Submitted:
11 September 2023
Posted:
13 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental field management and measurement of agronomic traits
2.3. Extraction of total genomic DNA from rice rhizosphere
2.4. Constriction of standard DNA for generating standard curves
2.5. Methanogen and methanotroph quantification
2.6. Statistical analysis
3. Results and Discussion
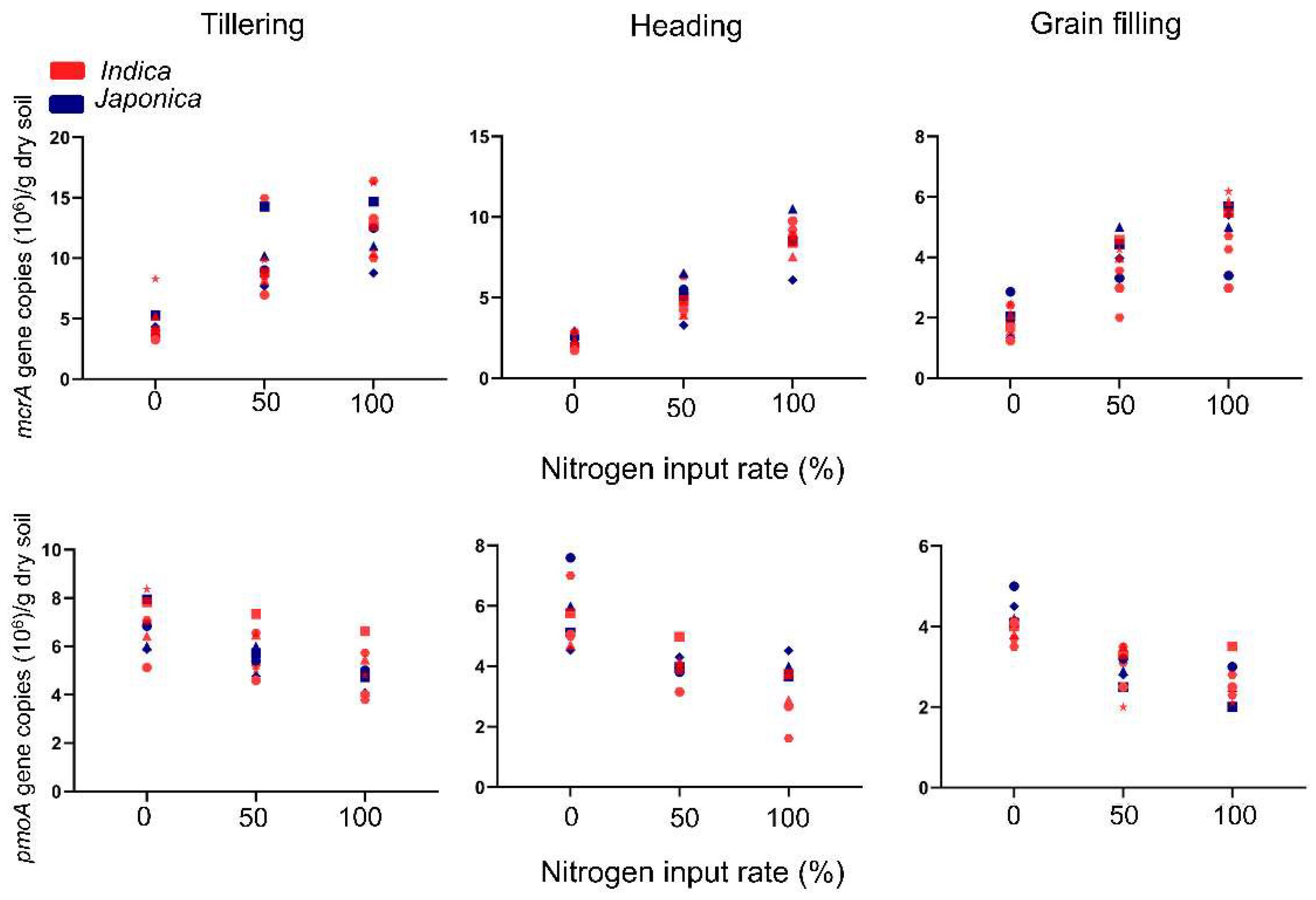
3.1. Methanogen and methanotroph abundance under different nitrogen levels and rice growth stages
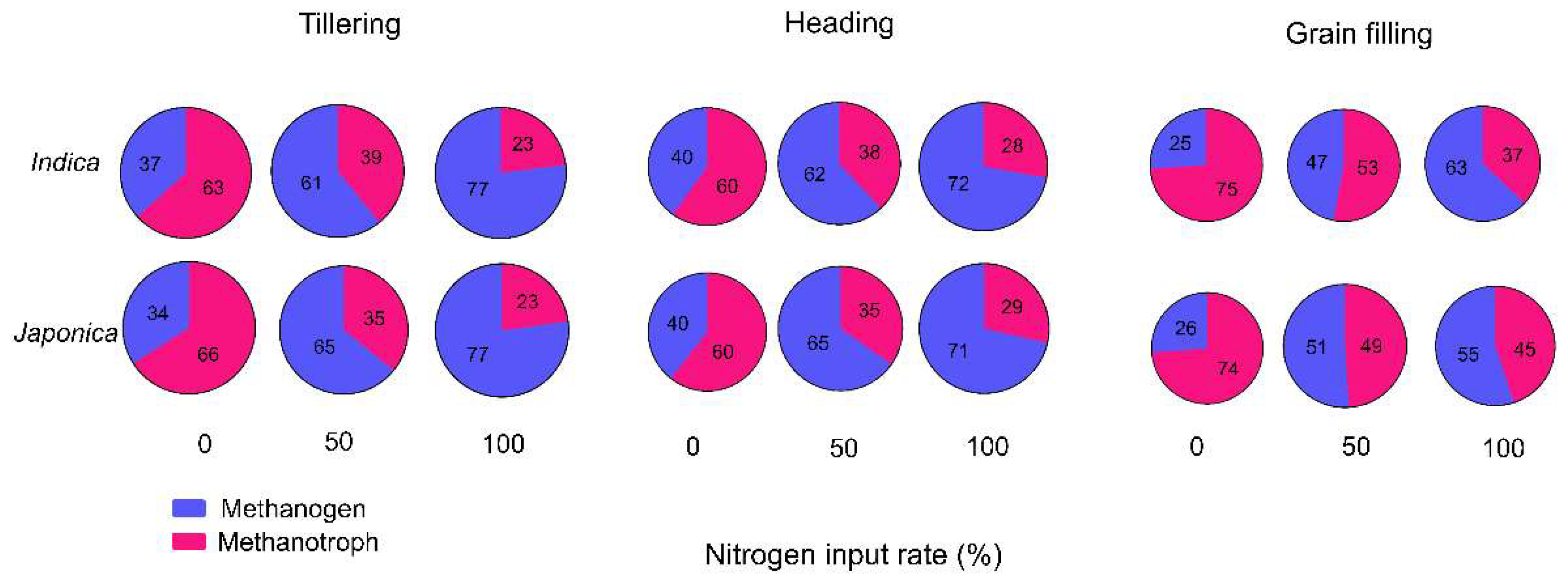
3.2. Ratio of methanogens/methanotrophs under different nitrogen levels and rice growth stages
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, S.; Jaffé, P.R.; Mauzerall, D.L. A process-based model for methane emission from flooded rice paddy systems. Ecol Modell 2007, 205, 475–491. [Google Scholar] [CrossRef]
- Change, I.P.O.C. The physical science basis. Agenda. Clim Change 2007, 6, 333. [Google Scholar]
- Solomon, S. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change 2007. (No Title) 2007. [Google Scholar]
- Wang, Z.; Xu, Y.; Li, Z.; Guo, Y.; Wassmann, R.; Neue, H.; Lantin, R.; Buendia, L.; Ding, Y.; Wang, Z. Methane emissions from irrigated rice fields in northern China (Beijing). Nutr Cycl Agroecosyst this issue. 2000. [Google Scholar]
- Win, K.T.; Nonaka, R.; Win, A.T.; Sasada, Y.; Toyota, K.; Motobayashi, T.; Hosomi, M. Comparison of methanotrophic bacteria, methane oxidation activity, and methane emission in rice fields fertilized with anaerobically digested slurry between a fodder rice and a normal rice variety. Paddy Water Environ 2012, 10, 281–289. [Google Scholar] [CrossRef]
- Bodelier, P.L.; Roslev, P.; Henckel, T.; Frenzel, P. Stimulation by ammonium-based fertilizers of methane oxidation in soil around rice roots. Nature 2000, 403, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Liesack, W.; Schnell, S.; Revsbech, N.P. Microbiology of flooded rice paddies. FEMS Microbiol Rev 2000, 24, 625–645. [Google Scholar] [CrossRef] [PubMed]
- Neue, H.-U. Methane emission from rice fields. J Bio Sci 1993, 43, 466–474. [Google Scholar] [CrossRef]
- Sigren, L.K.; Byrd, G.T.; Fisher, F.M.; Sass, R.L. Comparison of soil acetate concentrations and methane producton, transport, and emission in two rice cultivars. Global Biogeochem Cycles 1997, 11, 1–14. [Google Scholar] [CrossRef]
- Gogoi, N.; Baruah, K.K.; Gupta, P.K. Selection of rice genotypes for lower methane emission. Agron Sustain Dev 2008, 28, 181–186. [Google Scholar] [CrossRef]
- Linquist, B.A.; Marcos, M.; Adviento-Borbe, M.A.; Anders, M.; Harrell, D.; Linscombe, S.; Reba, M.L.; Runkle, B.R.K.; Tarpley, L.; Thomson, A. Greenhouse gas emissions and management practices that affect emissions in US rice systems. J Environ Qual 2018, 47, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.B.; Anders, M.; Adviento-Borbe, M.A.; van Kessel, C.; McClung, A.; Linquist, B.A. Seasonal methane and nitrous oxide emissions of several rice cultivars in direct-seeded systems. J Environ Qual 2015, 44, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; van Groenigen, K.J.; Huang, S.; Hungate, B.A.; van Kessel, C.; Hu, S.; Zhang, J.; Wu, L.; Yan, X.; Wang, L.; et al. Higher yields and lower methane emissions with new rice cultivars. Glob Chang Biol 2017, 23, 4728–4738. [Google Scholar] [CrossRef] [PubMed]
- Liechty, Z.; Santos-Medellín, C.; Edwards, J.; Nguyen, B.; Mikhail, D.; Eason, S.; Phillips, G.; Sundaresan, V. Comparative analysis of root microbiomes of rice cultivars with high and low methane emissions reveals differences in abundance of methanogenic archaea and putative upstream fermenters. mSystems 2020, 5, 00897–00819. [Google Scholar] [CrossRef]
- Ma, K.; Qiu, Q.; Lu, Y. Microbial mechanism for rice variety control on methane emission from rice field soil. Glob Change Biol 2010, 16, no. [Google Scholar] [CrossRef]
- Jiang, Y.; Qian, H.; Huang, S.; Zhang, X.; Wang, L.; Zhang, L.; Shen, M.; Xiao, X.; Chen, F.; Zhang, H.; et al. Acclimation of methane emissions from rice paddy fields to straw addition. Sci Adv 2019, 5, eaau9038. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, S.Y.; Kim, P.J.; Madsen, E.L.; Jeon, C.O. Methane emission and dynamics of methanotrophic and methanogenic communities in a flooded rice field ecosystem. FEMS Microbiol Ecol 2014, 88, 195–212. [Google Scholar] [CrossRef]
- Lee, H.J.; Jeong, S.E.; Kim, P.J.; Madsen, E.L.; Jeon, C.O. High resolution depth distribution of Bacteria, Archaea, methanotrophs, and methanogens in the bulk and rhizosphere soils of a flooded rice paddy. Front Microbiol 2015, 6, 639. [Google Scholar] [CrossRef]
- Ma, K.; Conrad, R.; Lu, Y. Responses of methanogen mcrA genes and their transcripts to an alternate dry/wet cycle of paddy field soil. Appl Environ Microbiol 2012, 78, 445–454. [Google Scholar] [CrossRef]
- Aulakh, M.S.; Wassmann, R.; Bueno, C.; Rennenberg, H. Impact of root exudates of different cultivars and plant development stages of rice (Oryza sativa L.) on methane production in a paddy soil. Plant Soil 2001, 230, 77–86. [Google Scholar] [CrossRef]
- Denier van Der Gon, H.A.; Kropff, M.J.; Van Breemen, N.; Wassmann, R.; Lantin, R.S.; Aduna, E.; Corton, T.M.; Van Laar, H.H. Optimizing grain yields reduces CH4 emissions from rice paddy fields. Proc Natl Acad Sci U S A 2002, 99, 12021–12024. [Google Scholar] [CrossRef]
- Inubushi, K.; Cheng, W.; Aonuma, S.; Hoque, M.M.; Kobayashi, K.; Miura, S.; Kim, H.Y.; Okada, M. Effects of free-air CO2 enrichment (FACE) on CH4 emission from a rice paddy field. Glob Change Biol 2003, 9, 1458–1464. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Papen, H.; Rennenberg, H. Impact of gas transport through rice cultivars on methane emission from rice paddy fields. Plant Cell Environ 1997, 20, 1175–1183. [Google Scholar] [CrossRef]
- Setyanto, P.; Makarim, A.K.; Fagi, A.M.; Wassmann, R.; Buendia, L.V. Crop management affecting methane emissions from irrigated and rainfed rice in Central Java (Indonesia). Nutr Cycl Agroecosystems 2000, 58, 85–93. [Google Scholar] [CrossRef]
- Wassmann, R.; Aulakh, M.S. The role of rice plants in regulating mechanisms of methane missions. Biol Fertil Soils 2000, 31, 20–29. [Google Scholar] [CrossRef]
- Alvarenga, P.; Mourinha, C.; Palma, P.; Cruz, N.; Rodrigues, S.M. Assessment of soil physicochemical characteristics and as, Cu, Pb and Zn contamination in non-active mines at the Portuguese sector of the Iberian pyrite belt. Environments 2022, 9, 105. [Google Scholar] [CrossRef]
- Luton, P.E.; Wayne, J.M.; Sharp, R.J.; Riley, P.W. The mcrA gene as an alternative to 16S rRNA in the phylogenetic analysis of methanogen populations in landfill. Microbiology (Reading) 2002, 148, 3521–3530. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.J.; Costello, A.; Lidstrom, M.E.; Murrell, J.C. Evidence that particulate methane monooxygenase and ammonia monooxygenase may be evolutionarily related. FEMS Microbiol Lett 1995, 132, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Costello, A.M.; Lidstrom, M.E. Molecular characterization of functional and phylogenetic genes from natural populations of methanotrophs in lake sediments. Appl Environ Microbiol 1999, 65, 5066–5074. [Google Scholar] [CrossRef]
- Gutierrez, J.; Kim, S.Y.; Kim, P.J. Effect of rice cultivar on CH4 emissions and productivity in Korean paddy soil. Field Crops Res 2013, 146, 16–24. [Google Scholar] [CrossRef]
- Kim, G.W.; Gutierrez-Suson, J.; Kim, P.J. Optimum N rate for grain yield coincides with minimum greenhouse gas intensity in flooded rice fields. Field Crops Res 2019, 237, 23–31. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
| Ratio (%) | Tillering | Heading | Grain-filling | |||||||
| 0% | 50% | 100% | 0% | 50% | 100% | 0% | 50% | 100% | ||
| Indica | Hanareum4 | 30 | 63 | 80 | 39 | 60 | 77 | 20 | 49 | 53 |
| Geumgang1 | 33 | 60 | 71 | 32 | 55 | 66 | 23 | 48 | 59 | |
| Milyang392 | 38 | 53 | 75 | 45 | 56 | 66 | 31 | 49 | 67 | |
| IR72 | 45 | 56 | 76 | 36 | 56 | 64 | 33 | 48 | 61 | |
| 93-11 | 40 | 66 | 81 | 50 | 66 | 77 | 33 | 51 | 63 | |
| IR64 | 33 | 67 | 80 | 33 | 74 | 81 | 17 | 34 | 74 | |
| Japonica | Saeilmi | 33 | 63 | 74 | 34 | 63 | 71 | 27 | 47 | 48 |
| Sobi | 32 | 67 | 81 | 40 | 71 | 76 | 29 | 53 | 61 | |
| Nampyeong | 41 | 69 | 81 | 42 | 63 | 69 | 25 | 56 | 56 | |
| Misojinmi | 29 | 54 | 71 | 42 | 62 | 68 | 23 | 47 | 54 | |
| Average | 35 | 62 | 77 | 39 | 63 | 71 | 26 | 48 | 60 | |
| Two-way ANOVA p-value |
Tillering | Heading | Grain-filling |
| Variety (V) | 0.0369 | 0.0428 | 0.0358 |
| Subspecies (S) | 0.5875 | 0.3642 | 0.3991 |
| Nitrogen level (N) | 0.0087 | 0.0151 | 0.0058 |
| S × N | 0.0344 | 0.0403 | 0.0378 |
| V × N | 0.0174 | 0.0235 | 0.0155 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





