Submitted:
10 September 2023
Posted:
12 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Molecular mechanisms of ferroptosis
2.1. Iron metabolism
2.2. Lipid metabolism
2.3. Antioxidant defenses
3. Ferroptosis in LUAD
3.1. Ferroptosis in LUAD tumorigenesis and progression
3.1.1. Ferroptosis in LUAD tumorigenesis
3.1.2. Ferroptosis in LUAD progression
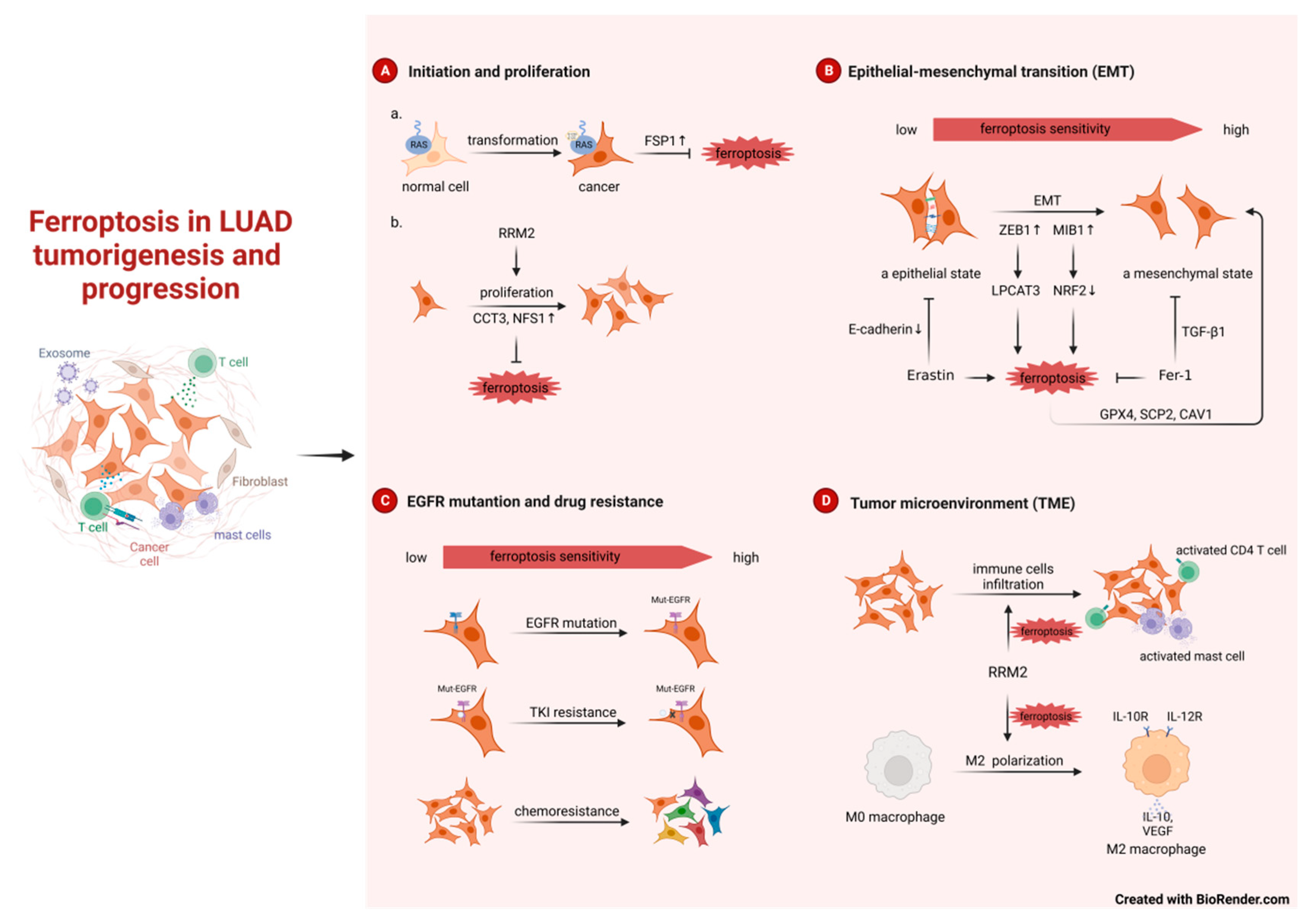
3.2. Regulation of ferroptosis in LUAD
3.2.1. Transcriptional regulation
NRF2
p53
YAP
Others
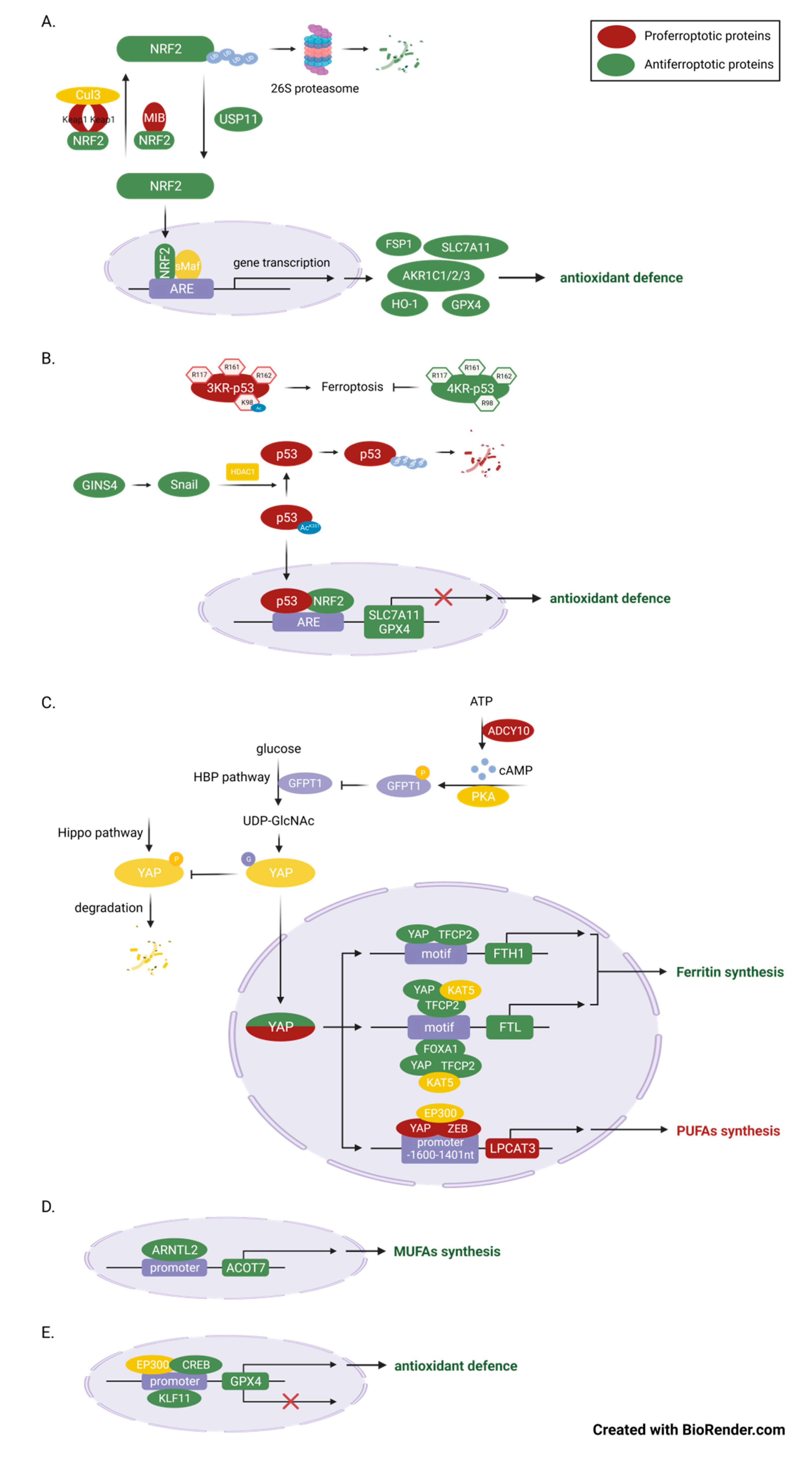
3.2.2. Epigenetic regulation
Histone modification
Non-coding RNA-mediated processes
- miRNAs
- CircRNAs
- LncRNAs
- m6A
- modification
| ncRNAs | Epigenetic mechanism | Functions in ferroptosis | Expression in LUAD patients | References |
|---|---|---|---|---|
| EP300 | histone modification | ①EP300/CREB complex: suppressor; increase GPX4 expression② EP300/YAP/ZEB complex: promoter; increase LPCAT3 expression | N/A | [19,79] |
| miR-27a-3p | miRNA | promoter; Inhibit SLC7A11 expression | downregulation | [88] |
| miR-324-3p | miRNA | promoter; Inhibit GPX4 expression | Downregulation in A549/DDP cells | [51] |
| P4HB | circRNA | Suppressor; sponge miRNA-1184 and increase SLC7A11 expression | Upregulation | [92] |
| circ_1010093 | circRNA | Suppressor; inhibit lipid (ACSL4, LPCAT3 and PLTP) | Upregulation | [94] |
| LINC00324 | lncRNA | Suppressor; sponge miR-200c-3p and promote TFAP2A-NRF2 axis | Upregulation | [37] |
| GSEC | lncRNA | Suppressor; sponge miR-101-3p and increase CISD1(a mitochondrial iron-sulfur protein) | Upregulation | [95] |
| Uc.339 | lncRNA | Suppressor; sponge pri-miR-339, inhibits the production of mature miR-339 and increase SLC7A11 expression | Upregulation | [96] |
| LINC00336 | lncRNA | Suppressor; sponge miR-6852 and increases cystathionine-β-synthase (CBS, involves in transsulfuration pathway and synthesizes cysteine ) expression | Upregulation | [97] |
| GMDS-AS1 and LINC01128 | lncRNA | Suppressor; sponge miR-6077 and promote KEAP1-NRF2-SLC7A11/NQO1 pathway | downregulation | [59] |
| LINC00551 | lncRNA | Promotor; sponge miR-4328 and increase DDIT4 expression,DDIT4 inhibits mTOR activity and promote autophagy-dependent ferroptosis | downregulation | [98] |
| METTL3 | m6A | Suppressor; enhance the stability and translation of SLC7A11 | upregulation | [104] |
| IGF2BP3 | m6A | Suppressor; enhance the stability and translation of anti-ferroptotic factors (GPX4, SLC3A2, ACSL3, and FTH1) | upregulation | [105] |
| YTHDC2 | m6A | Promotor; inhibit system Xc (directly inhibit SLC7A11 and indirectly inhibit SLC3A5 expression) | downregulation | [106] |
3.3. Ferroptosis in LUAD therapy
4. Conclusion and Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Herbst, R.S., D. Morgensztern, and C. Boshoff, The biology and management of non-small cell lung cancer. Nature. 2018, 553, 446–454. [Google Scholar] [CrossRef]
- Hirsch, F.R. , et al., Lung cancer: current therapies and new targeted treatments. Lancet. 2017, 389, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Kris, M.G. , et al., Adjuvant Systemic Therapy and Adjuvant Radiation Therapy for Stage I to IIIA Completely Resected Non-Small-Cell Lung Cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline Update. J Clin Oncol. 2017, 35, 2960–2974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. , et al., Elevated TRIM23 expression predicts cisplatin resistance in lung adenocarcinoma. Cancer Sci. 2020, 111, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Arbour, K.C. and G.J. Riely, Systemic Therapy for Locally Advanced and Metastatic Non-Small Cell Lung Cancer: A Review. JAMA. 2019, 322, 764–774. [Google Scholar] [CrossRef]
- Liu, S.-Y. , et al., Clinical characteristics and prognostic value of the KRAS G12C mutation in Chinese non-small cell lung cancer patients. Biomarker Research. 2020. 8(1). 2020. [Google Scholar]
- Huang, L. , et al., KRAS mutation: from undruggable to druggable in cancer. Signal Transduction and Targeted Therapy.2021. 6(1).
- Dixon, S.J. , et al., Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. , et al., Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol 2021, 18, 280–296. [Google Scholar] [CrossRef]
- Stockwell, B.R. , et al., Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- van Swelm, R.P.L., J. F.M. Wetzels, and D.W. Swinkels, The multifaceted role of iron in renal health and disease. Nat Rev Nephrol 2020, 16, 77–98. [Google Scholar] [CrossRef]
- Wang, Y. , et al., NEDD4L-mediated LTF protein degradation limits ferroptosis. Biochem Biophys Res Commun 2020, 531, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Song, N. , et al., Ferritin: A Multifunctional Nanoplatform for Biological Detection, Imaging Diagnosis, and Drug Delivery. Acc Chem Res 2021, 54, 3313–3325. [Google Scholar] [CrossRef] [PubMed]
- Mancias, J.D. , et al., Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 2014, 509, 105–109. [Google Scholar] [CrossRef]
- Zhang, X. , et al., Endogenous glutamate determines ferroptosis sensitivity via ADCY10-dependent YAP suppression in lung adenocarcinoma. Theranostics 2021, 11, 5650–5674. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.E. , et al., Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol 2017, 13, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S. , et al., Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A 2016, 113, E4966–E4975. [Google Scholar] [CrossRef]
- Zou, Y. , et al., Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nat Chem Biol 2020, 16, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Cui, J. , et al., LPCAT3 is transcriptionally regulated by YAP/ZEB/EP300 and collaborates with ACSL4 and YAP to determine ferroptosis sensitivity. Antioxid Redox Signal 2023.
- Wang, Y., et al., Transcriptional Repression of Ferritin Light Chain Increases Ferroptosis Sensitivity in Lung Adenocarcinoma. Front Cell Dev Biol 2021. 9: p. 719187.
- Yang, W.S. , et al., Regulation of ferroptotic cancer cell death by GPX4. Cell 2014. 156(1-2): p. 317-331.
- Doll, S. , et al., FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef]
- Kraft, V.A.N. , et al., GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent Sci 2020, 6, 41–53. [Google Scholar] [CrossRef]
- Bridges, R.J., N. R. Natale, and S.A. Patel, System xc(-) cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS. Br J Pharmacol 2012, 165, 20–34. [Google Scholar] [CrossRef]
- Muller, F. , et al., Elevated FSP1 protects KRAS-mutated cells from ferroptosis during tumor initiation. Cell Death Differ 2023, 30, 442–456. [Google Scholar] [CrossRef]
- Engelman, J.A. , et al., Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nature Medicine 2008, 14, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Downward, J. , Targeting RAS signalling pathways in cancer therapy. Nature Reviews Cancer 2003, 3, 11–22. [Google Scholar] [CrossRef]
- <56-6.pdf>.
- Zhang, Y. , et al., BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nature Cell Biology 2018, 20, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Wang, K., et al., Upregulation of CCT3 predicts poor prognosis and promotes cell proliferation via inhibition of ferroptosis and activation of AKT signaling in lung adenocarcinoma. BMC Molecular and Cell Biology 2022. 23(1).
- Alvarez, S.W. , et al., NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature 2017, 551, 639–643. [Google Scholar] [CrossRef]
- <14_2023 Aging (Albany NY)..pdf>.
- Gao, C., et al., Risk stratification of lung adenocarcinoma using a nomogram combined with ferroptosis-related LncRNAs and subgroup analysis with immune and N6-methyladenosine modification. BMC Medical Genomics 2022. 15(1).
- Shen, Y., et al., Cross-talk between cuproptosis and ferroptosis regulators defines the tumor microenvironment for the prediction of prognosis and therapies in lung adenocarcinoma. Frontiers in Immunology 2023. 13.
- Wang, Y., et al., A Novel Predictive Model Incorporating Ferroptosis-Related Gene Signatures for Overall Survival in Patients with Lung Adenocarcinoma. Medical Science Monitor 2021. 27.
- Zhang, W., et al., Molecular subtypes based on ferroptosis-related genes and tumor microenvironment infiltration characterization in lung adenocarcinoma. OncoImmunology 2021. 10(1).
- Zhu, G. , et al., Prognostic value of ferroptosis-related genes in patients with lung adenocarcinoma. Thoracic Cancer 2021, 12, 1890–1899. [Google Scholar] [CrossRef]
- Wang, H. , et al., The E3 Ligase MIB1 Promotes Proteasomal Degradation of NRF2 and Sensitizes Lung Cancer Cells to Ferroptosis. Mol Cancer Res 2022, 20, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.E. , et al., ZEB1 drives epithelial-to-mesenchymal transition in lung cancer. Journal of Clinical Investigation 2016, 126, 3219–3235. [Google Scholar] [CrossRef]
- Sun, L. , et al., Lipid Peroxidation, GSH Depletion, and SLC7A11 Inhibition Are Common Causes of EMT and Ferroptosis in A549 Cells, but Different in Specific Mechanisms. DNA and Cell Biology 2021, 40, 172–183. [Google Scholar] [CrossRef]
- Zhang, H., et al., ARNTL2 is an indicator of poor prognosis, promotes epithelial-to-mesenchymal transition and inhibits ferroptosis in lung adenocarcinoma. Transl Oncol 2022. 26: p. 101562.
- Yatabe, Y. , et al., EGFR Mutation Testing Practices within the Asia Pacific Region: Results of a Multicenter Diagnostic Survey. Journal of Thoracic Oncology 2015, 10, 438–445. [Google Scholar] [CrossRef]
- Han, B., et al., <em>EGFR</em> mutation prevalence in Asia-Pacific and Russian patients with advanced NSCLC of adenocarcinoma and non-adenocarcinoma histology: The IGNITE study. Lung Cancer 2017. 113: p. 37-44.
- Recondo, G. , et al. , Making the first move in EGFR-driven or ALK-driven NSCLC: first-generation or next-generation TKI? Nature Reviews Clinical Oncology 2018, 15, 694–708. [Google Scholar] [PubMed]
- Tan, C.-S., et al., Third generation EGFR TKIs: current data and future directions. Molecular Cancer 2018. 17(1).
- Chan, L.S. , et al., Selenite as a dual apoptotic and ferroptotic agent synergizes with EGFR and KRAS inhibitors with epigenetic interference. Clin Epigenetics 2023, 15, 36. [Google Scholar] [CrossRef]
- Wang, J. , et al., Intrinsic resistance to EGFR tyrosine kinase inhibitors in advanced non-small-cell lung cancer with activating EGFR mutations. OncoTargets and Therapy 2016.
- Tumbrink, H.L., A. Heimsoeth, and M.L. Sos, The next tier of EGFR resistance mutations in lung cancer. Oncogene 2020, 40, 1–11. [Google Scholar] [CrossRef]
- Reita, D., et al., Molecular Mechanism of EGFR-TKI Resistance in EGFR-Mutated Non-Small Cell Lung Cancer: Application to Biological Diagnostic and Monitoring. Cancers 2021. 13(19).
- Zhang, T. , et al., Targeting histone deacetylase enhances the therapeutic effect of Erastin-induced ferroptosis in EGFR-activating mutant lung adenocarcinoma. Transl Lung Cancer Res 2021, 10, 1857–1872. [Google Scholar] [CrossRef]
- Deng, S.H. , et al., miR-324-3p reverses cisplatin resistance by inducing GPX4-mediated ferroptosis in lung adenocarcinoma cell line A549. Biochem Biophys Res Commun 2021. 549: p. 54-60.
- Bi, G. , et al., miR-6077 promotes cisplatin/pemetrexed resistance in lung adenocarcinoma via CDKN1A/cell cycle arrest and KEAP1/ferroptosis pathways. Molecular Therapy - Nucleic Acids, 2022; 28, 366–386. [Google Scholar]
- Wang, P. Wang, and Y. Wang, Ferroptosis patterns modulate immunocyte communication in tumor microenvironments: clinical value and therapeutic guidance of lung adenocarcinoma. Functional & Integrative Genomics, 2023; 23(2). [Google Scholar]
- Deng, B. , et al., Identification and validation of a ferroptosis-related gene to predict survival outcomes and the immune microenvironment in lung adenocarcinoma. Cancer Cell International, 2022; 22(1). [Google Scholar]
- Luo, L. Chen, and F. Huang, Machine learning revealed ferroptosis features and ferroptosis-related gene-based immune microenvironment in lung adenocarcinoma. Chemico-Biological Interactions, 2023; 378, 110471. [Google Scholar]
- Tang, B. , et al., Identification of critical ferroptosis regulators in lung adenocarcinoma that RRM2 facilitates tumor immune infiltration by inhibiting ferroptotic death. Clinical Immunology 2021, 232, 108872. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y. , et al., Identification of a prognostic ferroptosis-related lncRNA signature in the tumor microenvironment of lung adenocarcinoma. Cell Death Discovery, 2021; 7(1). [Google Scholar]
- Feng, Z. , et al., Identification and Validation of a GPX4-Related Immune Prognostic Signature for Lung Adenocarcinoma. Journal of Oncology 2022, 2022, 1–24. [Google Scholar]
- Bi, G. , et al., miR-6077 promotes cisplatin/pemetrexed resistance in lung adenocarcinoma via CDKN1A/cell cycle arrest and KEAP1/ferroptosis pathways. Mol Ther Nucleic Acids 2022, 28, 366–386. [Google Scholar] [CrossRef]
- Jung, K.A. , et al., Identification of aldo-keto reductases as NRF2-target marker genes in human cells. Toxicol Lett 2013, 218, 39–49. [Google Scholar] [CrossRef]
- Bersuker, K. , et al., The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef]
- Yagoda, N. , et al., RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 2007, 447, 864–868. [Google Scholar] [CrossRef]
- Dai, E. , et al., Ferroptotic damage promotes pancreatic tumorigenesis through a TMEM173/STING-dependent DNA sensor pathway. Nat Commun 2020, 11, 6339. [Google Scholar] [CrossRef]
- Zhang, D.D. , et al., Keap1 Is a Redox-Regulated Substrate Adaptor Protein for a Cul3-Dependent Ubiquitin Ligase Complex. Molecular and Cellular Biology 2004, 24, 10941–10953. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. , Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 2013, 53, 401–26. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N. , Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014, 511, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Wohlhieter, C.A. , et al., Concurrent Mutations in STK11 and KEAP1 Promote Ferroptosis Protection and SCD1 Dependence in Lung Cancer. Cell Rep 2020, 33, 108444. [Google Scholar] [CrossRef] [PubMed]
- Arbour, K.C. , et al., Effects of Co-occurring Genomic Alterations on Outcomes in Patients with KRAS-Mutant Non-Small Cell Lung Cancer. Clin Cancer Res 2018, 24, 334–340. [Google Scholar] [CrossRef]
- Zhang, W. , et al., The RSL3 Induction of KLK Lung Adenocarcinoma Cell Ferroptosis by Inhibition of USP11 Activity and the NRF2-GSH Axis. Cancers (Basel) 2022. 14(21).
- Thompson, L.R. , et al., Distinct TP53 Mutation Types Exhibit Increased Sensitivity to Ferroptosis Independently of Changes in Iron Regulatory Protein Activity. International Journal of Molecular Sciences 2020. 21(18).
- Liu, D.S. , et al., Inhibiting the system xC−/glutathione axis selectively targets cancers with mutant-p53 accumulation. Nature Communications 2017. 8(1).
- Freire Boullosa, L. , et al., Auranofin reveals therapeutic anticancer potential by triggering distinct molecular cell death mechanisms and innate immunity in mutant p53 non-small cell lung cancer. Redox Biology 2021. 42.
- Wang, S.-J. , et al., Acetylation Is Crucial for p53-Mediated Ferroptosis and Tumor Suppression. Cell Reports 2016, 17, 366–373. [Google Scholar] [CrossRef]
- Proceedings of the National Academy of Sciences.
- Zhao, B. , et al., Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 2007, 21, 2747–2761. [Google Scholar] [CrossRef]
- Zhu, C. , et al., A Non-canonical Role of YAP/TEAD Is Required for Activation of Estrogen-Regulated Enhancers in Breast Cancer. Mol Cell 2019, 75, 791–806. [Google Scholar] [CrossRef]
- Thomas, C. , et al., LPCAT3 deficiency in hematopoietic cells alters cholesterol and phospholipid homeostasis and promotes atherosclerosis. Atherosclerosis 2018, 275, 409–418. [Google Scholar] [CrossRef]
- Wang, T. , et al., ARNTL2 upregulation of ACOT7 promotes NSCLC cell proliferation through inhibition of apoptosis and ferroptosis. BMC Mol Cell Biol 2023, 24, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. , et al., CREB stimulates GPX4 transcription to inhibit ferroptosis in lung adenocarcinoma. Oncol Rep 2021. 45(6).
- Zhao, G. , et al. , KLF11 regulates lung adenocarcinoma ferroptosis and chemosensitivity by suppressing GPX4. Commun Biol 2023, 6, 570. [Google Scholar]
- Logie, E. , et al., Ferroptosis Induction in Multiple Myeloma Cells Triggers DNA Methylation and Histone Modification Changes Associated with Cellular Senescence. Int J Mol Sci 2021. 22(22).
- Xu, Y. , et al., Ferroptosis-associated DNA methylation signature predicts overall survival in patients with head and neck squamous cell carcinoma. BMC Genomics 2022, 23, 63. [Google Scholar] [CrossRef]
- Zhang, X. , et al. , Homocysteine induces oxidative stress and ferroptosis of nucleus pulposus via enhancing methylation of GPX4. Free Radic Biol Med 2020, 160, 552–565. [Google Scholar] [PubMed]
- Tessarz, P. and T. Kouzarides, Histone core modifications regulating nucleosome structure and dynamics. Nat Rev Mol Cell Biol 2014, 15, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A. , et al., Bromodomain inhibition of the coactivators CBP/EP300 facilitate cellular reprogramming. Nat Chem Biol 2019, 15, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q. , et al., MnTE-2-PyP reduces prostate cancer growth and metastasis by suppressing p300 activity and p300/HIF-1/CREB binding to the promoter region of the PAI-1 gene. Free Radic Biol Med 2016, 94, 185–94. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y. , et al., Correction: miR-1260b, mediated by YY1, activates KIT signaling by targeting SOCS6 to regulate cell proliferation and apoptosis in NSCLC. Cell Death Dis 2020, 11, 261. [Google Scholar] [CrossRef] [PubMed]
- Lu, X. , et al. , MiR-27a-3p Promotes Non-Small Cell Lung Cancer Through SLC7A11-Mediated-Ferroptosis. Front Oncol 2021, 11, 759346. [Google Scholar]
- Wei, B. , et al., Comprehensive analysis of tumor immune infiltration associated with endogenous competitive RNA networks in lung adenocarcinoma. Pathol Res Pract 2019, 215, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Li, X. , et al., NUDT21 regulates circRNA cyclization and ceRNA crosstalk in hepatocellular carcinoma. Oncogene 2020, 39, 891–904. [Google Scholar] [CrossRef] [PubMed]
- Kong, X. , et al. , LncRNA-CDC6 promotes breast cancer progression and function as ceRNA to target CDC6 by sponging microRNA-215. J Cell Physiol 2019, 234, 9105–9117. [Google Scholar] [PubMed]
- Pan, C.F. , et al., CircP4HB regulates ferroptosis via SLC7A11-mediated glutathione synthesis in lung adenocarcinoma. Transl Lung Cancer Res 2022, 11, 366–380. [Google Scholar] [CrossRef]
- Zhou, W.Y. , et al., Circular RNA: metabolism, functions and interactions with proteins. Mol Cancer 2020, 19, 172. [Google Scholar] [CrossRef]
- Zhang, X. , et al., Essential roles of exosome and circRNA_101093 on ferroptosis desensitization in lung adenocarcinoma. Cancer Commun (Lond) 2022, 42, 287–313. [Google Scholar] [CrossRef]
- Jiang, X., et al., Systematic Analysis and Validation of the Prognosis, Immunological Role and Biology Function of the Ferroptosis-Related lncRNA GSEC/miRNA-101-3p/CISD1 Axis in Lung Adenocarcinoma. Front Mol Biosci 2021. 8: p. 793732.
- Zhang, N. , et al., LncRNA T-UCR Uc.339/miR-339/SLC7A11 Axis Regulates the Metastasis of Ferroptosis-Induced Lung Adenocarcinoma. J Cancer 2022, 13, 1945–1957. [Google Scholar] [CrossRef]
- Wang, M. , et al., Long noncoding RNA LINC00336 inhibits ferroptosis in lung cancer by functioning as a competing endogenous RNA. Cell Death Differ 2019, 26, 2329–2343. [Google Scholar] [CrossRef]
- Peng, X. , et al. , Overexpression of LINC00551 promotes autophagy-dependent ferroptosis of lung adenocarcinoma via upregulating DDIT4 by sponging miR-4328. PeerJ 2022, 10, e14180. [Google Scholar]
- Liu, J. , et al., Autophagy-Dependent Ferroptosis: Machinery and Regulation. Cell Chem Biol 2020, 27, 420–435. [Google Scholar] [CrossRef] [PubMed]
- Shi, H., J. Wei, and C. He, Where, When, and How: Context-Dependent Functions of RNA Methylation Writers, Readers, and Erasers. Mol Cell 2019, 74, 640–650. [Google Scholar] [CrossRef]
- Fu, Y. , et al., Gene expression regulation mediated through reversible m(6)A RNA methylation. Nat Rev Genet 2014, 15, 293–306. [Google Scholar] [CrossRef]
- Lan, Q. , et al., The Critical Role of RNA m(6)A Methylation in Cancer. Cancer Res 2019, 79, 1285–1292. [Google Scholar] [CrossRef]
- Song, Z., et al., Exosomal miR-4443 promotes cisplatin resistance in non-small cell lung carcinoma by regulating FSP1 m6A modification-mediated ferroptosis. Life Sci 2021. 276: p. 119399.
- Xu, Y. , et al., METTL3 promotes lung adenocarcinoma tumor growth and inhibits ferroptosis by stabilizing SLC7A11 m(6)A modification. Cancer Cell Int 2022, 22, 11. [Google Scholar] [CrossRef]
- Xu, X. , et al., IGF2BP3 is an essential N(6)-methyladenosine biotarget for suppressing ferroptosis in lung adenocarcinoma cells. Mater Today Bio, 2022; 17, 100503. [Google Scholar]
- Ma, L. , et al., Targeting SLC3A2 subunit of system X(C)(-) is essential for m(6)A reader YTHDC2 to be an endogenous ferroptosis inducer in lung adenocarcinoma. Free Radic Biol Med. 2021; 168, 25–43. [Google Scholar]
- <82-Lachaier.pdf>.
- He, H. , et al., KIF20A is associated with clinical prognosis and synergistic effect of gemcitabine combined with ferroptosis inducer in lung adenocarcinoma. Front Pharmacol. 2022; 13, 1007429. [Google Scholar]
- Mo, X. , et al., Tetrandrine citrate suppresses lung adenocarcinoma growth via SLC7A11/GPX4-mediated ferroptosis. Discov Oncol. 2023, 14, 85. [Google Scholar] [CrossRef]
- Huang, F., et al., Hedyotis diffusa injection induces ferroptosis via the Bax/Bcl2/VDAC2/3 axis in lung adenocarcinoma. Phytomedicine. 2022. 104: p. 154319.
- Lou, J.S., et al., Ginkgetin derived from Ginkgo biloba leaves enhances the therapeutic effect of cisplatin via ferroptosis-mediated disruption of the Nrf2/HO-1 axis in EGFR wild-type non-small-cell lung cancer. Phytomedicine. 2021. 80: p. 153370.
- Wang, W. , et al., Inhalable Biomimetic Protein Corona-Mediated Nanoreactor for Self-Amplified Lung Adenocarcinoma Ferroptosis Therapy. ACS Nano. 2022, 16, 8370–8387. [Google Scholar] [CrossRef] [PubMed]
- Luan, F., X. He, and N. Zeng, Tetrandrine: a review of its anticancer potentials, clinical settings, pharmacokinetics and drug delivery systems. J Pharm Pharmacol. 2020, 72, 1491–1512. [Google Scholar] [CrossRef]
- DeHart, D.N., et al., Opening of voltage dependent anion channels promotes reactive oxygen species generation, mitochondrial dysfunction and cell death in cancer cells. Biochem Pharmacol. 2018. 148: p. 155-162.
- Lipper, C.H. , et al., Redox-dependent gating of VDAC by mitoNEET. Proc Natl Acad Sci U S A. 2019, 116, 19924–19929. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, E.N. , et al., Voltage-dependent anion channels modulate mitochondrial metabolism in cancer cells: regulation by free tubulin and erastin. J Biol Chem. 2013, 288, 11920–11929. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




