Submitted:
11 September 2023
Posted:
14 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Animals
Tissue permeabilization
Mitochondrial respirometry
ATP quantification
Glutathione/glutathione disulfide redox potential analysis
Statistics
3. Results
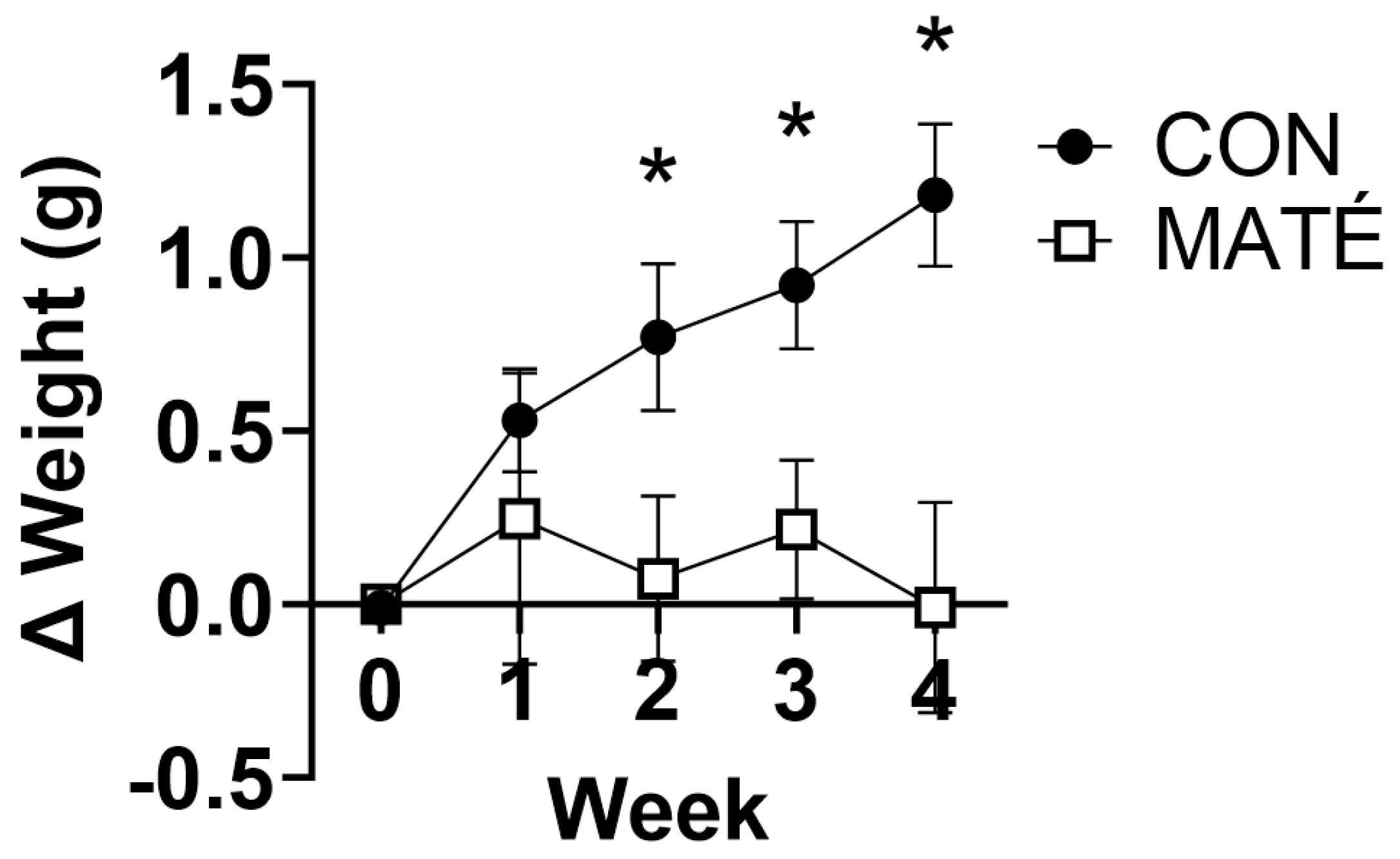
Yerba maté consumption prevents weight gain in male and female mice
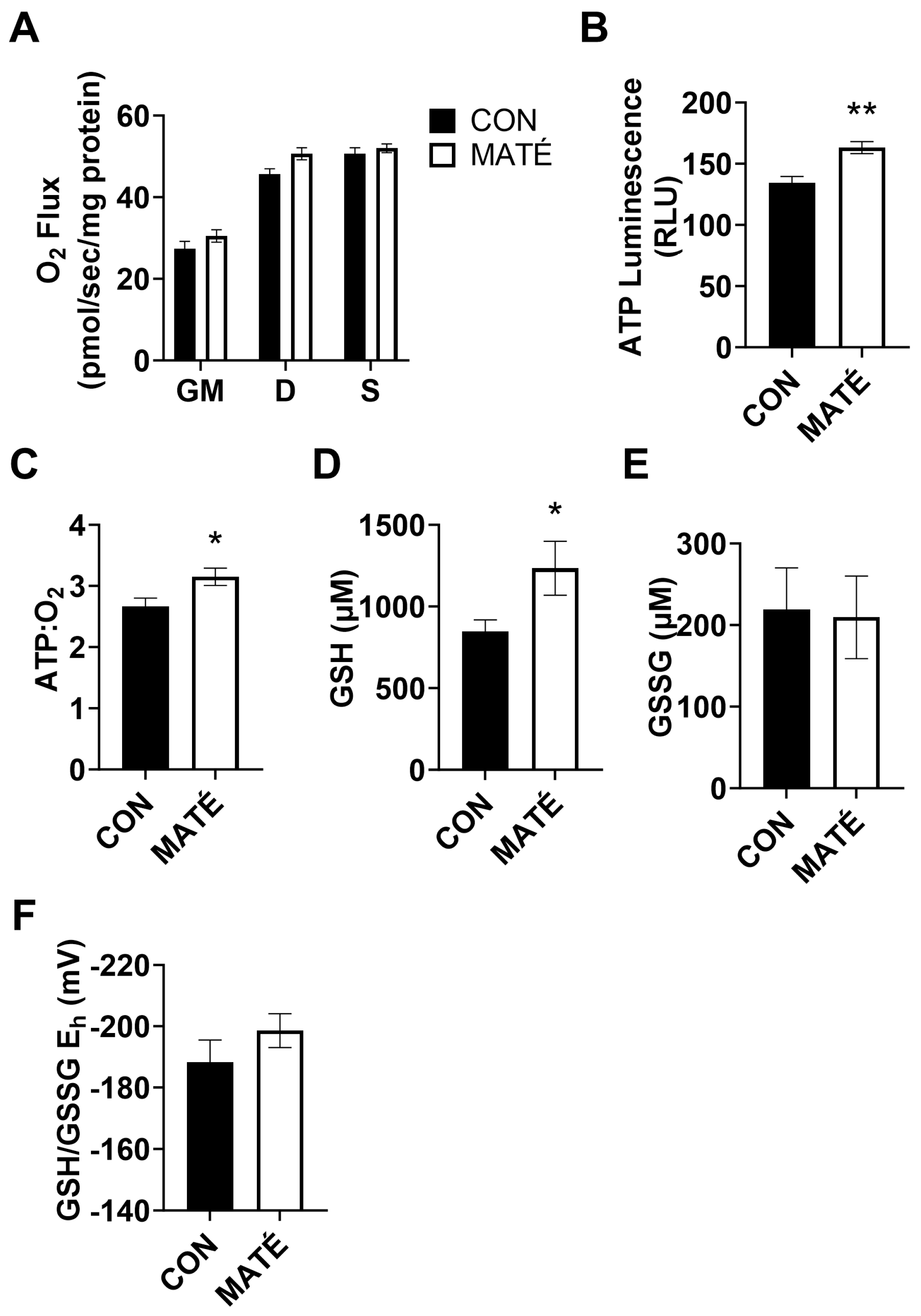
Yerba maté consumption increases mitochondrial efficiency in skeletal muscle
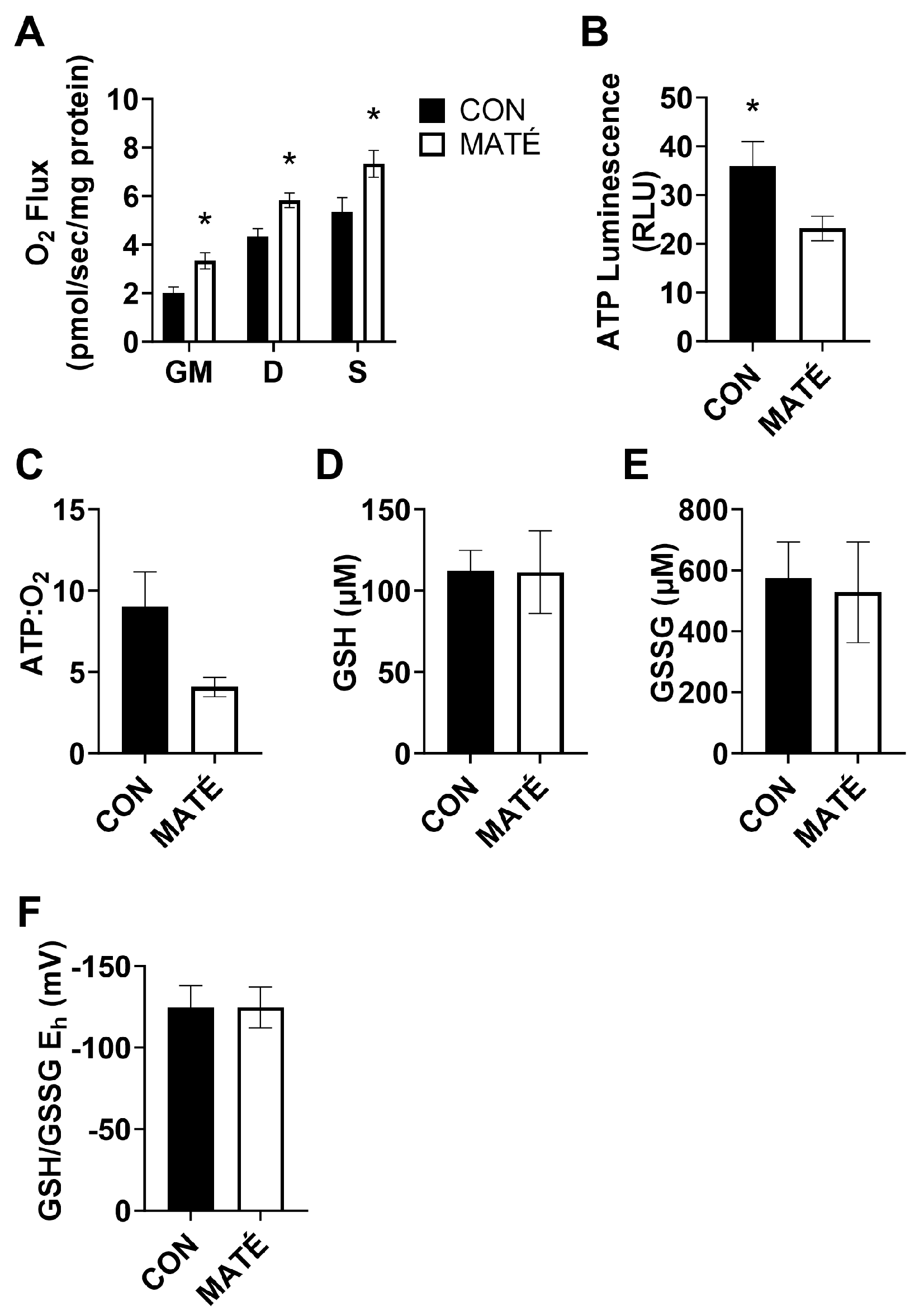
Yerba maté consumption decreases mitochondrial efficiency in white adipose tissue
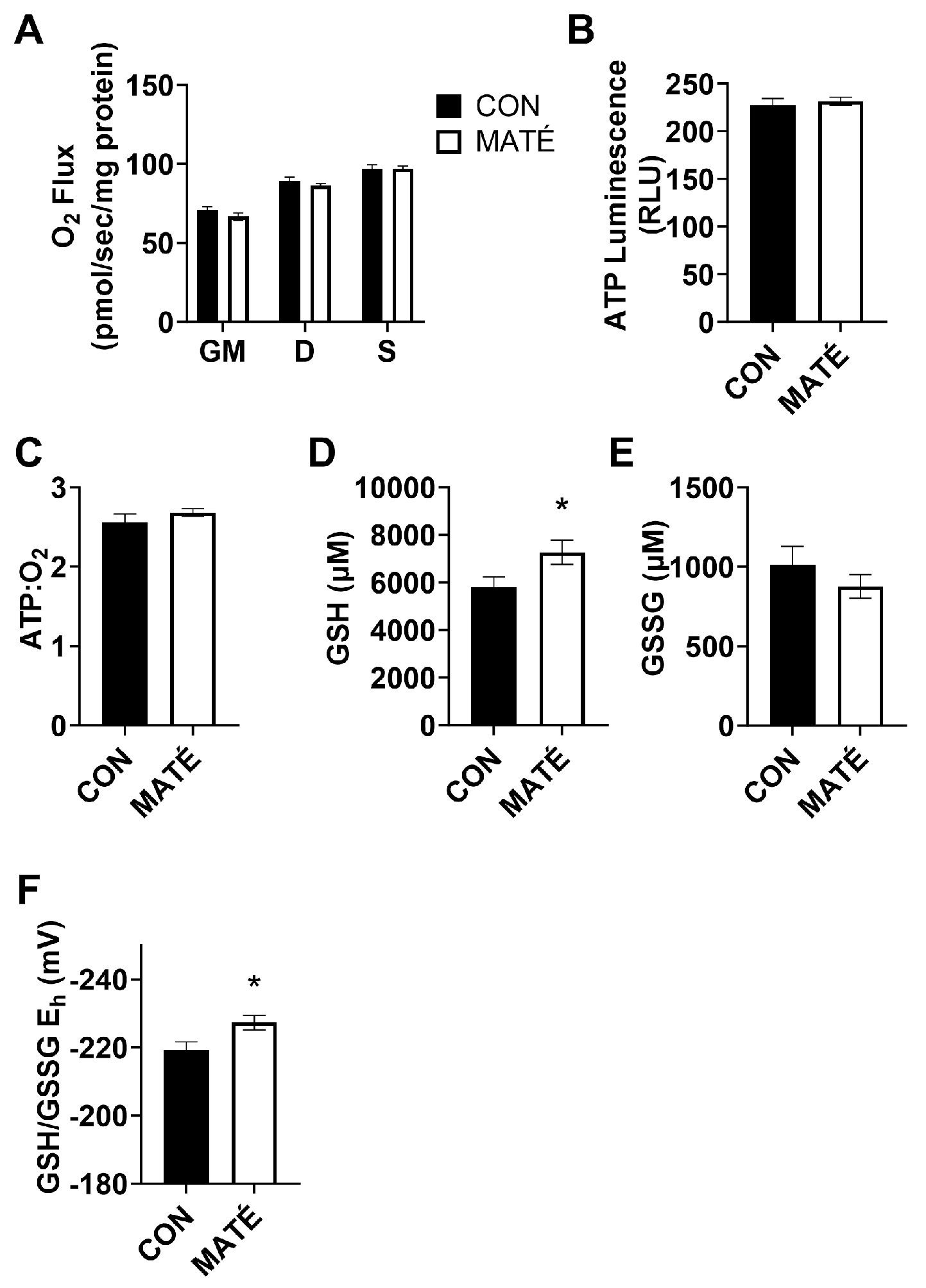
Yerba maté consumption alters hepatic redox potential but not mitochondrial efficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H. , et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. The New England journal of medicine 2008, 359, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, A.T.; Heilbronn, L.K. Metabolic impacts of altering meal frequency and timing - Does when we eat matter? Biochimie 2016, 124, 187–197. [Google Scholar] [CrossRef]
- Plotnick, G.D.; Corretti, M.C.; Vogel, R.A.; Hesslink, R., Jr.; Wise, J.A. Effect of supplemental phytonutrients on impairment of the flow-mediated brachial artery vasoactivity after a single high-fat meal. Journal of the American College of Cardiology 2003, 41, 1744–1749. [Google Scholar] [CrossRef]
- Vasanthi, H.R.; ShriShriMal, N.; Das, D.K. Phytochemicals from plants to combat cardiovascular disease. Curr Med Chem 2012, 19, 2242–2251. [Google Scholar] [CrossRef]
- Drašar, P.A.-O. Plant Secondary Metabolites Used for the Treatment of Diseases and Drug Development. 2022. [Google Scholar] [CrossRef]
- Miao, M.; Jiang, H.; Jiang, B.; Zhang, T.; Cui, S.W.; Jin, Z. Phytonutrients for controlling starch digestion: evaluation of grape skin extract. Food Chem 2014, 145, 205–211. [Google Scholar] [CrossRef]
- Weigel, H.J. Plant quality declines as CO2 levels rise. Elife 2014, 3, e03233. [Google Scholar] [CrossRef]
- Rowley, T.J.t.; Bitner, B.F.; Ray, J.D.; Lathen, D.R.; Smithson, A.T.; Dallon, B.W.; Plowman, C.J.; Bikman, B.T.; Hansen, J.M.; Dorenkott, M.R. , et al. Monomeric cocoa catechins enhance beta-cell function by increasing mitochondrial respiration. The Journal of nutritional biochemistry 2017, 49, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Galli, R.L.; Shukitt-Hale, B.; Youdim, K.A.; Joseph, J.A. Fruit polyphenolics and brain aging: nutritional interventions targeting age-related neuronal and behavioral deficits. Annals of the New York Academy of Sciences 2002, 959, 128–132. [Google Scholar] [CrossRef]
- Beecher, G.R. Phytonutrients' role in metabolism: effects on resistance to degenerative processes. Nutrition reviews 1999, 57, S3–6. [Google Scholar] [CrossRef] [PubMed]
- de Morais, E.C.; Stefanuto, A.; Klein, G.A.; Boaventura, B.C.; de Andrade, F.; Wazlawik, E.; Di Pietro, P.F.; Maraschin, M.; da Silva, E.L. Consumption of yerba mate ( Ilex paraguariensis ) improves serum lipid parameters in healthy dyslipidemic subjects and provides an additional LDL-cholesterol reduction in individuals on statin therapy. Journal of agricultural and food chemistry 2009, 57, 8316–8324. [Google Scholar] [CrossRef] [PubMed]
- Meinhart, A.D.; Damin, F.M.; Caldeirao, L.; da Silveira, T.F.F.; Filho, J.T.; Godoy, H.T. Chlorogenic acid isomer contents in 100 plants commercialized in Brazil. Food Res Int 2017, 99, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of chlorogenic Acid on regulating glucose and lipids metabolism: a review. Evid Based Complement Alternat Med 2013, 2013, 801457. [Google Scholar] [CrossRef]
- Kungel, P.; Correa, V.G.; Correa, R.C.G.; Peralta, R.A.; Sokovic, M.; Calhelha, R.C.; Bracht, A.; Ferreira, I.; Peralta, R.M. Antioxidant and antimicrobial activities of a purified polysaccharide from yerba mate (Ilex paraguariensis). Int J Biol Macromol 2018, 114, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Arçari, D.P.; Bartchewsky, W.; dos Santos, T.W.; Oliveira, K.A.; DeOliveira, C.C.; Gotardo, É.M.; Pedrazzoli, J.; Gambero, A.; Ferraz, L.F.C.; Carvalho, P.d.O. , et al. Anti-inflammatory effects of yerba maté extract (Ilex paraguariensis) ameliorate insulin resistance in mice with high fat diet-induced obesity. Molecular and Cellular Endocrinology 2011, 335, 110–115. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food chemistry 2019, 299, 125124. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Tippetts, T.S.; Winden, D.R.; Swensen, A.C.; Nelson, M.B.; Thatcher, M.O.; Saito, R.R.; Condie, T.B.; Simmons, K.J.; Judd, A.M.; Reynolds, P.R. Cigarette smoke increases cardiomyocyte ceramide accumulation and inhibits mitochondrial respiration. BMC cardiovascular disorders 2014, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Tippetts, T.; Anderson, M.; Holub, Z.; Moulton, E.; Swensen, A.; Prince, J.; Bikman, B. Insulin increases ceramide synthesis in skeletal muscle. Journal of diabetes research 2014, 2014. [Google Scholar] [CrossRef]
- Smith, Melissa E. ; Tippetts, Trevor S.; Brassfield, Eric S.; Tucker, Braden J.; Ockey, A.; Swensen, Adam C.; Anthonymuthu, Tamil S.; Washburn, Trevor D.; Kane, Daniel A.; Prince, John T., et al. Mitochondrial fission mediates ceramide-induced metabolic disruption in skeletal muscle. Biochemical Journal 2013, 456, 427–439. [Google Scholar] [CrossRef]
- Walton, C.M.; Jacobsen, S.M.; Dallon, B.W.; Saito, E.R.; Bennett, S.L.H.; Davidson, L.E.; Thomson, D.M.; Hyldahl, R.D.; Bikman, B.T. Ketones elicit distinct alterations in adipose mitochondrial bioenergetics. International Journal of Molecular Sciences 2020, 21, 6255. [Google Scholar] [CrossRef] [PubMed]
- Piorczynski, T.B.; Lapehn, S.; Ringer, K.P.; Allen, S.A.; Johnson, G.A.; Call, K.; Lucas, S.M.; Harris, C.; Hansen, J.M. NRF2 activation inhibits valproic acid-induced neural tube defects in mice. Neurotoxicology and Teratology 2022, 89, 107039. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P.; Carlson, J.L.; Mody Jr, V.C.; Cai, J.; Lynn, M.J.; Sternberg Jr, P. Redox state of glutathione in human plasma. Free Radical Biology and Medicine 2000, 28, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P. Redox potential of GSH/GSSG couple: assay and biological significance. In Methods in enzymology; Elsevier, 2002; Vol. 348, pp. 93–112. [Google Scholar]
- Saito, E.R.; Warren, C.E.; Hanegan, C.M.; Larsen, J.G.; du Randt, J.D.; Cannon, M.; Saito, J.Y.; Campbell, R.J.; Kemberling, C.M.; Miller, G.S. A novel ketone-supplemented diet improves recognition memory and hippocampal mitochondrial efficiency in healthy adult mice. Metabolites 2022, 12, 1019. [Google Scholar] [CrossRef]
- Andrade, V.M.d.M.; de Moura, A.F.; da Costa Chaves, K.; da Rocha, C.P.D.; de Andrade, C.B.V.; Trevenzoli, I.H.; Ortiga-Carvalho, T.M.; Barcellos, L.C.; Vaisman, M.; Salerno, V.P. Yerba mate consumption by ovariectomized rats alters white adipose tissue. Molecular and Cellular Endocrinology 2023, 564, 111881. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Long, Y.; Jiang, X.; Liu, Z.; Wang, D.; Zhao, Y.; Li, D.; Sun, B.-l. Beneficial effects of Yerba Mate tea (Ilex paraguariensis) on hyperlipidemia in high-fat-fed hamsters. Experimental Gerontology 2013, 48, 572–578. [Google Scholar] [CrossRef]
- Harrold, J.A.; Hughes, G.M.; O’Shiel, K.; Quinn, E.; Boyland, E.J.; Williams, N.J.; Halford, J.C.G. Acute effects of a herb extract formulation and inulin fibre on appetite, energy intake and food choice. Appetite 2013, 62, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Andersen, T.; Fogh, J. Weight loss and delayed gastric emptying following a South American herbal preparation in overweight patients. Journal of Human Nutrition and Dietetics 2001, 14, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Arçari, D.P.; Bartchewsky, W.; dos Santos, T.W.; Oliveira, K.A.; Funck, A.; Pedrazzoli, J.; de Souza, M.F.F.; Saad, M.J.; Bastos, D.H.M.; Gambero, A. , et al. Antiobesity Effects of yerba maté Extract (Ilex paraguariensis) in High-fat Diet–induced Obese Mice. Obesity 2012, 17, 2127–2133. [Google Scholar] [CrossRef]
- Silva, R.D.A.; Bueno, A.L.S.; Gallon, C.W.; Gomes, L.F.; Kaiser, S.; Pavei, C.; Ortega, G.G.; Kucharski, L.C.; Jahn, M.P. The effect of aqueous extract of gross and commercial yerba mate (Ilex paraguariensis) on intra-abdominal and epididymal fat and glucose levels in male Wistar rats. Fitoterapia 2011, 82, 818–826. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Oh, M.-R.; Kim, M.-G.; Chae, H.-J.; Chae, S.-W. Anti-obesity effects of Yerba Mate (Ilex Paraguariensis): a randomized, double-blind, placebo-controlled clinical trial. BMC Complementary and Alternative Medicine 2015, 15, 338. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Ko, J.; Storni, C.; Song, H.J.; Cho, Y.G. Effect of green mate in overweight volunteers: A randomized placebo-controlled human study. Journal of Functional Foods 2012, 4, 287–293. [Google Scholar] [CrossRef]
- Lobo, P.C.B.; da Silva, D.D.; Pimentel, G.D. Acute Supplementation of Yerba Mate Extract Did Not Change Muscle Strength in Physically Active Men Following the Strength Muscle Test: A Pilot Clinical Trial. Nutrients 2022, 14, 2619. [Google Scholar] [CrossRef] [PubMed]
- Panza, V.P.; Diefenthaeler, F.; Tamborindeguy, A.C.; de Quadros Camargo, C.; de Moura, B.M.; Brunetta, H.S.; Sakugawa, R.L.; de Oliveira, M.V.; de Oliveira Puel, E.; Nunes, E.A. Effects of mate tea consumption on muscle strength and oxidative stress markers after eccentric exercise. British Journal of Nutrition 2016, 115, 1370–1378. [Google Scholar] [CrossRef]
- Krolikowski, T.C.; Borszcz, F.K.; Panza, V.P.; Bevilacqua, L.M.; Nichele, S.; da Silva, E.L.; Amboni, R.D.M.C.; Guglielmo, L.G.A.; Phillips, S.M.; de Lucas, R.D. , et al. The Impact of Pre-Exercise Carbohydrate Meal on the Effects of Yerba Mate Drink on Metabolism, Performance, and Antioxidant Status in Trained Male Cyclists. Sports Medicine - Open 2022, 8, 93. [Google Scholar] [CrossRef]
- Alkhatib, A. Yerba Maté (Illex Paraguariensis) ingestion augments fat oxidation and energy expenditure during exercise at various submaximal intensities. Nutrition & Metabolism 2014, 11, 42. [Google Scholar] [CrossRef]
- Gawron-Gzella, A.A.-O.; Chanaj-Kaczmarek, J.A.-O.; Cielecka-Piontek, J.A.-O. Yerba Mate-A Long but Current History. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ruíz-Moreno, C.; Lara, B.; Brito de Souza, D.; Gutiérrez-Hellín, J.; Romero-Moraleda, B.; Cuéllar-Rayo, Á.; Del Coso, J. Acute caffeine intake increases muscle oxygen saturation during a maximal incremental exercise test. British Journal of Clinical Pharmacology 2020, 86, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Bracco, D.; Ferrarra, J.M.; Arnaud, M.J.; Jequier, E.; Schutz, Y. Effects of caffeine on energy metabolism, heart rate, and methylxanthine metabolism in lean and obese women. American Journal of Physiology-Endocrinology and Metabolism 1995, 269, E671–E678. [Google Scholar] [CrossRef]
- Jo, E.; Lewis, K.L.; Higuera, D.; Hernandez, J.; Osmond, A.D.; Directo, D.J.; Wong, M. Dietary Caffeine and Polyphenol Supplementation Enhances Overall Metabolic Rate and Lipid Oxidation at Rest and After a Bout of Sprint Interval Exercise. The Journal of Strength & Conditioning Research 2016, 30. [Google Scholar]
- Velickovic, K.; Wayne, D.; Leija, H.A.L.; Bloor, I.; Morris, D.E.; Law, J.; Budge, H.; Sacks, H.; Symonds, M.E.; Sottile, V. Caffeine exposure induces browning features in adipose tissue in vitro and in vivo. Scientific Reports 2019, 9, 9104. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, A.; Heeren, J. Adipose tissue browning and metabolic health. Nature Reviews Endocrinology 2014, 10, 24–36. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life sciences 2016, 148, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Rotariu, D.; Babes, E.E.; Tit, D.M.; Moisi, M.; Bustea, C.; Stoicescu, M.; Radu, A.-F.; Vesa, C.M.; Behl, T.; Bungau, A.F. Oxidative stress–Complex pathological issues concerning the hallmark of cardiovascular and metabolic disorders. Biomedicine & Pharmacotherapy 2022, 152, 113238. [Google Scholar]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria Journal of Medicine 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Biswas, S.K.; Rahman, I. Environmental toxicity, redox signaling and lung inflammation: the role of glutathione. 2010. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.; Hansen, J.M. Oxidative stress, thiols, and redox profiles. Developmental Toxicology: Methods and Protocols 2012, 325–346. [Google Scholar]
- Nogueira Silva Lima, M.T.; Boulanger, E.; Tessier, F.J.; Takahashi, J.A. Hibiscus, rooibos, and yerba mate for healthy aging: A review on the attenuation of in vitro and in vivo markers related to oxidative stress, glycoxidation, and neurodegeneration. Foods 2022, 11, 1676. [Google Scholar] [CrossRef]
- Lu, S.C. Dysregulation of glutathione synthesis in liver disease. Liver Research 2020, 4, 64–73. [Google Scholar] [CrossRef]
- Ballatori, N.; Krance Sm Fau - Notenboom, S.; Notenboom S Fau - Shi, S.; Shi S Fau - Tieu, K.; Tieu K Fau - Hammond, C.L.; Hammond, C.L. Glutathione dysregulation and the etiology and progression of human diseases. 2009. [Google Scholar] [CrossRef]
- Ulbrich, N.C.M.; do Prado, L.L.; Barbosa, J.Z.; Araujo, E.M.; Poggere, G.; Motta, A.C.V.; Prior, S.A.; Magri, E.; Young, S.D.; Broadley, M.R. Multi-elemental Analysis and Health Risk Assessment of Commercial Yerba Mate from Brazil. Biological Trace Element Research 2022, 200, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




