1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS–CoV–2) causing Covid–19 led to an urgent need for preventive measures and vaccination especially in high-risk populations. Several mRNA vaccines were developed and tested including BNT162b2, a lipid nanoparticle–formulated, nucleoside–modified RNA vaccine encoding a SARS–CoV–2 Spike protein (S protein). BNT162b2 was authorized for medical use based on a large, placebo controlled, randomized phase 3 study, which testified to the safety and efficacy of two 30–μg doses administered 21 days apart [
1,
2]. At a median of 10.8 months after the second dose, a third dose of BNT162b2 was administered [
3]. At a median follow–up of 2.5 months, the third dose of mRNA vaccine resulted in 95.5% effectiveness against Covid-19 without identification of any new safety signals.
Health–care facilities represent places with a high risk of acquiring infections and personal protective equipment (PPE) combined with vaccination became the strongest tools for protection against Covid–19. Real world data specifically confirmed the high efficacy of BNT162b2 in health–care workers (HCWs) as reported in randomized trials for general populations [
4]. Despite the high effectiveness of the vaccine, new Covid–19 cases enhanced over time and as a result, the issue of waning immunity arose. A prospective study involving vaccinated HCWs revealed a consistent decline of correlating IgG and neutralizing antibody titers over a half year of follow–up. Six months after the second vaccine dose administration, neutralizing antibodies were significantly lower in males, individuals of 65 years and older, and those with immunosuppression [
5]. Our previous project [
6] showed high BNT162b2 efficacy despite a rapid decline of IgG antibodies and a negative cell–mediated immunity response in the majority of enrolled HCWs. At 6 months, IgG antibodies and T cell activation had declined significantly in individuals with obesity.
Some authors speculate that vitamin D plays a complementary role in the efficacy of different vaccines developed against Covid–19 because it activates Toll-2 receptors, increases the synthesis of antimicrobial peptides and enzymes which degrade lysosomes in macrophages, and promotes autophagy, enhancing innate immunity. Moreover, vitamin D, through activation of T–dependent B cells, stimulates production of virus–specific IgG1 antibodies and interleukin 10 (IL–10), along with T helper 17 cell suppression and improving THαβ CD+ T lymphocytes [
7]. In the case of viral influenza infection, a meta–analysis investigating the influence of vitamin D deficiency on the sero–protection and –conversion rates following vaccination, showed no significant associations [
8]. When considering the high prevalence of vitamin D deficiency in an apparently healthy Slovak population [
9], although having improved during Covid–19 pandemic probably due to higher supplementation of vitamin D [
10], our interest in the relationship between Covid–19, serum neutralizing IgG and depleted levels of vitamin D in a vaccinated population became justified.
The purpose of the current study was to identify the efficacy of a third dose of BNT162b2 and its safety according to sex and body mass index (BMI) in the real–world setting of a medical facility (the National Cancer Institute, Bratislava, SK; NCI) and to explore plasma IgG antibodies and serum 25–hydroxyl (25–OH) vitamin D concentrations following vaccination and to correlate these by sex, BMI, number of adverse events (AEs), and the presence of Covid–19.
2. Methods
2.1. Study design, inclusion/exclusion criteria, study end points
This is a prospective, non–randomized, single–center observational study with the primary objective of determining the effectiveness of the third dose BNT162b2 and its safety by sex and BMI in a real–world scenario. Effectiveness was defined by the subsequent onset of Covid-19. Infection, or its absence, was confirmed in the laboratory by rapid antigen, rapid antigen real–time reverse transcriptase polymerase chain reaction (RT–PCR), RT–PCR, or loop–mediated isothermal amplification (LAMP) tests. A secondary objective was to reveal possible differences in plasma neutralizing IgG and serum 25–OH vitamin D following vaccination.
To be eligible for enrollment, participants had to be current employees of NCI, vaccinated with three doses of BNT162b2, and ≥ 18 years of age. Vitamin D supplementation and Covid-19 diagnosed between second and third administration of the vaccine were exclusion criteria. The protocol and amendments were reviewed by the Ethics committee of the NCI, the code of protocol was Covid19–SK001. The trial was conducted in accordance with the International Council for Harmonization of Good Clinical Practice Guidelines (ICH GCPG) and the principles of the Declaration of Helsinki. All participants provided written informed consent. Data relating to this study were entered by the investigators into electronic data files and validated by an independent investigator.
2.2. Process of vaccination
All subjects were vaccinated at the NCI. The process of BNT162b2 handling was described elsewhere [
6] and all steps conform to the manufacturer’s recommendations and the product license.
2.3. IgG neutralizing antibodies and vitamin (25OH) D measurement
Blood samples were obtained from fasting participants by 6 mL vein puncture in the morning to the BD Vacutainer® with citrate and tested by the Atellica
® IM SARS–CoV–2 IgG (sCOVG), a two-step sandwich immunoassay based on indirect chemiluminescent technology for the qualitative and quantitative determination of IgG neutralizing antibodies to the
receptor binding-domain (RBD) of the S1 antigen of the SARS–CoV–2 [
11]. Results were reported in index values as nonreactive < 1.00 index, which were negative for IgG, or reactive ≥ 1.00 index considered positive for IgG. The analytical measuring interval was of 0.5–150.0 index.
25–OH vitamin D was measured from blood samples taken to the 6 ml BD Vacutainer® SST™ II Advance and the Cobas e 411 was used for analysis [
12]. First, the blood sample (20 μl) was incubated with pretreatment reagents to release 25–OH vitamin D from the vitamin D binding protein (VDBP). Then, the pretreated sample was incubated with ruthenium labeled VDBP to form a complex between the 25–OH vitamin D and the ruthenylated VDBP. The 24, 25–dihydroxy vitamin D present in the sample was bound with a specific unlabeled antibody to inhibit cross–reactivity. Finally, after the addition of streptavidin–coated micro–particles and 25–OH vitamin D labeled with biotin, the unbound ruthenylated labeled VDBPs became occupied. A complex of the ruthenylated VDBP and the biotinylated 25–OH vitamin D was formed and became bound to the solid phase via interaction of biotin and streptavidin. The reaction mixture was aspirated into the measuring cell where the microparticles were magnetically captured onto an electrode surface. The unbound substances were then removed with ProCell/ProCell M and application of voltage to the electrode induced chemiluminescent emission which was measured by a photomultiplier. Results in ng/ml were determined via an instrument – specifically a calibration curve generated by 2–point calibration and a master curve provided via the reagent barcode. 25–OH vitamin D concentrations were measured in ng/ml and values of ≤ 30 ng/ml were classified as vitamin D deficiency.
2.4. Statistical Analyses
The cutoff date for this study was March 20, 2022. For continuous variables, data were summarized by mean ± standard deviation (SD) and range,
p values were calculated using the T test for normally distributed values or the Wilcoxon–Mann–Whitney test for non–normally distributed values. For categorical variables, data were summarized by frequency and
p values were calculated using χ
2 or Fisher’s exact test. Multivariate analysis (MVA) was carried out by multiple linear regression. A
p value of 0.05 was considered significant. All statistical analyses were performed using NCSS 23 Statistical Software, Kaysville, UT, USA [
13].
3. Results
3.1. Participants
Between October 2021 and March 2022, 273 eligible subjects who met the inclusion criteria were enrolled into this study. Of these, 232 were HCWs (48 doctors, 103 nurses, 8 pharmacists, 52 medical technicians, 1 psychologist, 2 physiotherapists, 18 other HCWs) and 41 were non–HCWs. Overall, the majority were females (N = 233, 85.3%) and the median of BMI was 24.8 kg/m
2 (range 16.3 to 44.6 kg/m
2). Fifty–three participants had a high BMI of ≥ 30 kg/m
2. All individuals had been vaccinated with 3 doses of BNT162b2. The median time from second to third dose of the vaccine was 8.5 months (range 4.9 to 10.9 months). Baseline characteristics are showed in
Table 1.
3.2. Effectiveness of vaccination
Thirty–eight participants contracted Covid–19 following vaccination. Therefore, the efficacy of the BNT162b2 third dose was of 86.1% at the median follow–up of 4.7 months (range 2.9 to 5.0 months). In Slovakia during spring 2022, the predominant circulating SARS–CoV–2 strain was the omicron variant (B.1.1.529). The median time from the BNT162b2 third dose to the diagnosis of Covid–19 was 1.1 months (range 0.1 to 4.4 months) and all patients had a mild course of infection.
3.2. Adverse events following the third dose of the BNT162b2
At least one AE had 258 (94.5%) participants and the median number of BNT162b2 adverse events (AEs) was 3 (range 0 to 13). Three or more AEs had one hundred and forty–three subjects (52.4%) had three or more AEs.
Pain at the injection site, fatigue, and limb pain were most frequent and all AEs are summarized in
Table 2 and
Table 3. In addition, 17 participants reported other AEs possibly related to the vaccination as herpes infection (3), shiver (3), vomitus (2), vomitus and diarrhea (1), diarrhea (1), tinnitus (1), dry cough (1), abdominal pain (1), loss of appetite (1), lower back pain (1), tachycardia (1), and eyelid edema (1). The median number of AEs was significantly higher in females compared to males (3.0 ± 2.8
vs. 1.5 ± 2.6,
p < 0.0108) and in participants with high BMI compared to those with low BMI (4.0 ± 3.0
vs. 2.0 ± 2.8,
p < 0.0117). No serious AEs (SAEs) were reported.
Regarding sex, the incidence of joint pain and limb pain was statistically significantly higher in females
vs. males (
p < 0.0271 and
p < 0.0091, respectively;
Table 2.). The participants with high BMI more frequently reported muscle pain, joint pain, swelling, redness and itching at the injection site (
p < 0.0164,
p < 0.0486,
p = 0.0237,
p < 0.0078, and
p < 0.0061, respectively;
Table 3.).
3.3. IgG and 25–OH vitamin D following vaccination
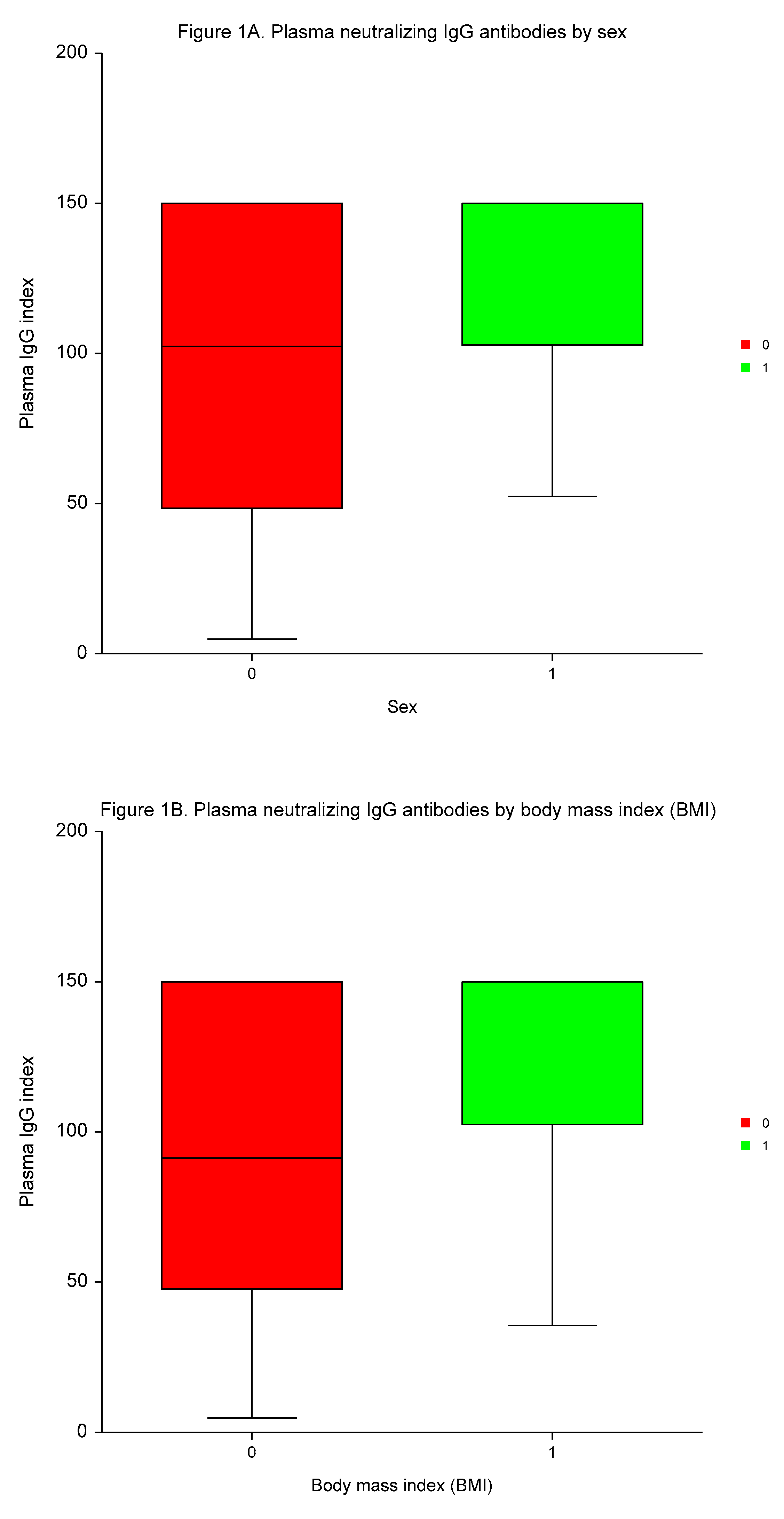
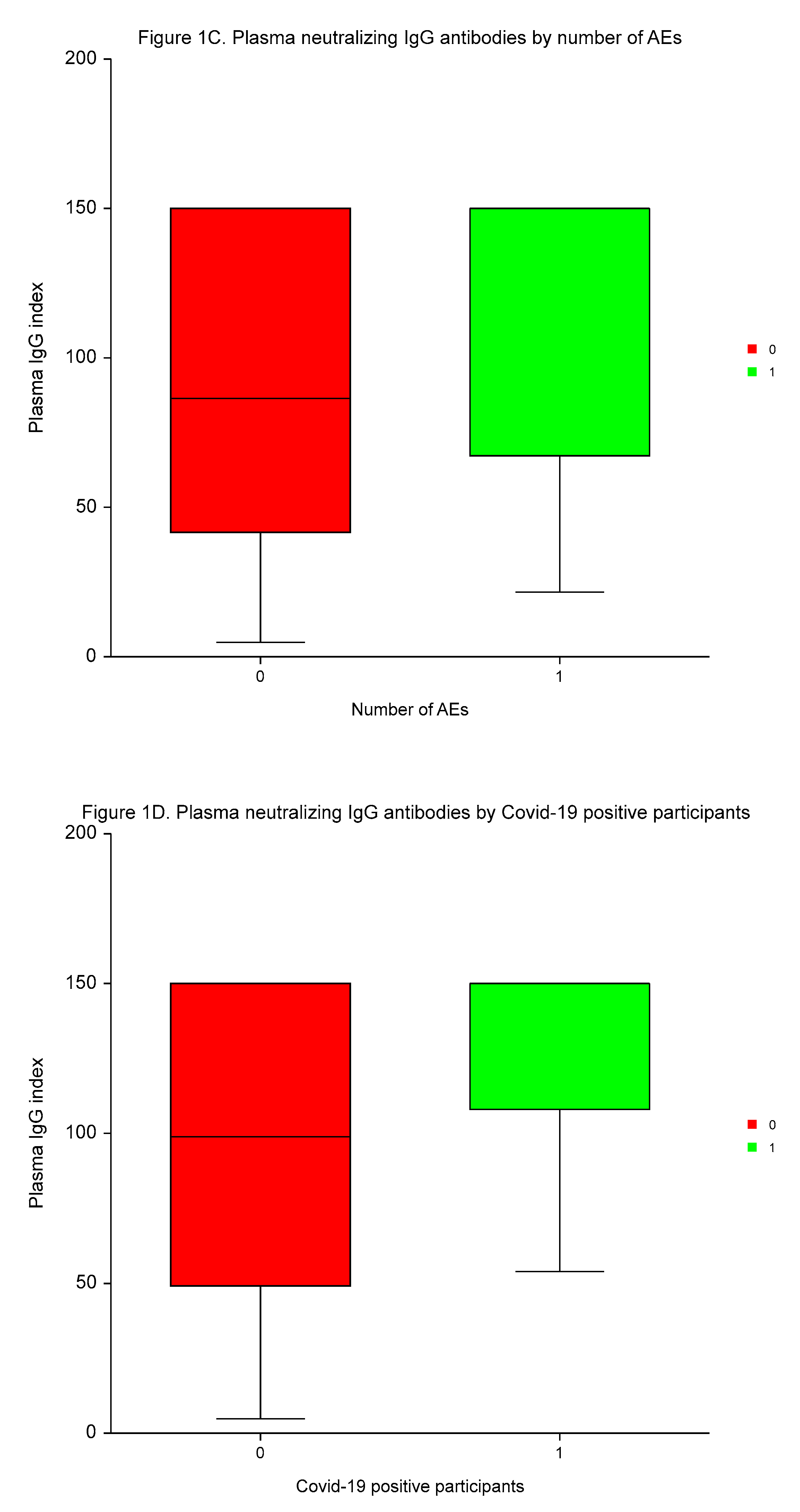
The median time to plasma IgG and serum 25–OH vitamin D measurement was 3.4 months (range 2.1 to 4.8 months). Vitamin D deficiency (25–OH vitamin D serum concentrations of ≤ 30 ng/ml) were present in 203 (74.4%) of participants. The medians of plasma IgG and serum 25–OH vitamin D values were 114.9 index (range 4.7 to 150.0 index), and 24.21 ng/ml (range 7.3 to 62.1 ng/ml), respectively.
Neutralizing IgG antibodies were significantly higher in males
vs. females, in participants with high BMI than low BMI, in individuals with a high number of AEs of ≥3
vs. a low number of AEs, and in patients testing positive for Covid–19
vs. individuals without Covid–19 (
p < 0.0004,
p < 0.0001,
p < 0.0003, and
p < 0.0005, respectively; (
Table 4,
Figure 1).
MVA demonstrated the independent predictive significance of sex, BMI, number of AEs, and Covid-19 for plasma concentrations of neutralizing IgG antibodies (p < 0.0001, p = 0.0095, p < 0.0001, and p = 0.0001, respectively).
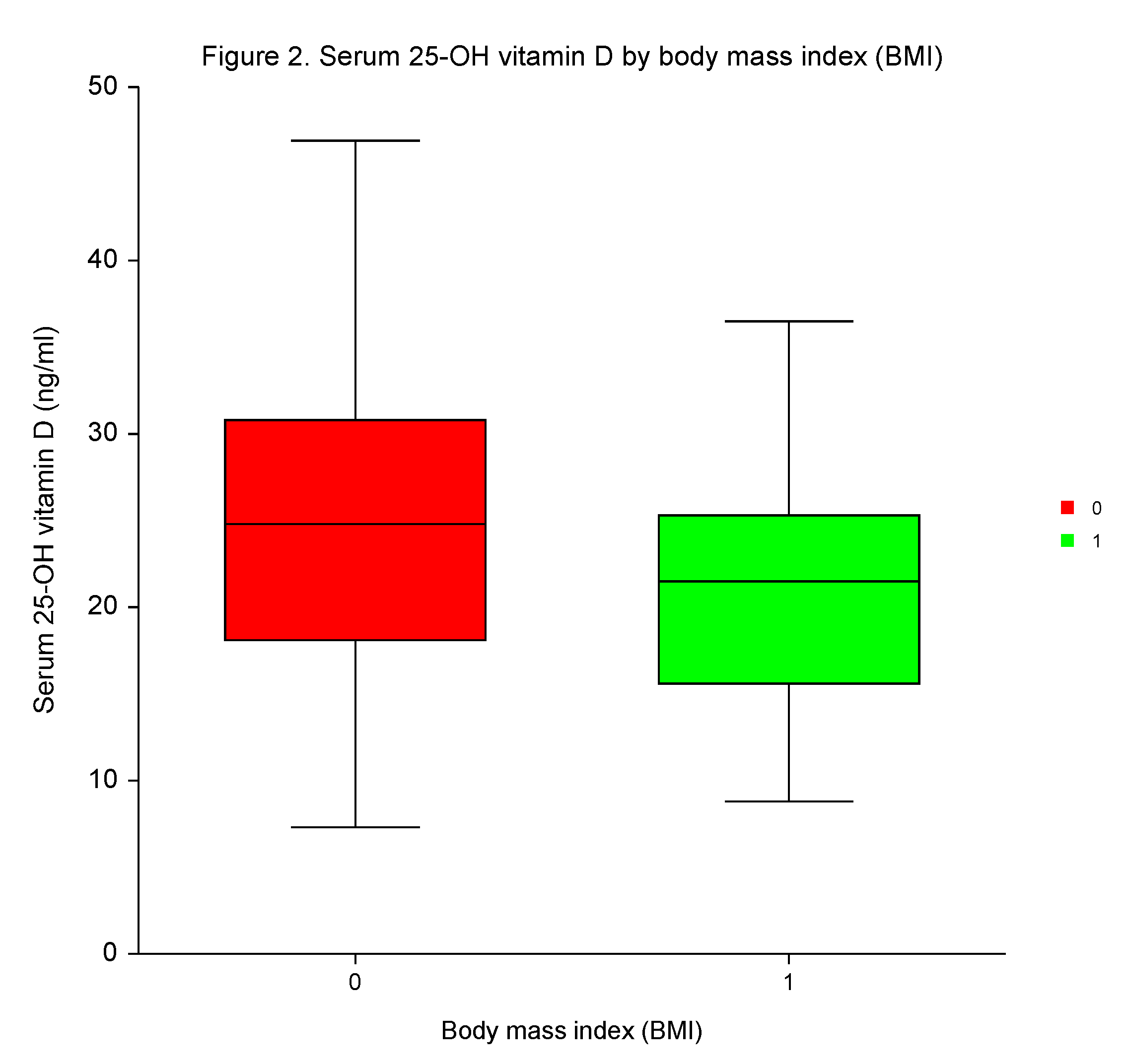
Following the third dose of the BNT162b2, the differences in 25–OH vitamin D levels were insignificant between the sexes, among patients with high or low incidences of AEs, and those with or without positivity for Covid-19.
However, participants with high BMI had significantly lower 25–OH vitamin D serum concentrations than those with low BMI (
p < 0.0144;
Table 5,
Figure 2). No independent predictive significance of sex, BMI, number of AEs, and Covid-19 for 25–OH vitamin D serum concentrations was shown in MVA (
p = 0.4090,
p = 0.1901,
p = 0.8960, and
p = 0.0934, respectively).
4. Discussion
In our prospective observational study, which included more than 270 participants, a third 30–µg dose of BNT162b2 received at a median of 8.5 months after the second dose was effective and safe. The efficacy of vaccination was 86.1% and 94.5% of participants had at least one AE. Among the most frequent AEs were injection site pain, referred to by 73.3% of participants, followed by fatigue (44.0%) and limb pain (33.0%). In the overall study population, statistically significant differences emerged in the incidence of pain (joints and limbs) in favor of males and pain (joints and muscles) with local reactions at the injection site in terms of swelling, itching and redness in favor of participants with low BMI. These observations corresponded with a significantly higher median number of adverse events in females compared to males and in participants with high vs. low BMI.
The effectiveness of BNT162b2 was slightly lower than in the phase 3 study [
3], in which the use of the third dose of this vaccine was authorized for persons who were 16 years of age or older as increased protection against Covid–19. The lower efficacy found in our study could be explained by the longer follow–up period and the circulation of a different predominant SARS–CoV–2 strain when efficacy was evaluated. An important fact may also be that the majority of this study population was represented by HCWs who are often more aware of clinically minor symptoms and signs that could lead to more frequent testing for SARS–CoV–2, and thus to a higher proportion of Covid–19 cases diagnosed. The higher overall incidence of AEs could also be explained by the structure of the study population consisting mostly of HCWs who are better informed about AEs and have a greater tendency to attribute newly developed difficulties to the administered vaccine.
The higher incidence of AEs in females than males and in those with high BMI compared to low BMI could stem from recognized germ line encoded differences in the innate responses evident in polymorphisms or variability in sex chromosomes and the autosomal genes encoding immunological proteins. Among the intersex differences are the varying number and activity of innate immune cells, CD4+, CD 8+ T and B lymphocyte subsets, the production of cytokines and chemokines. Finally, hormonal mediators, sex steroids as estradiol, progesterone, and androgens, play an important role in both innate and adaptive immunity. All these factors have an impact on vaccine efficacy. The effect of environmental factors as nutrition and microbiota cannot also be dismissed [
14]. Our results are consistent with those of a meta–analysis which included 46 studies and showed higher rates of adverse events in females rather than males following immunization after seasonal influenza vaccine [
15]. Differences in the incidence of adverse events following BNT162b2 mRNA vaccine in favor of males were shown in a further meta–analysis conducted by Green
et al. (16).
Furthermore, this study reveals statistically significant higher plasma neutralizing IgG antibodies following the third vaccine dose measured at the median time of 3.4 months in males, participants with high BMI and high number of AEs, and those with Covid–19. MVA showed all these factors were independent predictors of IgG values. Data relating to sex as a predictor of humoral immune response to vaccination are inconsistent. In some studies males emerged as low responders [
17,
18] but others have failed to confirm such a correlation [
19]. Papaioannidou
et al. did not establish any significant relationship between BMI and IgG antibodies after two doses of the BNT162b2 vaccine among HCWs in their hospital in Northern Greece [
20]. However, in our previous paper on dynamics of IgG following double BNT162b2 administration [
6], we provided evidence of high vaccine efficacy despite the rapid decline of plasma IgG and negative or borderline immune cell response in a specific population of employees of a medical facility. Certain significant differences in IgG were identified as being related to BMI, however these differences could have been a result of the small number of subjects in some subgroups. The associations between SARS–CoV–2 IgG antibody titer and the incidence of AEs following double vaccination with BNT162b2 [
21] and greater IgG levels in individuals who were infected after their booster mRNA vaccine compared to the uninfected subjects [
22] have been reported previously.
Covid–19 reduced mitochondrial bioenergy functions [
23] and vaccination against SARS–CoV–2 prevented the reduction of mitochondrial respiration and energy production in platelets [
24], hence hypothetical dysregulation in immune cell mitochondrial bioenergetics could constitute the mechanism of differing plasma IgG levels following vaccination in SARS–CoV–2 infected
vs. non–infected participants within this study.
In our study, most participants suffered from vitamin D deficiency, defined as 25–OH vitamin D serum concentrations of ≤ 30 ng/ml, which reflected the fact that previous vitamin D supplementation was an exclusion criterion and was fully compliant with a previously published paper [
9]. Despite the important roles of vitamin D in immunity, including interactions with innate and adaptive immune cells, co–regulation of their differentiations [
7], suppression of pro–inflammatory cytokines [
25], and induction of the transcription of the antimicrobial peptides cathelicidin and defensin [
26], we did not reveal any significant variances on 25–OH vitamin D concentrations in serum related to sex, AEs, and the infection caused by SARS–CoV–2 following vaccination. Even though the participants with low BMI had significantly higher 25–OH vitamin D levels, the predictive value of BMI was not confirmed in MVA.
The limitations of this study involve the lack of data on antibody levels prior to vaccination, chronic health conditions and their treatments, the upper limit of analytical measuring interval of 150.0 index, and various methods for confirming the diagnosis of Covid-19 including rapid antigen, rapid antigen RT–PCR, RT–PCR, and LAMP tests. However, our results brought real–world evidence on efficacy and safety of a third dose of the BNT162b2 mRNA vaccine against SARS–CoV–2 in a specific medical facility providing healthcare for patients with cancer diseases.
To conclude, this prospective, observational study confirmed the high effectiveness and safety of a third dose of BNT162b2 in employees of a national cancer center. A greater incidence of vaccine–related AEs was demonstrated in females and participants with high BMI. Furthermore, we revealed the dependance of neutralizing IgG antibodies in plasma on several factors including sex, frequency of vaccine adverse events, body mass index, and Covid–19 diagnosed following vaccination. As we believe that IgG titers are of great importance against Covid–19 infection disease outbreak, the knowledge obtained in this study could facilitate the setting up of vaccination programs and adaptation of the vaccine dosage and/or dose interval according to the characteristics of individual subjects.
Author Contributions
Conceptualization, P.P.; Data curation, P.P. and L.S.; Formal analysis, P.P.; Funding acquisition, P.P. and M.V.; Investigation, P.P., J.O. and K.R.; Methodology, P.P., A.S., and R.B.; Project administration, P.P.; Resources, P.P. and M.V.; Supervision, J.P.; Validation, M.M.; Visualization, P.P.; Writing-original draft, P.P.; Writing–review & editing, P.P. All authors have read and agreed to the published version of this manuscript.
Funding
National Cancer Institute, Bratislava, SK & OncoReSearch, Rovinka, SK. The funding bodies had no role in the manuscript writing.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
All participants provided written informed consent before enrollment.
Data Availability Statement
All the data presented in this study are available on request from the corresponding author.
Acknowledgments
Our gratitude goes to Katarína Zanchetta and Miroslava Augustínová from National Cancer Institute, Bratislava, SK for their excellent technical support, and Michael K Hill (Hill Long Associates, Teddington, UK) for his great assistance in improving the final version of this manuscript.
Conflicts of Interest
The authors declare no conflict of interests related to this study.
Abbreviations
| AE |
adverse event |
| BMI |
body mass index |
| HCWs |
health–care workers |
| ICHGCPG |
International Council for Harmonization Good Clinical Practice Guidelines |
| IL–10 |
interleukin 10 |
| LAMP test |
loop–mediated isothermal amplification tests |
| MVA |
multivariate analysis |
| N |
number of participants |
| NCI |
National Cancer Institute, Bratislava, SK |
| PPE |
personal protective equipment |
| RBD |
receptor binding-domain |
| RT–PCR test |
real–time reverse transcriptase polymerase chain reaction test |
| SAE |
serious adverse event |
| SARS–CoV–2 |
severe acute respiratory syndrome coronavirus 2 |
| SD |
standard deviation |
| SE |
standard error |
| S protein |
spike protein |
| VDBP |
vitamin D binding protein |
| 25–OH vitamin D |
25–hydroxyl vitamin D |
References
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; Bailey, R.; Swanson, K.A.; Roychoudhury, S.; Koury, K.; Li, P.; Kalina, W.V.; Cooper, D.; Frenck, R.W. Jr.; Hammitt, L.L.; Türeci, Ö.; Nell, H.; Schaefer, A.; Ünal, S.; Tresnan, D.B.; Mather, S.; Dormitzer, P.R.; Şahin, U.; Jansen, K.U.; Gruber, W.C.; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Moreira, E.D. Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; Bailey, R.; Swanson, K.A.; Xu, X.; Roychoudhury, S.; Koury, K.; Bouguermouh, S.; Kalina, W.V.; Cooper, D.; Frenck, R.W. Jr.; Hammitt, L.L.; Türeci, Ö.; Nell, H.; Schaefer, A.; Ünal, S.; Yang, Q.; Liberator, P.; Tresnan, D.B.; Mather, S.; Dormitzer, P.R.; Şahin, U.; Gruber, W.C.; Jansen, K.U.; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N Engl J Med 2021, 385, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Moreira, E.D. Jr.; Kitchin, N.; Xu, X.; Dychter, S.S.; Lockhart, S.; Gurtman, A.; Perez, J.L.; Zerbini, C.; Dever, M.E.; Jennings, T.W.; Brandon, D.M.; Cannon, K.D.; Koren, M.J.; Denham, D.S.; Berhe, M.; Fitz-Patrick, D.; Hammitt, L.L.; Klein, N.P.; Nell, H.; Keep, G.; Wang, X.; Koury, K.; Swanson, K.A.; Cooper, D.; Lu, C.; Türeci, Ö.; Lagkadinou, E.; Tresnan, D.B.; Dormitzer, P.R.; Şahin, U.; Gruber, W.C.; Jansen, K.U.; C4591031 Clinical Trial Group. Safety and Efficacy of a Third Dose of BNT162b2 Covid-19 Vaccine. N Engl J Med 2022, 386, 1910–1921. [Google Scholar] [CrossRef] [PubMed]
- Paris, C.; Perrin, S.; Hamonic, S.; Bourget, B.; Roué, C.; Brassard, O.; Tadié, E.; Gicquel, V.; Bénézit, F.; Thibault, V.; Garlantézec, R.; Tattevin, P. Effectiveness of mRNA-BNT162b2, mRNA-1273, and ChAdOx1 nCoV-19 vaccines against COVID-19 in healthcare workers: an observational study using surveillance data. Clin Microbiol Infect 2021, 27, 1699.e5–1699.e8. [Google Scholar] [CrossRef] [PubMed]
- Levin, E. G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; Rubin, C.; Freedman, L.; Kreiss, Y.; Regev-Yochay, G. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N Engl J Med 2021, 385, e84. [Google Scholar] [CrossRef]
- Palacka, P.; Poľanová, M.; Svobodová, A.; Žigmond, J.; Zanchetta, K.; Gombárová, V.; Vulganová, M.; Slopovský, J.; Obertová, J.; Drgoňa, Ľ.; Mego, M.; Pechan, J. Effectiveness, Adverse Events, and Immune Response Following Double Vaccination with BNT162b2 in Staff at the National Comprehensive Cancer Center (NCCC). Vaccines 2022, 10, 558. [Google Scholar] [CrossRef]
- Chiu, S.K.; Tsai, K.W.; Wu, C.C.; Zheng, C.M.; Yang, C.H.; Hu, W.C.; Hou, Y.C.; Lu, K.C.; Chao, Y.C. Putative Role of Vitamin D for COVID-19 Vaccination. Int J Mol Sci 2021, 22, 8988. [Google Scholar] [CrossRef]
- Lee, M.D.; Lin, C.H.; Lei, W.T.; Chang, H.Y.; Lee, H.C.; Yeung, C.Y.; Chiu, N.C.; Chi, H.; Liu, J.M.; Hsu, R.J.; Cheng, Y.J.; Yeh, T.L.; Lin, C.Y. Does Vitamin D Deficiency Affect the Immunogenic Responses to Influenza Vaccination? A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 409. [Google Scholar] [CrossRef]
- Sebekova, K.; Krivosikova, Z.; Gajdos, M.; Podracka, L. Vitamin D status in apparently healthy medication-free Slovaks: Association to blood pressure, body mass index, self-reported smoking status and physical activity. Bratisl Lek Listy 2016, 117, 702–709. [Google Scholar] [CrossRef]
- Smaha, J.; Jackuliak, P.; Kužma, M.; Max, F.; Binkley, N.; Payer, J. Vitamin D Deficiency Prevalence in Hospitalized Patients with COVID-19 Significantly Decreased during the Pandemic in Slovakia from 2020 to 2022 Which Was Associated with Decreasing Mortality. Nutrients 2023, 15, 1132. [Google Scholar] [CrossRef]
- SARS-CoV-2 IgG (sCOVG) Assay for the Detection of IgG Antibodies to SARS-CoV-2. Available online: https://www.fda.gov/media/146931/download (accessed on 26 August 2023).
- Vitamin D Total II. Available online: https://labogids.sintmaria.be/sites/default/files/files/ vit._d_total_ii_2017-11_v2.pdf (accessed on 26 August 2023).
- NCSS Statistical Software. NCSS, LLC; Kaysville, UT, USA: 2023. Available online: https://www.ncss.com/.
- Klein, S.L.; Flanagan, K.L. Sex Differences in Immune Responses. Nat Rev Immunol 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Tadount, F.; Doyon-Plourde, P.; Rafferty, E.; MacDonald, S.; Sadarangani, M.; Quach, C. Is there a Difference in the Immune Response, Efficacy, Effectiveness and Safety of Seasonal Influenza Vaccine in Males and Females? - A Systematic Review. Vaccine 2020, 38, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Green, M.S.; Peer, V.; Magid, A.; Hagani, N.; Anis, E.; Nitzan, D. Gender Differences in Adverse Events Following the Pfizer-BioNTech COVID-19 Vaccine. Vaccines 2022, 10, 233. [Google Scholar] [CrossRef]
- Wei, J.; Stoesser, N.; Matthews, P.C.; Ayoubkhani, D.; Studley, R.; Bell, I.; Newton, J.N.; Farrar, J.; Diamond, I.; Rourke, E.; et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat Microbiol 2021, 6, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Amodio, E.; Capra, G.; Casuccio, A.; De Grazia, S.; Genovese, D.; Pizzo, S.; Calamusa, G.; Ferraro, D.; Giammanco, G.M.; Vitale, F.; et al. Antibodies responses to SARS-CoV-2 in a large cohort of vaccinated subjects and seropositive patients. Vaccines 2021, 9, 714. [Google Scholar] [CrossRef] [PubMed]
- Đaković Rode, O.; Bodulić, K.; Zember, S.; Cetinić Balent, N.; Novokmet, A.; Čulo, M.; Rašić, Ž.; Mikulić, R.; Markotić, A. Decline of Anti-SARS-CoV-2 IgG Antibody Levels 6 Months after Complete BNT162b2 Vaccination in Healthcare Workers to Levels Observed Following the First Vaccine Dose. Vaccines 2022, 10, 153. [Google Scholar] [CrossRef]
- Papaioannidou, P.; Skoumpa, K.; Bostanitis, C.; Michailidou, M.; Stergiopoulou, T.; Bostanitis, I.; Tsalidou, M. Age, Sex and BMI Relations with Anti-SARS-CoV-2-Spike IgG Antibodies after BNT162b2 COVID-19 Vaccine in Health Care Workers in Northern Greece. Microorganisms 2023, 11, 1279. [Google Scholar] [CrossRef]
- Braun, E.; Horowitz, N.A.; Leiba, R.; Weissman, A.; Mekel, M.; Shachor-Meyouhas, Y.; Hussein, K.; Halberthal, M.; Azzam, Z.S.; Berger, G. Association between IgG antibody levels and adverse events after first and second Bnt162b2 mRNA vaccine doses. Clin Microbiol Infect 2022, 28, 1644–1648. [Google Scholar] [CrossRef]
- Ailsworth, S.M.; Keshavarz, B.; Richards, N.E.; Workman, L.J.; Murphy, D.D.; Nelson, M.R.; Platts-Mills, T.A.E.; Wilson, J.M. Enhanced SARS-CoV-2 IgG durability following COVID-19 mRNA booster vaccination and comparison of BNT162b2 with mRNA-1273. Ann Allergy Asthma Immunol 2023, 130, 67–73. [Google Scholar] [CrossRef]
- Sumbalova, Z.; Kucharska, J.; Palacka, P.; Rausova, Z.; Langsjoen, P.H.; Langsjoen, A.M.; Gvozdjakova, A. Platelet mitochondrial function and endogenous coenzyme Q10 levels are reduced in patients after COVID-19. Bratisl Lek Listy 2022, 123, 9–15. [Google Scholar] [CrossRef]
- Gvozdjáková, A.; Kucharská, J.; Rausová, Z.; Lopéz-Lluch, G.; Navas, P.; Palacka, P.; Bartolčičová, B.; Sumbalová, Z. Effect of Vaccination on Platelet Mitochondrial Bioenergy Function of Patients with Post-Acute COVID-19. Viruses 2023, 15, 1085. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, N.; Turan, H.; Bayraktar, M.; Ozturk, A.; Erdoğdu, H. Analysis of Serum Cytokine and Protective Vitamin D Levels in Severe Cases of COVID-19. J Med Virol 2021, 94, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, A.; Rustecka, A.; Lipińska-Opałka, A.; Piprek, R.P.; Kloc, M.; Kalicki, B.; Kubiak, J.Z. The Role of Vitamin D in COVID-19 and the Impact of Pandemic Restrictions on Vitamin D Blood Content. Front Pharmacol 2022, 13, 836738. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).