1. Introduction
The formation of the central nervous system (CNS) is one of the most complex developmental processes characterized by extremely precise and dynamic transitions between proliferative and differentiated cell states [
1,
2]. Another emerging and intriguing evidence is the tight correlation between early developmental cortical organization patterns and the progression of neurodegenerative diseases that are usually considered as late-onset disorders [
3,
4,
5]. Therefore, the understanding of key developmental processes and complex interplay between molecular cues as well as cell state transitions involved during neuro- and gliogenesis are of great importance for discovery of effective therapeutic approaches.
Among many existing and well-established in vitro developmental models, primary dissociated neuronal cultures derived from late embryonic or neonatal rodents’ brain (cortex or hippocampus) [
6,
7] were largely employed and have provided important insight into molecular mechanisms of neuronal polarization, axon guidance, network formation and many others. Despite simplified (compared to
in vivo) conditions, technologically advanced optical tools became available to image, manipulate and measure the cell activity at unprecedented precision in a more controlled experimental conditions [
7,
8,
9,
10].
We have recently established primary dissociated neuronal cultures derived from the cortex of postnatal gray South American short-tailed opossums,
Monodelphis domestica [
11,
12,
13]. We have demonstrated that they can offer a reliable CNS cell source and robust in vitro platform for investigating neuronal development, including neurogenesis, long-term differentiation and maturation as well as regeneration following experimental injury [
13]. Pioneer studies employed unique regenerative capabilities of
M. domestica to investigate spinal cord regeneration, possible in the first two postnatal weeks [
14,
15,
16], while regenerative properties of
M. domestica brain remain less explored. Valuable data regarding cortical histogenesis [
17,
18,
19], cellular composition [
20,
21] and neurotrophins receptors expression [
22,
23,
24] during their brain development is available. These and other studies using “unconventional” mammalian species (i.e., non-rodents) are expanding our knowledge regarding mammalian CNS development and contribute to a more effective translation to humans [
25,
26,
27].
In this work we examined the expression of neural stem cell markers SOX2 and SOX9 during early cortical development and neurogenesis which occur in
M. domestica almost entirely postnatally and extend beyond first postnatal month [
17]. We used three different postnatal age groups: P4-6, P16-18 and P30. According to recent developmental transcriptome analysis [
28], P4-6 opossums correspond to E16-19 rat or E14.5-16.5 mice embryos, P16-18 opossums are developmentally similar to neonatal (P1-2) rat or mice while P30 corresponds roughly to the postnatal age between P3 and P14 in rodents. P4-6 opossum cortex is enriched in cortical progenitors undergoing mitosis [
17]. P16-18 was chosen because at this age cortical gliogenesis starts [
17], neurogenesis still continues reaching peak between P14-24 [
20], while the regenerative capacity of the spinal cord following injury is no longer possible [
14,
15,
16]. Mid-frontal region of P30
M. domestica cortex is completing its neurogenesis [
17] while in the rest of the cortex it should be completed by P35 [
20] or even at later ages (P45) [
29].
SOX2 and SOX9 are members of the SOX2 (from sex-determining region Y protein (SRY) - high-mobility group (HMG) box) family of highly conserved transcription factors that are important developmental regulators [
30,
31,
32]. SOX2 is mainly associated with the maintenance of undifferentiated neural progenitor state [
33] and it is one of the factors responsible for induced pluripotency [
34]. SOX9 is also expressed in multipotent neural stem cells where it represses neuronal differentiation [
35]. Moreover, it was shown that SOX9 is involved in gliogenesis initiation [
36]. In the adult mice brain it was found to be expressed almost exclusively in astrocytes, except for the neurogenic regions [
37].
We confirmed the expression of SOX2 and SOX9 in non-neuronal cells of primary cortical cultures derived from P4-6
M. domestica pups. Interestingly, we identified (sub)population of cortical cells that are double-positive for SOX2 and a neuronal marker (NeuN or TUJ1). The coexpression of SOX2 with neuronal markers has been confirmed in both primary cultures at different days in vitro (DIV), as well as in tissue: by immunohistochemistry (IHC) of the tissue slices and isotropic fractionator method [
38,
39]. The isotropic fractionator method consists of fluorescent labelling of “isotropic suspension” of cell nuclei, obtained from previously fixed and homogenized brain tissue, and subsequently counted in a hemocytometer to obtain total cell number in the brain. This method was used to determine the total number of neurons in the adult human brain [
40,
41] as well as for dozens of other animal species, including 10 different species of marsupials [
20,
42].
SOX9 was exclusively expressed in SOX2-positive non-neuronal cells (i.e., negative for neuronal marker TUJ1) and in astrocytes, at later stages. Therefore, our findings showed that M. domestica express SOX2 and SOX9 in neurogenic regions of developing cortex, and that SOX2 is expressed also in a subset of neurons, implicating yet unexplored role of SOX2 in neuronal differentiation and maturation in marsupials.
2. Materials and Methods
Animals
In this work, South American grey short-tailed opossum (
Monodelphis domestica) pups of both sexes at postnatal days (P)4-6, P16-18 and P30 were used. The body weight and size of pups through postnatal ages used in this study are shown in
Supplementary Table S1. The
M. domestica colony was maintained at the animal house facility of the University of Trieste, in accordance with the guidelines of the Italian Animal Welfare Act, and their use was approved by the Local Veterinary Service, the Ethics Committee board and the National Ministry of Health (Permit Number: 1FF80.N.9Q3), in accordance with the European Union guidelines for animal care (d.1.116/92; 86/609/C.E.). The animals are housed in standard laboratory cages in a temperature- and humidity-controlled environment (27-28°C; 50-60% humidity) with a 12/12 h light/dark cycle and
ad libitum access to food and water. The body weight and size of animals for each postnatal age used in this study are shown in
Table S1.
Primary neuronal cultures
Dissociation protocol used for opossum cortical primary cultures was previously described [
11]. Briefly, cortices were isolated from P4-6 and P16-18
M. domestica pups and all efforts were made to minimize suffering and to reduce the number of animals used. Both left and right hemispheres from each animal were used while olfactory bulbs and remaining subcortical structures were removed. Dissection was performed in the ice-cold oxygenated (95% O
2 / 5% CO
2) dissection solution (113 mM NaCl, 4.5 mM KCl, 1 mM MgCl
2 x 6H
2O, 25 mM NaHCO
3, 1 mM NaH
2PO
4, 2 mM CaCl
2 x 2H
2O, 11 mM glucose and 0.5% w/v Penicillin/Streptomycin/Amphotericin B, pH 7.4, all from Sigma-Aldrich, St. Louis, MO, USA). After removal of meninges, the tissue was chopped into small pieces and washed three times in phosphate-buffered saline (PBS, 137 mM NaCl, 2.7 mM KCl, 10 mM Na
2HPO
4, 2 mM KH
2PO
4, all from Sigma-Aldrich). Enzymatic digestion was performed with prewarmed 0.5% w/v trypsin in PBS (Santa Cruz Biotechnology, SCBT, Dallas, TX, USA) for 10 min at 32.5°C for P4-6 pups. For P16-18 pups, trypsin concentration was raised to 2.5% and incubation time was increased to 15 min. After three washes in PBS, cells were triturated in Hank’s Balanced Salt Solution (HBSS) solution, w/o Ca
2+ and Mg
2+ (Pan-Biotech GmbH, Aidenbach, Germany) containing 10 µg/mL DNase I (Sigma-Aldrich), 1 mg/mL trypsin inhibitor (SCBT) and 1% w/v bovine serum albumin (BSA, PAN-Biotech GmbH). The supernatant was collected and layered on top of the 5% w/v BSA cushion in HBSS in the 5 mL tube. Cells were collected by centrifugation for 5 min at 100 g and resuspended in Dulbecco’s minimum essential medium (DMEM) with stable glutamine supplemented with 10% w/v fetal bovine serum (FBS) and 1% w/v Penicillin/Streptomycin (all from PAN-Biotech). Cell suspension was preplated on the plastic tissue culture dish for 5 min and plated on glass coverslips (12 mm diameter) precoated with 50 µg/mL poly-L-ornithine and 2 µg/mL laminin (all from Sigma-Aldrich) at the density of 5x10
4 cells per well in a 24-well plate. The next day, 2/3 of the medium was changed with the Neurobasal medium supplemented with B27 (both from Thermo Fisher Scientific, Waltham, MA, USA), 1 mM L-glutamine and 1% Penicillin/Streptomycin (both from PAN-Biotech). Half of the cell culture media was changed once per week with the fresh medium. The cortical cultures were maintained in an incubator at 32°C, 5% CO
2 and 95% relative humidity.
Immunocytochemistry
Cells were fixed for 20 minutes at room temperature (RT, 20-22°C) with 4% paraformaldehyde (PFA) containing 200 mM sucrose in PBS, pH 6.9 (all from Sigma-Aldrich). After fixation, cells were washed with PBS, saturated with 0.1 M glycine, permeabilized with 0.1% Triton X-100 (all from Sigma-Aldrich) in PBS, and washed with PBS, each step lasting 5 min. Cells were blocked with 0.5% w/v BSA (PAN-Biotech) in PBS for 30 min. Incubation with the primary antibodies was done in blocking solution in a wet chamber for 1 h, followed by the washing steps in PBS and incubation with the secondary antibodies. For every primary antibody used, the protein sequence similarity between opossum and immunogen was compared using the Universal Protein Resource (UniProt, available at
https://www.uniprot.org/).
Following antibodies were used: mouse monoclonal anti-SOX2 (immunoglobulin (Ig)G1 isotype, Abcam, Cambridge, UK, Cat#ab79351, RRID AB_10710406, 91.7% sequence similarity) and anti - β - Tubulin III (TUJ1, IgG2a isotype, Biolegend, Cat#801201, RRID AB_2313773, 99.8% similarity), rabbit monoclonal anti-NeuN (Abcam, Cat#ab177487, RRID AB_2532109, 84% similarity) and rabbit monoclonal anti-SOX9 (Abcam, Cat#ab185966, RRID AB_2728660, 96.7% similarity).
The secondary antibodies were goat anti-mouse Alexa Fluor® 555 (Thermo Fisher Scientific, Cat#A32732, RRID AB_2633281, 1:400), goat anti-mouse Alexa Fluor® 488 (Thermo Fisher Scientific, Cat#A32723, RRID AB_2633275, 1:400), goat anti-rabbit Alexa Fluor® 555 (Thermo Fisher Scientific, Cat#A32732, RRID AB_2633281, 1:400), goat anti-rabbit Alexa Fluor® 647 (Abcam, Cat# ab150083, RRID AB_2714032, 1:300), goat anti-mouse IgG1 Alexa Fluor® 488 (Thermo Fisher Scientific, Cat#A-21121, RRID AB_2535764, 1:300) and goat anti-mouse IgG2a Alexa Fluor® 555 (Thermo Fisher Scientific, Cat#A-21137, RRID AB_2535776, 1:300) and the incubation time was 30 min in the dark. Cell nuclei were stained with a 300 nM nuclear stain 4′,6-diamidino-2-phenylindole (DAPI, Thermo Fisher Scientific) and incubated with secondary antibodies. Finally, coverslips were washed with PBS and dH2O. Coverslips were mounted on a glass slide using the mounting medium (Vectashield, Vector Laboratories, Burlingame, CA, USA) and sealed with nail polish. All the incubations were performed at RT.
Immunohistochemistry
Immunohistochemistry (IHC) procedure was performed as previously described [
43]. Briefly, cortices were fixed overnight in 4% PFA in PBS at 4 ˚C followed by 24 h immersion in 30% sucrose in PBS for cryoprotection (all from Sigma-Aldrich). Cortices were cut coronally into 16 μm thin sections, using the sliding cryostat Leica CM1850 (Leica Biosystems, Germany) and mounted on Superfrost Plus microscope slides (Menzel-Glaser; Thermo Fisher Scientific). For immunostaining, the samples were first treated with blocking solution containing 3% normal goat serum, 3% BSA (both from PAN-Biotech) and 0.3% Triton X-100 (Sigma-Aldrich) in PBS for 1 h at RT and then incubated with primary antibodies in a solution containing 1% FBS, 1% BSA, 0.1% Triton X-100 overnight at 4°C. Following primary antibodies were used: mouse monoclonal anti-SOX2 (IgG
1 isotype, Abcam, Cat#ab79351, RRID AB_10710406, 91.7% sequence similarity), mouse monoclonal anti-GFAP (IgG
1 isotype, Sigma–Aldrich, Cat#G3893, RRID AB_477010, 84.9% sequence similarity), rabbit NeuN (Abcam, Cat#ab177487, 1:500, RRID AB_2532109, 84% similarity) and rabbit anti-SOX9 (Abcam, Cat#ab185966, RRID AB_2728660, 96.7% similarity).
The next day slides were washed 3x with PBS-T for 5 minutes and then incubated with secondary antibodies in PBS (goat anti-mouse Alexa Fluor 488 (Thermo Fisher Scientific, Cat# A32723, RRID AB_2633275, 1:500) and goat anti-rabbit Alexa Fluor 555 (Thermo Fisher Scientific, Cat# A32732, RRID AB_2633281, 1:500)) in a humid chamber for 2 hours at RT. Next, samples were incubated in a 1 μg/mL DAPI solution (Thermo Fisher Scientific, USA) for 20 min to visualize cell nuclei, mounted with Vectashield® mounting medium (Vector Laboratories) using 24x60 mm coverslip (Thermo Fischer Scientific) and sealed with a thin layer of nail polish. Slides were left to dry for 30 min at RT and stored short-term at 4˚C or long term at -20˚C, protected from light.
Isotropic fractionator
This method was performed as previously described [
38], with some modifications. Briefly, the cortices were dissected as described for primary cultures. After removal of meninges, sample was immediately fixed by immersion in 4% PFA in PBS for at least 2 h (for P4-6 cortices) or overnight (for P16-18 and P30 cortices) and stored at +4°C. Cortices were washed once with PBS, transferred to 15 mL glass tissue homogenizer (Tenbroeck tissue grinder, Wheaton, USA), suspended with 1 mL homogenization solution (40mM sodium citrate and 1% Triton X-100 in PBS, all from Sigma-Aldrich) and homogenized until the smallest visible fragments were dispersed. The homogenate was collected and centrifuged (10 min at 4000g and +4°C). Supernatant was transferred into a separate tube (and kept for the analysis of centrifugation efficiency). Pellet containing nuclei was resuspended in PBS containing 1% BSA (PAN-Biotech) and incubated for 15 min at RT. The final volumes of isotropic suspensions were 0.5 mL, 1 mL and 2 mL for P4-6, P18 and P30 cortices, respectively. For immunostaining, 150 μL aliquots were used and antigen retrieval was done by incubating the samples for 15 min at 60°C in homogenization solution. The same antibodies described for IHC were used. The incubation with primary antibodies (diluted in 1%BSA in PBS) was performed overnight at +4°C, while secondary antibodies were incubated for 1 h, in dark at RT. Between the two incubations with antibodies, samples were centrifuged (10 min at 4000g and +4°C) and washed twice by incubating with 1% BSA in PBS for 10 min at RT. To stain cell nuclei, Hoechst 33342 (2 μg/mL in PBS, incubated for 20 min in dark at RT) was used. After final washing step in dH
2O, pellet was resuspended with mounting medium (Vectashield
®, Vector Laboratories), mounted on a glass slide, covered with coverslip and sealed with nail polish. Control experiments for Hoechst 33342 staining efficiency as well as background staining are shown in
Supplementary Figure S1 and S2.
Imaging
Samples were analyzed using an Olympus IX83 inverted fluorescent microscope (Olympus, Tokyo, Japan) equipped with differential interference contrast (DIC) and fluorescence optics (mirror units: U-FUNA: EX360-370, DM410, EM420-460, U-FBW: EX460-495, DM505, EM510IF and U-FGW: EX530-550, DM570, EM575IF (Olympus) and Cy5 (EX620/60, DM660, EM700/75, Chroma, USA). Fluorescence images were acquired with Hamamatsu Orca R2 CCD camera (Hamamatsu Photonics, Hamamatsu, Japan) and CellSens software (Olympus). 10x 0.3 numerical aperture (NA) air and 20x 0.5 NA air, as well as 40x 1.4 NA and 60x 1.42 NA oil immersion objectives were used. For each image 15-30 frames were acquired with 1 µm (10x and 20x objectives) and 0.3-0.5 µm (40x and 60x objectives) slice spacing, and a maximum intensity projection was used. CellSens and ImageJ by W. Rasband (developed at the U.S. National Institutes of Health and available at
http://rsbweb.nih.gov/ij/) were used for image processing and analysis.
Statistics
All results have been obtained from at least three independent experiments and are presented as a bar graph with mean ± SEM. Statistical analysis was performed using GraphPad Prism 8.4 (GraphPad Software Inc., California, USA). To test normality of data, depending on the number of values tested, either D’Agostino-Pearson or Shapiro-Wilk normality test was used. Brown-Forsythe test was used to test the equality of variances. One-way ANOVA was used to compare three or more data groups, when data followed Gaussian distribution. Following the one-way ANOVA, Holm-Šídák test was performed for multiple comparisons between data groups. Data groups with different variances that fail a normality test were compared using the Kruskal-Wallis test. Following Kruskal-Wallis test, Dunn’s multiple comparisons test was performed for multiple comparisons between data groups. T test was used when comparing two normally distributed data groups with equal variances, and in case of unequal variances the Welch t test was used. The accepted level of significance was p < 0.05. p < 0.001 Very significant ***, 0.001 to 0.01 Very significant **, 0.01 to 0.05 Significant *, ≥ 0.05 Not significant.
3. Results
SOX2 expression in developing opossum cortex
SOX2 expression in primary dissociated cultures of P4-6 cortex at DIV1
Our previously established primary dissociated neuronal cultures derived from neonatal (P3-5)
Monodelphis domestica cortex and showed efficient differentiation capacity into long-term neuronal networks (surviving for over 1 month
in vitro), when cultured in serum-free conditions. Alternatively, progenitor/radial glia cells (RGC) proliferation is promoted using cell culture medium containing FBS. These cells can be further passaged, while maintaining their differentiation potential [
11,
12].
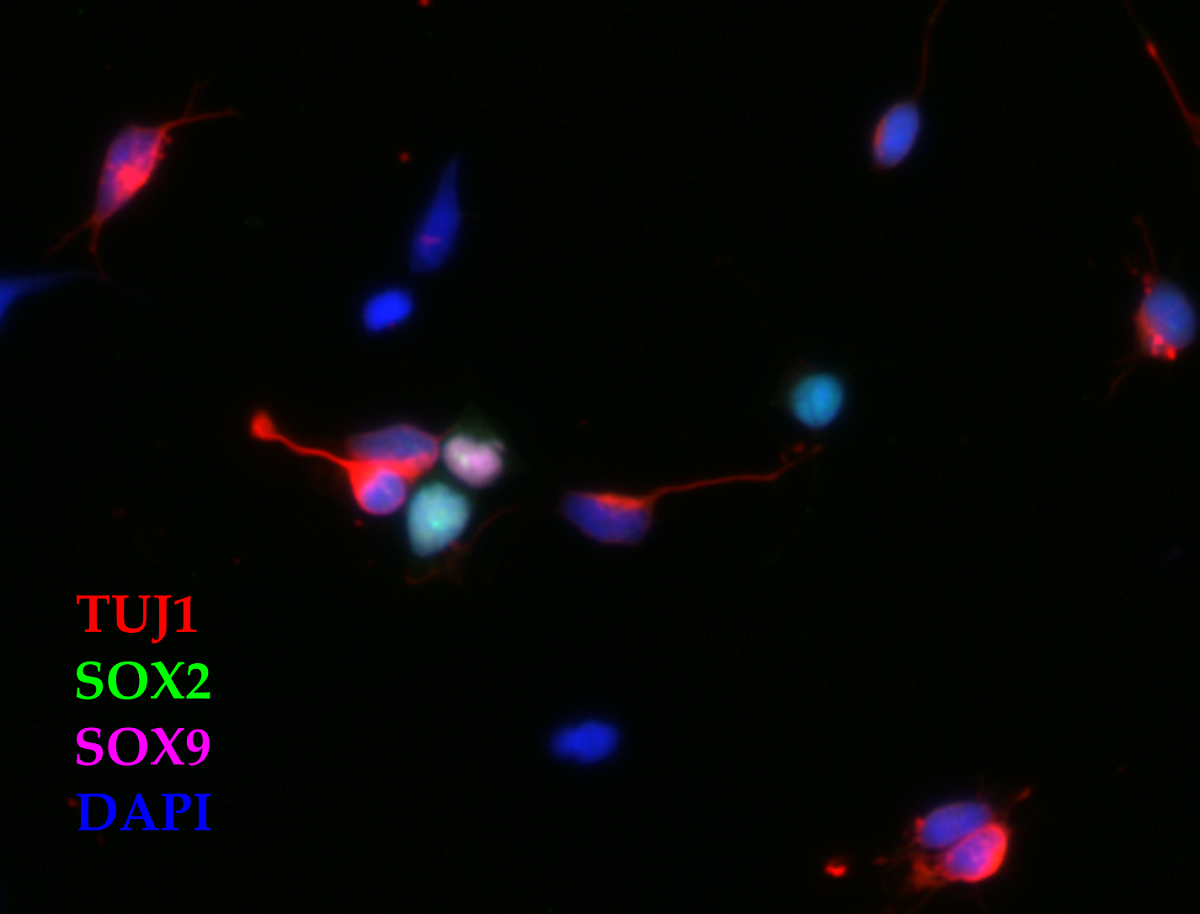
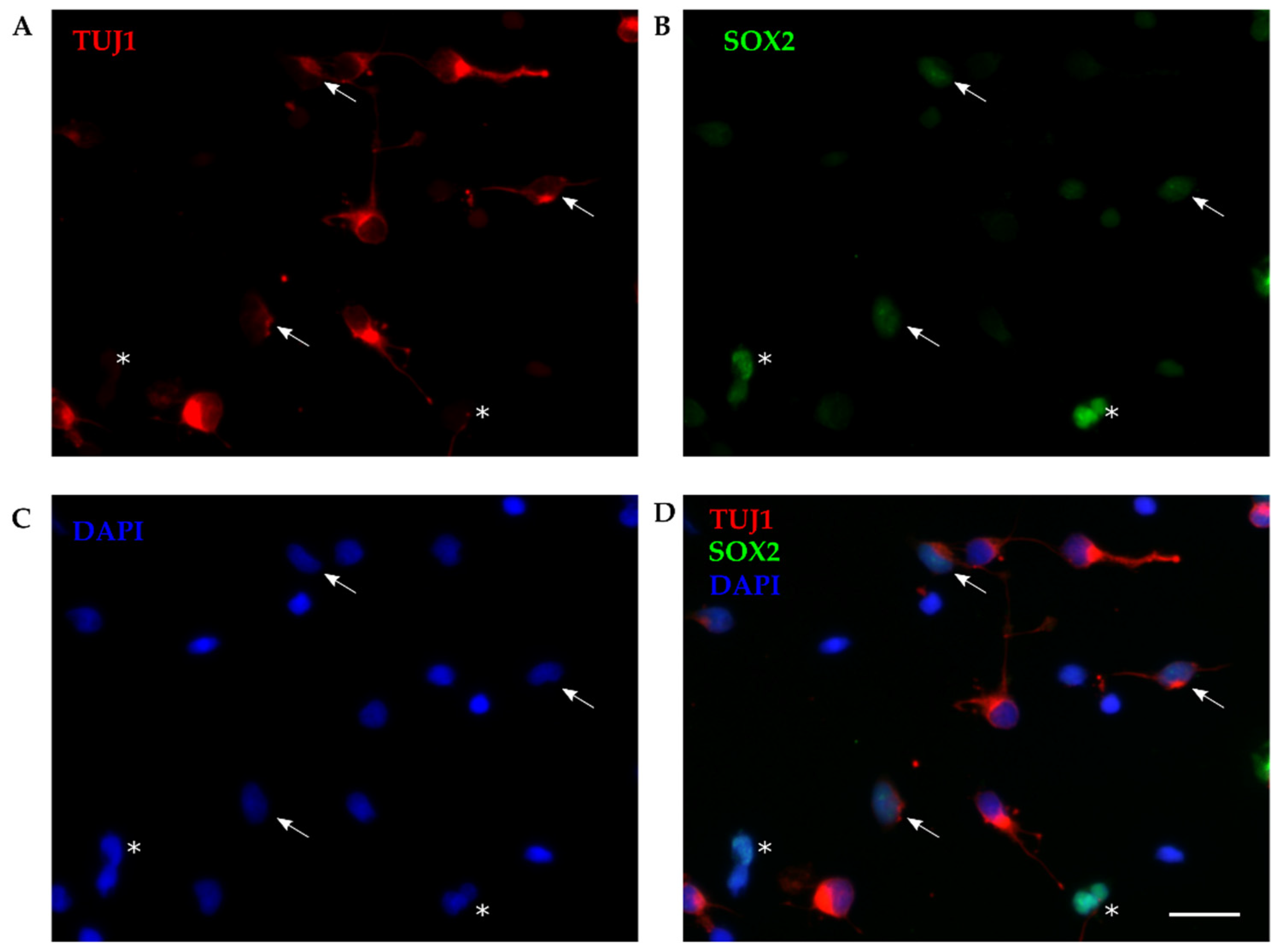
To further characterize the expression profile of these cultures, we used SOX2, a marker of multipotent neural stem/progenitor cells [
31,
32]. Cells were cultured in DMEM supplemented with 10% FBS, fixed 24h after plating (DIV1) and immunostained for neuronal marker β-tubulin III (TUJ1) and SOX2. As expected, SOX2 was detected in non-neuronal (TUJ1-negative) cells, showing bright staining and nuclear localization (
Figure 1, asterisks). 61.25 ± 2.80% of cells were shown to be SOX2-positive while 76.15 ± 2.03% (out of 394 cells analysed). Interestingly, SOX2 was also coexpressed in 49.02 ± 3.02% of TUJ1-positive neurons (as indicated by arrows in
Figure 1).
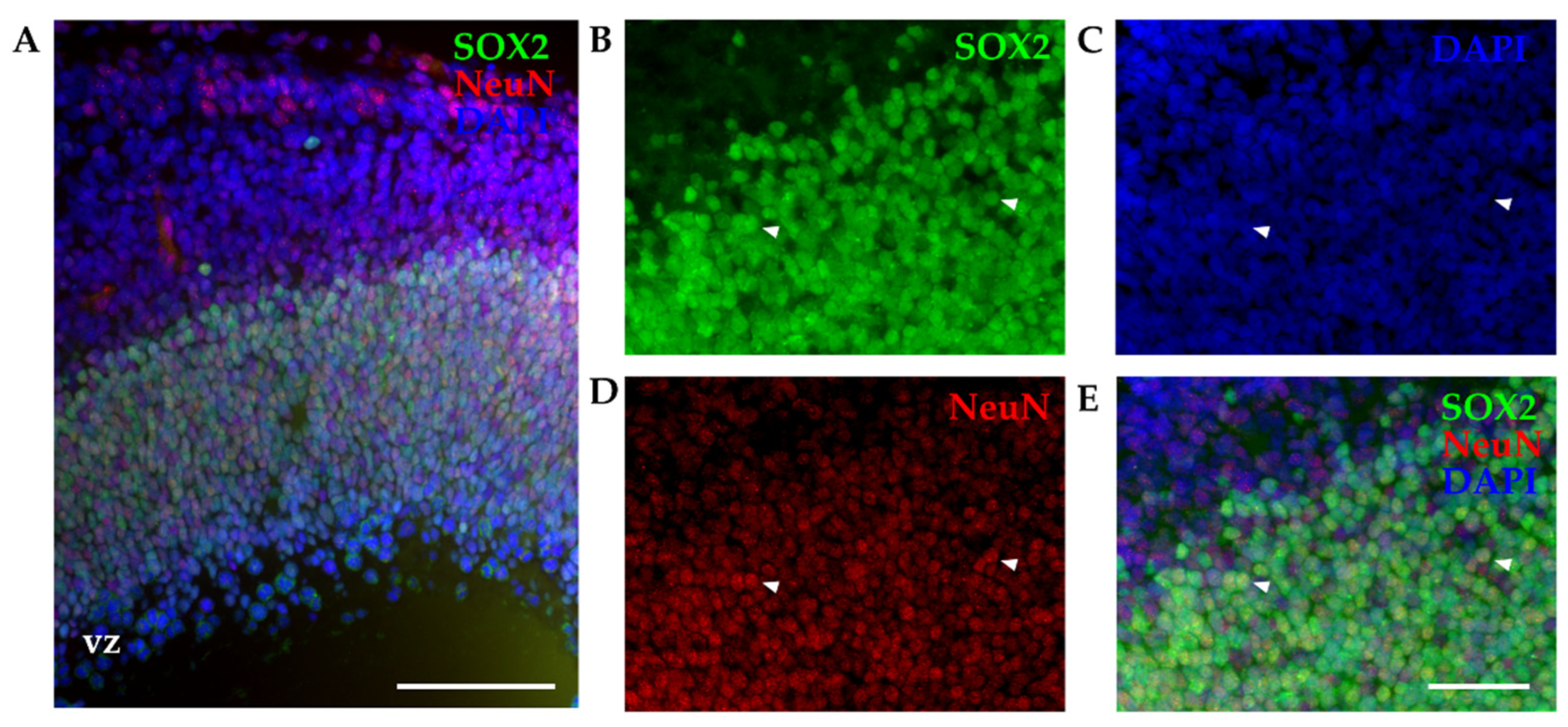
SOX2 expression by IHC
To exclude the possibility that coexpression of TUJ1 and SOX2 was induced by dissociation procedure (mechanical injury) and/or altered by in vitro conditions, we performed immunohistochemistry (IHC) analysis on cortical slices on opossum cortex of comparable age. These experiments allowed the reconstruction of the intact cortical structure and spatial location of developing cortical cells. As described in Methods, cortices of P6 opossums were isolated, dissected, fixed, sliced and stained for SOX2 (with the same antibody previously used on primary cultures). To facilitate the identification of individual neurons, neuronal nuclear marker NeuN was used. As shown in
Figure 2, SOX2 was expressed predominantly in ventricular region, while NeuN-positive cells were found distributed from the ventricular zone to the pial surface of developing opossum brain. Coexpression of SOX2 and NeuN was confirmed (
Figure 2, arrowheads).
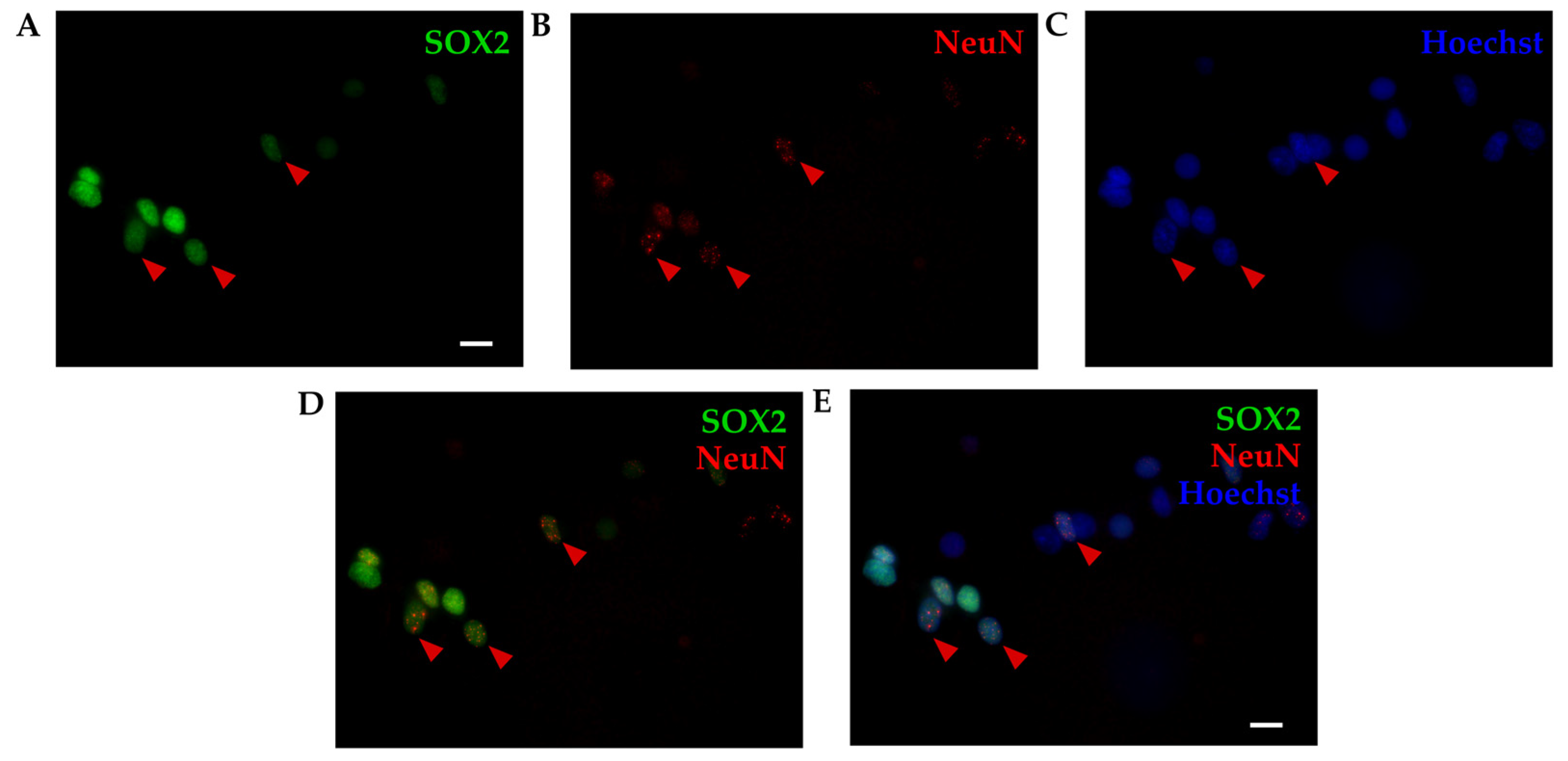
Isotropic fractionator
Due to high cell density of the tissue (
Figure 2), it was difficult to compare the percentage of SOX2-positive cells observed in vitro (
Figure 1) with IHC. Thus, we decided to use isotropic fractionator method [
38] following the original protocol, with some modifications. During the final step, instead of loading the immunolabelled nuclei fraction on cell counter (for which upright microscope is required), we mounted the sample on standard microscope slide with the addition of mounting medium, covered by glass coverslip and sealed with nail polish. This way we could visualize the stained nuclei on inverted fluorescence microscope, allowing long-term sample storage and more extensive imaging, i.e., using objectives with higher magnification and resolution. Detailed protocol is described in Methods section.
We used cortices from opossums of the same age (P4-6) and the same combination of two primary antibodies (SOX2 and NeuN), as for IHC and counted the percentage of stained nuclei for each marker relative to total cell number (counting Hoechst 33342-positive cells,
Figure 3). SOX2 and NeuN staining was efficient, which finally enabled quantification of different SOX2-positive cells present in cortices. Out of the 828 Hoechst 33342-positive nuclei counted, 64.67 ± 9.9% were SOX2-positive (
Figure 3A), 74.11 ± 2.93% were NeuN-positive (
Figure 3B), while 49.64 ± 9.77% were double-positive (SOX2
+/NeuN
+,
Figure 3D).
To verify the coexpression of SOX2 and NeuN during postnatal cortical development, we repeated the same experiments on P16-18 and P30 pups. P16-18 cortex had 41.38 ± 5.03% SOX2-positive cells, 46.5 ± 8.25% NeuN-positive and 33.88 ± 5.93% double-positive cells (n=1224). On the other hand, out of 1182 cell analysed, P30 opossums had 40.79 ± 5.09% SOX2-positive, 49.35 ± 4.94% NeuN-positive, and 34.68 ± 6.13% double-positive cells, respectively. These results confirmed the persistence of double-positive (SOX2+/NeuN+) cell population in the M. domestica cortex during the first postnatal month.
SOX9 expression in developing opossum cortex
SOX9 expression in primary dissociated cultures of P4-6 cortex at DIV1
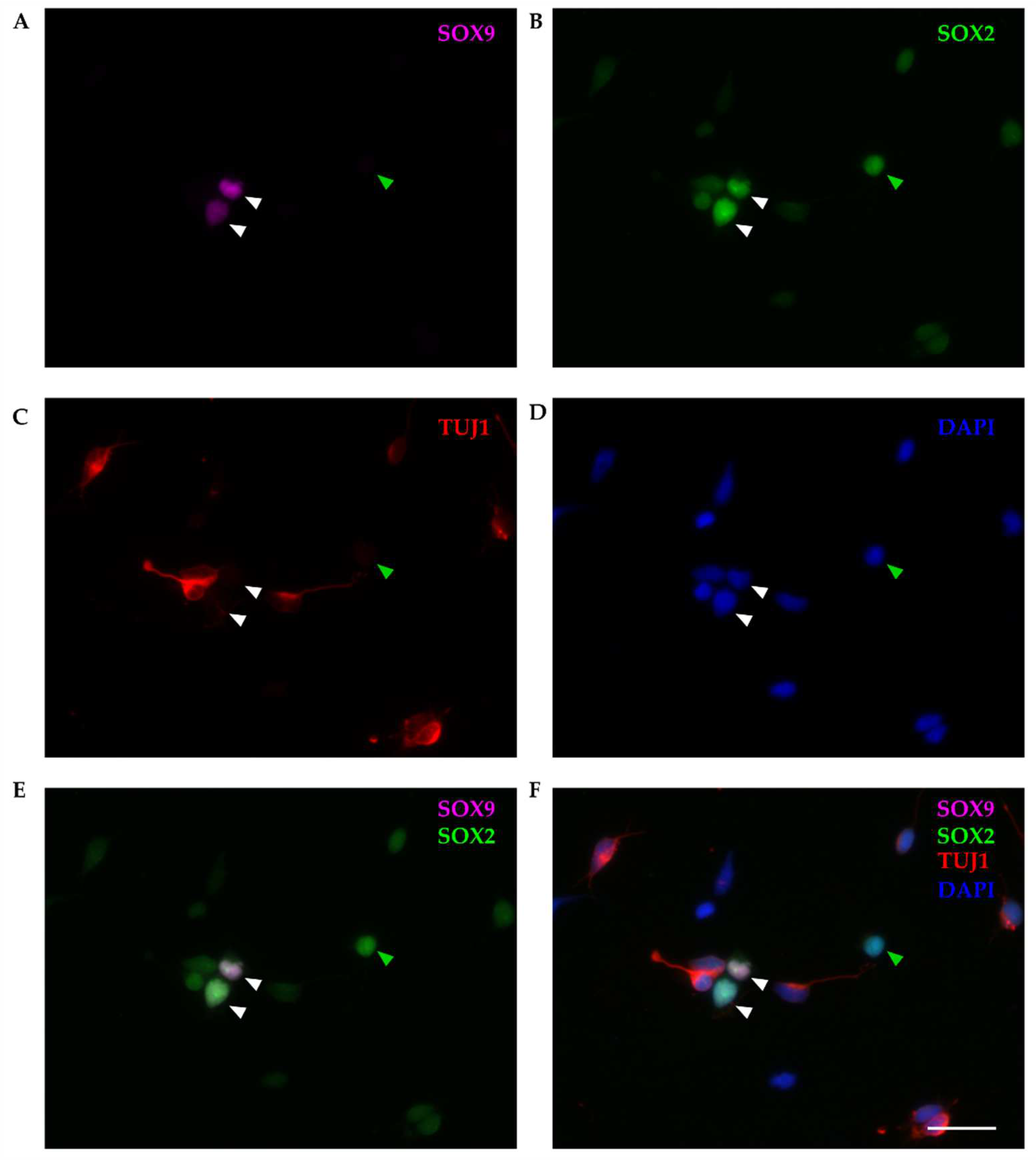
In addition to SOX2, we examined the expression pattern of SOX9 by immunocytochemistry. Primary dissociated neuronal cultures of P4-6 opossums were fixed at DIV1. Immunostaining was performed using appropriate combination of antibodies against β-tubulin III (TUJ1), SOX2 and SOX9 (
Figure 4).
SOX9 was exclusively stained in non-neuronal cells (TUJ1-negative), accounting for 11.47 ± 1.68% of total cells (n=303) and it was always coexpressed with SOX2 (
Figure 4E-F). However, not all non-neuronal cells were SOX9-positive (as indicated by green arrowhead in
Figure 4). Since P4-6 opossums correspond to late mouse or rat embryos, based on these results, we can conclude that at this stage, neural stem/progenitor cells are heterogeneous cell population [
44,
45] expressing SOX2, with SOX9 limited to a subset of neural stem cells.
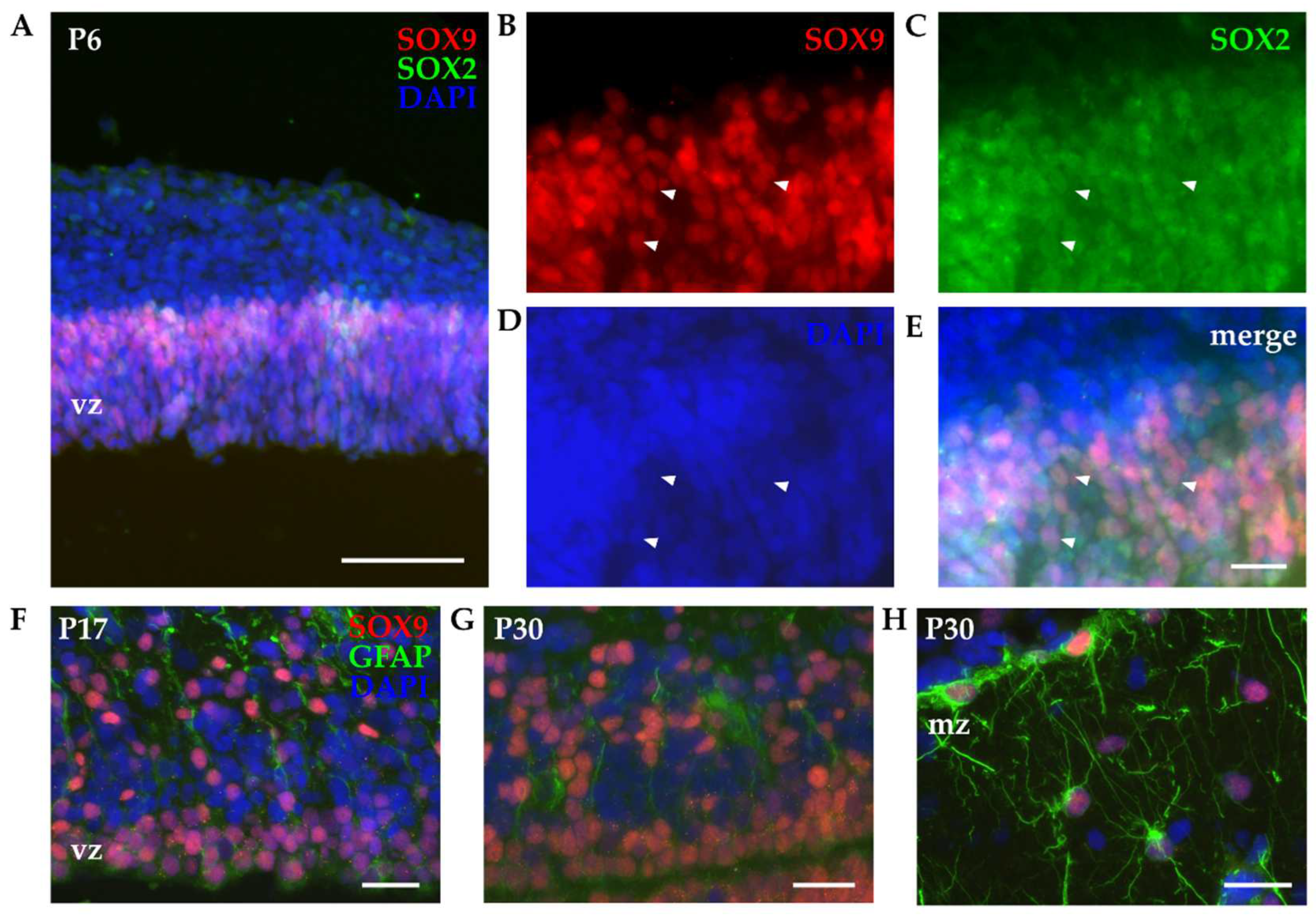
SOX9 expression by IHC
IHC experiments were conducted to examine the SOX9 expression and tissue localization in the developing opossum cortex. P6 cortices were isolated, dissected, sliced and stained for SOX9 in combination with SOX2. Neuronal markers were not used, given the SOX9 exclusive expression in non-neuronal cells. Similar to what observed with primary cortical cultures, nuclear localization of SOX9 was confirmed. As indicated in
Figure 5, SOX9 was detected exclusively within the ventricular region, although its prevalence was lower in comparison to SOX2. Coexpression of SOX2 and SOX9 was also confirmed (
Figure 5, arrowheads). Moreover, SOX2 had larger spatial expression with positive cells found in both ventricular zone and more distal layers of the developing cortex and it was present in both neuronal (
Figure 2) and non-neuronal cells (
Figure 5).
Since neural stem/progenitor cells express several common markers with astrocytes [
37,
46], we used SOX9 in combination with glial fibrillary acidic protein (GFAP,
Figure 5 F-H). In P17 and P30 opossum cortices SOX9 retained the expression in ventricular zone (
Figure 5 F-G) and coexpression of SOX9 with GFAP was observed. The presence of SOX9-positive cells with typical astrocyte’s stellate morphology was observed in marginal zone of P30 cortex (
Figure 5 H).
3. Discussion
In this work we examined the expression of transcription factors SOX2 and SOX9 during postnatal cortical development of
M. domestica. We used our recently established primary dissociated neuronal cultures derived from neonatal (P3-5) pups [
11] and we also showed that SOX2 is expressed in
M. domestica-derived neurospheres (at DIV7) as well as in primary cortical neuronal cultures following in vitro injury (made at DIV9) [
13].
We confirmed that SOX2 is widely expressed already at DIV1 in ~60% of cells that include both non-neuronal cells and neurons. The unexpected observation was that SOX2 is expressed in almost 50% of TUJ1-positive neurons at DIV1 (
Figure 1). Since we already reported that double-positive TUJ1
+/SOX2
+ neurons are present at the injury site following in vitro scratch test [
13], we performed IHC experiments on cortical tissue slices on
M. domestica pups of comparable age (P6). The presence of SOX2
+/NeuN
+ cell population was confirmed, demonstrating that the existence of these cells was not injury-induced or in vitro artefact. These cells are presumably newborn/immature neurons since neurogenesis in opossums occur mostly postnatally and at this developmental stage (P6 or younger age) the cortical layers are still forming [
17,
19,
29].
SOX2 is one of the key transcription factors responsible for early induction and maintenance of pluripotency as well as multipotency of neural stem cells [
30,
31,
32,
47]. When ectopically expressed in somatic cells such as fibroblasts (in combination with Oct3/4, c-Myc and Klf4, so-called Yamanaka factors) it can induce/reprogram them into pluripotent state [
34]. SOX2 function is dose-dependent: while ectopic expression inhibits neuronal differentiation, its reduction promotes the cell cycle exit and terminal differentiation [
32,
48]. Therefore, SOX2 downregulation occurs during neuronal differentiation [
32,
49]. For instance, in the mouse embryonic spinal cord, SOX2 coexpression with neuronal marker NeuN was observed in only a few cells (less than 2%) [
50], however retained expression in specific population of mature neurons such as thalamic projection neurons was reported as well [
51]. This and other studies [
52,
53] are demonstrating a new and emerging role for SOX2 in CNS differentiation and maturation.
In addition to many other species, isotropic fractionator method was used to determine the differential changes in number of neurons of the
M. domestica brain and its subregions, during postnatal development (from P18 to adult opossums) [
20,
21]. However, another study investigating cellular composition of marsupial brains from 10 different species found discrepancy (underestimates) with these findings, excluding it from the comparative analysis [
54].
In this work we have used SOX2 as additional marker and expanded the analysis with isotropic fractionator method to younger age groups (P4-6). To our knowledge, SOX2 was not previously used with this method, and neither on opossums. By modifying the protocol (mounting the samples on glass slide, see Methods) we were able to use this technique on inverted fluorescence microscope, allowing more extensive imaging, i.e., using objectives with higher magnification and resolution as well as long-term sample storage. This is usually not possible when hemocytometer is used, and the samples are discarded after counting. We have however tried to count the total number of cells with hemocytometer using inverted fluorescence microscope and long-working distance 20x and 0.45 NA objective (
Supplementary Table S2). Our results also differ from previous work on
M. domestica [
20,
21], suggesting that these experiments should be repeated by other groups in order to verify if opossums are outlier among marsupials [
54].
Finally, in addition to SOX2, we have checked the expression of SOX9 in primary cortical cultures derived from P4-6 opossum pups. At DIV1 SOX9 was exclusively stained in non-neuronal cell and it was always coexpressed with SOX2 (
Figure 4), but some non-neuronal, SOX9-negative cells were found as well. Our previous work showed that GFAP-positive astrocytes arise in vitro at later stages and at DIV7 they represent less than 6% of total cells in culture [
11]. Since P4-6 opossums correspond to late mouse or rat embryos, based on these results, we can conclude that at this stage, neural stem/progenitor cells are heterogeneous cell population [
44,
45] expressing SOX2, with SOX9 limited to a subset of neural stem cells. Additionally, IHC experiments on P16-18 and P30 cortical tissue slices showed the presence of SOX9
+/GFAP
+ astrocytes confirming the involvement of SOX9 during astrocytogenesis.
In conclusion, beside their well-known function in maintenance of stem cell state, SOX2 and SOX9 are showing emerging roles in cell differentiation as well. Better understanding of the SOX transcription factors-mediated developmental switches could provide precious insights into pathogenesis of neurodegenerative diseases.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, J.B.; isotropic fractionator method validation, Z.B. and J.B.; primary neuronal cultures, M.P., M.I., I.T., A.P.; immunofluorescence, M.P., M.I.; IHC, H.H.; fluorescence analysis, D.C.; data curation, D.C., M.P., Z.B.; writing—original draft preparation, J.B.; funding acquisition, M.M. and J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Croatian Science Foundation (Hrvatska Zaklada za Znanost; HRZZ/CSF) grant IP-2016-06-7060 to M.M., the financial support from the University of Rijeka (uniri-prirod-18-290-1463 to J.B., uniri-biomed-18-258- 6427, and uniri-sp-biomed-19-50-1560 to M.M.).
Institutional Review Board Statement
The animal study protocol was approved by the by the Local Veterinary Service, the Ethics Committee board and the National Ministry of Health (Permit Number: 1FF80.N.9Q3), in accordance with the European Union guidelines for animal care (d.1.116/92; 86/609/C.E.).
Data Availability Statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
This work is dedicated to prof. John G. Nicholls, FRS, PhD, MD. We will always be grateful for the inspiring discussions and his encouragement.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Xia, B.; Yanai, I. A Periodic Table of Cell Types. Development 2019, 146, dev169854. [Google Scholar] [CrossRef] [PubMed]
- Paridaen, J.T.M.L.; Huttner, W.B. Neurogenesis during Development of the Vertebrate Central Nervous System. EMBO Rep 2014, 15, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Shabani, K.; Hassan, B.A. The Brain on Time: Links between Development and Neurodegeneration. Development 2023, 150, dev200397. [Google Scholar] [CrossRef]
- Schaefers, A.T.U.; Teuchert-Noodt, G. Developmental Neuroplasticity and the Origin of Neurodegenerative Diseases. World J Biol Psychiatry 2016, 17, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.J.; Barker, R.A. Neurodegeneration: A Failure of Neuroregeneration? Lancet 2001, 358, 1174–1176. [Google Scholar] [CrossRef] [PubMed]
- Kaech, S.; Banker, G. Culturing Hippocampal Neurons. Nature Protocols 2006, 1, 2406–2415. [Google Scholar] [CrossRef]
- Beaudoin, G.M.J.; Lee, S.-H.; Singh, D.; Yuan, Y.; Ng, Y.-G.; Reichardt, L.F.; Arikkath, J. Culturing Pyramidal Neurons from the Early Postnatal Mouse Hippocampus and Cortex. Nat Protoc 2012, 7, 1741–1754. [Google Scholar] [CrossRef]
- Al-Ali, H.; Beckerman, S.R.; Bixby, J.L.; Lemmon, V.P. In Vitro Models of Axon Regeneration. Exp. Neurol. 2017, 287, 423–434. [Google Scholar] [CrossRef]
- Kaech, S.; Huang, C.-F.; Banker, G. General Considerations for Live Imaging of Developing Hippocampal Neurons in Culture. Cold Spring Harb Protoc 2012, 2012, pdbip068221. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Wu, J.; Zhang, D.; Wang, Y.; Zhai, J. Recent Progresses in Novel in Vitro Models of Primary Neurons: A Biomaterial Perspective. Front Bioeng Biotechnol 2022, 10, 953031. [Google Scholar] [CrossRef]
- Petrović, A.; Ban, J.; Tomljanović, I.; Pongrac, M.; Ivaničić, M.; Mikašinović, S.; Mladinic, M. Establishment of Long-Term Primary Cortical Neuronal Cultures From Neonatal Opossum Monodelphis Domestica. Front. Cell. Neurosci. 2021, 15. [Google Scholar] [CrossRef] [PubMed]
- Ban, J.; Mladinic, M. Monodelphis Domestica: A New Source of Mammalian Primary Neurons in Vitro. Neural Regen Res 2022, 17, 1726–1727. [Google Scholar] [CrossRef] [PubMed]
- Petrović, A.; Ban, J.; Ivaničić, M.; Tomljanović, I.; Mladinic, M. The Role of ATF3 in Neuronal Differentiation and Development of Neuronal Networks in Opossum Postnatal Cortical Cultures. Int J Mol Sci 2022, 23, 4964. [Google Scholar] [CrossRef] [PubMed]
- Mladinic, M.; Muller, K.J.; Nicholls, J.G. Central Nervous System Regeneration: From Leech to Opossum. J Physiol 2009, 587, 2775–2782. [Google Scholar] [CrossRef]
- Varga, Z.M.; Bandtlow, C.E.; Erulkar, S.D.; Schwab, M.E.; Nicholls, J.G. The Critical Period for Repair of CNS of Neonatal Opossum (Monodelphis Domestica) in Culture: Correlation with Development of Glial Cells, Myelin and Growth-Inhibitory Molecules. Eur J Neurosci 1995, 7, 2119–2129. [Google Scholar] [CrossRef]
- Nicholls, J.; Saunders, N. Regeneration of Immature Mammalian Spinal Cord after Injury. Trends Neurosci. 1996, 19, 229–234. [Google Scholar] [CrossRef]
- Puzzolo, E.; Mallamaci, A. Cortico-Cerebral Histogenesis in the Opossum Monodelphis Domestica: Generation of a Hexalaminar Neocortex in the Absence of a Basal Proliferative Compartment. Neural Dev 2010, 5, 8. [Google Scholar] [CrossRef]
- Cheung, A.F.P.; Kondo, S.; Abdel-Mannan, O.; Chodroff, R.A.; Sirey, T.M.; Bluy, L.E.; Webber, N.; DeProto, J.; Karlen, S.J.; Krubitzer, L.; et al. The Subventricular Zone Is the Developmental Milestone of a 6-Layered Neocortex: Comparisons in Metatherian and Eutherian Mammals. Cereb Cortex 2010, 20, 1071–1081. [Google Scholar] [CrossRef]
- Molnár, Z.; Knott, G.W.; Blakemore, C.; Saunders, N.R. Development of Thalamocortical Projections in the South American Gray Short-Tailed Opossum (Monodelphis Domestica). J Comp Neurol 1998, 398, 491–514. [Google Scholar] [CrossRef]
- Seelke, A.M.H.; Dooley, J.C.; Krubitzer, L.A. Differential Changes in the Cellular Composition of the Developing Marsupial Brain. J Comp Neurol 2013, 521, 2602–2620. [Google Scholar] [CrossRef]
- Seelke, A.M.H.; Dooley, J.C.; Krubitzer, L.A. The Cellular Composition of the Marsupial Neocortex. J Comp Neurol 2014, 522, 2286–2298. [Google Scholar] [CrossRef] [PubMed]
- Bartkowska, K.; Gajerska, M.; Turlejski, K.; Djavadian, R.L. Expression of TrkC Receptors in the Developing Brain of the Monodelphis Opossum and Its Effect on the Development of Cortical Cells. PLoS One 2013, 8, e74346. [Google Scholar] [CrossRef] [PubMed]
- Bartkowska, K.; Aniszewska, A.; Turlejski, K.; Djavadian, R.L. Distribution and Function of TrkB Receptors in the Developing Brain of the Opossum Monodelphis Domestica. Dev Neurobiol 2014, 74, 707–722. [Google Scholar] [CrossRef]
- Tepper, B.; Bartkowska, K.; Okrasa, M.; Ngati, S.; Braszak, M.; Turlejski, K.; Djavadian, R. Downregulation of TrkC Receptors Increases Dendritic Arborization of Purkinje Cells in the Developing Cerebellum of the Opossum, Monodelphis Domestica. Front Neuroanat 2020, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Bonfanti, L.; Peretto, P. Adult Neurogenesis in Mammals--a Theme with Many Variations. Eur. J. Neurosci. 2011, 34, 930–950. [Google Scholar] [CrossRef] [PubMed]
- Rodemer, W.; Gallo, G.; Selzer, M.E. Mechanisms of Axon Elongation Following CNS Injury: What Is Happening at the Axon Tip? Front Cell Neurosci 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- Herculano-Houzel, S. Mammalian Neurobiology: The Elephant (Brain) in the Room. Current Biology 2022, 32, R176–R178. [Google Scholar] [CrossRef]
- Cardoso-Moreira, M.; Halbert, J.; Valloton, D.; Velten, B.; Chen, C.; Shao, Y.; Liechti, A.; Ascenção, K.; Rummel, C.; Ovchinnikova, S.; et al. Gene Expression across Mammalian Organ Development. Nature 2019, 571, 505–509. [Google Scholar] [CrossRef]
- Saunders, N.R.; Adam, E.; Reader, M.; Møllgård, K. Monodelphis Domestica (Grey Short-Tailed Opossum): An Accessible Model for Studies of Early Neocortical Development. Anat Embryol (Berl) 1989, 180, 227–236. [Google Scholar] [CrossRef]
- Chew, L.-J.; Gallo, V. The Yin and Yang of Sox Proteins: Activation and Repression in Development and Disease. J Neurosci Res 2009, 87, 3277–3287. [Google Scholar] [CrossRef]
- Ellis, P.; Fagan, B.M.; Magness, S.T.; Hutton, S.; Taranova, O.; Hayashi, S.; McMahon, A.; Rao, M.; Pevny, L. SOX2, a Persistent Marker for Multipotential Neural Stem Cells Derived from Embryonic Stem Cells, the Embryo or the Adult. Dev Neurosci 2004, 26, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cui, W. Sox2, a Key Factor in the Regulation of Pluripotency and Neural Differentiation. World J Stem Cells 2014, 6, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Graham, V.; Khudyakov, J.; Ellis, P.; Pevny, L. SOX2 Functions to Maintain Neural Progenitor Identity. Neuron 2003, 39, 749–765. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.E.; Wynn, S.L.; Sesay, A.; Cruz, C.; Cheung, M.; Gomez Gaviro, M.-V.; Booth, S.; Gao, B.; Cheah, K.S.E.; Lovell-Badge, R.; et al. SOX9 Induces and Maintains Neural Stem Cells. Nat Neurosci 2010, 13, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.; Lee, H.K.; Glasgow, S.M.; Finley, M.; Donti, T.; Gaber, Z.B.; Graham, B.H.; Foster, A.E.; Novitch, B.G.; Gronostajski, R.M.; et al. Sox9 and NFIA Coordinate a Transcriptional Regulatory Cascade during the Initiation of Gliogenesis. Neuron 2012, 74, 79–94. [Google Scholar] [CrossRef]
- Sun, W.; Cornwell, A.; Li, J.; Peng, S.; Osorio, M.J.; Aalling, N.; Wang, S.; Benraiss, A.; Lou, N.; Goldman, S.A.; et al. SOX9 Is an Astrocyte-Specific Nuclear Marker in the Adult Brain Outside the Neurogenic Regions. J. Neurosci. 2017, 37, 4493–4507. [Google Scholar] [CrossRef]
- Herculano-Houzel, S.; Lent, R. Isotropic Fractionator: A Simple, Rapid Method for the Quantification of Total Cell and Neuron Numbers in the Brain. J Neurosci 2005, 25, 2518–2521. [Google Scholar] [CrossRef]
- Bahney, J.; von Bartheld, C.S. Validation of the Isotropic Fractionator: Comparison with Unbiased Stereology and DNA Extraction for Quantification of Glial Cells. J Neurosci Methods 2014, 222, 165–174. [Google Scholar] [CrossRef]
- Azevedo, F.A.C.; Carvalho, L.R.B.; Grinberg, L.T.; Farfel, J.M.; Ferretti, R.E.L.; Leite, R.E.P.; Jacob Filho, W.; Lent, R.; Herculano-Houzel, S. Equal Numbers of Neuronal and Nonneuronal Cells Make the Human Brain an Isometrically Scaled-up Primate Brain. J Comp Neurol 2009, 513, 532–541. [Google Scholar] [CrossRef]
- Herculano-Houzel, S. The Human Brain in Numbers: A Linearly Scaled-up Primate Brain. Frontiers in Human Neuroscience 2009, 3. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.E.D.; Porfirio, J.; Cunha, F.B. da; Manger, P.R.; Tavares, W.; Pessoa, L.; Raghanti, M.A.; Sherwood, C.C.; Herculano-Houzel, S. Cellular Scaling Rules for the Brains of Marsupials: Not as “Primitive” as Expected. BBE 2017, 89, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Petrović, A.; Kaur, J.; Tomljanović, I.; Nistri, A.; Mladinic, M. Pharmacological Induction of Heat Shock Protein 70 by Celastrol Protects Motoneurons from Excitotoxicity in Rat Spinal Cord in Vitro. Eur J Neurosci 2019, 49, 215–231. [Google Scholar] [CrossRef]
- Andreotti, J.P.; Silva, W.N.; Costa, A.C.; Picoli, C.C.; Bitencourt, F.C.O.; Coimbra-Campos, L.M.C.; Resende, R.R.; Magno, L.A.V.; Romano-Silva, M.A.; Mintz, A.; et al. Neural Stem Cell Niche Heterogeneity. Semin Cell Dev Biol 2019, 95, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Malatesta, P.; Appolloni, I.; Calzolari, F. Radial Glia and Neural Stem Cells. Cell Tissue Res. 2008, 331, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Kriegstein, A.; Alvarez-Buylla, A. The Glial Nature of Embryonic and Adult Neural Stem Cells. Annual Review of Neuroscience 2009, 32, 149–184. [Google Scholar] [CrossRef]
- Sarkar, A.; Hochedlinger, K. The Sox Family of Transcription Factors: Versatile Regulators of Stem and Progenitor Cell Fate. Cell Stem Cell 2013, 12, 15–30. [Google Scholar] [CrossRef]
- Pevny, L.H.; Nicolis, S.K. Sox2 Roles in Neural Stem Cells. The International Journal of Biochemistry & Cell Biology 2010, 42, 421–424. [Google Scholar] [CrossRef]
- Bylund, M.; Andersson, E.; Novitch, B.G.; Muhr, J. Vertebrate Neurogenesis Is Counteracted by Sox1-3 Activity. Nat Neurosci 2003, 6, 1162–1168. [Google Scholar] [CrossRef]
- Hoffmann, S.A.; Hos, D.; Küspert, M.; Lang, R.A.; Lovell-Badge, R.; Wegner, M.; Reiprich, S. Stem Cell Factor Sox2 and Its Close Relative Sox3 Have Differentiation Functions in Oligodendrocytes. Development 2014, 141, 39–50. [Google Scholar] [CrossRef]
- Mercurio, S.; Serra, L.; Motta, A.; Gesuita, L.; Sanchez-Arrones, L.; Inverardi, F.; Foglio, B.; Barone, C.; Kaimakis, P.; Martynoga, B.; et al. Sox2 Acts in Thalamic Neurons to Control the Development of Retina-Thalamus-Cortex Connectivity. iScience 2019, 15, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, M.; Mariani, J.; Lancini, C.; Latorre, E.; Caccia, R.; Gullo, F.; Valotta, M.; DeBiasi, S.; Spinardi, L.; Ronchi, A.; et al. Impaired Generation of Mature Neurons by Neural Stem Cells from Hypomorphic Sox2 Mutants. Development 2008, 135, 541–557. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, S.; Serra, L.; Nicolis, S.K. More than Just Stem Cells: Functional Roles of the Transcription Factor Sox2 in Differentiated Glia and Neurons. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, S.E.; Porfirio, J.; da Cunha, F.B.; Manger, P.R.; Tavares, W.; Pessoa, L.; Raghanti, M.A.; Sherwood, C.C.; Herculano-Houzel, S. Cellular Scaling Rules for the Brains of Marsupials: Not as “Primitive” as Expected. Brain Behav Evol 2017, 89, 48–63. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Primary cultures of P4-6 opossum cortex at DIV1. Cells were fixed 24 h after plating and stained for (A) β-tubulin III (TUJ1, red), (B) SOX2 (green), (C) DAPI nuclear stain (blue) and (D) merged. Images were acquired using 20x and 0.5 NA objective. Arrows indicate TUJ1/SOX2 double-positive neurons, while asterisks indicate non-neuronal SOX2-positive cells. Scale bar, 20μm.
Figure 1.
Primary cultures of P4-6 opossum cortex at DIV1. Cells were fixed 24 h after plating and stained for (A) β-tubulin III (TUJ1, red), (B) SOX2 (green), (C) DAPI nuclear stain (blue) and (D) merged. Images were acquired using 20x and 0.5 NA objective. Arrows indicate TUJ1/SOX2 double-positive neurons, while asterisks indicate non-neuronal SOX2-positive cells. Scale bar, 20μm.
Figure 2.
Immunohistochemistry of developing opossum cortex. (A) Coronal sections from P6 opossum cortex were immunostained for SOX2 (green), NeuN (red) and DAPI (blue) using 20x 0.5 NA objective. vz, ventricular zone. Scale bar, 100 µm. (B-E) Higher magnification image us-ing 40x 1.4 NA oil-immersion objective. Arrowheads indicate TUJ1/NeuN double-positive cells. Scale bar, 50 µm.
Figure 2.
Immunohistochemistry of developing opossum cortex. (A) Coronal sections from P6 opossum cortex were immunostained for SOX2 (green), NeuN (red) and DAPI (blue) using 20x 0.5 NA objective. vz, ventricular zone. Scale bar, 100 µm. (B-E) Higher magnification image us-ing 40x 1.4 NA oil-immersion objective. Arrowheads indicate TUJ1/NeuN double-positive cells. Scale bar, 50 µm.
Figure 3.
P6 cortex of Monodelphis domestica processed by isotropic fractionator. Obtained nuclei were stained for SOX2 (A, green), NeuN (B, red) and Hoechst 33342 (C, blue) and imaged using 60x 1.42 NA oil immersion objective. (D) Merging of SOX2 and NeuN. (E) Merging of SOX2, NeuN and Hoechst 33342. Red arrowheads show double-positive (SOX2/NeuN) nuclei. Scale bar, 10 µm.
Figure 3.
P6 cortex of Monodelphis domestica processed by isotropic fractionator. Obtained nuclei were stained for SOX2 (A, green), NeuN (B, red) and Hoechst 33342 (C, blue) and imaged using 60x 1.42 NA oil immersion objective. (D) Merging of SOX2 and NeuN. (E) Merging of SOX2, NeuN and Hoechst 33342. Red arrowheads show double-positive (SOX2/NeuN) nuclei. Scale bar, 10 µm.
Figure 4.
Primary cultures of P4-5 opossum cortex at DIV1. Cells were fixed 24 h after plating and stained for (A) SOX9 (magenta), (B) SOX2 (green), (C) β-tubulin III (TUJ1, red), (D) DAPI nuclear stain (blue). Images were acquired using 20x and 0.5 NA objective. (E) Merging of SOX2 and SOX9. (F) merging of all stainings. White aarrowheads indicate SOX2/SOX9 double-positive cells and green arrowheads indicate non-neuronal SOX2-positive, but SOX9-negative cell (TUJ1-/SOX9-/SOX2+). Scale bar, 25μm.
Figure 4.
Primary cultures of P4-5 opossum cortex at DIV1. Cells were fixed 24 h after plating and stained for (A) SOX9 (magenta), (B) SOX2 (green), (C) β-tubulin III (TUJ1, red), (D) DAPI nuclear stain (blue). Images were acquired using 20x and 0.5 NA objective. (E) Merging of SOX2 and SOX9. (F) merging of all stainings. White aarrowheads indicate SOX2/SOX9 double-positive cells and green arrowheads indicate non-neuronal SOX2-positive, but SOX9-negative cell (TUJ1-/SOX9-/SOX2+). Scale bar, 25μm.
Figure 5.
Immunohistochemistry of developing P6 opossum cortex. (A) Coronal sections from P6 opossum cortex were immunostained for SOX2 (green), SOX9 (red) and DAPI (blue) using 10x 0.3 NA objective. Scale bar, 100 µm. (B-E) Higher magnification image using 40x 1.4 NA oil-immersion objective. Arrowheads indicate SOX2/SOX9 double-positive cells. Scale bar, 25 µm. (F) P17 and (G-H) P30 cortex stained for GFAP (green), SOX9 (red) and DAPI (blue) using 40x 1.4 NA oil-immersion objective. vz, ventricular zone; mz, marginal zone. Scale bar, 25 µm.
Figure 5.
Immunohistochemistry of developing P6 opossum cortex. (A) Coronal sections from P6 opossum cortex were immunostained for SOX2 (green), SOX9 (red) and DAPI (blue) using 10x 0.3 NA objective. Scale bar, 100 µm. (B-E) Higher magnification image using 40x 1.4 NA oil-immersion objective. Arrowheads indicate SOX2/SOX9 double-positive cells. Scale bar, 25 µm. (F) P17 and (G-H) P30 cortex stained for GFAP (green), SOX9 (red) and DAPI (blue) using 40x 1.4 NA oil-immersion objective. vz, ventricular zone; mz, marginal zone. Scale bar, 25 µm.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).