1. Introduction
Ceramic glazes are a group of materials that contain, apart from the amorphous phase, crystalline phases. Their presence is significant due to the role they play in the material. The crystalline phases have greater mechanical strength than the glaze itself, increasing the mechanical strength of the entire system, as well as resistance to abrasion or chemical corrosion. [
1,
2]
The crystallization process in glazes is usually desirable, except for the group of transparent glazes, where the presence of crystalline phases could reduce the transparency of the glaze layer. Depending on what crystalline phase is planned in the finished product, it is necessary to conduct the design process and glaze production in a specific way. Some crystalline phases are formed in glazes very easily; others require meeting specific conditions related to the chemical composition of the glaze, i.e. the number of substrates for crystallization, firing temperature, holding temperature or viscosity of the system. [
3,
4,
5,
6]
Zirconium silicate as a crystalline phase is desirable in glazes with high brightness, increased mechanical parameters, and chemical resistance. The crystalline phase of this mineral is obtained by adding the raw material, zirconium silicate (rarely zirconium oxide), in the amount of at least 3% by mass. Adding a smaller amount may cause no crystallization and transition of the entire amount of zircon to the amorphous phase. [
1,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15]
The zirconium silicate crystallization process is described in numerous publications. The introduction of zirconium silicate into the system causes its partial decomposition in the temperature range of 1100°C-1150°C, and its recrystallization occurs above these temperatures. An important element here is the phase composition; in the case of a low molar ratio, only partial recrystallization is observed, while the remaining amount of zirconium in the fired product is present as zirconium oxide, usually in monoclinic form. [
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23]
As already mentioned, the crystallization processes are influenced by the chemical composition. An important element of glazes are fluxes, the main role of which is to reduce the firing temperature of glazes. Their actual participation in crystallization processes depends on the type of flux used. In addition to the most widely used oxides, such as potassium, sodium, calcium, or magnesium oxides, strontium oxide seems to be interesting. It is usually added in the form of carbonate. Its presence improves the gloss of the surface; however, the addition of a larger amount may cause crystallization on the surface of the glazes. [
1,
2,
3,
20,
21,
22,
23,
24,
25,
26,
27]
Strontium oxide also has a structure-depolymerizing effect. Its role is to break the aluminum and silica oxide bonds of the the amorphous phase of glaze. The depolymerization effect and loosening of the structure may favor crystallization processes. The mechanism was established for easier migration of substrates (through a looser structure) and a simultaneous reduction in the viscosity of the system. [
28,
29]
Zirconium silicate is added to the glazes to obtain the same crystalline phase after the firing process. As shown above, zirconium silicate may dissolve and partially transform into the amorphous phase, so its amount in the glaze after the firing process will be lower than the amount of zirconium silicate used as a raw material. Therefore, to obtain the designed amount of the crystalline phase of zirconium silicate, it will be necessary to add a larger amount of this raw material. [
30,
31,
32]
The final phase composition depends not only on the amount of zirconium silicate used but also on other glaze components. Fluxing raw materials play an important role; their presence and action can influence the crystallization processes of new phases, as the well as dissolution and recrystallization. Flux raw materials are one of the components of glaze, but their selection and quantity will have an actual impact on the phase composition of the fired glaze and the resulting properties. [
21,
22,
23,
24,
25,
33,
34,
35,
36,
37]
In summary, it can be clearly stated that the number of studies on the crystallization of zirconium silicate is significant, but most of the studies are based on frits. However, much of the focus is on the amount of this crystalline phase and the resulting properties of the glass-crystalline material. On the other hand, knowledge about fluxes is also discussed in many works. Still, it mainly focuses on the role of fluxes - reducing the firing temperature and their impact on the structure and resulting properties of the material. There are no publications in which the influence of changing the amount of feldspar on the phase composition and changes in the microstructure of the glass-crystalline material was examined in a wide range of compositions.
The aim of this experiment is to determine the effect of the addition of strontium oxide to zirconia glazes on the amount of crystalline phase of zirconium silicate obtained and the changes occurring in the microstructure of the analyzed glazes with a variable amount of strontium oxide. Glazes with a wide range of amounts of zirconium silicate and five different contents of strontium oxide were tested. Such activities will allow us to systematically trace the relationship between the amount of zirconium silicate and the added strontium oxide on the phase composition and microstructure of the analyzed glazing system.
2. Preparation
The compositions of the glazes have been designed on the basis of the composition of the production glaze used in the production of sanitary ceramics. The only variable in the compositions analyzed during the design process was the amount of strontium oxide added (five different amounts) and the varying amount of zirconia in the composition (four different amounts). The high molar fraction of SiO
2/Al
2O
3 is because zirconium oxide was introduced into the glaze as zirconium silicate, and it also introduced silicon oxide into the composition of the glaze. The simplified compositions of the analyzed glazes are presented in the
Table 1.
Glazes were prepared from natural raw materials such as quartz powder MK 40 (Strzeblowskie Kopalnie Surowców Mineralnych Sobótka), KOC kaolin (Surmin Kaolin), potassium feldspar Quantum DS (Sibelco Polska), wollastonite (Ottavi), talc (Luzenac), zirconium silicate (Kreuzonit) and strontium carbonate (Sigma Aldrich).
The weighted glazes were ground wet in a planetary mill for 30 minutes. This process was carried out wet with water with the ratio of water: grinding medium: weighted raw material = 1.5 : 1 : 1. After that, they were dried in a laboratory dryer at 110°C. The dried glazes were used to determine the characteristic temperatures and the maximum glaze firing temperature. Then 60 g of each glaze was placed in biscuit containers and fired according to a designated curve.
Characteristic temperatures were determined using a Misura 3 high temperature microscope, along with dedicated data analysis software. Each glaze was pressed into a pellet and then placed on a base made of aluminum oxide. Each sample was heated at a rate of 10°C/min to a temperature 20 degrees higher than the flow temperature. Characteristic temperatures were determined on the geometric dimensions of the samples - data obtained from measurements. Four temperatures were determined for the analyzed samples: sintering temperature, sphere temperature, hemisphere temperature and melting temperature.
The fired glazes were ground to a grain size of less than 63 µm. The grain size was controlled on a 63 µm sieve. This powder was used to determine the phase composition of the fired glaze. The phase composition (qualitative and quantitative) of the examined glazes was determined by X-ray diffraction. The study was performed using a Philips X'Pert Pro X-ray diffractometer. The qualitative identification of the phases was carried out by comparing the positions of the reflections and their intensity, obtained during the measurement, with the data collected in the JCPDS ICCD database (Join Committee for Powder Diffraction Standards, International Center for Diffraction Data). Quantitative determination of the phase composition, including the amount of amorphous phase, was performed using the internal standard method. Analysis of the diffractograms (quantitative and qualitative) was carried out using the HighScore Plus program. The quantitative share of the crystalline phases was determined by the Rietveld method. As an internal standard for quantitative analysis, zinc oxide (Huta Oława) was used, which was added to the samples in the amount of 5% by weight.
On the remaining pieces of glaze, microsections were made that were used to observe the microstructure of the fired glazes. These observations were carried out using the NOVA NANO SEM 200 scanning electron microscope, manufactured by FEI Europe, which allows the observation of the surface of materials in the backscattered electron detection (BSE) system with a resolution up to 2 nm. Microstructure observations were carried out each time at magnifications of 1000, 3000 and 5000 times, which greatly facilitates the comparison of sample images. For selected interesting crystalline phases, visual inspection was carried out at 10,000 times magnification. During the microscopic observations, the individual crystalline phases present in the image were also identified using the EDS X-ray microanalyzer by EDAX.
3. Results and Discussion
3.1. Hot stage microscopy (HSM).
| Glaze |
Characteristic temperatures [oC] |
Firingtemperature [oC]
|
| Sintering |
Sphere |
Halfsphere |
Melting |
| 1Zr0Sr |
1136 |
1278 |
1330 |
1365 |
1330 |
| 1Zr1Sr |
1120 |
1269 |
1316 |
1356 |
1315 |
| 1Zr3Sr |
1116 |
1242 |
1292 |
1328 |
1290 |
| 1Zr6Sr |
1122 |
1262 |
1304 |
1342 |
1305 |
| 1Zr12Sr |
1106 |
1182 |
1222 |
1246 |
1220 |
| 3Zr0Sr |
1139 |
1275 |
1320 |
1355 |
1320 |
| 3Zr1Sr |
1135 |
1261 |
1301 |
1331 |
1300 |
| 3Zr3Sr |
1126 |
1242 |
1290 |
1329 |
1290 |
| 3Zr6Sr |
1110 |
1219 |
1278 |
1323 |
1280 |
| 3Zr12Sr |
1130 |
1197 |
1244 |
1285 |
1245 |
| 6Zr0Sr |
1132 |
1282 |
1326 |
1359 |
1325 |
| 6Zr1Sr |
1122 |
1276 |
1319 |
1355 |
1320 |
| 6Zr3Sr |
1150 |
1270 |
1313 |
1352 |
1315 |
| 6Zr6Sr |
1125 |
1233 |
1290 |
1329 |
1290 |
| 6Zr12Sr |
1142 |
1218 |
1260 |
1306 |
1260 |
| 12Zr0Sr |
1180 |
1300 |
1346 |
1376 |
1345 |
| 12Zr1Sr |
1146 |
1285 |
1339 |
1376 |
1340 |
| 12Zr3Sr |
1135 |
1260 |
1311 |
1357 |
1310 |
| 12Zr6Sr |
1131 |
1221 |
1279 |
1320 |
1280 |
| 12Zr12Sr |
1124 |
1182 |
1224 |
1258 |
1225 |
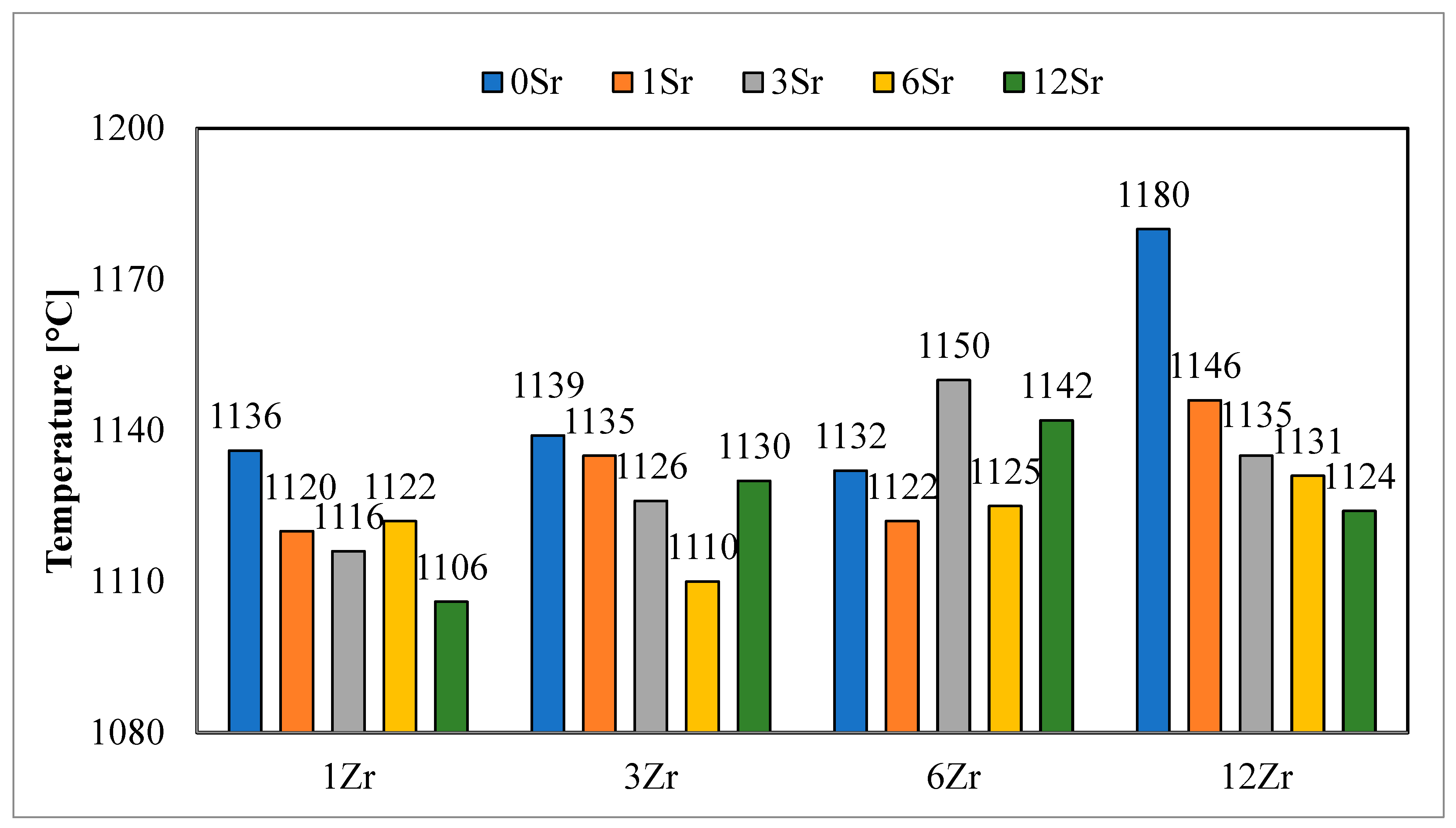
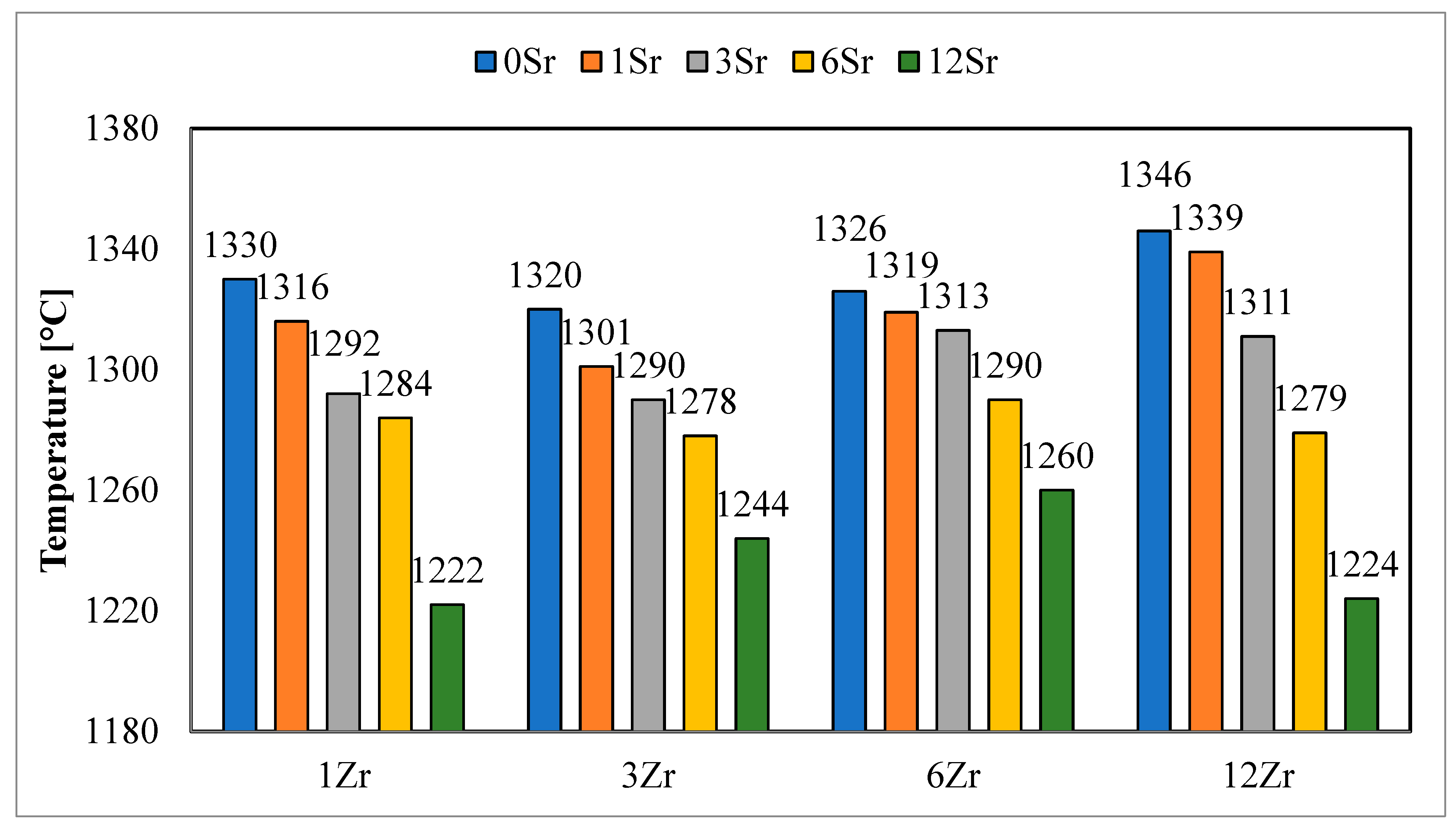
On the basis of the analysis of the obtained values of sintering temperatures (
Table 2,
Figure 1), it can be observed that the obtained glazes belong to the group of high-temperature glazes. The values obtained unequivocally indicate that all processes significant from the point of view of crystallization take place above the temperature of 1100°C.
The addition of strontium oxide to the glazes reduces the value of this temperature, which is consistent with data from the literature and the fluxing effect of this oxide. However, not in all cases, a decrease in the value of the sintering temperature was observed with the addition of strontium oxide. This may be due to the chemical composition of the glazes, which changes the rate of subsequent reactions in the glazes.
In terms of the the values of sintering temperatures for different contents of zirconium oxide in the glazes, it is difficult to notice any dependence. Both an increase and a decrease in the value of this temperature are observed with the same addition of strontium oxide.
The determined sphere temperatures (
Figure 2) for the analyzed glazes clearly indicate a decrease in the value of this temperature during the addition of strontium oxide. The higher the content of strontium oxide in the glazes, the lower the designated temperature of the hemisphere. The decrease in temperature shows a relationship with the amount of strontium oxide added to the glazes. For glazes with the same strontium oxide content, a slight increase in hemispheric temperatures is observed, along with a higher zirconium content in the glaze. This is especially noticeable with low-strontium oxide glazes. For a larger amount of strontium oxide in the glazes, this relationship is not observable.
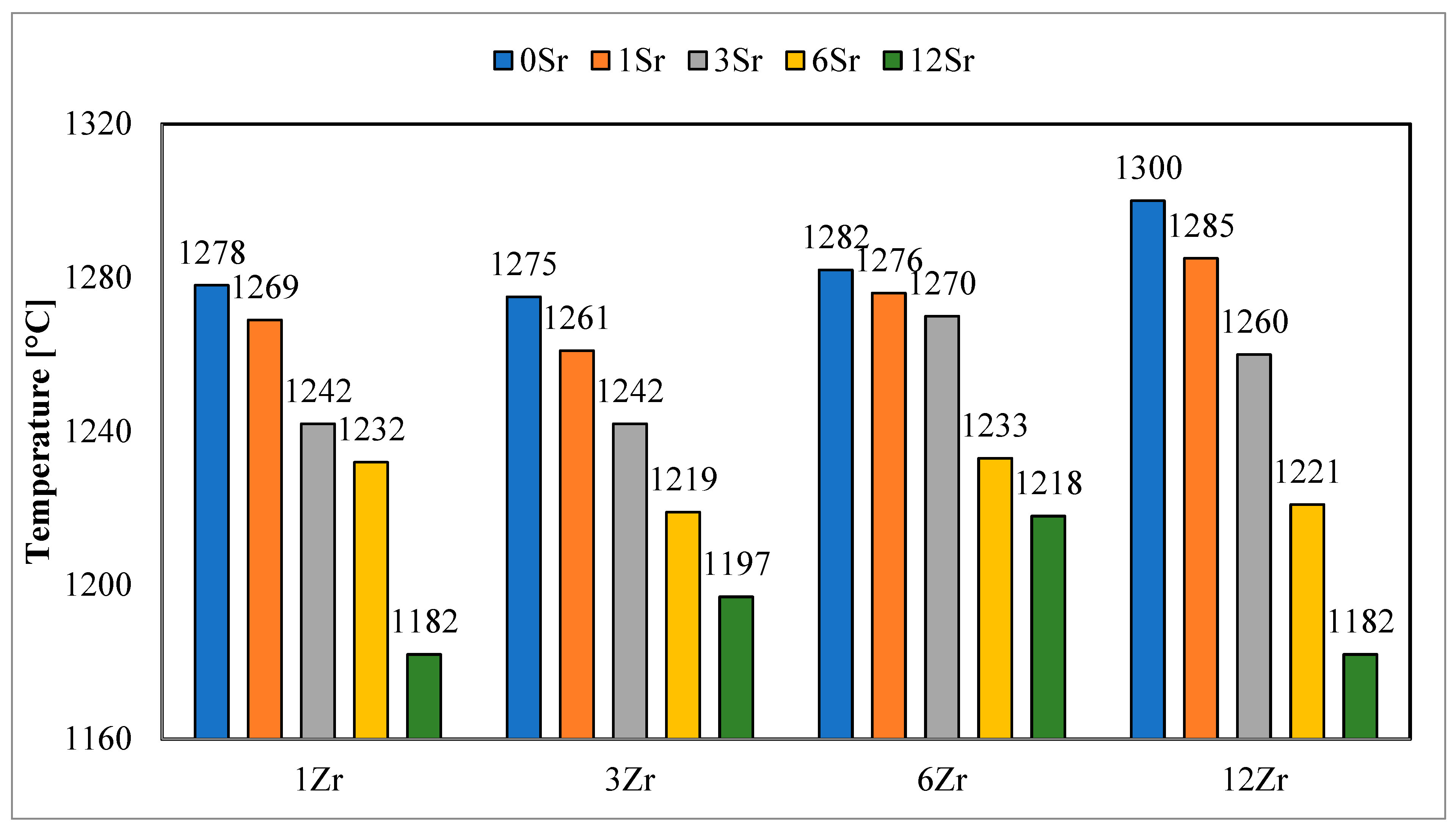
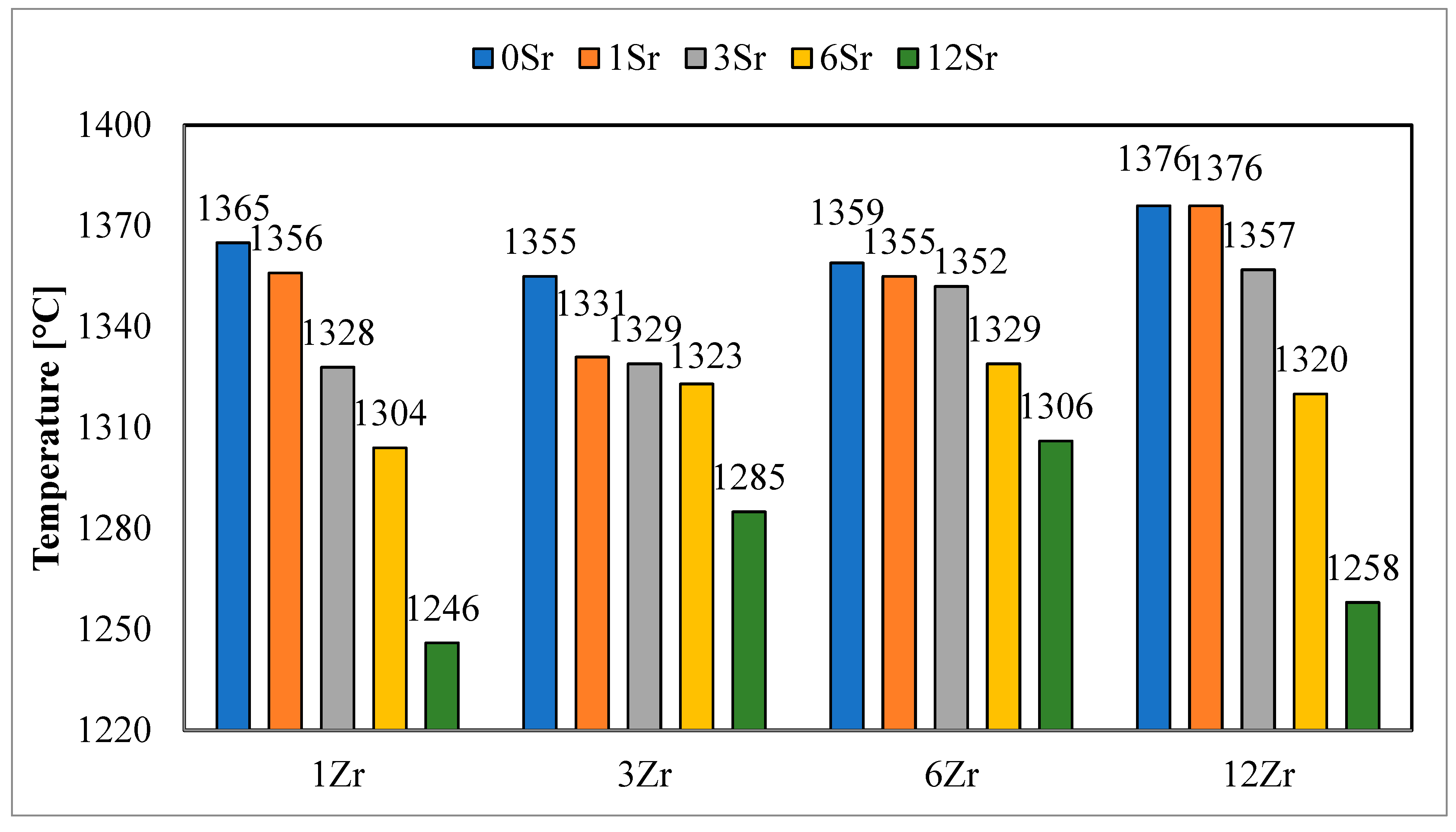
Similar results were observed for hemispheric temperatures (
Figure 3), and with an increase in the amount of strontium oxide added, a decrease in the determined hemispheric temperatures was observed. However, the hemispheric temperature values of glazes with a higher zirconium content have higher values only for glazes without the addition of strontium oxide. The addition of this oxide causes the determined hemispheric temperatures to show a clear upward or downward trend with the higher zirconium content in the glazes. This may indicate that the presence of strontium oxide in the glaze affects the processes that take place, while its fluxing effect is not directly proportional to the amount of SrO added and strongly depends on the chemical composition of the glaze.
The addition of strontium oxide to the glazes has a slightly different effect on the determined melting temperatures (
Figure 4). For the group of glazes with the lowest zirconium content, a decrease in the value of this temperature is observed with an increase in the amount of strontium oxide in the glaze. For glazes with higher zirconium oxide, a decrease in the value of the reflow temperature is observed, but it is less proportional than in the case of other characteristic temperatures. The decrease in the reflow temperature value is most visible for the highest addition of strontium oxide, compared to the glaze without the addition of SrO, with the same content of zirconium oxide.
Because the crystallization processes are also strongly influenced by the chemical composition of the glaze and its viscosity, the maximum firing temperatures were determined by the hemisphere temperatures, so that the conditions for the crystallization process were as close to each other as possible. According to the literature, the characteristic temperature of the hemisphere is the temperature at which the glaze sample assumes the shape of a hemisphere, and the geometric dimensions of the glaze sample at the temperature of the hemisphere, observed using a high-temperature microscope, are the following: the length of the base is equal to half the height of the sample, and the viscosity of the glazes for such a shape is 10
4 dPas. [
38,39]
The selected firing temperature based on the hemisphere temperature is not accidental. The maximum temperature used in the ceramics industry is the temperature between the characteristic temperature of the ball and the characteristic temperature of the flow. Because the firing process is intended to achieve a uniform baked glaze layer, it was decided that all glazes would be fired at the designated characteristic temperature of the hemisphere. The obtained results of characteristic temperatures show significant discrepancies; therefore, firing all glazes at one temperature would result in some glazes being fired at too high or too low a temperature. Because viscosity is an additional factor that influences the crystallization processes, it was decided that each glaze would be fired to a hemispherical temperature, which is characterized by constant viscosity.
3.2. Phase composition
The fired glazes were characterized by high transparency. This may indicate a high degree of amorphousness. The exception here was glazes from the 12Zr group, whose transparency was significantly low and can be classified as cloudy glazes. To determine the amount and type of amorphous phases, we subjected the glazes to X-ray radiation, and the results are presented in the
Table 3.
The results obtained indicate the presence of only two crystalline phases in varying amounts: zirconium silicate (ICSD 98-000-9582) and quartz (ICSD 98-000-0174).
When analyzing the content of the crystalline phase of zirconium silicate in fired glazes, greater amounts can be observed, especially in the glazes of the 6Zr and 12Zr series. In the glazes of the 1Zr and 3Zr series, the determined amount of zirconium silicate is small. This clearly suggests that the added zirconium silicate passed almost completely into the amorphous phase. Data from the literature indicate that, in the case of glazes to which zirconium silicate or zirconium oxide is added, there is no recrystallization of crystalline zirconium phases, and zirconium cations are present in the amorphous matrix. For the group of 6Zr and 12Zr glazes, a decrease in the amount of the crystalline phase of zirconium silicate is observed with an increase in the amount of strontium oxide added. This suggests the depolymerizing effect of strontium oxide, which breaks the bonds of the alumina and silica sublattices and loosens the structure, due to which large zirconium cations can fit into the amorphous phase of the glazes.
The second crystalline phase obtained is quartz. Its amount varies depending on the amount of zirconium silicate used and the addition of strontium oxide. For glazes of the 1Zr and 3Zr series, a decrease in the amount of quartz in the fired glaze is observed with the addition of strontium oxide in the amount of 1 and 3% by weight, and then the further addition of strontium oxide causes an increase in the amount of the crystalline phase of quartz in the glaze. For glazes of the 6Zr and 12Zr series, the determined amount of quartz in the glaze decreases with increasing strontium oxide, while in the case of glazes with the highest zirconium content, quartz appeared in a small amount only for glazes 12Zr0Sr and 12Zr1Sr. The fluxing and depolymerizing effect of strontium oxide will cause a faster dissolution of the quartz grains and a reduction of its amount in the fired glaze. However, the decomposition of zirconium silicate and partial dissolution of zirconium cations in the amorphous matrix will result in an incomplete dissolution of quartz grains due to their excess in the amorphous phase.
3.3. SEM observations
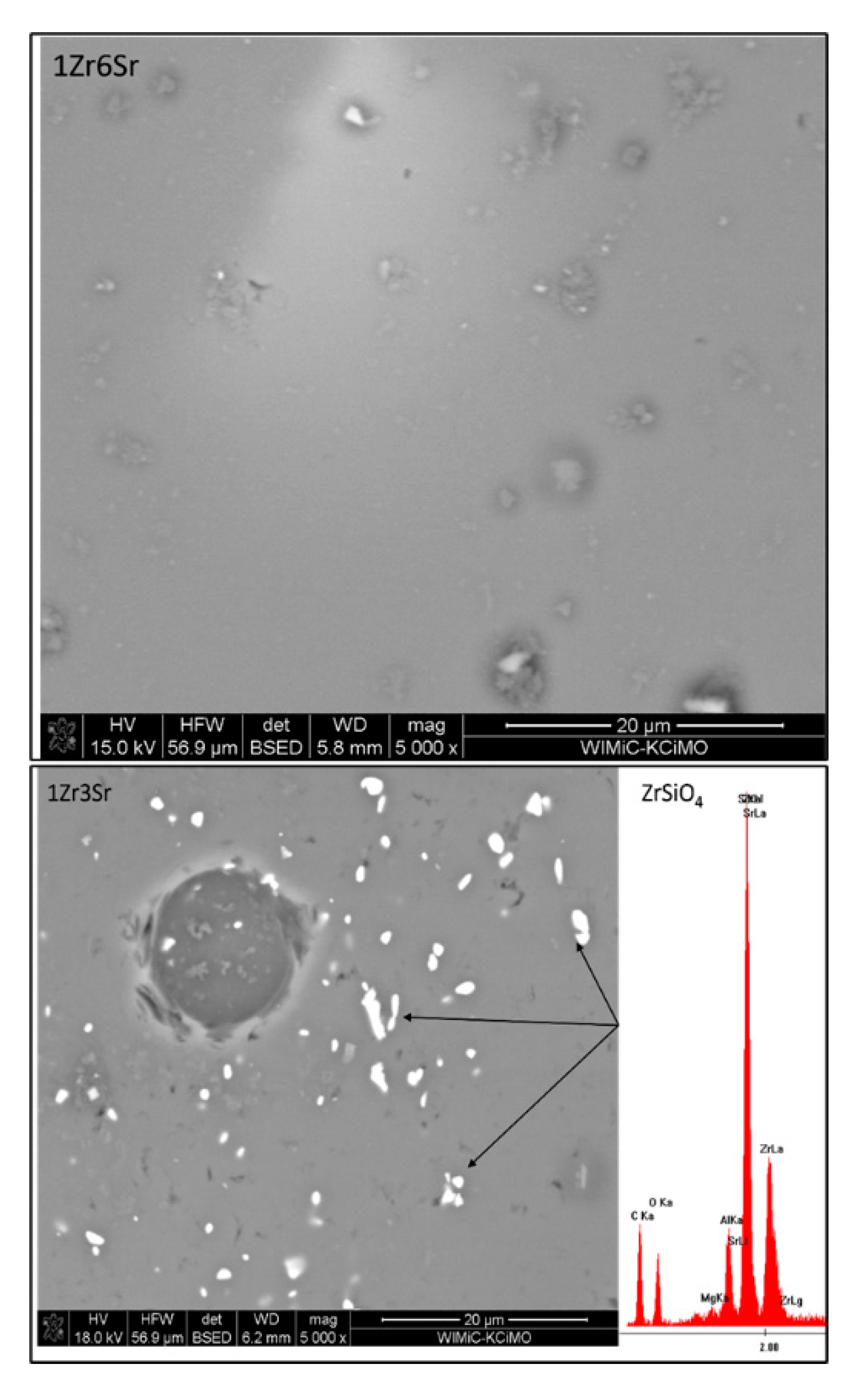
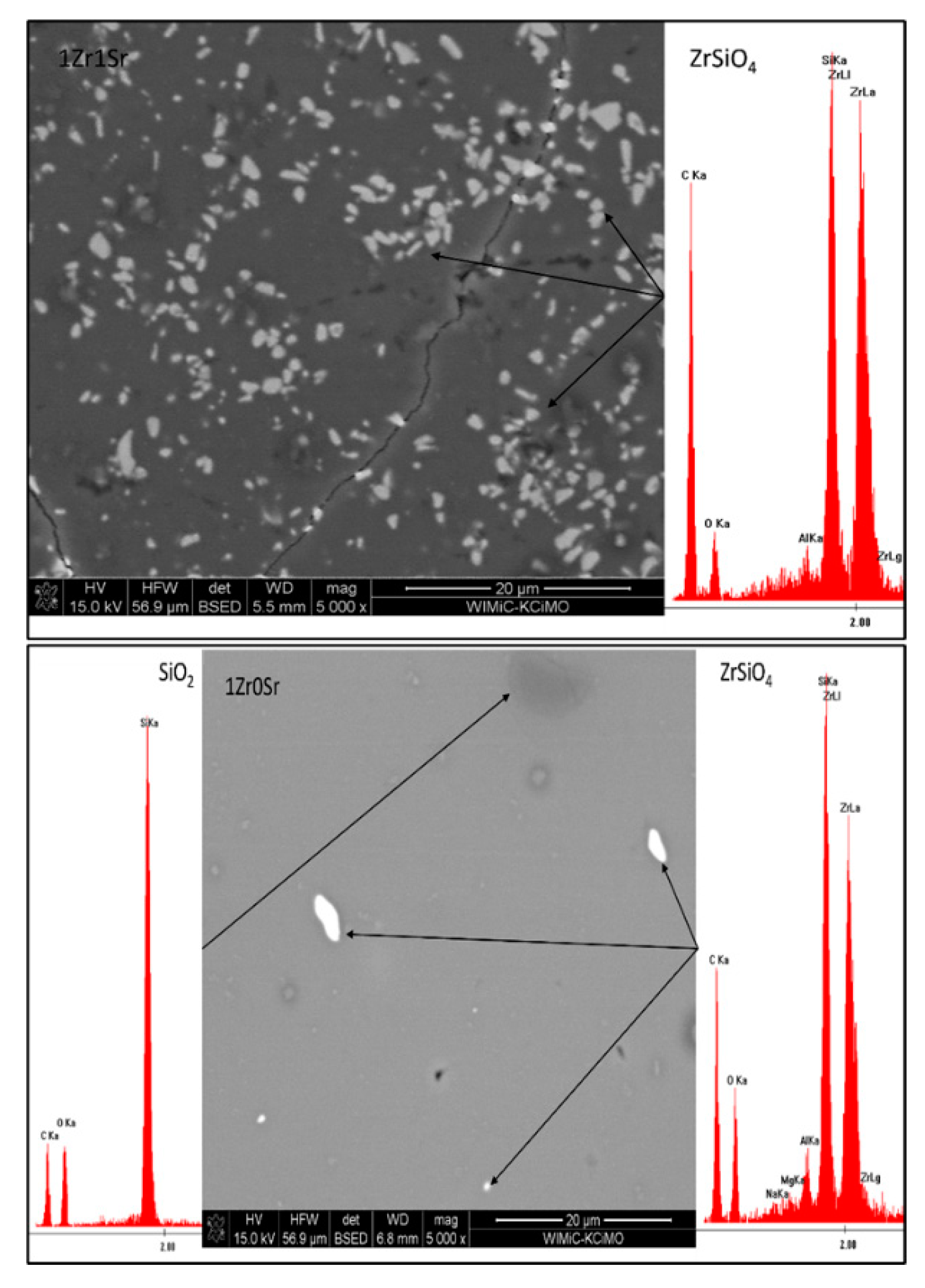
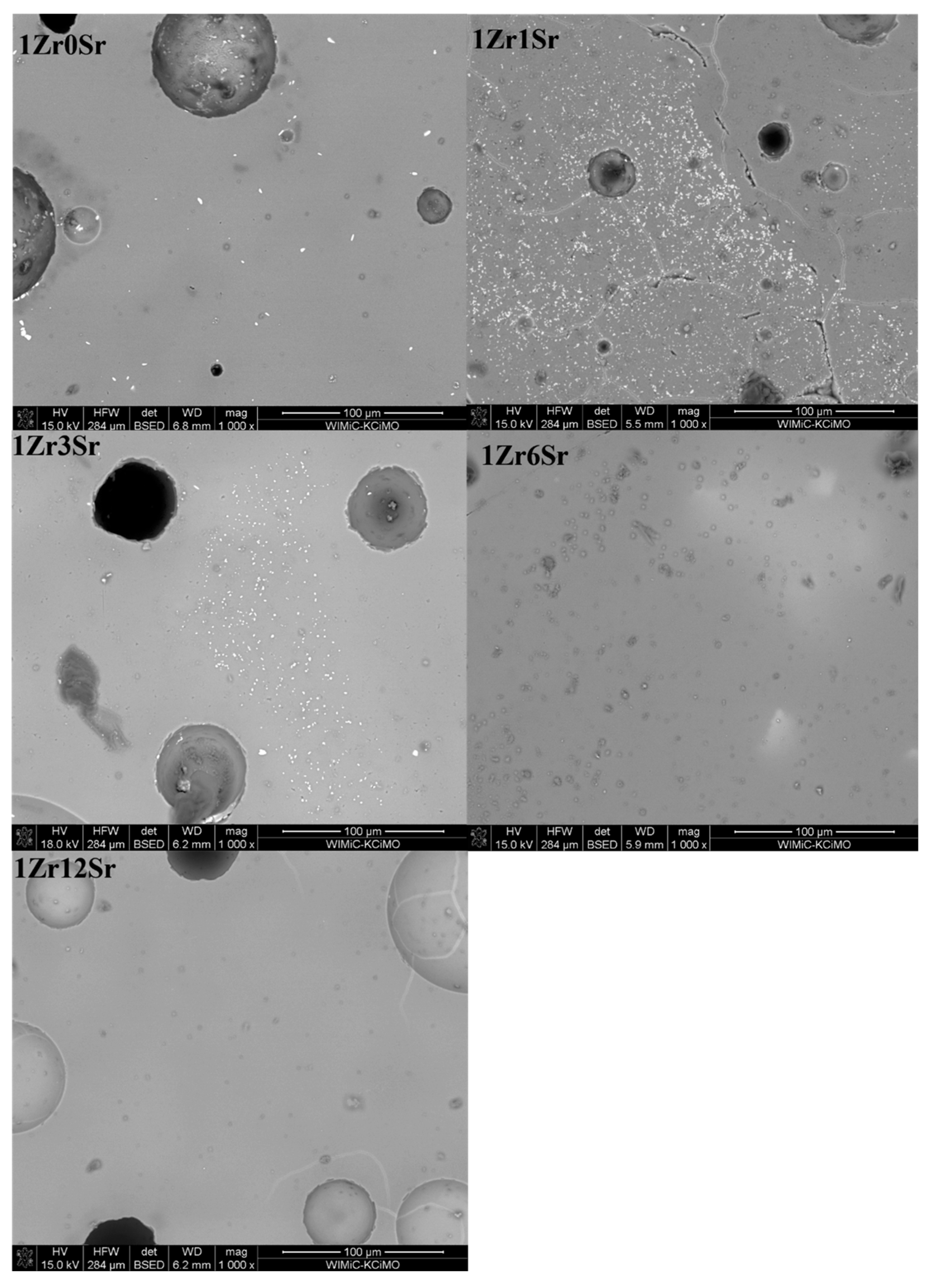
Observations of the microstructure glazes of the 1Zr series (
Figure 5) showed the presence of fine crystals of high brightness. EDS analysis confirmed that these are zirconium silicate crystals. Their presence was observed during the observation of the 0Sr, 1Sr, and 3Sr glazes, which is consistent with the results obtained from the composition of the analysis of the XRD phase. In the glaze without the addition of strontium oxide, the distribution of zirconium silicate is even over the entire surface. This is different for the microstructure of 1Sr and 3Sr glazes, for which clusters of zirconium silicate were observed, areas containing more of this crystalline phase, and areas completely without this crystalline phase. Glazes with a higher addition of strontium oxide show full amorphism, but no zirconia-crystalline phases were observed.
The microstructure of the glazes of the 1Zr series also showed the presence of cracks (
Figure 5). They are probably related to the stresses present in the glazes of this composition. Their occurrence was observed especially in 1Zr1Sr and 1Zr12Sr glazes. This indicates that significant stresses occur in these glazes. The occurrence of significant stresses that cause visible cracks may significantly reduce the mechanical strength and thermal resistance of such glazes. No cracks were observed in the remaining glazes in this series. This may be due to the chemical composition of the glaze, which does not cause stresses in the entire system during the processes that occur during cooling.
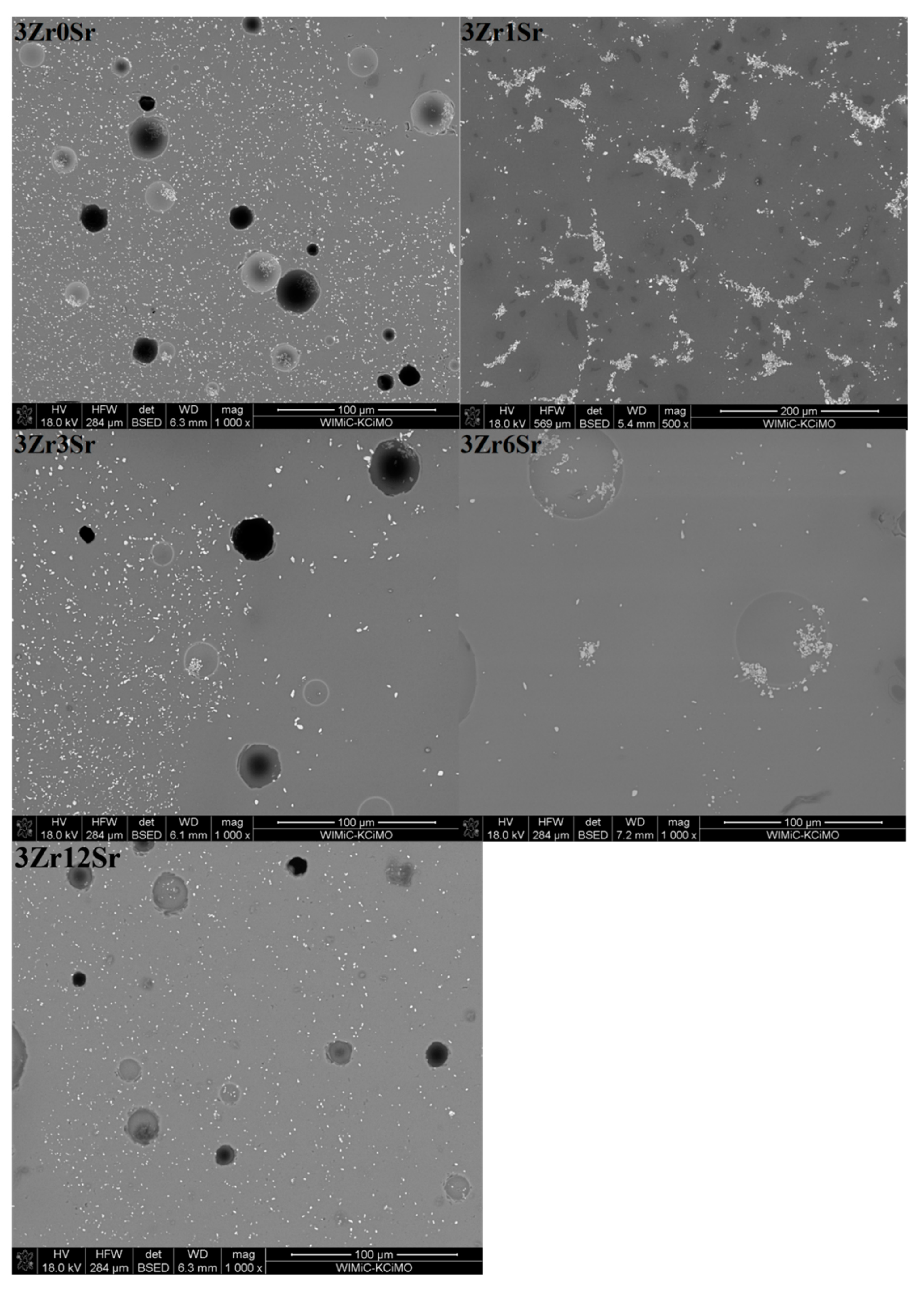
The microstructure of the glazes of the 3Zr series (
Figure 6) shows an uneven distribution of the crystalline zirconium phases. This is especially evident for glazes to which strontium oxide has been added. For glazes without the addition of this oxide, small areas devoid of zirconium silicate crystals are areas that, according to the EDS analysis, correspond to residual quartz crystals, although they do not have clearly marked intergrain boundaries. The addition of strontium oxide causes zirconium silicate to occur in clusters that constitute a larger or smaller area of the microstructure. For the 3Zr1Sr glaze, a photo magnified 500 times is shown, in which smaller and larger areas with the crystalline phase of zirconium silicate are visible. Residual quartz crystals and empty areas without any crystalline phase are also visible.
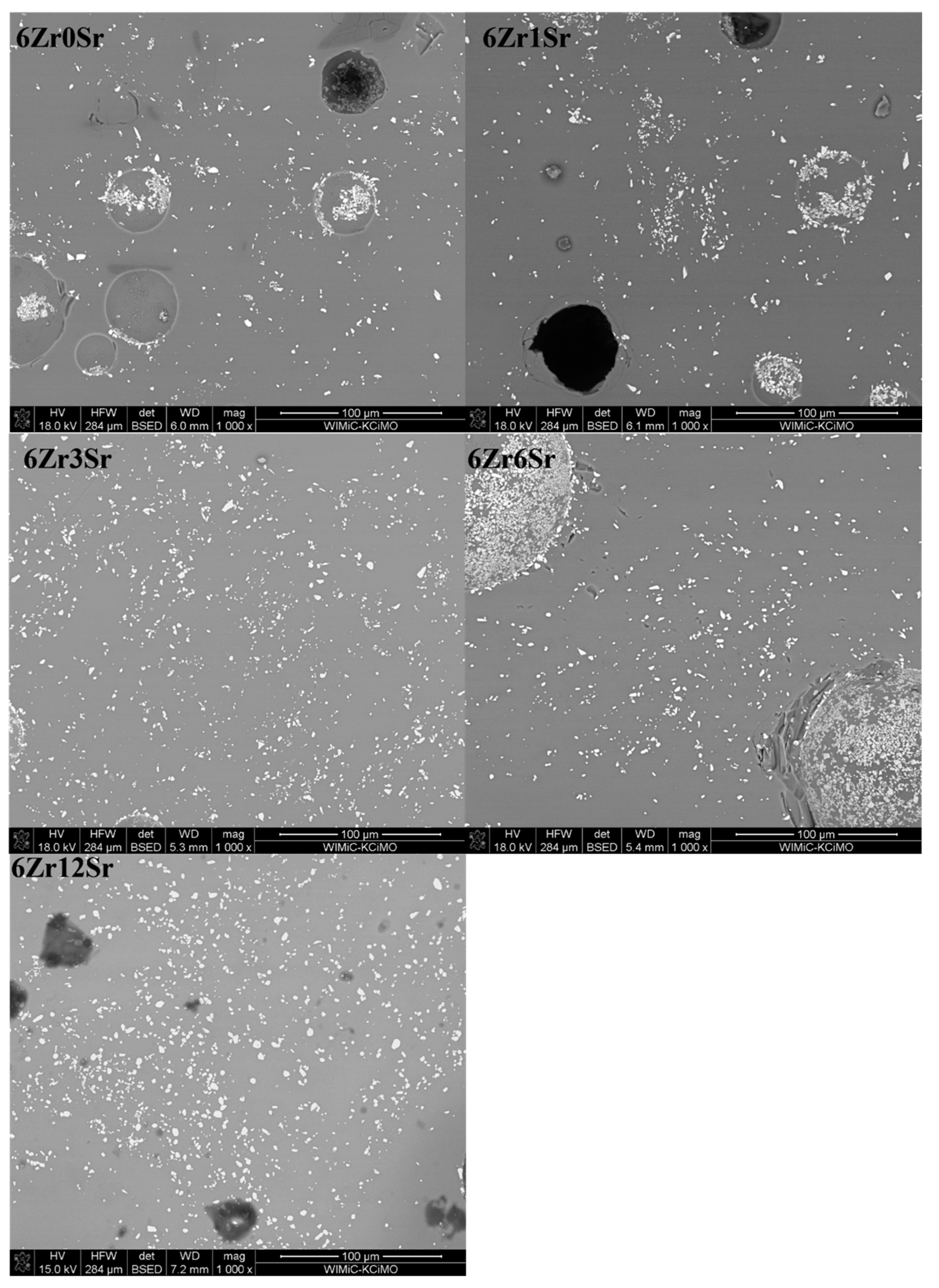
The glazes of the 6Zr series (
Figure 7) show much greater homogeneity than those of the series, with a lower zirconium content in the glaze. Areas with more crystals are still observed, but there are much fewer of them, and their amount decreases with increasing strontium oxide content in the glazes. However, there is greater diversity in terms of crystal size; There are also small ZrSiO
4 crystals, but slightly larger ones can be observed. The areas in which zirconium silicate crystals are not observed correspond to the composition of quartz.
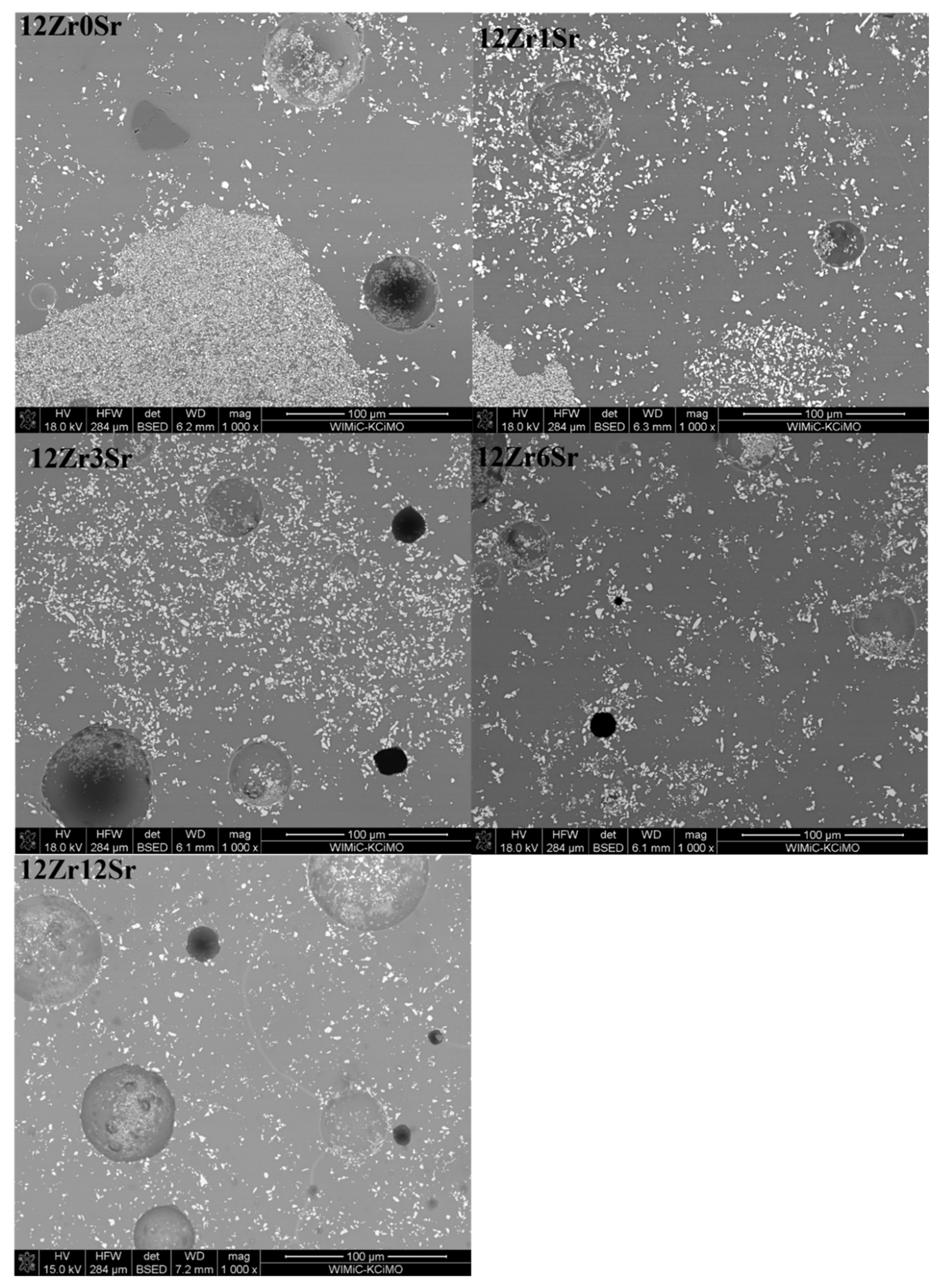
The 12Zr series of glazes (
Figure 8) shows considerable variation in the distribution of zirconium silicate crystals. An increase in the homogeneity of the microstructure can be observed with an increase in the amount of strontium oxide. Glaze 12Zr0Sr without the addition of strontium oxide shows significant inhomogeneity, which is visible microscopically - bright streaks on the glaze. The addition of strontium oxide causes this macroscopic inhomogeneity to decrease, and since the addition of 6Sr, the glazes appear uniform. The microstructure confirms this, since from the content of 3% mass % SrO, the glaze is more homogeneous than the reference glaze.
Observations of the microstructure of the glaze showed the presence of voids (
Figure 5,
Figure 6,
Figure 7 and
Figure 8). They can be divided into two types. Firstly, these are holes created during sample preparation, most likely grains torn out during surface polishing or chipping of glaze fragments, observed as black areas. The second type is spherical voids that were created during the firing of the samples. Due to the natural raw materials used, gases are released during heating. They are formed from the decomposition of kaolin, talc, and strontium carbonate. The presence of such voids is due to the inability to remove gases associated with the significant viscosity of the system. The result is these types of defects in the microstructure of glazes. In industrial processes, this is treated as a glaze defect, and efforts are made to remove or minimize its presence.
The different arrangement of the crystalline zirconia phases may be due to the difference in the viscosity of the glaze during the firing process. The inhomogeneity of glazes at the microscale results from the individual processes that take place with individual raw materials; some of them undergo reactions at a lower temperature, and others require a higher one. Thus, there are areas where the liquid phase appears much faster, and, in these areas, further reactions occur earlier than those where the liquid phase appears later.
The addition of strontium oxide causes a significant reduction in this inhomogeneity because of its fluxing effect, the appearance of the liquid phase, and the migration of system elements. Strontium oxide also has a depolymerizing effect, i.e. it loosens the structure and reduces the viscosity, which promotes mixing and homogenization of the entire system.
Due to the changes caused by the flux effect of strontium oxide and depolymerising structure, its presence significantly affects the obtained crystalline phases and the microstructure of glazes. Strontium oxide belongs to the group of fluxes that do not participate in crystallization themselves and do not create crystalline phases; therefore, its effect is more visible in terms of changes in the microstructure. Unlike fluxes with a similar effect, such as barium oxide, which have a much greater ability to crystallize, barium feldspar. In the case of strontium oxide, strontium feldspar does not occur in ceramic glazes. In some cases, the strontium cation may substitute cations in the structure of sodium or potassium feldspar, but such a phenomenon is observed very rarely because of the significant size of the strontium cation and its mismatch with the overall structure.
Despite the positive conclusion drawn from these studies, i.e. the increased homogenization of zirconium silicate crystals in the structure, the risks associated with the use of strontium oxide in glazes must be considered. The use of this oxide in larger quantities may result in a decrease in the mechanical properties of the glazes as a result of the significant depolymerizing properties of this oxide. This may reduce the hardness, abrasion resistance, and corrosion resistance of surfaces. The use of significant amounts of strontium oxide, in addition to lowering the firing temperature, can significantly reduce the viscosity and surface tension of the glaze, which can cause it to run off the surface.
3.4. SEM-EDS observations.
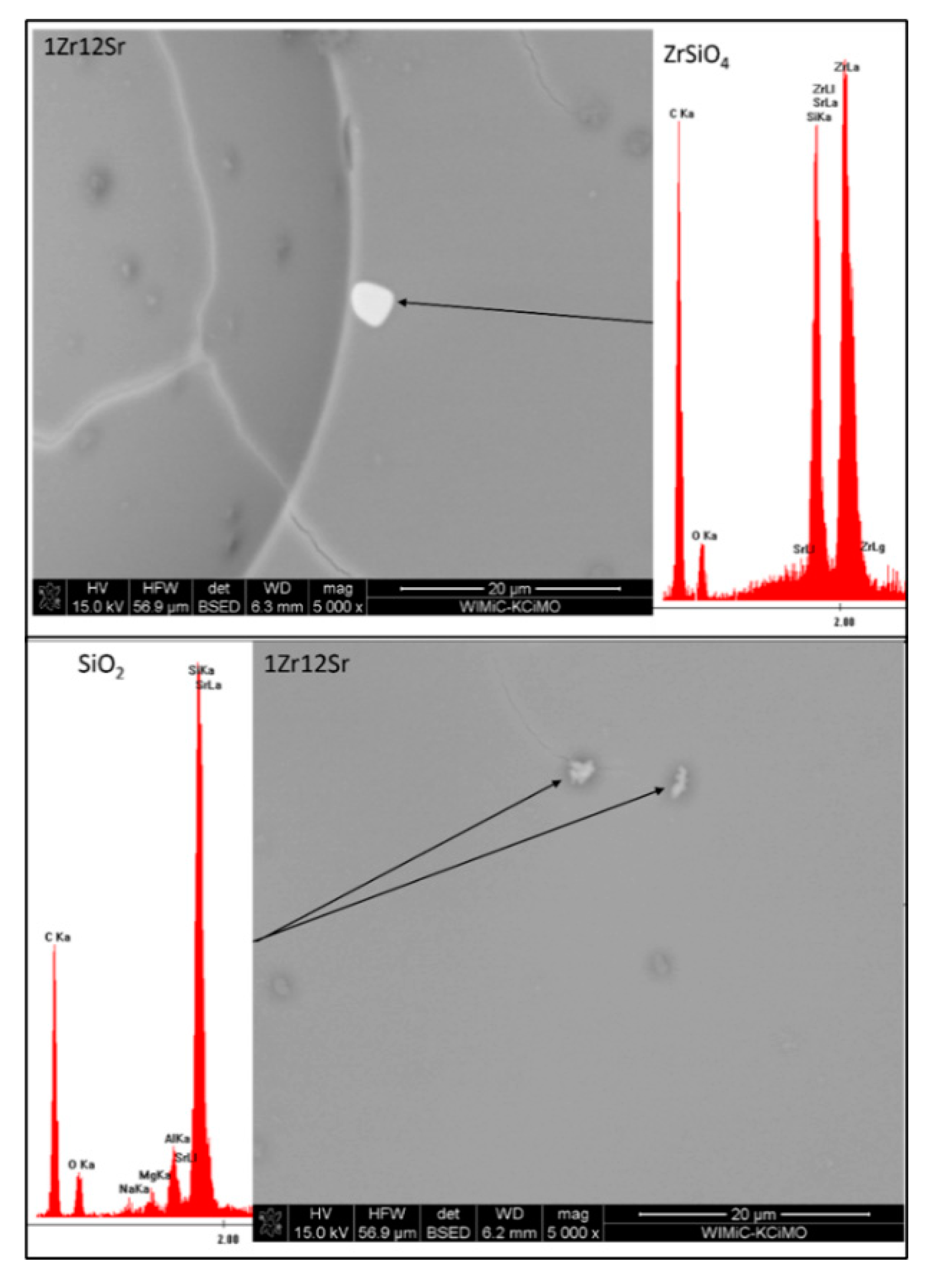
The main purpose of determining the chemical compositions by means of EDS of the visible crystallites, was to confirm the phase composition. XRD analysis of the phase composition showed the presence of only two crystalline phases (
Table 3), zirconium silicate and residual quartz. The low content of crystalline phases (4 to 18% vol.) made it difficult to find crystallites. The SEM and EDS photos on Figure X presented the 1Zr group with a variable amount (12; 6; 3; 1 and 0% mass) of SrO. All EDS measurements indicate the presence, in all sets of only quartz and zirconium silicate. This confirms the XRD results (
Table 3). The shell around the SiO2 crystallites confirms the residual form of the quartz crystals; it is a high concentration zone of SiO2 from the melting of the quartz crystals.
Figure 10.
Microphotographs from SEM-EDS observations of 1Zr series (ZrSiO4 – light crystals, SiO2 – dark-grey spot).
Figure 10.
Microphotographs from SEM-EDS observations of 1Zr series (ZrSiO4 – light crystals, SiO2 – dark-grey spot).
Figure 11.
Microphotographs from SEM-EDS observations of 1Zr series (ZrSiO4 – light crystals, SiO2 – dark-grey spot).
Figure 11.
Microphotographs from SEM-EDS observations of 1Zr series (ZrSiO4 – light crystals, SiO2 – dark-grey spot).
4. Conclusions
This paper presents the effect of the addition of strontium oxide on the recrystallization of zirconium silicate. The characteristic temperatures of the prepared glazes were measured, on the basis of which the maximum firing temperatures were determined on the determined temperature of the hemisphere. The results obtained from the measurement of characteristic temperatures indicated that strontium oxide exhibits a fluxing effect; however, it is not observed for each glaze with its addition and depends on the chemical composition of the entire system. Additionally, an increase in the amount of zirconium silicate in the glaze does not cause a proportional increase or decrease in the characteristic temperatures depending on the presence of other components in the glaze.
Analysis of the phase composition showed that some of the added zirconium silicate dissolves in the amorphous matrix, which is particularly evident at a lower zirconium content in the glazes. The amount of dissolved zirconium increases slightly with the addition of strontium oxide, but does not exceed 3% by mass. This is due to the structure and geometric dimensions of the zirconium cation.
Microstructure analysis showed that the addition of strontium oxide increased the homogeneity of the distribution of zirconium silicate crystals. This phenomenon is most desirable because of the lack of microareas of a purely amorphous nature. These areas will probably be characterized by reduced mechanical properties and the entire glaze as a material will be much weaker than one that is microstructurally homogeneous. The presence of crystalline phases affects the mechanical properties, whereas their uneven distribution, e.g. large areas with a predominance of the amorphous phase, will be characterized by a much lower mechanical strength and will constitute the weakest element of the entire system.
The information obtained constitutes essential information on the use of strontium oxide in glazes in which the final phase composition is zirconium silicate. The data obtained show how the addition of strontium oxide affects the phase composition and microstructure of glass-crystalline materials. The described crystal parameters and their arrangement, depending on the amount of zirconium silicate and strontium oxide, will significantly facilitate the design of this type of glaze.
References
- R. A. Eppler and D. R. Eppler, Glazes and glass coatings, Ohio: The American Ceramic Society, 2000.
- K. Shaw, Ceramic glazes, Elsevier Publishing Company, 1971.
- A. Escardino, “Crystalline glazes,” in Qualicer, Castellon, 1996.
- Meija J. F.: Understanding the role of fluxes in single-fire porcelain glaze development, Alfred University. Faculty of Ceramic Engineering. Kazuo Inamori School of Engineering, New York 2004.
- L. R. Kirk, “Function and action of opacifiers,” Journal of American Ceramic Society, vol. 15, no. 4, p. 226, 1932.
- J. K. Beam, “Effect of opacifiers on fused viscosity of feldspathic glazes,” Journal of American Ceramic Society, vol. 26, no. 7, pp. 205-212. [CrossRef]
- M. McCoy, W. E. Lee and A. H. Heuer, “Crystallization of MgO-Al2O3-SiO2-ZrO2 glasses,” Journal of the American Ceramic Society, vol. 69, no. 3, pp. 292-296, 1986.
- M. Dittmer, C. F. Yamamoto, C. Bocker and C. Russel, “Crystallization and mechanical properties of MgO/Al2O3/SiO2/ZrO2,” Solid State Sciences, vol. 13, pp. 2146-2153, 2011.
- R. J. Castilone, W. M. Carty, D. Sriram and R. L. Snyder, “Crystallization of zircon in stoneware glazes,” Journal of American Ceramic Society, vol. 82, no. 10, p. 2819, 1999. [CrossRef]
- K. Pasiut, J. Partyka, M. Leśniak, P. Jeleń, Z. Olejniczak: "Raw glass-ceramics glazes from SiO2–Al2O3–CaO–MgO–Na2O–K2O system modified by ZrO2 addition – Changes of structure, microstructure and surface properties", Open Ceramics, vol. 8, 2021.
- C. G. Harman, Howard R. Swept, Raw leadless whiteware glazes, Journal of the American Ceramic Society, vol 28, iss. 2, p. 48-52, 1945.
- J. Partyka, K. Pasiut, P. Jeleń, M. Leśniak, M. Sitarz, "Comparison of the impact of the addition of three alkaline earth metal oxides 𝐵𝑎𝑂, 𝑆𝑟𝑂 and 𝑍𝑛𝑂 on sintering of glass-ceramic glazes from the 𝑆𝑖𝑂2–𝐴𝑙2𝑂2–𝐶𝑎𝑂–𝑀𝑔𝑂–𝑁𝑎2𝑂–𝐾2𝑂 system", Journal of Thermal Analysis and Calorimetry, vol. 138 iss. 6, pp. 4341–4347, 2019.
- K. Pasiut, J. Partyka: "The influence of 𝑍𝑟𝑂2 addition on the thermal properties of glass-ceramic materials from 𝑆𝑖𝑂2−𝐴𝑙2𝑂3−𝑁𝑎2𝑂−𝐾2𝑂−𝐶𝑎𝑂 system", Journal of Thermal Analysis and Calorimetry, vol. 130 iss. 1, s. 343–350, 2017.
- K. Pasiut, J. Partyka: "Thermal properties of glass-ceramic glazes with zirconium oxide added to multicomponent system 𝑆𝑖𝑂2−𝐴𝑙2𝑂3−𝐶𝑎𝑂−𝑀𝑔𝑂−𝑁𝑎2𝑂", Journal of Thermal Analysis and Calorimetry vol. 148 iss. 5, pp. 1867–1874, 2023.
- K. Boudeghdegh, V. Diella, A. Bernasconi, A. Roula and Y. Amirouche, “Composition effects on the whiteness and physical-mechanical properties of traditional sanitary-ware glaze,” Journal of the European Ceramic Society, vol. 35, pp. 3735-3741, 2015. [CrossRef]
- M. Popa, M. Kakihana, M. Yoshimura and J. M. Calderon-Moreno, “Zircon formation from amorphous powder and melt in the silica-rich region of the alumina-silica-zirconia system,” Journal of Non-Crystalline Solids, vol. 352, pp. 5663-5669, 2006. [CrossRef]
- A. Levitskii, S. E. Barantseva, V. G. Lugin and A. I. Poznyak, “Optimization of the composition of the fritted component of the raw material mix of durable coatings,” Glass and Ceramics, vol. 67, no. 9-10, pp. 291-294, 2011. [CrossRef]
- M. G. Rasterio, T. Gassman, R. Santos and E. Antunes, “Crystalline phase characterization of glass-ceramic glazes,” Ceramics International, vol. 33, no. 3, pp. 345-354, 2007. [CrossRef]
- S. Wang, C. Peng, X. Huiyin and J. Wu, “Microstructural evolution and crystallization mechanism of zircon from frit glaze,” Journal of the European Ceramic Society, vol. 35, pp. 2671-2678, 2015. [CrossRef]
- C. W. F. Jacobs, “Opacifying crystalline phases present in zirconium-type glazes,” Jounal of the American Ceramic Society, vol. 37, pp. 216-220, 1954. [CrossRef]
- B. E. Yekta, P. Alizadeh and L. Rezazadeh, “Floor tile glass-ceramic glaze for improvement of glaze surface properties,” Journal of the European Ceramic Society, vol. 26, pp. 3809-3812, 2006. [CrossRef]
- L. Amoros, E. Blasco, C. Feliu, A. Moreno, Effect of particle size distribution on the evaluation of porous, microstructural, and dimensional characteristics during sinter-crystallisation of a glass-ceramic glaze, Journal of Non-Crystalline Solids, Vol. 572, 2021. [CrossRef]
- D. A. Earl and D. E. Clark, “Effects of glass frit oxides on crystallization and zircon pigment dissolution in whiteware coatings,” Journal of the American Ceramic Society, vol. 83, pp. 2170-2176, 2000. [CrossRef]
- B. Tiwari, A. Dixit, C. G. S. Pillai, S. C. Gadkari, G. P. Kothiyal: " Crystallization kinetics and mechanism of strontium zinc silicate glass", Journal of the American Ceramic Society, vol. 1-7, 2012.
- B. Karasu, M. Cable: "The chemical durability of SrO-MgO-ZrO2-SiO2 glasses in strongly alkaline environments", Journal of the European Ceramic Society, vol. 20, pp. 2499-2508, 2000.
- Kaczmarczyk, J. Partyka, K. Pasiut, J. Michałek, "Strontium carbonate in glazes from the 𝑆𝑖𝑂2–𝐴𝑙2𝑂3–𝐶𝑎𝑂–𝑀𝑔𝑂–𝑁𝑎2𝑂–𝐾2𝑂 system, sintering and surface properties", Open Ceramics, vol. 9 art. no. 100233, s. 1–8, 2022.
- M. Licheron, V. Montouillout, F. Millot, D. R. Neuville, Raman and 27Al NMR structure investigations of aluminate glasses: (1-x)Al2O3- x MO, with M = Ca, Sr, Ba and 0.5<x<0.75), Journal of Non-Crystalline Solids, vol. 357, p. 2796-2801, 2011.
- A. N. Novikov, D. R. Neuville, L. Hennet, Y. Gueguen, D. Thiaudière, T. Charpentier, P. Florian, Al and Sr environment in tectosilicate glasses and melts: Viscosity, Raman and NMR investigation, Chemical Geology, vol. 461, p. 115-127, 2017. [CrossRef]
- V. I. Voevodin, Unfritted opaque glaze for ceramic sanitaryware, Glass and Ceramics, vol. 57, pp. 23-24, 2000.
- Ch. Myung-Whun, Properties of glasses based on the CaO-MgO-SiO2 system for low-temperature co-fired ceramic, Ceramics International, vol. 35, p. 2513-2515, 2009.
- G. H. Beal, Refractory glass-ceramics based on alkaline earth aluminosilicates, Journal of the Ceramic Society, vol 29, p. 1211-1219, 2009. [CrossRef]
- D. Silva, A. M. Rodrigues, A. C. M. Rodrigues, M. J. Pascual, A. Durán, A. A. Cabral, Sintering and crystallization of SrO-CaO-B2O3-SiO2 glass-ceramics with different TiO2 contents, Journal of Non-Crystalline Solids, vol 473, p. 33-40, 2017.
- E. Enriquez, V. Fuertes, M. J. Cabrera, J. Seores, D. Munoz, B. Galiana, J. F. Fernandez, Study of the crystallization in fast sintered Na-rich plagioclase glass-ceramic, Ceramics International, vol. 45, 2019. [CrossRef]
- K. Thieme, Ch. Thieme, Determination of the crystallization mechanism of glasses in the system BaO/SrO/ZnO/SiO2 with differental scanning calorimetry, Journal of Thermal Analysis and Calorimetry, vol. 142, 2020.
- J. L. Amoros, E. Blasco, A. Moreno, N. Marin, C. Feliu, Effect of particle size distribution on the sinter-crystallizsation kinetics of a SiO2-Al2O3-CaO-MgO-Sro glass-ceramic glaze, Journal of Non-Crystalline Solids, Vol. 542, 2020.
- B. Karasu, Crystallisation in the SrO-MgO-ZrO2-SiO2 (SMZS) system glasses, Journal of materials Science, vol. 37, p. 271-285, 2002.
- Paganelli and D. Sighinolfi, “Understanding the behaviour of glazes: new possibilities using the automatic hot stage microscope Misura,” Industrial Ceramic, vol. 17, pp. 69-73, 1997.
- D. Sighinolfi, “Misura equipment, solving ceramic problems,” Industrial Ceramic, vol. 30, pp. 1-8, 2010.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).