Submitted:
09 September 2023
Posted:
13 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection
2.3. Data Extraction and Synthesis
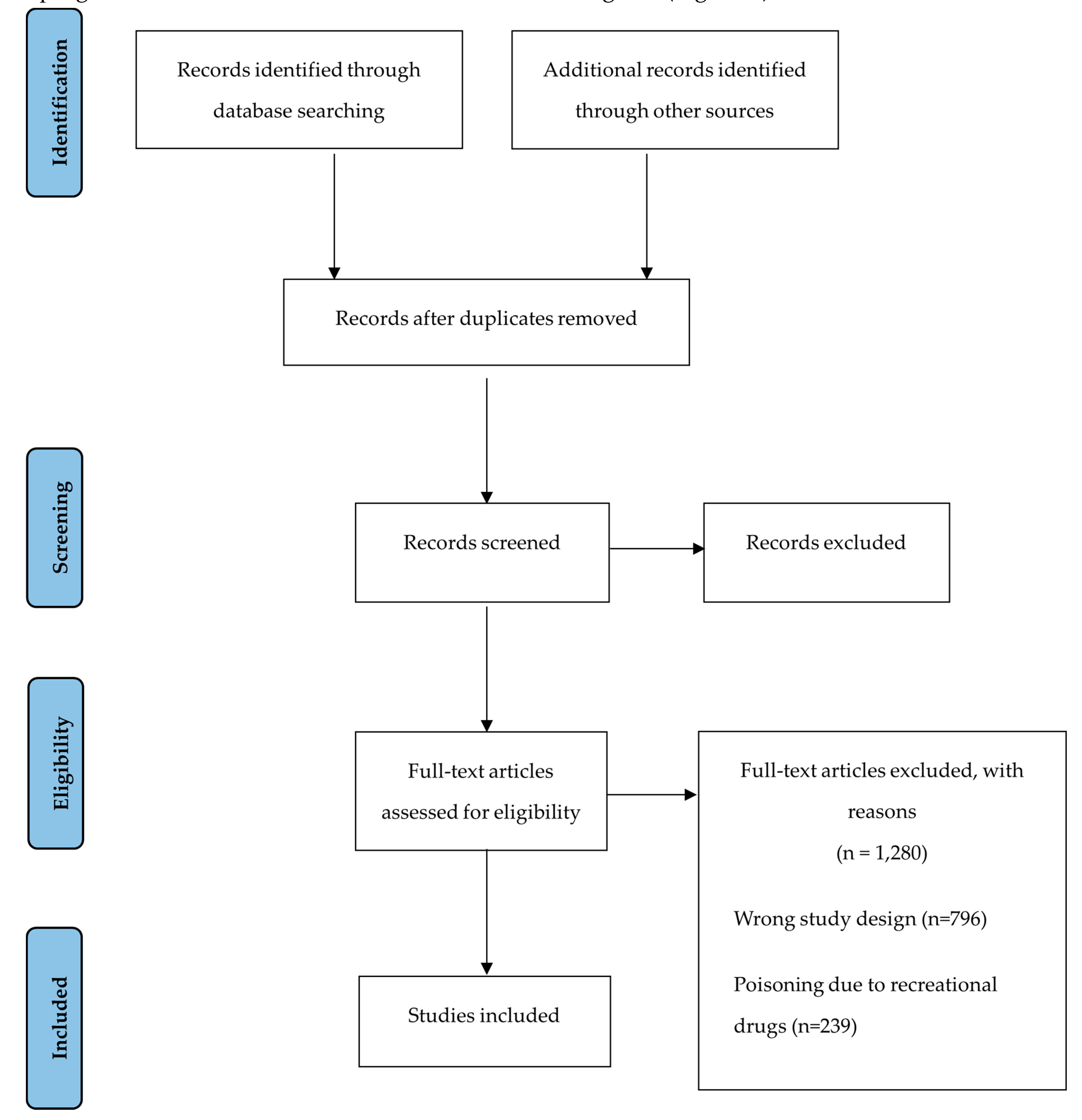
3. Results
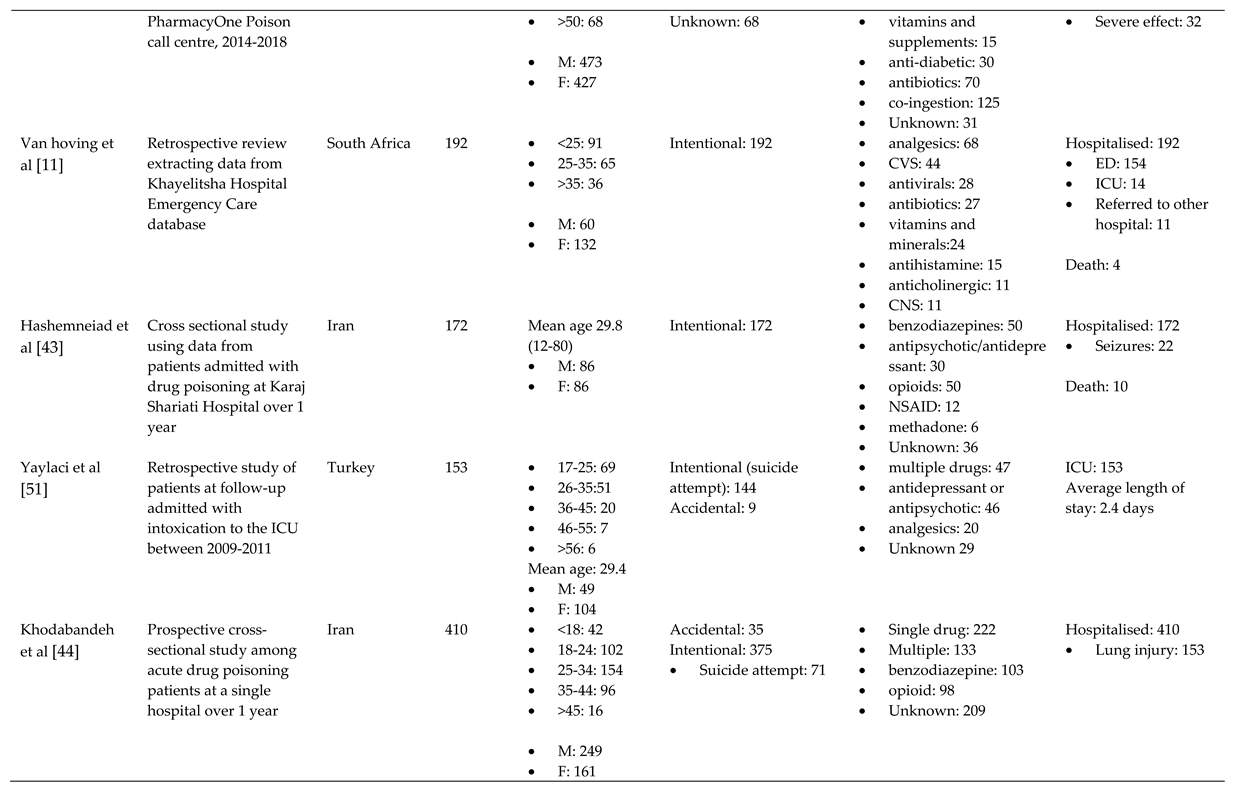
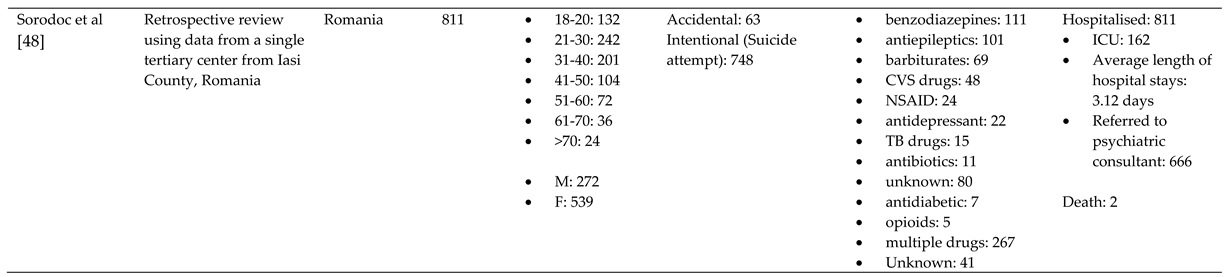
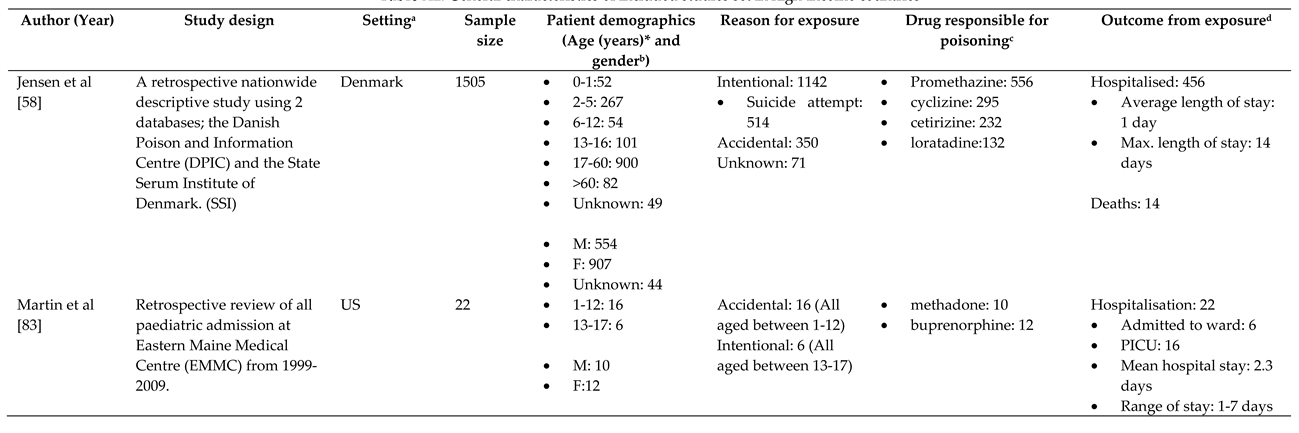
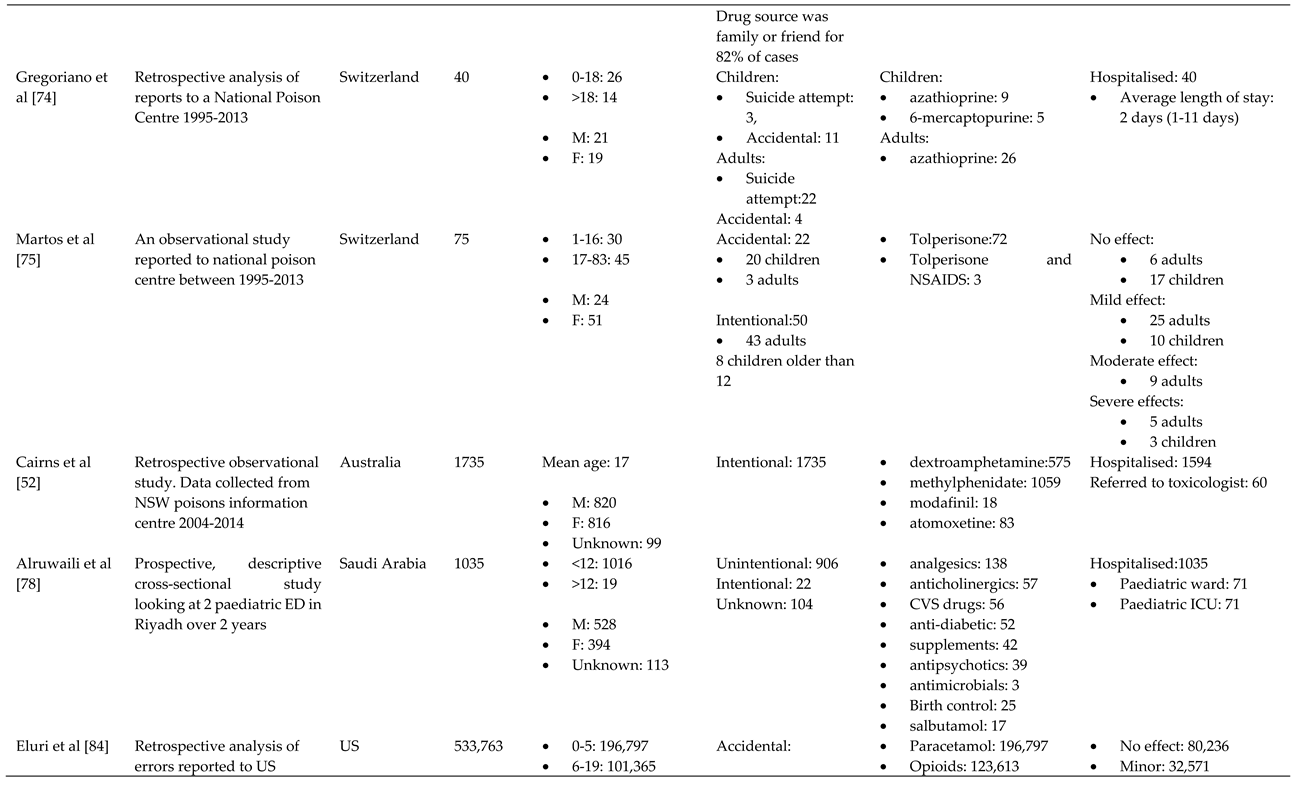
3.1. Characteristics of Included Studies
3.2. Overview
3.3. Trends
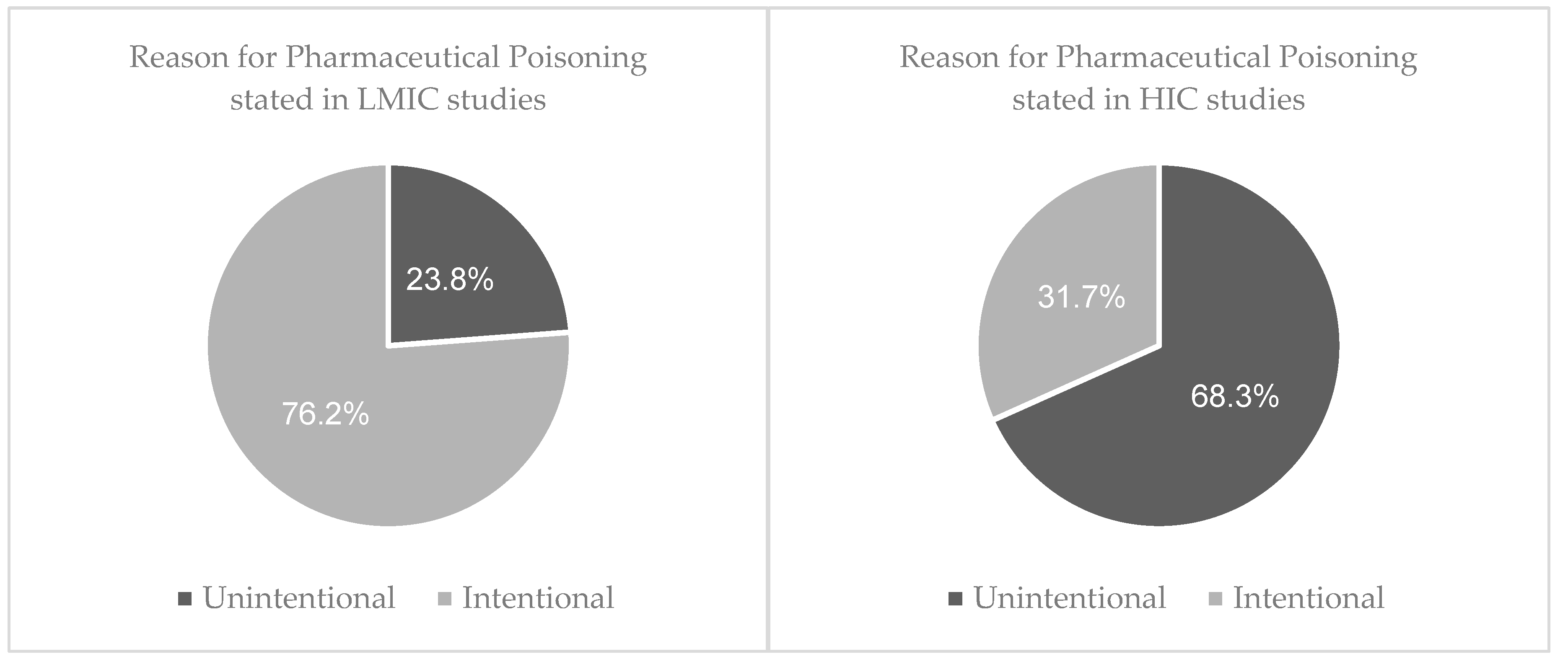
3.3.1. Reason behind toxic exposure
3.1.2. Types of pharmaceuticals responsible for poisoning
3.4. Outcome of pharmaceutical poisoning
4. Discussion
4.1. Reason Behind Toxic Exposure
4.2. Types of Pharmaceuticals Responsible for Poisoning
4.3. The Outcome of Pharmaceutical Poisoning
4.4. Future Research and Recommendations
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation list:
| ADR | Adverse drug reaction |
| AKI | Acute kidney injury |
| ATC | Anatomic Therapeutic Chemical |
| BB | Beta blocker |
| CCB | Calcium channel blocker |
| CVS | Cardiovascular |
| CNS | Central nervous system |
| DDD | Defined Daily Dose |
| ED | Emergency departments |
| ENT | Ear, nose, and throat |
| GI | Gastrointestinal |
| HICs | High-income countries |
| ICU | Intensive care unit |
| LMICs | Low-middle-income countries |
| MI | Myocardial infarction |
| MALA | Metformin associated lactic acidosis |
| NSAID | Non-steroidal anti-inflammatory |
| OTC | Over the counter |
| POU | Pyrexia of unknown origin |
| PSS | Poisoning Severity Score |
| SSRI | Selective serotonin reuptake inhibitor |
| TCA | Tricyclic antidepressant |
| TB | Tuberculosis |
| UK | United Kingdom |
| US | United States |
Appendix A. Search strategy
| 1. | Poison* .mp. |
| 2. | toxic.mp. |
| 3. | overdose.mp or intoxication/ |
| 4. | excessive.mp. |
| 5. | substance abuse/ |
| 6. | drug misuse/ |
| 7. | 1 or 2 or 3 or 4 or 5 or 6 |
| 8. | pharmaceutical.mp. |
| 9. | medicine/ |
| 10. | drug/ |
| 11. | opioid.mp. |
| 12. | 8 or 9 or 10 or 11 |
| 13. | 7 and 12 |
| 14. | limit 13 to (English and yr=2011-2020) |
Appendix B
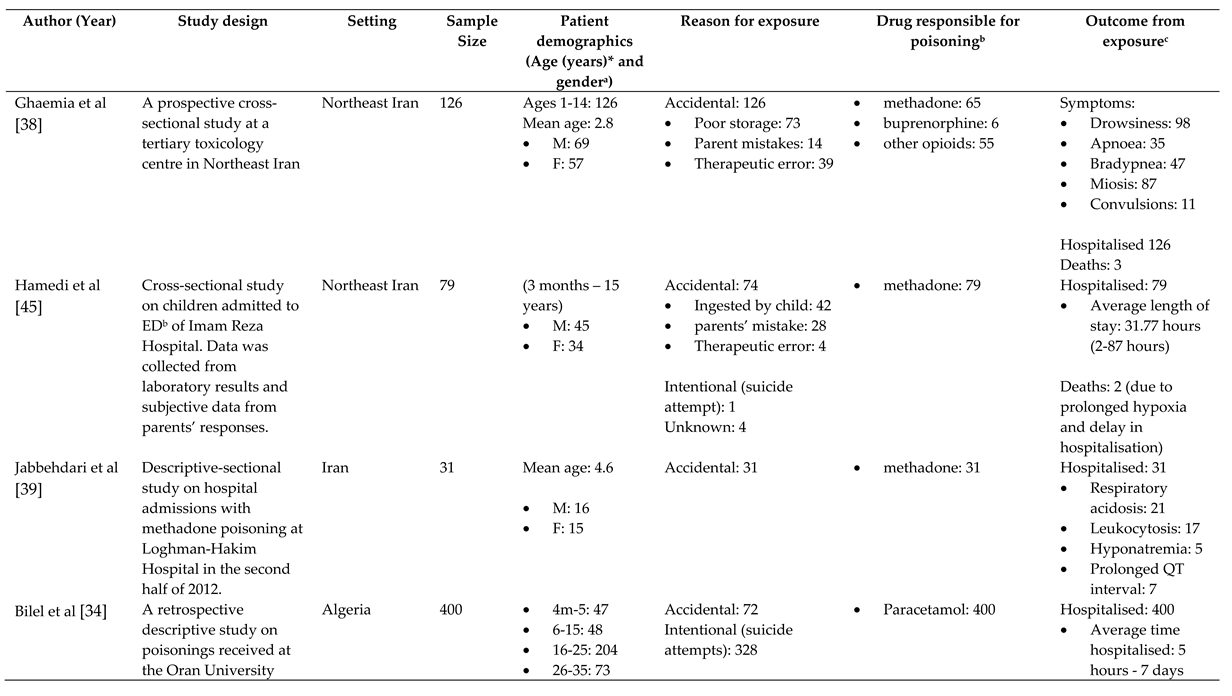
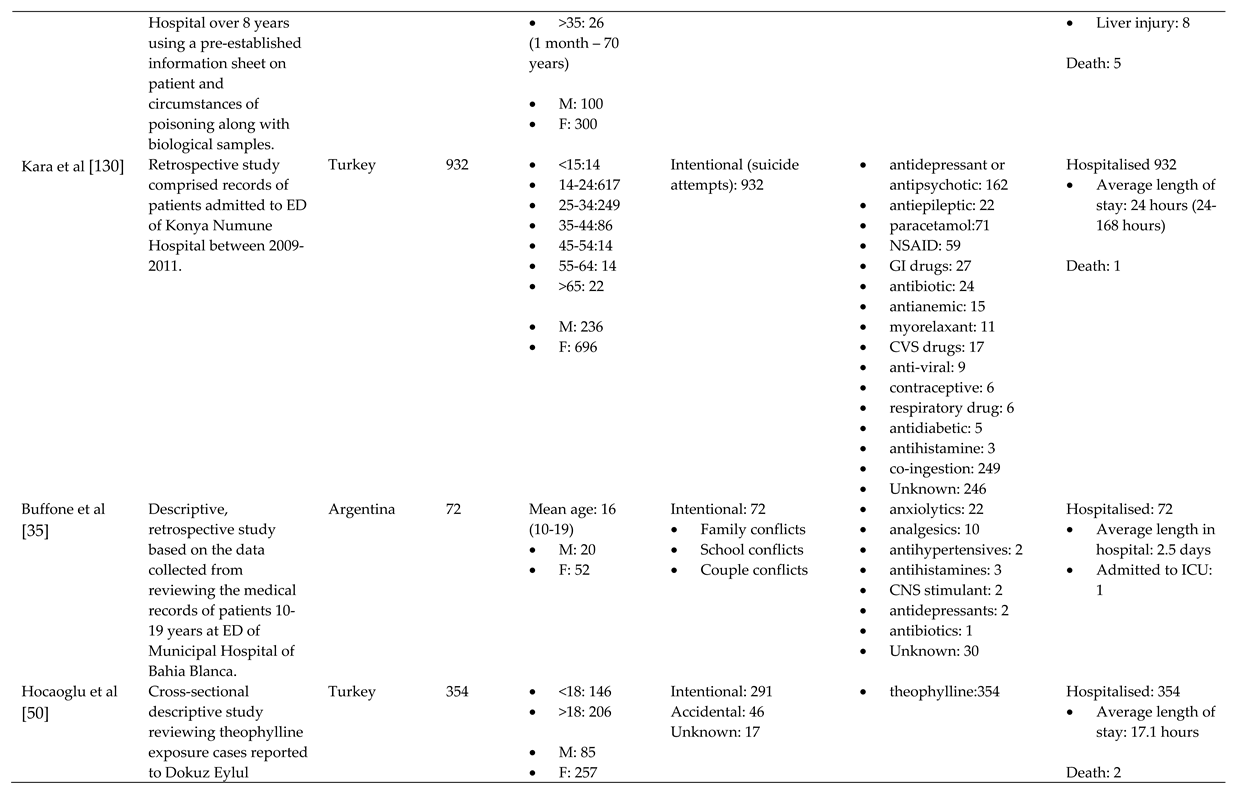
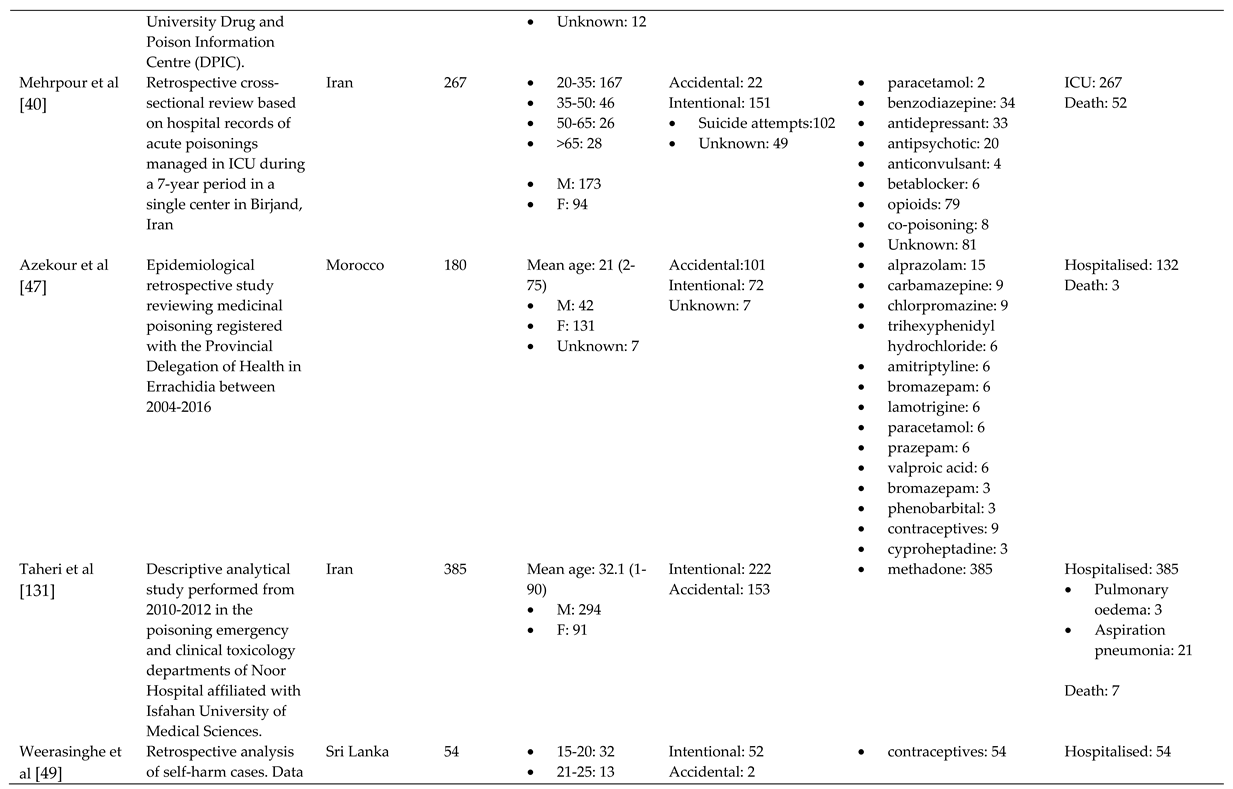
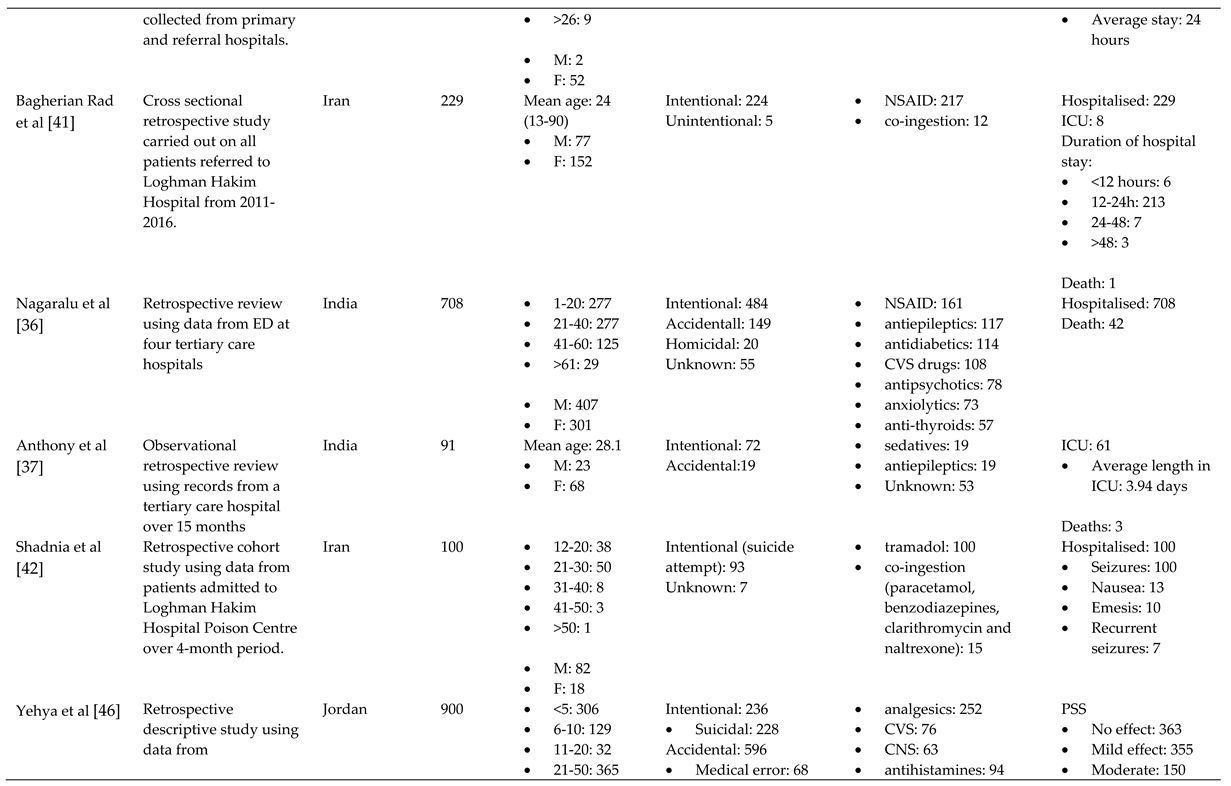
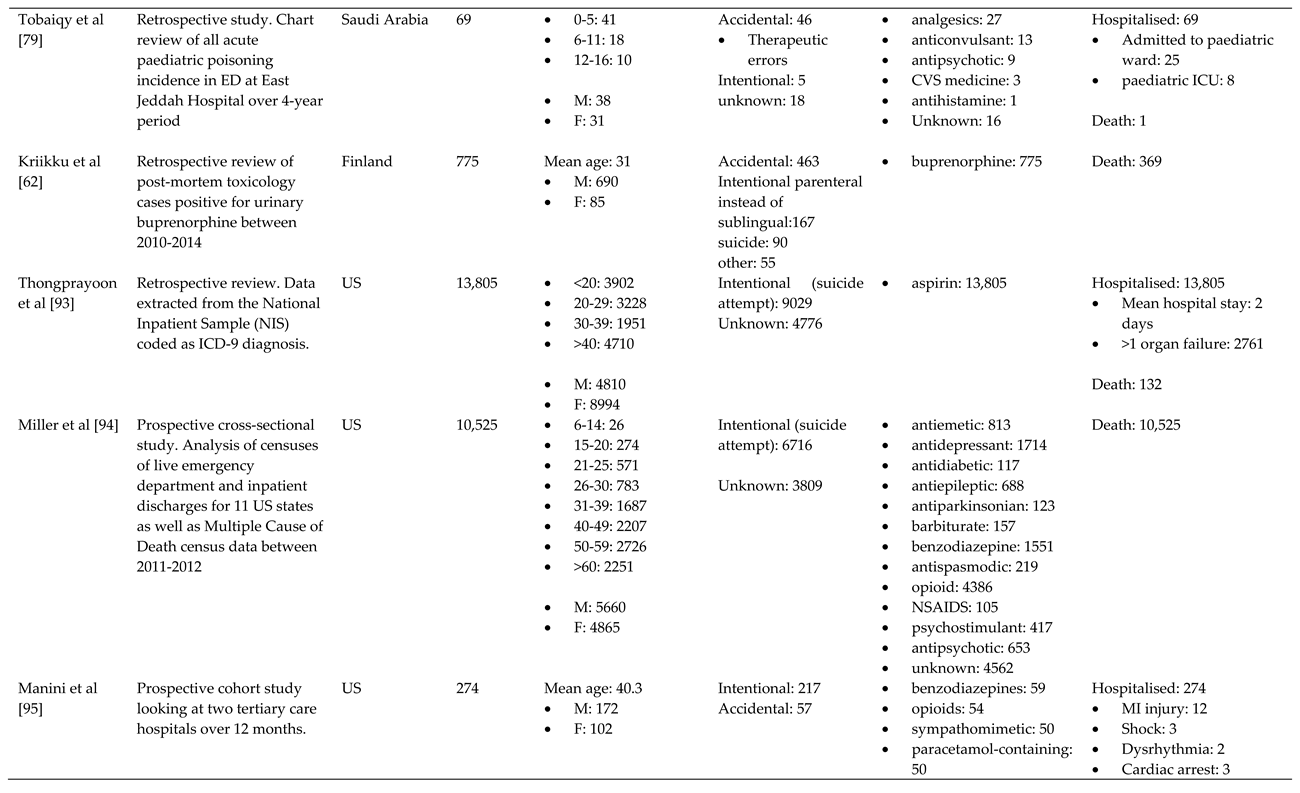
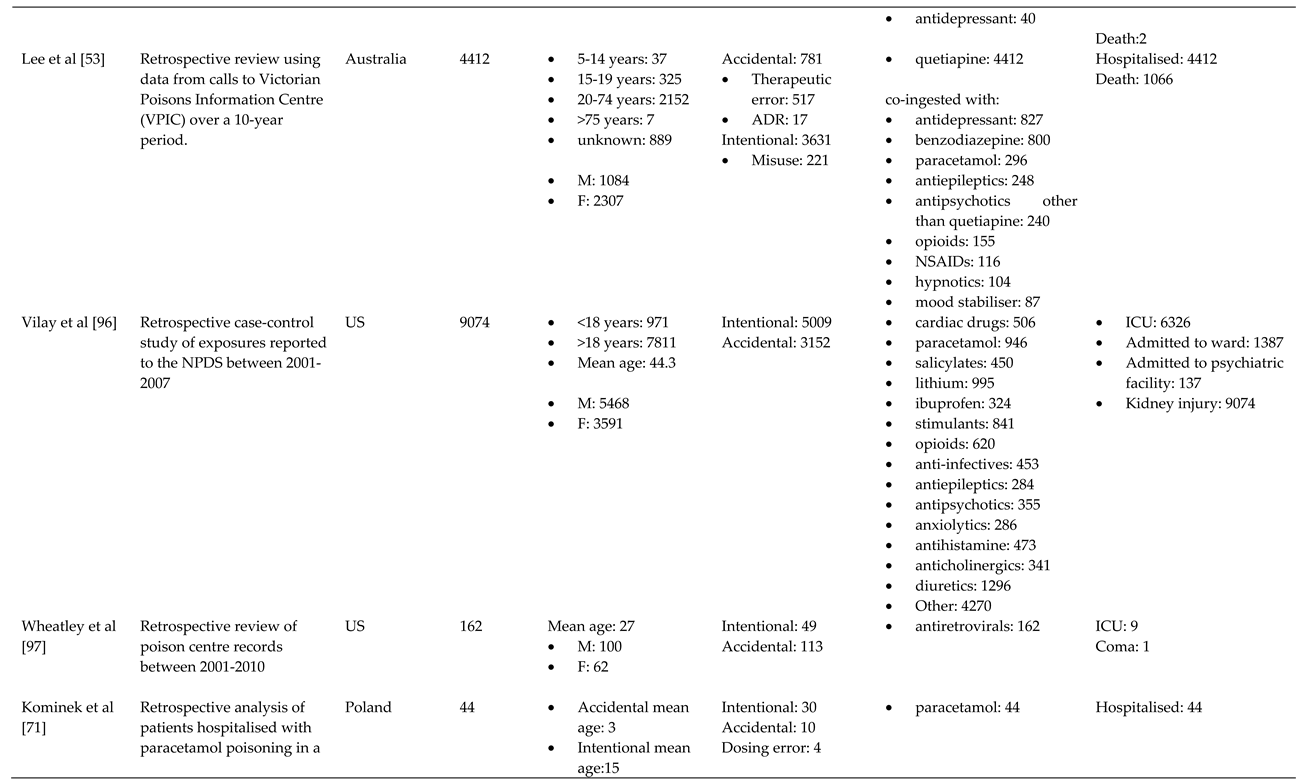
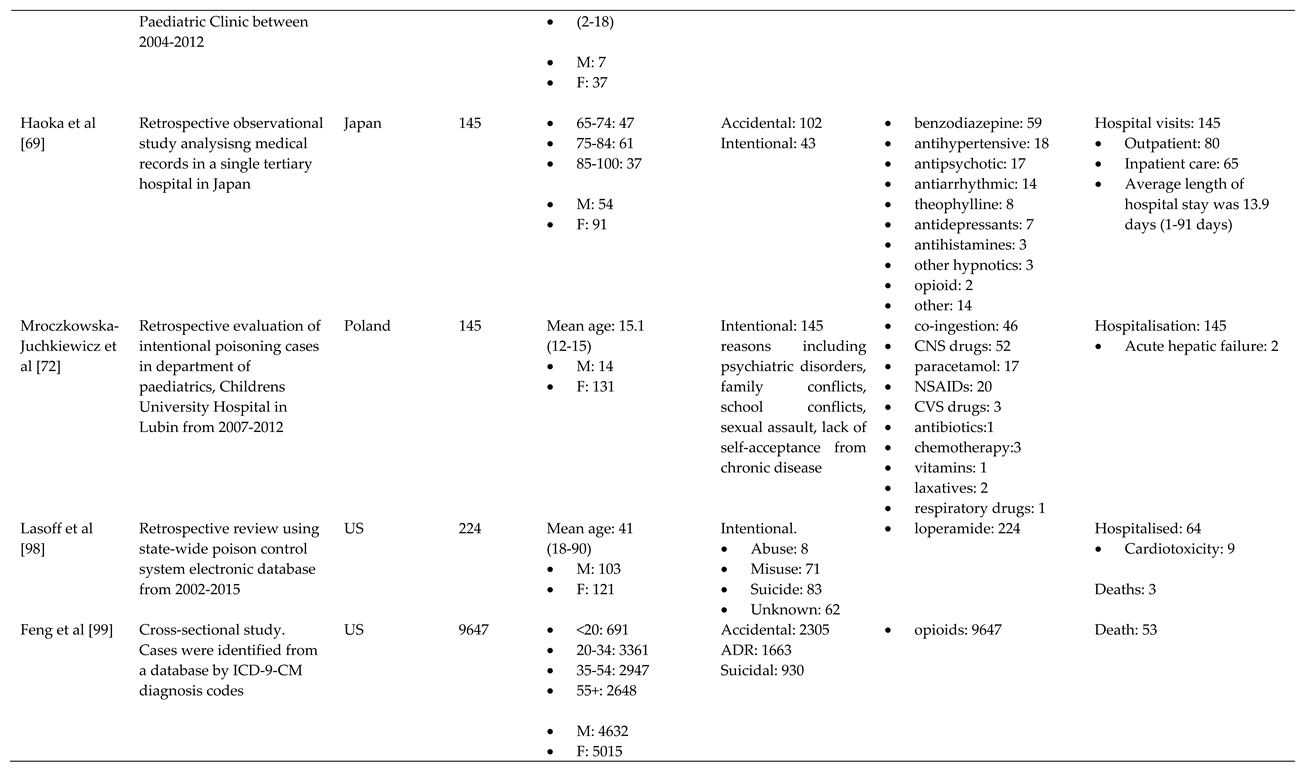
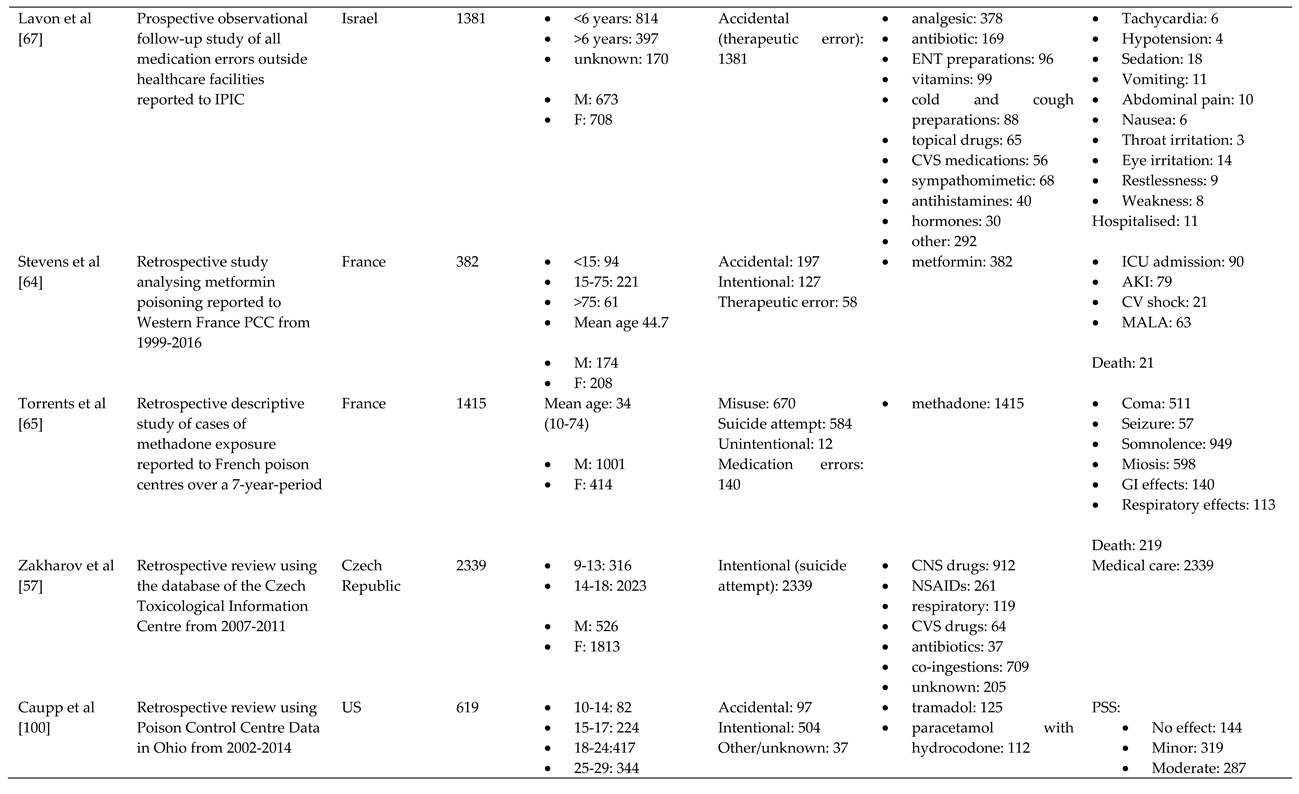
References
- Alwan, I.A.; Awadh, A.I.; Tangiisuran, B.; Khan, H.R.M.; Yahaya, N.; Majid, M.I. Pharmaceuticals Poisoning: Reported by the National Poison Centre in Malaysia between 2010 and 2015. J. Pharm. Bioallied Sci. 2020, 12, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Darke, S.; Mattick, R.P.; Degenhardt, L. The ratio of non-fatal to fatal heroin overdose. Addiction 2003, 98, 1169–1171. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.S.; Sampson, L.; Cerdá, M.; Galea, S. Worldwide Prevalence and Trends in Unintentional Drug Overdose: A Systematic Review of the Literature. Am. J. Public Health 2015, 105, e29–e49. [Google Scholar] [CrossRef] [PubMed]
- Thanacoody, R.; Anderson, M. Epidemiology of poisoning. Medicine 2020, 48, 153–155. [Google Scholar] [CrossRef]
- Okumura, Y.; Shimizu, S.; Ishikawa, K.B.; Matsuda, S.; Fushimi, K.; Ito, H. Comparison of emergency hospital admissions for drug poisoning and major diseases: a retrospective observational study using a nationwide administrative discharge database. BMJ Open 2012, 2, e001857. [Google Scholar] [CrossRef]
- Mintegi, S.; Esparza, M.J.; González, J.C.; Rubio, B.; Sánchez, F.; Vila, J.J.; Yagüe, F.; Benítez, M.T. Recommendations for the prevention of poisoning. Anales de Pediatría (English Edition) 2015, 83, 440.e441–440.e445. [Google Scholar] [CrossRef]
- Assar, S.; Hatami, S.; Lak, E.; Pipelzadeh, M.; Joorabian, M. Acute poisoning in children. Pak J Med Sci 2009, 25, 51–54. [Google Scholar]
- Kent, D.A. Out of Sight and Locked Up Tight: Pediatric Pharmaceutical Poisoning. BC Medical Journal 2013, 55, 33. [Google Scholar]
- Kurt, M.; Akdeniz, M.; Kavukcu, E. Assessment of Comorbidity and Use of Prescription and Nonprescription Drugs in Patients Above 65 Years Attending Family Medicine Outpatient Clinics. Gerontology & geriatric medicine 2019, 5, 2333721419874274–2333721419874274. [Google Scholar] [CrossRef]
- Duerden, M. What is the place for monitored dosage systems? Drug Ther. Bull. 2018, 56, 102. [Google Scholar] [CrossRef]
- van Hoving, D.J.; Hunter, L.D.; Gerber, R.E.J.; Lategan, H.J.; Marks, C.J. The burden of intentional self-poisoning on a district-level public Hospital in Cape Town, South Africa. Afr J Emerg Med 2018, 8, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Hedegaard, H.; Miniño, A.M.; Spencer, M.R.; Warner, M. Drug Overdose Deaths in the United States, 1999–2020. 2021, NCHS Data Brief, no 428. [CrossRef]
- Rehm, J.; Shield, K.D. Global Burden of Disease and the Impact of Mental and Addictive Disorders. Curr Psychiatry Rep 2019, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Mental Health ATLAS 2017. 2018, 68.
- Mak, K.K.; Ho, C.S.H.; Zhang, M.W.B.; Day, J.R.; Ho, R.C.M. Characteristics of overdose and non-overdose suicide attempts in a multi-ethnic Asian society. Asian J. Psychiatr. 2013, 6, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, J.; Smyth, R.S. 23 - Suicide and self-harm. In Companion to Psychiatric Studies (Eighth Edition), Johnstone, E.C., Owens, D.C., Lawrie, S.M., McIntosh, A.M., Sharpe, M., Eds.; Churchill Livingstone: St. Louis, 2010; pp. 693–713. [Google Scholar]
- Sharareh, N.; Sabounchi, S.S.; McFarland, M.; Hess, R. Evidence of Modeling Impact in Development of Policies for Controlling the Opioid Epidemic and Improving Public Health: A Scoping Review. Substance abuse : research and treatment 2019, 13, 1178221819866211–1178221819866211. [Google Scholar] [CrossRef]
- Keen, C.; Kinner, S.A.; Young, J.T.; Snow, K.; Zhao, B.; Gan, W.; Slaunwhite, A.K. Periods of altered risk for non-fatal drug overdose: a self-controlled case series. The Lancet Public Health 2021, 6, e249–e259. [Google Scholar] [CrossRef]
- Martin, J.; Cunliffe, J.; Décary-Hétu, D.; Aldridge, J. Effect of restricting the legal supply of prescription opioids on buying through online illicit marketplaces: interrupted time series analysis. BMJ 2018, 361, k2270. [Google Scholar] [CrossRef]
- Sanyal, C. Economic burden of opioid crisis and the role of pharmacist-led interventions. J. Am. Pharm. Assoc. (2003) 2021, 61, e70–e74. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. Drug-related deaths and mortality in Europe: update from the EMCDDA expert network; 2021.
- Dayasiri, K.; Jayamanne, S.F.; Jayasinghe, C.Y. Accidental and Deliberate Self-Poisoning with Medications and Medication Errors among Children in Rural Sri Lanka. Emerg. Med. Int. 2020, 2020 (no pagination). [CrossRef]
- Mariam, E.T. Global Epidemiology of Acute Poisoning with an Emphasis to Ethipia: Systematic Review International Journal of Pharma Sciences and Scientific Research 2016, 2, 161–171.
- Mittal, C.; Singh, S.; Kumar-M, P.; Varthya, S.B. Toxicoepidemiology of poisoning exhibited in Indian population from 2010 to 2020: a systematic review and meta-analysis. BMJ Open 2021, 11, e045182. [Google Scholar] [CrossRef]
- Peden M, O.K. , Ozanne-Smith J, et al. World Report on Child Injury Prevention Poisoning 2008, 6. [Google Scholar]
- Ndomondo-Sigonda, M.; Miot, J.; Naidoo, S.; Dodoo, A.; Kaale, E. Medicines Regulation in Africa: Current State and Opportunities. Pharmaceut. Med. 2017, 31, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Sithole, T.; Salek, S.; Mahlangu, G.; Walker, S. Comparison of the registration process of the medicines control authority of Zimbabwe with Australia, Canada, Singapore, and Switzerland: benchmarking best practices. Expert Rev. Clin. Pharmacol. 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Vu, H.; Xie, Z.; Chen, W.; Tang, S. Systematic review on irrational use of medicines in China and Vietnam. PLoS One, 2015; 10 (no pagination). [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O'Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Nada Hamadeh, C.V.R.a.E.M. New World Bank Country Classifications by Income Level: 2021-2022. Available online: https://blogs.worldbank.org/opendata/new-world-bank-country-classifications-income-level-2021-2022 (accessed on 31/12/21).
- World Health Organisation. Anatomical Therapeutic Chemical (ATC) Classidication. Available online: https://www.who.int/tools/atc-ddd-toolkit/atc-classification (accessed on 08/12/21).
- Cairns, R.; Buckley, N.A. The Poisoning Severity Score: If It Did Not Exist, We Would Have To Invent It. Journal of medical toxicology : official journal of the American College of Medical Toxicology 2017, 13, 131–134. [Google Scholar] [CrossRef] [PubMed]
- World Health Organsiation. Recognizing adolescence. Available online: https://apps.who.int/adolescent/second-decade/section2/page1/recognizing-adolescence.html (accessed on 03/01/2022).
- Bilel, C.; Zergui, A.; Rahmani, C.; Belmessabih, M.; Rezk-Kallah, H. Acute paracetamol poisonings received at the Oran University Hospital. Toxicology reports 2020, 7, 1172–1177. [Google Scholar] [CrossRef]
- Buffone, I.; Dejter, M.; Fortunatti, E.; García Elliot, F.; Irazabal, C.; Marlia, R.; Mujica, D.; Parrou, M.; Romano, M.; Speciale, G.; et al. Characterization of drug poisoning among adolescents seen at the municipal hospital of Bahía Blanca, Province of Buenos Aires, Argentina. Arch Argent Pediatr 2018, 116, 275–282. [Google Scholar] [CrossRef]
- Nagaraju, K.; Ganapathy, R.S. Pattern of pharmaceutical drug poisoning in south indian tertiary care hospitals. International Research Journal of Pharmacy 2016, 7, 44–47. [Google Scholar] [CrossRef]
- Anthony, L.; Kulkarni, C. Patterns of poisoning and drug overdosage and their outcome among in-patients admitted to the emergency medicine department of a tertiary care hospital. Indian J Crit Care Med 2012, 16, 130–135. [Google Scholar] [CrossRef]
- Ghaemi, N.; Alikhani, S.; Bagheri, S.; Sezavar, M. A Cross Sectional Study of Opioid Poisoning in Children at a Tertiary Center. Asia Pacific Journal of Medical Toxicology 2016, 5, 115–118. [Google Scholar] [CrossRef]
- Jabbehdari, S.; Farnaghi, F.; Shariatmadari, S.F.; Jafari, N.; Mehregan, F.-F.; Karimzadeh, P. Accidental children poisoning with methadone: an Iranian pediatric sectional study. Iran J Child Neurol 2013, 7, 32–34. [Google Scholar]
- Mehrpour, O.; Akbari, A.; Jahani, F.; Amirabadizadeh, A.; Allahyari, E.; Mansouri, B.; Ng, P.C. Epidemiological and clinical profiles of acute poisoning in patients admitted to the intensive care unit in eastern Iran (2010 to 2017). BMC Emergency Medicine 2018, 18, 30. [Google Scholar] [CrossRef] [PubMed]
- Bagherian Rad, N.; Rahimi, M. Pattern of NSAID Poisoning in a Referral Poisoning Center of Iran: Solutions to Reduce the Suicide. Iran J Pharm Res 2019, 18, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Shadnia, S.; Brent, J.; Mousavi-Fatemi, K.; Hafezi, P.; Soltaninejad, K. Recurrent Seizures in Tramadol Intoxication: Implications for Therapy Based on 100 Patients. Basic and Clinical Pharmacology and Toxicology 2012, 111, 133–136. [Google Scholar] [CrossRef]
- Hashmnejad, M.; Fatehi, R. Epidemiological Study of Poisoning in Patients of Karaj Shariati Hospital in 2011 to 2012. International Journal of Medical Toxicology and Forensic Medicine 2014, 4, 17–22. [Google Scholar] [CrossRef]
- Khodabandeh, F.; Agin, K. ASSESSMENT OF ASPIRATION-INDUCED LUNG INJURIES AMONG ACUTE DRUG POISONING PATIENTS; LOGHMAN HAKIM HOSPITAL, POISONING CENTER. INTERNATIONAL JOURNAL OF MEDICAL TOXICOLOGY AND FORENSIC MEDICINE 2016, 6, 209–216. [Google Scholar]
- Hamedi, A.; Ghahremani, S.; Nakhaei, A.A.; Balali, M.R.; Ghahremani, S. A Cross Sectional Study on Pediatric Methadone Poisoning in Northeast of Iran. Asia Pacific Journal Of Medical Toxicology 2016, 5, 75–78. [Google Scholar]
- Yehya, A.; Albals, D.; Issa, R.; Fawadleh, A. Retrospective assessment of acute poisoning incidents by pharmaceutical agents in Jordan: Data from Pharmacy OneTM Poison Call Center, 2014 to 2018-Part II. Pharmacology Research and Perspectives, 2020; 8 (no pagination). [Google Scholar] [CrossRef]
- Azekour, K.; Belamalem, S.; Soulaymani, A.; El Houate, B.; El Bouhali, B. Epidemiological Profile of Drug Overdose Reported in South-East Morocco from 2004 to 2016. Drugs Real World Outcomes 2019, 6, 11–17. [Google Scholar] [CrossRef]
- Sorodoc, V.; Jaba, I.M.; Lionte, C.; Mungiu, O.C.; Sorodoc, L. Epidemiology of acute drug poisoning in a tertiary center from Iasi County, Romania. Hum Exp Toxicol 2011, 30, 1896–1903. [Google Scholar] [CrossRef]
- Weerasinghe, M.; Konradsen, F.; Eddleston, M.; Pearson, M.; Agampodi, T.; Storm, F.; Agampodi, S. Overdose of oral contraceptive pills as a means of intentional self-poisoning amongst young women in Sri Lanka: considerations for family planning. J Fam Plann Reprod Health Care 2017, 43, 147–150. [Google Scholar] [CrossRef]
- Hocaoğlu, N.; Yıldıztepe, E.; Bayram, B.; Aydın, B.; Tunçok, Y.; Kalkan, Ş. Demographic and Clinical Characteristics of Theophylline Exposures between 1993 and 2011. Balkan medical journal 2014, 31, 322–327. [Google Scholar] [CrossRef]
- Yaylaci, S.; Genc, A.B.; Demir, M.V.; Cinemre, H.; Tamer, A. Retrospective evaluation of patients at follow-up with acute poisoning in Intensive Care Unit. Niger J Clin Pract 2016, 19, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Cairns, R.; Daniels, B.; Wood, D.A.; Brett, J. ADHD medication overdose and misuse: The NSW poisons information centre experience, 2004-2014. Medical Journal of Australia 2016, 204, 154.e151–154.e154. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Pilgrim, J.; Gerostamoulos, D.; Robinson, J.; Wong, A. Increasing rates of quetiapine overdose, misuse, and mortality in Victoria, Australia. Drug and Alcohol Dependence 2018, 187, 95–99. [Google Scholar] [CrossRef] [PubMed]
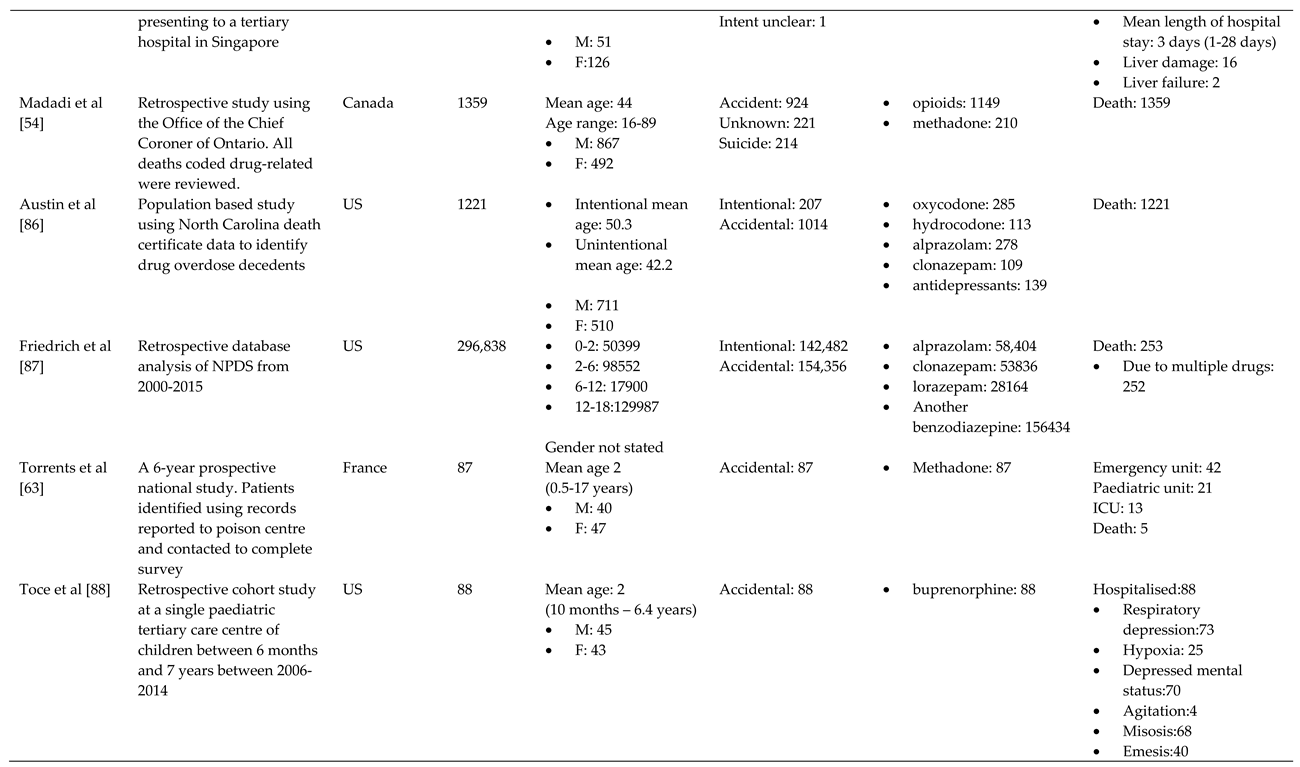
- Madadi, P.; Hildebrandt, D.; Lauwers, A.E.; Koren, G. Characteristics of Opioid-Users Whose Death Was Related to Opioid-Toxicity: A Population-Based Study in Ontario, Canada. PLoS One, 2013; 8 (no pagination). [Google Scholar] [CrossRef]
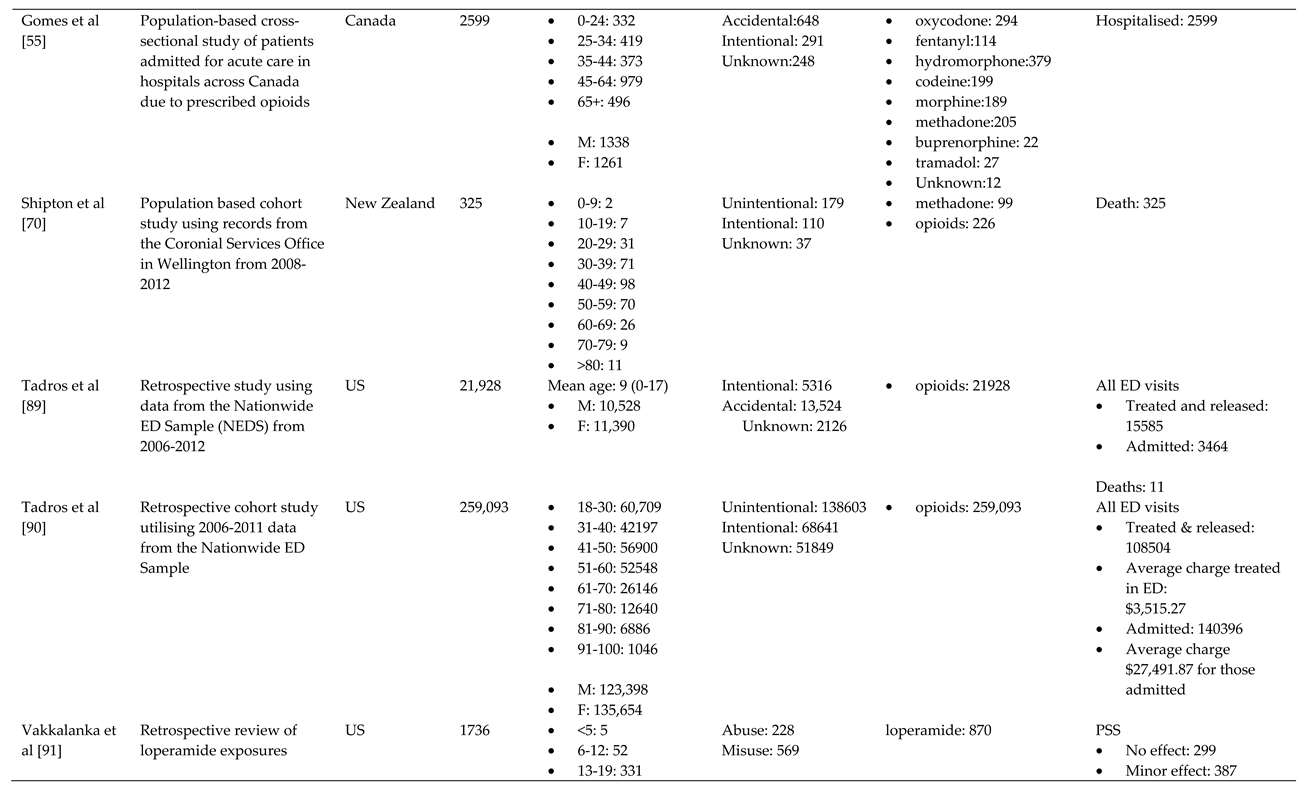
- Gomes, T.; Khuu, W.; Craiovan, D.; Martins, D.; Hunt, J.; Lee, K.; Tadrous, M.; Mamdani, M.; Paterson, J.; Juurlink, D. Comparing the contribution of prescribed opioids to opioid-related hospitalizations across Canada: A multi-jurisdictional cross-sectional study. Drug and Alcohol Dependence 2018, 191. [Google Scholar] [CrossRef]
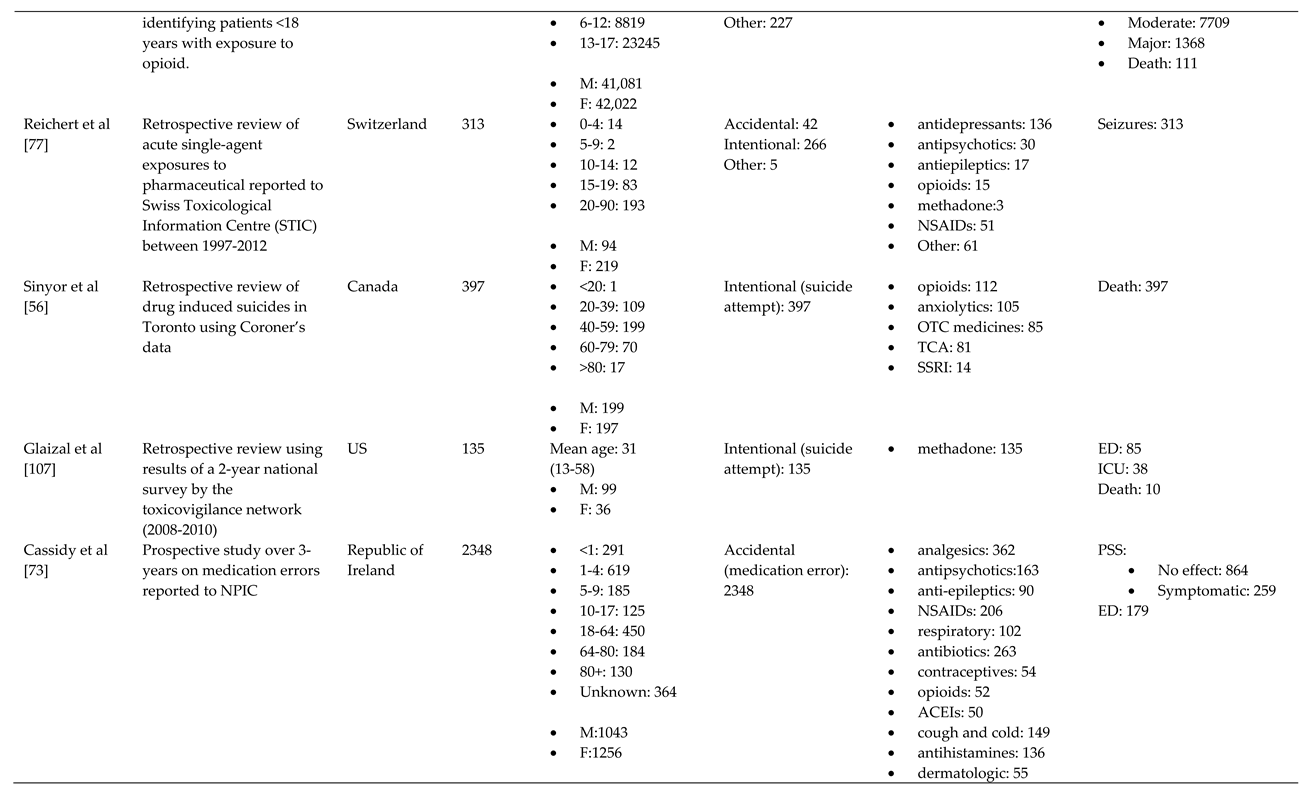
- Sinyor, M.; Howlett, A.; Cheung, A.H.; Schaffer, A. Substances used in completed suicide by overdose in Toronto: An observational study of coroner's data. Canadian Journal of Psychiatry 2012, 57, 184–191. [Google Scholar] [CrossRef]
- Zakharov, S.; Navratil, T.; Pelclova, D. Non-fatal suicidal self-poisonings in children and adolescents over a 5-year period (2007-2011). Basic Clin. Pharmacol. Toxicol. 2013, 112, 425–430. [Google Scholar] [CrossRef]
- Jensen, L.L.; Rømsing, J.; Dalhoff, K. A Danish Survey of Antihistamine Use and Poisoning Patterns. Basic Clin Pharmacol Toxicol 2017, 120, 64–70. [Google Scholar] [CrossRef]
- Christensen, M.B.; Petersen, K.M.; Bøgevig, S.; Al-Gibouri, S.; Jimenez-Solem, E.; Dalhoff, K.P.; Petersen, T.S.; Andersen, J.T. Outcomes following calcium channel blocker exposures reported to a poison information center. BMC Pharmacol Toxicol 2018, 19, 78. [Google Scholar] [CrossRef]
- Christensen, A.P.; Boegevig, S.; Christensen, M.B.; Petersen, K.M.; Dalhoff, K.P.; Petersen, T.S. Overdoses with Aripiprazole: Signs, Symptoms and Outcome in 239 Exposures Reported to the Danish Poison Information Centre. Basic Clin Pharmacol Toxicol 2018, 122, 293–298. [Google Scholar] [CrossRef]
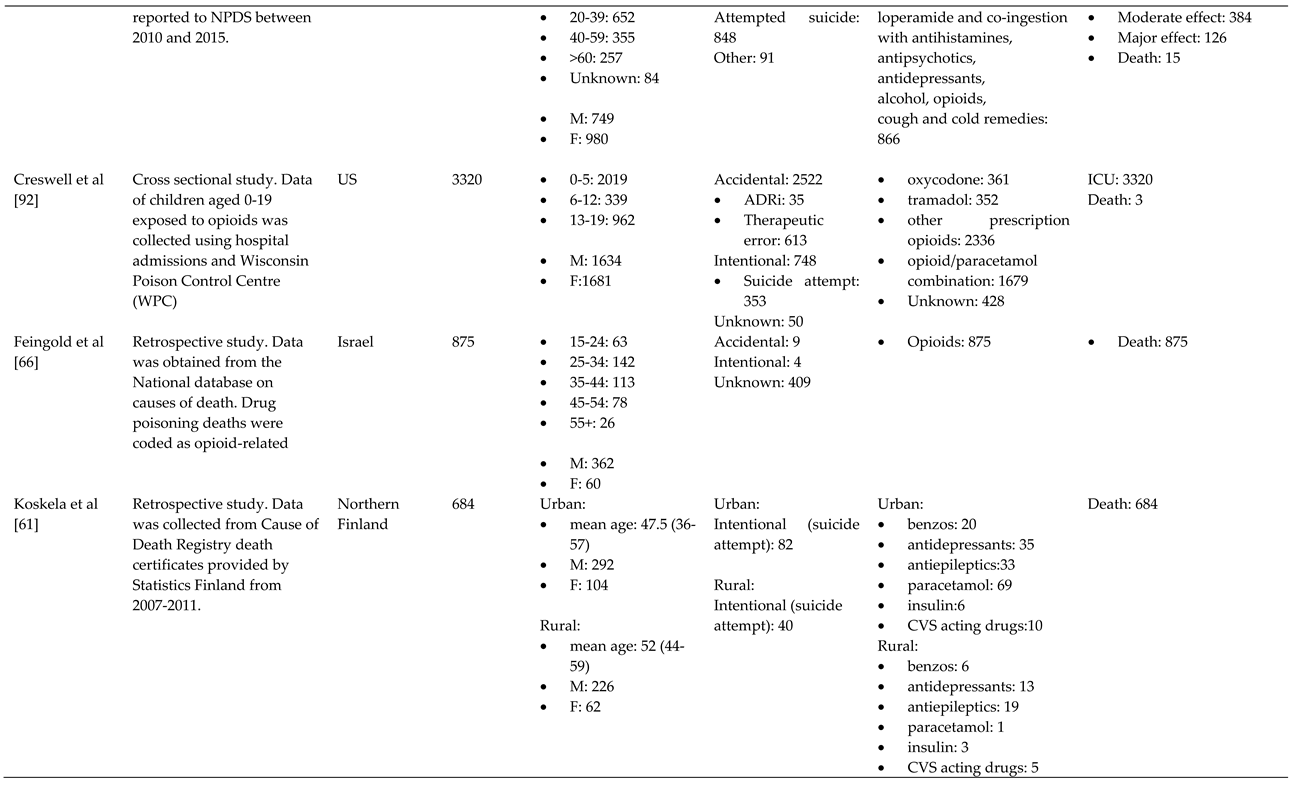
- Koskela, L.; Raatiniemi, L.; Bakke, H.K.; Ala-Kokko, T.; Liisanantti, J. Fatal poisonings in Northern Finland: causes, incidence, and rural-urban differences. Scand. J. Trauma Resusc. Emerg. Med. 2017, 25, 90. [Google Scholar] [CrossRef]
- Kriikku, P.; Hakkinen, M.; Ojanpera, I. High buprenorphine-related mortality is persistent in Finland. Forensic Science International 2018, 291, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Torrents, R.; Picot, C.; Glaizal, M.; Courne, M.A.; Schmitt, C.; Richard, N.; Simon, N.; Cardona, F.; De Haro, L. Child poisonings with methadone in France: A 6-year prospective national survey since the availability of capsules in 2008. Clin. Toxicol. 2015, 53, 819–822. [Google Scholar] [CrossRef]
- Stevens, A.; Hamel, J.F.; Toure, A.; Hadjadj, S.; Boels, D. Metformin overdose: A serious iatrogenic complication-Western France Poison Control Centre Data Analysis. Basic Clin Pharmacol Toxicol 2019, 125, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Torrents, R.; Glaizal, M.; Sinno-Tellier, S.; Richard, N.; Nisse, P.; Vodovar, D.; Bloch, J.; Simon, N.; de Haro, L. Methadone poisonings: a seven-year retrospective study of the French poison center network focusing on suicide attempts vs. misuses. Fundamental and Clinical Pharmacology 2020, 34, 290–295. [Google Scholar] [CrossRef]
- Feingold, D.; Goldberger, N.; Haklai, Z.; Lev-Ran, S. Fatal Overdoses of Opioids in Israel 2005-2014. Eur. Addict. Res. 2017, 23, 276–283. [Google Scholar] [CrossRef]
- Lavon, O.; Ben-Zeev, A.; Bentur, Y. Medication errors outside healthcare facilities: a national poison centre perspective. Basic Clin Pharmacol Toxicol 2014, 114, 288–292. [Google Scholar] [CrossRef]
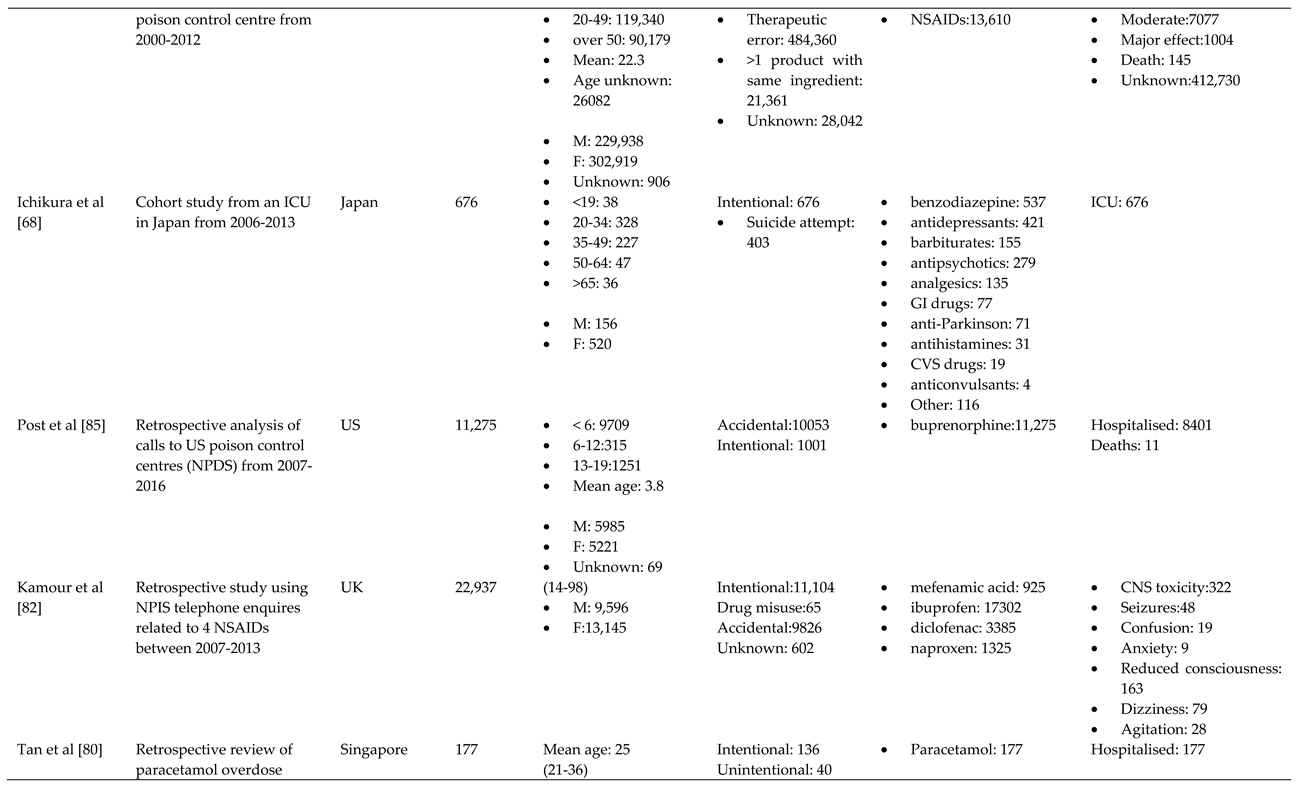
- Ichikura, K.; Okumura, Y.; Takeuchi, T. Associations of Adverse Clinical Course and Ingested Substances among Patients with Deliberate Drug Poisoning: A Cohort Study from an Intensive Care Unit in Japan. PLOS ONE 2016, 11, e0161996. [Google Scholar] [CrossRef]
- Haoka, T.; Sakata, N.; Okamoto, H.; Oshiro, A.; Shimizu, T.; Naito, Y.; Onishi, S.; Morishita, Y.; Nara, S. Intentional or unintentional drug poisoning in elderly people: retrospective observational study in a tertiary care hospital in Japan. Acute medicine & surgery 2019, 6, 252–258. [Google Scholar] [CrossRef]
- Shipton, E.E.; Shipton, A.J.; Williman, J.A.; Shipton, E.A. Deaths from Opioid Overdosing: Implications of Coroners' Inquest Reports 2008-2012 and Annual Rise in Opioid Prescription Rates: A Population-Based Cohort Study. Pain and Therapy 2017, 6, 203–215. [Google Scholar] [CrossRef]
- Kominek, K.; Pawłowska-Kamieniak, A.; Mroczkowska-Juchkiewicz, A.; Krawiec, P.; Pac-Kożuchowska, E. Intentional and accidental paracetamol poisoning in childhood - a retrospective analysis. Postepy Hig Med Dosw (Online) 2015, 69, 452–456. [Google Scholar] [CrossRef]
- Mroczkowska-Juchkiewicz, A.; Krawiec, P.; Pawłowska-Kamieniak, A.; Gołyska, D.; Kominek, K.; Pac-Kożuchowska, E. Intentional poisonings in urban and rural children - a 6-year retrospective single centre study. Ann. Agric. Environ. Med. 2016, 23, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, N.; Duggan, E.; Williams, D.J.P.; Tracey, J.A. The epidemiology and type of medication errors reported to the National Poisons Information Centre of Ireland. Clinical Toxicology 2011, 49, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Gregoriano, C.; Ceschi, A.; Rauber-Lüthy, C.; Kupferschmidt, H.; Banner, N.R.; Krähenbühl, S.; Taegtmeyer, A.B. Acute thiopurine overdose: analysis of reports to a National Poison Centre 1995-2013. PLoS One 2014, 9, e86390. [Google Scholar] [CrossRef] [PubMed]
- Martos, V.; Hofer, K.E.; Rauber-Lüthy, C.; Schenk-Jaeger, K.M.; Kupferschmidt, H.; Ceschi, A. Acute toxicity profile of tolperisone in overdose: Observational poison centre-based study. Clin. Toxicol. 2015, 53, 470–476. [Google Scholar] [CrossRef]
- Piotrowska, N.; Klukowska-Rötzler, J.; Lehmann, B.; Krummrey, G.; Haschke, M.; Exadaktylos, A.K.; Liakoni, E. Presentations Related to Acute Paracetamol Intoxication in an Urban Emergency Department in Switzerland. Emerg Med Int 2019, 2019, 3130843. [Google Scholar] [CrossRef]
- Reichert, C.; Reichert, P.; Monnet-Tschudi, F.; Kupferschmidt, H.; Ceschi, A.; Rauber-Luthy, C. Seizures after single-agent overdose with pharmaceutical drugs: Analysis of cases reported to a poison center. Clinical Toxicology 2014, 52, 629–634. [Google Scholar] [CrossRef]
- Alruwaili, N.D.; Halimeh, B.; Al-Omar, M.; Alhatali, B.; Sabie, II; Alsaqoub, M. An epidemiological snapshot of toxicological exposure in children 12 years of age and younger in Riyadh. Ann Saudi Med 2019, 39, 229–235. [Google Scholar] [CrossRef]
- Tobaiqy, M.; Asiri, B.A.; Sholan, A.H.; Alzahrani, Y.A.; Alkatheeri, A.A.; Mahha, A.M.; Alzahrani, S.S.; MacLure, K. Frequency and Management of Acute Poisoning Among Children Attending an Emergency Department in Saudi Arabia. Pharmacy (Basel) 2020, 8. [Google Scholar] [CrossRef]
- Tan, C.J.; Sklar, G.E. Characterisation and outcomes of adult patients with paracetamol overdose presenting to a tertiary hospital in Singapore. Singapore Med J 2017, 58, 695–702. [Google Scholar] [CrossRef]
- Lin, Y.R.; Liu, T.H.; Liu, T.A.; Chang, Y.J.; Chou, C.C.; Wu, H.P. Pharmaceutical poisoning exposure and outcome analysis in children admitted to the pediatric emergency department. Pediatr Neonatol 2011, 52, 11–17. [Google Scholar] [CrossRef]
- Kamour, A.; Crichton, S.; Cooper, G.; Lupton, D.J.; Eddleston, M.; Vale, J.A.; Thompson, J.P.; Thomas, S.H.L. Central nervous system toxicity of mefenamic acid overdose compared with other NSAIDs: an analysis of cases reported to the United Kingdom National Poisons Information Service. British journal of clinical pharmacology 2017, 83, 855–862. [Google Scholar] [CrossRef]
- Martin, T.C.; Rocque, M. Accidental and non-accidental ingestion of methadone and buprenorphine in childhood: A single center experience, 1999-2009. Current Drug Safety 2011, 6, 12–16. [Google Scholar] [CrossRef]
- Eluri, M.; Spiller, H.A.; Casavant, M.J.; Chounthirath, T.; Conner, K.A.; Smith, G.A. Analgesic-Related Medication Errors Reported to US Poison Control Centers. Pain Med. 2018, 19, 2357–2370. [Google Scholar] [CrossRef] [PubMed]
- Post, S.; Spiller, H.A.; Casavant, M.J.; Chounthirath, T.; Smith, G.A. Buprenorphine exposures among children and adolescents reported to us poison control centers. Pediatrics, 2018; 142 (no pagination). [Google Scholar] [CrossRef]
- Austin, A.E.; Proescholdbell, S.K.; Creppage, K.E.; Asbun, A. Characteristics of self-inflicted drug overdose deaths in North Carolina. Drug Alcohol Depend. 2017, 181, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, J.M.; Sun, C.; Geng, X.; Calello, D.P.; Gillam, M.; Medeiros, K.L.; Smith, M.; Ruck, B.; Mazer-Amirshahi, M. Child and adolescent benzodiazepine exposure and overdose in the United States: 16 years of poison center data. Clin. Toxicol. 2020, 58, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Toce, M.S.; Burns, M.M.; O'Donnell, K.A. Clinical effects of unintentional pediatric buprenorphine exposures: experience at a single tertiary care center. Clin. Toxicol. 2017, 55, 12–17. [Google Scholar] [CrossRef]
- Tadros, A.; Layman, S.M.; Davis, S.M.; Bozeman, R.; Davidov, D.M. Emergency department visits by pediatric patients for poisoning by prescription opioids. Am. J. Drug Alcohol Abuse 2016, 42, 550–555. [Google Scholar] [CrossRef]
- Tadros, A.; Layman, S.M.; Davis, S.M.; Davidov, D.M.; Cimino, S. Emergency Visits for Prescription Opioid Poisonings. J Emerg Med 2015, 49, 871–877. [Google Scholar] [CrossRef]
- Vakkalanka, J.P.; Charlton, N.P.; Holstege, C.P. Epidemiologic Trends in Loperamide Abuse and Misuse. Ann. Emerg. Med. 2017, 69, 73–78. [Google Scholar] [CrossRef]
- Creswell, P.D.; Gibson, C.; Theobald, J.; Meiman, J.G. Exposures to Opioids Among Wisconsin Children and Adolescents, 2002-2016. Wmj 2019, 118, 9–15. [Google Scholar]
- Thongprayoon, C.; Petnak, T.; Kaewput, W.; Mao, M.A.; Kovvuru, K.; Kanduri, S.R.; Boonpheng, B.; Bathini, T.; Vallabhajosyula, S.; Pivovarova, A.I.; et al. Hospitalizations for Acute Salicylate Intoxication in the United States. J Clin Med 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.R.; Swedler, D.I.; Lawrence, B.A.; Ali, B.; Rockett, I.R.H.; Carlson, N.N.; Leonardo, J. Incidence and Lethality of Suicidal Overdoses by Drug Class. JAMA network open 2020, 3, e200607–e200607. [Google Scholar] [CrossRef] [PubMed]
- Manini, A.F.; Nelson, L.S.; Stimmel, B.; Vlahov, D.; Hoffman, R.S. Incidence of adverse cardiovascular events in adults following drug overdose. Acad Emerg Med 2012, 19, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Vilay, A.; Wong, C.; Schrader, R.; Mercier, R.-C.; Seifert, S. Indicators for serious kidney complications associated with toxic exposures: An analysis of the National Poison Data System. Clinical toxicology (Philadelphia, Pa.) 2013, 51. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, M.A.; Shah, B.B.; Morgan, B.W.; Houry, D.; Kazzi, Z.N. Injury secondary to antiretroviral agents: retrospective analysis of a regional poison center database. The western journal of emergency medicine 2011, 12, 293–295. [Google Scholar]
- Lasoff, D.R.; Koh, C.H.; Corbett, B.; Minns, A.B.; Cantrell, F.L. Loperamide Trends in Abuse and Misuse Over 13 Years: 2002-2015. Pharmacotherapy 2017, 37, 249–253. [Google Scholar] [CrossRef]
- Feng, J.; Iser, J.; Yang, W. Medical encounters for opioid-related intoxications in Southern Nevada: Sociodemographic and clinical correlates. BMC Health Services Research 2016, 16. [Google Scholar] [CrossRef]
- Caupp, S.; Steffan, J.; Shi, J.; Wheeler, K.K.; Spiller, H.A.; Casavant, M.J.; Xiang, H. Opioid drug poisonings in Ohio adolescents and young adults, 2002-2014. Clin. Toxicol. (Phila.) 2018, 56, 765–772. [Google Scholar] [CrossRef]
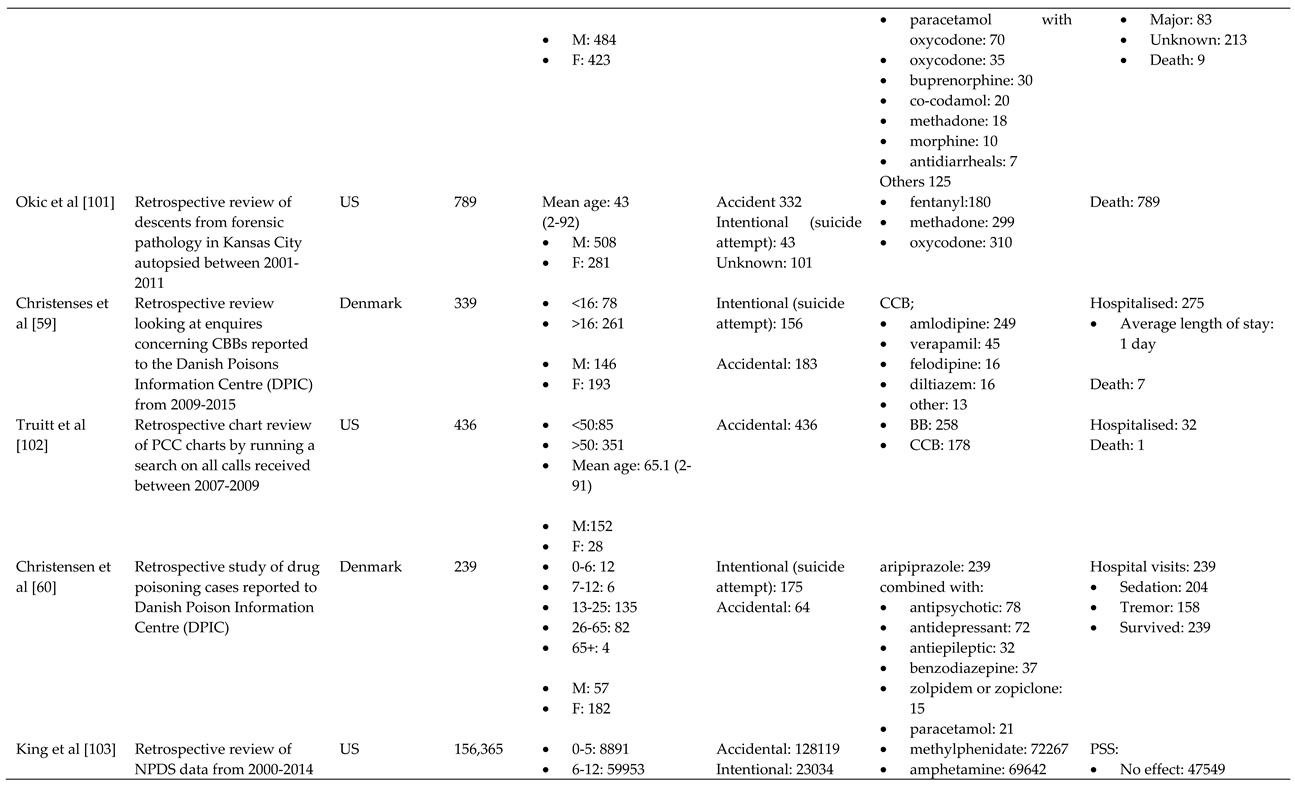
- Okic, M.; Cnossen, L.; Crifasi, J.A.; Long, C.; Mitchell, E.K. Opioid Overdose Mortality in Kansas, 2001–2011: Toxicologic Evaluation of Intent. J. Anal. Toxicol. 2013, 37, 629–635. [Google Scholar] [CrossRef]
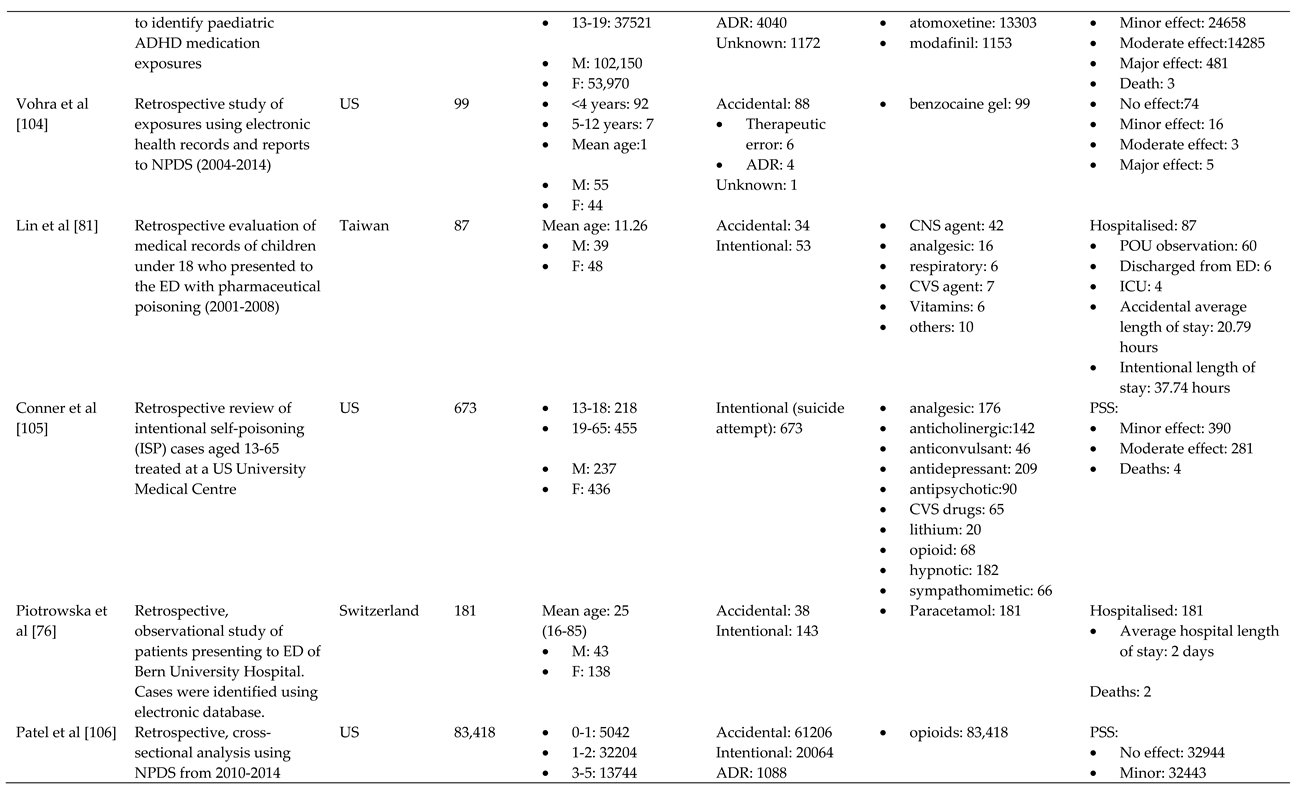
- Truitt, C.A.; Brooks, D.E.; Dommer, P.; LoVecchio, F. Outcomes of unintentional beta-blocker or calcium channel blocker overdoses: a retrospective review of poison center data. Journal of medical toxicology : official journal of the American College of Medical Toxicology 2012, 8, 135–139. [Google Scholar] [CrossRef]
- King, S.A.; Casavant, M.J.; Spiller, H.A.; Hodges, N.L.; Chounthirath, T.; Smith, G.A. Pediatric ADHD Medication Exposures Reported to US Poison Control Centers. Pediatrics 2018, 141. [Google Scholar] [CrossRef] [PubMed]
- Vohra, R.; Huntington, S.; Koike, J.; Le, K.; Geller, R.J. Pediatric Exposures to Topical Benzocaine Preparations Reported to a Statewide Poison Control System. West. J. Emerg. Med. 2017, 18, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Conner, K.R.; Wiegand, T.J.; Gorodetsky, R.; Schult, R.F.; Kaukeinen, K. Poisoning Severity Associated with a Range of Medications in Suicide Attempts by Ingestion. Suicide Life Threat. Behav. 2019, 49, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.M.; Wheeler, D.C.; Rose, S.R.; Nadpara, P.A.; Pakyz, A.L.; Carroll, N.V. Prevalence and Characteristics of Pediatric Opioid Exposures and Poisonings in the United States. J. Pediatr. 2019, 206, 148–155. [Google Scholar] [CrossRef]
- Glaizal, M.; Gazin, V.; Aymard, I.; Messina-Gourlot, C.; Richard, N.; Mallaret, M.; Saviuc, P.; De Haro, L. Suicidal poisonings with methadone in France: Results of a two year national survey by the Toxicovigilance Network. Clinical Toxicology 2012, 50, 841–846. [Google Scholar] [CrossRef]
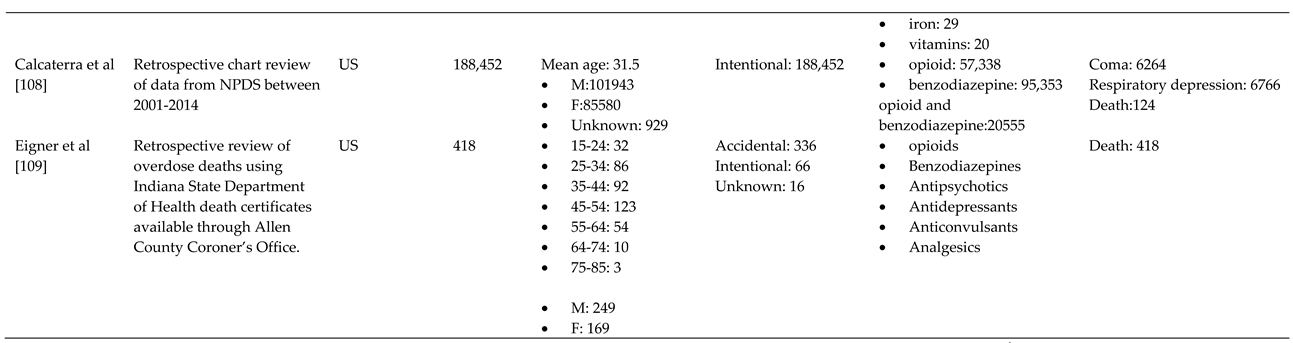
- Calcaterra, S.L.; Severtson, S.G.; Bau, G.E.; Margolin, Z.R.; Bucher-Bartelson, B.; Green, J.L.; Dart, R.C. Trends in intentional abuse or misuse of benzodiazepines and opioid analgesics and the associated mortality reported to poison centers across the United States from 2000 to 2014. Clinical Toxicology 2018, 56, 1107–1114. [Google Scholar] [CrossRef]
- Eigner, G.; Henriksen, B.; Huynh, P.; Murphy, D.; Brubaker, C.; Sanders, J.; McMahan, D. Who is Overdosing? An Updated Picture of Overdose Deaths From 2008 to 2015. Health Services Research and Managerial Epidemiology 2017, 4, 233339281772742. [Google Scholar] [CrossRef]
- Iemmi, V.; Bantjes, J.; Coast, E.; Channer, K.; Leone, T.; McDaid, D.; Palfreyman, A.; Stephens, B.; Lund, C. Suicide and poverty in low-income and middle-income countries: a systematic review. Lancet Psychiatry 2016, 3, 774–783. [Google Scholar] [CrossRef]
- Patel, I.; Balkrishnan, R. Medication Error Management around the Globe: An Overview. Indian J. Pharm. Sci. 2010, 72, 539–545. [Google Scholar] [CrossRef]
- Pawer, S.; Rajabali, F.; Zheng, A.; Pike, I.; Purssell, R.; Zargaran, A.; Babul, S. Socioeconomic factors and substances involved in poisoning-related emergency department visits in British Columbia, Canada. Health Promot Chronic Dis Prev Can 2021, 41, 211–221. [Google Scholar] [CrossRef]
- Getie, A.; Belayneh, Y.M. A Retrospective Study of Acute Poisoning Cases and Their Management at Emergency Department of Dessie Referral Hospital, Northeast Ethiopia. Drug Healthc Patient Saf 2020, 12, 41–48. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Social Determinants of Mental Health. 2014.
- Knaul, F.M.; Farmer, P.E.; Krakauer, E.L.; De Lima, L.; Bhadelia, A.; Jiang Kwete, X.; Arreola-Ornelas, H.; Gómez-Dantés, O.; Rodriguez, N.M.; Alleyne, G.A.O.; et al. Alleviating the access abyss in palliative care and pain relief-an imperative of universal health coverage: the Lancet Commission report. The Lancet 2018, 391, 1391–1454. [Google Scholar] [CrossRef] [PubMed]
- OECD. Addressing Problematic Opioid Use in OECD Countries, 2019.
- Gunnell, D.; Ho, D.; Murray, V. Medical management of deliberate drug overdose: A neglected area for suicide prevention? Emerg. Med. J. 2004, 21, 35. [Google Scholar] [CrossRef]
- Espinosa-Jovel, C.; Toledano, R.; Aledo-Serrano, Á.; García-Morales, I.; Gil-Nagel, A. Epidemiological profile of epilepsy in low income populations. Seizure 2018, 56, 67–72. [Google Scholar] [CrossRef]
- Zhao, P.; Li, S.; Liu, D. Unequable spatial accessibility to hospitals in developing megacities: New evidence from Beijing. Health Place 2020, 65, 102406. [Google Scholar] [CrossRef]
- Rathod, S.; Pinninti, N.; Irfan, M.; Gorczynski, P.; Rathod, P.; Gega, L.; Naeem, F. Mental Health Service Provision in Low- and Middle-Income Countries. Health services insights 2017, 10, 1178632917694350–1178632917694350. [Google Scholar] [CrossRef]
- World Health Organisation. Poison Control and Unintentional Poisoning 2021.
- National Academies of Sciences, E., and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Committee on Pain Management and Regulatory Strategies to Address Prescription Opioid Abuse; . 5. Evidence on Strategies for Addressing the Opioid Epidemic. Available from: . Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. 2017.
- Tabeefar, H.; Chang, F.; Cooke, M.; Patel, T. Community pharmacists and chronic pain: A qualitative study of experience, perception, and challenges. Can J Pain 2020, 4, 29–39. [Google Scholar] [CrossRef]
- Dasgupta, N.; Beletsky, L.; Ciccarone, D. Opioid Crisis: No Easy Fix to Its Social and Economic Determinants. Am. J. Public Health 2018, 108, 182–186. [Google Scholar] [CrossRef]
- Miller, R.; Goodman, C. Performance of retail pharmacies in low- and middle-income Asian settings: a systematic review. Health Policy Plan. 2016, 31, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Leisinger, K.M.; Garabedian, L.F.; Wagner, A.K. Improving access to medicines in low and middle income countries: corporate responsibilities in context. South Med Rev 2012, 5, 3–8. [Google Scholar] [PubMed]
- Hamid, H.; Masood, R.A.; Tariq, H.; Khalid, W.; Rashid, M.A.; Munir, M.U. Current pharmacy practices in low- and middle-income countries; recommendations in response to the COVID-19 pandemic. Drugs Ther Perspect 2020, 36, 355–357. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Guidelines for establishing a poison centre. 2020.
- World Health Organisation. Guidelines on the prevention of toxic exposures: Education and public awareness activities. 2004.
- Kara, H.; Bayir, A.; Degirmenci, S.; Akinci, M.; Ak, A.; Kayis, S.; Agacayak, A.; Azap, M. Causes of poisoning in patients evaluated in a hospital emergency department in Konya, Turkey. J Pak Med Assoc 2014, 64, 1042–1048. [Google Scholar]
- Taheri, F.; Yaraghi, A.; Sabzghabaee, A.M.; Moudi, M.; Eizadi-Mood, N.; Gheshlaghi, F.; Farajzadegan, Z. Methadone toxicity in a poisoning referral center. J Res Pharm Pract 2013, 2, 130–134. [Google Scholar] [CrossRef]
| Poison terms | Pharmaceutical terms |
|---|---|
| Poison* | Pharmaceutical |
| Toxic | Medicine |
| Overdose | Drug |
| Intoxication | Opioid |
| Excessive | |
| Substance abuse | |
| Drug Misuse |
| Low- Middle- Income Countries (n=21) | High- Income Countries* (n=58) |
|---|---|
| Algeria: 1[34] Argentina: 1[35] India: 2 [36,37] Iran: 9 [38,39,40,41,42,43,44,45] Jordan: 1 [46] Morocco: 1 [47] Romania: 1 [48] South Africa: 1 [11] Sri Lanka: 1 [49] Turkey: 3 [50,51] |
Australia: 2 [52,53] Canada: 3 [54,55,56] Czech Republic: 1 [57] Denmark: 3 [58,59,60] Finland: 2 [61,62] France: 3 [63,64,65] Israel: 2 [66,67] Japan: 2 [68,69] New Zealand: 1 [70] Poland: 2 [71,72] Republic of Ireland: 1 [73] Switzerland: 4 [74,75,76,77] Saudi Arabia: 2 [78,79] Singapore: 1 [80] Taiwan: 1 [81] UK: 1 [82] US: 27 [83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109] |
| ATC 1st level classification | LMIC (n) | HIC (n) |
|---|---|---|
|
336 | 2,721 |
|
15 | 29 |
|
193 | 2,947 |
|
0 | 219 |
| G. Genito urinary system and sex hormones | 60 | 79 |
| H. Systemic hormonal preparations, excluding sex hormones and insulins | 66 | 30 |
| J. Anti-infective for systemic use | 185 | 635 |
| L. Antineoplastic and immunomodulating agents | 0 | 43 |
| M. Musculo-skeletal system | 496 | 37,736 |
| N. Nervous System | 3,096 | 1,365,780 |
| R. Respiratory system | 478 | 2,775 |
| Combination of pharmaceuticals ingested | 844 | 27,168 |
| Nervous System | LMIC (n, %) | HIC (n, %) |
|---|---|---|
| Analgesics | 1236 (39.9) | 783,654 (57.3) |
| Antiepileptics | 287 (9.27) | 1,194 (0.87) |
| Anti-Parkinson drugs | 0 (0) | 194 (0.01) |
| Psycholeptics | 618 (20.0) | 105,036 (7.69) |
| Psychoanaleptics | 383 (12.4) | 461,019 (33.8) |
| Drugs used in opioid dependence | 572 (18.5) | 14,683 (10.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





