF endonuclease-mediated DNA damage responses. EMBO J 2015, 34, 326–343. [Google Scholar] [CrossRef]
- Nishi, R.; Wijnhoven, P.; le Sage, C.; Tjeertes, J.; Galanty, Y.; Forment, J.V.; Clague, M.J.; Urbe, S.; Jackson, S.P. Systematic characterization of deubiquitylating enzymes for roles in maintaining genome integrity. Nat Cell Biol 2014, 16, 1016–1026. [Google Scholar] [CrossRef]
- Sato, Y.; Yamagata, A.; Goto-Ito, S.; Kubota, K.; Miyamoto, R.; Nakada, S.; Fukai, S. Molecular basis of Lys-63-linked polyubiquitination inhibition by the interaction between human deubiquitinating enzyme OTUB1 and ubiquitin-conjugating enzyme UBC13. J Biol Chem 2012, 287, 25860–25868. [Google Scholar] [CrossRef]
- Butler, L.R.; Densham, R.M.; Jia, J.; Garvin, A.J.; Stone, H.R.; Shah, V.; Weekes, D.; Festy, F.; Beesley, J.; Morris, J.R. The proteasomal de-ubiquitinating enzyme POH1 promotes the double-strand DNA break response. EMBO J 2012, 31, 3918–3934. [Google Scholar] [CrossRef]
- Kakarougkas, A.; Ismail, A.; Katsuki, Y.; Freire, R.; Shibata, A.; Jeggo, P.A. Co-operation of BRCA1 and POH1 relieves the barriers posed by 53BP1 and RAP80 to resection. Nucleic Acids Res 2013, 41, 10298–10311. [Google Scholar] [CrossRef]
- Vincent, P.; Collette, Y.; Marignier, R.; Vuaillat, C.; Rogemond, V.; Davoust, N.; Malcus, C.; Cavagna, S.; Gessain, A.; Machuca-Gayet, I.; et al. A role for the neuronal protein collapsin response mediator protein 2 in T lymphocyte polarization and migration. J Immunol 2005, 175, 7650–7660. [Google Scholar] [CrossRef]
- Nakamura, F.; Ohshima, T.; Goshima, Y. Collapsin Response Mediator Proteins: Their Biological Functions and Pathophysiology in Neuronal Development and Regeneration. Front Cell Neurosci 2020, 14, 188. [Google Scholar] [CrossRef]
- Bedekovics, T.; Hussain, S.; Zhang, Y.; Ali, A.; Jeon, Y.J.; Galardy, P.J. USP24 Is a Cancer-Associated Ubiquitin Hydrolase, Novel Tumor Suppressor, and Chromosome Instability Gene Deleted in Neuroblastoma. Cancer Res 2021, 81, 1321–1331. [Google Scholar] [CrossRef]
- Thayer, J.A.; Awad, O.; Hegdekar, N.; Sarkar, C.; Tesfay, H.; Burt, C.; Zeng, X.; Feldman, R.A.; Lipinski, M.M. The PARK10 gene USP24 is a negative regulator of autophagy and ULK1 protein stability. Autophagy 2020, 16, 140–153. [Google Scholar] [CrossRef]
- Ma, X.; Qi, W.; Yang, F.; Pan, H. Deubiquitinase JOSD1 promotes tumor progression via stabilizing Snail in lung adenocarcinoma. Am J Cancer Res 2022, 12, 2323–2336. [Google Scholar]
- Yang, J.; Weisberg, E.L.; Liu, X.; Magin, R.S.; Chan, W.C.; Hu, B.; Schauer, N.J.; Zhang, S.; Lamberto, I.; Doherty, L.; et al. Small molecule inhibition of deubiquitinating enzyme JOSD1 as a novel targeted therapy for leukemias with mutant JAK2. Leukemia 2022, 36, 210–220. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Zhang, Y.; Zhao, P.; Qian, L.; Yuan, Y.; Liu, J.; Cheng, Q.; Xu, W.; Zuo, Y.; et al. JOSD1 Negatively Regulates Type-I Interferon Antiviral Activity by Deubiquitinating and Stabilizing SOCS1. Viral Immunol 2017, 30, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhou, J.; Tang, J.; Zhou, F.; He, Z.; Liu, T.; Liu, T. MINDY1 promotes bladder cancer progression by stabilizing YAP. Cancer Cell Int 2021, 21, 395. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Luo, Y.; Long, G.; Zhou, L. MINDY1 promotes breast cancer cell proliferation by stabilizing estrogen receptor alpha. Cell Death Dis 2021, 12, 937. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Heatmap and cluster analysis of differentially expressed DUBs in gliomas. Non-tumor, N = 23; Astrocytoma, N = 26; Glioblastoma, N = 77; Oligodendroglioma, N = 50).
Figure 1.
Heatmap and cluster analysis of differentially expressed DUBs in gliomas. Non-tumor, N = 23; Astrocytoma, N = 26; Glioblastoma, N = 77; Oligodendroglioma, N = 50).
Figure 2.
Differential expression of USP46 and ZRANB1 in adult glioma groups. USP46 (F = 42.34, p = 1.53e-20); ZRANB1 (F = 45.17, p = 1.40e-21) in the Sun dataset; Non tumor N = 23, Astrocytoma, N = 26, Glioblastoma, N = 77, Oligodendroglioma, N = 50).
Figure 2.
Differential expression of USP46 and ZRANB1 in adult glioma groups. USP46 (F = 42.34, p = 1.53e-20); ZRANB1 (F = 45.17, p = 1.40e-21) in the Sun dataset; Non tumor N = 23, Astrocytoma, N = 26, Glioblastoma, N = 77, Oligodendroglioma, N = 50).
Figure 3.
Heatmap and cluster analysis of DUB genes differentially expressed in GBM subtypes. On average, TNFAIP3 expression was higher in the GBM mesenchymal group, whereas the expression of the other 9 genes was greater in the proneural group. Classic, N = 17; Mesenchymal, N = 27; Proneural, N = 24).
Figure 3.
Heatmap and cluster analysis of DUB genes differentially expressed in GBM subtypes. On average, TNFAIP3 expression was higher in the GBM mesenchymal group, whereas the expression of the other 9 genes was greater in the proneural group. Classic, N = 17; Mesenchymal, N = 27; Proneural, N = 24).
Figure 4.
Differentially expressed DUBs identified under the GO category of Regulation of immune response in different glioma and non-tumor brain. *p < 0.001 compared to non-tumor group.
Figure 4.
Differentially expressed DUBs identified under the GO category of Regulation of immune response in different glioma and non-tumor brain. *p < 0.001 compared to non-tumor group.
Figure 5.
Differentially expressed DUB genes in the GO category of DNA repair. UCHL5, F = 21.83, p = 4.97e-120; OTUB1, F = 42.71, p = 1.12 E-20; USP3, F = 19.33, p = 7.56e-11; USP1, F = 16.99, p = 1.04e-09. * p < 0.001 compared to Non tumor group by t-test.
Figure 5.
Differentially expressed DUB genes in the GO category of DNA repair. UCHL5, F = 21.83, p = 4.97e-120; OTUB1, F = 42.71, p = 1.12 E-20; USP3, F = 19.33, p = 7.56e-11; USP1, F = 16.99, p = 1.04e-09. * p < 0.001 compared to Non tumor group by t-test.
Figure 6.
Heatmap and cluster analysis of DUB gene expression in ependymoma subtypes (Pf_Epn_a, N = 72; Pf_Epn-b, N = 39; Pf_se, N = 11; Sp_Epn, N = 11; Sp_Mpe, N = 8; St_Epn_Rela, N = 49; St_Epn_Yap1, N = 11; St_Se, N = 8).
Figure 6.
Heatmap and cluster analysis of DUB gene expression in ependymoma subtypes (Pf_Epn_a, N = 72; Pf_Epn-b, N = 39; Pf_se, N = 11; Sp_Epn, N = 11; Sp_Mpe, N = 8; St_Epn_Rela, N = 49; St_Epn_Yap1, N = 11; St_Se, N = 8).
Figure 7.
Differential expression of USP30 and STAMBPL1 in ependymoma molecular subgroups. USP30, F = 12.58, p = 2.38e-13; STAMBPL1, F = 54.29, p = 5.23e-43. This dataset is missing non-tumor control group for comparison by t test.
Figure 7.
Differential expression of USP30 and STAMBPL1 in ependymoma molecular subgroups. USP30, F = 12.58, p = 2.38e-13; STAMBPL1, F = 54.29, p = 5.23e-43. This dataset is missing non-tumor control group for comparison by t test.
Figure 8.
Heatmap and cluster analysis of DUB genes in craniopharyngioma. Normal, N = 27; Craniopharyngioma, N = 24.
Figure 8.
Heatmap and cluster analysis of DUB genes in craniopharyngioma. Normal, N = 27; Craniopharyngioma, N = 24.
Figure 9.
Heatmap and cluster analysis of the top 12 differentially expressed DUB genes for each of the four MB subgroups compared to non-tumor brain (Note that due to overlap the total is less than 48). The Swartling dataset was used to identify top genes differing from non-tumor controls. The Cavalli dataset was used for the construction of the heatmap depicting the expression of these genes. Based on gene expression in the MB subgroups, the most significantly elevated DUBs for each group were: Group 3 – USP2; Group 4 – USP20; SHH – EIF3H; WNT – USP5. Group 3, N = 144; Group 4, N = 326; SHH, N = 223; Wnt, N = 70).
Figure 9.
Heatmap and cluster analysis of the top 12 differentially expressed DUB genes for each of the four MB subgroups compared to non-tumor brain (Note that due to overlap the total is less than 48). The Swartling dataset was used to identify top genes differing from non-tumor controls. The Cavalli dataset was used for the construction of the heatmap depicting the expression of these genes. Based on gene expression in the MB subgroups, the most significantly elevated DUBs for each group were: Group 3 – USP2; Group 4 – USP20; SHH – EIF3H; WNT – USP5. Group 3, N = 144; Group 4, N = 326; SHH, N = 223; Wnt, N = 70).
Figure 10.
Gene expression of VCPIP1, USP49 and USP2 in medulloblastoma by age and subgroups. Orange – Group 3, Gray – Group 4, Blue – SHH, Yellow – Wnt. Of these genes USP2 expression was most specifically elevated in Group 3 MB infants and children compared to the other groups (Cavalli dataset) and compared to a non-tumor group (Swartling dataset).
Figure 10.
Gene expression of VCPIP1, USP49 and USP2 in medulloblastoma by age and subgroups. Orange – Group 3, Gray – Group 4, Blue – SHH, Yellow – Wnt. Of these genes USP2 expression was most specifically elevated in Group 3 MB infants and children compared to the other groups (Cavalli dataset) and compared to a non-tumor group (Swartling dataset).
Figure 11.
Differential expression of ERAD-associated DUBs in different medulloblastoma subgroups (Cavalli dataset). USP25, F = 138.98, p = 9.08e-72; USP 13, F = 49.90, p = 1.93e-29.
Figure 11.
Differential expression of ERAD-associated DUBs in different medulloblastoma subgroups (Cavalli dataset). USP25, F = 138.98, p = 9.08e-72; USP 13, F = 49.90, p = 1.93e-29.
Figure 12.
Treatment effects on DUB gene expression in Neuroblastoma. Intensive chemotherapy (ct) (N = 114), intermediate ct (N = 30), limited ct (N = 18), observation (no treatment control) (N =23), surgery (N = 43).
Figure 12.
Treatment effects on DUB gene expression in Neuroblastoma. Intensive chemotherapy (ct) (N = 114), intermediate ct (N = 30), limited ct (N = 18), observation (no treatment control) (N =23), surgery (N = 43).
Figure 13.
DUB expression in limited vs intensive chemotherapy of neuroblastoma from the Fischer dataset. Limited chemotherapy (ct), N = 18, intermediate ct, N = 30, intensive ct, N = 114. USP 24, F = 21.10, p = 7.44e-09; JOSD1, F = 18.44, p =6.26e-08; MINDY2, F = 21.61, p = 4.99e-09. *significantly different from limited ct group at p < 0.001 by t-test.
Figure 13.
DUB expression in limited vs intensive chemotherapy of neuroblastoma from the Fischer dataset. Limited chemotherapy (ct), N = 18, intermediate ct, N = 30, intensive ct, N = 114. USP 24, F = 21.10, p = 7.44e-09; JOSD1, F = 18.44, p =6.26e-08; MINDY2, F = 21.61, p = 4.99e-09. *significantly different from limited ct group at p < 0.001 by t-test.
Table 1.
Top ten differentially expressed DUB genes in gliomas by group compared to non-tumor group.
Table 1.
Top ten differentially expressed DUB genes in gliomas by group compared to non-tumor group.
| Astrocytoma1
|
DUB |
p value (corrected) |
Chromosome |
Versus non-tumor
group in the Sun dataset |
| CYLD |
Usp |
2.44E-13 |
16 |
Down |
| USP12 |
Usp |
3.73E-12 |
13 |
Down |
| USP3 |
Usp |
1.95E-11 |
15 |
Up |
| OTUD7A |
Otu |
1.26E-10 |
15 |
Down |
| OTUB1 |
Otu |
4.38E-10 |
11 |
Down |
| USP8 |
Usp |
1.69E-09 |
15 |
Up |
| USP33 |
Usp |
8.97E-09 |
1 |
Down |
| STAMBPL1 |
Jamm |
5.64E-08 |
10 |
Down |
| EIF3F |
Jamm |
5.94E-08 |
11 |
Up |
| EIF3H |
Jamm |
4.60E-07 |
8 |
Up |
| |
|
|
|
|
| Glioblastoma2 |
|
|
|
|
| USP12 |
Usp |
5.22E-20 |
13 |
Down |
| OTUD7A |
Otu |
1.13E-19 |
15 |
Down |
| ZRANB1 |
Otu |
2.37E-18 |
10 |
Down |
| USP46 |
Usp |
3.60E-18 |
4 |
Down |
| OTUB1 |
Otu |
4.24E-18 |
11 |
Down |
| CYLD |
Usp |
2.34E-16 |
16 |
Down |
| USP3 |
Usp |
1.20E-13 |
15 |
Up |
| USP27X |
Usp |
5.35E-12 |
X |
Down |
| USP30 |
Usp |
9.20E-12 |
12 |
Down |
| USP11 |
Usp |
1.39E-11 |
X |
Down |
| |
|
|
|
|
| Oligodendroglioma3 |
|
|
|
|
| USP12 |
Usp |
3.27E-11 |
13 |
Down |
| OTUD7A |
Otu |
3.55E-10 |
15 |
Down |
| USP48 |
Usp |
6.67E-09 |
1 |
Down |
| USP33 |
Usp |
1.37E-08 |
1 |
Down |
| CYLD |
Usp |
1.45E-08 |
16 |
Down |
| USP3 |
Usp |
1.50E-08 |
15 |
Up |
| EIF3F |
Jamm |
1.57E-08 |
11 |
Up |
| OTUD3 |
Otu |
2.46E-08 |
1 |
Down |
| USP14 |
Usp |
4.85E-08 |
18 |
Down |
| OTUB1 |
Otu |
6.68E-08 |
11 |
Down |
Table 2.
DUB gene expression and survival data of glioma patients (French dataset).
Table 2.
DUB gene expression and survival data of glioma patients (French dataset).
| DUB Gene |
Family |
Chromo-some |
Chi square Kaplan Meier |
p value |
Hazard Ratio (HR) |
HR p value |
Better survival |
| ZRANB1 |
Otu |
10 |
116.69 |
3.36e-27 |
0.21 |
4.6e-27 |
High |
| FAM188A |
Mindy |
10 |
68.95 |
1.01e-16 |
0.53 |
1.4e-13 |
High |
| USP34 |
Usp |
2 |
63.01 |
2.06e-15 |
0.30 |
4.4e-12 |
High |
| USP49 |
Usp |
6 |
63.46 |
1.63e-15 |
0.55 |
1.9e-16 |
High |
| Usp27X |
Usp |
X |
57.58 |
3.24e-14 |
0.43 |
1.6e-15 |
High |
| USP54 |
Usp |
10 |
51.46 |
7.30e-13 |
0.68 |
2.5e-11 |
High |
| USP51 |
Usp |
X |
49.10 |
2.43e-12 |
0.69 |
1.4e-11 |
High |
| USP30 |
Usp |
12 |
43.77 |
3.69e-11 |
0.47 |
1.5e-8 |
High |
| USP11 |
Usp |
X |
42.18 |
8.33e-11 |
0.52 |
2.6e-10 |
High |
| OTUD7A |
Otu |
15 |
38.15 |
6.54e-10 |
0.69 |
1.3e-9 |
High |
| EIF3H |
Jamm |
8 |
35.16 |
3.40e-09 |
0.47 |
7.1e-10 |
High |
| USP1 |
Usp |
1 |
33.71 |
6.39e-09 |
1.8 |
1.4e-8 |
Low |
| USP4 |
Usp |
3 |
33.07 |
8.90e-09 |
2.6 |
5.6e-8 |
Low |
| ATXN3 |
MJD |
14 |
33.10 |
8.75e-09 |
0.65 |
7.7e-6 |
High |
| USP43 |
Usp |
17 |
31.76 |
1.74e-08 |
0.74 |
1.1e-8 |
High |
| USP46 |
Usp |
4 |
31.45 |
2.05e-08 |
0.65 |
9.2e-8 |
High |
| TNFAIP3 |
Otu |
6 |
30.86 |
2.77e-08 |
1.3 |
6.0e-7 |
Low |
| OTUD1 |
Otu |
10 |
28.27 |
1.06e-07 |
0.54 |
2.0e-8 |
High |
| JOSD2 |
MJD |
19 |
25.88 |
3.62e-07 |
1.5 |
2.1e-4 |
Low |
| PSMD7 |
Jamm |
16 |
24.22 |
8.60e-07 |
1.9 |
1.3e-4 |
Low |
| STAMBPL1 |
Jamm |
10 |
23.87 |
1.03e-06 |
0.76 |
4.3e-5 |
High |
| USP14 |
Usp |
18 |
23.95 |
9.90e-07 |
2.1 |
1.0e-4 |
low |
Table 3.
Differential expression of DUBs in ERAD signaling in glioma.
Table 3.
Differential expression of DUBs in ERAD signaling in glioma.
| DUB gene |
Non-tumor
N = 23 |
Astrocytoma
N = 26 |
Glioblastoma
N = 77 |
Oligodendroglioma
N = 50 |
| USP14 |
851.87 ± 25.31 |
689.66 ± 25.63* |
738.5 ± 18.13* |
647.71 ± 16.28* |
| USP19 |
159.11 ± 6.47 |
206.93 ± 9.5* |
222.11 ± 6.09* |
226.03 ± 7.9* |
| USP25 |
314.57 ± 14.19 |
216.77 ± 9.34* |
216.88 ± 9.18* |
209.77 ± 7.59* |
| BAP1 |
295.14 ± 10.78 |
221.5 ± 7.97* |
244.66 ± 5.02* |
248.25 ± 6.88* |
Table 4.
Top ten differentially expressed DUB genes by MB subgroup compared to non-tumor group in the Swartling dataset.
Table 4.
Top ten differentially expressed DUB genes by MB subgroup compared to non-tumor group in the Swartling dataset.
Group 3
(N = 233) |
DUB |
p value vs
non-tumor |
Chromosome |
Versus non-tumor group in the Swartling dataset |
| USP46 |
Usp |
1.16e-57 |
4 |
Down |
| USP2 |
Usp |
5.71e-53 |
11 |
Up |
| PSMD14 |
Jamm |
4.25e-51 |
2 |
Up |
| USP49 |
Usp |
1.34e-36 |
6 |
Up |
| USP28 |
Usp |
2.41e-26 |
11 |
Down |
| USP30 |
Usp |
1.29e-25 |
12 |
Up |
| UCHL1 |
Uch |
1.72e-22 |
4 |
Down |
| OTUD7A |
Otu |
6.2e-22 |
15 |
Down |
| USP44 |
Usp |
6.39e-22 |
12 |
Down |
| COPS6 |
Jamm |
2.19E-21 |
7 |
Up |
| |
|
|
|
|
Group 4
(N = 530)
|
|
|
|
|
| USP20 |
Usp |
8.96e-65 |
9 |
Up |
| USP28 |
Usp |
2.28e-63 |
11 |
Down |
| USP22 |
Usp |
1.35e-61 |
17 |
Up |
| USP32 |
Usp |
1.21e-5 |
17 |
Up |
| USP3 |
Usp |
2.98e-54 |
15 |
Down |
| USP30 |
Usp |
2.19e-52 |
12 |
Up |
| USP49 |
Usp |
1.49e-43 |
6 |
Up |
| USP45 |
Usp |
9.56e-36 |
6 |
Down |
| USP36 |
Usp |
7.95e-29 |
17 |
Up |
| EIF3H |
Jamm |
1.59e-27 |
8 |
Down |
| |
|
|
|
|
SHH
(N = 405)
|
|
|
|
|
| EIF3H |
Jamm |
4.51e-135 |
8 |
Down |
| USP2 |
Usp |
1.17e-88 |
11 |
Down |
| USP20 |
Usp |
2.26e-69 |
9 |
Down |
| CYLD |
Usp |
7.13e-64 |
16 |
Down |
| USP25 |
Usp |
4.63e-40 |
21 |
Down |
| USP32 |
Usp |
4.97e-38 |
17 |
Down |
| USP33 |
Usp |
5.42e-36 |
1 |
Down |
| JOSD1 |
Mjd |
2.4e-30 |
22 |
Up |
| USP11 |
Usp |
3.32e-28 |
X |
Down |
| USP13 |
Usp |
1.03e-26 |
3 |
Up |
| |
|
|
|
|
WNT
(N = 118)
|
|
|
|
|
| UCHL1 |
Uch |
2.14e-82 |
4 |
Down |
| USP2 |
Usp |
3.18e-56 |
11 |
Down |
| USP20 |
Usp |
6.88e-50 |
9 |
Down |
| USP32 |
Usp |
2.23e-47 |
17 |
Down |
| USP5 |
Usp |
1.19e-38 |
12 |
Up |
| COPS6 |
Jamm |
2.5e-32 |
7 |
Up |
| EIF3H |
Jamm |
1.19e-29 |
8 |
Up |
| OTUD7A |
Otu |
1.48e-29 |
15 |
Down |
| USP33 |
Usp |
1.38e-28 |
1 |
Down |
| USP28 |
Usp |
6.05e-27 |
11 |
Down |
Table 5.
DUB gene expression and medulloblastoma survival data (Cavalli dataset).
Table 5.
DUB gene expression and medulloblastoma survival data (Cavalli dataset).
| DUB |
DUB family |
Chromo-some |
Chi square
Kaplan Meier |
p value |
Hazard Ratio |
p value for hazard ratio |
Better survival |
| VCPIP1 |
Otu |
8 |
28.14 |
1.13e-07 |
1.9 |
1.10E-05 |
Low |
| USP49 |
Usp |
6 |
26.73 |
2.34e-07 |
1.9 |
1.30E-05 |
Low |
| USP2 |
Usp |
11 |
21.82 |
2.99e-06 |
1.3 |
3.50E-05 |
Low |
| USP51 |
Usp |
X |
19.36 |
1.08e-05 |
0.44 |
1.00E-04 |
High |
| STAMBPL1 |
Jamm |
10 |
16.70 |
4.37e-05 |
0.73 |
3.10E-04 |
High |
| PSMD14 |
Jamm |
2 |
18.05 |
2.16e-05 |
1.4 |
4.20E-04 |
Low |
| ZRANB1 |
Otu |
10 |
17.11 |
3.52e-05 |
0.54 |
1.00E-03 |
High |
| OTUD3 |
Otu |
1 |
18.76 |
1.48e-05 |
2.4 |
1.30E-03 |
Low |
| PRPF8 |
Jamm |
17 |
18.77 |
1.47e-05 |
0.58 |
2.20E-03 |
High |
| USP15 |
Usp |
12 |
16.42 |
5.06e-05 |
2 |
3.00E-03 |
Low |
| USP45 |
Usp |
6 |
14.82 |
1.18e-04 |
1.5 |
3.90E-03 |
Low |
| USP26 |
Usp |
X |
22.77 |
1.82e-06 |
7.3 |
5.20E-03 |
Low |
| USP36 |
Usp |
17 |
22.07 |
2.63e-06 |
2.2 |
5.50E-03 |
Low |
| USPL1 |
Usp |
13 |
14.89 |
1.14e-04 |
2.1 |
5.80E-03 |
Low |
| USP25 |
Usp |
21 |
12.36 |
4.39e-04 |
1.6 |
8.70E-03 |
Low |
| EIF3H |
Jamm |
8 |
13.23 |
2.75e-04 |
1.5 |
9.40E-03 |
Low |
| COPS5 |
Jamm |
8 |
9.25 |
2.36e-03 |
1.9 |
9.90E-03 |
Low |
Table 6.
DUBs and GO category of Regulation of immune response by MB group compared to non-tumor group of Swartling dataset (n = 291).
Table 6.
DUBs and GO category of Regulation of immune response by MB group compared to non-tumor group of Swartling dataset (n = 291).
| DUB |
family |
p value corrected FDR |
Versus non-tumor group in Swartling dataset |
| Group 3 (n = 233) |
|
|
|
| PSMD14 |
Jamm |
8.74e-52 |
Up |
| OTUD7A |
Otu |
1.70e-22 |
Down |
| TNFAIP3 |
Otu |
6.26e-10 |
Up |
| CYLD |
Usp |
3.54e-08 |
Down |
| |
|
|
|
| Group 4 (n = 530) |
|
|
|
| PSMD14 |
Jamm |
2.23e-26 |
Up |
| OTUD7A |
Otu |
1.16e-13 |
Down |
| CYLD |
Usp |
7.04e-10 |
Up |
| TNFAIP3 |
Otu |
5.67e-09 |
Up |
| |
|
|
|
| SHH (n = 405) |
|
|
|
| CYLD |
Usp |
1.95e-64 |
Down |
| PSMD14 |
Jamm |
4.95e-09 |
Down |
| TNFAIP3 |
Otu |
2.95e-08 |
Up |
| PSMD7 |
Jamm |
5.19e-08 |
Up |
| |
|
|
|
| Wnt (n = 118) |
|
|
|
| OTUD7A |
Otu |
8.10e-30 |
Down |
| PSMD7 |
Jamm |
3.89e-25 |
Up |
| TNFAIP3 |
Otu |
2.40e-10 |
Down |
| CYLD |
Usp |
4.19e-04 |
Up |
| PSMD14 |
Jamm |
8.99e-04 |
Up |
Table 7.
DUBS and GO category of DNA repair in MB groups compared to non-tumor group.
Table 7.
DUBS and GO category of DNA repair in MB groups compared to non-tumor group.
| DUB |
family |
p value vs NT group Swartling |
Versus non-tumor group in Swartling dataset |
| Group 3 vs NT |
|
|
|
| PSMD14 |
Jamm |
2.27e-51 |
Up |
| USP28 |
Usp |
1.07e-26 |
Down |
| COPS6 |
Jamm |
1.30e-21 |
Up |
| USP47 |
Usp |
3.96e-15 |
Down |
| UCHL5 |
Uch |
6.12e-14 |
Up |
| COPS5 |
Jamm |
1.61e-10 |
Up |
| USP1 |
Usp |
2.53e-06 |
Up |
| OTUB1 |
Otu |
3.33e-05 |
Down |
| |
|
|
|
| Group 4 vs NT |
|
|
|
| Usp28 |
Usp |
8.12E-64 |
Down |
| USP3 |
Usp |
1.33E-54 |
Down |
| USP45 |
Usp |
4.54E-36 |
Down |
| PSMD14 |
Jamm |
1.45E-26 |
Up |
| COPS6 |
Jamm |
3.10E-24 |
Up |
| USP1 |
Usp |
1.48E-16 |
Up |
| OTUB1 |
Otu |
1.67E-14 |
Down |
| USP7 |
Usp |
2.65E-12 |
Up |
| COPS5 |
Jamm |
4.01E-06 |
Down |
| USP47 |
Usp |
2.16E-04 |
Down |
| UCHL5 |
Uch |
2.11E-03 |
Up |
| |
|
|
|
| SHH vs NT |
|
|
|
| USP10 |
Usp |
2.21E-15 |
Up |
| PSMD14 |
Jamm |
8.58E-09 |
Down |
| COPS5 |
Jamm |
1.16E-08 |
Up |
| USP45 |
Usp |
3.60E-07 |
Down |
| UCHL5 |
Uch |
1.30E-06 |
Down |
| USP3 |
Usp |
5.90E-06 |
Down |
| COPS6 |
Jamm |
5.98E-06 |
Down |
| USP1 |
Usp |
6.06E-06 |
Up |
| USP47 |
Usp |
6.68E-04 |
Down |
| USP7 |
Usp |
1.71E-03 |
Up |
| |
|
|
|
| WNT vs NT |
|
|
|
| COPS6 |
Jamm |
2.67E-32 |
Up |
| USP28 |
Usp |
5.39E-27 |
Down |
| USP3 |
Usp |
3.53E-25 |
Down |
| USP45 |
Usp |
2.61E-22 |
Down |
| UCHL5 |
Usp |
4.72E-14 |
Down |
| USP10 |
Usp |
7.73E-11 |
Up |
| COPS5 |
Jamm |
1.02E-03 |
Up |
| PSMD14 |
Jamm |
1.46E-03 |
Up |
| OTUB1 |
Otu |
2.89E-03 |
Up |
| USP1 |
Usp |
4.55E-03 |
Up |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).