Submitted:
15 September 2023
Posted:
19 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Geographic location
2.2. Study area
2.3. Material vegetal
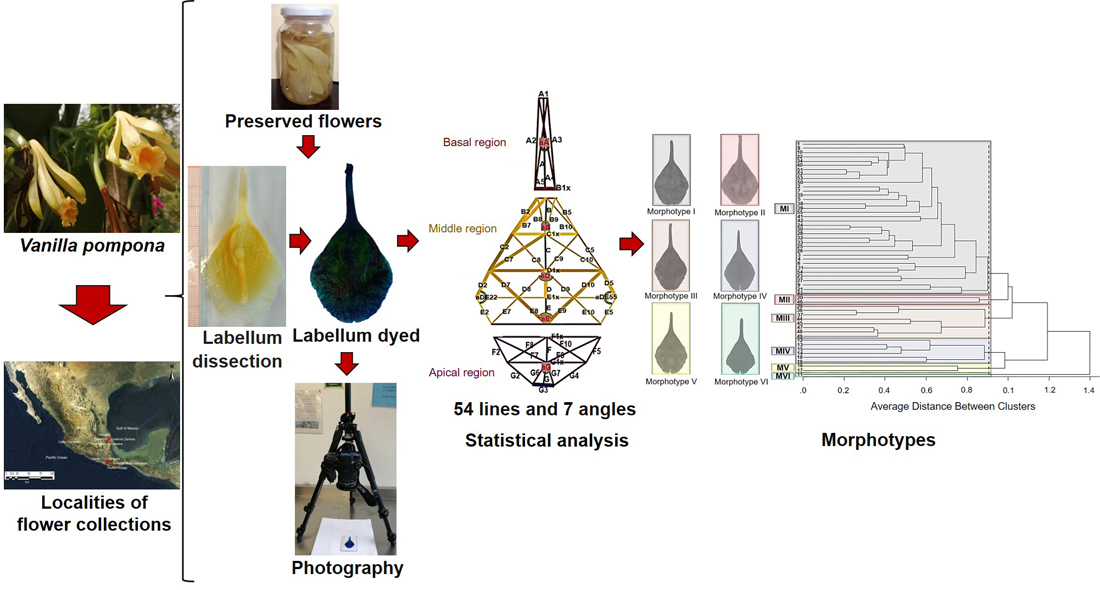
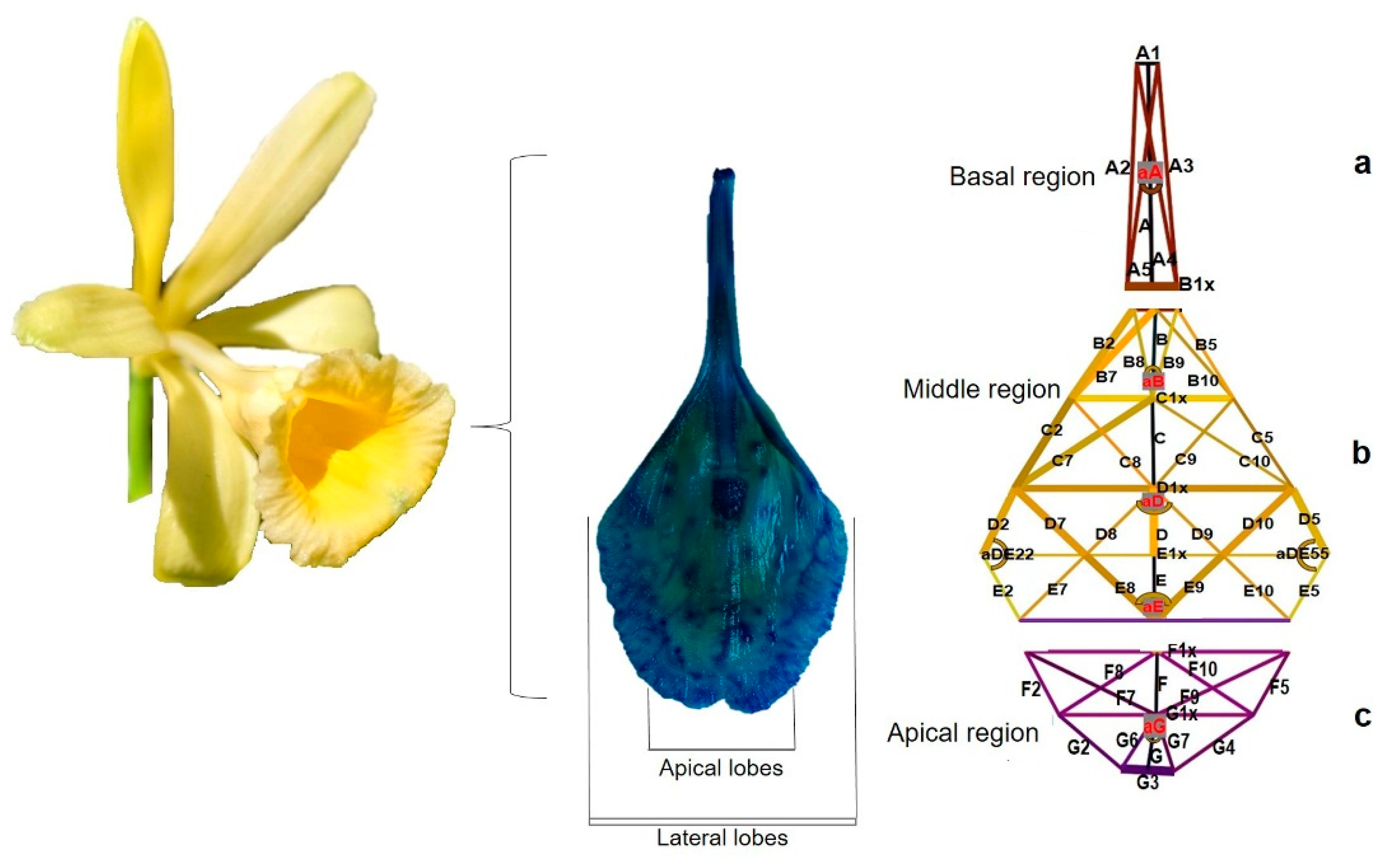
2.4. Morphological characterization of the labellum
2.5. Statistical analyses
3. Results
3.1. Characterization of the labellum
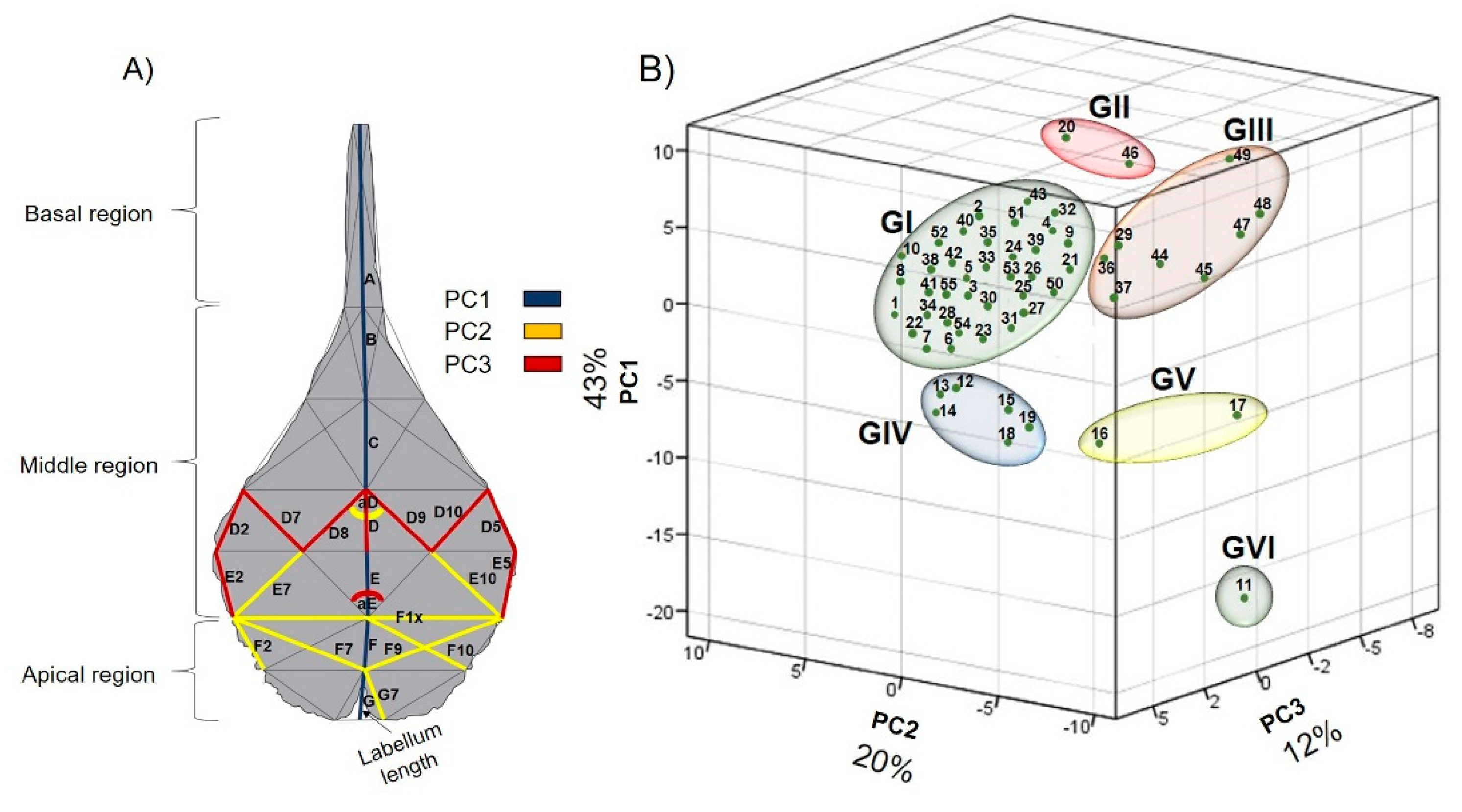
3.2. Distribution of the variation
3.3. Grouping diversity
4. Discussion
4.1. Characterization of the V. pompona labellum
4.2. Distribution of the variation
4.3. Morphotype grouping
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ehlers, D.; Pfister, M. Compounds of vanillons (Vanilla pompona Schiede). Essent. Oil Res. 1997, 9, 427–431. [Google Scholar] [CrossRef]
- Ranadive, A.S.; Havkinfrenkel, D.; Belanger, F.C. Quality control of vanilla beans and extracts. Handbook of vanilla science and technology 2011, 139–161. [Google Scholar] [CrossRef]
- Salazar-Rojas, V.M.; Herrera-Cabrera, B.E.; Delgado-Alvarado, A.; Soto-Hernández, M.; Castillo-González, F.; Cobos-Peralta, M. Chemotypical variation in Vanilla planifolia Jack. (Orchidaceae) from the Puebla-Veracruz Totonacapan region. Genet. Resour. Crop Evol. 2012, 59, 875–887. [Google Scholar] [CrossRef]
- Soto, M.A.; Dressler, R.L. A revision of the Mexican and Central American species of Vanilla Plumier ex Miller with a characterization of their ITS region of the nuclear ribosomal DNA. Lankesteriana 2010, 9, 285–354. [Google Scholar]
- Soto-Arenas, M. Filogeografía y Recursos Genéticos de las Vainillas de México. Informe Final SNIB-CONABIO Proyecto No. J101. Instituto Chinoín, A.C. Herbario de la Asociación Mexicana de Orquideología, A.C. México. 1999; pp. 1–106.
- Cameron, K.M. Vanilloid Orchids: Systematics and Evolution. In Vanilla. Medicinal and Aromatic Plants-Industrial Profiles; Odoux, E., Grisoni, M., Eds.; CRC Press Taylor and Francis Group: Boca Raton, Florida, 2010. [Google Scholar]
- Hernández-Hernández, J., Lubinsky, P. Vanilla Diseases. In Handbook of Vanilla Science and Technology; En Havkin- Frenkel, D., Belanger, F.C., Eds.; Wiley-Blackwell: 2011; pp. 26–38.
- Soto-Arenas, M.A. Recopilación y Análisis de la Información Existente sobre las Especies Mexicanas del Género Vanilla. Reporte Intermedio. CONACYT. México. 2009; 76 pp.
- Herrera-Cabrera, B.E.; Hernández, M.; Vega, M.; Wegier, A. Vanilla pompona (amended version of 2017 assessment). The IUCN Red List of Threatened Species 2020: 2020. e.T105878897A173977322. 1058. [Google Scholar] [CrossRef]
- Crymes, A.R., Salazer, I.V., Vásquez, J.V., Cubas, J.W.R., Labajos, H.V., Berdak, K.A. Patent No. US20200068817A1. 2020. Available online: https://patents.google.com/patent/US20200068817A1/en.
- Podolsky, R.H.; Holtsford, T.P. Population structure of morphological traits in Clarkia dudleyana. I. Comparison of FST between allozymes and morphological traits. Genetics 1995, 140, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Cabrera, B.E.; Trejo-Miranda, J.; Delgado-Alvarado, A. Conocimiento Tradicional, Predictores Climáticos y Diversidad Genética: Fitoindicadores, observaciones astronómicas y diversidad genética de haba en la agricultura. LAP LAMBERT Academic Publishing. 2010; 64 pp.
- Rudall, P.J.; Bateman, R.M. Roles of synorganisation, zygomorphy and heterotopy in floral evolution: The gynostemium and labellum of orchids and other lilioid monocots. Biol. Rev. 2002, 77, 403–441. [Google Scholar] [CrossRef]
- Pan, Z.J.; Chen, Y.Y.; Du, J.S.; Chen, Y.Y.; Chung, M.C.; Tsai, W.C.; Chen, H.H.; et al. Flower development of Phalaenopsis orchid involves functionally divergent SEPALLATA-like genes. New Phytol. 2014, 202, 1024–1042. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.F.; Hsu, W.H.; Lee, Y.I.; Mao, W.T.; Yang, J.Y.; Li, J.Y.; Yang, C.H. Model for perianth formation in orchids. Nat. Plants. 2015, 1, 1–8. [Google Scholar] [CrossRef]
- Gravendeel, B.; Dirks-Mulder, A. Floral development: Lip formation in orchids unravelled. Nat. plants. 2015, 1, 1–2. [Google Scholar] [CrossRef]
- Davies, K.L.; Stpiczyńska, M. Labellar micromorphology of two euglossine-pollinated orchid genera; Scuticaria Lindl. and Dichaea Lindl. and Dichaea Lindl. Ann. Bot. 2008, 102, 805–824. [Google Scholar] [CrossRef]
- Catling, P.M. Malaxis salazarii, a new species from Mexico and northern Mesoamerica. Orquidea 1990, 12, 93–104. [Google Scholar]
- Salazar-Rojas, V.M.; Herrera-Cabrera, B.E.; Soto-Arenas, M.Á.; Castillo-González, F. Morphological variation in Laelia anceps subsp. dawsonii f. chilapensis Soto-Arenas Orchidaceae in traditional home gardens of Chilapa, Guerrero, Mexico. Genet. Resour. Crop. Evol. 2010, 57, 543–552. [Google Scholar] [CrossRef]
- Andriamihaja, C.F.; Ramarosandratana, A.V.; Grisoni, M.; Jeannoda, V.; Besse, P. The leafless Vanilla species-complex from the South-West Indian Ocean Region: A taxonomic puzzle and a model for orchid evolution and conservation research. Diversity 2020, 12, 1–25. [Google Scholar] [CrossRef]
- Lima-Morales, M.; Herrera-Cabrera, B.E.; Delgado-Alvarado, A. Intraspecific variation of Vanilla planifolia (Orchidaceae) in the Huasteca region, San Luis Potosí, Mexico: Morphometry of floral labellum. Plant Syst. Evol. 2021, 307, 1–11. [Google Scholar] [CrossRef]
- Hernández-Ruíz, J.; Herrera-Cabrera, B.E.; Delgado-Alvarado, A. Variación morfológica del labelo de Vanilla pompona (Orchidaceae) en Oaxaca, México. Rev. Mex. Biodiversidad 2019, 90, 1–9. [Google Scholar] [CrossRef]
- Hernández-Ruíz, J.; Delgado-Alvarado, A.; Salazar-Rojas, V.M.; Herrera-Cabrera, B.E. Morphological variation of the labellum of Vanilla planifolia Andrews (Orchidaceae) in Oaxaca, Mexico. Rev. Fac. Cienc. Agrar. UNCuyo 2020, 52, 160–175. [Google Scholar]
- Maceda, A.; Delgado-Alvarado, A.; Salazar-Rojas, V.M.; Herrera-Cabrera, B.E. Vanilla planifolia Andrews (Orchidaceae): Labellum Variation and Potential Distribution in Hidalgo, Mexico. Diversity 2023, 15, 678. [Google Scholar] [CrossRef]
- Heywood, V.H.; Baste, I. Introducing biodiversity. In. Heywood, V.H and Watson, R.T (Eds). Global Biodiversity Assessment. University Press. Cambridge. 1995; pp 1-19.
- McNeely, J.A.; Miller, K.R.; Reid, W.V.; Mittermeier, R.A.; Werner, T.B. Conserving the world´s biological diversity. IUCN, World Resources Institute, Conservation International, WWF-US, World Bank, Washington. 1990; p. 193.
- Gliessman, S.R. Agroecología: Procesos ecológicos en agricultura sostenible. Catie. Costa Rica. 2002; pp 229-249.
- Piñero, D.; Caballero-Mellado, J.; Cabrera-Toledo, D.; Canteros, C.E.; Casas, A.; Castañeda, A.; Zúñiga, G.; et al. La diversidad genética como instrumento para la conservación y el aprovechamiento de la biodiversidad: Estudios en especies mexicanas, en Capital natural de México. Conocimiento actual de la biodiversidad. Conabio, México. 2008, 1, 437–494.
- Minoo, D.; Jayakumar, V.N.; Veena, S.S.; Vimala, J.; Basha, A.; Saji, K.V.; Peter, K.V. Genetic variations and interrelationships in Vanilla planifolia and few related species as expressed by RAPD polymorphism. Genet. Resour. Crop Evol. 2007, 55, 459–470. [Google Scholar] [CrossRef]
- Bory, S.; Catrice, O.; Brown, S.; Leitch, I.J.; Gigant, R.; Chiroleu, F.; Grisoni, M.; Duval, M.F.; Besse, P. Natural polyploidy in Vanilla planifolia (Orchidaceae). Genome 2008, 51, 816–826. [Google Scholar] [CrossRef]
- Herrera-Cabrera, B.E.; Salazar-Rojas, V.M.; Delgado-Alvarado, A.; Campos-Contreras, J.; Cervantes-Vargas, J. Use and conservation of Vanilla planifolia J. in the Totonacapan region, México. Eur. J. Environ. Sci 2012, 2, 43–50. [Google Scholar] [CrossRef]
- Hending, D.; Andrianiaina, A.; Maxfield, P.; Rakotomalala, Z.; Cotton, S. Floral species richness, structural diversity and conservation value of vanilla agroecosystems in Madagascar. Afr. J. Ecol. 2020, 58, 100–111. [Google Scholar] [CrossRef]
- Espinoza-Pérez, J.; Díaz-Bautista, M.; Barrales-Cureño, H.J.; Herrera-Cabrera, B.E.; Sandoval-Quintero, M.A.; Juárez-Bernabe; Reyes, C. Floristic biodiversity in Vanilla planifolia agroecosystems in the Totonacapan region of Mexico. Biocell 2019, 43, 440–452. [Google Scholar]
- Soto-Arenas, M. La vainilla: Retos y perspectivas de su cultivo. Biodiversitas 2006, 66, 1–9. [Google Scholar]
- Martin, D.A.; Andriafanomezantsoa, R.; Dröge, S.; Osen, K.; Rakotomalala, E.; Wurz, A.; Kreft, H. Bird diversity and endemism along a land-use gradient in Madagascar: The conservation value of vanilla agroforests. BioTROPICA. 2020, 53, 179–190. [Google Scholar] [CrossRef]
- Ordóñez-Blanco, J.C.; Parrado-Rosselli, Á. Relación fenología-clima de cuatro especies de orquídeas en un bosque altoandino de Colombia. Lankesteriana 2017, 17, 1–15. [Google Scholar] [CrossRef]
- Schaik, C.P.; Terborgh, J.W.; Wright, S.J. The phenology of tropical forests: Adaptive significance and consequences for primary consumers. Annu. Rev. Ecol. Syst. 1993, 24, 353–377. [Google Scholar] [CrossRef]
- CONABIO (Comisión Nacional para el Conocimiento y Uso de la Biodiversidad).. Portal de Geoinformación. 2012. (Consultado 04 febrero 2023). Disponible en linea. Available online: http://www.conabio.gob.mx/informacion/gis/.
- Sneath, P.H.A.; Sokal, R.R. Numerical taxonomy. The principles and practices of numerical classification. San Francisco: W. H. Freeman and Co. 1973.
- SAS (Statistical Analysis Systems). SAS/STAT Users guide, version 9. SAS Institute Inc., North Carolina 14. 2002; pp 421.
- Ketjarun, K.; Traiperm, P.; Suddee, S.; Watthana, S.; Gale, S.W. Labellar anatomy of the Nervilia plicata complex (Orchidaceae: Epidendroideae) in tropical Asia. Kew Bull. 2019, 74, 1–13. [Google Scholar] [CrossRef]
- González-Elizondo, M.S.; González-Elizondo, M.; Tena-Flores, J.A.; Ruacho-González, L.; López-Enríquez, I.L. Vegetación de la sierra madre occidental, México: Una síntesis. Acta Bot. Mex. 2012, 100, 351–403. [Google Scholar] [CrossRef]
- Challenger, A. Utilización y conservación de los ecosistemas terrestres de México: Pasado, presente y futuro. (primera edición). Ciudad de México: Conabio/ Instituto de Biología-UNAM/ Agrupación Sierra Madre S.C. 1988; pp. 269–292.
- Suárez-Mota, M.E.; Villaseñor, J.L.; López-Mata, L. Dominios climáticos de la Sierra Madre Oriental y su relación con la diversidad florística. Rev. Mex. biodiversidad 2017, 88, 224–233. [Google Scholar] [CrossRef]
- Han, L.X.; Jin, Y.; Zhang, J.L.; Li, X.L.; Chung, M.Y.; Herrando-Moraira, S.; Kawahara, T.; Yukawa, T.; Chung, S.W.; Chung, J.M.; Kim, Y.D.; López-Pujol, J.; Chung, M.G.; Tian, H.Z. Phylogeography of the endangered orchids Cypripedium japonicum and Cypripedium formosanum in East Asia: Deep divergence at infra-and interspecific levels. TAXON 2022, 71, 733–757. [Google Scholar] [CrossRef]
- Rzedowski, J. Vegetación de México. Editorial Limusa, México. 1978; p. 432.
- Evans, L.T. The Domestication of Crop Plants In: Crop evolution, adaptation and yield, Cambridge University Press. Cambridge. 1996; pp. 62–112.
- Mondragón-Palomino, M.; Theißen, G. Why are orchid flowers so diverse? Reduction of evolutionary constraints by paralogues of class B floral homeotic genes. Ann. Bot. 2009, 104, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Pansarin, E.R.; Ferreira, A.W.C. Evolutionary disruption in the pollination system of Vanilla (Orchidaceae). Plant Biology 2022, 24, 157–167. [Google Scholar] [CrossRef]
- Pansarin, E.R. Non-species-specific pollen transfer and double-reward production in euglossine-pollinated Vanilla. Plant Biology 2023, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Watteyn, C.; Scaccabarozzi, D.; Muys, B.; Van der Schueren, N.; Van Meerbeek, K.; Guizar Amador, M.F.; Karremans, A.P.; et al. Trick or treat? Pollinator attraction in Vanilla pompona (Orchidaceae). BioTROPICA 2021, 54, 268–274. [Google Scholar] [CrossRef]
- Ferreira, A.W.C.; de Oliveira, M.S.; Silva, E.O.; Campos, D.S.; Pansarin, E.R.; Guarçoni, E.A.E. Vanilla bahiana Hoehne and Vanilla pompona Schiede (Orchidaceae, Vanilloideae): Two new records from Maranhão state, Brazil. Check List. 2017, 13, 1131. [Google Scholar] [CrossRef]
- Householder, E.; Janovec, J.; Mozambite, A.B.; Maceda, J.H.; Wells, J.; Valega, R.; Christenson, E.; et al. Diversity, natural history, and conservation of Vanilla (Orchidaceae) in Amazonian wetlands of Madre de Dios, Peru. J. Bot. Res. Inst. Texas. 2010, 4, 227–243. [Google Scholar]



| State | Municipality | Locality | Altitude (m) |
|---|---|---|---|
| Veracruz | Papantla | Cazuelas | 69 |
| Gutiérrez Zamora | Paso de Barriles | 29 | |
| Tihuatlán | La Pasadita | 159 | |
| Puebla | Tuzamapan de Galeana | Reyes de Vallarta | 365 |
| Jalisco | Cabo Corrientes | Cabo Corrientes | 379 |
| Oaxaca | Sta. Cruz Itundujia | Hidalgo | 1075 |
| Morelos | 1668 | ||
| Primavera | 651 | ||
| Sta. Ma. Chimalapa | Sta. Ma. Chimalapa | 362 | |
| Pluma Hidalgo | Pluma Hidalgo | 1010 |
| Locality | Climate | Mean annual precipitation (mm) | Mean annual temperature (°C) | Soil moisture regime | Ecological zone |
|---|---|---|---|---|---|
| Cazuelas | Warm subhumid | 800-1200 | >22 | Ustic | Humid tropical |
| Paso de Barriles |
Warm subhumid | 1500-2000 | >22 | Udic type II | Humid tropical |
| La Pasadita | Warm subhumid | 1200-1500 | >22 | Ustic | Humid tropical |
| Reyes de Vallarta |
Warm humid | 2500-4000 | >22 | Udic type I | Humid tropical |
| Cabo Corrientes |
Warm subhumid | 1500-2000 | >22 | Xeric | Subhumid tropical |
| Hidalgo | Semi-warm subhumid | 2000-2500 | >22 | Xeric | Subhumid temperate |
| Morelos | Semi-warm subhumid | 2000-2500 | >18 | Xeric | Humid temperate |
| Primavera | Semi-warm subhumid | 2000-2500 | >22 | Xeric | Subhumid temperate |
| Sta. Ma. Chimalapa |
Warm humid | 1500-2000 | >22 | Ustic | Humid tropical |
| Pluma Hidalgo |
Warm subhumid | 1500-2000 | >22 | Ustic | Humid temperate |
| Variable | Mean (mm) | Coefficient of variation | Mean squares | ||||
|---|---|---|---|---|---|---|---|
| Error | Locality | Collection | |||||
| I. Labellum basal region | |||||||
| A | 23.33 | 3.80 | 0.79 | 34.08 | *** | 3.64 | *** |
| A1 | 2.18 | 10.63 | 0.05 | 1.53 | *** | 0.30 | *** |
| A2 | 23.37 | 3.85 | 0.81 | 33.58 | *** | 3.64 | *** |
| A3 | 23.38 | 3.82 | 0.80 | 33.17 | *** | 3.57 | *** |
| A4 | 23.59 | 4.15 | 0.96 | 34.14 | *** | 3.61 | *** |
| A5 | 23.57 | 4.13 | 0.95 | 34.86 | *** | 3.73 | *** |
| aA | 17.17 | 8.19 | 1.98 | 50.91 | *** | 5.15 | *** |
| B1x | 4.87 | 8.03 | 0.15 | 3.09 | *** | 0.51 | *** |
| II. Labellum middle region | |||||||
| B | 11.63 | 4.75 | 0.31 | 8.64 | *** | 0.93 | *** |
| B2 | 13.25 | 4.72 | 0.39 | 9.58 | *** | 1.14 | *** |
| B5 | 13.40 | 4.72 | 0.40 | 8.06 | *** | 1.12 | *** |
| B7 | 14.57 | 4.58 | 0.45 | 8.69 | *** | 1.15 | *** |
| B8 | 11.92 | 4.54 | 0.29 | 7.36 | *** | 0.83 | *** |
| B9 | 11.87 | 4.57 | 0.29 | 8.09 | *** | 0.97 | *** |
| B10 | 14.76 | 4.56 | 0.45 | 6.77 | *** | 1.16 | *** |
| aB | 23.67 | 8.16 | 3.73 | 153.82 | *** | 16.61 | *** |
| C | 11.62 | 4.78 | 0.31 | 8.65 | *** | 0.93 | *** |
| C1x | 17.78 | 7.07 | 1.58 | 18.58 | *** | 4.88 | *** |
| C2 | 13.86 | 5.38 | 0.56 | 13.41 | *** | 1.57 | *** |
| C5 | 13.95 | 5.42 | 0.57 | 9.71 | *** | 1.96 | *** |
| C7 | 20.08 | 5.26 | 1.11 | 20.64 | *** | 2.26 | ** |
| C8 | 14.69 | 4.65 | 0.47 | 5.90 | *** | 1.02 | ** |
| C9 | 14.62 | 4.51 | 0.44 | 9.45 | *** | 1.17 | *** |
| C10 | 20.23 | 5.00 | 1.02 | 14.39 | *** | 2.97 | *** |
| D | 8.35 | 7.37 | 0.38 | 5.29 | *** | 1.57 | *** |
| D1x | 32.90 | 5.95 | 3.84 | 57.94 | *** | 9.48 | *** |
| D2 | 8.74 | 6.87 | 0.36 | 4.43 | *** | 1.25 | *** |
| D5 | 8.77 | 6.74 | 0.35 | 4.31 | *** | 1.28 | *** |
| D7 | 11.84 | 7.37 | 0.76 | 11.70 | *** | 2.37 | *** |
| D8 | 11.61 | 5.66 | 0.43 | 6.49 | *** | 0.93 | ** |
| D9 | 11.64 | 5.25 | 0.37 | 6.61 | *** | 0.84 | ** |
| D10 | 11.84 | 7.27 | 0.74 | 11.37 | *** | 2.53 | *** |
| aD | 88.25 | 4.53 | 16.00 | 230.14 | *** | 102.01 | *** |
| aDE22 | 146.00 | 4.32 | 39.71 | 321.71 | *** | 118.57 | *** |
| aDE55 | 145.98 | 4.47 | 42.55 | 348.61 | *** | 120.61 | *** |
| E | 8.12 | 6.39 | 0.27 | 4.75 | *** | 0.78 | *** |
| E1x | 37.45 | 4.67 | 3.05 | 78.02 | *** | 6.58 | ** |
| E2 | 8.65 | 6.33 | 0.30 | 3.59 | *** | 0.92 | *** |
| E5 | 8.63 | 6.37 | 0.30 | 4.25 | *** | 1.03 | *** |
| E7 | 11.37 | 5.98 | 0.46 | 14.08 | *** | 1.49 | *** |
| E8 | 11.47 | 4.53 | 0.27 | 7.35 | *** | 0.51 | ** |
| E9 | 11.43 | 4.65 | 0.28 | 7.81 | *** | 0.54 | ** |
| E10 | 11.41 | 6.32 | 0.52 | 13.18 | *** | 1.48 | *** |
| aE | 89.69 | 4.25 | 14.55 | 76.97 | *** | 62.19 | *** |
| III. Labellum apical region | |||||||
| F | 6.22 | 7.45 | 0.22 | 5.62 | *** | 0.70 | *** |
| F1x | 32.02 | 5.06 | 2.62 | 96.25 | *** | 11.02 | *** |
| F2 | 7.25 | 7.82 | 0.32 | 5.76 | *** | 0.94 | *** |
| F5 | 7.20 | 8.11 | 0.34 | 6.49 | *** | 0.88 | *** |
| F7 | 17.20 | 5.64 | 0.94 | 28.32 | *** | 3.39 | *** |
| F8 | 13.82 | 6.07 | 0.70 | 21.96 | *** | 2.64 | *** |
| F9 | 13.92 | 6.27 | 0.76 | 19.98 | *** | 3.15 | *** |
| F10 | 17.13 | 5.33 | 0.83 | 28.96 | *** | 2.92 | *** |
| G | 6.25 | 9.31 | 0.34 | 5.55 | *** | 1.23 | *** |
| G1x | 24.77 | 6.14 | 2.31 | 65.83 | *** | 10.69 | *** |
| G2 | 11.30 | 9.46 | 1.14 | 17.49 | *** | 4.06 | *** |
| G3 | 6.16 | 12.84 | 0.63 | 22.79 | *** | 3.90 | *** |
| G4 | 11.20 | 9.40 | 1.11 | 26.84 | *** | 4.78 | *** |
| G6 | 6.92 | 9.75 | 0.46 | 7.23 | *** | 1.53 | *** |
| G7 | 6.98 | 9.60 | 0.45 | 5.43 | *** | 1.19 | *** |
| aG | 51.99 | 10.80 | 31.55 | 1168.06 | *** | 221.56 | *** |
| Labellum length | 75.47 | 3.29 | 6.17 | 286.36 | *** | 21.27 | *** |
| Variable | Principal component (PC) | Variable | principal Component (PC) | |||||
|---|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | |||
| I. Labellum basal region | D9 | 0.150 | -0.047 | 0.205 | ||||
| A | 0.156 | -0.123 | -0.133 | D10 | 0.097 | -0.135 | 0.217 | |
| A1 | 0.101 | -0.055 | -0.026 | aD | 0.011 | 0.205 | -0.232 | |
| A2 | 0.156 | -0.123 | -0.134 | aDE22 | 0.047 | -0.055 | -0.052 | |
| A3 | 0.156 | -0.122 | -0.134 | aDE55 | 0.034 | -0.092 | -0.075 | |
| A4 | 0.159 | -0.121 | -0.130 | E | 0.129 | 0.092 | 0.180 | |
| A5 | 0.158 | -0.122 | -0.130 | E1x | 0.139 | 0.137 | 0.084 | |
| aA | -0.062 | 0.083 | 0.107 | E2 | 0.117 | 0.025 | 0.251 | |
| B1x | -0.025 | 0.073 | 0.069 | E5 | 0.117 | 0.038 | 0.249 | |
| II. Labellum middle region | E7 | 0.125 | 0.196 | 0.029 | ||||
| B | 0.157 | -0.118 | -0.133 | E8 | 0.161 | 0.124 | 0.115 | |
| B2 | 0.168 | -0.116 | -0.065 | E9 | 0.159 | 0.125 | 0.123 | |
| B5 | 0.158 | -0.113 | -0.066 | E10 | 0.126 | 0.196 | 0.021 | |
| B7 | 0.165 | -0.101 | -0.033 | aE | -0.006 | 0.017 | -0.188 | |
| B8 | 0.156 | -0.119 | -0.132 | III. Labellum apical region | ||||
| B9 | 0.159 | -0.115 | -0.128 | F | 0.121 | 0.182 | 0.047 | |
| B10 | 0.151 | -0.099 | -0.026 | F1x | 0.125 | 0.209 | -0.058 | |
| aB | -0.093 | 0.104 | 0.110 | F2 | 0.110 | 0.191 | 0.065 | |
| C | 0.157 | -0.118 | -0.133 | F5 | 0.109 | 0.175 | 0.046 | |
| C1x | 0.064 | -0.013 | 0.146 | F7 | 0.125 | 0.211 | -0.036 | |
| C2 | 0.166 | -0.098 | -0.088 | F8 | 0.132 | 0.186 | -0.067 | |
| C5 | 0.153 | -0.080 | -0.091 | F9 | 0.125 | 0.195 | -0.050 | |
| C7 | 0.170 | -0.077 | 0.020 | F10 | 0.129 | 0.205 | -0.050 | |
| C8 | 0.160 | -0.096 | -0.033 | G | 0.113 | 0.176 | -0.087 | |
| C9 | 0.161 | -0.105 | -0.029 | G1x | 0.126 | 0.185 | -0.084 | |
| C10 | 0.149 | -0.054 | 0.020 | G2 | 0.092 | 0.148 | -0.066 | |
| D | 0.095 | -0.137 | 0.264 | G3 | 0.034 | 0.111 | 0.040 | |
| D1x | 0.132 | -0.025 | 0.092 | G4 | 0.122 | 0.119 | -0.097 | |
| D2 | 0.110 | -0.084 | 0.274 | G6 | 0.104 | 0.181 | -0.064 | |
| D5 | 0.109 | -0.077 | 0.275 | G7 | 0.105 | 0.187 | -0.056 | |
| D7 | 0.101 | -0.143 | 0.208 | aG | -0.027 | 0.027 | 0.072 | |
| D8 | 0.148 | -0.042 | 0.214 | Long_lab | 0.187 | -0.044 | -0.029 | |
| Eigenvalues | 26.126 | 12.174 | 7.401 | |||||
| Proportion | 0.43 | 0.20 | 0.12 | |||||
| Accumulated | 0.43 | 0.63 | 0.75 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





