1. Introduction
Rice, as the world's most important food, stands out among cereals for being an aquatic crop. Currently, around 131 million hectares of rice paddies are flooded by irrigation or rainwater during the growing season[
1]. Nevertheless, these rice paddies are also considered a major source of anthropogenic methane (CH4) emissions, contributing to 12% of the total anthropogenic CH
4 budget (IPCC, 2007)[
2]. Recent estimates of CH
4 emissions from rice fields vary between 39 and 112 Tg CH4 per year[
3]. Unfortunately, due to the increasing rice demand from the world's rapidly growing population, rice cultivation is expected to continue rising in the coming decades[
4], leading to a significant increase in CH
4 emissions.
Methane emissions from paddy fields result from the production and subsequent oxidation of CH
4 by methanogenic and methanotrophic bacteria, respectively[
5]. CH
4 fluxes in rice paddy soils are influenced by various factors, including the use of inorganic and organic fertilizers, water management practices, the physicochemical and geochemical properties of the soil, air and soil temperature, the composition and activity of soil microorganisms, and the physiological characteristics of rice cultivars[
6]. Methane is produced in the anaerobic conditions typical of flooded rice fields, providing a favorable environment for methanogens, which rely on reduced conditions and soil carbon sources produced by the rice plant[
7]. To quantify methanogens in rice paddy soils and other methanogenic environments, the
mcrA gene, responsible for encoding the alpha subunit of methyl coenzyme M reductase (MCR), has been commonly used due to its high conservation and specificity to methanogens (paddies). Meanwhile, methanotrophic bacteria depend on methane produced by methanogens and can oxidize up to 60-70% of the methane in paddy soils, significantly reducing methane production potential from rice agriculture[
8,
9]. The variation in methane emissions observed among rice cultivars[
10,
11,
12] indicates the potential for mitigating methane production from rice cultivation through breeding low-emitting rice varieties. However, the genetic differences impacting the rhizosphere microbial communities, which are ultimately reflected in methane emissions from rice paddy soils, remain poorly understood. Studies have shown that cultivar differences affect the composition of methanogens and methanotrophs in the rice rhizosphere[
13,
14,
15,
16,
17,
18,
19]. Methanogens interact with other members of the microbial community, influencing the observed methane emissions from rice fields. Consequently, variations in cultivars may play a major role in regulating CH
4 emissions from rice fields. In addition, Nitrogen fertilizers play a significant role in stimulating crop growth and providing additional carbon substrates, such as organic root exudates and sloughed-off cells, to methanogens for CH4 production[
20,
21,
22]. However, despite research in this area, no single consensus has been reached regarding the net impacts of nitrogen fertilizers on methanogen abundance in rice soils. In other words, while it is well-established that nitrogen fertilizers can enhance crop growth and contribute to the production of methane by providing more carbon substrates to methanogens, there is no universally agreed-upon conclusion regarding the overall effect of nitrogen fertilizers on the abundance of methanogens in rice soils. Further research is needed to fully understand the complex interactions between nitrogen fertilizers and methanogen abundance in rice cultivation. Likewise, widespread observation of differences in CH
4 emissions among rice cultivars[
23,
24,
25] few studies have attempted to examine CH
4 emission control mechanisms, such as the abundance of methanogens and methanotrophs, and apparent plant growth properties, using different cultivars.
The purpose of this study was evaluation and quantification of methanogen and methanotroph under different nitrogen levels and cultivars on rhizosphere. To this end, we suggested that there is synergetic or additive effect with variety and nitrogen level to reduce methanogen and enhance methanotroph inhabitance by two-way ANOVA.
2. Materials and Methods
2.1. Experimental field management and measurement of agronomic traits
The experimental fields were situated in Miryang, South Korea (35° 29' 32.2872" N, 128° 44' 32.1972" E). Prior to transplanting, conventional tillage was carried out, and no straw or organic matter was applied. The rice varieties utilized were Hanareum4, Geumgang1, Milyang392, IR72, 93-11, IR64 (Indica), Saeilmi, Sobi, Nampyeong, and Misojinmi (Japonica), which were transplanted 30 days after sowing. The seedlings were simultaneously transplanted in both experimental fields with a spacing of 30 cm x 15 cm. Each field (8m x 70m) consisted of three replicate plots. A chemical fertilizer (N-P2O5-K2O: 21-17-17) was applied as 0, 50% and 100%. 100% (conventional: N-P2O5-K2O: 9.0-4.5-5.9) fertilization was applied at a total rate of 90 kg N ha−1 during rice cultivation. This fertilizer was split into three applications: 54 kg N ha−1 as basal application before transplanting, 18 kg N ha−1 at 20 days after transplanting (DAT), and another 18 kg N ha−1 at 65 DAT, under normal nitrogen conditions. In the low nitrogen plots, only half (50%) the amount of nitrogen used in the normal plots was applied. The normal nitrogen, low nitrogen, and no nitrogen fields have been maintained for 20 years. The Miryang field was continuously flooded until 110 DAT as part of field management practices. Throughout the rice growing season, continuous flooding was maintained to keep the water level consistently at 5-7 cm above the soil surface. The plant height and tiller number were measured during the tillering, heading, grain filling stages, and prior to harvesting. Agronomic traits such as milled rice yield, spikelet number per panicle, and thousand grain weight were measured before harvesting.
2.2. Chemical characterization of testbed soil
Soil analysis was conducted following the methods[
26]. The pH of the soil was measured using a soil-to-distilled water ratio of 1:5, and the resulting suspension was measured using a pH meter (720, ORION, USA) based on the glass electrode method. Organic matter was determined using the Tyurin method, while available phosphorus was extracted and analyzed using the Lancaster method with a spectrophotometer (CINTRA6, GBC, Australia) (NIAST, 2000). Nitrogen within the soil was extracted with 2 M KCl and analyzed using a nitrogen analyzer (K-314, Buchi, Switzerland). For the analysis of exchangeable cations, the soil was extracted with 1 N-NH
4OAc (pH 7), and the quantification was performed using an Inductively Coupled Plasma-Atomic Emission Spectrometer (ICP-AES, GBC Intergra XM2, Australia).
2.3. Extraction of total genomic DNA from rice rhizosphere
Rhizospheric soils from rice roots were collected from each experimental plot 5 replicates on tillering, heading and grain filling stages. 1g of rhizospheric soils were dried for 12hrs. Extraction of total genomic DNAs were performed using DNeasy PowerSoil Pro Kit (Qiagen, Germany), as followed provided protocol.
To ensure that the beads have settled at the bottom, briefly spin the PowerBead Pro Tube. After that, add up to 250 mg of soil and 800 µl of Solution CD1, and vortex briefly to mix. Please note that once the sample has been loaded into the PowerBead Pro Tube, the next step involves homogenization and lysis. The PowerBead Pro Tube contains a buffer that serves multiple purposes: (a) dispersing the soil particles, (b) beginning the dissolution of humic acids, and (c) protecting nucleic acids from degradation. By gently vortexing, the components in the PowerBead Pro Tube are mixed, and the sample starts dispersing in the buffer. If using a Vortex Adapter for 1.5–2 ml tubes (cat. no. 13000-V1-24), secure the PowerBead Pro Tube horizontally and vortex at maximum speed for 10 minutes. If more than 12 preps are being done simultaneously using the Vortex Adapter, increase the vortexing time by 5–10 minutes. Avoid using tape, as it may become loose and result in reduced homogenization efficiency and inconsistent results. Centrifuge the PowerBead Pro Tube at 15,000 x g for 1 minute. Transfer the supernatant, which may still contain some soil particles, to a clean 2 ml Microcentrifuge Tube Add 200 µl of Solution CD2 to the supernatant and vortex for 5 seconds. Solution CD2 contains IRT, a reagent that can precipitate non-DNA organic and inorganic materials, including humic substances, cell debris, and proteins. Removing these contaminants is crucial to ensure DNA purity and prevent inhibition of downstream DNA applications. Centrifuge at 15,000 x g for 1 minute. Carefully transfer up to 700 µl of the supernatant to a clean 2 ml Microcentrifuge Tube. Note that the pellet at this stage contains non-DNA organic and inorganic materials, such as humic acids, cell debris, and proteins. For optimal DNA yields and quality,
2.4. Constriction of standard DNA for generating standard curves
To quantify methanogen and methanotroph on rhizosphere, we contructed standard DNA. Specific primers were used for amplification. For the key enzyme Methyl-Coenzyme M Reductase (MCR) of methanogenic bacteria, the primers MLf (5'-GGT GGT GTM GGA TTC ACA CARTAY GCW ACA GC-3') and MLr (5'-TTC ATT GCR TAG TTW GGR TAG TT-3') were used[
27]. For the major enzyme particulate Methane monooxygenase (pMMO) of methanotrophic bacteria, the primers A189f (5'-GGNGAC TGG GAC TTC TGG-3') and mb661r (5'-CCG GMG CAA CGT CGT CYT TAC C-3') were used[
28,
29]. PCR amplification was performed with each primer set. For MCR gene amplification, the reaction included an initial denaturation at 94°C for 5 minutes, followed by 32 cycles of denaturation at 94°C for 45 seconds, annealing at 55°C for 45 seconds, extension at 72°C for 45 seconds, and a final extension at 72°C for 10 minutes. For pMMO gene amplification, the reaction included an initial denaturation at 94°C for 5 minutes, followed by 32 cycles of denaturation at 94°C for 45 seconds, annealing at 60°C for 45 seconds, extension at 72°C for 45 seconds, and a final extension at 72°C for 10 minutes. After amplification, the MCR and pMMO genes were purified, and TOPO TA cloning kit (Invitrogen, MA, USA) was used for transformation into E. coli. Colonies containing the inserted plasmids were obtained on LB + km (kanamycin, 50 ppm) + X-gal + IPTG agar plates. The sequences of the inserted genes in the obtained colonies were analyzed for confirmation, and the plasmids were used as standards. The plasmids prepared as standards were extracted using the Plasmid Mini Extraction Kit (Bioneer, Korea), and their concentrations were measured using an ELISA reader with the Take3 multivolume plate. A standard curve was generated and gene copy numbers were calculated.
2.5. Quantification of methanogen and methanotroph
The qPCR was performed using the QuantiFast SYBR Green PCR kit (Qiagen, Germany). The reaction mixture consisted of 0.3 μM of primers, 50 nl of genomic DNA, and 7.8 μl of D.W, resulting in a total volume of 20 μl. For the standards, the plasmid DNA prepared earlier was diluted from 102 to 107 for mcrA and from 102 to 108 for pmoA, to be used for quantification. The prepared mixture was subjected to real-time PCR using the QuantStudio 5 real-time PCR system (Applied Biosystems, USA). The PCR amplification was carried out with the same conditions as those used for standard preparation, with 50 cycles to confirm specific amplification. Additionally, a melting curve analysis was performed by increasing the temperature from 65°C to 95°C with a 0.5°C increment per cycle to determine if PCR amplification occurred specifically. The results of the real-time PCR were calculated using the QuantStudio Design and Analysis software (Applied Biosystems, USA) to quantify the copy numbers of methanogenic and methanotrophic bacteria present in the genomic DNA.
2.6. Statistical analysis
Statistical analyses, two-way ANOVA and correlation analysis, were performed using SAS Enterprise (version 7.15 HF8).
3. Results and Discussion
3.1. Abundance of methanogen and methanotroph under different nitrogen levels and growth stages
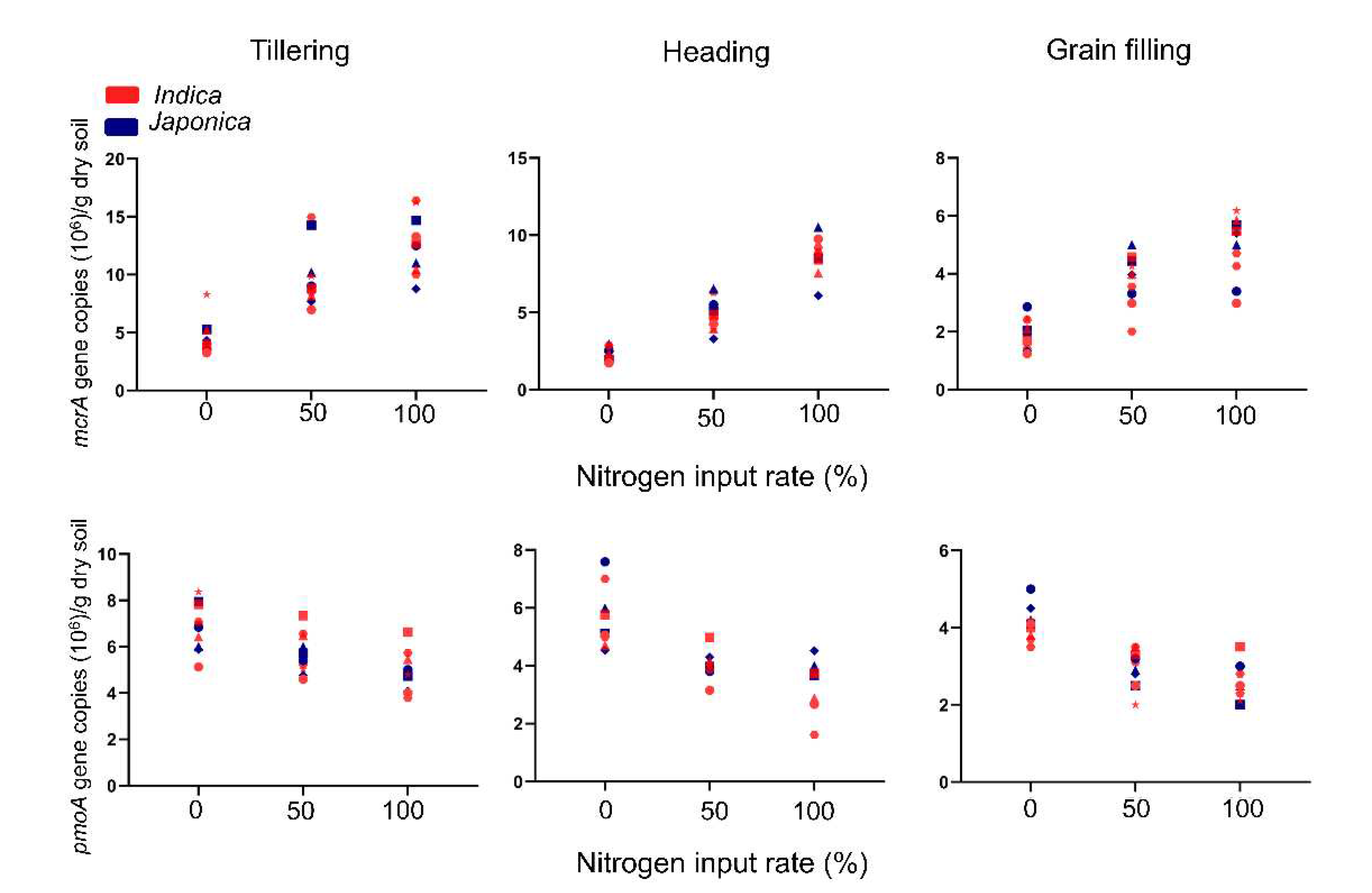
To explore abundances of rhizospheric methanogen and methanothroph under different nitrogen levels and growth stages, we conducted qPCR using 6 Indica and 4 Japonica subspecies, 0%, 50%, 100% (conventional level) and tillering, heading and grain filling stages collected samples. The abundances of methanogens and methanotrophs appeared to be highly affected by the nitrogen levels during all growth stages. On low nitrogen (LN) conditions, the abundances of methanogens were 3.8, 9.8, and 4.7 x 10
6 mcrA gene copies/g dry soil during the tillering, heading, and grain filling stages, respectively. Methanotroph abundances on LN were 4.0, 5.7, and 2.9 x 10
6 pmoA gene copies/g dry soil during the same stages, respectively. On the other hand, under normal nitrogen (NN) conditions, the abundances of methanogens were 4.9, 12.6, and 8.6 x 10
6 mcrA gene copies/g dry soil, while methanotroph abundances on NN were 3.3, 4.9, and 2.5 x 10
6 pmoA gene copies/g dry soil during the corresponding stages. First of all, quantitive abuncances of methanogen and methanotroph were observed as the highest at tillering stage and gradually decreased untill grain filling stage. Next, nitrogen application levels were higly affected to abundance of methanogen and methanotroph. The more nitrogen apply, the more methanogen and less methanotroph were inhabited on rice rhizosphere (
Figure 1).
Interestingly, the abundances were different depend on rice varieties. Otherwise, there was no different between Indica and Japonica subspecies. It means that inhabitance of methanogen and methanotrophs prefer different genotype rather than sub-species.
3.2. The ratio of methanogen/methanotroph under different nitrogen levels and growth stages
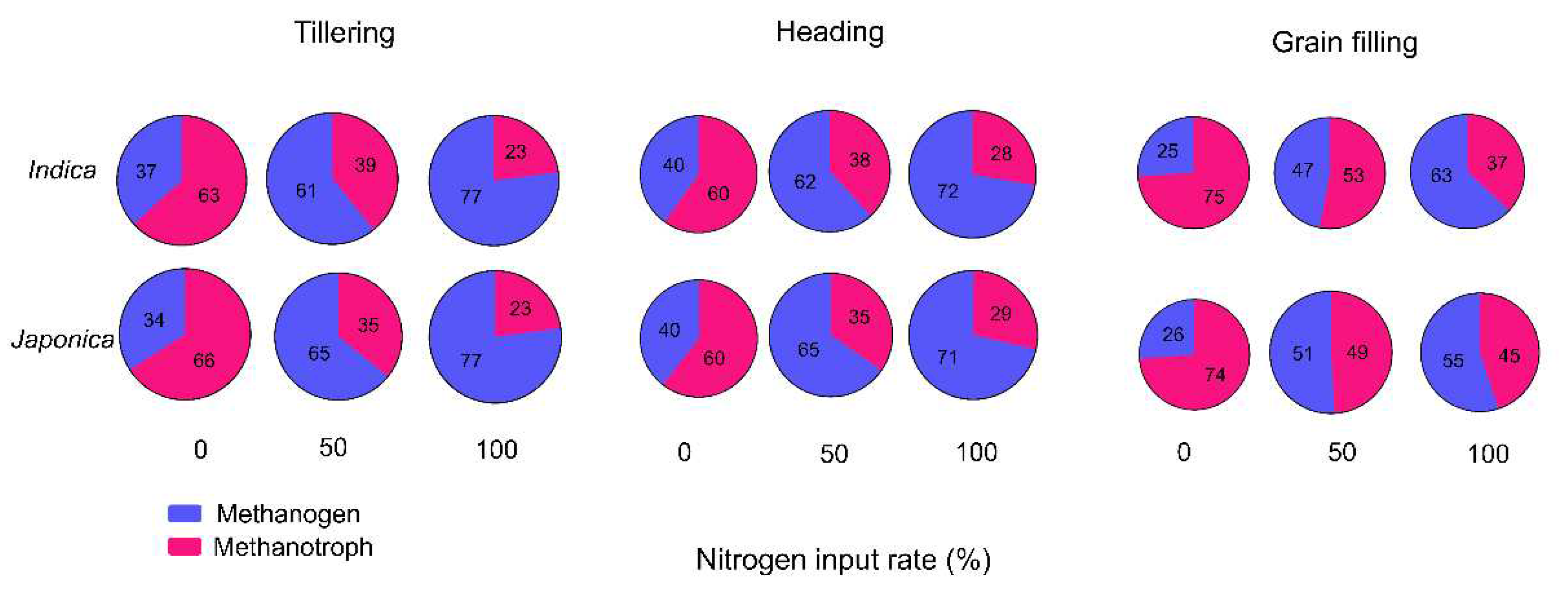
The ratio of methanogen and methanotroph were highly different under nitrogen levels and growth stages. On tillering stage, methanogen/methanotroph ratio were 35, 62 and 77% under 0, 50 and 100% nitrogen levels respectively. On heading and grain filing stages, ratio were 40, 63, 71% and 26, 48, 59% respectively (
Figure 2).
The Methanogen/Methanotroph ratio was found to vary depending on the rice genotypes studied. Geumgang1, which exhibits low panicle numbers but high rice yield, showed a low level of inhabited methanogens. On the other hand, IR64, a genotype highly influenced by nitrogen levels and with varying tiller numbers, demonstrated significant changes in methanogen abundances during the study (as shown in
Table 1 and
Table S1). These findings indicate that reducing nitrogen fertilizer application can potentially lead to lower methane emissions by maintaining lower levels of methanogens in the soil. This aligns with the global adoption of low-input regimes aimed at mitigating greenhouse gas emissions. Moreover, it is crucial to focus on reducing methanogen abundances during specific stages of rice growth, particularly the tillering and heading stages. Methanogen levels tend to remain high during these stages, which contributes significantly to greenhouse gas production. By addressing methanogen levels during these critical stages, efforts can be made to implement effective strategies for reducing greenhouse gas emissions. In summary, the study highlights the importance of understanding the methanogen/methanotroph ratio in different rice genotypes and emphasizes the potential benefits of reducing nitrogen fertilizer use to lower methane emissions. Targeting and managing methanogen levels during key growth stages can be a valuable approach in greenhouse gas reduction efforts.
In our investigation, we conducted a Two-way ANOVA using the SAS enterprise program to explore the combined effect of rice varieties and nitrogen levels on methanogen abundance. The p-values obtained from the one-way ANOVA of varieties and nitrogen levels with methanogen abundance were found to be significantly different at all stages. However, the sub-species factor did not show a significant effect. On the other hand, the Two-way ANOVA of S X N (Sub-species X Nitrogen) and V X N (Variety X Nitrogen) interactions demonstrated significant differences. These results suggest that both rice genotypes and nitrogen levels can influence and moderate methanogen inhabitance. Furthermore, combining specific rice varieties with low nitrogen levels may result in a synergistic or additive effect, potentially leading to a reduction in methanogen abundance. Previous studies focusing on mitigating greenhouse gas emissions have often examined individual factors such as rice varieties and nitrogen levels[
30,
31]. For instance, Kim et al. 2019 [
31] described that low nitrogen input could reduce methane emission without quantifying methane emission related microbes. Lee et al. 2014 [
17] revealed that the characteristics and behaviors of methanogens and methanotrophs in rice paddies can vary depending on the specific rice varieties being grown. In other words, different rice varieties can have distinct impacts on these microbial groups and their activities in the soil.
Furthermore, there is a correlation between methanogen abundances and agronomic traits. Notably, yield, spikelet number, and thousand grain weight demonstrated significant correlation coefficients of -0.81, -0.88, and -0.85, respectively. Consequently, we propose that by enhancing yield potential, methanogens would have a reduced presence in the rhizosphere (
Table 3 and
Table S1).
In conclusion, our study highlights the importance of considering the combined effects of rice varieties and nitrogen levels in modulating methanogen abundance. Indeed, this approach provides additional evidence supporting and expanding on previous research that focused on the effects of nitrogen and differentiation among rice varieties. By understanding these interactions, we can develop more effective strategies for mitigating greenhouse gas emissions from rice cultivation
5. Conclusions
In this study, we investigated the abundances of rhizospheric methanogens and methanotrophs under different nitrogen levels and growth stages in rice paddies. Using qPCR, we analyzed samples collected from six Indica and four Japonica subspecies at 0%, 50%, and 100% (conventional level) nitrogen levels during the tillering, heading, and grain filling stages. Our results showed that nitrogen levels significantly affected the abundances of both methanogens and methanotrophs across all growth stages. Under low nitrogen conditions, methanogen abundances were 3.8, 9.8, and 4.7 x 106 mcrA gene copies/g dry soil during the tillering, heading, and grain filling stages, respectively, while methanotroph abundances were 4.0, 5.7, and 2.9 x 106 pmoA gene copies/g dry soil during the same stages. On the other hand, under normal nitrogen conditions, methanogen abundances were 4.9, 12.6, and 8.6 x 106 mcrA gene copies/g dry soil, and methanotroph abundances were 3.3, 4.9, and 2.5 x 106 pmoA gene copies/g dry soil during the corresponding stages. Methanogen and methanotroph abundances were highest at the tillering stage and gradually decreased until the grain filling stage. The application of nitrogen had a significant impact on methanogen and methanotroph abundances, with higher nitrogen levels resulting in more methanogens and fewer methanotrophs in the rice rhizosphere. Interestingly, the abundances varied depending on rice varieties, while there was no significant difference between Indica and Japonica subspecies, suggesting that methanogen and methanotroph inhabitance may prefer specific rice genotypes rather than subspecies. The Methanogen/Methanotroph ratio was highly different under different nitrogen levels and growth stages, with higher ratios observed under higher nitrogen levels. Our findings indicate that reducing nitrogen fertilizer application can potentially lower methane emissions by maintaining lower levels of methanogens in the soil, aligning with low-input regimes to mitigate greenhouse gas emissions. Additionally, targeting and managing methanogen levels during specific growth stages, particularly tillering and heading, can be instrumental in greenhouse gas reduction strategies. Additionally, improving yield related agronomic traits may mitigate CH4 emission. This study emphasizes the importance of understanding the methanogen/methanotroph ratio in different rice genotypes and highlights the potential benefits of reducing nitrogen fertilizer use to lower methane emissions from rice cultivation. By considering the combined effects of rice varieties and nitrogen levels, more effective strategies for mitigating greenhouse gas emissions can be developed.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: Agronomic traits of 10 rice varieties under different nitrogen levels.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, J.-H.L. and Y.K.; methodology, Y.K and S.-M.L.; software, J.-K.C.; validation, H.P., D.-S.P. and N.R.K; writing—original draft preparation, Y.K.; writing—review and editing, J.-H.L.; supervision, J.-H.L., Y.-S.K. and K.-W.O.; project administration, K.-W.O.; funding acquisition, Y.K, J.-H.L. and K.-W.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out with the support of "Cooperative Research Program for Agriculture Science and Technology Development (Project No. RS-2022-RD010405)" Rural Development Administration, Republic of Korea.
Data Availability Statement
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, S.; Jaffé, P.R.; Mauzerall, D.L. A process-based model for methane emission from flooded rice paddy systems. ecological modelling 2007, 205, 475–491. [Google Scholar] [CrossRef]
- Change, I.P.O.C. Climate change 2007: The physical science basis. Agenda 2007, 6, 333. [Google Scholar]
- Solomon, S. Contribution of Working Group I to the fourth assessment report of the Intergovernmental Panel on Climate Change 2007. (No Title), 2007. [Google Scholar]
- Wang, Z.; Xu, Y.; Li, Z.; Guo, Y.; Wassmann, R.; Neue, H.; Lantin, R.; Buendia, L.; Ding, Y.; Wang, Z. Methane emissions from irrigated rice fields in northern China (Beijing). Nutr Cycling Agroecosyst, this issue 2000.
- Win, K.; Nonaka, R.; Win, A.; Sasada, Y.; Toyota, K.; Motobayashi, T.; Hosomi, M. Comparison of methanotrophic bacteria, methane oxidation activity, and methane emission in rice fields fertilized with anaerobically digested slurry between a fodder rice and a normal rice variety. Paddy and Water Environment 2012, 10, 281–289. [Google Scholar] [CrossRef]
- Bodelier, P.L.; Roslev, P.; Henckel, T.; Frenzel, P. Stimulation by ammonium-based fertilizers of methane oxidation in soil around rice roots. Nature 2000, 403, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Liesack, W.; Schnell, S.; Revsbech, N.P. Microbiology of flooded rice paddies. FEMS Microbiology Reviews 2000, 24, 625–645. [Google Scholar] [CrossRef] [PubMed]
- Neue, H.-U. Methane emission from rice fields. Bioscience 1993, 43, 466–474. [Google Scholar] [CrossRef]
- Sigren, L.; Byrd, G.; Fisher, F.; Sass, R. Comparison of soil acetate concentrations and methane producton, transport, and emission in two rice cultivars. Global Biogeochemical Cycles 1997, 11, 1–14. [Google Scholar] [CrossRef]
- Gogoi, N.; Baruah, K.; Gupta, P.K. Selection of rice genotypes for lower methane emission. Agronomy for sustainable development 2008, 28, 181–186. [Google Scholar] [CrossRef]
- Linquist, B.A.; Marcos, M.; Adviento-Borbe, M.A.; Anders, M.; Harrell, D.; Linscombe, S.; Reba, M.L.; Runkle, B.R.; Tarpley, L.; Thomson, A. Greenhouse gas emissions and management practices that affect emissions in US rice systems. Journal of environmental quality 2018, 47, 395–409. [Google Scholar] [CrossRef]
- Simmonds, M.B.; Anders, M.; Adviento-Borbe, M.A.; van Kessel, C.; McClung, A.; Linquist, B.A. Seasonal methane and nitrous oxide emissions of several rice cultivars in direct-seeded systems. Journal of Environmental Quality 2015, 44, 103–114. [Google Scholar] [CrossRef]
- Jiang, Y.; van Groenigen, K.J.; Huang, S.; Hungate, B.A.; van Kessel, C.; Hu, S.; Zhang, J.; Wu, L.; Yan, X.; Wang, L. Higher yields and lower methane emissions with new rice cultivars. Global Change Biology 2017, 23, 4728–4738. [Google Scholar] [CrossRef]
- Liechty, Z.; Santos-Medellín, C.; Edwards, J.; Nguyen, B.; Mikhail, D.; Eason, S.; Phillips, G.; Sundaresan, V. Comparative analysis of root microbiomes of rice cultivars with high and low methane emissions reveals differences in abundance of methanogenic archaea and putative upstream fermenters. Msystems 2020, 5, 10–1128. [Google Scholar] [CrossRef]
- Ma, K.; Qiu, Q.; Lu, Y. Microbial mechanism for rice variety control on methane emission from rice field soil. Global Change Biology 2010, 16, 3085–3095. [Google Scholar] [CrossRef]
- Jiang, Y.; Qian, H.; Huang, S.; Zhang, X.; Wang, L.; Zhang, L.; Shen, M.; Xiao, X.; Chen, F.; Zhang, H. Acclimation of methane emissions from rice paddy fields to straw addition. Science advances 2019, 5, eaau9038. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, S.Y.; Kim, P.J.; Madsen, E.L.; Jeon, C.O. Methane emission and dynamics of methanotrophic and methanogenic communities in a flooded rice field ecosystem. FEMS Microbiology Ecology 2014, 88, 195–212. [Google Scholar] [CrossRef]
- Lee, H.J.; Jeong, S.E.; Kim, P.J.; Madsen, E.L.; Jeon, C.O. High resolution depth distribution of Bacteria, Archaea, methanotrophs, and methanogens in the bulk and rhizosphere soils of a flooded rice paddy. Frontiers in microbiology 2015, 6, 639. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Conrad, R.; Lu, Y. Responses of methanogen mcrA genes and their transcripts to an alternate dry/wet cycle of paddy field soil. Applied and Environmental Microbiology 2012, 78, 445–454. [Google Scholar] [CrossRef]
- Aulakh, M.S.; Wassmann, R.; Bueno, C.; Rennenberg, H. Impact of root exudates of different cultivars and plant development stages of rice (Oryza sativa L.) on methane production in a paddy soil. Plant and Soil 2001, 230, 77–86. [Google Scholar] [CrossRef]
- Denier van Der Gon, H.; Kropff, M.; Van Breemen, N.; Wassmann, R.; Lantin, R.; Aduna, E.; Corton, T.; Van Laar, H. Optimizing grain yields reduces CH4 emissions from rice paddy fields. Proceedings of the National Academy of Sciences 2002, 99, 12021–12024. [Google Scholar] [CrossRef]
- Inubushi, K.; Cheng, W.; Aonuma, S.; Hoque, M.; Kobayashi, K.; Miura, S.; Kim, H.Y.; Okada, M. Effects of free-air CO2 enrichment (FACE) on CH4 emission from a rice paddy field. Global Change Biology 2003, 9, 1458–1464. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Papen, H.; Rennenberg, H. Impact of gas transport through rice cultivars on methane emission from rice paddy fields. Plant, Cell & Environment 1997, 20, 1175–1183. [Google Scholar]
- Setyanto, P.; Makarim, A.; Fagi, A.; Wassmann, R.; Buendia, L. Crop management affecting methane emissions from irrigated and rainfed rice in Central Java (Indonesia). Nutrient cycling in agroecosystems 2000, 58, 85–93. [Google Scholar] [CrossRef]
- Wassmann, R.; Aulakh, M.S. The role of rice plants in regulating mechanisms of methane missions. Biology and Fertility of Soils 2000, 31, 20–29. [Google Scholar] [CrossRef]
- Alvarenga, P.; Mourinha, C.; Palma, P.; Cruz, N.; Rodrigues, S.M. Assessment of Soil Physicochemical Characteristics and As, Cu, Pb and Zn Contamination in Non-Active Mines at the Portuguese Sector of the Iberian Pyrite Belt. Environments 2022, 9, 105. [Google Scholar] [CrossRef]
- Luton, P.E.; Wayne, J.M.; Sharp, R.J.; Riley, P.W. The mcrA gene as an alternative to 16S rRNA in the phylogenetic analysis of methanogen populations in landfill. Microbiology 2002, 148, 3521–3530. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.J.; Costello, A.; Lidstrom, M.E.; Murrell, J.C. Evidence that participate methane monooxygenase and ammonia monooxygenase may be evolutionarily related. FEMS microbiology letters 1995, 132, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Costello, A.M.; Lidstrom, M.E. Molecular characterization of functional and phylogenetic genes from natural populations of methanotrophs in lake sediments. Applied and environmental microbiology 1999, 65, 5066–5074. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.; Kim, S.Y.; Kim, P.J. Effect of rice cultivar on CH4 emissions and productivity in Korean paddy soil. Field Crops Research 2013, 146, 16–24. [Google Scholar] [CrossRef]
- Kim, G.W.; Gutierrez-Suson, J.; Kim, P.J. Optimum N rate for grain yield coincides with minimum greenhouse gas intensity in flooded rice fields. Field Crops Research 2019, 237, 23–31. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).