Preprint
Article
Atherogenic Index of Plasma Predicts Obstructive Coronary Artery Disease in Patients with Stable Angina Pectoris
Altmetrics
Downloads
113
Views
49
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
13 September 2023
Posted:
13 September 2023
You are already at the latest version
Alerts
Abstract
Aims: Chronic coronary syndrome is associated with several risk factors, such as dyslipidemia and hypertension. AIP has been demonstrated to be a biochemical risk factor for coronary artery disease (CAD). This study aimed to determine whether AIP is an effective parameter for estimating obstructive CAD.
Methods and Results: A total of 345 patients (mean age 62,2±10,3; 63% male) who underwent coronary angiography were included in this study. Obstructive CAD is defined as having one or more vessels with a stenosis of≥50%. Depending on the presence of obstructive CAD, all patients were divided into two groups.
The AIP was significantly higher in the obstructive coronary artery group (AIP; 0,49±0,26 vs. 0,58±0,27, p=0,002). In univariate analysis, AIP was significantly associated with obstructive coronary artery disease [OD:3,74 (CI95% 1,62-8,64), P=0,020]. The AIP was further adjusted for confounding risk factors in three multivariate analysis models. Therefore, all three models showed a significant association. According to ROC analysis, 0.49 is the cut-off value for AIP, and a value above 0.49 indicates 50% coronary artery stenosis
Conclusions: AIP may be used in the assessment of cardiovascular risk in patients with stable angina pectoris, and it may also be used to estimate obstructive CAD.
Keywords:
Subject: Medicine and Pharmacology - Cardiac and Cardiovascular Systems
1. Introduction
Coronary Artery Disease (CAD) also named Ischemic Heart Disease is one of the most common causes of mortality and morbidity worldwide [1]. Atherosclerotic plaques in the coronary arteries are the main feature of the disease. CAD can be acute or chronic according to its onset and duration and categorized as either acute coronary syndromes (ACS) or chronic coronary syndromes (CCS) [2,3]. The most common clinical forms of CCS are stable angina and/or dyspnea, newly onset heart failure, and/or left ventricular dysfunction. Additionally, asymptomatic and symptomatic patients who had ACS within one year or patients with recent revascularization in one year and/or > 1-year revascularization. Moreover, patients suspected of having vasospastic or microvascular disease as well as those with CAD who are detected at screening comprise the other forms of CCS [3].
CCS risk factors including a family history of CVD, dyslipidemia, metabolic syndrome, diabetes, hypertension, and smoking are well established in recent guidelines, and risk stratifications for CAD have been created using those factors [4]. Particularly, dyslipidemia has been highly investigated and thought that lipid-lowering treatment was the main risk modifier for CAD. Dyslipidaemia is identified as an increase in low-density lipoprotein cholesterol (LDL-C), total cholesterol, and triglyceride (TG) and a reduction in high-density lipoprotein cholesterol (HDL-C). Recent guidelines focus on the LDL-C lowering treatment to decrease CAD risk [4,5]. Nevertheless, about half of the residual cardiovascular risks continued even though the LDL-C was decreased to its recommended level. The atherogenic index of plasma (AIP) has been demonstrated as a new biochemical risk factor for CAD development as a result of several laboratory findings [6]. By using the formula log (TG/HDL-C), AIP is a measure of plasma atherogenicity based on a positive correlation between cholesterol esterification rates, remnant lipoproteinemia, and lipoprotein particle size. AIP is a powerful predictor of atherosclerosis and coronary heart disease, as well as accurately representing the link between protective and atherogenic lipoproteins. [7].
In this study, we investigated whether AIP could predict CAD in patients with stable angina pectoris. In addition, we sought to determine the AIP as a non-invasive parameter for estimating obstructive coronary artery disease.
2. Materials and Methods
Patient Data. A total of three hundred nighty-four patients suspicious of CAD and underwent coronary angiography were retrospectively reviewed. Three hundred forty-five of those who had coronary angiography images and laboratory results were included into our study. The medical histories, including all clinical and demographic data including CAD risk factors such as hypertension (HT), diabetes mellitus(DM), dyslipidemia, smoking, family history, and body mass index were obtained from the electronic medical records. None of the patients had established CAD or ACS before. Two hundred forty-seven of the patients had stable angina with typical symptoms that refractory to medical treatment and ninety-eight of whom has atypical symptoms had performed coronary angiography. Laboratory results were received within 24 hrs before coronary angiography. Patients with acute coronary syndrome, unstable coronary artery disease, previous coronary stent implantation and established coronary artery disease, severe valvular diseases, malignancies, cardiomyopathies, thyroid diseases, and familial hypercholesterolemia were excluded from our study. The study protocol was reviewed and approved by the ethical committee.
Coronary Angiography: Coronary angiography was performed in accordance with the standard procedure by an interventional cardiologist. We used femoral artery or radial artery access and obtained standard left anterior oblique (LAO) and right anterior oblique (RAO) projections with cranial and caudal angulations for the assessment of the left coronary arteries and LAO and RAO projections for the assessment of the right coronary artery [8,9].
QCA Calculation: QCA was performed according to a standard protocol, in which the maximum stenosis was determined from two orthogonal views. The angiograms were read by two blinded expert interventional cardiologists. The angiograms were carefully evaluated and QCAs were measured on any possible obstructions by the primary reader. A secondary reader ensured the quality and accuracy of the QCA. A patient with obstructive CAD is defined as having a stenosis of 50% or greater in one or more vessels [10,11].
AIP calculation: Triglyceride (TG) and High-density lipoprotein levels were obtained from laboratory results which were held before 24 hrs. of a coronary angiogram. The AIP is a logarithmically converted ratio of TG to HDL-C in molar concentration (millimole per liter). AIP was calculated as a log10 (TG/HDLC) [12].
Statistical Analysis
Baseline characteristics are presented as the mean ± SD for continuous variables and were compared using Student’s t-test, or percentages for categorical variable differences were compared using the chi-square test. A p-value < 0.05 was defined as statistically significant. Univariate and multivariate analyses based on the logistic regression model were performed to identify the relationship between cardiovascular risk factors and coronary artery stenosis. In order to avoid multicollinearity, parameters with a strong correlation with AIP (TG, HDL-C r > 0,7) did not enter into the multivariable analysis. Other variables in the univariable analysis were entered into the multivariable analysis. Parameters with p-value < 0,05 in multivariable analysis were defined as predictors of coronary artery stenosis. Using the receiver operating characteristic (ROC) curves based on the logistic regression method a cutoff value of AIP was also found. All data were analyzed using IBM SPSS Statistics version 26 (SPSS Inc., Chicago, Illinois)
3. Results
Baseline characteristics. Three hundred forty-five patients who underwent coronary angiography with suspicion of CCS (mean age 62,2±10,3 63% male) were included in the study. A diagnosis of obstructive coronary artery disease is defined as 50% or greater stenosis in at least one coronary artery. Having one or more vessels with a stenosis of 50% or greater was defined as obstructive coronary artery disease and all patients were divided into two groups based on having obstructive coronary artery disease or not. The ≥ 50% group (190 patients; 55%) was defined as the obstructive coronary artery group, and the < 50% group (155 patients, 45%) was defined as the non-obstructive coronary artery group. Among those with obstructive coronary arteries, 32 patients had left main coronary artery (LMCA) stenosis, 154 patients had left anterior descending artery [13] stenosis, 112 patients had circumflex artery (CX) stenosis and 126 patients had right coronary artery stenosis [8]. Additionally, 36% (n=124) of the patients in the obstructive coronary artery group had multi-vessel coronary artery stenosis. Among the groups, we compared demographic and clinical characteristics, laboratory results, and medication use (Table 1). The obstructive coronary artery group was older (age; 60,6±10,5 vs. 63,2±10,1, p=0,002) and a higher percentage of males were found [male 85 (55,5%) vs. 152 (80%), p<0,0001]. There were no differences in medication use. Similarly, laboratory results (obtained within 24 hrs. prior to coronary angiography), including complete blood count, creatine, glomerular filtration ratio (GFR), and fasting plasma glucose(FPG) levels were also similar between groups. Further, low-density cholesterol level (LDL-C) (LDL-C; 114.1±35.9 vs. 108,8±4,8, p=0.2) and total cholesterol (TC) (TC; 180.8±41.3 vs. 186,8±48,9, p=0,23) were not different between the two groups. In contrast, high-density cholesterol level (HDL-C) (HDL-C; 43,4±11,3 vs. 40,4±11,4, p=0.01) was significantly lower, and TG (TG; 147,0±78,5 vs. 168,7±100,9, p=0.03) was significantly higher in the group with obstructive coronary arteries. The risk factors for coronary artery disease, such as diabetes mellitus, and family history, were similar between the two groups, with the exception of HT, which was significantly more prevalent in the group with obstructive coronary artery disease [HT; 78 (50,3%) vs. 120 (63,2%), p=0.02].
Atherogenic Index of Plasma: The AIP calculated based on log (TG/HDL-C) was significantly higher in the obstructive coronary artery group (AIP; 0,49±0,26 vs. 0,58±0,27, p=0,002) (Table 1).
Univariable and multivariable analyses of parameters associated with ≥50% coronary artery stenosis. We entered the parameters in Table 1 with a p-value of <0.05 into the univariable and multivariable logistic regression models to identify how these variables are associated with obstructive coronary artery disease. Due to the strong correlation (r>0,7) between the TG, HDL-C, and AIP we did not include them in multivariable analysis.
In order to evaluate their relationship with coronary artery occlusion, variables with p < 0.05 in Table 1 (age, gender, BMI, HT,TG, HDL-C, AIP) were included in the univariable analysis. In univariable modeling (Table 2) there were significant relationships between AIP and obstructive coronary artery disease [OD:3.74 (CI95% 1.62-8.64), P=0.002]. In addition, age [OD:1.02 (CI95% 1-1.04), P=0.04] and gender [OD:3,2 (CI95% 1.99-5.16), P<0.000] were also predictors of obstructive coronary artery disease. Having hypertension [OD:1.69 (CI95% 1.09-2.6), P=0,02] and an increased Body mass index (BMI kg/cm2) [OD:1.15 (CI95% 1.0-1.33), P=0,04] were highly associated with coronary artery stenosis. Furthermore, higher TG levels [OD:1.31 (CI95% 1.02-1.7), P=0.03] and lover HDL-C levels [OD:0.98 (CI95% 0.96-1.0), P=0.02] which are the parameters used to calculate AIP were predictors of obstructive coronary artery disease (Table 2).
In the univariate analysis, all parameters were individually associated with coronary artery occlusion. Therefore, each parameter was entered one by one into the multivariate analysis in order to determine the most accurate predictor of coronary artery occlusion. There was a significant correlation between AIP and coronary artery occlusion as classic risk factors for coronary artery disease. Consequently, the AIP was adjusted for confounding risk factors in a multivariable analysis in order to evaluate its impact on coronary artery occlusions. Each classical risk factor has been added to the multivariable analysis one by one. Following this, we developed three separate models to analyze the relationship between AIP and coronary artery occlusion (Table 3). Model 1 was adjusted for age and gender and found a significant association between AIP and obstructive coronary artery disease [OD:3,39 (CI95% 1,41-8,13) P=0,003]. Based on the model 1 results, age [OD:1,0 (CI95% 1.01-1.06), P=0,0001] and gender [OD:3.53 (CI95% 2.1-5.9), P=0,0000] were also independent predictors of coronary artery occlusion. Model 2 was adjusted for BMI in addition to Model 1, and Model 3 was adjusted for HT in addition to the confounders of Model 2. The association of AIP with coronary artery occlusion remained significant under Model 2 [OD:3,34 (CI95% 1,37-8,13) P=0,008]. Furthermore, although age [OD:1.04 (CI95% 1.02-1.06), P<0,001] and gender [OD:3.48 (CI95% 2.09-5.81), P<0,0000] were found to be independent predictors of coronary artery occlusion, BMI [OD:1.16 (CI95% 0.99-1.35), P=0,06] was not associated with coronary artery occlusion in the multivariable analysis of Model 2.
Additionally, in Model 3, AIP was highly correlated with coronary artery occlusion [OD:3,07 (CI95% 1,25-7,54) P=0,01] Moreover, age [OD:1.03 (CI95% 1.01-1.06), P=0,006] and gender [OD:3.6 (CI95% 2.14-5.98), P<0,000] were strongly correlated with coronary artery occlusion, whereas HT [OD:1.50 (CI95% 0.93-2.44), P=0,1] and BMI [OD:1.15 (CI95% 0.99-1.35), P=0,06] did not correlate with coronary artery occlusion in Model 3.As a result of adjusting for classical risk factors separately in all three models, AIP has been identified as a significant predictor of coronary artery occlusion.
ROC Analysis
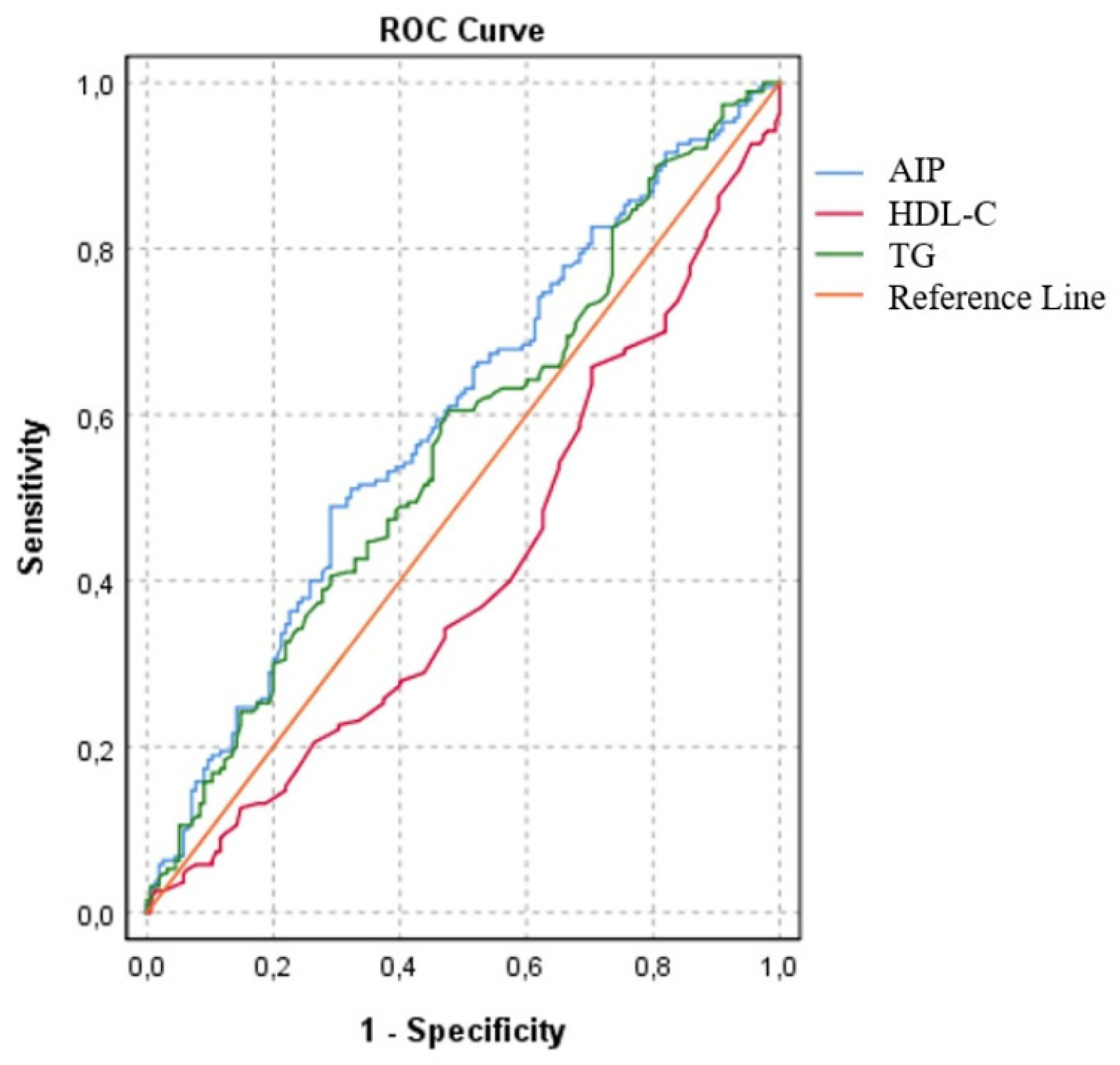
In the present study, we investigated the individual effects of the parameters used to calculate AIP and found that HDL-C had lower accuracy [AUC: 0,41 (CI 95%: 0,35-0,47; p=0.06), specificity 52%, and sensitivity 37%] than TG [AUC: 0,56(CI 95%: 0,50-0,62; p=0,04), specificity 55%, and sensitivity 54%. Although TG and HDL-C were used to calculate AIP, it was found that AIP had a higher accuracy for estimating obstructive coronary artery disease [AUC: 0,60; CI 95%: 0,53-0,65; p=0,002), specificity 51% and sensitivity 62% The cut-off value for AIP were 0.49, and an AIP above 0.49 was estimated to have 50% coronary artery stenosis (figure 1).
Figure 1.
Receiver operating curves of estimated obstructive coronary artery disease. Cut off point of 0,49% for AIP AUC = 0,60; (CI 95%: 0,53-0,65); p=0,002.
Figure 1.
Receiver operating curves of estimated obstructive coronary artery disease. Cut off point of 0,49% for AIP AUC = 0,60; (CI 95%: 0,53-0,65); p=0,002.
4. Discussion
The results of this study confirmed that higher TG levels and lower HDL-C levels were significantly associated with coronary artery disease. The major finding of this study was that AIP levels [the log of TG/HDL-C] increased in patients with CAD. Further, the AIP was found to be a significant predictor of CAD even after adjusting for confounding risk factors. In addition, AIP showed a better correlation with CAD than the parameters that were used in its formula (HLD-C and TC).
Cardiovascular disease still represents the greatest burden of disease, with its high mortality and morbidity rates [1,2] In this regard, cardiac risk stratification is essential for improving preventive and therapeutic measures [4]. The main cause and modifiable risk factors of atherosclerosis are blood apolipoprotein-B-containing lipoproteins, high blood pressure, smoking, adiposity, and diabetes mellitus. Additionally, there are a number of other relevant risk factors and clinical conditions such as gender, age, and ethnicity. These factors are taken into account when estimating an individual's cardiovascular risk [14]. According to previous European Society of Cardiology (ESC) guidelines, the Systematic Coronary Risk Estimation (SCORE) method was developed and updated in the 2021 guidelines to evaluate 10 years of CVD risk estimation based on several risk factors mentioned above [14,15]. In estimating CVD risk, LDL-C is one of the most commonly used and emphasized variables, so LDL-C lowering treatments are typically designed primarily to prevent CVD [5].
In the presence of endothelial dysfunction, small TG-rich lipoproteins and their remnant particles, known as ApoB-containing lipoproteins, have a tendency to cross the endothelial barrier, resulting in lipid deposition and atherosclerosis development. The SCORE risk algorithm utilizes non-high-density lipoprotein cholesterol (HDL-C) value to estimate the coronary heart disease risk, which includes all atherogenic lipoproteins (apo-B contained) as LDL-C and TG, and is calculated as Non-HDL-C=TC - HDL-C. Non-HDL-C identifies the apo-B-containing proteins which are highly associated with atheroma formation. Therefore, elevated plasma TG levels indicate an increase in ApoB-containing proteins and, consequently, an increase in the risk of atherosclerotic cardiovascular disease (ASCVD) [5,14]. As a result of this information, TG levels can be evaluated as well as plasma LDL levels, in order to determine whether the lipid-lowering treatment reduces the risk of atherogenicity. Therefore, the treatment can be adjusted by taking into account the TG value in addition to the LDL-C value. Our study found that although the LDL-C levels were similar between the two groups of patients, the TG levels were significantly higher and the HDL-C levels were significantly lower in the patients with obstructive coronary artery disease. Furthermore, the level of AIP was significantly elevated in the group with obstructive coronary artery disease. Based on this information, it can be concluded that LDL-lowering treatments should be regulated in accordance with both TG and LDL levels in order to achieve optimum results.
Several biomarkers associated with CAD have been identified over the past few years, including inflammatory biomarkers such as C-reactive protein [16] and fibrinogen [17] and lipid-related biomarkers such as lipoprotein-associated phospholipase A2 [18] and lipoprotein A [19]. Nevertheless, recent guidelines do not recommend the use of biomarkers in risk stratification since they could result in confusion in risk assessment. In the recent guideline ApoB analysis is recommended as a tool for risk assessment, particularly in patients with high TG levels, diabetes, obesity, metabolic syndrome, or very low LDL-C levels. According to the 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk, ApoB measurement is recommended as a class I indication instead of LDL-C for primary screening, diagnosis, and management. Furthermore, ApoB may be used instead of non-HDL-C in individuals with high TG, diabetes, obesity, or very low LDL-C levels. We believe that using easy-to-measure lipid biomarkers may provide useful information about the levels of ApoB-containing lipoproteins in assessing CVD risk. Atherogenic index of plasma (AIP) is a novel biomarker that includes a logarithmically transformed ratio of triglycerides to high-density lipoprotein (HDL)-cholesterol in molar concentrations [20]. Additionally, AIP can be calculated using only a standard lipid profile, making it an easily accessible biomarker [21]. Despite the lack of widespread availability and cost-effectiveness of ApoB measurement, AIP measurement is an easy and inexpensive method, and it is not accompanied by additional costs in addition to cholesterol measurement. Due to the fact that AIP is calculated using TG, which is an ApoB-containing lipoprotein, it may indirectly provide information regarding the amount of ApoB. We believe that using the AIP along with the LDL-C value may be an effective method for estimating ApoB.
It is well known that the low HDL level, a component of AIP, is known as an independent risk factor for coronary artery disease. Moreover, as a biomarker, HDL-C is an effective tool for refining SCORE2 risk estimation. Additionally, TG, an ApoB-containing lipoprotein that is highly associated with ASCVD mentioned above, is another component of AIP. Moreover, one of the major benefits of AIP is the combination of hypertriglyceridemia and low HDL levels as independent markers of coronary artery disease in order to enhance their predictive ability [4,6,21,22]. As a matter of fact, AIP's correlation with lipoprotein particle size is most likely to explain the nature of the relationship between AIP and CVD incidence [21]. There is an inverse relationship between the diameter of LDL-C and the amount of AIP, which is a substitute for minute-dense LDL particles. Thus, an increase in AIP indicates that oxidized particles are more likely to produce foamy cells, resulting in an increase in LDL-C and oxidized apoprotein B combinations, which have been shown to be highly atherogenic. The overexpression of adhesion molecules and the activation of oxygen radicals have been directly linked to endothelial dysfunction due to the promotion of lipid peroxidation by AIP [22]. Furthermore, HDL-C is a component of AIP, which transports cholesterol from peripheral tissues to the liver and contains antioxidant enzymes [23]. Clinical studies have confirmed these theoretical findings by demonstrating a strong association between AIP and carotid artery intima-media thickness [23], arterial stiffness [24], and coronary artery calcification [25,26].
Generally, metabolic syndrome (MetS) is defined as a group of cardiometabolic risk factors (CMR). There is no doubt that MetS is a leading cause of type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD), which are still among the leading causes of morbidity and mortality, as well as some of the most prevalent healthcare concerns. Recently, numerous studies have concluded that certain CMR factors have a positive relationship with the atherogenic index of plasma (AIP) [27-29]. Moreover, studies have shown that AIP values below 0.11 were associated with low CVD risk, whereas values between 0.11 and 0.21 as well as greater than 0.21 were associated with intermediate and greater CVD risk, respectively [28,29]. An analysis of 32 articles revealed that high waist circumference (WC), triglycerides (TG), insulin resistance (IR), and low high-density lipoprotein cholesterol (HDLC) concentrations were strongly correlated with increased AIP. There were a few studies that examined blood pressure (BP), and the results were inconsistent [30]. Based on these studies, it can be concluded that AIP is associated with WC, TG, IR, and HDL-C. There is no clear evidence linking AIP to blood pressure. Due to the retrospective nature of our study, we were not able to obtain WC and IR values for the patients; however, the BMI values of patients with obstructive coronary artery disease were higher. In spite of the fact that the BMI value does not meet the definition of obesity, the increased AIP value and BMI value in this group support the findings of the study. Considering all of the findings presented above, it is reasonable to conclude that an increased AIP value indicates an increased risk for metabolic syndrome among patients, which eventually causes an increase in the risk of cardiovascular disease (CVD).
A number of studies have found a link between AIP and the progression of patients with acute coronary syndromes and myocardial infarctions. A Turkish study has demonstrated that AIP is independently associated with no reflow following primary percutaneous coronary intervention in patients with ST-elevated myocardial infarctions [31]. According to another study, patients after myocardial infarction with lower AIP values (0.24%) exhibited almost four times higher hospital mortality than those with higher AIP values [32]. Another study examining the relationship between acute coronary syndrome and AIP in patients under 35 years of age found AIP to be independently associated with the presence and severity of coronary artery disease in young patients [33]. According to our study, there was a correlation between AIP and obstructive coronary artery disease. There were no ST-T changes detected in the electrocardiogram (ECG) and no troponin elevations were observed in the patients. Therefore, acute coronary syndrome was excluded. However, due to the lack of opportunity to analyse the plaque structure, plaque vulnerability could not be determined. The relationship between AIP and vulnerable plaque needs to be evaluated in further research.
In many studies, the relationship between AIP major cardiovascular events and prognosis has been investigated. For instance, the ACCORD study with a large-scale analysis demonstrated that the higher AIP levels were the independent predictor of survival in patients with Type 2 DM [34]. In addition, some studies have shown that higher AIP levels are highly associated with major cardiovascular events (MACEs) in patients with and without diabetes [11, 16, 21, 23]. Furthermore, Khosravi et al. found that AIP was an independent biomarker that can differentiate unstable from stable plaques with 89.70% sensitivity and 34% specificity [35]. AIP has also been found to be associated with CAD severity in some studies. For instance, Mangalesh et.al. found that AIP was highly associated with both MACE within 3 years and the severity of CAD in patients with established coronary artery disease detected by coronary computed tomography angiography. [36]. Moreover, Balci et. al. demonstrated that the increased plasma AIP levels was strongly associated with decreased FFR values in chronic coronary syndrome patients with intermediate coronary artery stenosis [37]. Another study with a large population noted a strong correlation between increased AIP and in-stent restenosis [38].According to these studies, coronary artery disease severity is generally assessed in patients who already have coronary artery disease. However, in our study, we sought to determine whether or not AIP could be used to detect obstructive coronary artery disease in patients with suspected chronic coronary syndromes. As a consequence, we were able to demonstrate a high association between AIP and obstructive coronary artery disease. Furthermore, the risk was further increased by higher levels of AIP. According to these findings, AIP may be an important marker for the early detection of this disease. Therefore, AIP may be used as a biomarker to investigate coronary artery stenosis in order to improve the efficiency of non-invasive imaging techniques. As a result of our findings, LDL-lowering treatments may be adjusted in accordance with the AIP cut-off value to minimize CVD risk by evaluating the decrease in AIP value, such as a decrease in LDL value, since AIP value is more closely correlated with the presence of obstructive coronary arteries than TG and HDL values in our study.
Study Limitation
There are several limitations to our study, including the fact that it was a single-center study of a retrospective nature. The second limitation was that we were unable to determine how the other risk factors, such as diabetes mellitus, hypertension, and smoking, affected the plaque burden because we did not know their onsets or durations of exposure. Furthermore, due to the retrospective nature of our study, we were unable to determine the duration of patient use of risk modifiers. Prospective studies with a larger number of patients will be essential to demonstrate the efficacy of AIP in the estimation of obstructive coronary artery disease.
5. Conclusion
Atherogenic Index of Plasma, which is calculated as log10 (TG-c/HDL-c), has been identified as a coronary artery risk factor in several studies. Moreover, a high level of AIP is associated with a poor prognosis in patients with coronary artery disease. This study confirmed that the AIP is an independent predictor of CAD in patients suspected of having CCS. We also observed that increased AIP levels are highly related to obstructive stenosis. Therefore, we believe that AIP can provide an additional contribution to the CVD risk algorithm. In addition, the reduction of AIP, including the lowering of LDL, may also be an important component of lipid-lowering therapies.
Author Contributions
Methodology, Haci Ali Kurklu and Emir Baskovski; Formal analysis, Emir Baskovski and Cagdas Ozdol; Investigation, Nil Ozyuncu; Data curation, Haci Ali Kurklu; Writing – original draft, Nil Ozyuncu; Writing – review & editing, Turkan Seda Tan; Supervision, Cagdas Ozdol. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Ankara University School of Medicine (protocol code İ6-460-21 and the date of approval 03.08.2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [TST], upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ASCVD | Atherosclerotic cardiovascular disease |
| ACS | Acute coronary syndrome |
| AUC | Area Under the curve |
| CAD | Coronary artery disease |
| CCS | Chronic coronary syndrome |
| CVD | Cardio vascular disease |
| DM | Diabetes mellitus |
| HT | Hypertension |
| HDL-C | Hight-density lipoprotein cholesterol |
| LDL-C | Low-density lipoprotein cholesterol |
| TG-C | Triglyceride cholesterol |
References
- Andersson, C.; Nayor, M.; Tsao, C.W.; Levy, D.; Vasan, R.S. Framingham Heart Study: JACC Focus Seminar, 1/8. J Am Coll Cardiol 2021, 77, 2680–2692. [Google Scholar] [CrossRef] [PubMed]
- Ralapanawa, U.; Sivakanesan, R. Epidemiology and the Magnitude of Coronary Artery Disease and Acute Coronary Syndrome: A Narrative Review. J Epidemiol Glob Health 2021, 11, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). European Heart Journal 2019, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Lu, Z.; Zhu, L.; Ouyang, X.; Yang, Y.; He, W.; Feng, Y.; Yi, F.; Song, Y. Lipoprotein ratios are better than conventional lipid parameters in predicting coronary heart disease in Chinese Han people. Kardiol Pol 2015, 73, 931–938. [Google Scholar] [CrossRef]
- Nwagha, U.I.; Ikekpeazu, E.J.; Ejezie, F.E.; Neboh, E.E.; Maduka, I.C. Atherogenic index of plasma as useful predictor of cardiovascular risk among postmenopausal women in Enugu, Nigeria. Afr Health Sci 2010, 10, 248–252. [Google Scholar]
- Bussani, R.; Castrichini, M.; Restivo, L.; Fabris, E.; Porcari, A.; Ferro, F.; Pivetta, A.; Korcova, R.; Cappelletto, C.; Manca, P.; et al. Cardiac Tumors: Diagnosis, Prognosis, and Treatment. Curr Cardiol Rep 2020, 22, 169. [Google Scholar] [CrossRef]
- Collet, C.; Grundeken, M.J.; Asano, T.; Onuma, Y.; Wijns, W.; Serruys, P.W. State of the art: coronary angiography. EuroIntervention 2017, 13, 634–643. [Google Scholar] [CrossRef]
- Brown, B.G.; Bolson, E.; Frimer, M.; Dodge, H.T. Quantitative coronary arteriography: estimation of dimensions, hemodynamic resistance, and atheroma mass of coronary artery lesions using the arteriogram and digital computation. Circulation 1977, 55, 329–337. [Google Scholar] [CrossRef]
- Shah, R.; Yow, E.; Jones, W.S.; Kohl, L.P., 3rd; Kosinski, A.S.; Hoffmann, U.; Lee, K.L.; Fordyce, C.B.; Mark, D.B.; Lowe, A.; et al. Comparison of visual assessment of coronary stenosis with independent quantitative coronary angiography: Findings from the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) trial. Am Heart J 2017, 184, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Zhou, K.; Li, Y.; Cheng, W.; Wang, Z.; Wang, J.; Gao, F.; Yang, L.; Xu, Y.; Wu, Y.; et al. The atherogenic index of plasma plays an important role in predicting the prognosis of type 2 diabetic subjects undergoing percutaneous coronary intervention: results from an observational cohort study in China. Cardiovasc Diabetol 2020, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Roşca, M.; Călin, A.; Beladan, C.C.; Enache, R.; Mateescu, A.D.; Gurzun, M.M.; Varga, P.; Băicuş, C.; Coman, I.M.; Jurcuţ, R.; et al. Right ventricular remodeling, its correlates, and its clinical impact in hypertrophic cardiomyopathy. J Am Soc Echocardiogr 2015, 28, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). European Heart Journal 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- Casas, J.P.; Shah, T.; Hingorani, A.D.; Danesh, J.; Pepys, M.B. C-reactive protein and coronary heart disease: a critical review. J Intern Med 2008, 264, 295–314. [Google Scholar] [CrossRef] [PubMed]
- Morange, P.E.; Bickel, C.; Nicaud, V.; Schnabel, R.; Rupprecht, H.J.; Peetz, D.; Lackner, K.J.; Cambien, F.; Blankenberg, S.; Tiret, L. Haemostatic factors and the risk of cardiovascular death in patients with coronary artery disease: the AtheroGene study. Arterioscler Thromb Vasc Biol 2006, 26, 2793–2799. [Google Scholar] [CrossRef] [PubMed]
- Khuseyinova, N.; Imhof, A.; Rothenbacher, D.; Trischler, G.; Kuelb, S.; Scharnagl, H.; Maerz, W.; Brenner, H.; Koenig, W. Association between Lp-PLA2 and coronary artery disease: focus on its relationship with lipoproteins and markers of inflammation and hemostasis. Atherosclerosis 2005, 182, 181–188. [Google Scholar] [CrossRef]
- Rusnak, J.; Fastner, C.; Behnes, M.; Mashayekhi, K.; Borggrefe, M.; Akin, I. Biomarkers in Stable Coronary Artery Disease. Curr Pharm Biotechnol 2017, 18, 456–471. [Google Scholar] [CrossRef] [PubMed]
- Dobiásová, M.; Frohlich, J. [The new atherogenic plasma index reflects the triglyceride and HDL-cholesterol ratio, the lipoprotein particle size and the cholesterol esterification rate: changes during lipanor therapy]. Vnitr Lek 2000, 46, 152–156. [Google Scholar]
- Ulloque-Badaracco, J.R.; Hernandez-Bustamante, E.A.; Alarcon-Braga, E.A.; Mosquera-Rojas, M.D.; Campos-Aspajo, A.; Salazar-Valdivia, F.E.; Valdez-Cornejo, V.A.; Benites-Zapata, V.A.; Herrera-Añazco, P.; Valenzuela-Rodríguez, G.; et al. Atherogenic index of plasma and coronary artery disease: A systematic review. Open Med (Wars) 2022, 17, 1915–1926. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Nicholls, S.J.; Sakuma, I.; Zhao, D.; Koh, K.K. Hypertriglyceridemia and Cardiovascular Diseases: Revisited. Korean Circ J 2016, 46, 135–144. [Google Scholar] [CrossRef]
- Yildiz, G.; Duman, A.; Aydin, H.; Yilmaz, A.; Hür, E.; Mağden, K.; Cetin, G.; Candan, F. Evaluation of association between atherogenic index of plasma and intima-media thickness of the carotid artery for subclinic atherosclerosis in patients on maintenance hemodialysis. Hemodial Int 2013, 17, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.S.; Kim, M.K.; Park, K.; Choi, A.; Kang, S.; Ahn, C.W.; Park, J.S. The Plasma Atherogenic Index is an Independent Predictor of Arterial Stiffness in Healthy Koreans. Angiology 2022, 73, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Won, K.B.; Heo, R.; Park, H.B.; Lee, B.K.; Lin, F.Y.; Hadamitzky, M.; Kim, Y.J.; Sung, J.M.; Conte, E.; Andreini, D.; et al. Atherogenic index of plasma and the risk of rapid progression of coronary atherosclerosis beyond traditional risk factors. Atherosclerosis 2021, 324, 46–51. [Google Scholar] [CrossRef]
- Kim, S.H.; Cho, Y.K.; Kim, Y.-J.; Jung, C.H.; Lee, W.J.; Park, J.-Y.; Huh, J.H.; Kang, J.G.; Lee, S.J.; Ihm, S.-H. Association of the atherogenic index of plasma with cardiovascular risk beyond the traditional risk factors: a nationwide population-based cohort study. Cardiovascular Diabetology 2022, 21, 81. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-W.; Kao, T.-W.; Chang, P.-K.; Chen, W.-L.; Wu, L.-W. Atherogenic index of plasma as predictors for metabolic syndrome, hypertension and diabetes mellitus in Taiwan citizens: a 9-year longitudinal study. Scientific Reports 2021, 11, 9900. [Google Scholar] [CrossRef]
- Dobiásová, M.; Frohlich, J.; Sedová, M.; Cheung, M.C.; Brown, B.G. Cholesterol esterification and atherogenic index of plasma correlate with lipoprotein size and findings on coronary angiography. J Lipid Res 2011, 52, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.R.; Song, S.; Cho, J.A.; Ly, S.Y. Atherogenic Index of Plasma and Its Association with Risk Factors of Coronary Artery Disease and Nutrient Intake in Korean Adult Men: The 2013-2014 KNHANES. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- Lioy, B.; Webb, R.J.; Amirabdollahian, F. The Association between the Atherogenic Index of Plasma and Cardiometabolic Risk Factors: A Review. Healthcare (Basel) 2023, 11. [Google Scholar] [CrossRef]
- Süleymanoğlu, M.; Rencüzoğulları, İ.; Karabağ, Y.; Çağdaş, M.; Yesin, M.; Gümüşdağ, A.; Çap, M.; Gök, M.; Yıldız, İ. The relationship between atherogenic index of plasma and no-reflow in patients with acute ST-segment elevation myocardial infarction who underwent primary percutaneous coronary intervention. Int J Cardiovasc Imaging 2020, 36, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Hartopo, A.B.; Arso, I.A.; Setianto, B.Y. Low Plasma Atherogenic Index Associated with Poor Prognosis in Hospitalized Patients with Acute Myocardial Infarction. Acta Med Indones 2016, 48, 106–113. [Google Scholar] [PubMed]
- Cai, G.; Liu, W.; Lv, S.; Wang, X.; Guo, Y.; Yan, Z.; Du, Y.; Zhou, Y. Gender-specific associations between atherogenic index of plasma and the presence and severity of acute coronary syndrome in very young adults: a hospital-based observational study. Lipids Health Dis 2019, 18, 99. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhou, Y.; Sun, J.; Zhu, Z.; Xing, Z.; Zhou, S.; Wang, Y.; Tai, S. Atherogenic index of plasma is associated with major adverse cardiovascular events in patients with type 2 diabetes mellitus. Cardiovasc Diabetol 2021, 20, 201. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.; Sadeghi, M.; Farsani, E.S.; Danesh, M.; Heshmat-Ghahdarijani, K.; Roohafza, H.; Safaei, A. Atherogenic index of plasma: A valuable novel index to distinguish patients with unstable atherogenic plaques. J Res Med Sci 2022, 27, 45. [Google Scholar] [CrossRef]
- Mangalesh, S.; Yadav, P.; Dudani, S.; Mahesh, N.K. Atherogenic index of plasma predicts coronary artery disease severity and major adverse cardiac events in absence of conventional risk factors. Coron Artery Dis 2022, 33, 523–530. [Google Scholar] [CrossRef]
- Balci, M.M.; Balci, K.G.; Ocak, K.; Ekici, E.; Çetin, E.H.; Selçuk, H.; Selçuk, T.; Maden, O. Predictive Value of Resting Fractional Flow Reserve and Atherogenic Index of Plasma for Evaluation of Physiologically Significant Coronary Artery Lesions. Angiology 2023, 74, 282–287. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, M.; Liu, K.; Gao, A.; Kong, X.; Liu, Y.; Han, H.; Li, H.; Zhu, H.; Zhang, J.; et al. Atherogenic Index of Plasma and the Risk of In-Stent Restenosis in Patients with Acute Coronary Syndrome beyond the Traditional Risk Factors. J Atheroscler Thromb 2022, 29, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
Table 1.
Baseline Characteristics divided by ≥50% coronary artery stenosis.
| Characteristics | < 50% Coronary Stenosis Group (n=155) |
≥ 50% Coronary Stenosis Group (n=190) |
P value |
|---|---|---|---|
| Age, year | 60,6±10,5 | 63,2±10,1 | 0.02 |
| Gender | |||
| Male% | 85 (55,5) | 152 (80) | < 0,0001 |
| Female% | 69 (44,5) | 38(20) | <0,0001 |
| BMI kg/cm2 | 23,1±1,5 | 23,5±1,51 | 0.04 |
| HT (%) | 78 (50,3) | 120 (63,2) | 0.02 |
| DM (%) | 57 (36,8) | 63 (33,2) | 0.48 |
| HL (%) | 35 (22,6) | 51 (26,8) | 0.36 |
| Smoking % | 70 (45,2) | 103(54,2) | 0,09 |
| Family History % | 49(31,6) | 65(34,2) | 0,61 |
| Medication | |||
| ACE inhibitors (%) | 69 (44,5) | 86 (45,3) | 0.9 |
| ARB (%) | 17(11) | 21(11) | 1 |
| Beta Blocker (%) | 133 (70) | 118 (76) | 0.2 |
| Any Dihydropyridine | 42(21,1) | 56(29,5) | 0,60 |
| Statin (%) | 68(43,9) | 71(37,4) | 0,20 |
| Aldosterone inhibitors (%) | 31(20) | 28(14,7) | 0,2 |
| Diuretic (%) * | 79 (51) | 109 (57,4) | 0.23 |
| Metformin | 63(40,6) | 61(32,1) | 0,1 |
| Any SGLT2 inhibitor | 61(39,4) | 69(36,3) | 0,6 |
| Insulin | 48(31) | 56(29,5) | 0,8 |
| Laboratory Result | |||
| FPG mg/dl | 121,3±52,9 | 112±44,37 | 0,07 |
| Hemoglobin g/dl | 13,7±2,1 | 14,1±1,7 | 0.93 |
| Leukocyte | 8,5±2,5 | 8,6±2,4 | 0,65 |
| Neutrophil | 5,25±2,1 | 7,5±19,8 | 0,18 |
| Lymphocyte | 2,4±2 | 2,4±1,5 | 0,9 |
| Platelet | 256±71,5 | 256±66 | 0.9 |
| Creatine (mg/dl) | 0.86±0.35 | 0.94±0.38 | 0.06 |
| GFR | 86,3±19,6 | 83,6±18,9 | 0,2 |
| TC, mg/dl | 180,8±41,3 | 186,8±48,9 | 0,23 |
| TG, mg/dl | 147,0±78,5 | 168,7±100,9 | 0.03 |
| HDL-C, mg/dl | 43,4±11,3 | 40,4±11,4 | 0,01 |
| LDL-C, mg/dl | 114,1±35,9 | 108,8±4,8 | 0,2 |
| AIP | 0,49±0,26 | 0,58±0,27 | 0,002 |
Data are expressed as mean± SD. (%).* Including thiazide and indapamide.; BMI: body mass index HT: hypertension; DM: Diabetes Mellitus; HL: Hyperlipidemia; ACE: angiotensin converting enzyme; ARB=Aldosterone receptor antagonist; ACE: angiotensin converting enzyme; ARB=Aldosterone receptor antagonist; SGLT2 inhibitor: Sodium glucose cotransporter type 2 inhibitor FPG: Fasting plasma glucose; ALT: alanine amino transferase; AST: aspartate amino transferase; TC: total cholesterol; TG: Triglyceride; HDL-C: high-density cholesterol level; LDL-C: low-density cholesterol level. p=probability.
Table 2.
Univariable analysis of CAD risk parameters and AIP associated with coronary artery stenosis score (n= 190).
Table 2.
Univariable analysis of CAD risk parameters and AIP associated with coronary artery stenosis score (n= 190).
| Variable | Univariable Analysis | ||
|---|---|---|---|
| OR | (95%CI) | P-value | |
| Age | 1.02 | (1.0-1.04) | 0.04 |
| Gender % | 3.20 | (1.99-5.16) | <0.0001 |
| BMI kg/cm2 | 1.15 | (1.0- 1.33) | 0.04 |
| HT% | 1.69 | (1.09-2.6) | 0.02 |
| TG mg/dl (1%) * | 1.31 | (1.02-1.7) | 0.03 |
| HDL-C mg/dl | 0.98 | (0.96-1.0) | 0.02 |
| AIP | 3.74 | (1.62-8.64) | 0.002 |
* 1% increased of TG OR= odds ratio, CI= confidence interval, other abbreviations as in Table 1.
Table 3.
Multivariable analysis of AIP in patients with coronary artery stenosis.
| Variables | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95%CI) | p-value | OR (95%CI) | p-value | OR (95%CI) | p-value | |
| Age | 1,0 (1,01-1,06) 0,001 | 1,04 (1,02-1,06) 0,001 | 1,03 (1,01-1,06) 0,006 | |||
| Gender % | 3,53 (2,1-5,9) <0,000 | 3,48 (2,09-5,81) <0,000 | 3,6 (2,14-5,98) <0,000 | |||
| BMI kg/cm2 | 1,16 (0,99-1,35) 0,06 | 1,15 (0,99-1,35) 0,06 | ||||
| HT (%) | 1,50 (0,93-2,44) 0,1 | |||||
| AIP | 3,39 (1,41-8,13) 0,003 | 3,34 (1,37-8,13) 0,008 | 3,07 (1,25-7,54) 0,01 | |||
Model 1: Adjusted for Age, gender; Model2: Adjusted for age, gender BMI; Model 3: adjusted for age, gender, BMI, and HT Multicollinearity r> 0,7 (TG-C and HDL-C ).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated





