Submitted:
08 September 2023
Posted:
12 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methodology
- 1)
- What drives the research interest on the application of A. ferrooxidans in metallurgical processes?
- 2)
- By which mechanism does the A. ferrooxidans interacts with PGMs-bearing sulphides?
- 3)
- What are the reported applications of A. ferrooxidans for PGMs-bearing sulphides concentration processes?
3. Results and discussion
3.1. The Drivers for the Research Interest on the Application of A. ferrooxidans in the Metallurgical Processes
- a)
- b)
- The refractoriness of gold locked in sulphides [28], and
- c)
- a)
- Interactions between metal-bearing minerals and micro-organisms,
- b)
- Bio-flotation of base metal sulphides,
- c)
- Removal of silicates and chromite during bio-flotation,
- d)
- Biotechnological recovery of heavy minerals from secondary sources by means of bioleaching, bio-oxidation of sulphides minerals and
- e)
- Optimization of bio-beneficiation processes.
- a)
- The adhesion of microbial cells to the mineral surface,
- b)
- Oxidation reactions brought by the micro-organisms and their metabolites onto the minerals,
- c)
- The adhesion of bacterial proteins and exopolysaccharides to mineral surfaces and/or the chemical reaction between mineral surfaces and metabolite products.
3.2. Application of Acidithiobacillus ferrooxidans in PGMs processing
| References | Category | Approach and Objective | Main findings |
| [28,33,34,35,36,37,38,39] | Biooxidation of Refractory gold | Quantitative. The main objective was to improve gold recovery by oxidizing the gold-bearing sulfidic minerals, mainly pyrite and arsenopyrite using A. ferrooxidans thereby liberating gold from the sulphide matrix prior cyanidation. |
The gold recovery was strongly related to the extent of sulphide biooxidation, with the highest recoveries ranging from 85 to 98% depending on the operational parameters. |
| [40,41,42,43,44,45,46,47,48,49] | Bioleaching of Cu, Ni, Co from base metal sulphides | Quantitative. The aim was to investigate the efficacy of bioleaching of copper, Ni, Co using A. ferrooxidans (mesophile and moderately thermophile). |
Together with the results from chemical leaching, the data indicated that Cu was mainly leached by sulphuric acid (bio-generated and traditional acid leaching) while a high Co extraction required Fe (II)-oxidizing microbial activity (bioleaching). Adding sulphuric acid reduces the needed time to reach the possible maximal recovery of metals. As it is obvious, the maximum recovery of Cu and Ni in both bioleaching and hydrometallurgy processes is competitive and excellent |
| [50,51,52,53,54,55,56,57,58] | Bioflotation of base metal sulphides | Quantitative. Most research on bioflotation investigated the use of A. ferrooxidans as a pyrite depressant. This was applied in selective flotation of chalcopyrite and pyrite. It was also used in coal processing. The other objective was to ascertain the role of the phenomena in the biomodification of sulphides by Acidithiobacillus ferrooxidans culture (cells and growth media) and their impact in bioflotation. |
The main results showed that A. ferrooxidans can facilitate the depression of pyrite while promoting the flotation of other base metal sulphides. It was further concluded that elemental sulphur -concentration increased because of the oxidation generated by bacterial cells, the effect is intensified by the Fe (III) left in the culture and by galvanic contact. |
| [42,59,60,61,62,63] | Bioprocessing/pre- concentration of PGMs from secondary sources /waste | Quantitative. The aim was to investigate the use of A. ferrooxidans in metal extraction from spent catalytic converters. |
It was concluded that the bacteria may concentrate Cu, Cd, Zn and Pb in that way pre-concentrating PGMs prior conventional hydrometallurgy methods and reducing reagents consumption. |
3.2.1. Application of A. ferrooxidans in Biohydrometallurgy: Biooxidation and Bioleaching
3.2.2. The Application of A. ferrooxidans in Bio-Flotation
- a)
- Fe- sulphides
- b)
- Cu-Fe-sulphides
- c)
- Other sulphides
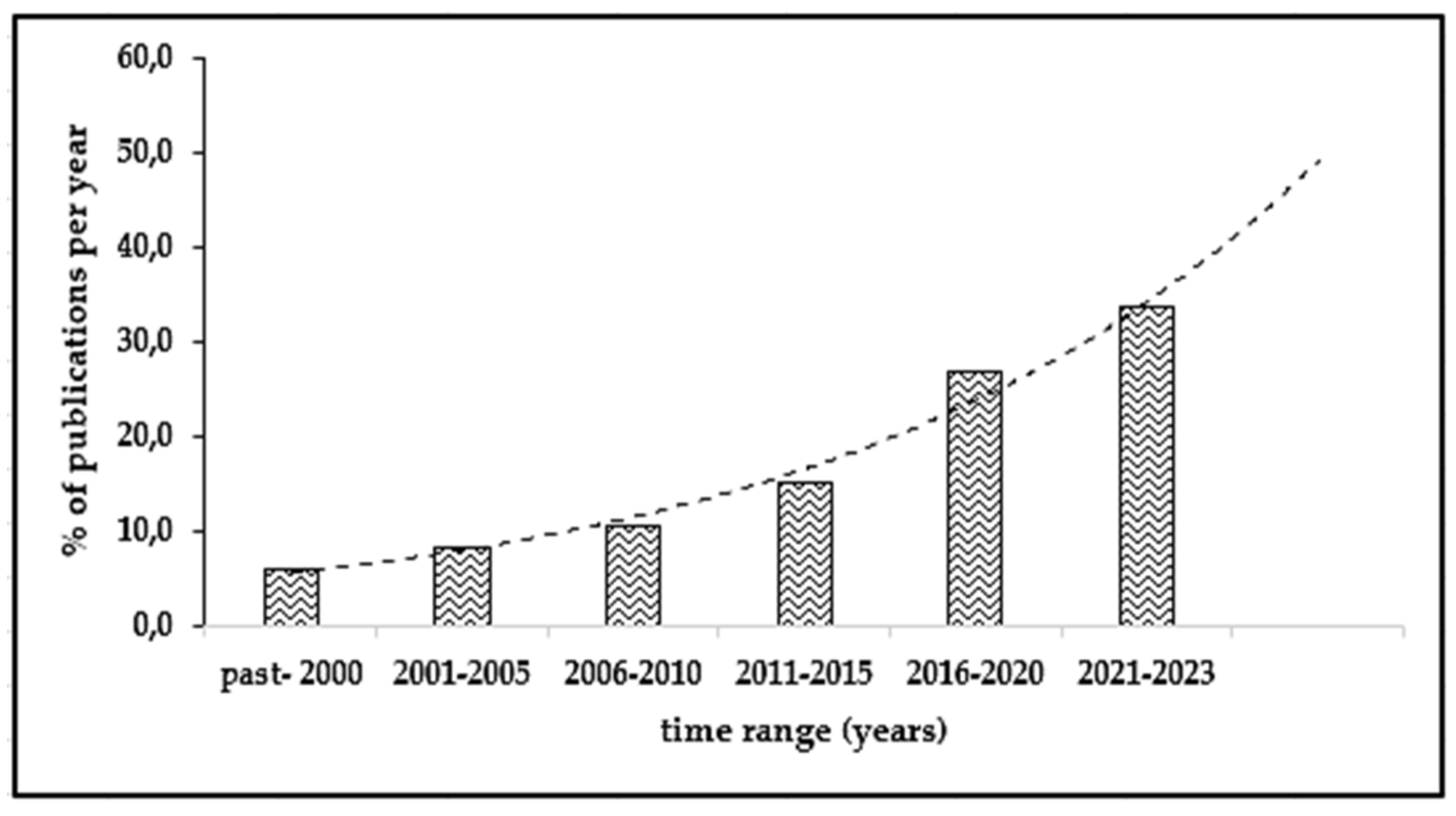
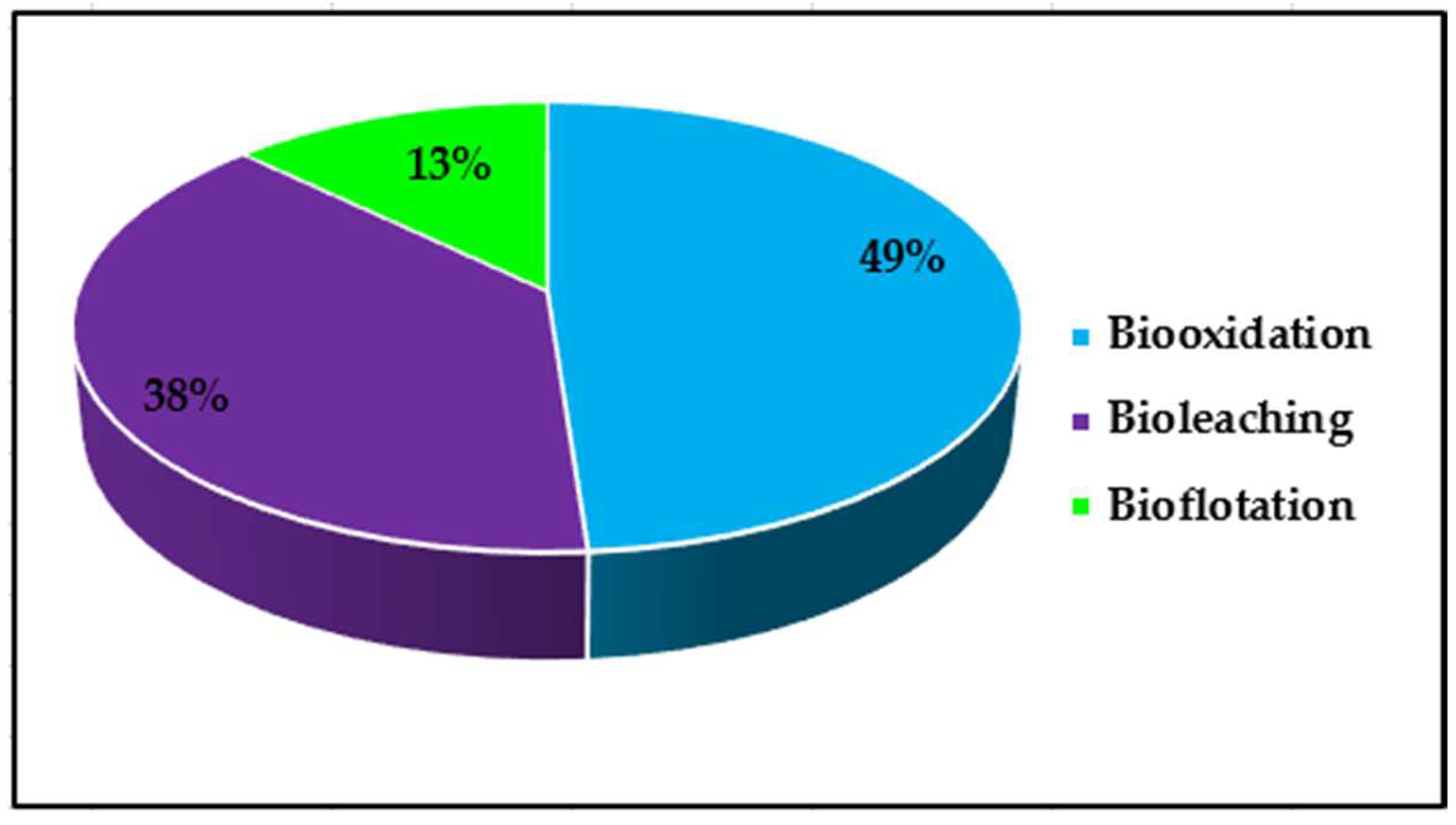
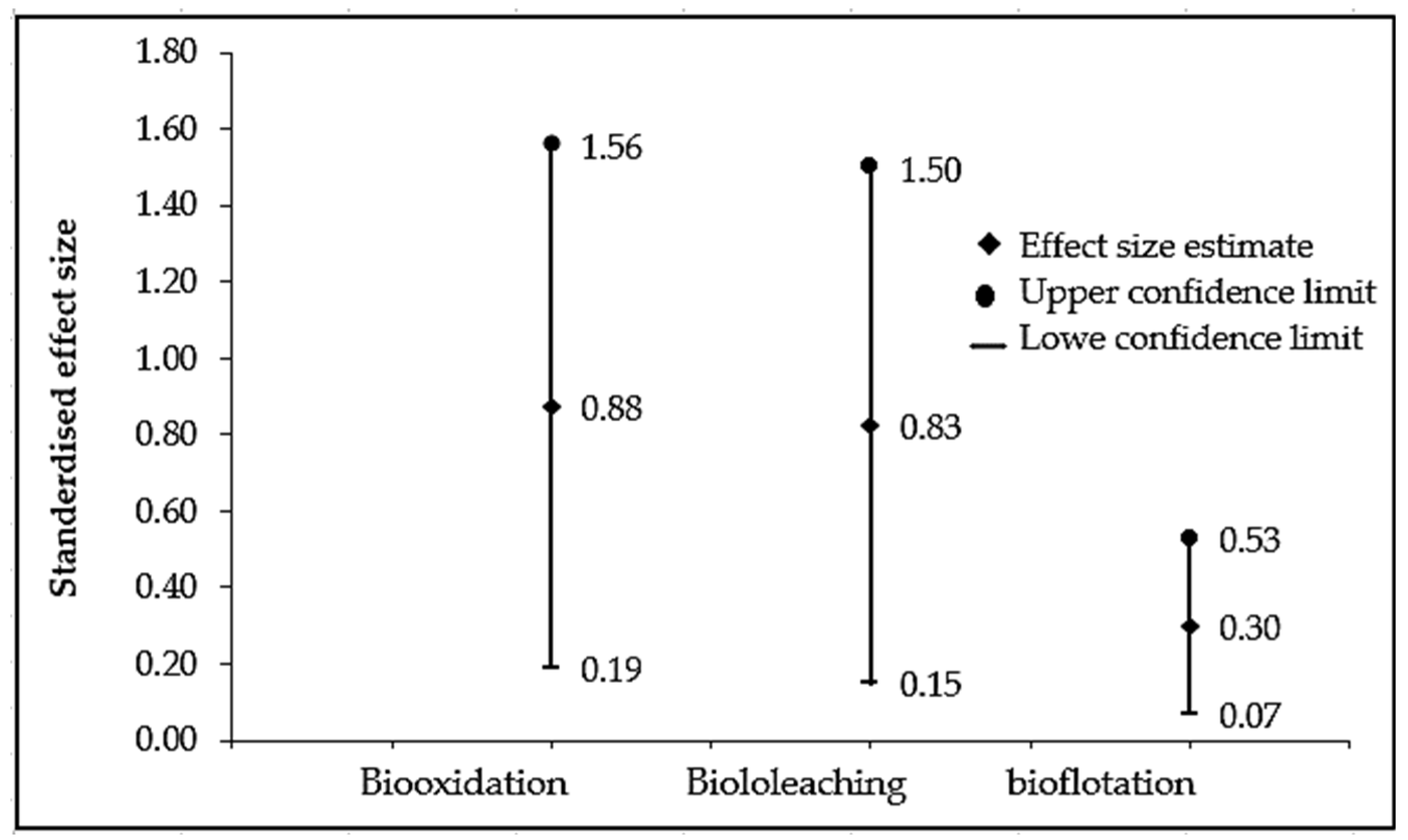
3.3. Statistical Analysis
4. Conclusion
- A. ferrooxidans have been successfully applied in gold processing as a bio-oxidant which serves to liberate gold prior to leaching. It was concluded that the recovery of gold is directly proportional to the rate of bio-oxidation. It was further discovered that when A. ferrooxidans was used for oxidation, reagents consumption decrease, in this case, cyanide consumption was related to the extent at which the mineral surfaces were oxidized.
- It was concluded that A. ferrooxidans could be used where selective flotation is desired due to the different responses shown by different sulphides. These conclusions were drawn from the fact that the bacteria can depress Fe-sulphides, while promoting the flotation of Cu- sulphides.
- For secondary PGMs sources, it was concluded that the A. ferrooxidans may concentrate Cu, Cd, Zn and Pb through leaching in that way pre-concentrating PGMs prior conventional hydrometallurgy methods and reducing reagents consumption.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- David Davis Platinum Analysis (Ore Grades &, S. African PGM Mines’ Geological Structure) Available online:. Available online: https://auctusmetals.com/platinum-analysis-ore-grades-s-african-pgm-mines-geological-structure-part-ii-by-dr-david-davis/ (accessed on 21 January 2023).
- Thethwayo, B.M. Extraction of Platinum Group Metals. In Noble and Precious Metals - Properties, Nanoscale Effects and Applications; InTech: 2018.
- Chipise, L.; Ndlovu, S.; Shemi, A. Towards the Biobeneficiation of PGMs: Reviewing the Opportunities. Minerals 2022, 12. [Google Scholar] [CrossRef]
- Kesari, K.K.; Siddiqui, M.H.; Kumar, A.; Arif, J.M. Biomining-A Useful Approach Toward Metal Extraction. Am.-Eurasian j agron 2009, 2, 84–88. [Google Scholar]
- Johnson, D.B. Biomining-Biotechnologies for Extracting and Recovering Metals from Ores and Waste Materials. Curr Opin Biotechnol 2014, 30, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Jerez, C.A. Bioleaching and Biomining for the Industrial Recovery of Metals. In Comprehensive Biotechnology; Elsevier, 2019; pp. 758–771a. [Google Scholar]
- Martínez-Bellange, P.; von Bernath, D.; Navarro, C.A.; Jerez, C.A. Biomining of Metals: New Challenges for the next 15 Years. Microb Biotechnol 2022, 15, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Panda, S.; Akcil, A.; Dembele, S. Biotechnological Avenues in Mineral Processing: Fundamentals, Applications and Advances in Bioleaching and Bio-Beneficiation. Miner Process Extr Metall 2022, 44, 22–51. [Google Scholar] [CrossRef]
- Brown, R.M.; Mirkouei, A.; Reed, D.; Thompson, V. Current Nature-Based Biological Practices for Rare Earth Elements Extraction and Recovery: Bioleaching and Biosorption. Renewable and Sustainable Energy Reviews 2023, 173. [Google Scholar] [CrossRef]
- Karim, S.; Ting, Y.P. Bioleaching of Platinum, Palladium, and Rhodium from Spent Automotive Catalyst Using Bacterial Cyanogenesis. Bioresour Technol Rep 2022, 18. [Google Scholar]
- Sanwani, E.; Chaerun, S.; Mirahati, R.; Wahyuningsih, T. Bioflotation: Bacteria-Mineral Interaction for Eco-Friendly and Sustainable Mineral Processing. Procedia Chem 2016, 19, 666–672. [Google Scholar] [CrossRef]
- Pecina-Treviño, E.T.; Ramos-Escobedo, G.T.; Gallegos-Acevedo, P.M.; López-Saucedo, F.J.; Orrantia-Borunda, E. Bioflotation of Sulfide Minerals with Acidithiobacillus Ferrooxidans in Relation to Copper Activation and Surface Oxidation. Can J Microbiol 2012, 58. [Google Scholar] [CrossRef]
- Hosseini, T.R.; Kolahdoozan, M.; Tabatabaei, Y.S.M.; Oliazadeh, M.; Noaparast, M.; Eslami, A.; Manafi, Z.; Alfantazi, A. Bioflotation of Sarcheshmeh Copper Ore Using Thiobacillus Ferrooxidans Bacteria. Miner Eng 2005, 18, 371–374. [Google Scholar] [CrossRef]
- Vilinska, A.; Rao, K.H. Leptosririllum Ferrooxidans-Sulfide Mineral Interactions with Reference to Bioflotation Nad Bioflocculation. Trans Nonferrous Met Soc 2008, 18. [Google Scholar] [CrossRef]
- Bahaj, A.S.; Ellwood, D.C.; Watson, J.H.P. Extraction of Heavy Metals Using Microorganisms and High Gradient Magnetic Separation. IEEE Trans Magn 1991, 27, 5371–5374. [Google Scholar] [CrossRef]
- Watson, J.H.P.; Ellwood, D.C. Biomagnetic Separation and Extraction Process for Heavy Metals From Solution. Miner Eng 1994, 7, 1017–1028. [Google Scholar] [CrossRef]
- Hoque, M.E.; Philip, O.J. Biotechnological Recovery of Heavy Metals from Secondary Sources-An Overview. Mater Sci Eng C 2011, 31, 57–66. [Google Scholar] [CrossRef]
- Clark, M.E.; Batty, J.D.; van Buuren, C.B.; Dew, D.W.; Eamon, M.A. Biotechnology in Minerals Processing: Technological Breakthroughs Creating Value. Hydrometallurgy 2006, 83, 3–9. [Google Scholar] [CrossRef]
- Jerez, C.A. Bioleaching and Biomining for the Industrial Recovery of Metals. In Comprehensive Biotechnology 2019, 758–771b. [Google Scholar]
- Roberto, F.F.; Schippers, A. Progress in Bioleaching: Part B, Applications of Microbial Processes by the Minerals Industries. Appl Microbiol Biotechnol 2022, 106, 5913–5928. [Google Scholar] [CrossRef] [PubMed]
- Vera, M.; Schippers, A.; Hedrich, S.; Sand, W. Progress in Bioleaching: Fundamentals and Mechanisms of Microbial Metal Sulfide Oxidation – Part A. Appl Microbiol Biotechnol 2022, 106, 6933–6952. [Google Scholar] [CrossRef] [PubMed]
- Li, R.C.B.; Sasaki, K.; Ohmura, N. Does Aporusticyanin Mediate the Adhesion of Thiobacillus Ferrooxidans to Pyrite. Hydrometallurgy 2001, 59, 357–372a. [Google Scholar]
- Chandraprabha, M.N.; Natarajan, K.A. Surface Chemical and Flotation Behaviour of Chalcopyrite and Pyrite in the Presence of Acidithiobacillus Thiooxidans. Hydrometallurgy 2006, 83, 146–152. [Google Scholar] [CrossRef]
- Zulu, N.G.; Thethwayo, B.M.; Madiba, M.S. Application of Acidithiobacillus Ferrooxidans in Biomodification of Base Metal Sulphides from Upper Group 2 Prior Flotation. In Proceedings of the Biomining’23, Falmouth, United Kingdom, 5-6 June 2023. [Google Scholar]
- Asgari, K.; Huang, Q.; Khoshdast, H.; Hassanzadeh, A. A Review on Bioflotation of Coal and Minerals: Classification, Mechanisms, Challenges, and Future Perspectives. Miner Process Extr Metall 2022, 43, 1–32. [Google Scholar] [CrossRef]
- Yin, S.; Wang, L.; Kabwe, E.; Chen, X.; Yan, R.; An, K.; Zhang, L.; Wu, A. Copper Bioleaching in China: Review and Prospect. Minerals 2018, 8. [Google Scholar] [CrossRef]
- Kinnunen, P.; Miettinen, H.; Bomberg, M. Review of Potential Microbial Effects on Flotation. Minerals 2020, 10. [Google Scholar] [CrossRef]
- Ciftci, H.; Akcil, A. Effect of Biooxidation Conditions on Cyanide Consumption and Gold Recovery from a Refractory Gold Concentrate. Hydrometallurgy 2010, 104, 142–149. [Google Scholar] [CrossRef]
- Yakoumis, I.; Panou, M.; Moschovi, A.M.; Panias, D. Recovery of Platinum Group Metals from Spent Automotive Catalysts: A Review. Clean Eng Technol 2021, 3. [Google Scholar] [CrossRef]
- Maass, D.; Yannick das Neves Vasconcellos Brandão, I.; Akihiro Munakata, A.; Antonio Lourenço, L. How Biomining Has Been Used to Recover Metals from Ores and Waste? A Review. Int j earth environ sci 2021, 6, 1–11. [Google Scholar]
- Adetunji, A.I.; Oberholster, P.J.; Erasmus, M. Bioleaching of Metals from E-Waste Using Microorganisms: A Review. Minerals 2023, 13, 828. [Google Scholar] [CrossRef]
- Seifelnassr, A.A.S.; Abouzeid, A.-Z.M. Exploitation of Bacterial Activities in Mineral Industry and Environmental Preservation: An Overview. J Min 2013, 1–13. [Google Scholar]
- Mubarok, M.Z.; Winarko, R.; Chaerun, S.K.; Rizki, I.N.; Ichlas, Z.T. Improving Gold Recovery from Refractory Gold Ores through Biooxidation Using Iron-Sulfur-Oxidizing/Sulfur-Oxidizing Mixotrophic Bacteria. Hydrometallurgy 2017, 168, 69–75. [Google Scholar] [CrossRef]
- de Carvalho, L.C.; da Silva, S.R.; Giardini, R.M.N.; de Souza, L.F.C.; Leão, V.A. Bio-Oxidation of Refractory Gold Ores Containing Stibnite and Gudmundite. Environ Technol Innov 2019, 15. [Google Scholar] [CrossRef]
- Chandraprabha, M.N.; Modak, J.M.; Natarajan, K.A.; Raichur, A.M. Strategies for Efficient Start-up of Continuous Biooxidation Process for Refractory Gold Ores. Miner Eng 2001, 15, 751–753. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, Y. li; Li, H. ran Enhancement of Bio-Oxidation of Refractory Arsenopyritic Gold Ore by Adding Pyrolusite in Bioleaching System. Trans Nonferrous Met Soc 2016, 26, 2479–2484. [Google Scholar] [CrossRef]
- Wadsworth, M.E.; Zhu, X.; Thompson, J.S.; Pereira, C.J. Gold Dissolution and Activation in Cyanide Solution: Kinetics and Mechanism. Hydrometallurgy 2000, 57, 1–11a. [Google Scholar] [CrossRef]
- Corkhill, C.L.; Wincott, P.L.; Lloyd, J.R.; Vaughan, D.J. The Oxidative Dissolution of Arsenopyrite (FeAsS) and Enargite (Cu3AsS4) by Leptospirillum Ferrooxidans. Geochim Cosmochim Acta 2008, 72, 5616–5633. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Zhang, Y.; Jiang, T.; Yang, Y.; Xu, B.; He, Y. Electrochemical Behaviour of the Dissolution and Passivation of Arsenopyrite in 9K Culture Medium. Appl Surf Sci 2020, 508. [Google Scholar] [CrossRef]
- Zhang, R.; Schippers, A. Stirred-Tank Bioleaching of Copper and Cobalt from Mine Tailings in Chile. Miner Eng 2022, 180. [Google Scholar] [CrossRef]
- Rodrigues, M.L.M.; Lopes, K.C.S.; Leôncio, H.C.; Silva, L.A.M.; Leão, V.A. Bioleaching of Fluoride-Bearing Secondary Copper Sulphides: Column Experiments with Acidithiobacillus Ferrooxidans. J Chem Eng 2016, 284, 1279–1286. [Google Scholar] [CrossRef]
- Arshadi, M.; Yaghmaei, S. Advances in Bioleaching of Copper and Nickel from Electronic Waste Using Acidithiobacillus Ferrooxidans: Evaluating Daily PH Adjustment. Chemical Papers 2020, 74, 2211–2227. [Google Scholar] [CrossRef]
- Jung, H.; Inaba, Y.; Vardner, J.T.; West, A.C.; Banta, S. Overexpression of the Licanantase Protein in Acidithiobacillus Ferrooxidans Leads to Enhanced Bioleaching of Copper Minerals. ACS Sustain Chem Eng 2022, 10, 10888–10897. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Liu, X.; Yin, H.; Yang, Y.; Xu, B.; Jiang, T.; He, Y. The Catalytic Effect of Copper Ion in the Bioleaching of Arsenopyrite by Acidithiobacillus Ferrooxidans in 9K Culture Medium. J Clean Prod 2020, 256. [Google Scholar] [CrossRef]
- Behera, S.K.; Panda, S.K.; Mulaba-Bafubiandi, A.F. Valorization of Copper Smelter Slag through the Recovery of Metal Values by a Synergistic Bioprocess System of Bio-Flotation and Bio-Leaching. Environmental Quality Management 2022, 32, 233–241. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Malverde, S.; Quezada, V. A Sustainable Bioleaching of a Low-Grade Chalcopyrite Ore. Minerals 2022, 12. [Google Scholar] [CrossRef]
- Sand, W.; Gehrke, T.; Jozsa, P.-G.; Schippers, A. Biochemistry of Bacterial Leaching-Direct vs. Indirect Bioleaching. Hydrometallurgy 2001, 59, 159–175. [Google Scholar] [CrossRef]
- Pogliani, C.; Donati, E. The Role of Exopolymers in the Bioleaching of a Non-Ferrous Metal Sulphide. J Ind Microbiol Biotechnol 1999, 22, 88–92. [Google Scholar] [CrossRef]
- Xin, Y.; Guo, X.; Chen, S.; Wang, J.; Wu, F.; Xin, B. Bioleaching of Valuable Metals Li, Co, Ni and Mn from Spent Electric Vehicle Li-Ion Batteries for the Purpose of Recovery. J Clean Prod 2016, 116, 249–258. [Google Scholar] [CrossRef]
- Pecina-Treviño, E.T.; Ramos-Escobedo, G.T.; Gallegos-Acevedo, P.M.; López-Saucedo, F.J.; Orrantia-Borunda, E. Bioflotation of Sulfide Minerals with Acidithiobacillus Ferrooxidans in Relation to Copper Activation and Surface Oxidation. Can J Microbiol 2012, 58. [Google Scholar] [CrossRef]
- Sanwani, E.; Chaerun, S.; Mirahati, R.; Wahyuningsih, T. Bioflotation: Bacteria-Mineral Interaction for Eco-Friendly and Sustainable Mineral Processing. Procedia Chem 2016, 19, 666–672. [Google Scholar] [CrossRef]
- Firkala, T.; Lederer, F.; Pollmann, K.; Rudolph, M. Investigation of a Bioflotation Interface with Infrared Spectroscopy. In Proceedings of the Solid State Phenomena; Trans Tech Publications Ltd; 2017; 262, pp. 537–540. [Google Scholar]
- Li, R.C.B.; Sasaki, K.; Ohmura, N. Does Aporusticyanin Mediate the Adhesion of Thiobacillus Ferrooxidans to Pyrite. Hydrometallurgy 2001, 59, 357–372b. [Google Scholar]
- San Martín, F.; Kracht, W.; Vargas, T. Biodepression of Pyrite Using Acidithiobacillus Ferrooxidans in Seawater. Miner Eng 2018, 117, 127–131. [Google Scholar] [CrossRef]
- Mehrabani, J. V.; Noaparast, M.; Mousavi, S.M.; Dehghan, R.; Rasooli, E.; Hajizadeh, H. Depression of Pyrite in the Flotation of High Pyrite Low-Grade Lead-Zinc Ore Using Acidithiobacillus Ferrooxidans. Miner Eng 2010, 23, 10–16. [Google Scholar] [CrossRef]
- San Martín, F.; Kracht, W.; Vargas, T.; Rudolph, M. Mechanisms of Pyrite Biodepression with Acidithiobacillus Ferrooxidans in Seawater Flotation. Miner Eng 2020, 145. [Google Scholar] [CrossRef]
- Attia, Y.A.; Elzeky, M.; Ismail, M. Enhanced Separation of Pyrite from Oxidized Coal by Froth Flotation Using Biosurface Modification. Int J Miner Process 1993, 37, 61–77. [Google Scholar] [CrossRef]
- Chandraprabha, M.N.; Natarajan, K.A. Surface Chemical and Flotation Behaviour of Chalcopyrite and Pyrite in the Presence of Acidithiobacillus Thiooxidans. Hydrometallurgy 2006, 83, 146–152. [Google Scholar] [CrossRef]
- Maluckov, BS. The Catalytic Role of Acidithiobacillus Ferrooxidans for Metals Extraction from Mining - Metallurgical Resource. Biodivers Int J 2017, 1. [Google Scholar] [CrossRef]
- García-Balboa, C.; Martínez-Alesón García, P.; López-Rodas, V.; Costas, E.; Baselga-Cervera, B. Microbial Biominers: Sequential Bioleaching and Biouptake of Metals from Electronic Scraps. Microbiologyopen 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B. Biomining-Biotechnologies for Extracting and Recovering Metals from Ores and Waste Materials. Curr Opin Biotechnol 2014, 30, 24–31. [Google Scholar] [CrossRef]
- Karim, S.; Ting, Y.P. Bioleaching of Platinum, Palladium, and Rhodium from Spent Automotive Catalyst Using Bacterial Cyanogenesis. Bioresour Technol Rep 2022, 18. [Google Scholar]
- Ilyas, S.; Srivastava, R.R.; Kim, H. Electronic Supplementary Information Mobilization of Platinum and Palladium from Exhausted Catalytic Converters Using Bio-Cyanide and Ionic-Liquid as Mass Transport Carriers. R Soc Chem 2022, 1–10. [Google Scholar]
- Moosakazemi, F.; Ghassa, S.; Jafari, M.; Chelgani, S.C. Bioleaching for Recovery of Metals from Spent Batteries–A Review. Miner Process Extr Metall 2022, 44, 1–12. [Google Scholar] [CrossRef]
- Mubarok, M.Z.; Winarko, R.; Chaerun, S.K.; Rizki, I.N.; Ichlas, Z.T. Improving Gold Recovery from Refractory Gold Ores through Biooxidation Using Iron-Sulfur-Oxidizing/Sulfur-Oxidizing Mixotrophic Bacteria. Hydrometallurgy 2017, 168, 69–75. [Google Scholar] [CrossRef]
- Wadsworth, M.E.; Zhu, X.; Thompson, J.S.; Pereira, C.J. Gold Dissolution and Activation in Cyanide Solution: Kinetics and Mechanism. Hydrometallurgy 2000, 57, 1–11b. [Google Scholar] [CrossRef]
- de Carvalho, L.C.; da Silva, S.R.; Giardini, R.M.N.; de Souza, L.F.C.; Leão, V.A. Bio-Oxidation of Refractory Gold Ores Containing Stibnite and Gudmundite. Environ Technol Innov 2019, 15. [Google Scholar] [CrossRef]
- Miller, P.; Brown, A.R.G. Bacterial Oxidation of Refractory Gold Concentrates. In Gold Ore Processing; Elsevier, 2016; pp. 359–372. [Google Scholar]
- Thomas, K.G.; Cole, A.P. Roasting Developments - Especially Oxygenated Roasting. Dev miner process 2005, 15, 403–432. [Google Scholar]
- Zhang, Y.; Li, Q.; Liu, X.; Yin, H.; Yang, Y.; Xu, B.; Jiang, T.; He, Y. The Catalytic Effect of Copper Ion in the Bioleaching of Arsenopyrite by Acidithiobacillus Ferrooxidans in 9K Culture Medium. J Clean Prod 2020, 256. [Google Scholar] [CrossRef]
- Xin, Y.; Guo, X.; Chen, S.; Wang, J.; Wu, F.; Xin, B. Bioleaching of Valuable Metals Li, Co, Ni and Mn from Spent Electric Vehicle Li-Ion Batteries for the Purpose of Recovery. J Clean Prod 2016, 116, 249–258. [Google Scholar] [CrossRef]
- Rodrigues, M.L.M.; Lopes, K.C.S.; Leôncio, H.C.; Silva, L.A.M.; Leão, V.A. Bioleaching of Fluoride-Bearing Secondary Copper Sulphides: Column Experiments with Acidithiobacillus Ferrooxidans. J Chem Eng 2016, 284, 1279–1286. [Google Scholar] [CrossRef]
- Roberto, F.F.; Schippers, A. Progress in Bioleaching: Part B, Applications of Microbial Processes by the Minerals Industries. Appl Microbiol Biotechnol 2022, 106, 5913–5928. [Google Scholar] [CrossRef]
- Martins, F.L.; Leão, V.A. Chalcopyrite Bioleaching in Chloride Media: A Mini-Review. Hydrometallurgy 2023, 216. [Google Scholar] [CrossRef]
- Asgari, K.; Huang, Q.; Khoshdast, H.; Hassanzadeh, A. A Review on Bioflotation of Coal and Minerals: Classification, Mechanisms, Challenges, and Future Perspectives. Miner Process Extr Metall 2022, 43, 2–33. [Google Scholar] [CrossRef]
- Misra, O.M.; Bukka, K.; Chen, S. The Effect of Growth Medium of Thiobacillus Ferrooxidans on Pyrite Flotation. Miner Eng 1996, 9, 167–168. [Google Scholar] [CrossRef]
- Dwyer, R.; Bruckard, W.J.; Rea, S.; Holmes, R.J. Bioflotation and Bioflocculation Review: Microorganisms Relevant for Mineral Beneficiation. Miner Process Extr Metall 2012, 121, 65–71. [Google Scholar] [CrossRef]
- San Martín, F.; Kracht, W.; Vargas, T. Biodepression of Pyrite Using Acidithiobacillus Ferrooxidans in Seawater. Miner Eng 2018, 117, 127–131. [Google Scholar] [CrossRef]
- Chandraprabha, M.N.; Natarajan, K.A. Microbially Induced Mineral Beneficiation. Miner Process Extr Metall 2010, 31, 1–29. [Google Scholar] [CrossRef]
- Sanwani, E.; Chaerun, S.; Mirahati, R.; Wahyuningsih, T. Bioflotation: Bacteria-Mineral Interaction for Eco-Friendly and Sustainable Mineral Processing. Procedia Chem 2016, 19, 666–672. [Google Scholar] [CrossRef]
- Kinnunen, P.; Miettinen, H.; Bomberg, M. Review of Potential Microbial Effects on Flotation. Minerals 2020, 10. [Google Scholar] [CrossRef]
- Santhiya, D.; Subramanian, S.; Natarajan, K. Surface Chemical Studies on Galena and Sphalerite in the Presence of Thiobacillus Thiooxidans with Reference to Mineral Beneficiation. Miner Eng 2000, 13, 747–763. [Google Scholar] [CrossRef]
- Jefferson, K.K. What Drives Bacteria to Produce a Biofilm? FEMS Microbiol Lett 2004, 236, 163–173. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and Reporting Effect Sizes to Facilitate Cumulative Science: A Practical Primer for t-Tests and ANOVAs. Front Psychol 2013, 4. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




