1. Introduction
Human epidermal growth factor receptor 2 (HER2) is included in the receptor tyrosine kinase family of human epidermal growth factor receptors. To activate the downstream signaling, HER2 must either form heterodimers with other HER members and their specific ligands or self-assemble into ligand-independent homodimers when overexpressed [
1]. The HER2 overexpression is observed in approximately 20% of breast cancers [
2] and 20% of gastric cancers [
3], which are associated with higher rates of recurrence, poor prognosis, and shorter overall survival. A monoclonal antibody (mAb) against HER2, trastuzumab, exhibited an anti-proliferating effect
in vitro and a potent antitumor effect
in vivo [
4,
5]. The addition of trastuzumab to chemotherapy improves objective response rates, progression-free survival, and overall survival in HER2-positive breast cancer patients with metastasis [
6]. Trastuzumab has become the standard treatment for HER2-positive breast cancers [
7] and HER2-positive gastric cancers [
8]. Trastuzumab has been the most effective therapy for HER2-positive breast cancer for more than 20 years [
9].
The major adverse effect associated with anti-HER2 therapeutic mAbs is cardiotoxicity, thereby necessitating routine cardiac monitoring in clinics [
10]. Furthermore, mice lacking
ErbB2 (ortholog of HER2) displayed embryonic lethal due to the dysfunctions associated with a lack of cardiac trabeculae [
11]. Ventricular-restricted
ErbB2-deficient mice showed the features of dilated cardiomyopathy [
12]. These results indicate that HER2 is vital for normal heart development and homeostasis. Therefore, more selective anti-HER2 mAbs against tumors, which can reduce heart failures are required.
We previously established several anti-HER2 mAbs, such as H
2Mab-19 (IgG
2b, kappa) [
13], H
2Mab-41 (IgG
2b, kappa) [
14], H
2Mab-77 (IgG
1, kappa) [
15], H
2Mab-119 (IgG
1, kappa) [
16], H
2Mab-139 (IgG
1, kappa) [
17], H
2Mab-181 (IgG
1, kappa) [
18], H
2Mab-193 (IgG
1, kappa), and H
2Mab-215 (IgG
1, kappa) by the immunization of HER2 ectodomain (HER2ec). We further engineered the mAbs into the mouse IgG
2a type (H
2Mab-77-mG
2a, H
2Mab-119-mG
2a, and H
2Mab-139-mG
2a, respectively), and produced the core fucose-deficient types (H
2Mab-77-mG
2a-f, H
2Mab-119-mG
2a-f, and H
2Mab-139-mG
2a-f, respectively) to potentiate the antibody-dependent cellular cytotoxicity (ADCC) and antitumor effect
in vivo [
19,
20,
21]. In this study, we developed and characterized a novel HER2 mAb, named H
2Mab-250/H
2CasMab-2.
2. Materials and Methods
2.1. Cell culture
Chinese hamster ovary (CHO)-K1, BT-474, SK-BR-3, MDA-MB-468, MCF 10A, hTERT TIGKs, HBEC3-KT, hTERT-HME1, RPTEC/TERT1, and P3X63Ag8U.1 (P3U1) were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Human keratinocyte HaCaT was purchased from Cell Lines Service GmbH (Eppelheim, Germany). hTCEpi, hTEC/SVTERT24-B, and HCEC-1CT were purchased from EVERCYTE (Vienna, Austria).
The cDNA of HER2 (wild type; WT) and deletion mutants (dN218, dN342, and dN511) were cloned into the pCAG-nPA16 vector. A HER2 point mutant (W614A) and HER2 WT were cloned into the pCAG-nPA-cRAPMAP vector. CHO-K1 cells were transfected with the above-mentioned vectors using a Neon transfection system (Thermo Fisher Scientific Inc., Waltham, MA). A few days after transfection, PA tag-positive cells were sorted by the cell sorter (SH800; Sony Corp., Tokyo, Japan) using NZ-1, which was originally developed as an anti-human PDPN mAb [
22]. Finally, CHO/HER2 and CHO/HER2 (dN218, dN342, and dN511) cell lines were established.
CHO-K1, CHO/HER2 (WT, deletion, and point mutants), and P3U1 were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Nacalai Tesque, Inc., Kyoto, Japan), and BT-474, SK-BR-3, MDA-MB-468, HEK293T, and HaCaT were cultured in Dulbecco’s Modified Eagle Medium (DMEM) medium (Nacalai Tesque, Inc.), supplemented with 10% heat-inactivated fetal bovine serum (FBS; Thermo Fisher Scientific Inc.), 100 units/mL of penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B (Nacalai Tesque, Inc.). Mammary epithelial cell line, MCF 10A was cultured in Mammary Epithelial Cell Basal Medium BulletKitTM (Lonza, Basel, Switzerland) supplemented with 100 ng/mL cholera toxin (Sigma-Aldrich Corp., St. Louis, MO).
Immortalized normal epithelial cell lines were maintained, as follows; hTERT TIGKs, Dermal Cell Basal Medium and Keratinocyte Growth Kit (ATCC); HBEC3-KT, Airway Epithelial Cell Basal Medium and Bronchial Epithelial Cell Growth Kit (ATCC); hTERT-HME1, Mammary Epithelial Cell Basal Medium BulletKitTM without GA-1000 (Lonza); hTCEpi, KGMTM-2 BulletKitTM (Lonza); hTEC/SVTERT24-B, OptiPROTM SFM and GlutaMAXTM-I (Thermo Fisher Scientific Inc.); RPTEC/TERT1, DMEM/F-12 and hTERT Immortalized RPTEC Growth Kit with supplement A and B (ATCC); HCEC-1CT, DMEM / M199 (4:1, Thermo Fisher Scientific Inc.), 2 % Cosmic Calf Serum (Cytiva, Marlborough, MA), 20 ng/mL hEGF (Sigma-Aldrich Corp.), 10 μg/mL insulin (Sigma-Aldrich Corp.), 2 μg/mL apo-transferrin (Sigma-Aldrich Corp.), 5 nM sodium-selenite (Sigma-Aldrich Corp.), 1 μg/mL hydrocortisone (Sigma-Aldrich Corp.).
All cell lines were cultured at 37°C in a humidified atmosphere with 5% CO2 and 95% air.
2.2. Development of hybridomas
Anti-HER2 mAbs, such as H
2Mab-119 (IgG
1, kappa) [
16], H
2Mab-77 (IgG
1, kappa) [
15], H
2Mab-139 (IgG
1, kappa) [
17], H
2Mab-193 (IgG
1, kappa), H
2Mab-215 (IgG
1, kappa), H
2Mab-19 (IgG
2b, kappa) [
13], H
2Mab-181 (IgG
1, kappa) [
18], and H
2Mab-41 (IgG
2b, kappa) [
14] were established previously. H
2Mab-250 (IgG
1, kappa) was established by the same strategy. Briefly, BALB/c mice were immunized with recombinant HER2ec produced by LN229 cells together with Imject Alum (Thermo Fisher Scientific, Inc.). After several additional immunizations, spleen cells were fused with P3U1 cells. The culture supernatants of hybridomas were screened using enzyme-linked immunosorbent assay (ELISA) with recombinant HER2ec and flow cytometry with cell lines.
2.3. Production of recombinant mAb
To generate recombinant H2Mab-250 and H2Mab-119, their VH cDNAs and the CH cDNA of mouse IgG1 were cloned into the pCAG-Ble vector (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan). The VL cDNAs and CL cDNA of the mouse kappa light chain were also cloned into the pCAG-Neo vector (FUJIFILM Wako Pure Chemical Corporation). The vectors were transfected into ExpiCHO-S cells using the ExpiCHO Expression System (Thermo Fisher Scientific, Inc.), and Ab-Capcher (ProteNova, Kagawa, Japan) was used to purify the recombinant H2Mab-250 and H2Mab-119.
To generate a mouse IgG2a type of trastuzumab (tras-mG2a-f), the VH cDNA of trastuzumab and the CH cDNA of mouse IgG2a were cloned into the pCAG-Neo vector, and the VL cDNA of trastuzumab and the CL cDNA of mouse kappa light chain were cloned into the pCAG-Ble vector.
To generate the recombinant PMab-231, we cloned heavy and light chains of PMab-231 [
23] into the pCAG-Neo and pCAG-Ble vectors, respectively. To produce the defucosylated form (H
2Mab-250-mG
2a-f, tras-mG
2a-f, and PMab-231-f), the vectors were transfected into BINDS-09 (fucosyltransferase 8-knockout ExpiCHO-S) cells using the ExpiCHO Expression System. H
2Mab-250-mG
2a-f, tras-mG
2a-f, and PMab-231-f were purified using Ab-Capcher.
2.4. Flow cytometry
Cells were collected using 0.25% trypsin and 1 mM ethylenediamine tetraacetic acid (EDTA; Nacalai Tesque, Inc.). The cells (1 × 105 cells/sample) were treated with primary mAbs (10 μg/mL) or blocking buffer [control; 0.1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS)] for 30 min at 4˚C. Next, the cells were treated with Alexa Fluor 488-conjugated anti-mouse IgG (1:2,000; Cell Signaling Technology, Danvers, MA) for 30 min at 4˚C. The fluorescence data was collected using EC800 or SA3800 Cell Analyzer (Sony Corp), and the data were analyzed using FlowJo (BD Biosciences, Franklin Lakes, NJ).
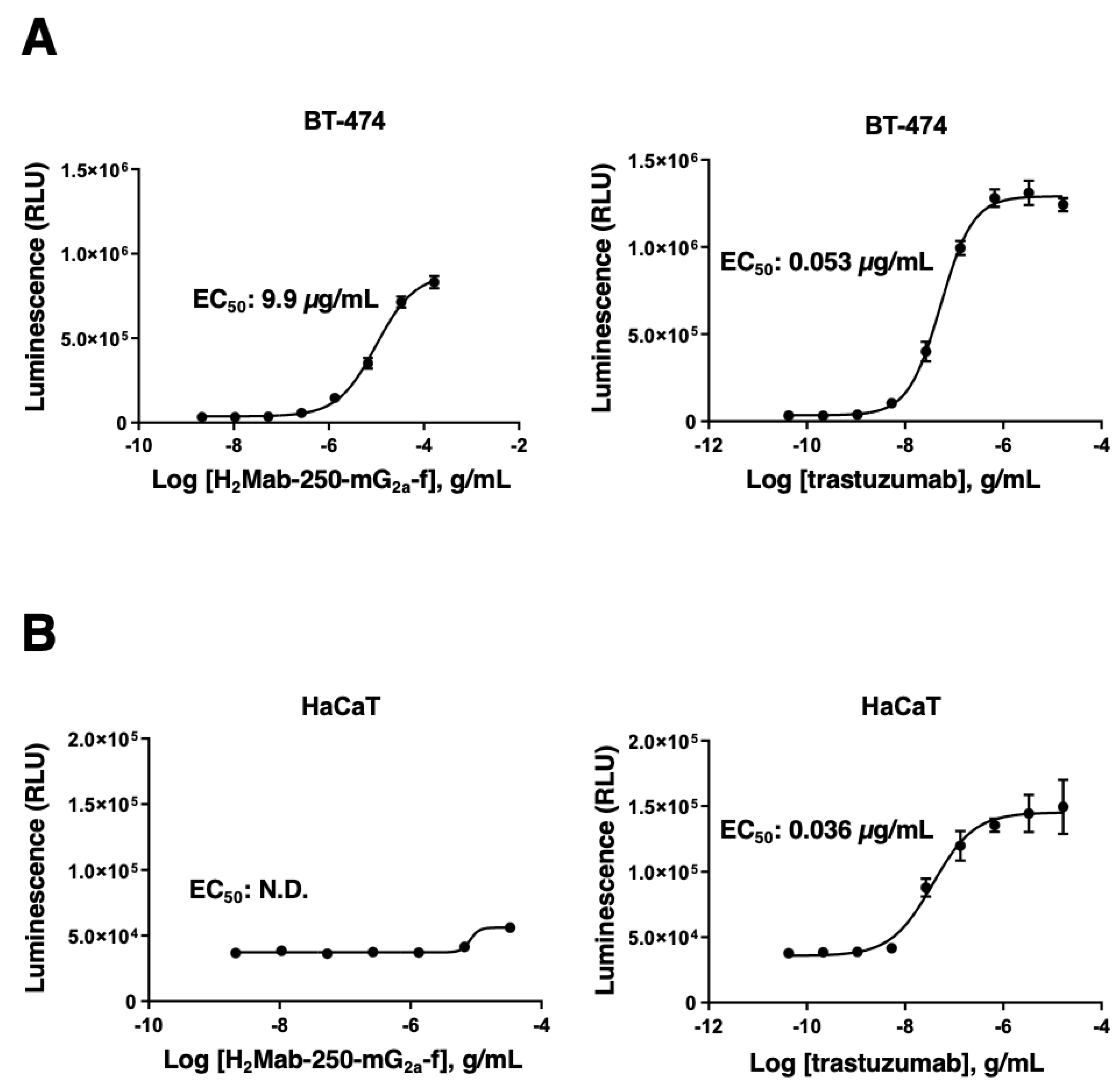
2.5. ADCC reporter bioassay
The ADCC reporter bioassay was performed using an ADCC Reporter Bioassay kit (Promega Corporation, Madison, WI), according to the manufacturer’s instructions. Target cells (BT-474 and HaCaT, 12,500 cells per well) were cultured in a 96-well white solid plate. H2Mab-250-mG2a-f and trastuzumab (Herceptin; Chugai Pharmaceutical Co., Ltd, Tokyo, Japan) were serially diluted and added to the target cells. Jurkat cells stably expressing the human FcγRIIIa receptor and a nuclear factor of activated T-cells (NFAT) response element driving firefly luciferase, were used as effector cells. The engineered Jurkat cells (75,000 cells in 25 μl) were then added and co-cultured with antibody-treated target cells at 37˚C for 6 h. Luminescence using the Bio-Glo Luciferase Assay System was measured using a GloMax luminometer (Promega Corporation).
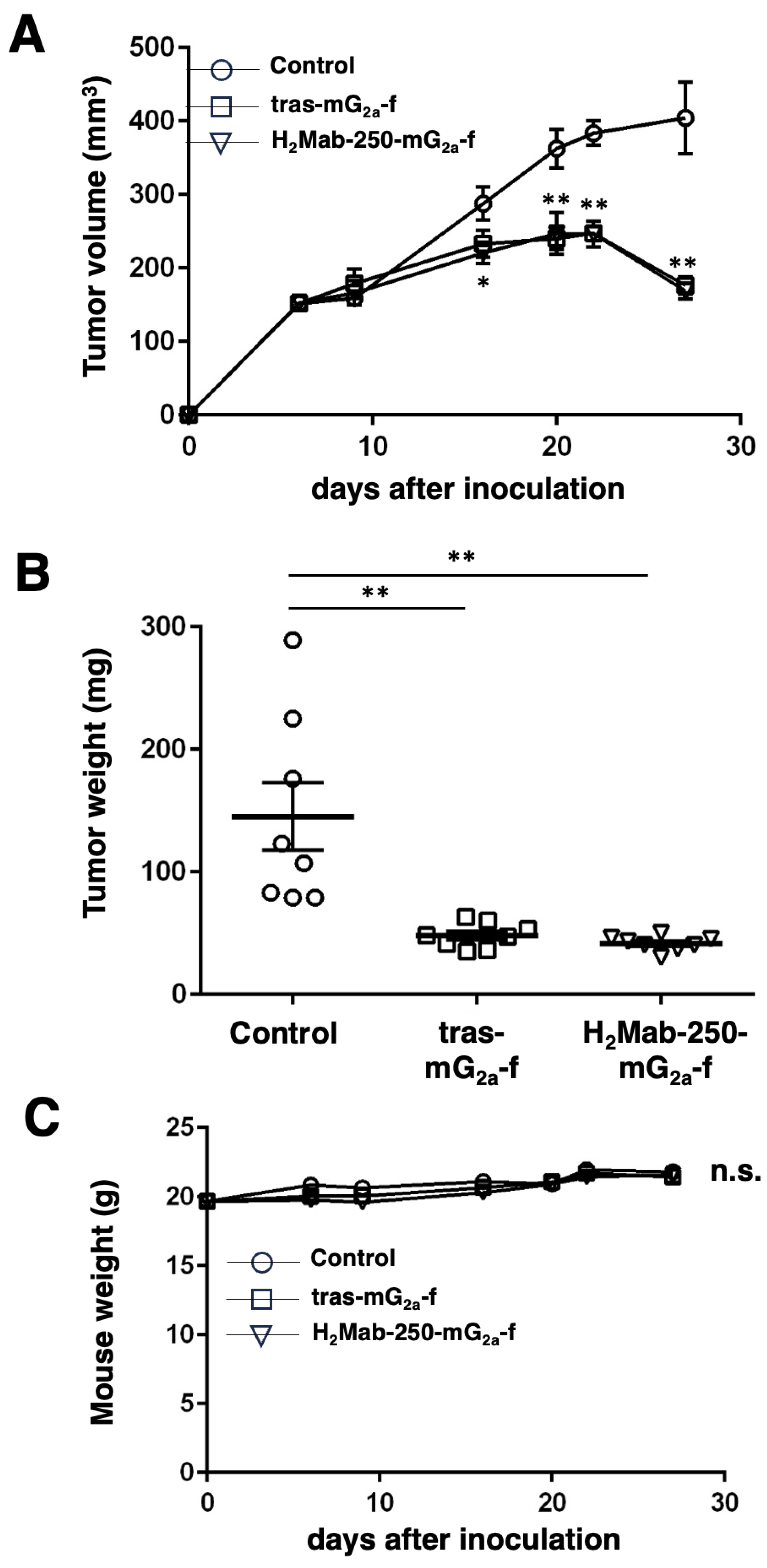
2.6. Antitumor activities of H2Mab-250-mG2a-f in breast cancer xenografts
To examine the antitumor effect of H2Mab-250-mG2a-f, animal experiments were approved by the Institutional Committee for Experiments of the Institute of Microbial Chemistry (approval no. 2023-060). During the experimental period, we monitored mice maintained in a pathogen-free environment on 11 h light/13 h dark cycle with food and water supplied ad libitum. Mice were monitored for health and weight every one or five days. We identified body weight loss exceeding 25% and maximum tumor size exceeding 3000 mm3 as humane endpoints, and terminated the experiments.
We resuspended BT-474 (5 × 106 cells) in DMEM and mixed them with BD Matrigel Matrix Growth Factor Reduced (BD Biosciences, Franklin Lakes, NJ). We injected them subcutaneously into the left flank of BALB/c nude mice (female, 5 weeks old, Jackson Laboratory Japan, Kanagawa, Japan). On day 6 post-inoculation, 100 μg of H2Mab-250-mG2a-f (n = 8), tras-mG2a-f (n = 8), or control (PMab-231-f; n = 8) in 100 µL PBS were intraperitoneally injected. On days 13 and 20, additional antibody injections were performed. The tumor volume was measured on days 6, 9, 16, 20, 22, and 27 after the inoculation of cells.
2.7. Immunohistochemical analysis
Formalin-fixed paraffin-embedded (FFPE) tissue of HER2-positive breast cancer was obtained from Sendai Medical Center [
15]. Informed consent for sample procurement and subsequent data analyses was obtained from the patient or the patient’s guardian at Sendai Medical Center. Normal tissues were purchased from BioChain Institute Inc. (Eureka Drive Newark, CA) or Cybrdi Inc. (Frederick, MD). The tissue sections were autoclaved in citrate buffer (pH 6.0; Nichirei Biosciences, Inc., Tokyo, Japan) for 20 min. The blocking was performed using SuperBlock T20 (Thermo Fisher Scientific Inc.). The sections were incubated with H
2Mab-250 (1, 0.5, or 0.1 μg/mL) and tras-mG
2a-f (1 μg/mL), and then treated with the EnVision+ Kit for mouse (Agilent Technologies, Inc., Santa Clara, CA). The chromogenic reaction was performed using 3,3′-diaminobenzidine tetrahydrochloride (DAB; Agilent Technologies, Inc.). Counterstaining was performed using hematoxylin (FUJIFILM Wako Pure Chemical Corporation) and Leica DMD108 (Leica Microsystems GmbH, Wetzlar, Germany) was used to obtain images and examine the sections.
2.8. ELISA
HER2ec was immobilized on Nunc Maxisorp 96−well immunoplates (Thermo Fisher Scientific Inc.) at a concentration of 1 µg/mL for 30 min at 37°C. After washing with PBS containing 0.05% (v/v) Tween 20 (PBST; Nacalai Tesque, Inc.), wells were blocked with 1% (w/v) bovine serum albumin (BSA)−containing PBST for 30 min at 37°C. The serially diluted H2Mab-250 and trastuzumab (0.0006–10 µg/mL) were added to each well, followed by peroxidase−conjugated anti−mouse immunoglobulins (1:3000 diluted; Agilent Technologies Inc., Santa Clara, CA, USA) and anti-human immunoglobulins (1:3000 diluted; Sigma-Aldrich Corp.). Enzymatic reactions were conducted using ELISA POD Substrate TMB Kit (Nacalai Tesque, Inc.) followed by the measurement of the optical density at 655 nm, using an iMark microplate reader (Bio−Rad Laboratories, Inc., Berkeley, CA, USA). The binding isotherms were fitted into the built-in, one-site binding model in GraphPad PRISM 6 (GraphPad Software, Inc., La Jolla, CA) to calculate the dissociation constant (KD).
Synthesized peptides covering the HER2 extracellular domain IV and point mutant peptides were synthesized by Sigma-Aldrich Corp. The peptides (10 µg/mL) were immobilized on Nunc Maxisorp 96-well immunoplates (Thermo Fisher Scientific Inc.). Plate washing was performed with PBS containing 0.05% (v/v) Tween 20 (PBST; Nacalai Tesque, Inc.). After blocking with 1% (w/v) BSA in PBST, H2Mab-250 (10 µg/mL) was added to each well. Then, the wells were further incubated with peroxidase-conjugated anti-mouse immunoglobulins (1:2000 dilution; Agilent Technologies, Inc). Enzymatic reactions were conducted using One-Step Ultra TMB. The optical density at 655 nm was measured using an iMark microplate reader.
2.9. Determination of KD via surface plasmon resonance (SPR)
Measurement of KD between H2Mab-250 and the HER2 peptides was performed using SPR. H2Mab-250 was immobilized on the sensor chip CM5 in accordance with the manufacturer’s protocol by Cytiva. Immobilization of H2Mab-250 (10 μg/mL in acetate buffer (pH 4.0); Cytiva) was carried out using an amine coupling reaction. The surface of the flow cell 2 of the sensor chip CM5 was treated with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide and N-hydroxysuccinimide (NHS), followed by the injection of H2Mab-250. The KD between H2Mab-250 and the peptides was determined using Biacore X100 (Cytiva). The binding signals were measured using a single-cycle kinetics method. The data were analyzed by 1:1 binding kinetics using Biacore X100 evaluation software (Cytiva) to determine the association rate constant (ka) and dissociation rate constant (kd) and KD. The affinity constant (KA) at equilibrium was calculated as 1/KD.
4. Discussion
In this study, we developed a cancer-specific mAb targeting HER2. H
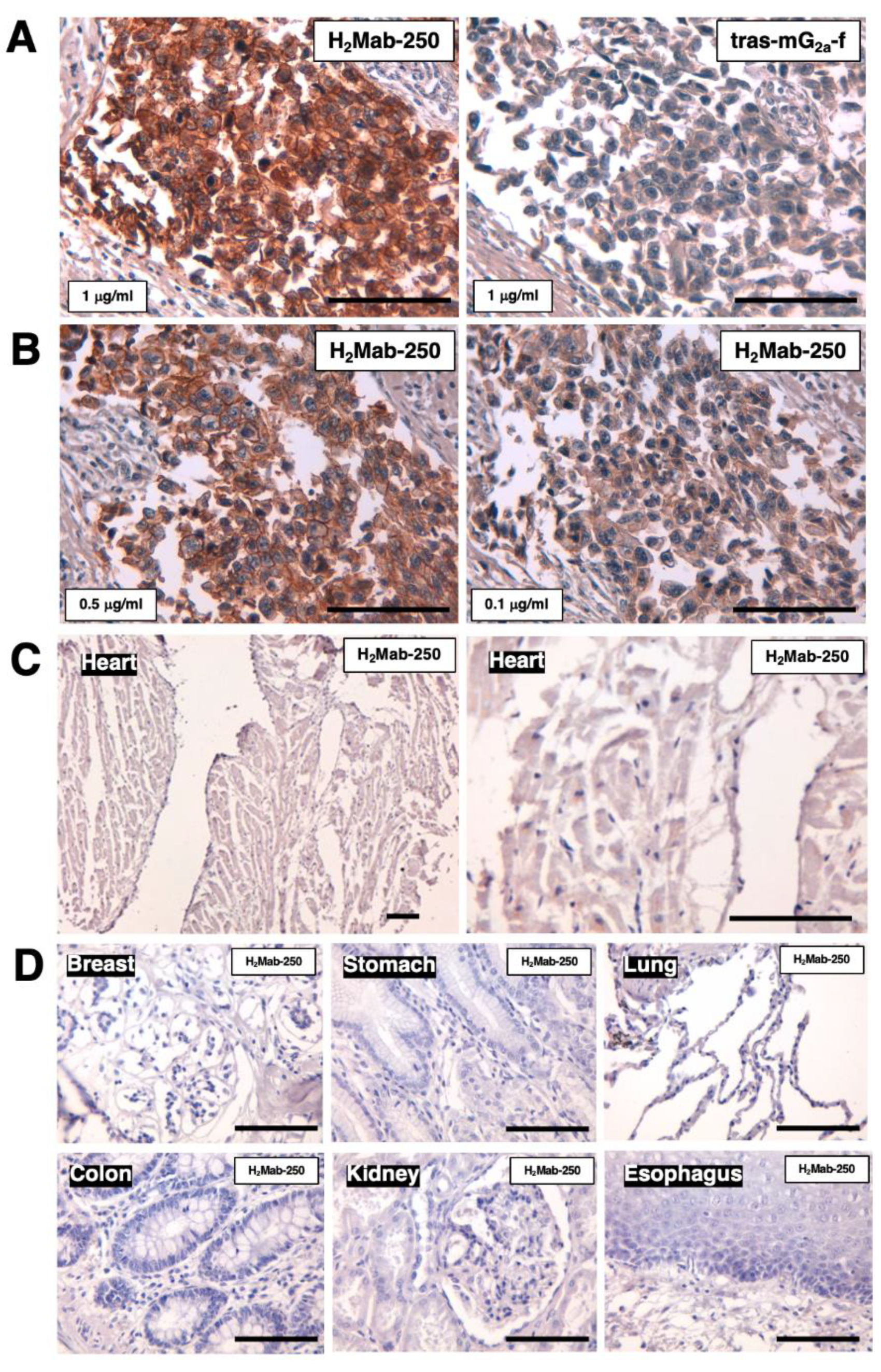
2Mab-250 can recognize breast cancer cells, but not normal cells in flow cytometry (
Figure 1) and immunohistochemistry (
Figure 6). H
2Mab-250-mG
2a-f could activate ADCC against breast cancer cells, but not against normal epithelial cells (
Figure 4). We also identified the H
2Mab-250 epitope sequence (
613-IWKFP
-617) by SPR analysis (
Figure 7). The
613-IWKFP
-617 sequence is partially included with the wider binding epitope of trastuzumab (residues 579-625) [
25]. Furthermore, no reaction was observed between H
2Mab-250 and CHO/HER2 W614A in flow cytometry (
Figure 7), indicating that Trp614 plays a central role in recognition by H
2Mab-250. Although H
2Mab-250 possesses a high affinity to epitope-containing peptide (603–622) in SPR analysis, the recognition in flow cytometry using cell lines was lower than that of trastuzumab (
Figure 1). In contrast, H
2Mab-250 exhibited a higher reactivity than tras-mG
2a-f in the immunohistochemical analysis using breast cancer tissues (
Figure 6). This discrepancy might be induced by the possibility that the epitope sequence is partially exposed in cancer cells, but not in normal cells in clinical cancer tissues. The mechanism of recognition by H
2Mab-250 should be further investigated in future studies.
For the clinical treatment of metastatic breast cancer, trastuzumab is administered in patients with HER2-overexpressing tumors, which are defined by strong and complete IHC membranous staining of more than 10% of cells (IHC 3+) and/or
in situ hybridization (ISH)-amplified. Furthermore, trastuzumab-based antibody-drug conjugates (ADCs) such as trastuzumab-deruxtecan (T-DXd) have been evaluated in various clinical trials. Based on the studies, T-DXd has been approved in not only HER2-positive breast cancers [
26,
27], but also HER2-mutant lung cancer [
28] and HER2-low (IHC 1+ or IHC 2+ / ISH-non-amplified) advanced breast cancer [
29]. A significant number of patients can benefit from T-DXd therapy since approximately half of all breast cancers are classifiable as HER2-low [
30]. Meanwhile, cardiotoxicity is the most significant toxicity associated with T-DXd [
31]. Further studies are essential to evaluate
in vivo toxicities of H
2Mab-250.
Figure 7.
Epitope identification for H2Mab-250. (A) Epitope determination of H2Mab-250 and H2Mab-119 using flow cytometry. The schematic representation of HER2 and the deletion mutants (left). Flow cytometry using H2Mab-250 (10 μg/mL; Red line) and H2Mab-119 (10 μg/mL; Blue line) against CHO/HER2 (WT and deletion mutants). The cell surface expression was confirmed by an anti-PA tag mAb, NZ-1 (10 μg/mL; Green). The black line represents the negative control (blocking buffer). (B and C) Determination of H2Mab-250 epitope by ELISA. Five synthesized peptides that cover the HER2 domain IV (B), alanine-substituted peptides of HER2 domain IV (603–622) (C), HER2ec, or buffer control (NC) were immobilized on immunoplates. The plates were incubated with H2Mab-250 (10 μg/mL), followed by incubation with peroxidase-conjugated anti-mouse immunoglobulins. Optical density was measured at 655 nm. Error bars represent means ± SDs. (D) Flow cytometry using H2Mab-250 (10 μg/mL; Red line) against CHO/HER2 (WT and W614A). The cell surface expression was confirmed by an anti-PA tag mAb, NZ-1 (10 μg/mL; Red line). The black line represents the negative control (blocking buffer). (E) Surface plasmon resonance analysis between H2Mab-250 and HER2 domain IV (603–622) peptides. The affinity constant (KA) at equilibrium was calculated as 1/KD.
Figure 7.
Epitope identification for H2Mab-250. (A) Epitope determination of H2Mab-250 and H2Mab-119 using flow cytometry. The schematic representation of HER2 and the deletion mutants (left). Flow cytometry using H2Mab-250 (10 μg/mL; Red line) and H2Mab-119 (10 μg/mL; Blue line) against CHO/HER2 (WT and deletion mutants). The cell surface expression was confirmed by an anti-PA tag mAb, NZ-1 (10 μg/mL; Green). The black line represents the negative control (blocking buffer). (B and C) Determination of H2Mab-250 epitope by ELISA. Five synthesized peptides that cover the HER2 domain IV (B), alanine-substituted peptides of HER2 domain IV (603–622) (C), HER2ec, or buffer control (NC) were immobilized on immunoplates. The plates were incubated with H2Mab-250 (10 μg/mL), followed by incubation with peroxidase-conjugated anti-mouse immunoglobulins. Optical density was measured at 655 nm. Error bars represent means ± SDs. (D) Flow cytometry using H2Mab-250 (10 μg/mL; Red line) against CHO/HER2 (WT and W614A). The cell surface expression was confirmed by an anti-PA tag mAb, NZ-1 (10 μg/mL; Red line). The black line represents the negative control (blocking buffer). (E) Surface plasmon resonance analysis between H2Mab-250 and HER2 domain IV (603–622) peptides. The affinity constant (KA) at equilibrium was calculated as 1/KD.
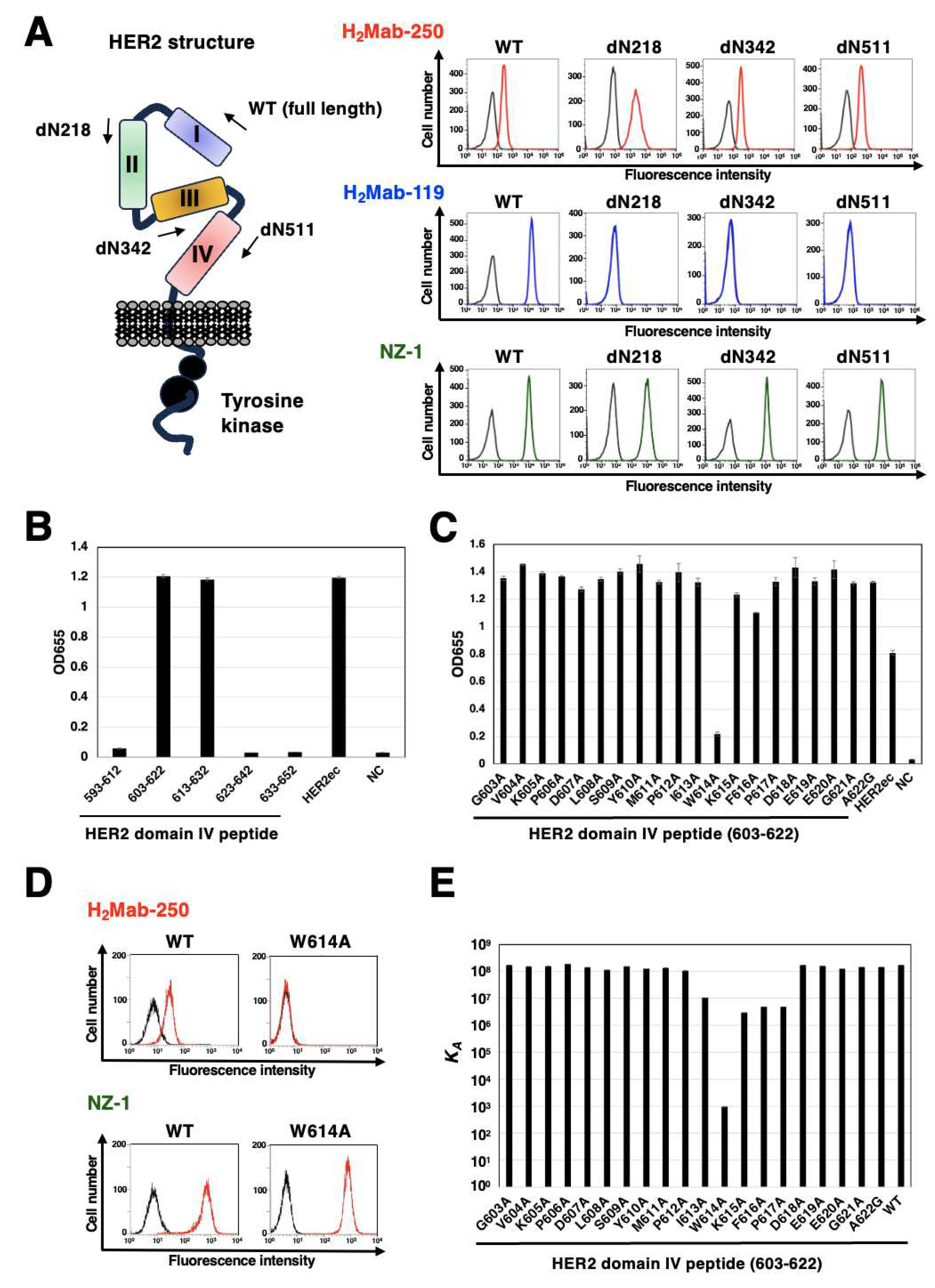
Table 1.
Identification of H2Mab-250 epitope using point mutants by Biacore.
Table 1.
Identification of H2Mab-250 epitope using point mutants by Biacore.
| Peptide |
Sequence |
KD (M) |
| 603-622 (WT) |
GVKPDLSYMPIWKFPDEEGA |
5.8 × 10-9
|
| G603A |
AVKPDLSYMPIWKFPDEEGA |
5.9 × 10-9
|
| V604A |
GAKPDLSYMPIWKFPDEEGA |
6.5 × 10-9
|
| K605A |
GVAPDLSYMPIWKFPDEEGA |
6.5 × 10-9
|
| P606A |
GVKADLSYMPIWKFPDEEGA |
5.3 × 10-9
|
| D607A |
GVKPALSYMPIWKFPDEEGA |
7.1 × 10-9
|
| L608A |
GVKPDASYMPIWKFPDEEGA |
8.8 × 10-9
|
| S609A |
GVKPDLAYMPIWKFPDEEGA |
6.5 × 10-9
|
| Y610A |
GVKPDLSAMPIWKFPDEEGA |
7.9 × 10-9
|
| M611A |
GVKPDLSYAPIWKFPDEEGA |
7.5 × 10-9
|
| P612A |
GVKPDLSYMAIWKFPDEEGA |
9.5 × 10-9
|
| I613A |
GVKPDLSYMPAWKFPDEEGA |
9.4 × 10-8
|
| W614A |
GVKPDLSYMPIAKFPDEEGA |
1.1 × 10-3
|
| K615A |
GVKPDLSYMPIWAFPDEEGA |
3.4 × 10-7
|
| F616A |
GVKPDLSYMPIWKAPDEEGA |
2.0 × 10-7
|
| P617A |
GVKPDLSYMPIWKFADEEGA |
2.1 × 10-7
|
| D618A |
GVKPDLSYMPIWKFPAEEGA |
5.8 × 10-9
|
| E619A |
GVKPDLSYMPIWKFPDAEGA |
6.3 × 10-9
|
| E620A |
GVKPDLSYMPIWKFPDEAGA |
8.0 × 10-9
|
| G621A |
GVKPDLSYMPIWKFPDEEAA |
6.9 × 10-9
|
| A622G |
GVKPDLSYMPIWKFPDEEGG |
6.9 × 10-9
|
The benefit of low-affinity mAbs for therapeutic applications has been discussed. A low affinity anti-EGFR mAb (
KD: 3.4 × 10
−7 M) was efficiently taken up by cancer cells but not normal cells, which results in sufficient efficacy against tumor cells, but low toxicity against normal keratinocytes [
32]. An anti-HER3 mAb, Ab562, possesses ~ 10-fold lower affinity (
KD: 2~3 × 10
−8 M) than patritumab. The ADC, AMT-562 exhibited sufficient antitumor effects with minimizing potential toxicity [
33]. Since H
2Mab-250 possesses lower affinity to HER2ec than tras-mG
2a-f (
Figure 3) and exhibited no reactivity to normal epithelial cells (
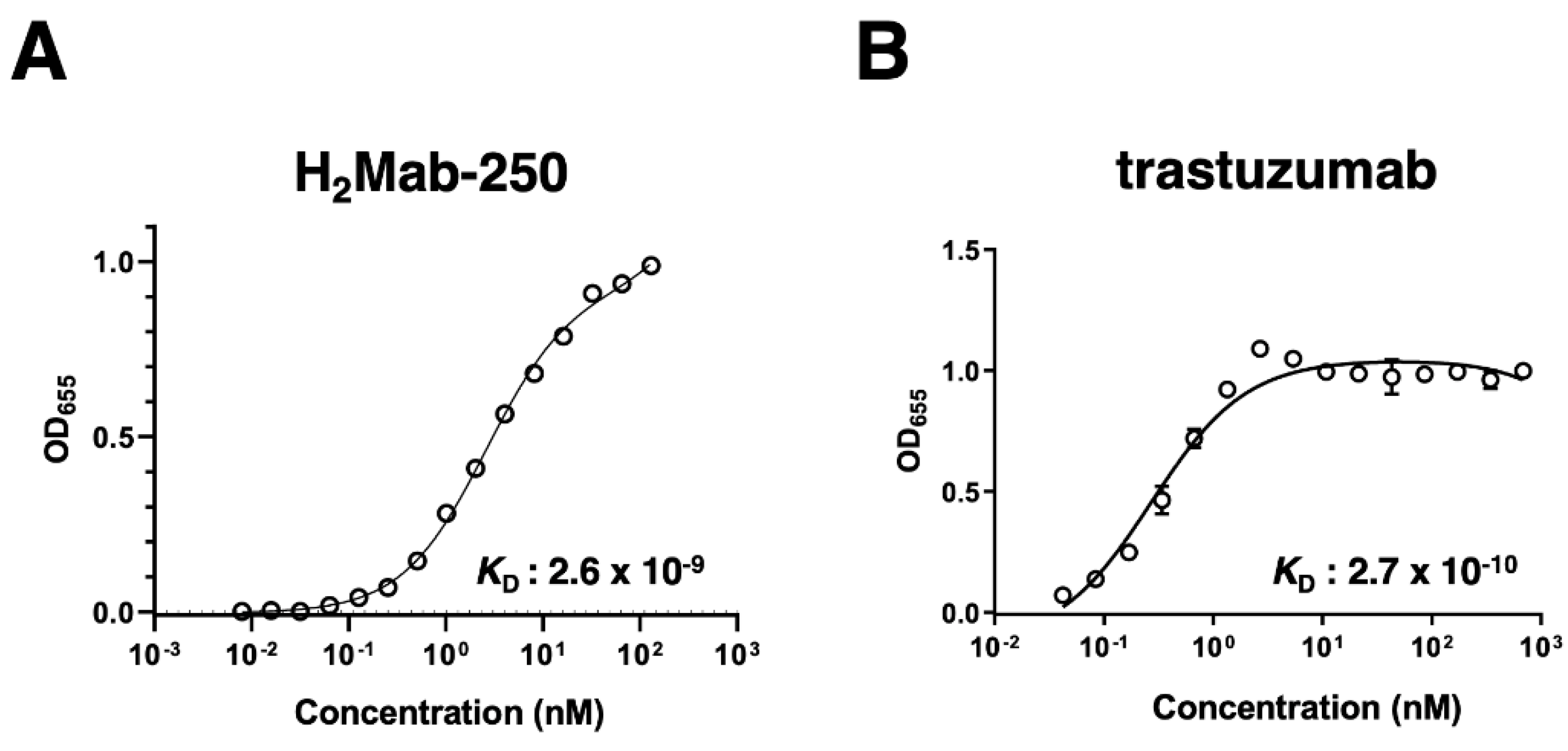
Figure 1 and 2), H
2Mab-250 or H
2Mab-250-ADC could exhibit antitumor efficacy with lower side effects.
H
2Mab-250-mG
2a-f could trigger the ADCC activity to BT-474 selectively (
Figure 4). Although the effect of H
2Mab-250-mG
2a-f is lower than that of trastuzumab, we should consider that effector Jurkat cells express human FcγRIIIa receptor. In contrast, H
2Mab-250 exhibited a superior reactivity to HER2-positive breast cancer tissue sections in immunohistochemistry (
Figure 6). Since the similar
in vivo efficacy of H
2Mab-250-mG
2a-f with tras-mG
2a-f against BT-474 xenograft was shown in
Figure 5, clinical application of H
2Mab-250 is expected. Furthermore, chimeric antigen receptor (CAR)-T cell therapy against HER2 has been evaluated in clinical studies [
30]. It would be worthwhile to investigate the cancer specificity of H
2Mab-250 scFv and the efficacy of CAR-T against HER2-positive tumors in future studies.
CAR-T cells against CD19 have improved outcomes for patients with B cell lymphoma. However, disease relapse is commonly occurred in many patients [
34,
35]. Although different mechanisms of immune escape have been demonstrated [
36], the tumor cells from many relapsed patients did not exhibit the features of immune escape. Recently, additional mechanisms, such as trogocytosis, have been proposed [
37]. When CAR-T cells, possessing the high-affinity anti-CD19 FMC63-based CAR, are co-cultured with CD19-positive lymphoma cells, the CAR-T cells strip CD19 from lymphoma cells and incorporate it into their own plasma membrane [
37]. This is called “trogocytosis” which results in the emergency of antigen-negative target cells. Furthermore, the CAR-T cells that acquired CD19 by trogocytosis can be killed by the other CD19 CAR-T cells [
37]. A promising approach to limit CAR-T cell-mediated trogocytosis is the reduction of CAR affinity [
38]. CD19-targeting CAR with ~ 40-fold lower affinity (
KD: 1.4 × 10
−8 M) than the clinically approved FMC63-based CAR (
KD: 3.3 × 10
−10 M) was developed [
39]. The reduced affinity CAR-T cells exhibited higher efficacy and persistence than FMC63-based CAR-T cells in a murine model [
39], as well as robust antitumor efficacy and persistence in two clinical trials [
39,
40]. These data show that it is possible to significantly limit trogocytosis by reducing CAR affinity while maintaining antitumor activity as well as clinical efficacy. The property of H
2Mab-250 could contribute to the future development of HER2-targeting CAR-T cells by limiting trogocytosis and maintaining cancer specificity.
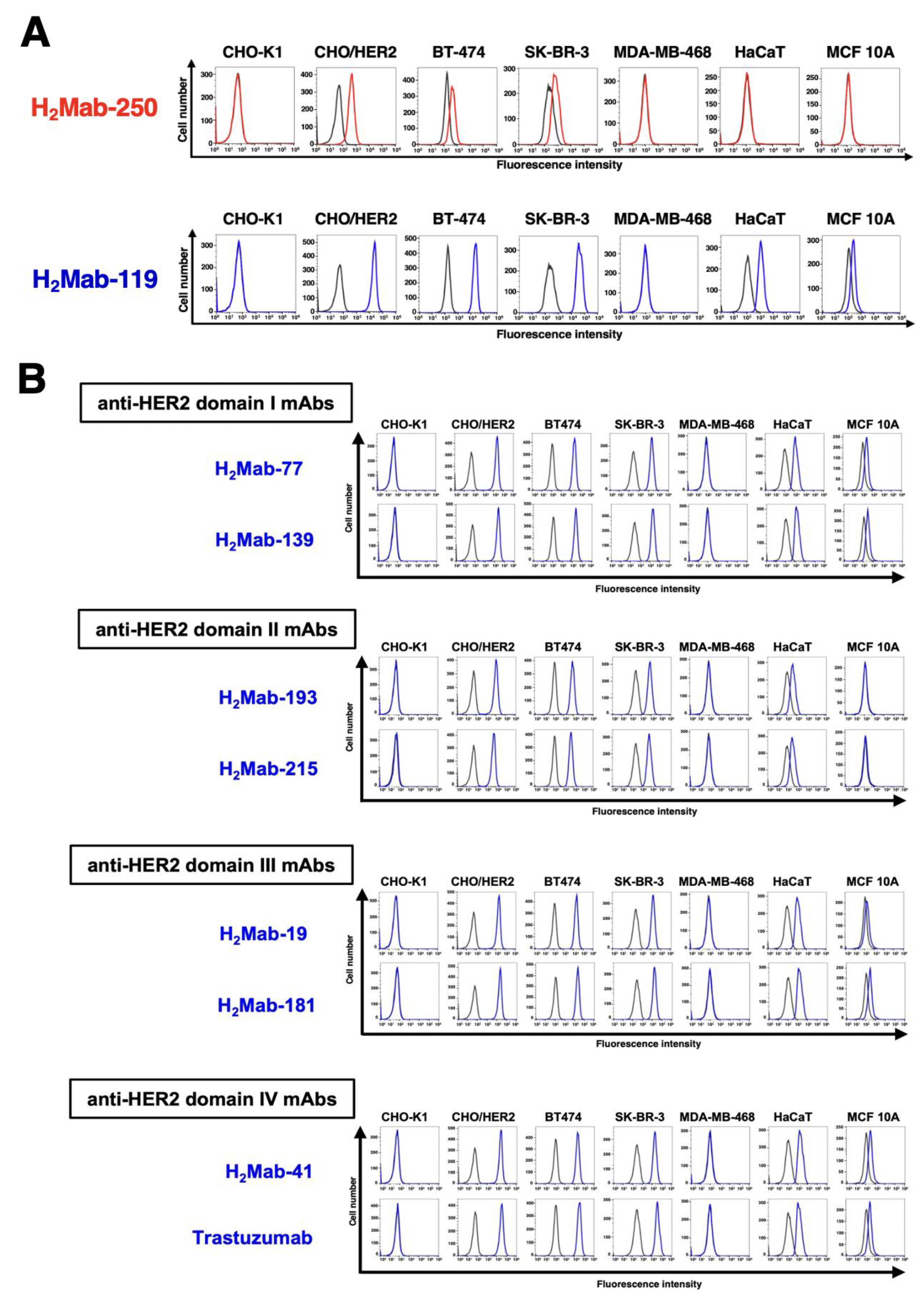
Figure 1.
Flow cytometry using anti-HER2 mAbs against HER2-overexpressed cells, breast cancer cell lines, and non-transformed normal epithelial cell lines. (A) Flow cytometry using H2Mab-250 (10 μg/mL; Red line) and H2Mab-119 (10 μg/mL; Blue line) against CHO-K1, CHO/HER2, HER2-positive breast cancers (BT-474 and SK-BR-3), a triple-negative breast cancer (MDA-MB-468), and non-transformed normal epithelial cells (HaCaT and MCF 10A). (B) Flow cytometry using H2Mab-77 (10 μg/mL), H2Mab-139 (10 μg/mL), H2Mab-193 (10 μg/mL), H2Mab-215 (10 μg/mL), H2Mab-19 (10 μg/mL), H2Mab-181 (10 μg/mL), H2Mab-41 (10 μg/mL), and trastuzumab (10 μg/mL) against CHO-K1, CHO/HER2, HER2-positive breast cancers (BT-474 and SK-BR-3), a triple-negative breast cancer (MDA-MB-468), non-transformed normal epithelial cells (HaCaT and MCF 10A). The black line represents the negative control (blocking buffer).
Figure 1.
Flow cytometry using anti-HER2 mAbs against HER2-overexpressed cells, breast cancer cell lines, and non-transformed normal epithelial cell lines. (A) Flow cytometry using H2Mab-250 (10 μg/mL; Red line) and H2Mab-119 (10 μg/mL; Blue line) against CHO-K1, CHO/HER2, HER2-positive breast cancers (BT-474 and SK-BR-3), a triple-negative breast cancer (MDA-MB-468), and non-transformed normal epithelial cells (HaCaT and MCF 10A). (B) Flow cytometry using H2Mab-77 (10 μg/mL), H2Mab-139 (10 μg/mL), H2Mab-193 (10 μg/mL), H2Mab-215 (10 μg/mL), H2Mab-19 (10 μg/mL), H2Mab-181 (10 μg/mL), H2Mab-41 (10 μg/mL), and trastuzumab (10 μg/mL) against CHO-K1, CHO/HER2, HER2-positive breast cancers (BT-474 and SK-BR-3), a triple-negative breast cancer (MDA-MB-468), non-transformed normal epithelial cells (HaCaT and MCF 10A). The black line represents the negative control (blocking buffer).
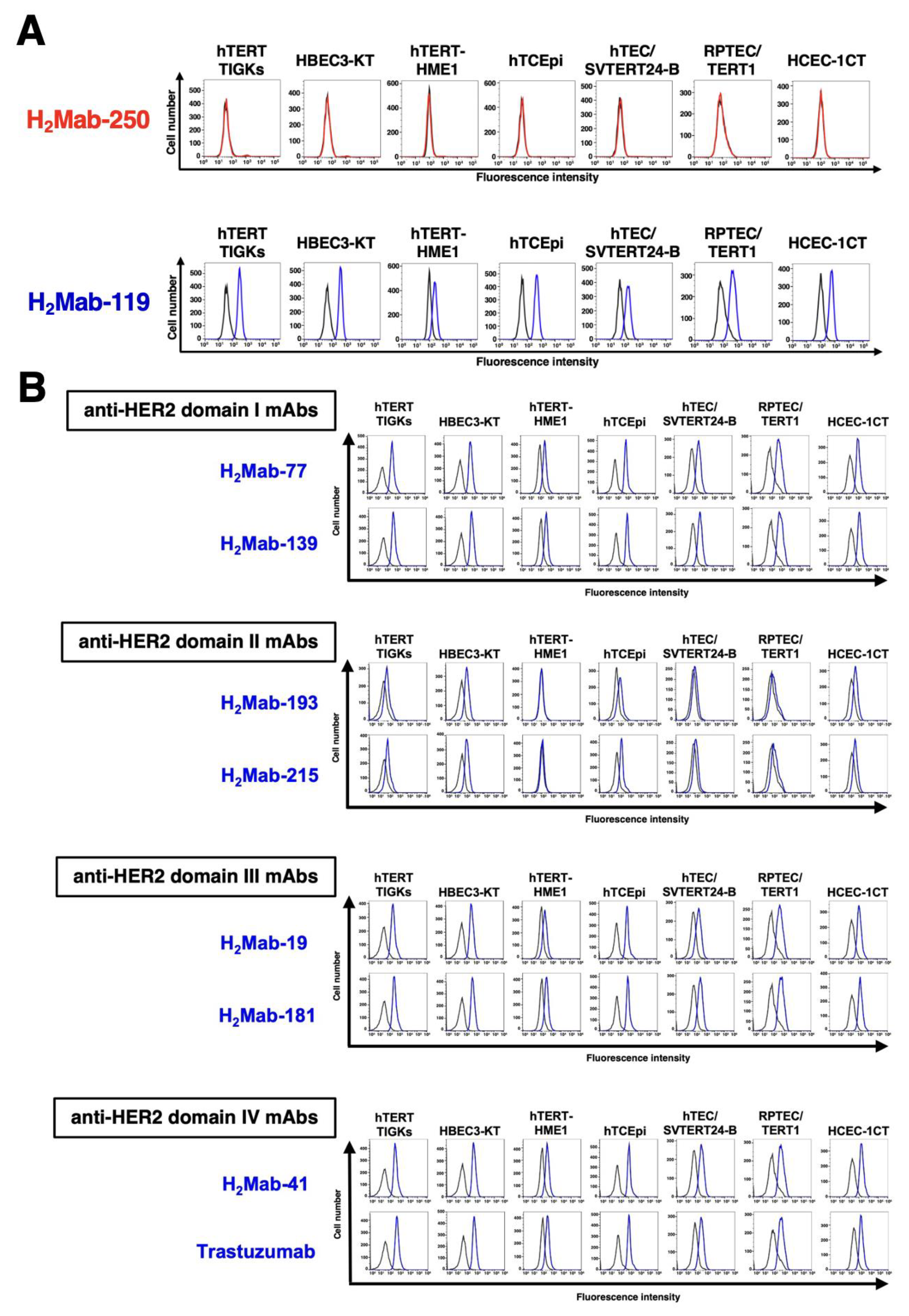
Figure 2.
Flow cytometry using anti-HER2 mAbs against immortalized normal epithelial cells. (A) Flow cytometry using H2Mab-250 (10 μg/mL; Red line) and H2Mab-119 (10 μg/mL; Blue line) against immortalized normal epithelial cells including hTERT TIGKs (gingiva), HBEC3-KT (lung bronchus), hTERT-HME1 (mammary gland), hTCEpi (corneal), hTEC/SVTERT24-B (thymus), RPTEC/TERT1 (kidney proximal tubule), and HCEC-1CT (colon). The black line represents the negative control (blocking buffer). (B) Flow cytometry using H2Mab-77 (10 μg/mL), H2Mab-139 (10 μg/mL), H2Mab-193 (10 μg/mL), H2Mab-215 (10 μg/mL), H2Mab-19 (10 μg/mL), H2Mab-181 (10 μg/mL), H2Mab-41 (10 μg/mL), and trastuzumab (10 μg/mL) against immortalized normal epithelial cells, including hTERT TIGKs (gingiva), HBEC3-KT (lung bronchus), hTERT-HME1 (mammary gland), hTCEpi (corneal), hTEC/SVTERT24-B (thymus), RPTEC/TERT1 (kidney proximal tubule), and HCEC-1CT (colon). The black line represents the negative control (blocking buffer).
Figure 2.
Flow cytometry using anti-HER2 mAbs against immortalized normal epithelial cells. (A) Flow cytometry using H2Mab-250 (10 μg/mL; Red line) and H2Mab-119 (10 μg/mL; Blue line) against immortalized normal epithelial cells including hTERT TIGKs (gingiva), HBEC3-KT (lung bronchus), hTERT-HME1 (mammary gland), hTCEpi (corneal), hTEC/SVTERT24-B (thymus), RPTEC/TERT1 (kidney proximal tubule), and HCEC-1CT (colon). The black line represents the negative control (blocking buffer). (B) Flow cytometry using H2Mab-77 (10 μg/mL), H2Mab-139 (10 μg/mL), H2Mab-193 (10 μg/mL), H2Mab-215 (10 μg/mL), H2Mab-19 (10 μg/mL), H2Mab-181 (10 μg/mL), H2Mab-41 (10 μg/mL), and trastuzumab (10 μg/mL) against immortalized normal epithelial cells, including hTERT TIGKs (gingiva), HBEC3-KT (lung bronchus), hTERT-HME1 (mammary gland), hTCEpi (corneal), hTEC/SVTERT24-B (thymus), RPTEC/TERT1 (kidney proximal tubule), and HCEC-1CT (colon). The black line represents the negative control (blocking buffer).
Figure 3.
The binding affinity of H2Mab-250 and trastuzumab. HER2ec was immobilized on immunoplates and then incubated with the serially diluted H2Mab-250 (A) and trastuzumab (B), followed by peroxidase−conjugated anti−mouse immunoglobulins and anti-human immunoglobulins, respectively. Enzymatic reactions were conducted and the optical density at 655 nm was measured. The binding isotherms were fitted into the built-in, one-site binding model in GraphPad PRISM 6 to calculate the binding affinity.
Figure 3.
The binding affinity of H2Mab-250 and trastuzumab. HER2ec was immobilized on immunoplates and then incubated with the serially diluted H2Mab-250 (A) and trastuzumab (B), followed by peroxidase−conjugated anti−mouse immunoglobulins and anti-human immunoglobulins, respectively. Enzymatic reactions were conducted and the optical density at 655 nm was measured. The binding isotherms were fitted into the built-in, one-site binding model in GraphPad PRISM 6 to calculate the binding affinity.
Figure 6.
Immunohistochemical analysis of H2Mab-250 and tras-mG2a-f in breast cancer and normal epithelium. (A) The HER2-positive breast cancer tissue sections were treated with H2Mab-250 (1 µg/mL) or tras-mG2a-f (1 µg/mL). (B) The HER2-positive breast cancer tissue sections were treated with H2Mab-250 (0.5 µg/mL) or H2Mab-250 (0.1 µg/mL). (C) A normal heart section was treated with H2Mab-250 (1 µg/mL). (D) Sections of normal breast, stomach, lung, colon, kidney, and esophagus were treated with H2Mab-250 (0.1 µg/mL). The sections were then treated with the Envision+ kit. The chromogenic reaction was performed using DAB, and the sections were counterstained with hematoxylin. Scale bar = 100 µm.
Figure 6.
Immunohistochemical analysis of H2Mab-250 and tras-mG2a-f in breast cancer and normal epithelium. (A) The HER2-positive breast cancer tissue sections were treated with H2Mab-250 (1 µg/mL) or tras-mG2a-f (1 µg/mL). (B) The HER2-positive breast cancer tissue sections were treated with H2Mab-250 (0.5 µg/mL) or H2Mab-250 (0.1 µg/mL). (C) A normal heart section was treated with H2Mab-250 (1 µg/mL). (D) Sections of normal breast, stomach, lung, colon, kidney, and esophagus were treated with H2Mab-250 (0.1 µg/mL). The sections were then treated with the Envision+ kit. The chromogenic reaction was performed using DAB, and the sections were counterstained with hematoxylin. Scale bar = 100 µm.