Submitted:
13 September 2023
Posted:
14 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
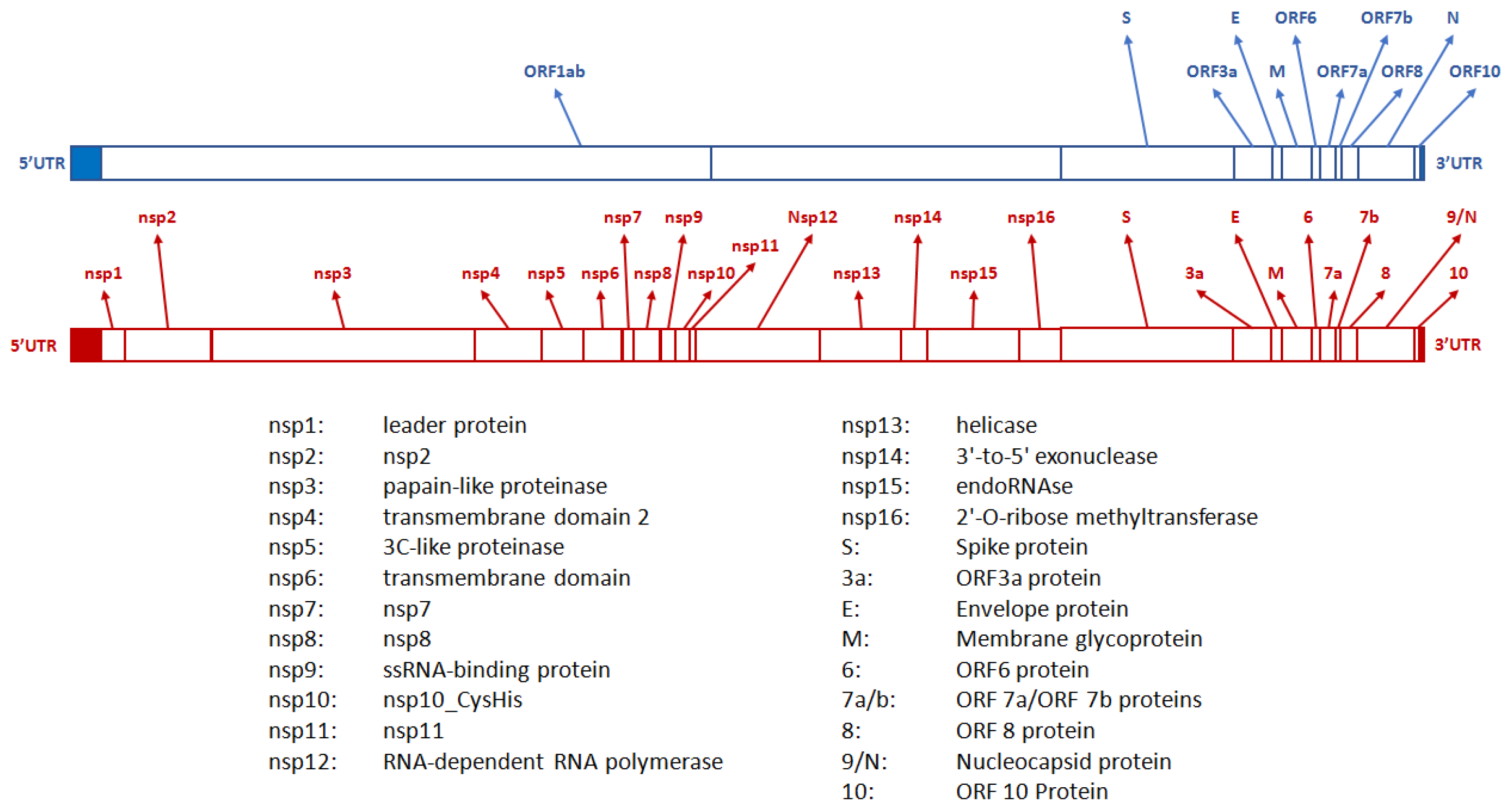
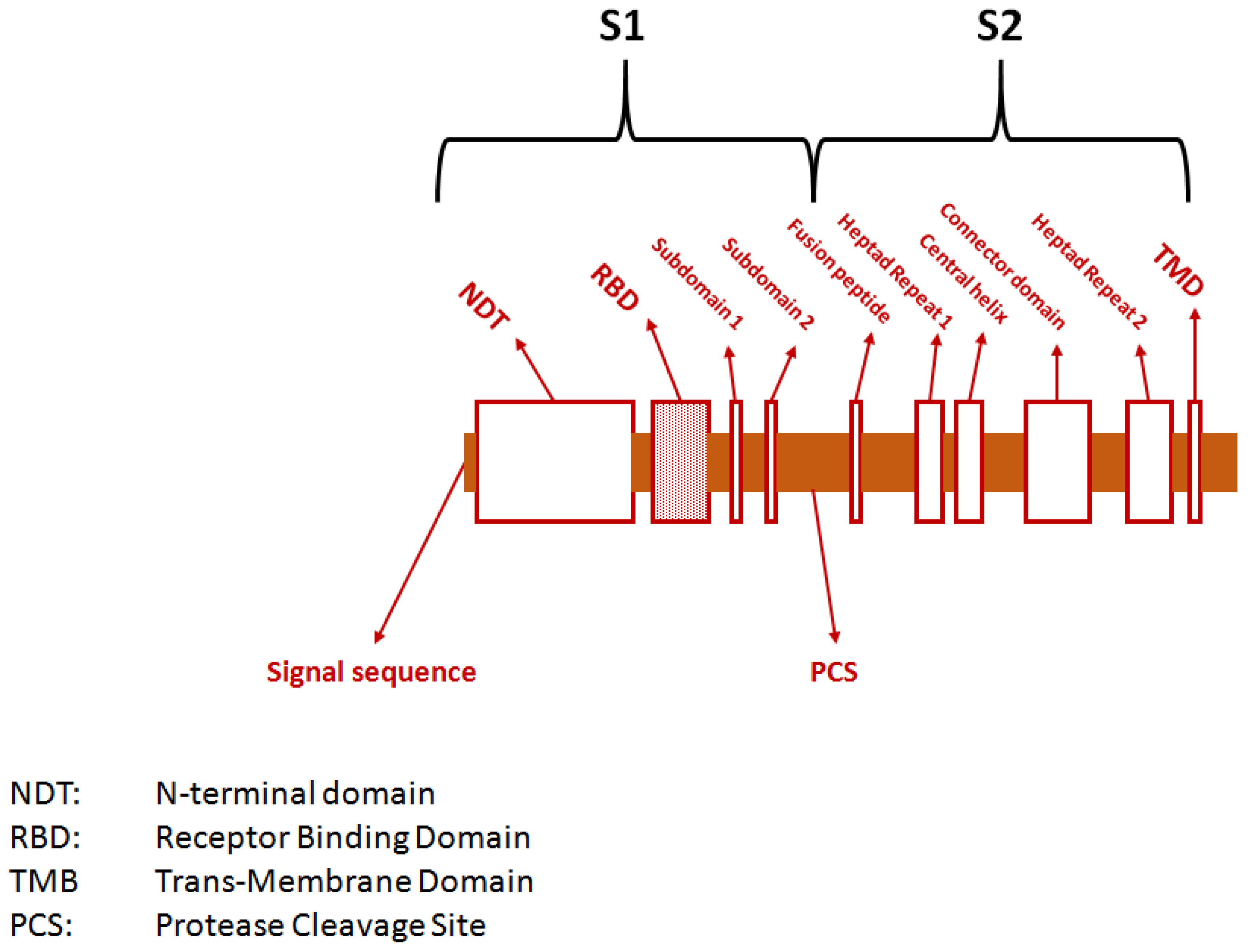
2. The pathogen
3. Biological characteristics of SARS-CoV-2 in humans
3.1. Incubation
3.2. Viral shedding
3.3. Infectivity
3.4. The role of super-spreaders
4. SARS-CoV-2 evolution
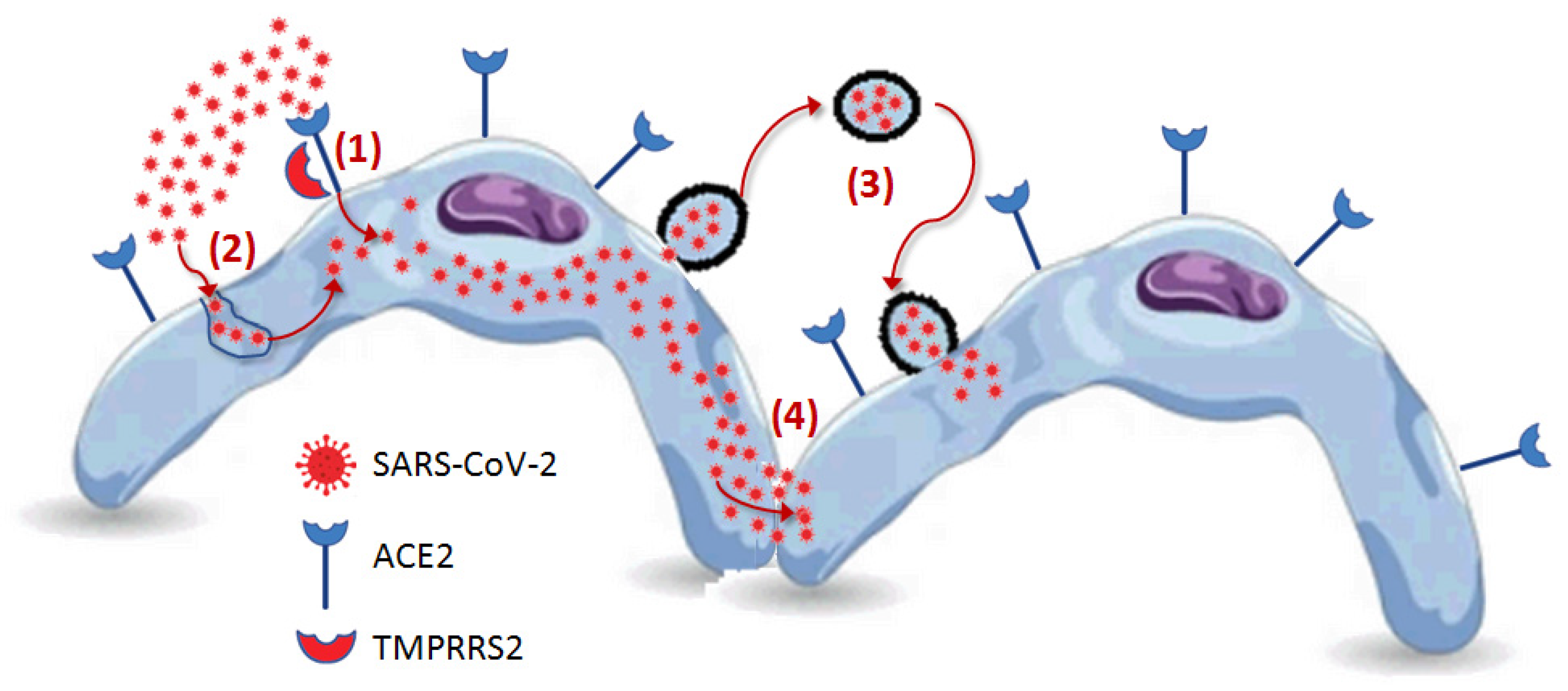
5. Human host cell penetration
5.1. Receptor-mediated penetration
5.2. Cathepsin L-mediated endocytosis
5.3. SARS-CoV-2 bearing extracellular particles
5.4. Cell to cell propagation
6. Intracellular processing
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sampath S, Khedr A, Qamar S, Tekin A, Singh R, Green R, Kashyap R. Pandemics Throughout the History. Cureus. 2021 Sep 20;13(9):e18136. [CrossRef] [PubMed] [PubMed Central]
- Lippi G, Mattiuzzi C, Henry BM. Uncontrolled confounding in COVID-19 epidemiology. Diagnosis (Berl). 2022 Dec 7;10(2):200-202. [CrossRef] [PubMed]
- Wise, J. Covid-19: WHO declares end of global health emergency. BMJ. 2023 May 9;381:1041. [CrossRef] [PubMed]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available at: https://covid19.who.int/. Last accessed: September 10, 2023.
- Jones JM, Manrique IM, Stone MS, Grebe E, Saa P, Germanio CD, Spencer BR, Notari E, Bravo M, Lanteri MC, Green V, Briggs-Hagen M, Coughlin MM, Stramer SL, Opsomer J, Busch MP. Estimates of SARS-CoV-2 Seroprevalence and Incidence of Primary SARS-CoV-2 Infections Among Blood Donors, by COVID-19 Vaccination Status - United States, April 2021-September 2022. MMWR Morb Mortal Wkly Rep. 2023 Jun 2;72(22):601-605. [CrossRef] [PubMed] [PubMed Central]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020 Apr;5(4):536-544. Epub 2020 Mar 2. [CrossRef] [PubMed] [PubMed Central]
- Lippi G, Mattiuzzi C, Bovo C, Plebani M. Current laboratory diagnostics of coronavirus disease 2019 (COVID-19). Acta Biomed. 2020 May 11;91(2):137-145. [CrossRef] [PubMed] [PubMed Central]
- Mariano G, Farthing RJ, Lale-Farjat SLM, Bergeron JRC. Structural Characterization of SARS-CoV-2: Where We Are, and Where We Need to Be. Front Mol Biosci. 2020 Dec 17;7:605236. [CrossRef] [PubMed] [PubMed Central]
- Machitani M, Yasukawa M, Nakashima J, Furuichi Y, Masutomi K. RNA-dependent RNA polymerase, RdRP, a promising therapeutic target for cancer and potentially COVID-19. Cancer Sci. 2020 Nov;111(11):3976-3984. Epub 2020 Sep 12. [CrossRef] [PubMed] [PubMed Central]
- Sakkiah S, Guo W, Pan B, Ji Z, Yavas G, Azevedo M, Hawes J, Patterson TA, Hong H. Elucidating Interactions Between SARS-CoV-2 Trimeric Spike Protein and ACE2 Using Homology Modeling and Molecular Dynamics Simulations. Front Chem. 2021 Jan 5;8:622632. [CrossRef] [PubMed] [PubMed Central]
- Ke Z, Oton J, Qu K, Cortese M, Zila V, McKeane L, Nakane T, Zivanov J, Neufeldt CJ, Cerikan B, Lu JM, Peukes J, Xiong X, Kräusslich HG, Scheres SHW, Bartenschlager R, Briggs JAG. Structures and distributions of SARS-CoV-2 spike proteins on intact virions. Nature. 2020 Dec;588(7838):498-502. Epub 2020 Aug 17. [CrossRef] [PubMed] [PubMed Central]
- Jaimes JA, André NM, Chappie JS, Millet JK, Whittaker GR. Phylogenetic Analysis and Structural Modeling of SARS-CoV-2 Spike Protein Reveals an Evolutionary Distinct and Proteolytically Sensitive Activation Loop. J Mol Biol. 2020 May 1;432(10):3309-3325. Epub 2020 Apr 19. [CrossRef] [PubMed] [PubMed Central]
- Lippi G, Henry BM, Sanchis-Gomar F. The real origin of SARS-CoV-2: does it really matter? J Lab Precis Med 2021;6:9. [CrossRef]
- Wu Y, Kang L, Guo Z, Liu J, Liu M, Liang W. Incubation Period of COVID-19 Caused by Unique SARS-CoV-2 Strains: A Systematic Review and Meta-analysis. JAMA Netw Open. 2022 Aug 1;5(8):e2228008. Erratum in: JAMA Netw Open. 2022 Sep 1;5(9):e2235424. [CrossRef] [PubMed] [PubMed Central]
- Salvagno GL, Henry BM, Pighi L, De Nitto S, Montagnana M, Lippi G. SARS-CoV-2 Omicron infection is associated with high nasopharyngeal viral load. J Infect. 2022 Jun;84(6):834-872. Epub 2022 Feb 26. [CrossRef] [PubMed] [PubMed Central]
- Woodbridge Y, Amit S, Huppert A, Kopelman NM. Viral load dynamics of SARS-CoV-2 Delta and Omicron variants following multiple vaccine doses and previous infection. Nat Commun. 2022 Nov 7;13(1):6706. [CrossRef] [PubMed] [PubMed Central]
- Tan KS, Ong SWX, Koh MH, Tay DJW, Aw DZH, Nah YW, Abdullah MRB, Coleman KK, Milton DK, Chu JJH, Chow VTK, Tambyah PA, Tham KW. SARS-CoV-2 Omicron variant shedding during respiratory activities. Int J Infect Dis. 2023 Jun;131:19-25. Epub 2023 Mar 21. [CrossRef] [PubMed] [PubMed Central]
- Yan D, Zhang X, Chen C, Jiang D, Liu X, Zhou Y, Huang C, Zhou Y, Guan Z, Ding C, Chen L, Lan L, Fu X, Wu J, Li L, Yang S. Characteristics of Viral Shedding Time in SARS-CoV-2 Infections: A Systematic Review and Meta-Analysis. Front Public Health. 2021 Mar 19;9:652842. [CrossRef] [PubMed] [PubMed Central]
- Owens K, Esmaeili-Wellman S, Schiffer JT. Heterogeneous SARS-CoV-2 kinetics due to variable timing and intensity of immune responses. medRxiv [Preprint]. 2023 Aug 29:2023.08.20.23294350. [CrossRef] [PubMed] [PubMed Central]
- Marc A, Kerioui M, Blanquart F, Bertrand J, Mitjà O, Corbacho-Monné M, Marks M, Guedj J. Quantifying the relationship between SARS-CoV-2 viral load and infectiousness. Elife. 2021 Sep 27;10:e69302. [CrossRef] [PubMed] [PubMed Central]
- SeyedAlinaghi S, Karimi A, Mojdeganlou H, Pashaei Z, Mirzapour P, Shamsabadi A, Barzegary A, Afroughi F, Dehghani S, Janfaza N, Fakhfouri A, Khodaei S, Mehraeen E, Dadras O. Minimum infective dose of severe acute respiratory syndrome coronavirus 2 based on the current evidence: A systematic review. SAGE Open Med. 2022 Aug 11;10:20503121221115053. [CrossRef] [PubMed] [PubMed Central]
- Hamner L, Dubbel P, Capron I, Ross A, Jordan A, Lee J, Lynn J, Ball A, Narwal S, Russell S, Patrick D, Leibrand H. High SARS-CoV-2 Attack Rate Following Exposure at a Choir Practice - Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020 May 15;69(19):606-610. [CrossRef] [PubMed]
- Park M, Pawliuk C, Nguyen T, Griffitt A, Dix-Cooper L, Fourik N, Dawes M. Determining the communicable period of SARS-CoV-2: A rapid review of the literature, March to September 2020. Euro Surveill. 2021 Apr;26(14):2001506. [CrossRef] [PubMed] [PubMed Central]
- Ceulemans LJ, Khan M, Yoo SJ, Zapiec B, Van Gerven L, Van Slambrouck J, Vanstapel A, Van Raemdonck D, Vos R, Wauters E, Wauters J, Carmeliet P, Mombaerts P. Persistence of SARS-CoV-2 RNA in lung tissue after mild COVID-19. Lancet Respir Med. 2021 Aug;9(8):e78-e79. Epub 2021 Jun 9. [CrossRef] [PubMed] [PubMed Central]
- Takahashi K, Ishikane M, Ujiie M, Iwamoto N, Okumura N, Sato T, Nagashima M, Moriya A, Suzuki M, Hojo M, Kanno T, Saito S, Miyamoto S, Ainai A, Tobiume M, Arashiro T, Fujimoto T, Saito T, Yamato M, Suzuki T, Ohmagari N. Duration of Infectious Virus Shedding by SARS-CoV-2 Omicron Variant-Infected Vaccinees. Emerg Infect Dis. 2022 May;28(5):998-1001. Epub 2022 Mar 15. [CrossRef] [PubMed] [PubMed Central]
- Alimohamadi Y, Taghdir M, Sepandi M. Estimate of the Basic Reproduction Number for COVID-19: A Systematic Review and Meta-analysis. J Prev Med Public Health. 2020 May;53(3):151-157. Epub 2020 Mar 20. [CrossRef] [PubMed] [PubMed Central]
- Nishiura H, Ito K, Anzai A, Kobayashi T, Piantham C, Rodríguez-Morales AJ. Relative Reproduction Number of SARS-CoV-2 Omicron (B.1.1.529) Compared with Delta Variant in South Africa. J Clin Med. 2021 Dec 23;11(1):30. [CrossRef] [PubMed] [PubMed Central]
- Omer SB, Yildirim I, Forman HP. Herd Immunity and Implications for SARS-CoV-2 Control. JAMA. 2020 Nov 24;324(20):2095-2096. [CrossRef] [PubMed]
- Tamura T, Ito J, Uriu K, Zahradnik J, Kida I, Anraku Y, Nasser H, Shofa M, Oda Y, Lytras S, Nao N, Itakura Y, Deguchi S, Suzuki R, Wang L, Begum MM, Kita S, Yajima H, Sasaki J, Sasaki-Tabata K, Shimizu R, Tsuda M, Kosugi Y, Fujita S, Pan L, Sauter D, Yoshimatsu K, Suzuki S, Asakura H, Nagashima M, Sadamasu K, Yoshimura K, Yamamoto Y, Nagamoto T, Schreiber G, Maenaka K; Genotype to Phenotype Japan (G2P-Japan) Consortium; Hashiguchi T, Ikeda T, Fukuhara T, Saito A, Tanaka S, Matsuno K, Takayama K, Sato K. Virological characteristics of the SARS-CoV-2 XBB variant derived from recombination of two Omicron subvariants. Nat Commun. 2023 May 16;14(1):2800. [CrossRef] [PubMed] [PubMed Central]
- Hosseini M, Poon LLM, Chin AWH, Ducker WA. Effect of Surface Porosity on SARS-CoV-2 Fomite Infectivity. ACS Omega. 2022 May 23;7(22):18238-18246. [CrossRef] [PubMed] [PubMed Central]
- Mattiuzzi C, Henry BM, Lippi G. Regional Association between Mean Air Temperature and Case Numbers of Multiple SARS-CoV-2 Lineages throughout the Pandemic. Viruses. 2022 Aug 30;14(9):1913. [CrossRef] [PubMed] [PubMed Central]
- Greenhalgh T, Jimenez JL, Prather KA, Tufekci Z, Fisman D, Schooley R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet. 2021 May 1;397(10285):1603-1605. Epub 2021 Apr 15. Erratum in: Lancet. 2021 May 15;397(10287):1808. [CrossRef] [PubMed] [PubMed Central]
- Lau MSY, Grenfell B, Thomas M, Bryan M, Nelson K, Lopman B. Characterizing superspreading events and age-specific infectiousness of SARS-CoV-2 transmission in Georgia, USA. Proc Natl Acad Sci U S A. 2020 Sep 8;117(36):22430-22435. Epub 2020 Aug 20. [CrossRef] [PubMed] [PubMed Central]
- Zhou J, Singanayagam A, Goonawardane N, Moshe M, Sweeney FP, Sukhova K, Killingley B, Kalinova M, Mann AJ, Catchpole AP, Barer MR, Ferguson NM, Chiu C, Barclay WS. Viral emissions into the air and environment after SARS-CoV-2 human challenge: a phase 1, open label, first-in-human study. Lancet Microbe. 2023 Aug;4(8):e579-e590. Epub 2023 Jun 9. Erratum in: Lancet Microbe. 2023 Aug;4(8):e576. [CrossRef] [PubMed] [PubMed Central]
- Callaway, E. The coronavirus is mutating - does it matter? Nature. 2020 Sep;585(7824):174-177. [CrossRef] [PubMed]
- Corey L, Beyrer C, Cohen MS, Michael NL, Bedford T, Rolland M. SARS-CoV-2 Variants in Patients with Immunosuppression. N Engl J Med. 2021 Aug 5;385(6):562-566. [CrossRef] [PubMed] [PubMed Central]
- Losos, JB. Convergence, adaptation, and constraint. Evolution. 2011 Jul;65(7):1827-40. Epub 2011 Apr 7. [CrossRef] [PubMed]
- Boyle L, Hletko S, Huang J, Lee J, Pallod G, Tung HR, Durrett R. Selective sweeps in SARS-CoV-2 variant competition. Proc Natl Acad Sci U S A. 2022 Nov 22;119(47):e2213879119. Epub 2022 Nov 3. [CrossRef] [PubMed] [PubMed Central]
- Markov PV, Ghafari M, Beer M, Lythgoe K, Simmonds P, Stilianakis NI, Katzourakis A. The evolution of SARS-CoV-2. Nat Rev Microbiol. 2023 Jun;21(6):361-379. Epub 2023 Apr 5. [CrossRef] [PubMed]
- Kawasaki Y, Abe H, Yasuda J. Comparison of genome replication fidelity between SARS-CoV-2 and influenza A virus in cell culture. Sci Rep. 2023 Aug 11;13(1):13105. [CrossRef] [PubMed] [PubMed Central]
- Tay JH, Porter AF, Wirth W, Duchene S. The Emergence of SARS-CoV-2 Variants of Concern Is Driven by Acceleration of the Substitution Rate. Mol Biol Evol. 2022 Feb 3;39(2):msac013. [CrossRef] [PubMed] [PubMed Central]
- Shen Z, Xiao Y, Kang L, Ma W, Shi L, Zhang L, Zhou Z, Yang J, Zhong J, Yang D, Guo L, Zhang G, Li H, Xu Y, Chen M, Gao Z, Wang J, Ren L, Li M. Genomic Diversity of Severe Acute Respiratory Syndrome-Coronavirus 2 in Patients With Coronavirus Disease 2019. Clin Infect Dis. 2020 Jul 28;71(15):713-720. Erratum in: Clin Infect Dis. 2021 Dec 16;73(12):2374. [CrossRef] [PubMed] [PubMed Central]
- Li J, Du P, Yang L, Zhang J, Song C, Chen D, Song Y, Ding N, Hua M, Han K, Song R, Xie W, Chen Z, Wang X, Liu J, Xu Y, Gao G, Wang Q, Pu L, Di L, Li J, Yue J, Han J, Zhao X, Yan Y, Yu F, Wu AR, Zhang F, Gao YQ, Huang Y, Wang J, Zeng H, Chen C. Two-step fitness selection for intra-host variations in SARS-CoV-2. Cell Rep. 2022 Jan 11;38(2):110205. Epub 2021 Dec 16. [CrossRef] [PubMed] [PubMed Central]
- Armero A, Berthet N, Avarre JC. Intra-Host Diversity of SARS-Cov-2 Should Not Be Neglected: Case of the State of Victoria, Australia. Viruses. 2021 Jan 19;13(1):133. [CrossRef] [PubMed] [PubMed Central]
- Pathak AK, Mishra GP, Uppili B, Walia S, Fatihi S, Abbas T, Banu S, Ghosh A, Kanampalliwar A, Jha A, Fatma S, Aggarwal S, Dhar MS, Marwal R, Radhakrishnan VS, Ponnusamy K, Kabra S, Rakshit P, Bhoyar RC, Jain A, Divakar MK, Imran M, Faruq M, Sowpati DT, Thukral L, Raghav SK, Mukerji M. Spatio-temporal dynamics of intra-host variability in SARS-CoV-2 genomes. Nucleic Acids Res. 2022 Feb 22;50(3):1551-1561. [CrossRef] [PubMed] [PubMed Central]
- Laskar R, Ali S. Differential mutation profile of SARS-CoV-2 proteins across deceased and asymptomatic patients. Chem Biol Interact. 2021 Sep 25;347:109598. Epub 2021 Jul 23. [CrossRef] [PubMed] [PubMed Central]
- Al-Khatib HA, Smatti MK, Ali FH, Zedan HT, Thomas S, Ahmed MN, El-Kahlout RA, Al Bader MA, Elgakhlab D, Coyle PV, Abu-Raddad LJ, Al Thani AA, Yassine HM. Comparative analysis of within-host diversity among vaccinated COVID-19 patients infected with different SARS-CoV-2 variants. iScience. 2022 Nov 18;25(11):105438. Epub 2022 Oct 25. [CrossRef] [PubMed] [PubMed Central]
- He Y, Ma W, Dang S, Chen L, Zhang R, Mei S, Wei X, Lv Q, Peng B, Chen J, Kong D, Sun Y, Tang X, Wu W, Chen Z, Li S, Wan J, Zou X, Li M, Feng T, Ren L, Wang J. Possible recombination between two variants of concern in a COVID-19 patient. Emerg Microbes Infect. 2022 Dec;11(1):552-555. [CrossRef] [PubMed] [PubMed Central]
- Mohapatra RK, Kandi V, Tuli HS, Chakraborty C, Dhama K. The recombinant variants of SARS-CoV-2: Concerns continues amid COVID-19 pandemic. J Med Virol. 2022 Aug;94(8):3506-3508. Epub 2022 Apr 27. [CrossRef] [PubMed] [PubMed Central]
- Scarpa F, Ciccozzi M. On the SARS-CoV-2 BA.2.86 lineage: a mutation point of view. J Med Virol. 2023 Sep;95(9):e29079. [CrossRef] [PubMed]
- Wang Q, Iketani S, Li Z, Liu L, Guo Y, Huang Y, Bowen AD, Liu M, Wang M, Yu J, Valdez R, Lauring AS, Sheng Z, Wang HH, Gordon A, Liu L, Ho DD. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell. 2023 Jan 19;186(2):279-286.e8. Epub 2022 Dec 14. [CrossRef] [PubMed] [PubMed Central]
- Mykytyn AZ, Fouchier RA, Haagmans BL. Antigenic evolution of SARS coronavirus 2. Curr Opin Virol. 2023 Aug 28;62:101349. Epub ahead of print. [CrossRef] [PubMed]
- Mattiuzzi C, Lippi G. Timeline analysis of clinical severity of COVID-19 in the general population. Eur J Intern Med. 2023 Apr;110:97-98. Epub 2022 Dec 16. [CrossRef] [PubMed] [PubMed Central]
- Perez-Guzman PN, Knock E, Imai N, Rawson T, Elmaci Y, Alcada J, Whittles LK, Thekke Kanapram D, Sonabend R, Gaythorpe KAM, Hinsley W, FitzJohn RG, Volz E, Verity R, Ferguson NM, Cori A, Baguelin M. Epidemiological drivers of transmissibility and severity of SARS-CoV-2 in England. Nat Commun. 2023 Jul 17;14(1):4279. [CrossRef] [PubMed] [PubMed Central]
- Schwab C, Merle U, Schirmacher P, Longerich T. Lethality of SARS-CoV-2 infection-a comparative autopsy study focusing on COVID-19 development and virus variants. Histopathology. 2023 Aug;83(2):242-251. Epub 2023 May 5. [CrossRef] [PubMed]
- Nocini R, Henry BM, Mattiuzzi C, Lippi G. Improving Nasal Protection for Preventing SARS-CoV-2 Infection. Biomedicines. 2022 Nov 17;10(11):2966. [CrossRef] [PubMed] [PubMed Central]
- Lippi G, Lavie CJ, Henry BM, Sanchis-Gomar F. Do genetic polymorphisms in angiotensin converting enzyme 2 (ACE2) gene play a role in coronavirus disease 2019 (COVID-19)? Clin Chem Lab Med. 2020 Jun 29;58(9):1415-1422. [CrossRef] [PubMed]
- Kishimoto M, Uemura K, Sanaki T, Sato A, Hall WW, Kariwa H, Orba Y, Sawa H, Sasaki M. TMPRSS11D and TMPRSS13 Activate the SARS-CoV-2 Spike Protein. Viruses. 2021 Feb 28;13(3):384. [CrossRef] [PubMed] [PubMed Central]
- Anand P, Puranik A, Aravamudan M, Venkatakrishnan AJ, Soundararajan V. SARS-CoV-2 strategically mimics proteolytic activation of human ENaC. Elife. 2020 May 26;9:e58603. [CrossRef] [PubMed] [PubMed Central]
- Johnson BA, Xie X, Bailey AL, Kalveram B, Lokugamage KG, Muruato A, Zou J, Zhang X, Juelich T, Smith JK, Zhang L, Bopp N, Schindewolf C, Vu M, Vanderheiden A, Winkler ES, Swetnam D, Plante JA, Aguilar P, Plante KS, Popov V, Lee B, Weaver SC, Suthar MS, Routh AL, Ren P, Ku Z, An Z, Debbink K, Diamond MS, Shi PY, Freiberg AN, Menachery VD. Loss of furin cleavage site attenuates SARS-CoV-2 pathogenesis. Nature. 2021 Mar;591(7849):293-299. Epub 2021 Jan 25. [CrossRef] [PubMed] [PubMed Central]
- Evans JP, Liu SL. Role of host factors in SARS-CoV-2 entry. J Biol Chem. 2021 Jul;297(1):100847. Epub 2021 May 28. [CrossRef] [PubMed] [PubMed Central]
- Lim S, Zhang M, Chang TL. ACE2-Independent Alternative Receptors for SARS-CoV-2. Viruses. 2022 Nov 16;14(11):2535. [CrossRef] [PubMed] [PubMed Central]
- Cantuti-Castelvetri L, Ojha R, Pedro LD, Djannatian M, Franz J, Kuivanen S, van der Meer F, Kallio K, Kaya T, Anastasina M, Smura T, Levanov L, Szirovicza L, Tobi A, Kallio-Kokko H, Österlund P, Joensuu M, Meunier FA, Butcher SJ, Winkler MS, Mollenhauer B, Helenius A, Gokce O, Teesalu T, Hepojoki J, Vapalahti O, Stadelmann C, Balistreri G, Simons M. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020 Nov 13;370(6518):856-860. Epub 2020 Oct 20. [CrossRef] [PubMed] [PubMed Central]
- Lempp FA, Soriaga LB, Montiel-Ruiz M, Benigni F, Noack J, Park YJ, Bianchi S, Walls AC, Bowen JE, Zhou J, Kaiser H, Joshi A, Agostini M, Meury M, Dellota E Jr, Jaconi S, Cameroni E, Martinez-Picado J, Vergara-Alert J, Izquierdo-Useros N, Virgin HW, Lanzavecchia A, Veesler D, Purcell LA, Telenti A, Corti D. Lectins enhance SARS-CoV-2 infection and influence neutralizing antibodies. Nature. 2021 Oct;598(7880):342-347. Epub 2021 Aug 31. [CrossRef] [PubMed]
- Cai Y, Zhang J, Xiao T, Peng H, Sterling SM, Walsh RM Jr, Rawson S, Rits-Volloch S, Chen B. Distinct conformational states of SARS-CoV-2 spike protein. Science. 2020 Sep 25;369(6511):1586-1592. Epub 2020 Jul 21. [CrossRef] [PubMed] [PubMed Central]
- Koch J, Uckeley ZM, Doldan P, Stanifer M, Boulant S, Lozach PY. TMPRSS2 expression dictates the entry route used by SARS-CoV-2 to infect host cells. EMBO J. 2021 Aug 16;40(16):e107821. Epub 2021 Jul 13. [CrossRef] [PubMed] [PubMed Central]
- Iwata-Yoshikawa N, Kakizaki M, Shiwa-Sudo N, Okura T, Tahara M, Fukushi S, Maeda K, Kawase M, Asanuma H, Tomita Y, Takayama I, Matsuyama S, Shirato K, Suzuki T, Nagata N, Takeda M. Essential role of TMPRSS2 in SARS-CoV-2 infection in murine airways. Nat Commun. 2022 Oct 15;13(1):6100. [CrossRef] [PubMed] [PubMed Central]
- Kongsomros S, Pongsakul N, Panachan J, Khowawisetsut L, Somkird J, Sangma C, Kanjanapruthipong T, Wongtrakoongate P, Chairoungdua A, Pattanapanyasat K, Newburg DS, Morrow AL, Hongeng S, Thitithanyanont A, Chutipongtanate S. Comparison of viral inactivation methods on the characteristics of extracellular vesicles from SARS-CoV-2 infected human lung epithelial cells. J Extracell Vesicles. 2022 Dec;11(12):e12291. [CrossRef] [PubMed] [PubMed Central]
- Xia B, Pan X, Luo RH, Shen X, Li S, Wang Y, Zuo X, Wu Y, Guo Y, Xiao G, Li Q, Long XY, He XY, Zheng HY, Lu Y, Pang W, Zheng YT, Li J, Zhang LK, Gao Z. Extracellular vesicles mediate antibody-resistant transmission of SARS-CoV-2. Cell Discov. 2023 Jan 6;9(1):2. [CrossRef] [PubMed] [PubMed Central]
- Ning B, Huang Z, Youngquist BM, Scott JW, Niu A, Bojanowski CM, Zwezdaryk KJ, Saba NS, Fan J, Yin XM, Cao J, Lyon CJ, Li CZ, Roy CJ, Hu TY. Liposome-mediated detection of SARS-CoV-2 RNA-positive extracellular vesicles in plasma. Nat Nanotechnol. 2021 Sep;16(9):1039-1044. Epub 2021 Jul 22. [CrossRef] [PubMed] [PubMed Central]
- Zeng C, Evans JP, King T, Zheng YM, Oltz EM, Whelan SPJ, Saif LJ, Peeples ME, Liu SL. SARS-CoV-2 spreads through cell-to-cell transmission. Proc Natl Acad Sci U S A. 2022 Jan 4;119(1):e2111400119. [CrossRef] [PubMed] [PubMed Central]
- Li X, Yuan H, Li X, Wang H. Spike protein mediated membrane fusion during SARS-CoV-2 infection. J Med Virol. 2023 Jan;95(1):e28212. Epub 2022 Oct 25. [CrossRef] [PubMed] [PubMed Central]
- Martin-Sancho L, Lewinski MK, Pache L, Stoneham CA, Yin X, Becker ME, Pratt D, Churas C, Rosenthal SB, Liu S, Weston S, De Jesus PD, O'Neill AM, Gounder AP, Nguyen C, Pu Y, Curry HM, Oom AL, Miorin L, Rodriguez-Frandsen A, Zheng F, Wu C, Xiong Y, Urbanowski M, Shaw ML, Chang MW, Benner C, Hope TJ, Frieman MB, García-Sastre A, Ideker T, Hultquist JF, Guatelli J, Chanda SK. Functional landscape of SARS-CoV-2 cellular restriction. Mol Cell. 2021 Jun 17;81(12):2656-2668.e8. Epub 2021 Apr 13. [CrossRef] [PubMed] [PubMed Central]
- Lu S, Ye Q, Singh D, Cao Y, Diedrich JK, Yates JR 3rd, Villa E, Cleveland DW, Corbett KD. The SARS-CoV-2 nucleocapsid phosphoprotein forms mutually exclusive condensates with RNA and the membrane-associated M protein. Nat Commun. 2021 Jan 21;12(1):502. [CrossRef] [PubMed] [PubMed Central]
- Lei X, Dong X, Ma R, Wang W, Xiao X, Tian Z, Wang C, Wang Y, Li L, Ren L, Guo F, Zhao Z, Zhou Z, Xiang Z, Wang J. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat Commun. 2020 Jul 30;11(1):3810. [CrossRef] [PubMed] [PubMed Central]
- Xia H, Cao Z, Xie X, Zhang X, Chen JY, Wang H, Menachery VD, Rajsbaum R, Shi PY. Evasion of Type I Interferon by SARS-CoV-2. Cell Rep. 2020 Oct 6;33(1):108234. Epub 2020 Sep 19. [CrossRef] [PubMed] [PubMed Central]
- Wise, J. Covid-19: WHO declares end of global health emergency. BMJ. 2023 May 9;381:1041. [CrossRef] [PubMed]
- Lippi G, Plebani M. COVID-19: the global health emergency is over for the WHO, but not yet for laboratory medicine. J Lab Precis Med 2023;8:17. [CrossRef]
- Callaway, E. COVID's future: mini-waves rather than seasonal surges. Nature. 2023 May;617(7960):229-230. [CrossRef] [PubMed]
- Ruaño G, Ha T. Living with respiratory viruses: The next saga in human/viral coexistence? Bioessays. 2021 Apr;43(4):e2000321. Epub 2021 Jan 6. [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




