Submitted:
11 September 2023
Posted:
12 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Ethical considerations
2.2. Design
2.3. Studied variables
2.4. Inclusion and exclusion criteria
2.5. Statistical analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pisano M, Allievi N, Gurusamy K, Borzellino G, Cimbanassi S, Boerna D, et al. 2020 World Society of Emergency Surgery updated guidelines for the diagnosis and treatment of acute calculus cholecystitis. World J Emerg Surg. 2020, 15, 1–26. [Google Scholar]
- Wakabayashi G, Iwashita Y, Hibi T, Takada T, Strasberg SM, Asbun HJ, et al. Tokyo Guidelines 2018: surgical management of acute cholecystitis: safe steps in laparoscopic cholecystectomy for acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018, 25, 73–86. [Google Scholar] [CrossRef] [PubMed]
- De, U. Evolution of cholecystectomy: A tribute to Carl August Langenbuch. Indian J Surg. 2004, 66, 97–100. [Google Scholar]
- Coccolini F, Solaini L, Binda C, Catena F, Chiarugi M, Fabbri C, et al. Laparoscopic Cholecystectomy in Acute Cholecystitis: Refining the Best Surgical Timing Through Network Meta-Analysis of Randomized Trials. Surg Laparosc Endosc Percutan Tech. 2022, 32, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Brooks K, Scarborough J, Vaslef S, Shapiro M. No need to wait: An analysis of the timing of cholecystectomy during admission for acute cholecystitis using the American College of Surgeons National Surgical Quality Improvement Program database. J Trauma Acute Care Surg. 2012, 74. [Google Scholar]
- Tzovaras G, Zacharoulis D, Liakou P, Theodoropoulos T, Paroutoglou G, Hatzitheofilou C. Timing of laparoscopic cholecystectomy for acute cholecystitis: A prospective non randomized study. World J Gastroenterol. 2006, 12, 5528–5531. [Google Scholar] [CrossRef] [PubMed]
- Borzellino G, Khuri S, Pisano M, Mansour S, Allievi N, Ansaloni L, et al. Timing of early laparoscopic cholecystectomy for acute calculous cholecystitis: a meta-analysis of randomized clinical trials. World J Emerg Surg. 2021, 16, 1–12. [Google Scholar]
- Al-Mulhim, AA. Timing of early laparoscopic cholecystectomy for acute cholecystitis. J Soc Laparoendosc Surg. 2008, 12, 282–287. [Google Scholar]
- Wiggins T, Markar SR, MacKenzie H, Faiz O, Mukherjee D, Khoo DE, et al. Optimum timing of emergency cholecystectomy for acute cholecystitis in England: population-based cohort study. Surg Endosc [Internet]. 2019, 33, 2495–2502. [Google Scholar] [CrossRef] [PubMed]
- Alore EA, Ward JL, Todd SR, Wilson CT, Gordy SD, Hoffman MK, et al. Ideal timing of early cholecystectomy for acute cholecystitis: An ACS-NSQIP review. Am J Surg [Internet]. 2019, 218, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Brunée L, Hauters P, Closset J, Fromont G, Puia-Negelescu S. Assessment of the optimal timing for early laparoscopic cholecystectomy in acute cholecystitis: a prospective study of the Club Coelio. Acta Chir Belg [Internet]. 2019, 119, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Chandler CF, Lane JS, Ferguson P, Thompson JE, Ashley SW. Prospective evaluation of early versus delayed laparoscopic cholecystectomy for treatment of acute cholecystitis. Am Surg. 2000, 66, 896–900. [Google Scholar] [CrossRef]
- Arslan onuk zinet asuman. The timing of laparoscopic cholecystectomy in acute cholecystitis: importance of first 72 hours and oxidative stress markers. Turkish J Trauma Emerg Surg. 2019, 25, 440–446. [Google Scholar]
- Jan Y, Shah M, Hussain S, Din W, Khan A. Variables affecting outcome of laparoscopic cholecystectomy in acute cholecystitis. Pak J Surg. 2016, 32, 16–21. [Google Scholar]
- Gurusamy KS, Davidson C, Gluud C, Davidson B. Early versus delayed laparoscopic cholecystectomy for people with acute cholecystitis (Review). Cochrane Database Syst Rev. 2013;(6).
- Fugazzola P, Cobianchi L, Di Martino M, Tomasoni M, Dal Mas F, Abu-Zidan FM, Agnoletti V, Ceresoli M, Coccolini F, Di Saverio S, Dominioni T, Farè CN, Frassini S, Gambini G, Leppäniemi A, Maestri M, Martín-Pérez E, Moore EE, Musella V, Peitzman AB, de la Hoz Rodríguez Á, Sargenti B, Sartelli M, Viganò J, Anderloni A, Biffl W, Catena F, Ansaloni L; S.P.Ri.M.A.C.C. Collaborative Group. Prediction of morbidity and mortality after early cholecystectomy for acute calculous cholecystitis: results of the S.P.Ri.M.A.C.C. study. World J Emerg Surg. 2023 Mar 18;18(1):20. PMID: 36934276; PMCID: PMC10024826. [CrossRef]
- Liao Y, Cai Q, Zhang X, Li F. Single-stage intraoperative ERCP combined with laparoscopic cholecystectomy versus preoperative ERCP Followed by laparoscopic cholecystectomy in the management of cholecystocholedocholithiasis: A meta-analysis of randomized trials. Med (United States). 2022, 101, E29002. [Google Scholar]
| Onset of Symptoms | ||||
|---|---|---|---|---|
| Variables | 0-3 days n=656 |
4-7 days n=360 |
8-10 days n=101 |
P value |
| Age (years) | 59 (46-72) | 62 (50-74) | 61 (46-74) | 0.14 |
| BMI | 26.7 (24.3-29.4) | 26.7 (24.2-29.4) | 26.3 (23.6-28.9) | 0.4 |
| ACC severity grade | 2 (1-2)* | 2 (2-2)* | 2 (1-2)* | <0.001 |
| POSSUM physiological score | 19 (15-24) | 19 (16-24) | 21(17-26) | 0.012 |
| Days from admission to surgery | 1 (0-1) | 2 (0-3) | 6 (2-8) | <0.001 |
| Onset of Symptoms | ||||
|---|---|---|---|---|
| Variables | 0-3 days n=656 |
4-7 days n=360 |
8-10 days n=101 |
P value |
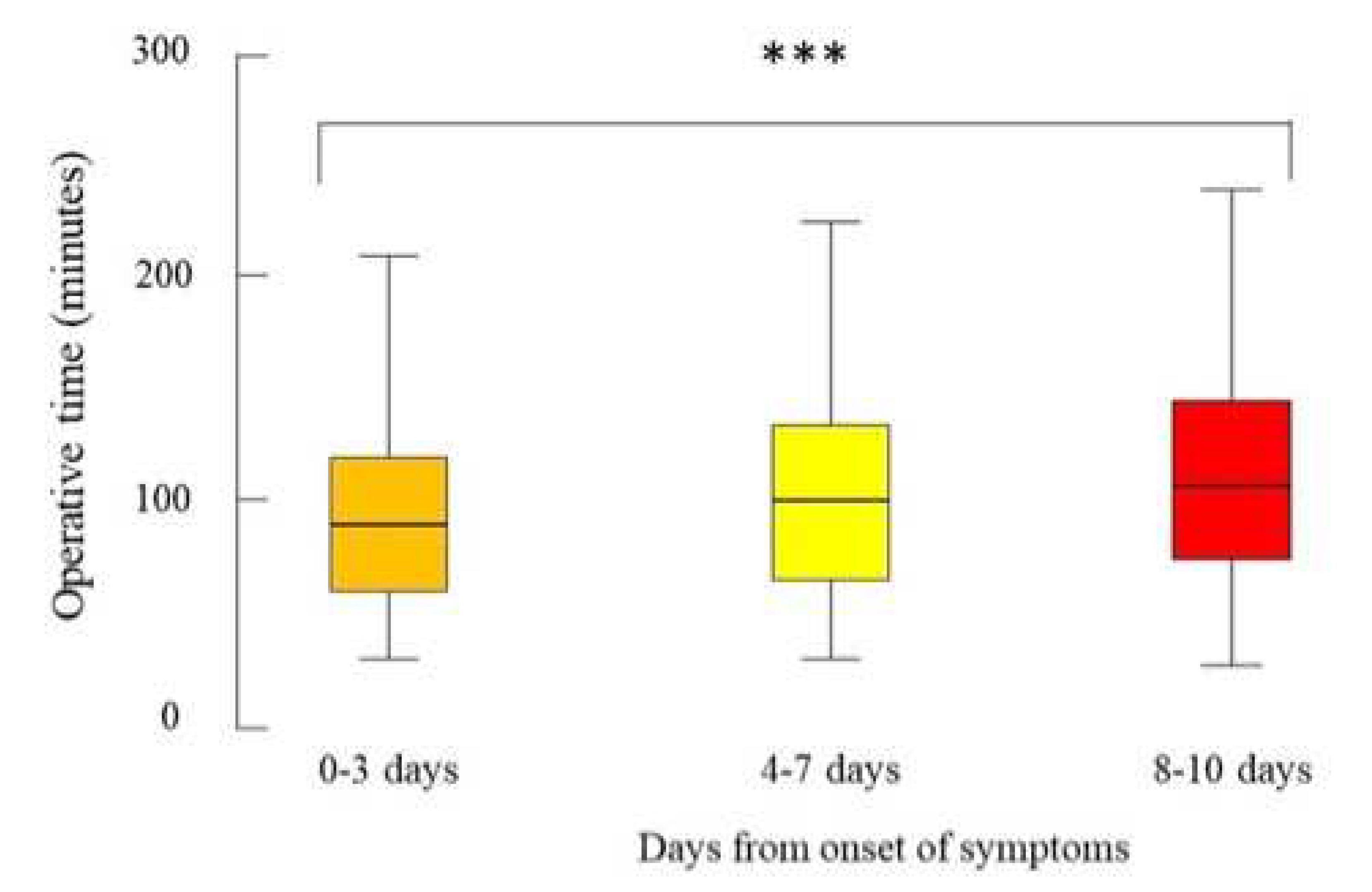
| Operative time (minutes) | 90 (60-120) | 100 (65-134.5) | 107 (74-145) | <0.001 |
| Conversion to open surgery | 48 (7.9%) | 32 (9.9%) | 9 (9.5%) | 0.54 |
|
Bail-out procedure: Subtotal cholecystectomy Fundus-first technique Drainage only |
45 (6.9%) 18 (2.7%) 34 (5.2%) 1 (0.2%) |
35 (9.7%) 20 (5.6%) 24 (6.7%) 0 (0.0%) |
14 (13.9%) 11 (10.9%) 7 (6.9%) 0 (0%) |
0.037 <0.001 0.52 0.99 |
| Intraoperative complications | 18 (2.8%) | 20 (5.6%) | 8 (7.9%) | 0.01 |
| Onset of Symptoms | ||||
|---|---|---|---|---|
| Variables | 0-3 days n=656 |
4-7 days n=360 |
8-10 days n=101 |
P value |
| Reintervention | 16 (2.4%) | 6 (1.7%) | 0 (0%) | 0.29 |
| Inhospital major complication | 38 (5.8%) | 15 (4.2%) | 6 (5.9%) | 0.49 |
| 30-day major complications | 48 (7.3%) | 22 (6.2%) | 7 (7.1%) | 0.78 |
| Inhospital mortality | 5 (0.8%) | 5 (1.4%) | 1 (1%) | 0.52 |
| 30-day mortality | 5 (0.8%) | 7 (1.9%) | 1 (1%) | 0.25 |
| LOS>10 days | 43 (6.6%) | 38 (10.6%) | 25 (24.8) | <0.001 |
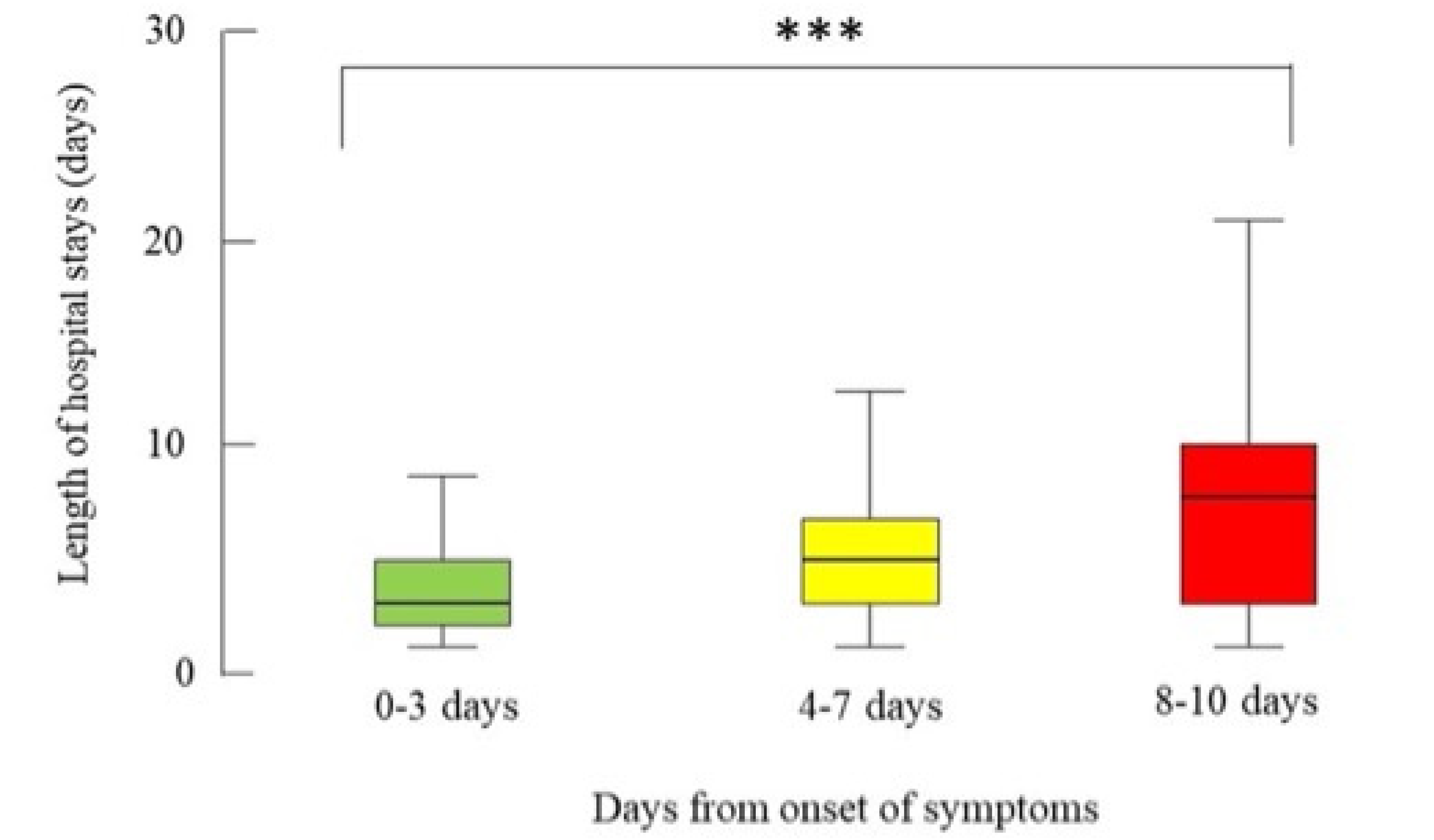
| LOS (days) | 3 (2-5) | 5 (3-7) | 8 (3-11) | <0.001 |
| Length of stay | Days from admission to EC | POSSUM PS | Operative time | ||
|---|---|---|---|---|---|
| Days from onset to EC | Correlation | 0.26 | 0.53 | 0.01 | 0.14 |
| P value | <.001 | <.001 | 0.002 | <.001 | |
| Length of stay | Correlation | ------- | 0.35 | 0.31 | 0.33 |
| P value | ------- | <.001 | <.001 | <.001 | |
| Days from admission | Correlation | ------- | ------- | 0.06 | .167 |
| P value | ------- | ------- | 0.049 | <.001 | |
| POSSUM score | Correlation | ------- | ------- | ------- | 0.16 |
| P value | ------- | ------- | ------- | <.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





