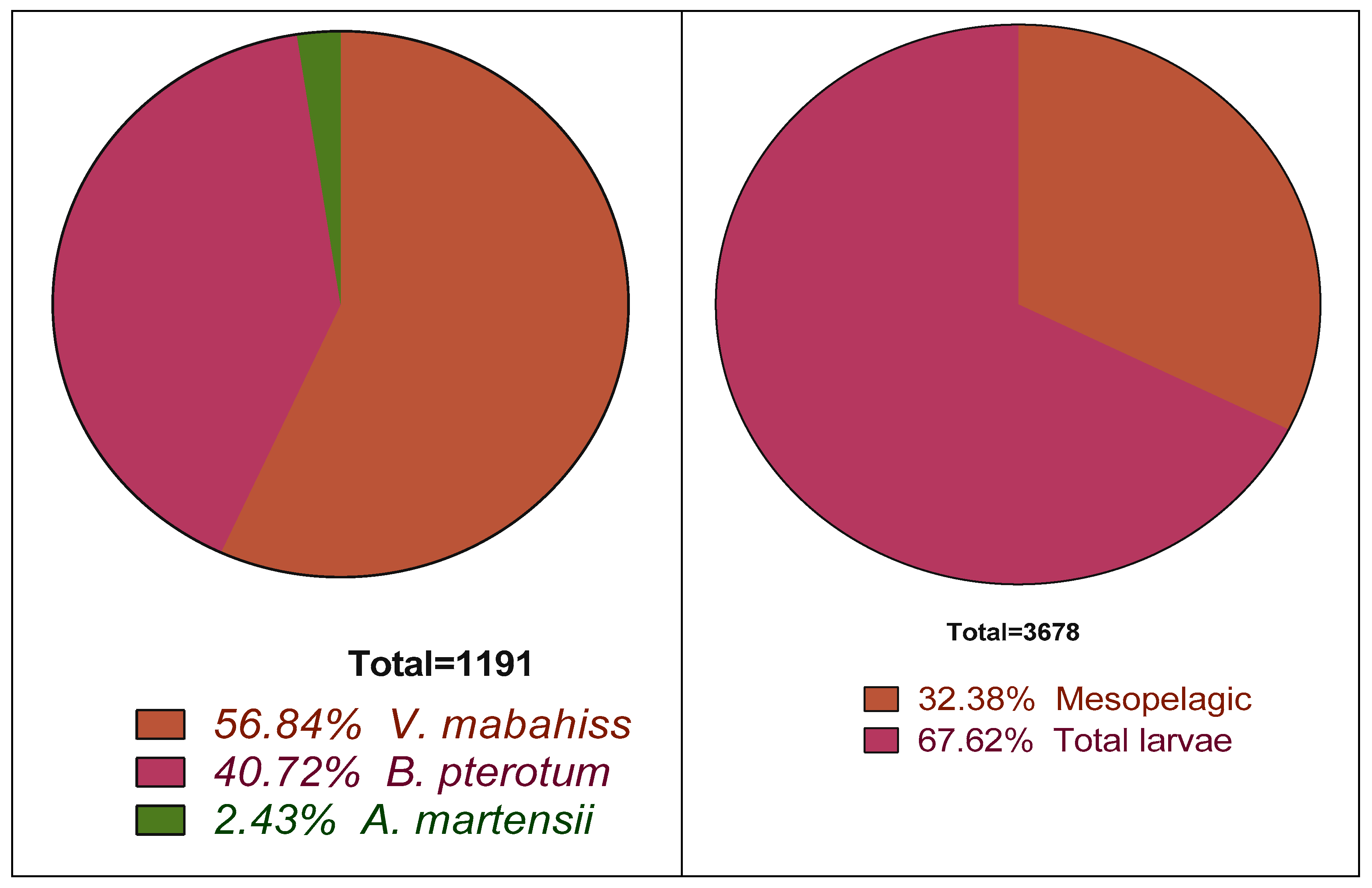1. Introduction
Mesopelagic (or midwater) fish live in the intermediate pelagic water masses between the euphoric zone at 100 m and the deep bathypelagic zone at 1000 m where no light is found (Kaiser et al., 2011; Clavel-Henry et, 2020). Most mesopelagic species undergo vertical migration into the epipelagic zone at night to feed and later return to mesopelagic depths during the day (Olivar et al., 2012).
Mesopelagic species are the most abundant marine vertebrates in the world oceans (Mann, 1984) with a total biomass of billions of tons of which about 300 million tons occur in the Indian Ocean. These estimates represent approximately 10 times the biomass of the world's total fish catch. Due to the high proportion of wax-esters of limited nutritional value, however, only a few species, such as Benthosema glaciale in the Oman Sea (Valinassab et al., 2007), are potentially abundant enough for commercial purposes. Although most species lack commercial importance, mesopelagic fishes play essential roles in the ocean’s pelagic and benthic food webs and are able to affect the dynamics of their zooplankton prey (Williams et al., 2001; Saunders et al., 2019), and predators, such as some commercially important pelagic fishes (Würtz, 2010), marine mammals (Giménez et al., 2017), seabirds (Barrett et al., 2002), and benthic predators (Herring, 2002), and are important in transferring energy between ocean ecosystems.
Due to their small size, mesopelagic fish are characterized by low fecundity, and average from a few hundred to few thousand eggs over the spawning season. Most species are oviparous with plankton eggs and larvae and some exhibit batch-spawning characteristics (i.e., spawn eggs in batches over several months). Eggs are released either during the day in mesopelagic waters, or at night in the epipelagic zone where larvae hatch and may remain until metamorphosis during which the light organs or photophores develop; thereafter, they return to deeper water juvenile and adult habitat. Some mesopelagic species are distributed worldwide, and many are circumpolar, especially in the Southern Hemisphere (Salvanes and Kristofersen, 2001).
Much of the research on the distribution and natural history of mesopelagic fishes was conducted in the 1970s’ when FAO (Food and Agriculture Organization) searched for new unexplored commercial resources. Interest in mesopelagic fishes has been renewed since the 1990’s after the discovery that the sound-scattering layers (SSL’s) in the ocean was high densities of mesopelagic species, which formed the basis for studies of the life history and adaptations of mesopelagic fish in the context of general ecological theory.
Investigations of the midwaters of the Red Sea have shown that the deep-water fauna in general is quite limited (Marshall, 1963; Marshall and Bourne, 1964; Aron and Goodyear, 1969; Halim, 1969; Weikert, 1982) due to the unusually isothermal structure of the waters below the sill level that makes the Red Sea unsuitable for all but a very few adaptable species.
Sixteen species of mesopelagic fish occur in the Red Sea (
Table 1) with little known about their spawning seasonality. This work aims to describe the developmental stages of three mesopelagic species whose larvae are abundant near coral reefs in the Red Sea. Larvae of two species,
V. mabahiss and A. martensii are described for the first time.
2. Material and methods
2.1. Study area
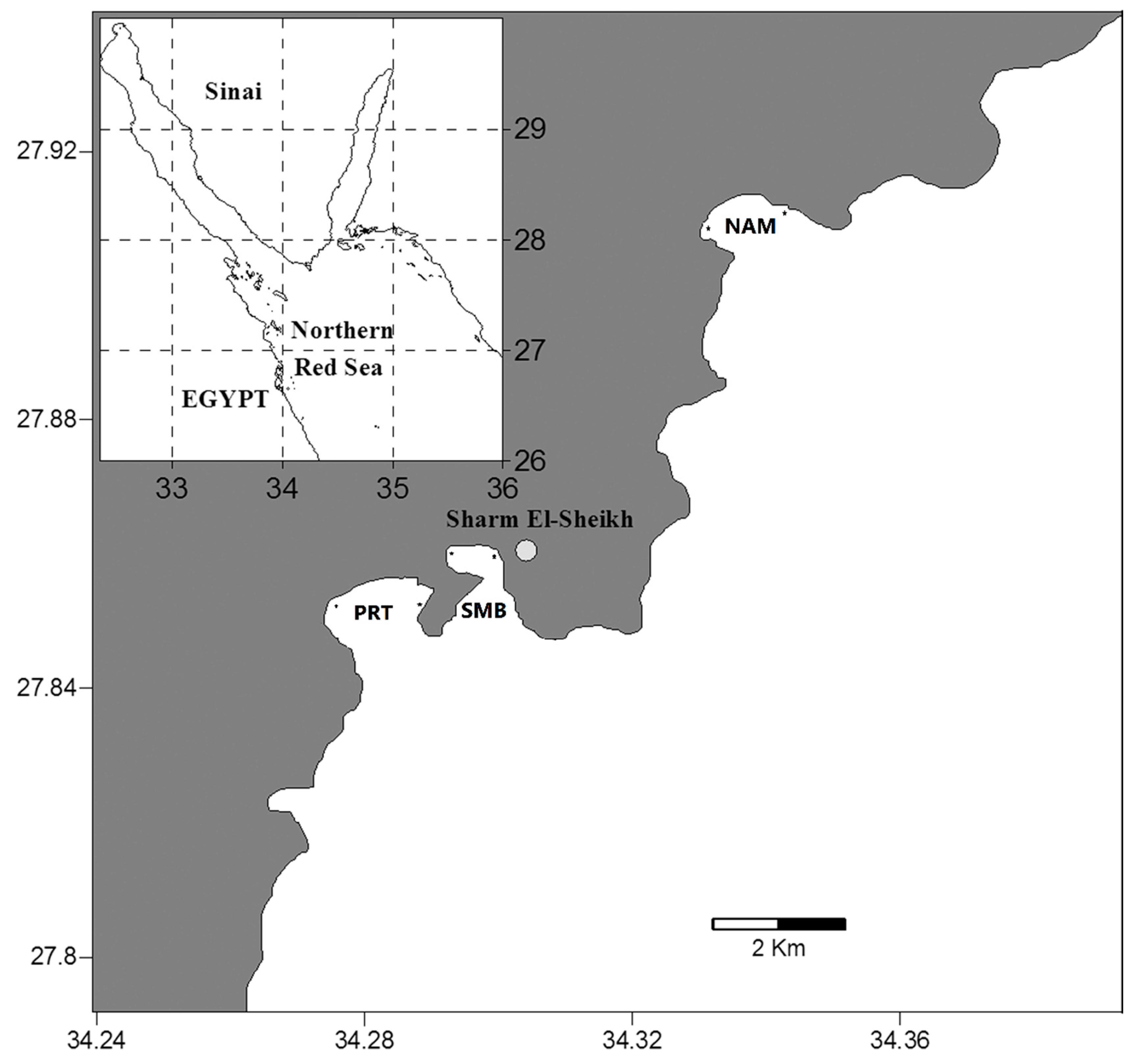
The present study was carried out at three coastal sites along Gulf of Aqaba coast (
Figure 1), Naama Bay (NAM), Sharm El-Maya Bay (SMB) and Port Bay (PRT), each with different ecological conditions. Naama Bay is located approximately 15 km south of the Strait of Tiran at 27° 55' N and 34° 20' E with a maximum depth of 100 m and is exposed to touristic stress from its many resorts, sailing, and diving activities. This bay is bordered by a fringing reefs characteristics of the Red Sea. Sharm El-Maya Bay is a semi-enclosed bay at 27° 51.8' N and 34° 18.1' E and is divided into two water bodies, a small, near-shore, sub-tidal area (mean depth: 9 m) and a larger area with a maximum depth of 90 m connected to the Red Sea through an opening 200 m wide. The bottom is covered mainly with seagrass patches. Port Bay lies at the entrance of Sharm El-Sheikh City at 27° 51' N and 34° 16.7' E with a maximum depth of 35 m and is subjected to less stress by tourism and other human activities than the other two sites. Borders of this bay are characterized by coral reefs whereas the bottom is sandy with many reef and seagrass patches.
2.2. Field work
Each site was sampled with a 100 cm mouth diameter and 500 µm mesh plankton net. The net was hauled horizontally parallel to the reef with the top of the net at the water’s surface for 10 minutes at a speed of 1.5 to 2.5 knots. Two stations were sampled monthly with three replicates each site from January to December 2015, which resulted in collection of 216 plankton samples. The sampling process was conducted in the morning just before sunrise and in the afternoon just before sunset. A flowmeter fitted in the mouth of the net was used to estimate the volume of water filtered, which was calculated. Samples were fixed in buffered 5% formalin solution in seawater on board ship and later preserved in 90% ethanol for further examination in the laboratory.
2.3. Laboratory work
The samples were examined under a stereomicroscope and larvae were sorted and separated by group. The larvae of mesopelagic fishes were separated, counted, and measured under the stereomicroscope with an eyepiece micrometer. Measurements were expressed as proportions of the body length (BL) and rounded to the nearest 0.1 mm. Here, we consider BL equivalent to standard length (SL) and use both interchangeably. Larvae were identified to species level, where possible, using relevant identification guides (Abu El-Regal, 2000 and 2008), and were divided into preflexion, flexion and postflexion stages based on degree of notochord flexion. Larvae were placed in separate labeled vials by life stage and preserved in 70% ethanol for further examination and description.
2.4. Data analysis
Abundance of fish larvae was expressed as number of larvae in 1000 m
3 based on the following equation:
where ‘A’ is the abundance of larvae of a given taxon; ‘N’ is the number of larvae of said taxon collected; and ‘V’ is the volume of water filtered. A correlation between the standard length and other measurements was tested by SPSS 22. Univariate statistics were done in SPSS 22 using analysis of variance (ANOVA) to determine differences in number of individuals and number of species between months and sites. All data were tested for homogeneity of variance. Where the samples were not homogeneous, data were either transformed or the non-parametric Kruskal-Wallis test was used (Zar, 1999; Dytham, 2003). Graphs were illustrated in GraphPad Prism 8.
2.5. Ethical statement
Samples are collected by plankton net, and they are only plankton samples and there are no rules to follow.
3. Results
3.1. General abundance of larvae of mesopelagic fish
Mesopelagic fish in the current study were represented by four species in four families and three orders, Stomiiformes, Myctophifiormes, and Scombriformes. Order Stomiiformes was represented by two species in two families (Phosichthyidae,
V. mabahiss, and Stomiidae,
A. martensii). Order Myctophiformes was represented by
B. pterotum (Myctophidae). Scombriformes were represented by one family, Trichiuridae, and one species, likely
T. auriga. The larvae of mesopelagic fishes constituted about 32% of all larvae (1191 of 3678 total larvae collected). The most abundant mesopelagic species was
Vinciguerria mabhaiss with 677 larvae, which represents 18% of all fish larvae collected and 5
7% of larvae of mesopelagic species, followed by
Benthosema pterotum with 485 larvae (13% of all larvae and 41% of all mesopelagic larvae) (
Table 2). The larvae of
Astronesthes martensii (n = 29) accounted for 2% of all mesopelagic larvae (
Figure 2). A 5 mm trichiurid larva (likely
Trichiurus auriga) was also collected in Sharm El-Maya Bay during July but was excluded from further analyses.
The ANOVA showed that the abundance of V. mabahiss differed significantly among months (F=4.94, P<0.05), but not among bays (F=0.89, P>0.05). The abundance of B. pterotum also differed significantly among months (F=4, P<0.05), but not among bays (F=1.2, P>0.05), as did A. martensii among months (F=2.4, P<0.05), but not among bays (F=0.1, P>0.05).
3.2. Vinciguerria mabahiss
3.2.1. Description of larvae
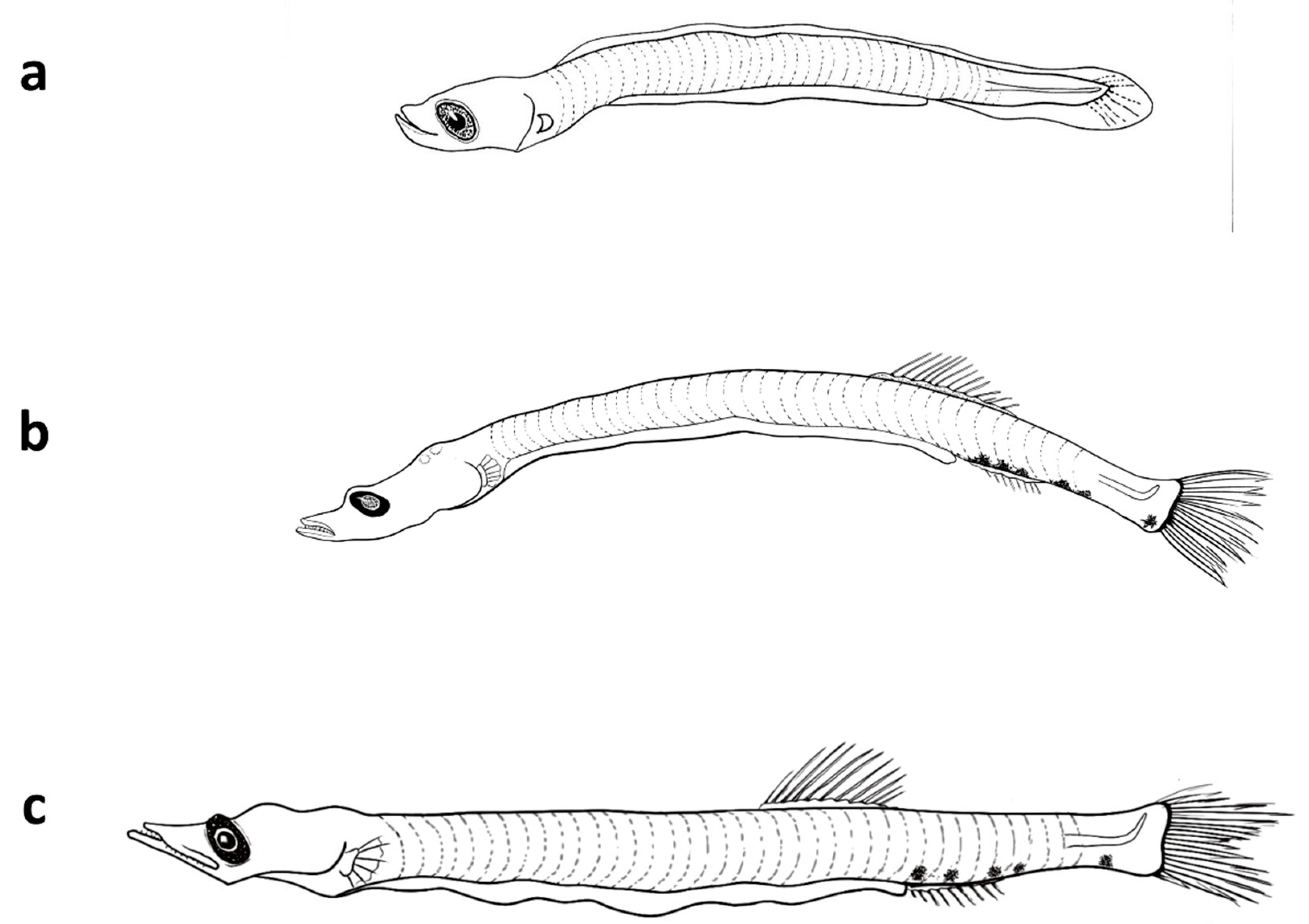
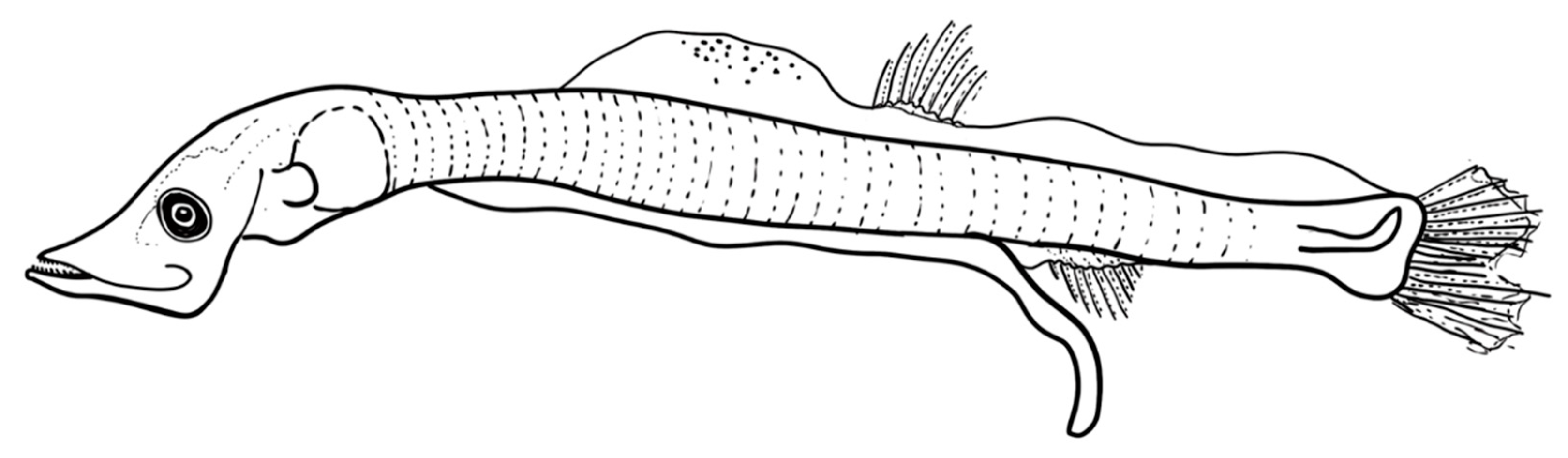
The larvae are elongate with 37-39 myomeres and have a long gut (70%-80% BL). The head has an elongated snout, large mouth, and elliptical eye; larvae also lack fin and head spines (
Table 3). Preflexion larvae of 5 mm have pectoral and pelvic fin buds, but lack anlage in the dorsal, anal, and caudal fins. Head length is about 20% BL, and body depth is 8% BL at this size (
Figure 3, Table 4). Notochord flexion occurs between 6 mm and 7 mm, after which early postflexion larvae have a well-formed caudal fin. By 8 mm, rays begin to develop in the dorsal fin. By about 9 mm, rays begin to develop in the anal fin, which originates below mid-base of the dorsal fin; canine teeth are also visible in both jaws at this size, but pectoral fin rays remain undeveloped. Larvae also have pigment at the base of the caudal fin (hypurals area), and three melanophores along the ventral margin of the caudal peduncle. By 12.5 mm, the dorsal and anal fins have 14 rays each, and rays are developing in the pectoral fins. At this size,
V. mabahiss typically have a melanophore above the cleithrum, one above the hindgut near the vent, one to three melanophores along the ventral margin of the caudal peduncle, and pigment dorsally above the caudal fin base.
Distinguishing characters of V. mabahiss include an elongate body; elliptical eyes and duck-like snout; the anal fin originates below the middle of the dorsal fin base; myomere count of 37-39; and unusual pigmentation pattern. V. mabahiss can be confused with larvae of members of Clupeiformes; however, clupeids and engraulids have round eyes and a longer preanal length (typically >80% compared to <80% in V. mabahiss) and differ in pigmentation pattern and in the relative positions of the dorsal and anal fins. Clupeids and engraulids also lack the canine teeth of V. mabahiss. Larvae closely resemble those of V. nimbaria (Ahlstrom et al., 1984; Watson, 1996a), which occur in the Indian Ocean, but not Red Sea.
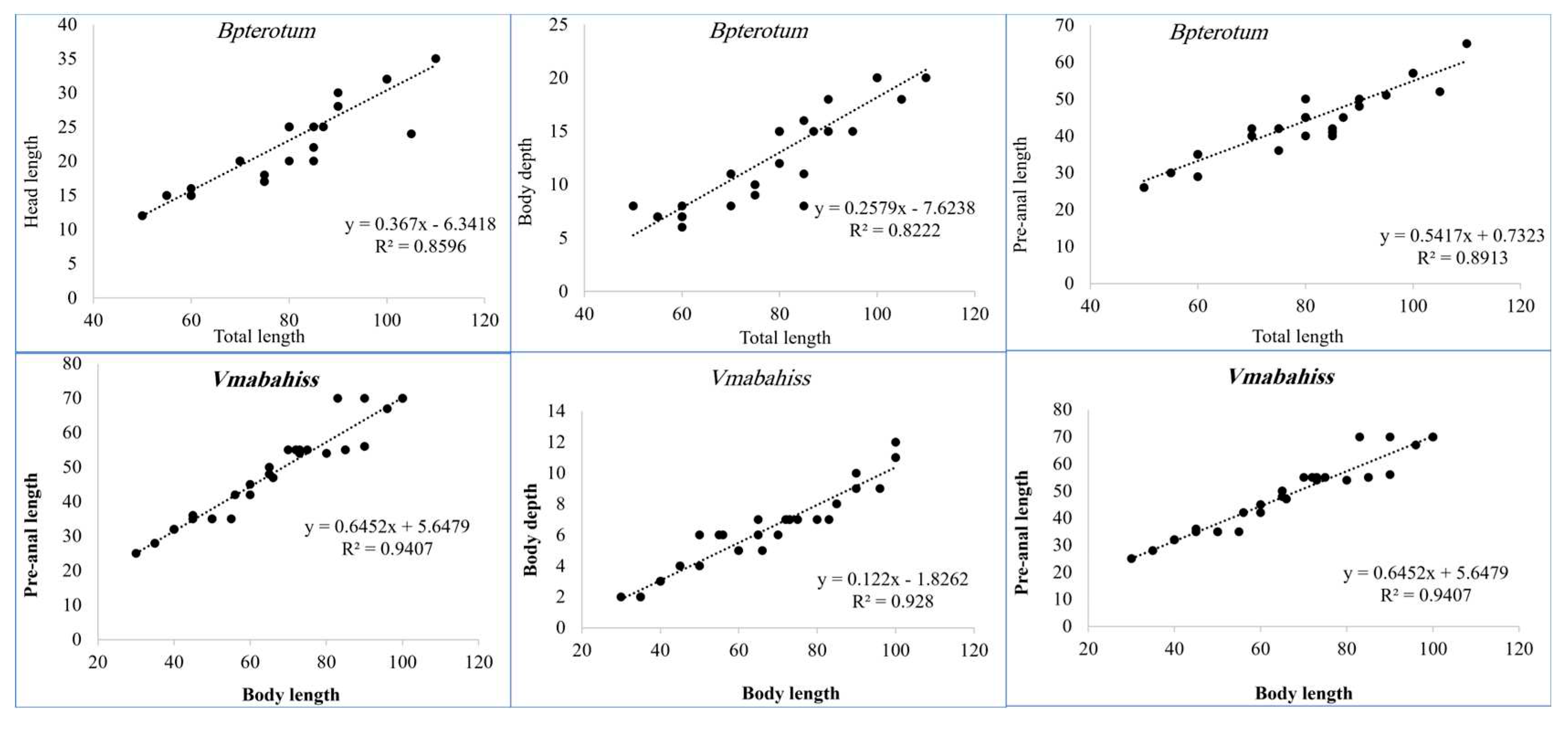
The study of morphometrics showed that the ratios of head length (HL), body depth (BD, preanal length (PAL) and pre-dorsal length (PDL) increase linearly with the standard length (
Figure 4,
Table 4).
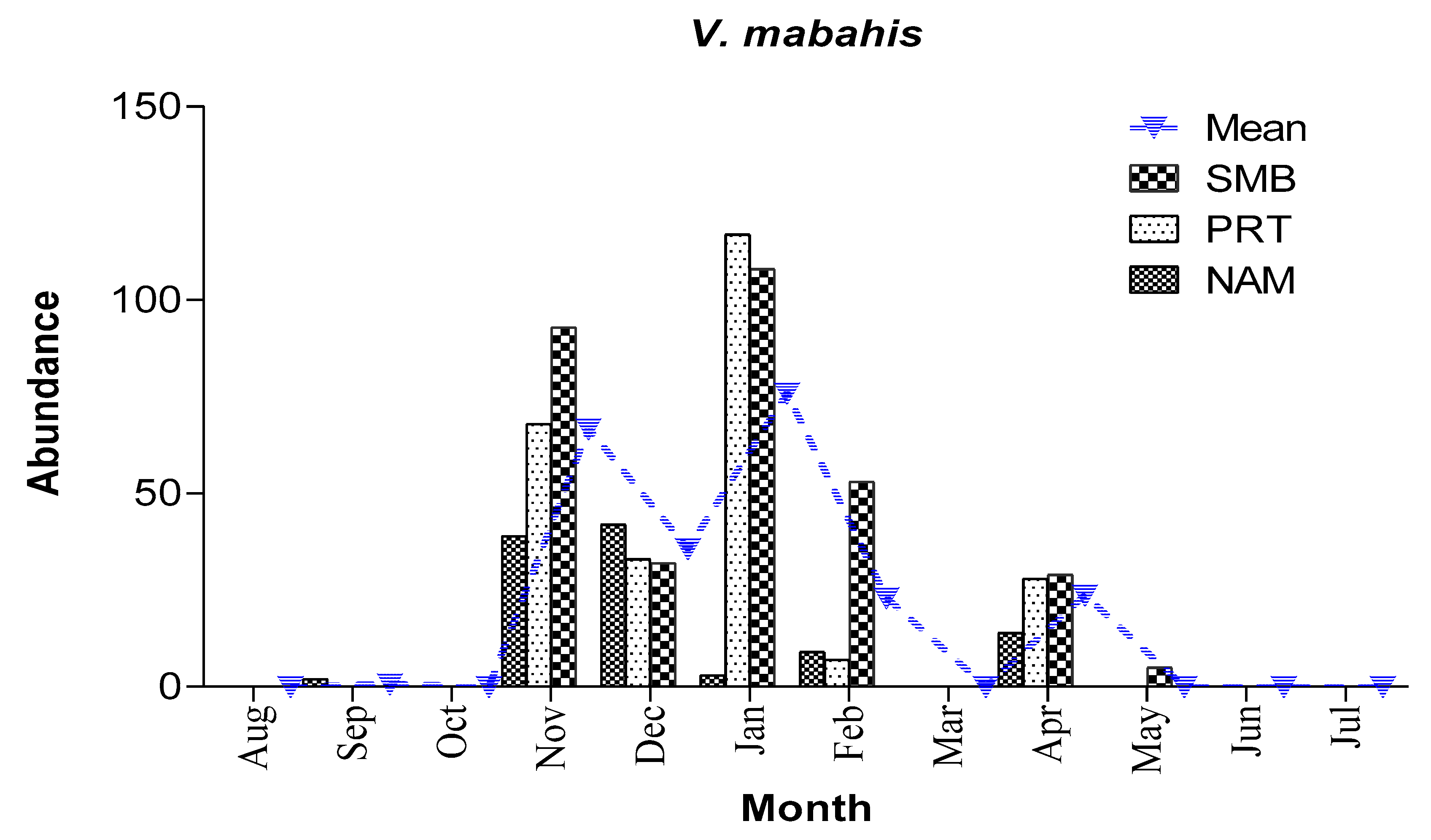
3.2.2. Abundance and spawning season of V. mabahiss
Larvae occurred in 39% of all samples collected and were the most abundant larvae of any mesopelagic species collected with an overall abundance of 19/1000 m
3). Larvae occurred mostly during the cooler months from November to April with peaks of abundance during November (200/1000 m
3) and January (228/1000 m
3). Larvae were rare in September (2/1000 m
3) and totally absent during the summer month (June-August) and in October and March. Larvae were collected at all sites sampled but were most abundant in Sharm El Maya Bay (315/1000 m
3) and least abundant in Naama Bay (109/1000 m
3). Larvae reached their maximum abundance in Port Bay (PRT)in January (192/1000 m
3) and Sharm El-Maya Bay (SMB) in November (140/1000 m
3) (
Figure 5).
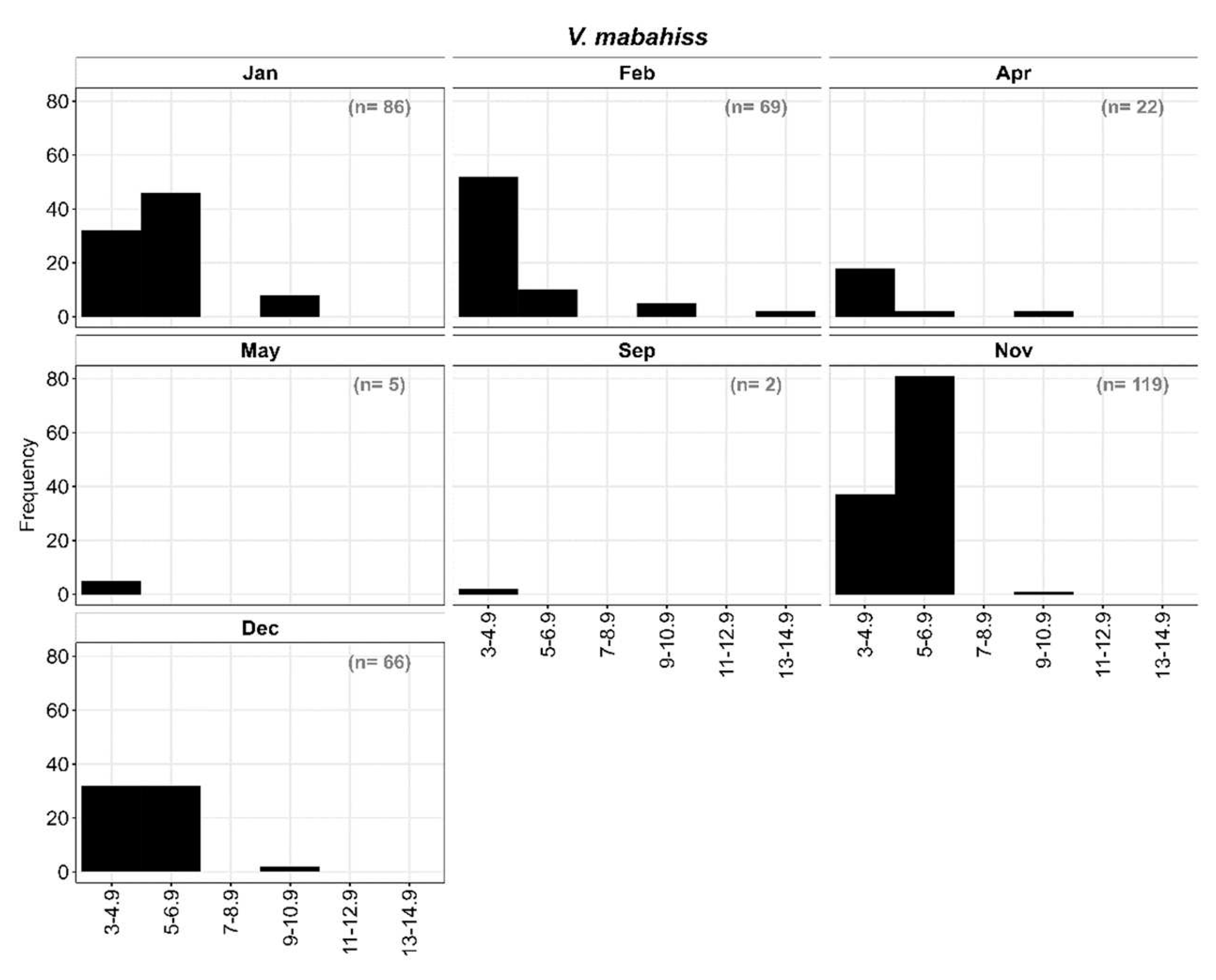
3.2.3. Length frequency distribution of V. mabahiss larvae:
The larvae of
V. mabahiss occurred primarily from September through May with a total of 339 larvae collected (
Figure 6)
V. mabahiss larvae ranged in size from 3 to 13 mm SL and were divided into six size classes at two-millimeter (mm) length intervals: I: 3-4.9 mm; II: 5-6.9 mm; III: 7-8.9 mm, IV: 9-10.9 mm, V: 11-12.9 mm; and VI: 13-14.9 mm. Overall, most larvae were preflexion stage (
Figure 6) with size classes I-III (<8.9 mm) accounting for about 97% of all
V. mabahiss larvae collected, while larvae >8.9 mm only contributed 3%. Although
V. mabahiss larvae collected in November (n = 119) ranged in size from 3 to 10.9 mm SL, most were in size classes I or II with one larva > 9 mm SL. December contained 66 larvae between 3 and 8.9 mm SL, but 48% were in the two smallest size classes, while January collections contained 86 larvae between 3 and 12.9 mm SL with 48% in size class II (5-6.9 mm); 9% of
V. mabahiss larvae collected in January were >8.9 mm SL (
Figure 6). During February, 89% of all larvae were in size classes I and II (<6.9 mm) with two larvae in the largest between 13 and 14.9 mm SL. Of the 22 larvae collected in April between 3- and 10.9-mm SL, 86% were <4.9 mm SL. All larvae collected in May (n = 5) were <4.9 mm SL. Collection of larvae <4.9 mm SL during all months in which
V. mabahiss occurred suggests that spawning may have occurred near the reef at those locations.
3.2.4. Description of larvae
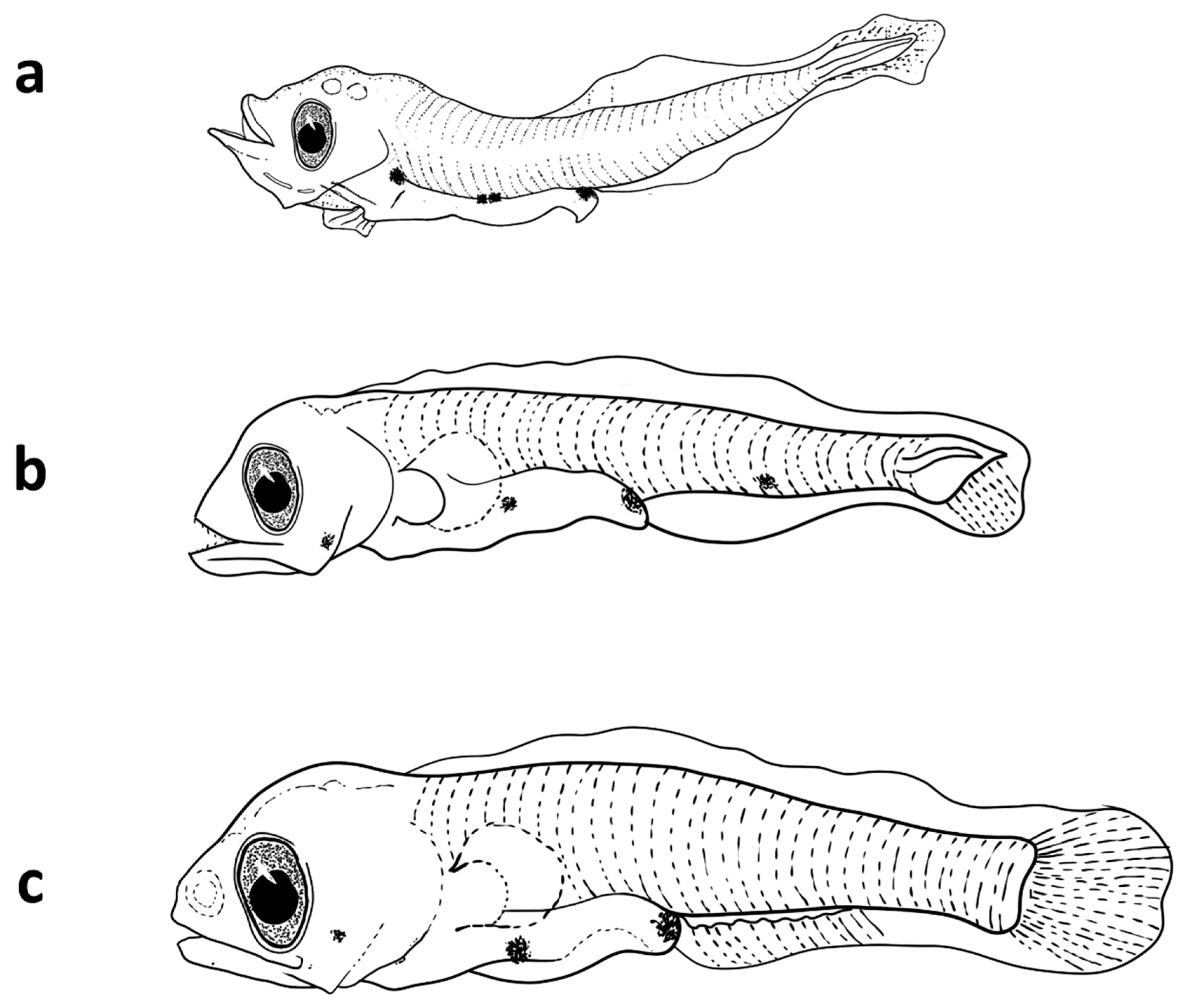
The larvae of
Astronesthes martensii ranged in size from 6 to 13.5 mm SL with all larvae <8 mm preflexion stage. In general, larvae of
A. martensii are elongate and moderately slender with >50 myomeres, and have a small head with small elliptical eyes, and both jaws have fang-like teeth. The long gut extends about 70% SL and the posterior portion of the hindgut trails the body. The finfold along the dorsal and ventral margins of the body are peppered with small melanophores, and stellate, brown melanophores line the dorsal and ventral margins of the tail and extend forward onto the trunk. Head length is about 16% SL and body depth about 6% SL. Notochord flexion begins at about 8 mm and is completed by 13.5 mm when the caudal fin is well-formed, and the dorsal and anal fins are beginning to form; however, the pectoral fins still lack rays. The anal fin originates well behind the dorsal fin and is slightly longer than the dorsal fin base (
Figure 7). The larvae of
A. martensii are distinguished from those of other mesopelagic fish in the Red Sea by the trailing gut, finfold pigmentation, relative position of the dorsal and anal fins, and high myomere count.
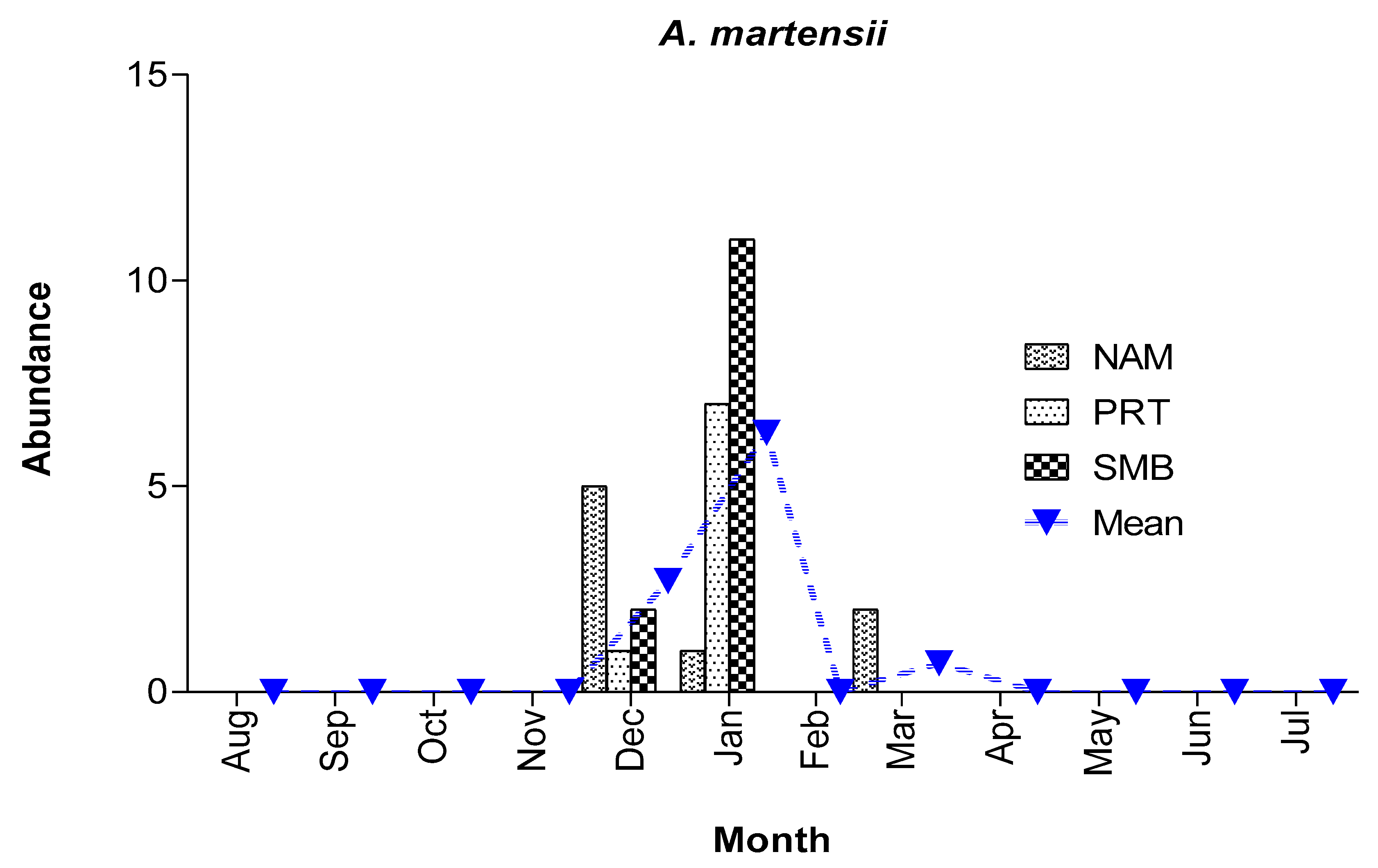
3.2.5. Abundance and spawning season of A. martensii
To our knowledge, information on the spawning seasonality of
A. martensii has not been reported, until now. All 29 larvae were collected during December, January, and March with a peak in January (
Figure 8), whereas March samples contained only two larvae. A. martensii accounted for <2% of all larvae of mesopelagic fishes, at a mean overall abundance of 1/1000 m
3. Larvae occurred in all bays sampled but were most abundant in SMB (
Figure 8). Even though collections contained few larvae, our data suggest that
A. martensii spawn during the winter months, although we cannot exclude the possibility that they may also spawn during other months and seasons.
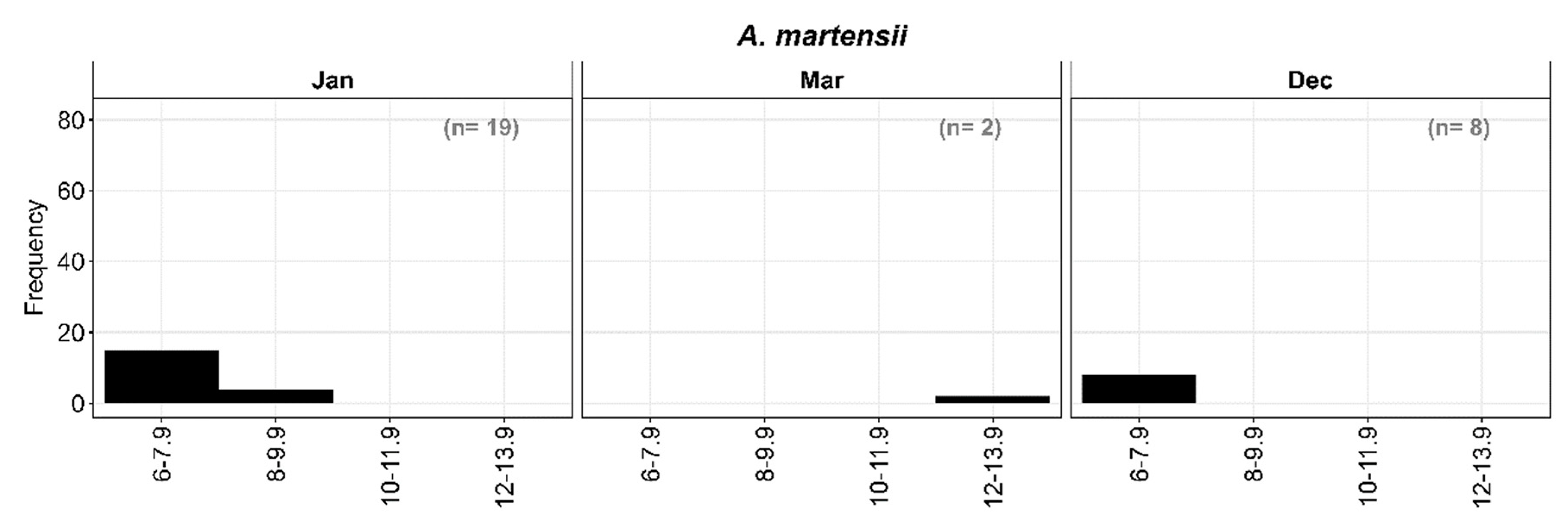
3.2.6. Length frequency distribution of A. martensii
Larvae were divided into four size classes at two mm length intervals: I: 6-7.9 mm; II: 8-9.9 mm, III: 10-11.9 mm, and IV: 12-13.9 mm SL with most preflexion larvae in the smallest size class. December collections contained eight preflexion larvae, as were 15 of 19 larvae collected in January. Both larvae collected during March, however, were between 12- and 13.9-mm SL, which is also consistent with primary spawning during the winter months (
Figure 9).
3.3. Benthosema pterotum
3.3.1. Description of larvae:
The larvae of
B. pterotum (
Figure 10) have robust bodies, elliptical eyes with a small, lunate choroid mass ventrally, a sigmoid-shaped gut with transverse mucosal folds that extend to mid-body. A gap between the anus and anal fin origin closes between 9- and 10-mm SL as the anal fin develops. The body becomes shorter and deeper as larvae develop, and the gut narrows posteriorly. The sequence of fin formation is as follows: pectoral-primary caudal-anal and dorsal-secondary caudal-pelvic fin rays. Larvae begin to transform at about 10 mm SL.
Small preflexion larvae have 12-15 preanal myomeres and 30-32 total myomeres. The gut terminates near mid-body (preanal length about 55% SL) and larvae have pigment along the ventral margin of the gut and over the dorsal margin of the vent. The larvae have a relatively large head (25% SL), and moderately deep body (depth 15% SL), which deepens to 19% by 4 mm SL. The notochord begins to flex by 5 mm, and flexion is complete by about 5.5 mm SL or shortly thereafter. Rays begin to develop in the dorsal and anal fins of early postflexion larvae, and by 7.5 mm SL, all fins have a full complement of primary elements. Postflexion larvae lack pigment ventrally on the body, except on the ventral surface of head, and on the dorsal and ventral margins of the pectoral fin base.
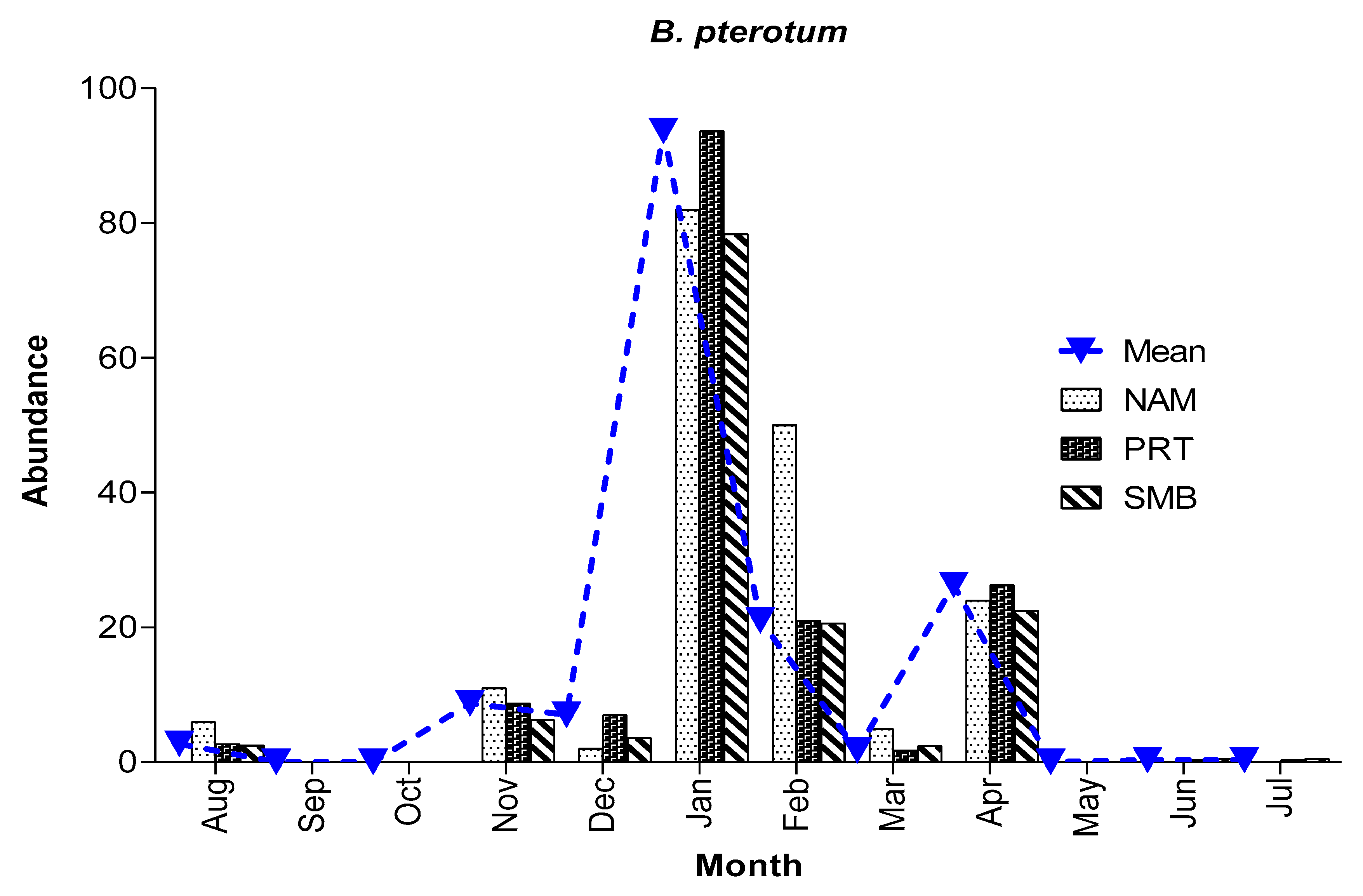
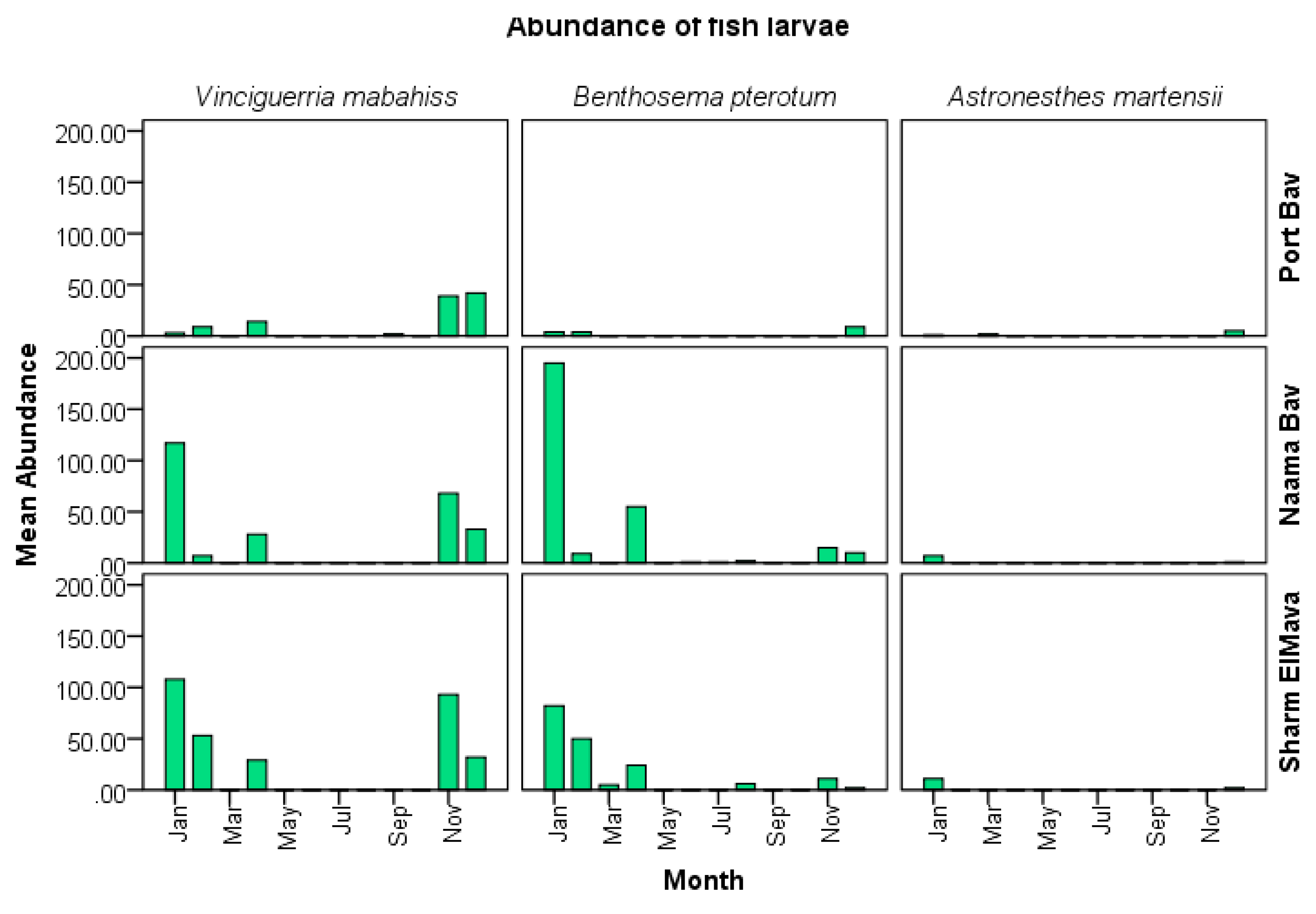
3.3.2. Abundance and Spawning season of B. pterotum
Larvae occurred in 43% of all samples taken and accounted for 14.8% of all fish larvae collected at an
overall average concentration of 14/1000 m
3. Larvae were collected during all months, except September and October, and most abundant between November and April with a peak in January (
Figure 11). In fact, January accounted for >50% of all
B. pterotum larvae collected (255 of
485 larvae). Although not significantly different among bays, abundance was somewhat higher in Naama Bay (180/1000 m
3), than Port Bay (161/1000 m
3), and Sharm El-Maya Bay (137/1000 m
3).
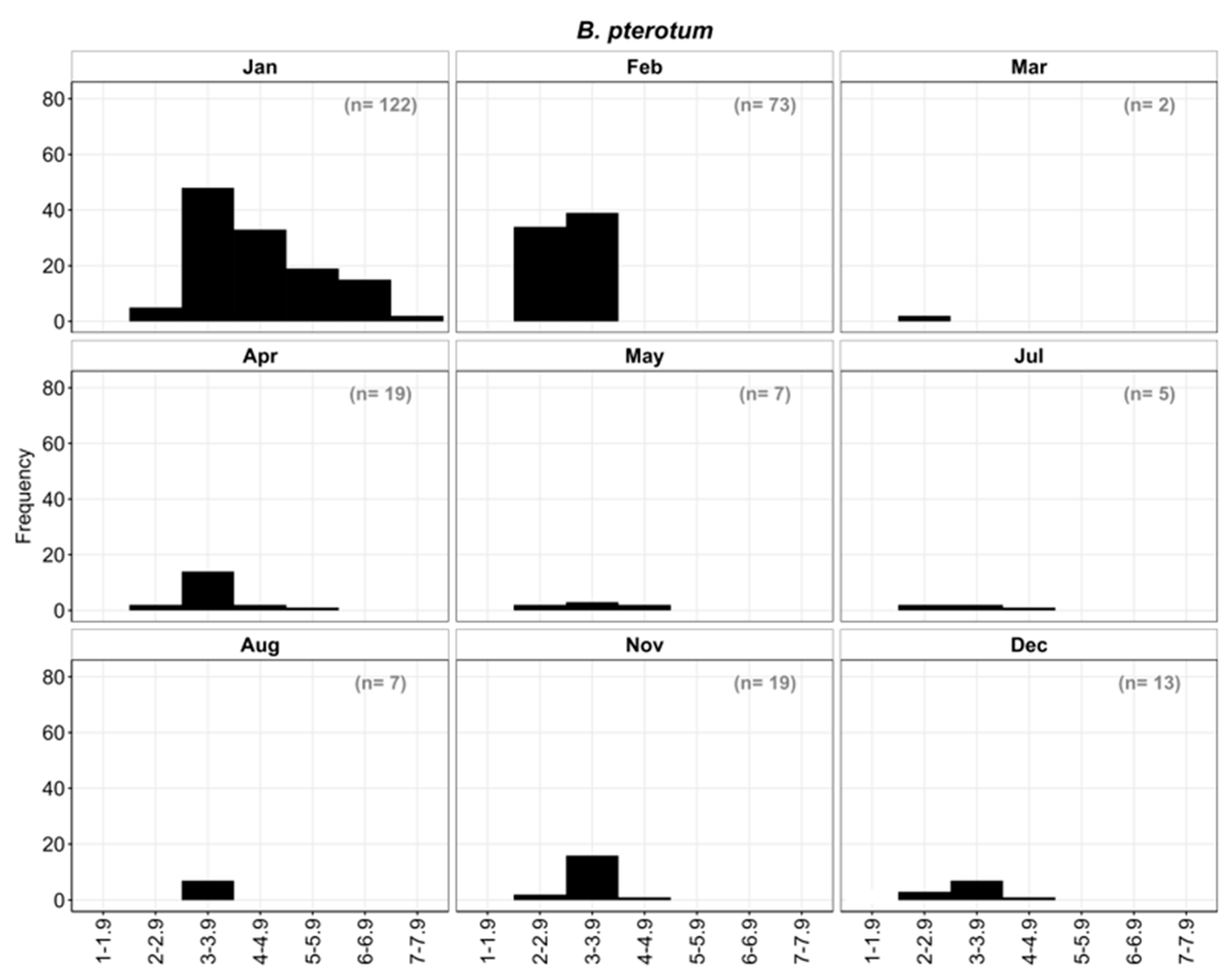
3.3.3. Length frequency distribution of B. pterotum larvae:
Larvae ranged in size from 2.3 to 7.5 mm SL and were divided into six size classes in one mm length intervals from I: 2-2.9 mm to VI: 6-6.9 mm. Larvae <5 mm (i.e., size classes I, II, III) accounted for nearly 75% of all
B. pterotum collected, and those between 5 and 6.9 mm for about 25%. The smallest larva was collected during December in Port Bay as was the largest larva during January. Based on monthly length frequency distributions, small larvae were collected in every month, except September and October, which suggests that spawning may occur year-round with peak spawning in January and February, and at much lower levels during late spring through early fall (
Figure 12).
In August, seven larvae of the same size class III (3-3.9 mm) were collected. In November, the length varied between 2 mm to 4.9 mm with nearly 93% of all larvae in size classes I and II (2-3.9 mm). In December, 13 larvae were collected, all <5 mm SL (preflexion). About 54% of these larvae were in size class II (3-3.9 mm). In January, 122 larvae representing all size classes (I-VI) were collected with about 70% <5 mm SL. In February, one peak was observed representing size groups I, II with 47% and 53% respectively. The March sample contained only two larvae, both of similar length. A total of 19 larvae were collected in April and ranged in size between 2-5.9 mm. SL with most in size class II (3-3.9 mm). The seven larvae collected in May ranged from 2-4.9 mm SL and belonged to size classes I, II, III, as did the five larvae taken in July.
4. Discussion
4.1. Diversity and distribution of mesopelagic fish larvae
The Red Sea, an area characterized by high endemism (Klevjer et al.,
2012), contains 16 species of mesopelagic fishes in 9 families and 6 orders (
Table 1). Despite the unusual environmental conditions of its mesopelagic waters (warm, stable water temperatures (~ 22°C); high salinities (40.5 ppt); and low zooplankton concentrations (Dypvik and Kaartvedt,
2013), the Red Sea reportedly has abundant populations of some mesopelagic fishes during the day (Klevjer et al.,
2012) dominated by
V. mabahiss at ~ 500 m (Isari et al.
2017). These aggregations of mesopelagic fishes later disperse as they migrate vertically into warmer near-surface waters in the early evening to feed and return to cooler waters before sunrise.
Although patterns of abundance vary by location, larvae of V. mabahiss and B. pterotum dominate collections of mesopelagic fishes in the Red Sea (Abu El-Regal et al., 2008; Abu El-Regal et al., 2014; Isari et al. 2017; this study) and are important components of the ichthyoplankton community (Isari et al. 2017). We found small V. mabahiss larvae most abundant from November through April with peaks in November and January and are absent from collections taken during June through August. By comparison, Isari et al. (2017) showed a gradual increase in abundance for V. mabahiss at the offshore station from January through April, a decline in abundance thereafter through August, and a low secondary peak in September. At the inshore station, monthly estimated abundances were consistently low throughout the year with a minor peak during June (Isari et al 2017). However, Vinciguerria mabahiss was weakly represented and B. pterotum was completely absent in a coastal reef lagoon at Hurghada coast Red Sea (Abu El-Regal, 2013) compared to the current results and previous ichthyoplankton surveys in the area. Differences in sampling methodologies and locations may help to explain this discrepancy in seasonal abundance patterns because Gulf of Aqaba waters were sampled off Egypt by horizontal tows within one meter of the sea surface in this study, whereas Isari et al. (2017) sampled waters down to 50 m off central Saudi Arabia with oblique tows. Otolith-derived back-calculated hatch dates between June and October for V. mabahiss larvae collected in the central Red Sea (Aldanondo et al. 2023), however, when combined with our findings and those by Isari et al. (2017) is consistent with the suggestion that V. mabahiss spawn continuously throughout the year (Isari et al. 2017) at least in some parts of the Red Sea.
Although larvae of V. mabahiss were the most abundant mesopelagic species collected in this study, and second in total abundance among all taxa collected near coral reefs in the Indo-Pacific (Leis, 1991), this was a somewhat surprising result. The Island Mass Effect may help to explain these findings, however, as physical processes near island and other underwater structures can enhanced input of nutrients in the euphotic layer, which favors biological productivity, as nutrient rich deeper waters are brought up towards the surface through upwelling and mixing (De Falco et al. 2022).
Larvae of Astronesthes martensii were the least abundant mesopelagic species collected in this study (n = 29 total larvae) and are rare to absent from other ichthyoplankton collections (Abu El-Regal et al. 2014; Isari et al. 2017), as are juveniles and adults in deep-water trawl collections taken in the southeast Arabian Sea (Renjith at al. 2020). This finding should not be surprising, as Astronesthes martensii typically inhabit waters at depths >1000 m and ichthyoplankton surveys rarely sample waters at that depth.
Larvae of B. pterotum were collected during all months sampled in this study, except September and October, with larvae most abundant between November and April, and peak abundance during January; larvae were collected at relatively low abundance levels from May through October. By comparison, Isari et al. (2017) collected larvae in the smallest size classes during all months, except during July and August. Although larvae were consistently less abundant in collections taken by Isari et al. (2015) than in this study, Isari et al. (2017) show a small spike in abundance about every three months throughout the year at their offshore stations. Back-calculated hatch dates for B. pterotum in the Sea of Oman suggest a primary spawning season from May to September (Hosseini-Shekarabi et al. 2015), which is generally consistent with that of B. pterotum in the East China Sea that spawn primarily during August and September (Gjّsوter & Tilseth, 1988; Sassa et al. 2015), although based on the gonadosomatic index (GSI), a small percentage of mature females occur from May through January based on the gonadosomatic index (GSI; (Sassa et al., 2014). Dalpadado and Gjesaeter, )1987) studied the reproduction of B. pterotum in the Red Sea and found no direct evidence of a second spawning in the Red Sea, although in a few females a second mode of ova was observed, which is similar to the ones observed by Dalpadado (1985) from other areas.
Benthosema pterotum eggs are spawned in deep waters (200 to 300 m in the Gulf of Oman); yolk sac larvae were found between 100 and 300m, and older larvae were generally found between 5 and 200m (Gjّs
وter and Tilseth, 1983). If time to hatching is about 10-12 hrs at water temperature of 21°C as is common for the Red Sea, and larvae hatch at about 1.5 mm SL (Gjّs
وter & Tilseth 1988), if daily growth rates approach those for
B. pterotum larvae in the East China Sea (mean absolute growth rate 0.26 mm; Sassa et al. 2015), larvae in the two smallest size classes (2-3.9 mm SL) in this study suggest collection within 7 to 10 of spawning. A mean absolute growth rate of 0.26 mm (Sassa et al. 2015) appears reasonable given an overall mean absolute growth rate of 0.21 mm per day based on growth rates for eight other species of myctophids (see Sassa et al. 2015;
Table 4). Therefore, an estimated hatch date for the largest
B. pterotum larva collected in this study (7.5 mm SL) is 23-25 days prior to collection date, which is consistent with an estimated larval duration period of 42 days for the smallest juvenile (12 mm SL) collected by Sassa et al. (2015).
4.2. The diagnostic features of Vinciguerria species, B. pterotum and A. martensii
V. mabahiss, endemic to the Red Sea and Gulf of Aqaba (Johnson and Feltes, 1984), are one of five species of Vinciguerria currently described. V. mabahiss is most similar to its congeners, V. nimbaria and V. lucetia from the Indian Ocean and elsewhere as all three species possess a pair of symphyseal photophores (just behind the mandibular symphysis), which V. attenuate and V. poweriae lack (Johnson and Feltes 1984). V. mabahiss differs from V. nimbaria and V. lucetia without overlap by having 58 to 63 total photophores (vs. 64-73) and 37 to 38 vertebrae (vs. 39-44, typically 40-41).
All species of Vinciguerria larvae have an enlarged pigment medially or near the ventral margin of the caudal complex. Vinciguerria mabahiss larvae like those of V. nimbaria and V. lucetia have pigment near the ventral margin of the tail and pigment above the anal fin base, whereas V. poweriae have the caudal pigment medially and lack pigment above the anal fin. V. attenuata also has pigment above the gas bladder (Johnson and Feltes 1984). V. mabahiss larvae lack pigment typically found at three locations in V. nimbaria and V. lucetia: post-pectoral, anal, and precaudally, whereas larvae of all three species have pigment in front of the pectoral fin base, along the base of the anal fin, on the tail, and pigment at the base of upper and lower caudal rays (Ahlstrom & Counts, 1958; Johnson and Feltes 1984).
Only two species within Myctophidae (Benthosema pterotum and Diaphus coeruleus) are reported in the Red Sea (Goren & Dor, 1994), and all collected in this study were identified as B. pterotum based on the pigment patterns and myomere counts. Myctophid larvae are characterized by the presence of an adipose fin and can be distinguished by differences in the number and placement of photophores and the size at which photophores develop. Larvae collected in this study (2.3 to 7.5 mm SL) are generally consistent with the description of the smallest B. pterotum larva (5.75 mm SL) by Lee et al. (2020), who observed pigment on the gut, and on the dorsal margin of the hindgut near the vent as noted here. Lee et al. (2020) also suggests that metamorphosis and photophore development is complete and the juvenile life stage attained by 12–13 mm SL in B. pterotum. Benthosema pterotum larvae can be confused with the larvae of other myctophids due to the elliptical eye in some species, and with some scarids, but scarids have <27 myomeres and a row of melanophores along or beneath the base of the anal fin that typically extend onto the caudal peduncle, which B. pterotum lack. Larvae of A. martensii are highly diverse and have a great number of morphological specializations; however, no complete developmental series, with transformation specimens, has been described and identifications in the literature are tentative (Kawaguchi and Moser 1984).
Larvae of mesopelagic fishes collected during this study from the Red Sea have some common characteristics including the absence of spines both in fins and head, the early forming teeth in one or both jaws the long snout and the elliptical eyes. The elongated body and elongate gut in V. mabahiss and A. martensii characterized them from larvae of many other families such as Clupeidae. The trailing gut of A. martensii is a very distinctive feature of the species.
5. Conclusion
This study contains valuable information about the early stages of mesopelagic fish, which have rarely been described worldwide in general and in the Red Sea in particular. The larvae of V. mabahiss and A. martensii are described for the first time in the Red Sea. Moreover, the spawning seasons and grounds of these deepwater fish are described for the first time. The presence of their larvae in large numbers, which outnumber the larvae of coastal fish, indicates that sea currents play a major role in transporting these larvae. However, more studies on the life history of mesopelagic fish are required.
Author Contributions
Methodology, Mohamed Ahmed Abu El-Regal; Investigation, Mohamed Ahmed Abu El-Regal and James G. Ditty; Data curation, Mohamed Ahmed Abu El-Regal; Writing – original draft, Mohamed Ahmed Abu El-Regal and James G. Ditty; Writing – review & editing, Mohamed Ahmed Abu El-Regal.
Institutional Review Board Statement
Samples are collected by plankton net, and they are only plankton samples and there are no rules to follow.
Acknowledgments
This research work was funded by the institutional Fund Projects under grant no. (IFPIP 222-150-1443). The authors gratefully acknowledge technical and financial support provided by the Ministry of Education and King Abdulaziz university, DSR, Jeddah, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
References
- Abu El-Regal, M. A.; Ahmed, A. I.; El-Etreby, S. G.; El-Komi, M.; Elliott, M. Abundance and diversity of coral reef fish larvae at Hurghada, Egyptian Red Sea. Egyptian Journal of Aquatic Biology and Fisheries 2008, 12, 17–33. [Google Scholar] [CrossRef]
- Abu El-Regal, M.A. Adult and larval reef fish communities in coastal reef lagoon at Hurghada, Red Sea, Egypt. International Journal of Environmental Science and Engineering 2013, 4, 39–49. [Google Scholar]
- Abu El-Regal, M.A. 2000. Ecological and Biological Studies on the larvae of coral reef fishes in Sharm El-Sheikh (Gulf of Aqaba-Red Sea). MSc Thesis. Marine Science Department, faculty of Science, Suez Canal University. 190pp.
- Abu El-Regal, M.A. 2008. Ecological Studies on the Ichthyoplankton of Coral Reef Fishes in Hurghada, Red Sea, Egypt. PhD thesis. Marine Science Department, faculty of Science, Suez Canal University. 178pp.
- Abu El-Regal, M.A.; Hellal, A.M.; Abu Zeid, M.M.; Maaty, M.M. Abundance and Diversity of Reef Fish Larvae in Mabahiss Bay, on the Egyptian Red Sea Coast. Egypt. J. Aquat. Biol. Fish. 2014, 18, 63–79. [Google Scholar] [CrossRef]
- Aldanondo, N.; Kaartvedt, S.; Irigoien, X. Growth patterns of two Red Sea mesopelagic fishes. Mar. Biol. 2022, 170, 1–9. [Google Scholar] [CrossRef]
- Barrett, R.T.; Anker-Nilssen, T.; Gabrielsen, G.W.; Chapdelaine, G. Food consumption by seabirds in Norwegian waters. ICES J. Mar. Sci. 2002, 59, 43–57. [Google Scholar] [CrossRef]
- Clavel-Henry M, Piroddi C, Quattrocchi F, Macias D and Christensen V (2020) Spatial.
- Dalpadado, P.; Gjøsaeter, J. Observations on mesopelagic fish from the Red Sea. Mar. Biol. 1987, 96, 173–183. [Google Scholar] [CrossRef]
- Dalpadado, P. (1985). Biology of the lanternfish Benthosema pterotum from the Indian Ocean, Ph.D thesis, 127 pp. Bergen: University of Bergen.
- De Falco, C.; Desbiolles, F.; Bracco, A.; Pasquero, C. Island Mass Effect: A Review of Oceanic Physical Processes. Front. Mar. Sci. 2022, 9, 1–21. [Google Scholar] [CrossRef]
- Distribution and Abundance of Mesopelagic Fish Biomass in the Mediterranean Sea.
- Dypvik, E.; Kaartvedt, S. Vertical migration and diel feeding periodicity of the skinnycheek lanternfish (Benthosema pterotum) in the Red Sea. Deep. Sea Res. Part I: Oceanogr. Res. Pap. 2013, 72, 9–16. [Google Scholar] [CrossRef]
- Dytham, C. (2003). Choosing and using statistics. A biologist’s guide. Blackwell Publishing, Oxford, 248pp.
- Fisher, J.P.; Pearcy, W.G. Reproduction, growth and feeding of the mesopelagic fish Tactostoma macropus (Melanostomiatidae). Mar. Biol. 1983, 74, 257–267. [Google Scholar] [CrossRef]
- Clavel-Henry, M.; Piroddi, C.; Quattrocchi, F.; Macias, D.; Christensen, V. Spatial Distribution and Abundance of Mesopelagic Fish Biomass in the Mediterranean Sea. Front. Mar. Sci. 2020, 7, 573986. [Google Scholar] [CrossRef]
- Giménez, J.; Marçalo, A.; García-Polo, M.; García-Barón, I.; Castillo, J.J.; Fernández-Maldonado, C.; Saavedra, C.; Santos, M.B.; de Stephanis, R. Feeding ecology of Mediterranean common dolphins: The importance of mesopelagic fish in the diet of an endangered subpopulation. Mar. Mammal Sci. 2017, 34, 136–154. [Google Scholar] [CrossRef]
- Tilseth, S.; Gjøsæter, J. Spawning behaviour, egg and larval development of the myctophid fish Benthosema pterotum. Mar. Biol. 1988, 98, 1–6. [Google Scholar] [CrossRef]
- Golani, D.; Bogorodsky, S.V. The Fishes of the Red Sea—Reappraisal and Updated Checklist. Zootaxa 2010, 2463, 1–100. [Google Scholar] [CrossRef]
- Goren, M. & Dor, M. (1994). An updated checklist of the fishes of the Red Sea CLOFRE SII. Israel Academy for Sciences and Humanities. The Israel Academy of Sciences and Humanities. Jerusalem, 120 pp.
- Halim, Y. (1969). Plankton of the Red Sea. In: Oceanography and marine biology. An annual review, Vol. 8, pp 231-275. Ed. By H. Barnes. London: George Allen and Unwin Ltd.
- Herring, P. (2002). The Biology of the Deep Ocean. Oxford: Oxford University Press.
- Hosseini-Shekarabi, S.P.; Valinassab, T.; Bystydzieńska, Z.; Linkowski, T. Age and growth of Benthosema pterotum (Alcock, 1890) (Myctophidae) in the Oman Sea. J. Appl. Ichthyol. 2014, 31, 51–56. [Google Scholar] [CrossRef]
- Lee, H.-L.; Kim, J.-K.; Yu, H.-J.; Kim, J.-N. Ontogenetic comparison of larvae and juveniles of Diaphus garmani and Benthosema pterotum (Myctophidae, Pisces) collected from Korea. Fish. Aquat. Sci. 2020, 23, 1–10. [Google Scholar] [CrossRef]
- Isari, S.; Pearman, J.K.; Casas, L.; Michell, C.T.; Curdia, J.; Berumen, M.L.; Irigoien, X. Exploring the larval fish community of the central Red Sea with an integrated morphological and molecular approach. PLOS ONE 2017, 12, e0182503. [Google Scholar] [CrossRef]
- Johnson, R.K.; Feltes, R.M. A new speices of Vinciguerria (Salmoniformes: Photichthyidae) from the Red Sea and the Gulf of Acaba, with comments on depauperacy of the Red Sea mesopelagic fauna. Fieldiana Zoology 1984, 22, 1353. [Google Scholar]
- Kaiser, M. J., Attrill, M. J., Jennings, S., Thomas, D. N., Barnes, D. K. A., Brierley, S. A., et al. (2011). Marine ecology: processes, systems, and impacts. Oxford: Oxford University Press.
- Klevjer, T.A.; Torres, D.J.; Kaartvedt, S. Distribution and diel vertical movements of mesopelagic scattering layers in the Red Sea. Mar. Biol. 2012, 159, 1833–1841. [Google Scholar] [CrossRef]
- Leis, J. M. (1991). The pelagic stage of reef fishes: The larval biology of coral reefs. In: Sale, P. F. the ecology of fishes on coral reefs. Academic press,. pp 183-230.
- Leis, J. M. (1991a).
- Mann, K. H. (1984). “Fish production in open ocean ecosystems,” in Flows of energy and materials in marine ecosystems: theory and practice, ed. M. J. R. Fasham (New York,NY: Plenum Press), 435–458. [CrossRef]
- Marshall, N. B.; Bourne, D. W. A photographic survey of benthic fishes in the Red Sea and Gulf of Aden, with observations on their population density, diversity and habits. Bull. Mus. Comp. Zool. Harvard Univ. 1964, 132, 223–244. [Google Scholar]
- Marshall, N. B. (1963). Diversity, distribution and speciation of deep-sea fishes, pp 181-185. Systematics Assoc. Publ. no. 5, Speciation in the sea.
- Olivar, M.P.; Bernal, A.; Molي, B.; Peٌa, M.; Balbيn, R.; Castellَn, A.; Miquel, J.; Massutي, E. Vertical distribution, diversity and assemblages of mesopelagic fishes in the western Mediterranean. Deep-Sea Research Part I 2012, 62, 53–69.
- Renjith, R.K.; Jha, P.N.; Chinnadurai, S.; Bineesh, M.; Remesan, M.P. Length weight relationship of five deep sea fishes from Kerala, southwest coast of India. J. Appl. Ichthyol. 2020, 36, 259–260. [Google Scholar] [CrossRef]
- Salvanes, A. G., & Kristoffersen, J. B. (2001). Mesopelagic fish. Cambridge: Academic Press. 1711–1717. [CrossRef]
- Sassa, C.; Ohshimo, S.; Tanaka, H.; Tsukamoto, Y. Reproductive biology of Benthosema pterotum (Teleostei: Myctophidae) in the shelf region of the East China Sea. J. Mar. Biol. Assoc. United Kingd. 2013, 94, 423–433. [Google Scholar] [CrossRef]
- Sassa, C.; Takahashi, M.; Tsukamoto, Y. Distribution, hatch-date, growth, and mortality of larval Benthosema pterotum (Pisces: Myctophidae) in the shelf region of the East China Sea. J. Mar. Biol. Assoc. United Kingd. 2014, 95, 161–174. [Google Scholar] [CrossRef]
- Saunders, R.A.; Hill, S.L.; Tarling, G.A.; Murphy, E.J. Myctophid Fish (Family Myctophidae) Are Central Consumers in the Food Web of the Scotia Sea (Southern Ocean). Front. Mar. Sci. 2019, 6, 530. [Google Scholar] [CrossRef]
- The Manihine Expedition to the Gulf of Aqaba, Part IX, Fishes, by N. B. MARSHALL. 1952, Bulletin Brit Mus Nat Hist Zool 1(8): 221-252.
- Thesis. Marine Science Department, faculty of Science, Suez Canal University. 190pp.
- Valinassab, T.; Pierce, G.J.; Johannesson, K. Lantern fish (Benthosema pterotum) resources as a target for commercial exploitation in the Oman Sea. J. Appl. Ichthyol. 2007, 23, 573–577. [Google Scholar] [CrossRef]
- Weikert, H. The Vertical Distribution of Zooplankton in Relation to Habitat Zones in the Area of the Atlantis II Deep, Central Red Sea. Mar. Ecol. Prog. Ser. 1982, 8, 129–143. [Google Scholar] [CrossRef]
- Williams, A.; Koslow, J. A.; Terauds, A.; Haskard, K. Feeding ecology of five fishes from the mid-slope micronekton community off southern Tasmania. Mar. Biol. 2001, 139, 1177–1192. [Google Scholar] [CrossRef]
- Williams, I.; Polunin, N.; Hendrick, V. Limits to grazing by herbivorous fishes and the impact of low coral cover on macroalgal abundance on a coral reef in Belize. Mar. Ecol. Prog. Ser. 2001, 222, 187–196. [Google Scholar] [CrossRef]
- Zar, J.H. (1999). Biostatistical Analysis. 4th Edition, Prentice Hall, Upper Saddle River.
Figure 1.
Sampling sites of fish larvae.
Figure 1.
Sampling sites of fish larvae.
Figure 2.
Percent contribution of mesopelagic larvae to all fish larvae collected.
Figure 2.
Percent contribution of mesopelagic larvae to all fish larvae collected.
Figure 3.
Larvae of V. mabahiss collected form the Red Sea. A.5 mm, B. 9 mm, C. 13 mm.
Figure 3.
Larvae of V. mabahiss collected form the Red Sea. A.5 mm, B. 9 mm, C. 13 mm.
Figure 4.
Morphometrics of V. mabahiss and B. pterotum collected from the Red Sea.
Figure 4.
Morphometrics of V. mabahiss and B. pterotum collected from the Red Sea.
Figure 5.
Mean monthly abundance of V. mabahiss (number/ 1000 m3) at different bays throughout the sampling period.
Figure 5.
Mean monthly abundance of V. mabahiss (number/ 1000 m3) at different bays throughout the sampling period.
Figure 6.
Length frequency distribution of V. mabahiss larvae.
Figure 6.
Length frequency distribution of V. mabahiss larvae.
Figure 7.
Larvae of A. martensii collected from Sharm El-Sheikh during the sampling period.
Figure 7.
Larvae of A. martensii collected from Sharm El-Sheikh during the sampling period.
Figure 8.
Mean monthly abundance of A. martensii (no/1000 m3) at different bays throughout the sampling period.
Figure 8.
Mean monthly abundance of A. martensii (no/1000 m3) at different bays throughout the sampling period.
Figure 9.
Length frequency distribution of A. martensii.
Figure 9.
Length frequency distribution of A. martensii.
Figure 10.
larvae of B. pterotum. A. 2.1 mm Benthosema pterotum, B. 4 mm and C. 6 mm.
Figure 10.
larvae of B. pterotum. A. 2.1 mm Benthosema pterotum, B. 4 mm and C. 6 mm.
Figure 11.
Mean monthly abundance of B. pterotum (per 1000 m3) at different bays throughout the sampling period.
Figure 11.
Mean monthly abundance of B. pterotum (per 1000 m3) at different bays throughout the sampling period.
Figure 12.
Length frequency distribution of B. pterotum.
Figure 12.
Length frequency distribution of B. pterotum.
Table 1.
Orders, families, and species of mesopelagic fish in the Red Sea.
Table 1.
Orders, families, and species of mesopelagic fish in the Red Sea.
| Order |
Family |
Species |
| Stomiiformes |
Phosichthyidae |
Vinciguerria mabahiss Johnson & Feltes 1984 |
| Stomiidae |
|
| Subfam: Astroneshthinae |
Astronesthes martensii Klunzinger 1871 |
| Subfam: Stomiinae |
Chauliodus sloani Bloch & Schneider 1801 |
|
Stomias affinis Günther 1887 |
| Sternoptychidae |
Maurolicus mucronatus Klunzinger 1871 |
| Myctophiformes |
Myctophidae |
Benthosema fibulatum (Gilbert & Cramer 1897) |
|
Benthosema pterotum (Alcock 1890) |
|
Diaphus coeruleus (Klunzinger 1871) |
| Ateleopodiformes |
Ateleopodididae |
Ateleopus japonicus Bleeker 1853 |
| Scombriformes |
Trichiuridae |
Evoxymetopon moricheni
Fricke, Golani & Appelbaum-Golani 2014 |
|
Tentoriceps cristatus (Klunzinger 1884) |
|
Trichiurus auriga Klunzinger 1884 |
|
Trichiurus lepturus Linnaeus 1758 |
| Gempylidae |
Thyrsitoides marleyi Fowler 1929 |
| Aulopiformes |
Paralepididae |
Lestidiops jayakari (Boulenger 1889) |
|
Lestrolepis luetkeni (Ege 1933) |
Table 2.
General abundance of larvae of mesopelagic fish from Sharm El-Shaeikh during the sampling period from January to December 2015.
Table 2.
General abundance of larvae of mesopelagic fish from Sharm El-Shaeikh during the sampling period from January to December 2015.
| Month |
V. mabahiss |
B. Pterotum |
A. martensii |
| NAM |
PRT |
SMB |
NAM |
PRT |
SMB |
NAM |
PRT |
SMB |
| Aug |
0 |
0 |
0 |
0 |
2 |
6 |
0 |
0 |
0 |
| Sep |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Oct |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Nov |
39 |
68 |
93 |
0 |
15 |
11 |
0 |
0 |
0 |
| Dec |
42 |
33 |
32 |
9 |
10 |
2 |
5 |
1 |
2 |
| Jan |
3 |
117 |
108 |
4 |
195 |
82 |
1 |
7 |
11 |
| Feb |
9 |
7 |
53 |
4 |
9 |
50 |
0 |
0 |
0 |
| Mar |
0 |
0 |
0 |
0 |
0 |
5 |
2 |
0 |
0 |
| Apr |
14 |
28 |
29 |
0 |
55 |
24 |
0 |
0 |
0 |
| May |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Jun |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
| Jul |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
| Sum |
109 |
253 |
315 |
17 |
288 |
180 |
8 |
8 |
13 |
Table 3.
Meristics of mesopelagic species collected from the Red Sea.
Table 3.
Meristics of mesopelagic species collected from the Red Sea.
| Meristics |
V. mabahiss |
B. pterotum |
A. martensii |
| Myomeres |
37 |
30 |
50 |
| Preanal |
25 |
19 |
35 |
| Postanal |
12 |
11 |
15 |
| Doral fin spines |
0 |
0 |
0 |
| Doral fin rays |
13 |
12 |
10-21*
|
| Anal fin spines |
0 |
0 |
0 |
| Anal fin rays |
13 |
18 |
12-22*
|
| Pelvic |
7 |
8 |
5-9*
|
| Pectoral |
10 |
19 |
5-9*
|
| Caudal |
19 |
|
NA |
Table 4.
Morphometrics of the mesopelagic species collected from the Red Sea.
Table 4.
Morphometrics of the mesopelagic species collected from the Red Sea.
| |
V. mabahiss |
B. pterotum |
A. martensii |
| |
Preflexion |
Postflexion |
Preflexion |
Postflexion |
Preflexion |
Postflexion |
| Snl/HL |
37% |
33-40% |
33-40% |
25-30% |
25% |
30% |
| ED/HL |
25% |
22-26% |
33-40% |
16-20% |
18% |
35% |
| HL/BL |
26% |
16-20% |
20-25% |
25-26% |
16% |
36% |
| PAL/BL |
83% |
74-77% |
50-60% |
55-60% |
trailing gut |
trailing gut |
| PDL/BL |
--- |
61-63% |
--- |
44-50% |
---- |
61% |
| BD/BL |
|
|
13-15% |
15-17% |
% |
6% |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).